Short abstract
Emerging evidence showed that hyperpolarization-activated cation channels (HCN) participate in the development of inflammatory and neuropathic pain. However, the role of HCN2 in oxaliplatin-induced neuropathic pain remains unknown. Here, we found that HCN2 expression was upregulated in a rat model of oxaliplatin-induced neuropathic pain. Intrathecal injection of ZD7288, an HCN specific inhibitor, decreased the HCN2 level, as well as weakened the neuropathic pain behaviors compared to naive rats. Besides, mechanistic studies revealed that the expression of the spinal N-methyl-D-aspartate receptor subunit 2B was increased after oxaliplatin administration and was reduced by ZD7288 administration. The nociceptive behaviors were reversed by NR2B antagonist Ro 25–6981 in HCN2-overexpression rats. Furthermore, the underlying cellular mechanism demonstrated that ZD7288 administration restrained the enhanced activation of the neuronal calcium–calmodulin-dependent kinase II (CaMKII)/cyclic adenosine monophosphate response element-binding protein cascade after oxaliplatin administration. Moreover, pretreatment of CaMKII inhibitor KN-93 suppressed the nociceptive behaviors, as well as NR2B upregulation induced by overexpression of HCN2. In a word, HCN2 is conducive to oxaliplatin-induced neuropathic pain by activating the neuronal CaMKII/CREB cascade.
Keywords: HCN2, oxaliplatin, neuropathic pain, NR2B, CaMKII/CREB
Introduction
Oxaliplatin, a third-generation organoplatinum compound, is a first-line treatment for metastatic colorectal cancer.1 In spite of its satisfactory clinical efficacy, the application of oxaliplatin is often limited by its side effects, including neuropathy. The progression of neuropathy induced by oxaliplatin chemotherapy can limit doses and frequently results in treatment interruption.2 However, the exact mechanisms of oxaliplatin-induced neuropathy remain unclear.
Hyperpolarization-activated, cyclic nucleotide-gated (HCN) cation channels are widely expressed in cardiac and neuronal tissues and have been recently reported to contribute to nociceptive excitability.3 HCN channels are usually activated by hyperpolarization within a range of 60 to 90 mV and differ from other voltage-gated cation channels, which are activated by depolarization. There are four HCN subtypes (HCN1 to HCN4) expressed in sensory neurons,4 and over half of small nociceptive neurons express HCN2 channels.5 Recently, HCN2 has drawn considerable attention as a target of a potential analgesic drug that treats pathological pain. Deactivating HCN2 in primary sensory neurons has been found to weaken tactile hypersensitivity in chronic inflammation, while leaving thermal hyperalgesia unaffected.6 HCN2 could be directly activated by cyclic adenosine monophosphate and has been thought to contribute to nociceptor sensitization in inflammatory pain sensitization.7 Studies have found that neuropathic pain is initiated by an HCN2-driven action potential firing in NaV1.8-expressing nociceptors.5 However, the function of HCN2 in oxaliplatin-induced neuropathic pain remains unclear.
In this study, we created a rat model of neuropathic pain using oxaliplatin administration. Through this model, we verified that HCN2 is involved in oxaliplatin-induced neuropathic pain through the upregulation of NR2B expression, which is modulated by the activation of the CaMKII/CREB cascade.
Materials and methods
Ethics and animals
Male Sprague-Dawley rats weighing 200–220 g were housed in cages with corncob bedding and were maintained on a 12-h light/dark cycle with ad libitum access to food and water. The animals were habituated to the housing facilities for at least one week prior to the behavioral test. All procedures were approved by the Ethics Committee of Jinling Hospital and were performed in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health.
Drugs
Oxaliplatin (Eloxatin, Sanofi-Aventis, Laboratoires Thissen, Belgium) was dissolved in a 5% glucose solution. Oxaliplatin (4 mg/kg body weight) was injected intraperitoneally (i.p.) into each rat twice a week for four weeks.8 ZD7288 (Tocris Bioscience, Ellisville, MO) solubilized in 0.9% saline was intrathecally administrated with a 10-μl microinjection syringe into the intervertebral space between L4 and L6 after the rat was anesthetized with isoflurane/carbogen. Ro 25–6981 (Tocris Bioscience), an NR2B activation antagonist, and KN-93 (Tocris Bioscience), a selective CaMKII antagonist, were both administered via intrathecal injection.
Behavioral tests
All the behavioral tests were conducted 5 h after drug treatment and performed blindly with respect to drug administration. Rats were acclimatized to the testing environment for 30 min prior to data collection.
Paw withdrawal threshold (PWT) was measured with the Electro Von Frey Aneshesiometer (Model 2390CE, IITC Life Science Inc, USA) according to a modification of a previously described method9 before the first drug administration (day 0), and on days 7, 14, and 21, and 5 h after drug administration in a quiet, temperature-controlled room. Rats were placed individually beneath an inverted, ventilated Plexiglas cage on a metal-mesh floor in order to allow access to the plantar surface of the hind paw. After acclimatization, a calibrated von Frey hair of incremental stiffness (maximum 200g) was applied perpendicularly to the dorsal surface of the bilateral hind paw. Each trial consisted of five applications of the hair to the dorsal surface of the bilateral hind paw perpendicularly every 4 s for 3 to 4 s. Foot withdrawals in response to the mechanical stimulus of the von Frey hair were considered positive. Five trials for each paw were conducted every 5 min and the applied force (g) was recorded.
Paw withdrawal thermal latency (PWTL) was determined using a modified Hargreaves device (UARDG of UCSD, La Jolla, CA). Rats were placed in boxes with a wire mesh floor. An infrared light beam was focused on the plantar surface of the hind paw. Withdrawal latency (s) was automatically recorded when the rat withdrew its paw. Each paw was measured three times with intervals of 3 min.
Cold sensitivity was assessed using the acetone test previously described.10 Rats were placed in a clear plastic box with a metal grid and habituated for 30 min before testing. A 50-μl drop of acetone (Wako Pure Chemical Ltd., Osaka, Japan) was applied to the plantar skin of each hind paw three times with a syringe fitted with a blunted needle. The duration of lifting/licking (s) of the hind paw was recorded for 40 s from the start of the acetone spray.
Western blot analysis
All rats were deeply anesthetized with sodium pentobarbital (60 mg/kg), and the L4-L6 spinal cord was quickly harvested and stored at −80°C before being subjected to the following procedure. Samples were homogenized in Tris buffer with a cocktail of proteinase inhibitors and phosphatase inhibitors and were subjected to SDS-PAGE, followed by electrophoretic transfer onto polyvinylidene fluoride (PVDF) membranes. The membranes were placed in the block buffer at room temperature for 1 h and incubated with one of the following primary antibodies: anti-HCN2 (Proteintech, Wuhan, China), anti-NR2B (Proteintech), anti-pCaMKII (Cell Signalling Technology, USA); anti-CaMKII (Cell Signalling Technology); anti-pCREB (Cell Signalling Technology); anti-CREB (Cell Signalling Technology) at 4°C for 12 h, and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (Proteintech) for 1 h. Quantification of the band for each protein was performed with Image J software.
qRT-PCR analysis
Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. First-strand cDNA synthesis was performed using the Verso cDNA synthesis kit (Thermo Scientific, ABgene, Surrey, UK). qRT-PCR was performed using SYBR Green Master Mix (Takara, Dalian, China) on ABI 7900HT Fast Real-Time PCR System-Applied Bio-systems. Relative mRNA levels were calculated using the 2−ΔΔCT method. Gene expression was normalized using GAPDH levels. Each analysis was performed in triplicate.
Lentiviral vector production and intrathecal injection
The full-length sequence of the HCN2 cDNA was amplified and cloned into the lentiviral vector pCDH-CMV-MCS-EF1-coGFP (System Biosciences, Palo Alto, CA) using the Gateway system™. According to the manufacturer’s instructions, Lentivirus particles were harvested 48 h after pCDH-HCN2 cotransfection with the packaging plasmid psPAX2 and pMD into HEK 293T cells using Lipofectamine® 2000. The intraspinal injection was performed as described previously.11 The needle was inserted into the intervertebral space of a conscious rat between the L4 and L6 regions of the spinal cord after the rat was anesthetized with isoflurane.
Statistical analysis
Data are presented as mean ± SD. Differences between two groups or more than two groups were determined using a Student’s t test or a one-way analysis of variance, respectively. Statistical evaluation was performed with Graphpad Prism 5.0 software (San Diego, CA, http://www.graphpad.com/). *P < 0.05 was considered statistically significant.
Results
HCN2 is upregulated in a rat model of oxaliplatin-induced neuropathic pain
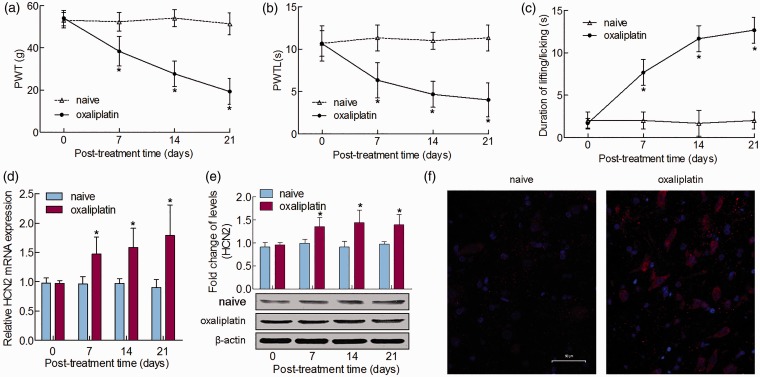
In order to investigate the involvement of HCN2 in oxaliplatin-induced neuropathic pain, we first established a rat neuropathic pain model using intraperitoneal injection of oxaliplatin (4 mg/kg). As shown in Figure 1(a) and (b), significant decreases in the PWT and PWTL were detected in rats beginning day 7 and persisting to day 21 after intraperitoneal injection of oxaliplatin compared to vehicle administration. In the acetone test, the oxaliplatin-treated rats showed an increase in the duration of lifting/licking behaviors over the same period (Figure 1(c)). These results revealed that the rats that were subjected to oxaliplatin developed mechanical hyperalgesia, heat hypoalgesia, and cold allodynia. To examine the role of HCN2 in oxaliplatin-induced neuropathic pain, we detected HCN2 mRNA and protein expression in the spinal cord on days 0, 7, 14, and 21 after oxaliplatin administration. The qRT-PCR and Western blot results indicated that HCN2 expression increased remarkably and remained at a high, stable level starting at day 21 after oxaliplatin injection, compared to that of the naive rats (Figure 1(d) and (e)). We performed immunostaining to further determine HCN2 expression pattern changed in neuropathic pain. The images showed an increase in the number of HCN2-positive neurons in oxaliplatin rats compared with the naive rats (Figure 1(f)). All findings suggested that HCN2 might be involved in oxaliplatin-induced neuropathic pain.
Figure 1.
HCN2 is upregulated in oxaliplatin-induced neuropathic pain. A single intraperitoneal administration of oxaliplatin induced pain-related behavior in the spinal dorsal horn. (a) Mechanical allodynia was measured by PWT. (b) Thermal hyperalgesia was measured by PWTL. (c) Cold allodynia was measured by the acetone test on days 0, 7, 14, and 21 after oxaliplatin administration. (d) mRNA and (e) protein levels of HCN2 in the spinal dorsal horn after single oxaliplatin treatment. Tissues were collected on days 0 and 21 after oxaliplatin treatment or no treatment. (f) Immunofluorescence staining of HCN2 immunoreactive neurons in the spinal cord of rat treatment with oxaliplatin or not was shown. Bar = 50 μm. *P < 0.05.
Role of HCN2 in oxaliplatin-induced neuropathic pain behaviors
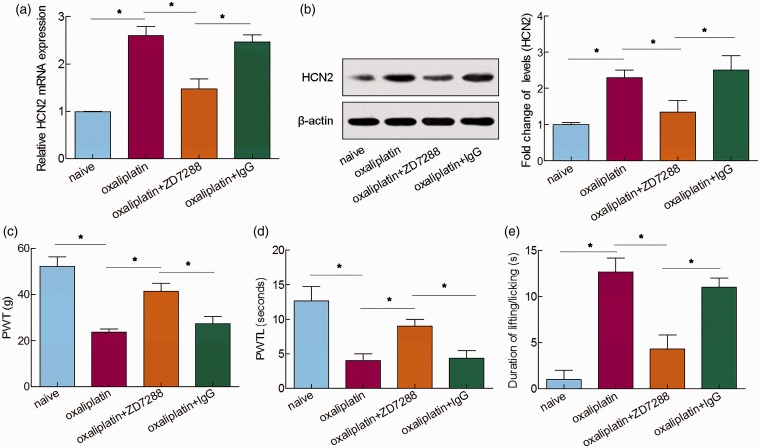
To elucidate the role of HCN2 in oxaliplatin-induced neuropathic pain, an HCN-specific blocker ZD7288 was intrathecally injected on day 14 after the first injection of oxaliplatin. qRT-PCR and Western blot analysis showed that the upregulated expression of HCN2 was dramatically decreased by the treatment with ZD7288 (Figure 2(a) and (b)). Furthermore, oxaliplatin-induced mechanical allodynia, thermal hyperalgesia, and cold allodynia were observably reduced after intrathecal injection of ZD7288 when compared to naive rats (Figure 2(c) to (e)). Together, these results indicated that the HCN2 blockage eased the oxaliplatin-induced neuropathic pain behaviors, illustrating the important role of HCN2 in oxaliplatin-induced neuropathic pain.
Figure 2.
Role of HCN2 in oxaliplatin-induced neuropathic pain behaviors. HCN2 expression was detected by (a) qRT-PCR and (b) Western blot analysis 5 h after ZD7288 or IgG administration. (c) Mechanical allodynia was measured by PWT. (d) Thermal hyperalgesia was measured by PWTL. (e) Cold allodynia was measured by the acetone test 5 h after ZD7288 or IgG administration. *P < 0.05.
Role of HCN2 in the upregulation of NR2B expression in oxaliplatin-induced neuropathic pain model
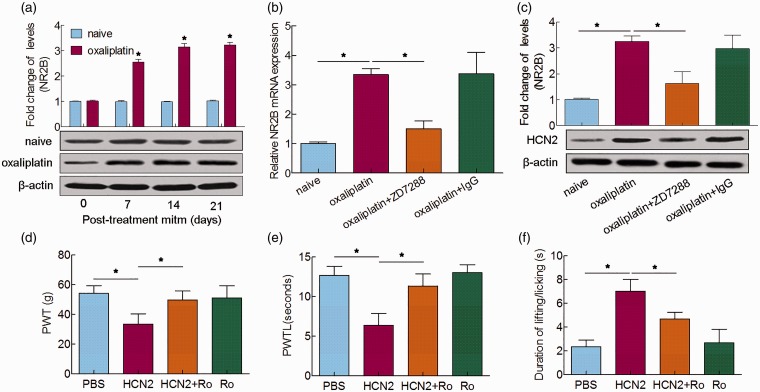
The N-methyl-D-aspartate (NMDA) receptor in the superficial dorsal horn has been found to greatly contribute to pain transmission sensitization.12 Inhibition of the NMDA receptor by its antagonist reduces the excitatory nociceptive transmission that results from exposure to noxious stimuli and chronic pain in experimental animals with spinal cord injuries.13 Recently, NR2B (a membrane-spanning subunit of the NMDA receptor) has gained much attention, and we previously reported that NR2B was upregulated during oxaliplatin-induced neuropathic pain.9 Congruously, we found that the expression of spinal NR2B was increased after oxaliplatin injection (Figure 3(a)). Next, we detected the expression of NR2B after ZD7288 injection to determine whether the increase in NR2B was modulated by HCN2. qRT-PCR and Western blot analysis indicated that the enhanced expression of NR2B was decreased by the administration of ZD7288 on the 14th day after oxaliplatin administration (Figure 3(b) and (c)). In addition, pretreatment with Ro 25–6981, an NR2B antagonist, observably weakened the mechanical, cold allodynia and thermal hyperalgesia induced by overexpression of HCN2 (Figure 3(d) to (f)). Collectively, these data demonstrated that HCN2 is able to regulate the upregulation of NR2B expression in oxaliplatin-induced neuropathic pain.
Figure 3.
Role of HCN2 in the upregulation of NR2B expression in oxaliplatin-induced neuropathic pain. (a) Western blot analysis of NR2B in the spinal cord 0, 7, 14, and 21 days after oxaliplatin treatment. (b) qRT-PCR and (c) Western blot analysis of NR2B 5 h after ZD7288 or IgG administration. (d) Mechanical allodynia was measured by PWT. (e) Cold allodynia was measured by PWTL. (f) Thermal hyperalgesia was measured by the acetone test 5 h after administration of Ro25–6981 in naïve rats with induced HCN2 overexpression. *P < 0.05.
Involvement of the spinal neuronal CaMKII–CREB cascade in HCN2-mediated, oxaliplatin-induced neuropathic pain
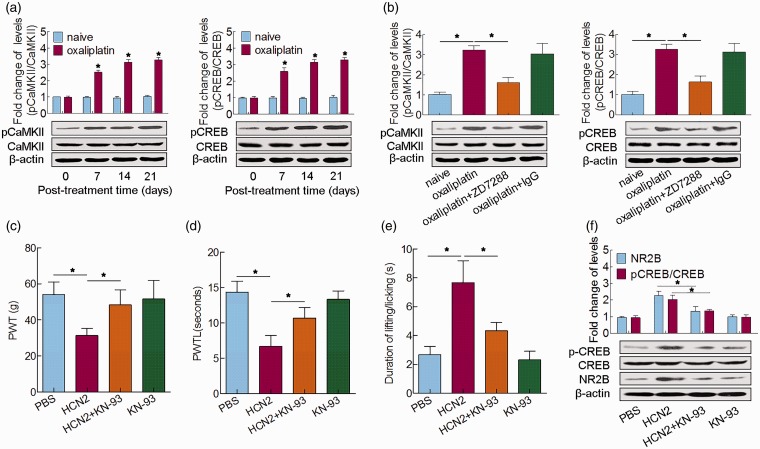
Next, we examined the underlying mechanisms of HCN2-mediated, oxaliplatin-induced neuropathic pain. It has been illustrated that Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-AMP-responsive element binding protein (CREB) in spinal neurons contribute to the development of neuropathic pain.14 Here, the expressions of pCaMKII/CaMKII and pCREB/CREB were both increased after oxaliplatin administration and maintained at a high level until the 21st day, when compared to those of naive rats (Figure 4(a)). After intrathecal administration of ZD7288 on the 14th day after the first injection of oxaliplatin, the expressions of both pCaMKII/CaMKII and pCREB/CREB were notably decreased (Figure 4(b)). Pretreatment with CaMKII inhibitor KN-93 notably reversed the mechanical and cold allodynia and thermal hyperalgesia induced by overexpression of HCN2 (Figure 4(c) to (e)), as well as restrained the CREB activation and NR2B upregulation (Figure 4(f)). These results jointly indicated that the spinal neuronal CaMKII/CREB cascade was modulated by HCN2 and was involved in oxaliplatin-induced neuropathic pain.
Figure 4.
Involvement of the spinal neuronal CaMKII–CREB cascade in HCN2-mediated, oxaliplatin-induced neuropathic pain. (a) Western blot analysis of pCaMKII/CaMKII and pCREB/CREB in the spinal cord 0, 7, 14, and 21 days after oxaliplatin treatment. (b) Western blot analysis of pCaMKII/CaMKII and pCREB/CREB 5 h after ZD7288 or IgG administration. (c) Mechanical allodynia was measured by PWT. (d) Cold allodynia was measured by PWTL. (e) Thermal hyperalgesia was measured by the acetone test 5 h after administration of KN-93 in naïve rats infected with HCN2 overexpression. (f) Western blot analysis of pCREB/CREB and NR2B in the spinal cord 5 h after cotreatment of KN-93 and HCN2 in naïve rats. *P < 0.05.
Discussion
In this study, we investigated the function of HCN2 in oxaliplatin-induced neuropathic pain. We found that HCN2 was upregulated after oxaliplatin administration, and HCN2 inhibition by ZD7288 could ease neuropathic pain. In addition, ZD7288 reduced the increased expression of NR2B. Moreover, the neuronal CaMKII–CREB cascade was also upregulated after oxaliplatin administration, and injection of ZD7288 could restrain the increased expression of pCaMKII/CaMKII and pCREB/CREB. Furthermore, we found that treatment with the CaMKII inhibitor KN-93 significantly reversed the nociceptive behaviors provoked by HCN2 overexpression, and also suppressed the CREB activation and NR2B upregulation.
HCN2 plays a vital role in many diseases. It has been illustrated that the loss-of-function mutation in the HCN2 channel leads to enhanced neuronal excitability in a patient with idiopathic generalized epilepsy.15 Overexpression of HCN2 decreases the repolarization reserve of the ventricular action potential and enhances arrhythmogenic potential under pathological conditions, such as excessive β-adrenergic stimulation.16 Genetic deletion of HCN2 in nociceptive neurons prevents mice from developing diabetic pain.17 Resta et al.18 have found a decrease of HCN2 expression level without alteration of HCN1 expression in rat treated with Oxaliplatin (2.4 mg/kg), administered intraperitoneally (i.p.) for five consecutive days every week for two weeks (10 i.p. injections). In this regard, it must be noticed that our animal model is deeply different, consisting in a chronic treatment that Oxaliplatin (4 mg/kg) was injected intraperitoneally (i.p.) into each rat twice a week for four weeks. We showed that HCN2 was upregulated in oxaliplatin-induced neuropathic pain, and blocking HCN2 using ZD7288 increased the PWT and PWTL but decreased the duration of lifting/licking behaviors induced by oxaliplatin. These results implied the important role of HCN2 in the development of oxaliplatin-induced neuropathic pain.
Recently, NR2B has gained much attention for neuropathic pain modulation. Research has found that NR2B is regulated by Caveolin-1 in the anterior cingulate cortex neurons in order to modulate chronic neuropathic pain.19 Injection of Ro25–6981, a NR2B-containing NMDA blocker, reduced the SNL-induced mechanical allodynia in rats.20,21 In our current study, we showed that NR2B was also increased after oxaliplatin injection, and the increased expression of NR2B was restrained in the presence of ZD7288. In addition, pretreatment with Ro25–6981 attenuated the mechanical hyperalgesia, heat hypoalgesia, and cold allodynia provoked by HCN2 overexpression in naïve rats. The above results illustrated that HCN2 modulated the oxaliplatin-induced neuropathic pain via regulation of NR2B.
CaMKII is a serine/threonine protein kinase, which regulates Ca2+ signaling and affects synaptic function through phosphorylation of membrane receptors. CREB is crucial for the regulation of pro-nociceptive genes and maintenance of persistent pain sensitization.22 It has been proved that CaMKII could induce the CREB phosphorylation that is related to hyperalgesia and central sensitization in some pain models.23,24 Here, we showed that the expressions of pCaMKII/CaMKII and pCREB/CREB were both increased after oxaliplatin injection and were both decreased in the presence of ZD7288. Pretreatment with KN-93 markedly restrained the upregulation of NR2B and relieved pain behaviors caused by overexpression of HCN2. Hence, we suggested that HCN2 maintains neuropathic pain through the CaMKII/CREB cascade in spinal neurons.
In conclusion, HCN2 is significantly elevated after oxaliplatin-induced neuropathic pain. HCN2 contributes to neuropathic pain through the upregulation of NR2B and the activation of the CaMKII/CREB cascade in spinal neurons. Blockage of HCN2 provides a novel therapeutic approach for the treatment of oxaliplatin-induced neuropathic pain.
Author Contributions
JT and WL designed research. XL and LZ performed experiments. LJ and YT wrote the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81603151), Natural Science Foundation of Jiangsu Province (BK20160608), and China Postdoctoral Science Foundation (2015M582899).
References
- 1.André T Boni C Navarro M Tabernero J Hickish T Topham C Bonetti A Clingan P Bridgewater J andRivera F.. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Balibrea E Martinez-Cardus A Gines A Ruiz de Porras V Moutinho C Layos L Manzano JL Buges C Bystrup S Esteller M andAbad A.. Tumor-related molecular mechanisms of oxaliplatin resistance. Mol Cancer Ther 2015; 14: 1767–1776. [DOI] [PubMed] [Google Scholar]
- 3.Emery EC andMcnaughton PA.. HCN2 ion channels: an emerging role as the pacemakers of pain. Trends Pharmacol Sci 2012; 33: 456–463. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig A Zong X Jeglitsch M Hofmann F andBiel M.. A family of hyperpolarization-activated mammalian cation channels. Nature 1998; 393: 587–591. [DOI] [PubMed] [Google Scholar]
- 5.Emery EC Young GT Berrocoso EM Chen L andMcNaughton PA.. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011; 333: 1462–1466. [DOI] [PubMed] [Google Scholar]
- 6.Schnorr S Eberhardt M Kistner K Rajab H Kasser J Hess A Reeh P Ludwig A andHerrmann S.. HCN2 channels account for mechanical (but not heat) hyperalgesia during long-standing inflammation. Pain 2014; 155: 1079–1090. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann S Rajab H Christ I Schirdewahn C Hofler D Fischer MJM Bruno A Fenske S Gruner C Kramer F Wachsmann T Wahl-Schott C Stieber J Biel M andLudwig A.. Protein kinase A regulates inflammatory pain sensitization by modulating HCN2 channel activity in nociceptive sensory neurons. Pain 2017; 158: 2012–2024. [DOI] [PubMed] [Google Scholar]
- 8.Mihara Y Egashira N Sada H Kawashiri T Ushio S Yano T Ikesue H andOishi R.. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol Pain 2011; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X Zhang G Dong L Wang X Sun H Shen J Li W andXu J.. Repeated administration of mirtazapine attenuates oxaliplatin-induced mechanical allodynia and spinal NR2B up-regulation in rats. Neurochem Res 2013; 38: 1973–1979. [DOI] [PubMed] [Google Scholar]
- 10.Flatters SJL andBennett GJ.. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 2004; 109: 150–161. [DOI] [PubMed] [Google Scholar]
- 11.Liu S Mi WL Li Q Zhang MT Han P Hu S Mao-Ying QL andWang YQ.. Spinal IL-33/ST2 signaling contributes to neuropathic pain via neuronal CaMKII-CREB and astroglial JAK2-STAT3 cascades in mice. Anesthesiology 2015; 123: 1154–1169. [DOI] [PubMed] [Google Scholar]
- 12.Larsson M andBroman J.. Synaptic plasticity and pain: role of ionotropic glutamate receptors. Neuroscientist 2011; 17: 256–273. [DOI] [PubMed] [Google Scholar]
- 13.Bennett AD Everhart AW andHulsebosch CE.. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res 2000; 859: 72–82. [DOI] [PubMed] [Google Scholar]
- 14.Yao CY Weng ZL Zhang JC Feng T Lin Y andYao S.. Interleukin-17A acts to maintain neuropathic pain through activation of CaMKII/CREB signaling in spinal neurons. Mol Neurobiol 2016; 53: 3914–3926. [DOI] [PubMed] [Google Scholar]
- 15.DiFrancesco JC Barbuti A Milanesi R Coco S Bucchi A Bottelli G Ferrarese C Franceschetti S Terragni B Baruscotti M andDiFrancesco D.. Recessive loss-of-function mutation in the pacemaker HCN2 channel causing increased neuronal excitability in a patient with idiopathic generalized epilepsy. J Neurosci 2011; 31: 17327–17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshita K Itoh M Hirashima S Kuwabara Y Ishihara K Kuwahara K Nakao K Kimura T Nakamura K Ushijima K andTakano M.. Ectopic automaticity induced in ventricular myocytes by transgenic overexpression of HCN2. J Mol Cell Cardiol 2015; 80: 81–89. [DOI] [PubMed] [Google Scholar]
- 17.Tsantoulas C Lainez S Wong S Mehta I Vilar B andMcNaughton PA.. Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci Transl Med 2017; 9: eaam6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resta F Micheli L Laurino A Spinelli V Mello T Sartiani L Di Cesare Mannelli L Cerbai E Ghelardini C Romanelli MN Mannaioni G andMasi A.. Selective HCN1 block as a strategy to control oxaliplatin-induced neuropathy. Neuropharmacology 2018; 131: 403–413. [DOI] [PubMed] [Google Scholar]
- 19.Yang JX Hua L Li YQ Jiang YY Han D Liu H Tang QQ Yang XN Yin C Hao LY Yu L Wu P Shao CJ Ding HL Zhang YM andCao JL.. Caveolin-1 in the anterior cingulate cortex modulates chronic neuropathic pain via regulation of NMDA receptor 2B subunit. J Neurosci 2015; 35: 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai C Hsieh M Ho Y Lee A Wang H Cheng J Chau Y andPeng H.. Growth arrest and DNA-damage-inducible protein 45β-mediated DNA demethylation of voltage-dependent T-type calcium channel 3.2 subunit enhances neuropathic allodynia after nerve injury in rats. Anesthesiology 2017; 126: 1077–1095. [DOI] [PubMed] [Google Scholar]
- 21.Wang W Mei X Wei Y Zhang M Zhang T Wang W Xu L Wu S andLi Y.. Neuronal NR2B-containing NMDA receptor mediates spinal astrocytic c-Jun N-terminal kinase activation in a rat model of neuropathic pain. Brain Behav Immun 2011; 25: 1355–1366. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki Y Zhang L Cheng JK andJi RR.. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008; 28: 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki Y Kohno T Zhuang ZY Brenner GJ Wang H Van Der MC Befort K Woolf CJ andJi RR.. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci 2004; 24: 8310–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva K Paszcuk A Passos G Silva E Bento A Meotti F andCalixto J.. Activation of cannabinoid receptors by the pentacyclic triterpene α,β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain 2011; 152: 1872–1887. [DOI] [PubMed] [Google Scholar]