Abstract
Alcohol consumption likely induces gastric carcinogenesis through deregulation of RNA polymerase (Pol) III genes and oxidative damage. Transcription factor IIB-related factor 1 (BRF1) overexpression alleviates RNA Pol III transcription inhibition through breast cancer susceptibility gene 1 (BRCA1). Myeloperoxidase (MPO) involvement in cancer is induced by alcohol-mediated oxidative damage. BRCA1/2 and MPO play key roles in DNA repair. BRCA1 and BRCA2 exert different roles in homologous recombination repair. By using human gastric cancer (GC) biopsies, we investigated the prognostic value of these proteins upon alcohol induction. In total, high expression of BRF1 (P = 0.010) and positive cell infiltration of MPO (P = 0.004) in tumor tissues as well as positive expression of BRCA1 (P < 0.001) in para-tumor tissues were more frequent in GC patients with hazardous or harmful alcohol consumption habits. BRF1 (P = 0.021), BRCA2 (P < 0.001), and MPO (P = 0.039) were independent prognostic factors for disease-free survival. BRCA1 (P = 0.005) and BRCA2 (P < 0.001) also were identified as independent prognostic factors for overall survival. Furthermore, BRCA2 was an independent unfavorable prognostic factor for disease-free survival and overall survival (P < 0.001) in GC patients who underwent platinum-based adjuvant chemotherapy. BRF1, BRCA1/2, and MPO are DNA repair–related biomarkers, induced by alcohol with prognostic value in GC patients.
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-related death worldwide.1 Specifically, GC carries high morbidity and mortality in China. It is significant to note that upper gastrointestinal malignancies, such as GC, are related to alcohol consumption. A strong effect of alcohol consumption on GC risk was observed through a meta-analysis that indicated that the risk of GC was increased by 5% per 10-g/day increment of alcohol consumption.2 Alcohol consumption is thought to induce carcinogenesis through various mechanisms, such as enhanced RNA polymerase III (Pol III) transcription3, 4, 5, 6, 7 and oxidative damage.8, 9, 10, 11
RNA Pol III–dependent genes (Pol III genes) are involved in mRNA processing (5S rRNA and U6 snRNA) and protein translation (tRNA), which play crucial roles in tumorigenesis.3 Proper initiation by RNA Pol III requires transcription factor III B (TFIIIB).4 The TFIIIB complex is made up of the TATA box binding protein, TFIIB-related factor 1 (BRF1), TFIIB-related factor 2 (BRF2), and B double prime 1. Among these proteins, BRF1 is a key transcription factor that specifically modulates Pol III gene transcription.5 Up-regulation of BRF1 expression leads to an increase in the translational capacity to promote cell transformation and tumor formation in hepatocellular carcinoma (HCC) and breast cancer cell lines.3, 6 In addition, a recent study showed that BRF1 expression is increased in HCC patients, which is correlated with poorer survival. Moreover, BRF1 expression in HCC patients who partake in alcohol consumption is higher than in non-HCC patients.7 However, it remains to be determined whether BRF1 is overexpressed in GC patients. Furthermore, RNA Pol III transcription, through TFIIIB, is subject to repression by tumor suppressor proteins such as breast cancer susceptibility gene 1 (BRCA1).12 BRCA1 is a well-known gene implicated in breast and ovarian cancer predisposition. This protein contains an acidic carboxyl-terminal domain comprising two BRCA1 C-terminal regions, which interact with BRF1.12 Furthermore, BRF1 overexpression was shown to alleviate the inhibition of U6 snRNA and tRNA transcription through BRCA1 in HeLa and HCC1937 cell lines,12 and BRCA1 expression in the MCF-7 cell line decreases the induction of Pol III genes due to alcohol consumption.13 However, it remains unclear whether the correlation between BRCA1 expression and GC is mediated by alcohol consumption. In addition, it remains to be determined whether BRCA2, another important member of the BRCA family, is involved in alcohol-mediated transcription by Pol III.
Oxidative damage induced by alcohol plays a key tumor-promoting role in malignancies, and it has been shown that neutrophil infiltration plays a critical role in the development of oxidative damage. The accumulation of neutrophils in gastric tissues can be indicated by the measurement of myeloperoxidase (MPO).14 MPO is a cationic heme-containing enzyme found in the azurophilic granules of neutrophils that catalyzes the production of hypochlorous in the presence of hydrogen peroxide. In mouse models, alcohol intake generated gastric inflammation and tissue damage, and gastric tissue MPO activity was enhanced significantly.8, 9 Thus, MPO might be an important determinant of inflammation and diet in colon cancer risk via its effect on oxidants.10 At the same time, MPO catalytic activity is critical during the early stages of tumor development in a lung cancer model.11 Therefore, it has been shown that MPO is involved in cancer caused by alcohol-mediated inflammation. However, whether alcohol consumption mediates MPO expression in GC remains unclear.
It is worth noting that BRCA1/2 and MPO also play key roles in DNA repair. The BRCA1/2 proteins are necessary for efficient homologous recombination DNA repair,15 and neutrophils catalyze the formation of hypochlorous via MPO, both of which affect the nucleotide excision repair pathway.16 Moreover, the benefits of platinum combined with 5-fluorouracil chemotherapy have been confirmed in GC. Platinum generates platinum-DNA adducts that block transcription, leading to cytotoxicity and cell death, and the tumor cell amplification response to DNA repair plays an important role in the chemotherapeutic efficacy of platinum. Recent studies have reported that patients with decreased BRCA1 protein expression may benefit from platinum-based adjuvant chemotherapy in GC.17, 18 However, it remains uncertain whether BRCA1 expression may be a prognostic factor in GC. Moreover, the correlation between BRCA2/MPO protein expression and the survival of patients who have received platinum-based adjuvant chemotherapy in GC remains unknown.
Therefore, this clinical study sought to determine whether BRF1, BRCA1/2, and MPO may serve as biomarkers in GC patients. Specifically, our aims were as follows: i) to assess the correlation between protein expression and clinicopathologic features, especially alcohol consumption; ii) to compare protein expression between GC tumor and para-tumor tissues; iii) to examine the correlation and co-localization between related protein expression; iv) to evaluate the correlation between protein expression and prognosis; and v) to show the correlation between protein expression and the survival of patients with GC who have received platinum-based adjuvant chemotherapy.
Materials and Methods
Patients and Treatment
The ethics committee at the First Affiliated Hospital of Anhui Medical University approved this study. In total, 77 tumor and 69 para-tumor tissues (at a distance of at least 2 cm from the tumor edge) were isolated from 77 primary gastric adenocarcinoma resections performed at the First Affiliated Hospital of Anhui Medical University from May 2009 to September 2015. Each subject signed an informed consent form before entry into this study. All samples were obtained before radiotherapy or chemotherapy. Cancer TNM stage was defined based on the American Joint Committee on Cancer Staging Manual.19 Clinicopathologic data, including age (> or < age 59 years, using 59 years as the median age), sex, surgery (radical and palliative), clinical stage (II and III + IV), pathology tumor stage (1 + 2 and 3 + 4), tumor location (proximal and distal), lymph node metastasis, differentiation (poor, moderate, and well), and survival data, were evaluated by reviewing the patients' medical records. Furthermore, the Alcohol Use Disorders Identification Test [https://www.drugabuse.gov/sites/default/files/files/AUDIT.pdf, last accessed December 27, 2017 (authors used Chinese version)] was a screening questionnaire developed by the World Health Organization to identify alcohol consumption. The 10-item questionnaire was scored on a scale from zero to four points per item, with a maximum score of 40. Patients were divided into two groups (Alcohol Use Disorders Identification Test cut-off scores were < 8 and ≥8). An Alcohol Use Disorders Identification Test score between 0 and 7 was defined as abstinence or low-risk drinking, and scores ≥8 indicated a tendency to hazardous or harmful drinking.20, 21
Adjuvant chemotherapy was administered at the discretion of the physician and agreement of the patients and consisted of six to eight courses administered as follows: oxaliplatin [100 mg/m2 day (D)]/cisplatin (20 mg/m2 D1 to D5), and capecitabine (1000 mg/m2 twice daily D1 to D14)/tegafur gimeracil oteracil potassium capsule (40 mg/m2 twice daily D1 to D14) every 3 weeks. In this study, 57 of the patients who underwent radical surgery received platinum-based adjuvant chemotherapy.
Analyses for overall survival (OS) and disease-free survival (DFS) also were performed. OS was defined as the time period from the study initiation until the date of mortality, regardless of the cause, or until the most recently documented follow-up evaluation. DFS was calculated from the time of diagnosis to disease recurrence or the last follow-up evaluation. In this study, the follow-up period ended in May 2017.
Immunohistochemistry
BRF1 immunohistochemistry was performed using the primary BRF1 antibody provided by BETHYL Laboratories, Inc (Montgomery, TX) (1:500 dilution). BRCA1/2 antibodies were obtained from ZSGB Biotech Co., Ltd. (Beijing, China). The antibody against MPO was obtained from Biocare Medical (Martinez, CA).
Formalin-fixed, paraffin-embedded GC and para-cancer tissue microarrays were cut into 4-μm–thick sequential sections and baked at 65°C for 30 minutes. Section deparaffinization was performed twice with xylene at different alcohol concentrations before rehydration. The sections then were boiled in citrate/EDTA antigen retrieval solution for 20 minutes in a pressure cooker for antigen retrieval followed by immersion in 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase activity at room temperature. Next, the sections were incubated with the BRF1/BRCA1/BRCA2/MPO antibodies overnight at 4°C, followed by incubation with the secondary antibody (Elivision super HRP IHC Kit; Maixin, Fujian, China) for 30 minutes at 37°C. After the sections were incubated with diaminobenzidine (Maixin) for 3 to 5 minutes for protein detection, the sections were counterstained with hematoxylin to stain the nucleus, and finally were dehydrated and mounted.
The immunohistochemistry results were scored independently by two pathologists. A histochemical score (H-score) was used to estimate protein expression. Tumor cell percentages were scored as 0% to 100% positive tumor cells, and positive expression was defined by positive staining in 5% or more of tumor cells. To evaluate the intensity, we used a score index of 0, 1, 2, or 3 that corresponded to no staining, weak staining, moderate staining, and strong staining, respectively. Finally, the H-score was calculated as the sum of the intensity value multiplied by the percentage of positive tumor cells, with values ranging from 0% to 300%.22 An optimal cut-off value for high and low expression was determined by the median H-score for protein-positive expression.
Immunofluorescence
The BRF1/BRCA1 pair was investigated in paraffin-embedded, 3-μm–thick sections. The sections were blocked for 30 minutes in 10% goat serum at room temperature. After an overnight incubation at 4°C with the primary antibody, the slides were incubated with the secondary antibodies for 1 hour at room temperature [goat anti-rabbit IgG heavy chain and light chain (Alexa Fluor 488; green) and goat anti-mouse IgG heavy chain and light chain (Alexa Fluor 647; red); Abcam, Cambridge, UK]. DAPI was used to stain the cell nuclei (blue) (ZSGB Biotech Co., Ltd.). Finally, the slides were analyzed with a confocal laser scanning microscope (TCS-SP5; Leica, Wetzlar, Germany).
Statistical Analysis
Statistical analysis was performed using SPSS version 18.0 (SPSS, Chicago, IL) and GraphPad Prism software version 6.0 (GraphPad, La Jolla, CA). The χ2 test was used to evaluate the associations between BRF1/BRCA1/BRCA2/MPO expression and the existing clinicopathologic factors, specifically alcohol consumption. The associations between tumor and para-tumor tissue protein expression were tested using U-tests. The correlation between the related protein H-scores was assessed by the Pearson correlation coefficient. The Kaplan-Meier method was used to estimate the survival distributions, and the log-rank test was used to compare the survival distributions. Multivariate analysis was performed by Cox proportional hazards regression modeling. P < 0.05 was considered statistically significant.
Results
Characteristics and Clinical Outcomes of Patients
The characteristics of the 77 patients are presented in Table 1. In total, 69 para-tumor tissues were obtained from the 77 patients, and 66 patients received radical surgery. Among them, disease recurrence occurred in 62 (93.9%) patients. Fifty-seven cases received platinum-based adjuvant chemotherapy. OS was recorded for 69 (89.6%) patients. The time range for follow-up evaluation in the 77 patients was 1 to 90 months, with a median follow-up time of 18 months. The median OS and DFS were 20 and 14 months, respectively.
Table 1.
Clinicopathologic Characteristics of 77 GC Patients
| Characteristics | 77 cases of tumor tissues |
69 cases of para-tumor tissues |
||
|---|---|---|---|---|
| Cases, n | % | Cases, n | % | |
| Age, years | ||||
| ≥59 | 43 | 55.8 | 39 | 56.5 |
| <59 | 34 | 44.2 | 30 | 43.5 |
| Sex | ||||
| Male | 49 | 63.6 | 43 | 62.3 |
| Female | 28 | 36.4 | 26 | 37.7 |
| Stage | ||||
| II | 20 | 30.0 | 19 | 27.5 |
| III + IV | 57 | 70.0 | 50 | 72.5 |
| Pathology tumor stage | ||||
| T1 + T2 | 11 | 14.3 | 11 | 15.9 |
| T3 + T4 | 66 | 85.7 | 58 | 84.1 |
| Tumor location | ||||
| Distal | 42 | 54.5 | 38 | 55.1 |
| Proximal | 35 | 45.5 | 31 | 44.9 |
| Lymph node metastasis | ||||
| Yes | 63 | 81.8 | 56 | 81.2 |
| No | 14 | 18.2 | 13 | 18.8 |
| Differentiation | ||||
| Poor | 51 | 66.2 | 45 | 65.2 |
| Moderate and well | 26 | 33.8 | 24 | 34.8 |
| Alcohol consumption | ||||
| Hazardous or harmful | 25 | 32.5 | 23 | 33.3 |
| Abstinence or low risk | 52 | 67.5 | 46 | 66.7 |
Expression of Each Biomarker and Its Correlation with Clinicopathologic Parameters
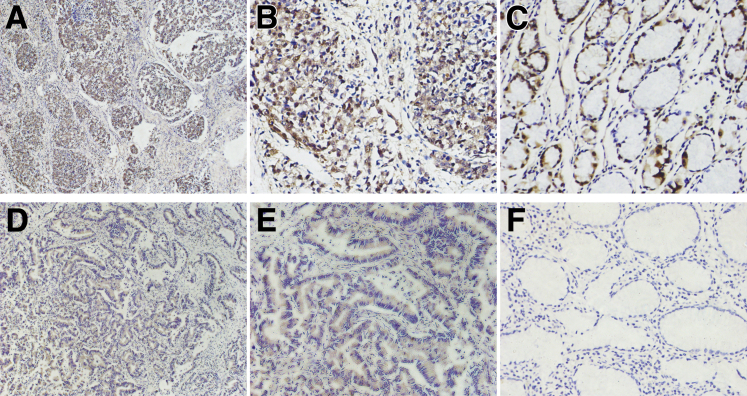
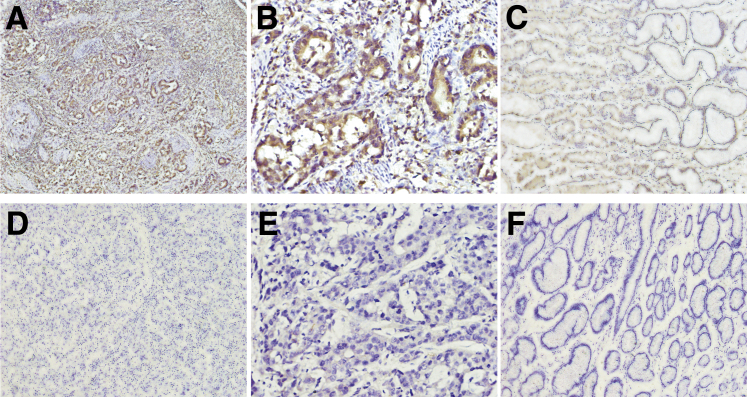
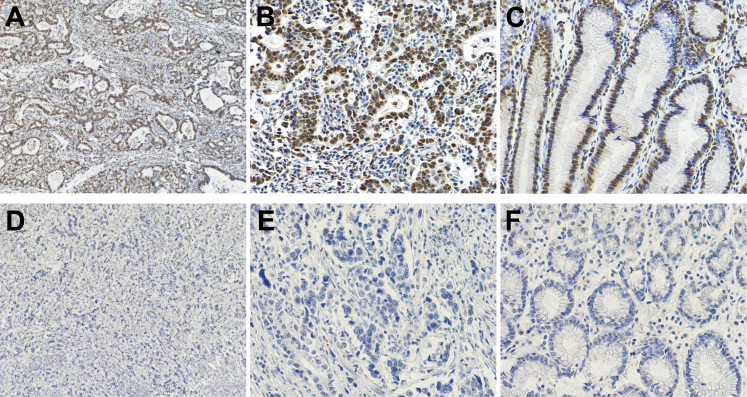
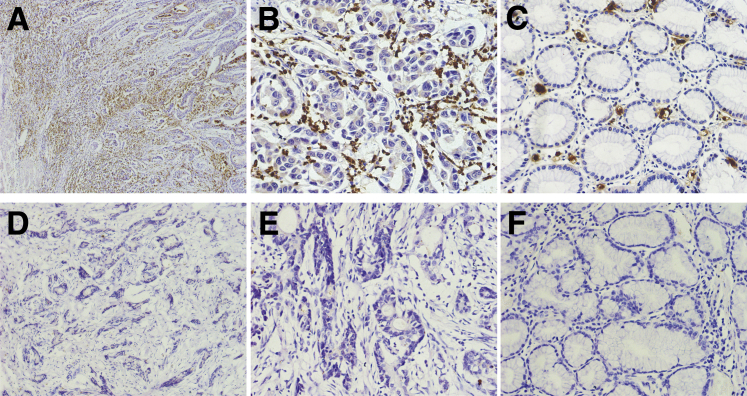
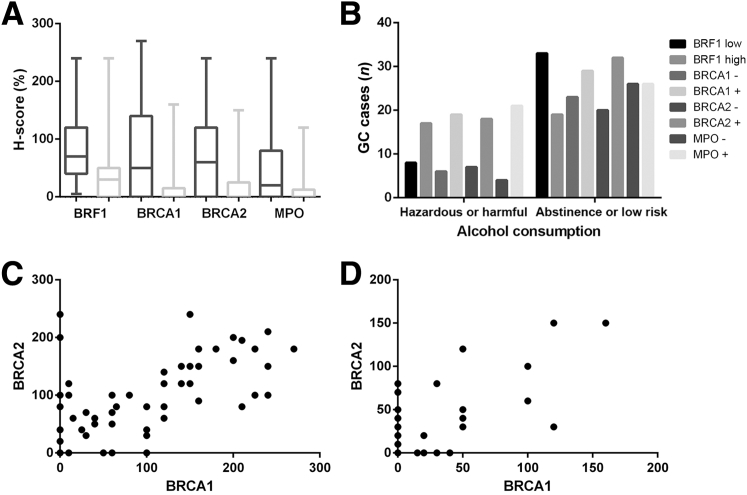
In GC tissues, BRF1 (Figure 1, A, B, D and E) and BRCA2 (Figure 2, A and B) proteins were located in the tumor cell nucleus and cytoplasm. BRCA1 (Figure 3, A and B) protein staining was detected in the tumor cell nucleus. Negative BRCA1 and BRCA2 expressions are shown in Figure 3, D and E, and Figure 2, D and E, respectively. However, MPO protein was not located in either the tumor cell nucleus or the cytoplasm. Intriguingly, MPO-positive inflammatory cell infiltration was discovered in the GC tissues (Figure 4, A and B), and absence of MPO-positive inflammatory cell infiltration is shown in Figure 4, D and E. BRF1 staining was observed in all 77 GC tissues, and the positive BRCA1 and BRCA2 expression rates were 62.3% (48 of 77) and 64.9% (50 of 77). MPO-positive inflammatory cell infiltration was discovered in 61.0% (47 of 77) of GC tissues. Furthermore, as optimal cut-off values for high and low expressions, the median positive H-scores for BRF1, BRCA1, and BRCA2 were 70% (5% to 240%), 120% (10% to 270%), and 100% (20% to 240%), respectively. High BRF1 and BRCA1/2 expressions were observed in 46.8% (36 of 77), 26.0% (20 of 77), and 28.6% (22 of 77) of GC tissues, respectively.
Figure 1.
Immunohistochemical staining of BRF1 in gastric cancer (GC). A and B: High transcription factor II B–related factor 1 (BRF1) expression in GC tissue. C: Positive BRF1 expression in GC para-tumor tissue. D and E: Low BRF1 expression in GC tissue. F: Negative BRF1 expression in GC para-tumor tissue. Original magnification: ×100 (A and D); ×400 (B, C, E, and F).
Figure 2.
Immunohistochemical staining of BRCA2 in gastric cancer (GC). A and B: Positive BRCA2 expression in GC tissue. C: Positive BRCA2 expression in GC para-tumor tissue. D and E: Negative BRCA2 expression in GC tissue. F: Negative BRCA2 expression in GC para-tumor tissue. Original magnification: ×100 (A and D); ×200 (C and F); ×400 (B and E).
Figure 3.
Immunohistochemical staining of BRCA1 in gastric cancer (GC). A and B: Positive BRCA1 expression in GC tissue. C: Positive BRCA1 expression in GC para-tumor tissue. D and E: Negative BRCA1 expression in GC tissue. F: Negative BRCA1 expression in GC para-tumor tissue. Original magnification: ×100 (A and D); ×400 (B, C, E, and F).
Figure 4.
Immunohistochemical staining of myeloperoxidase (MPO) in gastric cancer (GC). A–C: MPO-positive inflammatory cell infiltration is discovered in GC (A and B) tissues and para-tumor tissue (C). D and E: Absence of MPO-positive inflammatory cell infiltration in GC tissue. F: Absence of MPO-positive inflammatory cell infiltration in GC para-tumor tissue. Original magnification: ×100 (A and D); ×400 (B, C, E, and F).
The clinicopathologic characteristics of the 77 GC patients are described according to BRF1/BRCA1/BRCA2/MPO expression. BRF1 expression was associated significantly with tumor stage (P = 0.023) and lymph node metastasis (P = 0.005); BRCA2 expression also was associated significantly with lymph node metastasis (P = 0.004). There were no relationships between BRCA1/MPO and clinicopathologic factors including age, sex, clinical stage, T stage, lymph node metastasis, tumor location, and degree of differentiation. Moreover, high BRF1 (P = 0.010) expression and MPO-positive (P = 0.004) cell infiltration were more frequent in GC patients with hazardous or harmful alcohol consumption habits (Table 2).
Table 2.
Alcohol Consumption of GC Patients according to BRF1/BRCA1/BRCA2 Expression and MPO + Cell Infiltration
| GC patients | Alcohol consumption | BRF1 expression, n |
χ2 (P) | BRCA1 expression, n |
χ2 (P) | |||
|---|---|---|---|---|---|---|---|---|
| Low: 41 | High: 36 | Negative: 29 | Positive: 48 |
|||||
| Low: 28 | High: 20 | |||||||
| 77 tumor tissues | Hazardous or harmful | 8 | 17 | 6.713 (0.010) | 6 | 11 | 8 | 2.946 (0.229) |
| Abstinence or low risk | 33 | 19 | 23 | 17 | 12 | |||
| Negative: 18 | Positive: 51 | Negative: 51 | Positive: 18 | |||||
| 69 para-tumor tissues | Hazardous or harmful | 5 | 18 | 0.338 (0.561) | 5 | 18 | 48.706 (<0.001) | |
| Abstinence or low risk | 13 | 33 | 46 | 0 | ||||
| Alcohol consumption | BRCA2 expression, n |
MPO+ cell infiltration, n |
||||||
|---|---|---|---|---|---|---|---|---|
| Negative: 27 | Positive 50 |
Absence: 30 | Presence: 47 | |||||
| Low: 28 | High: 22 | |||||||
| 77 tumor tissues | Hazardous or harmful | 7 | 8 | 10 | 2.413 (0.299) | 4 | 21 | 8.207 (0.004) |
| Abstinence or low risk | 20 | 20 | 12 | 26 | 26 | |||
| Negative: 48 | Positive: 21 | Absence: 51 | Presence: 18 | |||||
| 69 para-tumor tissues | Hazardous or harmful | 15 | 8 | 0.308 (0.579) | 16 | 7 | 0.338 (0.561) | |
| Abstinence or low risk | 33 | 13 | 35 | 11 | ||||
In para-tumor tissues, positive BRF1 (Figure 1C), BRCA1 (Figure 3C), and BRCA2 (Figure 2C) expression rates of 73.9% (51 of 69), 26.1% (18 of 69), and 30.4% (21 of 69), respectively, were observed. On the other hand, negative BRF1, BRCA1 and BRCA2 expressions showed in Figure 1F, Figure 2F, and Figure 3F, respectively. The MPO-positive (Figure 4C) cell infiltration rate was 26.1% (18 of 69), and absence of MPO-positive inflammatory cell infiltration was in Figure 4F. There were no relationships between BRF1/BRCA1/BRCA2/MPO in para-tumor tissues from GC patients and clinicopathologic factors including age, sex, clinical stage, T stage, lymph node metastasis, tumor location, and degree of differentiation. In particular, positive BRCA1 expression in para-tumor tissues was more frequent in GC patients with hazardous or harmful alcohol consumption habits (P < 0.001) (Table 2 and Figure 5).
Figure 5.
A: Histochemical scores (H-scores) for the four biomarkers in gastric cancer (GC) tissues (dark gray) are higher than those in GC para-tumor tissues (light gray) (P < 0.001). B: High transcription factor II B–related factor 1 (BRF1) (P = 0.010) expression and myeloperoxidase (MPO)-positive (P = 0.004) cell infiltration are more frequent in GC patients with hazardous or harmful alcohol consumption habits. C and D: There is a statistically significant correlation between the H-scores for BRCA1/2 in tumor tissues (r = 0.696, P < 0.001; C) and para-tumor tissues (r = 0.715, P < 0.001; D) in GC patients.
Expression of Biomarkers between Tumor and Para-Tumor Tissues
The H-scores for BRF1 (Z = −5.162; P < 0.001), BRCA1 (Z = −5.120; P < 0.001), and BRCA2 (Z = −4.164; P < 0.001) in GC patient tumor tissues were significantly higher than those in para-tumor tissues. MPO-positive cell infiltration degrees (Z = −4.083; P < 0.001) in GC patient tumor tissues were significantly higher than those in para-tumor tissues (Figure 5).
Association of Nuclear BRF1 and BRCA1 in GC Tissues
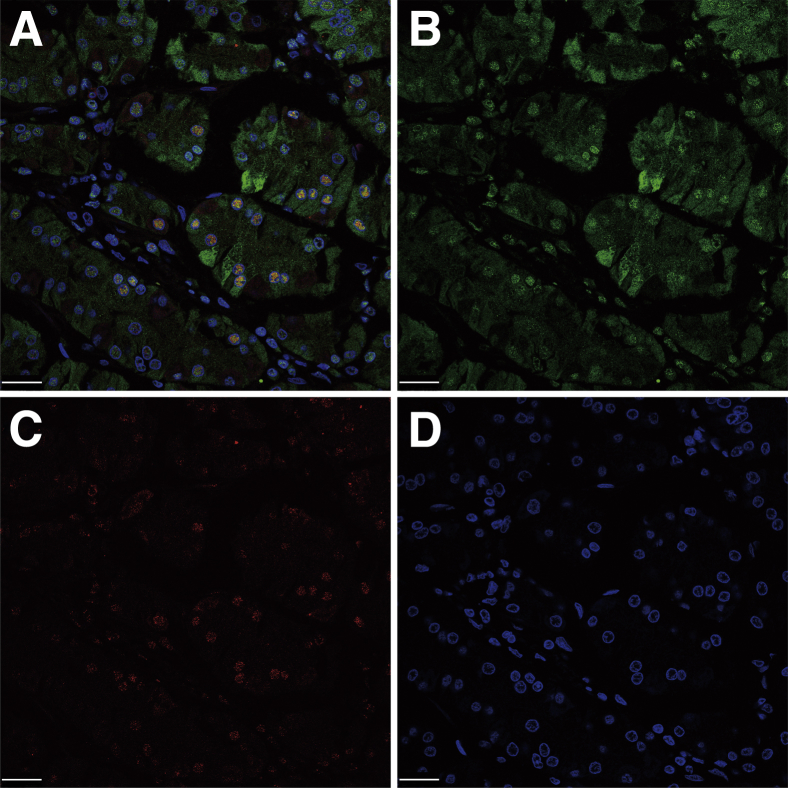
In GC tissues, BRF1 protein was located in the tumor cell nucleus and cytoplasm, whereas BRCA1 protein showed staining in the tumor cell nucleus. Co-localization of BRF1 and BRCA1 in the nucleus was confirmed by immunofluorescence (Figure 6).
Figure 6.
A: Co-localization of BRF1 and BRCA1 in tumor cell nuclei in gastric cancer (GC). B: Transcription factor II B–related factor 1 (BRF1) is located in the tumor cell nucleus and cytoplasm. C: BRCA1 was stained in the tumor cell nucleus. D: DAPI. Scale bars: 25 μm.
The Correlation between BRCA1 and BRCA2 Expression
The correlation between BRCA1 and BRCA2 expression was determined using Pearson coefficient analysis. The correlation was statistically significant regarding the expression of BRCA1/2 in GC patient tumor tissues (r = 0.696; P < 0.001). There was also a statistically significant correlation regarding BRCA1/2 expression in GC patient para-tumor tissues (r = 0.715; P < 0.001) (Table 3 and Figure 5).
Table 3.
Expression of BRCA1 and BRCA2 in Tumor Tissues and Para-Tumor Tissues from GC Patients
| GC tissues | BRCA2 expression |
Total, n | ||
|---|---|---|---|---|
| High | Low | Negative | ||
| BRCA1 expression | ||||
| High | 16 | 4 | 0 | 20 |
| Low | 4 | 19 | 5 | 28 |
| Negative | 2 | 5 | 22 | 29 |
| Total, n |
22 |
28 |
27 |
77 |
| Para-GC tissues | BRCA1 expression | Total, n | ||
| Positive |
Negative |
|||
| BRCA2 expression | ||||
| Positive | 11 | 10 | 21 | |
| Negative | 7 | 41 | 48 | |
| Total, n | 18 | 51 | 69 | |
H-Scores for Proteins and Prognoses
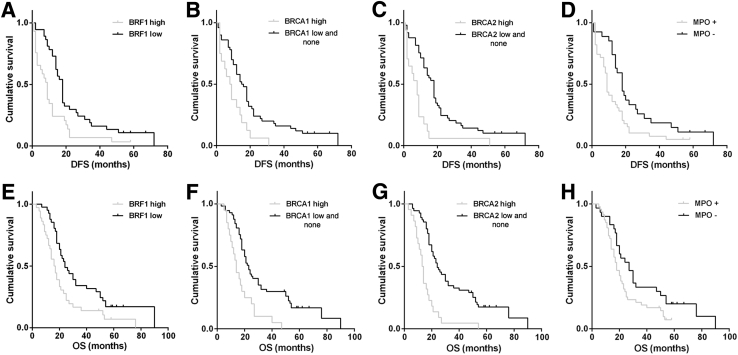
Patients with low BRF1 expression displayed better DFS (18 versus 9 months; P = 0.003) compared with patients with high BRF1 expression. The DFS of patients with high BRCA1/2 expression was significantly shorter than patients with low and negative BRCA1/2 expression (BRCA1: 8 versus 15 months, P = 0.005; and BRCA2: 8 versus 18 months, P < 0.001). In addition, the presence of MPO-positive cell infiltration was related significantly to poor DFS compared with the absence of MPO-positive cell infiltration (9 versus 18 months; P = 0.013). Patients with low BRF1 expression displayed better OS (24 versus 16 months; P = 0.006) compared with patients with high BRF1 expression. Patients with high BRCA1/2 expression showed worse OS than patients with low and no BRCA1/2 expression (BRCA1: 14 versus 23 months, P < 0.001; and BRCA2: 13 versus 24 months, P < 0.001). MPO-positive cell infiltration was associated significantly with poor OS (18 versus 27 months; P = 0.034) (Figure 7).
Figure 7.
Kaplan-Meier survival analysis for transcription factor II B–related factor 1 (BRF1) (A and E), BRCA1 (B and F), and BRCA2 expression (C and G), and myeloperoxidase (MPO)-positive cell infiltration (D and H). Patients with high BRF1 (P = 0.003; A)/BRCA1 (P = 0.005; B)/BRCA2 (P < 0.001; C) expression and MPO-positive cell infiltration (P = 0.013; D) show a shorter disease-free survival (DFS), and patients with high BRF1 (P = 0.006; E)/BRCA1 (P < 0.001; F)/BRCA2 (P < 0.001; G) expression and MPO-positive cell infiltration (P = 0.034; H) show a worse overall survival (OS).
Univariate Cox proportional hazard regression analysis showed that BRF1 expression (P = 0.021), BRCA2 expression (P < 0.001), and MPO-positive inflammatory cell infiltration (P = 0.039) were independent prognostic factors in GC patients and were associated significantly with DFS. BRCA1 (P = 0.005) and BRCA2 (P < 0.001) expression levels also were independent prognostic factors for OS. In contrast, the prognostic value of BRCA1 was not significant for DFS. In addition, BRF1 and MPO were not independent prognostic factors for OS (Table 4).
Table 4.
Multivariate Analysis of Factors Related to DFS and OS in GC Patients
| GC patients |
77 cases |
57 cases who received platinum-based adjuvant chemotherapy |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | DFS |
OS |
DFS |
OS |
||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| BRF1 expression high versus low | 1.997 (1.109–3.598) | 0.021 | 1.741 (0.968–3.133) | 0.064 | – | – | – | – |
| BRCA1 expression high versus low + negative | 1.921 (0.971–3.799) | 0.061 | 2.409 (1.299–4.467) | 0.005 | 2.063 (0.963–4.423) | 0.063 | 2.237 (0.998–5.017) | 0.051 |
| BRCA2 expression high versus low + negative | 3.450 (1.731–6.877) | <0.001 | 3.269 (1.730–6.177) | <0.001 | 4.446 (2.047–9.657) | <0.001 | 6.090 (2.601–14.255) | <0.001 |
| MPO + cell infiltration presence versus absence | 2.028 (1.037–3.964) | 0.039 | 1.153 (0.612–2.174) | 0.659 | 2.009 (1.000–4.035) | 0.050 | 1.432 (0.705–2.908) | 0.320 |
–, no correlation; HR, homologous recombination.
The Benefit of Platinum-Based Adjuvant Chemotherapy according to Related Biomarkers
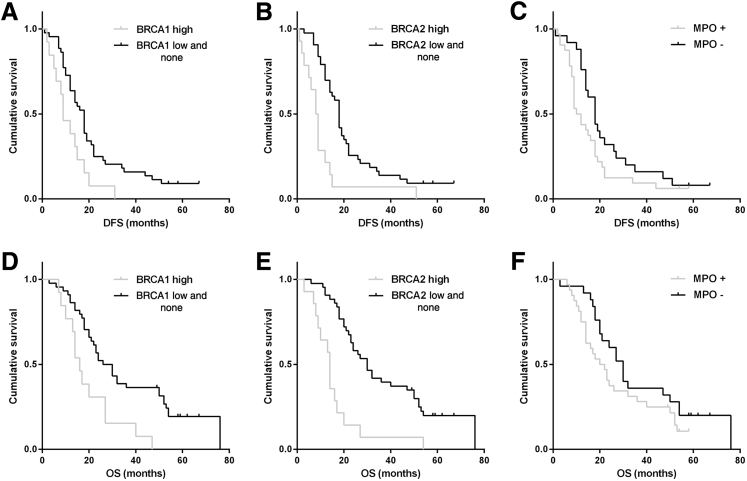
Patients who had received platinum-based adjuvant chemotherapy and who showed negative or low BRCA1/2 expression had significantly longer DFS (BRCA1: 18 versus 9 months, P = 0.010; and BRCA2: 18 versus 8 months, P < 0.001) and OS (BRCA1: 26 versus 16 months, P = 0.003; and BRCA2: 30 versus 14 months, P < 0.001) compared with patients with high BRCA1/2 expression. The benefits of platinum-based adjuvant chemotherapy for DFS and OS were enhanced in BRCA1/2-negative and BRCA1/2-low-expression tumors, respectively. Moreover, patients who received platinum-based adjuvant chemotherapy and had no MPO-positive inflammatory cell infiltration showed longer DFS (18 versus 10 months; P = 0.080) and OS (30 versus 20 months; P = 0.137) compared with patients with MPO-positive inflammatory cell infiltration in GC tissues. Unfortunately, this trend did not translate to a significant improvement in survival (Figure 8).
Figure 8.
Kaplan-Meier survival analysis for BRCA1 (A and D) and BRCA2 expression (B and E), and myeloperoxidase (MPO)-positive cell infiltration (C and F). Patients who received platinum-based adjuvant chemotherapy and showed negative or low BRCA1/2 expression showed significantly longer disease-free survival (DFS) (BRCA1: P = 0.010, A; BRCA2: P < 0.001, B) and overall survival (OS) (BRCA1: P = 0.003, D; BRCA2: P < 0.001, E).
In the multivariate analysis, BRCA2 was an independent prognostic factor for DFS (P < 0.001) and OS (P < 0.001) in GC patients who received platinum-based adjuvant chemotherapy. However, the prognostic value of BRCA1/MPO for DFS and OS did not remain significant (Table 4).
Discussion
Alcohol has been classified as carcinogenic to humans,23 and the target sites for alcohol consumption–related carcinogenesis in humans include the stomach, liver, breast, and many other organs. RNA Pol III transcribes genes that play a crucial role in alcohol-mediated tumorigenesis.3 Normally, RNA Pol III genes are transcribed in the cell nucleus. Tumor cells show consistent nucleolar hypertrophy,24 which provides a common mechanism for alcohol-mediated cancers in humans through the regulation of BRF1, BRCA, and Pol III genes. In this study, BRF1 and BRCA1 were located in the nucleus/cytoplasm and the nucleus of tumor cells, respectively. However, it remains unknown whether BRCA1 and BRF1 are co-expressed in GC tumor cells. Because overexpression of BRF1 alleviates the inhibition of RNA Pol III transcription through BRCA1 in HeLa cells,12 BRF1/BRCA1 may inter-relate in GC cells. Therefore, co-expression of BRF1/BRCA1 was determined in GC using an immunofluorescence technique. BRF1 and BRCA1 were found to co-localize in the tumor cell nucleus. This finding further shows that BRF1/BRCA1 may interact in the alcohol-mediated transcription of RNA Pol III.3, 12, 24 The H-scores for BRF1, BRCA1, BRCA2, and MPO in tumor tissues from GC patients were significantly higher than those in para-tumor tissues, suggesting that these proteins play important roles in tumorigenesis and the development of GC.
A previous study indicated that the estrogen receptor might play a critical role in the alcohol-induced deregulation of Pol III genes through BRF1 in estrogen receptor–positive breast cancer development.25 In this way, tamoxifen may inhibit alcohol-induced BRF1 expression through the estrogen receptor to down-regulate Pol III gene transcription.6 Moreover, a study on HCC showed that the induction of BRF1 by alcohol in hepatoma cells was higher than that in nontumor cells and that alcohol consumption enhanced BRF1 expression to promote cell transformation.7 Together, these data show a correlation between BRF1 and alcohol consumption in breast cancer and HCC. High BRF1 expression was more frequent in GC patients with hazardous or harmful alcohol consumption habits. Consequently, it also was confirmed that alcohol mediates BRF1 expression in GC as well as in breast cancer and HCC. Furthermore, MPO-positive inflammatory cell infiltration was more frequent in GC patients with hazardous or harmful alcohol consumption habits, which is consistent with the results of previous studies in mice. In these studies, alcohol generated gastric inflammation and tissue damage in mice, whereas MPO activity was enhanced significantly in gastric tissue.8, 9 Intriguingly, this study discovered that positive BRCA1 expression in para-tumor tissues was more frequent in GC patients with hazardous or harmful alcohol consumption habits. According to this result, RNA Pol III gene transcription might be enhanced in para-tumor tissues as a result of alcohol-induced carcinogenesis. Up-regulation of BRCA1 may inhibit RNA Pol III transcription as a self-protective mechanism,12 although this hypothesis remains to be confirmed in further studies. In the past, there were no good markers to indicate alcohol consumption in GC tissue. In this study, we identified the different expression of some alcohol-related proteins in GC tissues between the patients with hazardous or harmful drinking habits and abstinence or low-risk drinkers. The expression of BRF1/BRCA1 and MPO-positive cell infiltration were closely related to the increase of alcohol consumption. BRF1, MPO, and BRCA1 may indicate alcohol consumption in tumor or para-tumor tissue samples of GC. In contrast, there was no correlation between the expression of BRCA2 in para-tumor tissues and hazardous or harmful alcohol consumption habits. However, a strong correlation was found between BRCA1/2 expression in para-tumor tissues in GC patients.
Based on the results from Kaplan-Meier and multivariate Cox regression analyses, patients with high BRF1 expression showed significantly shorter DFS times than patients with low BRF1 expression, indicating that BRF1 served as an independent predictor for DFS in GC patients. This result is similar to the study on BRF1 expression in HCC patients.7 Furthermore, BRCA showed stronger prognostic value for DFS (BRCA2) and OS (BRCA1/2) in GC patients. In the past, many studies have focused on the relationship between BRCA1 expression and GC patient prognosis.17, 18, 26 However, similar studies on BRCA2 rarely have been reported. In a recent study, BRCA2 gene mutations, which were found in 8% of The Cancer Genome Atlas GC cohort, were associated significantly with longer survival.27 Because of the role of BRCA2 protein as a custodian of chromosome integrity during the cell cycle, BRCA2 protein expression is decreased because of this gene mutation. Therefore, these data are consistent with the results from this study regarding high BRCA2 protein expression and worse survival. In addition, the relationship between MPO-positive cell infiltration in tumor tissues and cancer patient prognosis remains controversial. In colorectal cancer, the high density of MPO-positive cell infiltration is associated with significantly improved survival,28 and MPOlow and CD8low cell densities were associated independently with an unfavorable prognosis.29 However, in another study on colorectal cancer, higher levels of oxidation and MPO correlated with lower survival rates.30 This study provides additional insight into the favorable prognostic relevance of no MPO-positive cell infiltration for DFS. Furthermore, similar to findings in colorectal cancer, MPO was not expressed in tumor cells, but rather in infiltrating inflammatory cells in GC.28, 29
BRCA1/2 and MPO also play key roles in DNA damage repair. Tumor tissues with little to no expression of these proteins show a diminished capacity to repair damaged DNA induced by platinum-based chemotherapy. Some studies have reported that patients with negative BRCA1 protein expression may benefit from platinum-based adjuvant chemotherapy in GC.17, 18 In this study, similar results were found for BRCA1 through the Kaplan-Meier method. However, in the Cox multivariate analysis, the prognostic value of BRCA1 for platinum-based adjuvant chemotherapy in GC remains insignificant. In contrast, BRCA2 was found to be an independent unfavorable prognostic factor for DFS and OS in GC patients who had received platinum-based adjuvant chemotherapy. Nevertheless, a strong correlation also was found with BRCA1/2 expression in tumor tissues in GC patients. There are some differences between BRCA1 and BRCA2 in homologous recombination repair. First, BRCA1 primarily acts at proximal steps that signal the presence of these lesions and helps initiate their repair via homologous recombination, whereas BRCA2 stabilizes the structure at the replication-associated lesions and works directly to resolve the lesions with homologous recombination by controlling the activity and assembly of the essential recombination enzyme RAD51.31, 32 On the other hand, findings on the relationship between MPO and nucleotide excision repair have not yet been extended to the clinic. It was reported that neutrophils that catalyze the formation of hypochlorous via MPO are inhibiting factors in the nucleotide excision repair pathway.16 Moreover, in steatotic livers, high MPO immunoreactivity is paralleled by a significant decrease in nucleotide excision repair capacity.33 In the present study, patients who received platinum-based adjuvant chemotherapy and displayed absence of MPO-positive inflammatory cell infiltration displayed longer DFS and OS. Unfortunately, this trend did not translate to a significant improvement in survival.
In this study, the association between these proteins and survival/prognosis in approximately 57 patients who received platinum-based chemotherapy. A total of 77 patients were analyzed, including the 57 patients mentioned above, but also patients who had not received platinum-based adjuvant chemotherapy or radical surgery. In particular, the implication of long-term survival of platinum-based adjuvant chemotherapy is different from overall patients' survival. In the former, it reflects the effect of chemotherapy on survival significantly. However, the latter emphasizes the influence of protein expression on survival and the prognosis of total patients.
In conclusion, the H-scores for BRF1, BRCA1, and BRCA2 in tumor tissues from GC patients were significantly higher than those in para-tumor tissues. And MPO-positive cell infiltration degrees in GC patient tumor tissues were significantly higher than those in para-tumor tissues. High BRF1 expression and MPO-positive cell infiltration in tumor tissues as well as positive BRCA1 expression in para-tumor tissues were more frequent in GC patients with hazardous or harmful alcohol consumption habits. It had statistical correlation about expression of BRCA1/2 in tumor tissues of GC patients. BRF1 and BRCA1 were co-localized in GC tumor cell nuclei. BRF1, BRCA2, and MPO were independent prognostic factors in GC patients and were associated significantly with DFS. Moreover, BRCA1 and BRCA2 were independent prognostic factors for OS, and BRCA2 was an independent unfavorable prognostic factor for DFS and OS in GC patients who had received platinum-based adjuvant chemotherapy. Our results suggest that BRF1, BRCA1/2, and MPO, which are DNA repair–related biomarkers induced by alcohol, have prognostic value in GC patients. These innovative findings are of great significance, and additional larger clinical studies are warranted.
Acknowledgments
Y.Z., H.Wa., and K.G. conceived the hypothesis and overall study design; S.Z. participated in the experimental design; Y.Z. and F.Y. performed immunohistochemistry and immunofluorescence analysis; Y.Z. and H.Wa. performed the statistical analysis and wrote the manuscript; and H.Wu., J.N., M.L., and C.Z. assisted in the collection of the clinical data; all authors read and approved the final manuscript.
Footnotes
Supported by National Natural Science Foundation of China grants 81522009 (H.Wa.) and 81372577 (H.Wa.), and Anhui Provincial Science and Technology Plan Project 1604b0602027 (K.G.).
Disclosures: None declared.
Contributor Information
Kangsheng Gu, Email: 13805692145@163.com.
Hua Wang, Email: wanghua@ahmu.edu.cn.
References
- 1.McLean M.H., El-Omar E.M. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 2.Fang X., Wei J., He X., An P., Wang H., Jiang L., Shao D., Liang H., Li Y., Wang F., Min J. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820–2832. doi: 10.1016/j.ejca.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Johnson S.A., Johnson D.L. Enhanced RNA polymerase III-dependent transcription is required for oncogenic transformation. J Biol Chem. 2008;283:19184–19191. doi: 10.1074/jbc.M802872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong S., Johnson D.L. The JNKs differentially regulate RNA polymerase III transcription by coordinately modulating the expression of all TFIIIB subunits. Proc Natl Acad Sci U S A. 2009;106:12682–12687. doi: 10.1073/pnas.0904843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong S., Machida K., Tsukamoto H., Johnson D.L. Alcohol induces RNA polymerase III-dependent transcription through c-Jun by co-regulating TBP and BRF1 expression. J Biol Chem. 2011;286:2393–2401. doi: 10.1074/jbc.M110.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong Q., Shi G., Zhang Q., Lu L., Levy D., Zhong S. Tamoxifen represses alcohol-induced transcription of RNA polymerase III-dependent genes in breast cancer cells. Oncotarget. 2014;5:12410–12417. doi: 10.18632/oncotarget.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong Q., Xi S., Liang J., Shi G., Huang Y., Zhang Y., Levy D., Zhong S. The significance of BRF1 overexpression in human hepatocellular carcinoma. Oncotarget. 2015;7:6243–6254. doi: 10.18632/oncotarget.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W., Huang H., Niu X., Fan T., Mu Q., Li H. Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol Appl Pharmacol. 2013;272:21–29. doi: 10.1016/j.taap.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Amirshahrokhi K., Khalili A.R. The effect of thalidomide on ethanol-induced gastric mucosal damage in mice: involvement of inflammatory cytokines and nitric oxide. Chem Biol Interact. 2015;225:63–69. doi: 10.1016/j.cbi.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Al-Salihi M., Reichert E., Fitzpatrick F.A. Influence of myeloperoxidase on colon tumor occurrence in inflamed versus non-inflamed colons of Apc(Min/+) mice. Redox Biol. 2015;6:218–225. doi: 10.1016/j.redox.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rymaszewski A.L., Tate E., Yimbesalu J.P., Gelman A.E., Jarzembowski J.A., Zhang H., Pritchard K.A., Jr., Vikis H.G. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers (Basel) 2014;6:1111–1127. doi: 10.3390/cancers6021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veras I., Rosen E.M., Schramm L. Inhibition of RNA polymerase III transcription by BRCA1. J Mol Biol. 2009;387:523–531. doi: 10.1016/j.jmb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Q., Shi G., Zhang Y., Lu L., Levy D., Zhong S. Alteration of BRCA1 expression affects alcohol-induced transcription of RNA Pol III-dependent genes. Gene. 2015;556:74–79. doi: 10.1016/j.gene.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott S.N., Wallace J.L. Neutrophil-mediated gastrointestinal injury. Can J Gastroenterol. 1998;12:559–568. doi: 10.1155/1998/398384. [DOI] [PubMed] [Google Scholar]
- 15.Venkitaraman A.R. Functions of BRCA1 and BRCA2 in the biological response to DNA damage. J Cell Sci. 2001;114:3591–3598. doi: 10.1242/jcs.114.20.3591. [DOI] [PubMed] [Google Scholar]
- 16.Gungor N., Godschalk R.W., Pachen D.M., Van Schooten F.J., Knaapen A.M. Activated neutrophils inhibit nucleotide excision repair in human pulmonary epithelial cells: role of myeloperoxidase. FASEB J. 2007;21:2359–2367. doi: 10.1096/fj.07-8163com. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.W., Cho H.J., Kim M., Lee K.H., Kim M.A., Han S.W., Oh D.Y., Lee H.J., Im S.A., Kim T.Y., Yang H.K., Kim W.H., Bang Y.J. Differing effects of adjuvant chemotherapy according to BRCA1 nuclear expression in gastric cancer. Cancer Chemother Pharmacol. 2013;71:1435–1443. doi: 10.1007/s00280-013-2141-x. [DOI] [PubMed] [Google Scholar]
- 18.Chen W., Wang J., Li X., Li J., Zhou L., Qiu T., Zhang M., Liu P. Prognostic significance of BRCA1 expression in gastric cancer. Med Oncol. 2013;30:423. doi: 10.1007/s12032-012-0423-5. [DOI] [PubMed] [Google Scholar]
- 19.Washington K. 7th Edition of the AJCC Cancer Staging Manual: Stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 20.Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsai M.C., Tsai Y.F., Chen C.Y., Liu C.Y. Alcohol Use Disorders Identification Test (AUDIT): establishment of cut-off scores in a hospitalized Chinese population. Alcohol Clin Exp Res. 2005;29:53–57. doi: 10.1097/01.alc.0000151986.96710.e0. [DOI] [PubMed] [Google Scholar]
- 22.Detre S., Saclani Jotti G., Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogliani V.J., Baan R., Straif K., Grosse Y., Lauby-Secretan B., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Wild C.P. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White R.J. RNA polymerase III transcription and cancer. Oncogene. 2004;23:3208–3216. doi: 10.1038/sj.onc.1207547. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q., Jin J., Zhong Q., Yu X., Levy D., Zhong S. ERα mediates alcohol-induced deregulation of Pol III genes in breast cancer cells. Carcinogenesis. 2013;34:28–37. doi: 10.1093/carcin/bgs316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z.Z., Liu Y.J., Yin X.L., Zhan P., Gu Y., Ni X.Z. Loss of BRCA1 expression leads to worse survival in patients with gastric carcinoma. World J Gastroenterol. 2013;19:1968–1974. doi: 10.3748/wjg.v19.i12.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen K., Yang D., Li X., Sun B., Song F., Cao W., Brat D.J., Gao Z., Li H., Liang H., Zhao Y., Zheng H., Li M., Buckner J., Patterson S.D., Ye X., Reinhard C., Bhathena A., Joshi D., Mischel P.S., Croce C.M., Wang Y.M., Raghavakaimal S., Li H., Lu X., Pan Y., Chang H., Ba S., Luo L., Cavenee W.K., Zhang W., Hao X. Mutational landscape of gastric adenocarcinoma in Chinese: implications for prognosis and therapy. Proc Natl Acad Sci U S A. 2015;112:1107–1112. doi: 10.1073/pnas.1422640112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Droeser R.A., Hirt C., Eppenberger-Castori S., Zlobec I., Viehl C.T., Frey D.M., Nebiker C.A., Rosso R., Zuber M., Amicarella F., Iezzi G., Sconocchia G., Heberer M., Lugli A., Tornillo L., Oertli D., Terracciano L., Spagnoli G.C. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One. 2013;8:e64814. doi: 10.1371/journal.pone.0064814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Däster S., Eppenberger-Castori S., Hirt C., Soysal S.D., Delko T., Nebiker C.A., Weixler B., Amicarella F., Iezzi G., Governa V., Padovan E., Mele V., Sconocchia G., Heberer M., Terracciano L., Kettelhack C., Oertli D., Spagnoli G.C., von Holzen U., Tornillo L., Droeser R.A. Absence of myeloperoxidase and CD8 positive cells in colorectal cancer infiltrates identifies patients with severe prognosis. Oncoimmunology. 2015;4:e1050574. doi: 10.1080/2162402X.2015.1050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crespo-Sanjuán J., Calvo-Nieves M.D., Aguirre-Gervás B., Herreros-Rodríguez J., Velayos-Jiménez B., Castro-Alija M.J., Muñoz-Moreno M.F., Sánchez D., Zamora-González N., Bajo-Grañeras R., García-Centeno R.M., Largo Cabrerizo M.E., Bustamante M.R., Garrote-Adrados J.A. Early detection of high oxidative activity in patients with adenomatous intestinal polyps and colorectal adenocarcinoma: myeloperoxidase and oxidized low-density lipoprotein in serum as new markers of oxidative stress in colorectal cancer. Lab Med. 2015;46:123–135. doi: 10.1309/LMZJJU6BC86WUDHW. [DOI] [PubMed] [Google Scholar]
- 31.Venkitaraman A.R. Cancer suppression by the chromosome custodians, BRCA1 and BRCA2. Science. 2014;343:1470–1475. doi: 10.1126/science.1252230. [DOI] [PubMed] [Google Scholar]
- 32.Rajendra E., Venkitaraman A.R. Two modules in the BRC repeats of BRCA2 mediate structural and functional interactions with the RAD51 recombinase. Nucleic Acids Res. 2010;38:82–96. doi: 10.1093/nar/gkp873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schults M.A., Nagle P.W., Rensen S.S., Godschalk R.W., Munnia A., Peluso M., Claessen S.M., Greve J.W., Driessen A., Verdam F.J., Buurman W.A., van Schooten F.J., Chiu R.K. Decreased nucleotide excision repair in steatotic livers associates with myeloperoxidase-immunoreactivity. Mutat Res. 2012;736:75–81. doi: 10.1016/j.mrfmmm.2011.11.001. [DOI] [PubMed] [Google Scholar]