Abstract
Background:
Despite colonoscopic screening, colorectal cancer (CRC) remains frequent in patients with Lynch syndrome (LS). The objective of this study was to evaluate the impact of an optimized colorectal screening program within a French dedicated network.
Methods:
All LS patients followed at our institution were consecutively included in the Prédisposition au Cancer Colorectal-Ile de France (PRED-IdF) network. Patients were offered an optimized screening program allowing an adjustment of the interval between colonoscopies, depending on bowel preparation, chromoendoscopy achievement and adenoma detection. Colonoscopies were defined as optimal when all the screening criteria were respected. We compared colonoscopy quality and colonoscopy detection rate before and after PRED-IdF inclusion, including polyp detection rate (PDR), adenoma detection rate (ADR) and cancer detection rate (CDR).
Results:
Between January 2010 and January 2016, 144 LS patients were consecutively included (male/female = 50/94, mean age = 51 ± 13 years and mutations: MLH1 = 39%, MSH2 = 44%, MSH6 = 15%, PMS2 = 1%). A total of 564 colonoscopies were analyzed, 353 after inclusion and 211 before. After PRED-IdF inclusion, 98/144 (68%) patients had optimal screening colonoscopies versus 33/132 (25%) before (p < 0.0005). The optimal colonoscopy rate was 304/353 (86%) after inclusion versus 87/211 (41%) before, (p < 0.0001). PRED-IdF inclusion was associated with a reduction of CRC occurrence with a CDR of 1/353 (0.3%) after inclusion versus 6/211 (2.8%) before (p = 0.012). ADR and PDR were 99/353 (28%) versus 60/211 (28.8%) (p > 0.05) and 167/353 (48.1%) versus 90/211 (42.2%) (p > 0.05), respectively after and before inclusion.
Conclusions:
An optimized colonoscopic surveillance program in LS patients seems to improve colonoscopic screening quality and might possibly decrease colorectal interval cancer occurrence. Long-term cohort studies are needed to confirm these results.
Keywords: Lynch syndrome, colorectal cancer, colorectal screening program, PRED-IdF network, adenoma detection rate, cancer detection rate
Introduction
Lynch syndrome (LS) is an autosomal dominant disorder due to a DNA germline mismatch mutation, involving 1,000,000 people in the European Union.1,2 It is responsible for 3% of all colorectal cancers (CRCs).1 Currently, there are four main genes (MLH1, MSH2, MSH6 and PMS2) responsible for its genesis, with variable malignancy risks.2 This syndrome is characterized by an accelerated carcinogenesis (median age at diagnosis of CRC of 45 years old) and a high cumulative cancer risk (38% for men and 31% for women at the age of 70 years old).1,3,4
Colonoscopic screening is effective in reducing both the incidence and mortality of CRC by 65% among individuals with LS.1,5–7 Guidelines recommend a CRC screening program with colonoscopies performed every 1–2 years, starting at the age of 20–25 years old.6,8–10 The time between colonoscopic exams in patients with LS is often longer than recommended and may place them at risk for interval cancers. Recent studies have shown that colonoscopy using indigo carmine chromoendoscopy in patients with LS markedly improved adenoma detection, including flat adenomas.11–13 Moreover, providing genetic counseling and an intensive surveillance within a specialized network may improve CRC prevention while increasing patient compliance.7
Based on these findings, the Prédisposition au Cancer Colorectal-Ile de France (PRED-IdF) network, a French specialized network, developed an optimized CRC screening program for LS patients. The objective of this study was to assess the network impact on colonoscopy quality at our center and its consequences regarding lesion detection rate, including CRC detection rate, adenoma detection rate (ADR) and polyp detection rate (PDR).
Methods
Network description
Supported by the French National Cancer Institute (INCa), the PRED-IdF network was created in January 2010 in Paris, France. This multidisciplinary specialized network was built to offer patients with hereditary predisposition to CRC, including LS, an optimized cancer surveillance program. Initially developed in three hospitals, the PRED-IdF now encompasses seven centers: five university hospitals (European Georges Pompidou Hospital, Cochin Hospital, Saint-Antoine Hospital, Pitié Salpêtrière Hospital and Avicenne Hospital) and two oncology dedicated hospitals (Gustave Roussy Institute and the Curie Institute). All patients followed in the network were included after expert medical consultation and written informed consent. LS was confirmed if a mutation in one of the four mismatch repair (MMR) genes was present. After network inclusion, all patients were offered a personal screening program, which included an optimized CRC screening colonoscopy as described below.
Screening program
Patients with LS were enrolled in an optimized screening program as follows: a complete colonoscopy with indigo carmine chromoendoscopy that was performed from 20 years of age, with a 2-year interval. Total colon chromoendoscopy was performed using 0.2% indigo carmine that was either sprayed with a catheter or administered through the air/water channel of the endoscope.14
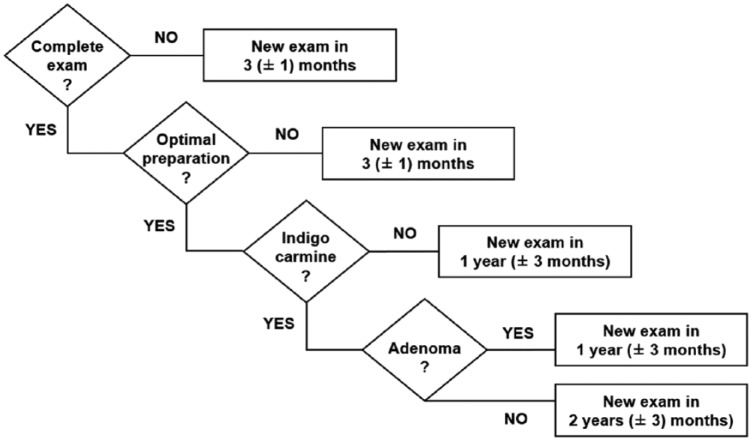
In cases of adenoma detection, the frequency between colonoscopies was increased to 1 year (± 3 months). If chromoendoscopy was not performed, the interval between colonoscopies was also increased to 1 year (± 3 months). During colonoscopy, if bowel preparation was poor or insufficient (according to the endoscopist’s interpretation or according to the Boston scale with scores <7 or a Boston underscore per segment <2), a 3-month interval colonoscopy was then required (± 1 month). (Figure 1).
Figure 1.
Starting at the age of 20, colonoscopy with blue indigo carmine is scheduled every 2 years. In cases of incomplete colonoscopy, insufficient bowel preparation, absence of chromoendoscopy achievement or adenoma detection, the interval between screening colonoscopies was adjusted.
Exam denotes colonoscopy, complete exam denotes caecal intubation, optimal preparation denotes a sufficient bowel preparation according to endoscopist appreciation or defined as a Boston scale >6 with an underscore per segment >2, indigo carmine denotes indigo carmine chromoendoscopy achievement and adenoma denotes adenoma detection during colonoscopy.
Colonoscopies
LS patients followed at the European Georges Pompidou Hospital were consecutively included. Inclusion criteria were: a genetically confirmed MMR mutation (MLH1, MSH2, MSH6 or PMS2) in a patient aged between 20 and 80 years old, a member of the PRED-IdF network with written informed consent and presenting at least two colonoscopies including one after network inclusion. Exclusion criteria were: CRC detected at the first screening colonoscopy or total colectomy (ileo-anal anastomosis or ileo-rectal anastomosis).
We collected retrospective and prospective colonoscopy data. Retrospective and prospective colonoscopies were defined as colonoscopies performed before and after the patient’s inclusion in the network, respectively. Retrospective colonoscopy data were collected from medical reports.
Colonoscopy evaluation
To evaluate the impact of network inclusion on colonoscopic quality, we compared retrospective colonoscopies with prospective colonoscopies (before and after design). Overall, two different types of colonoscopies were then considered: optimal colonoscopy when all criteria for optimized surveillance program were met and nonoptimal colonoscopy when at least one screening criterion was not reached.
Cancer detection rate (CDR), ADR and PDR were defined as the proportion of colonoscopies in which at least one CRC, adenoma or polyp was detected. According to Sanduleanu and colleagues,15 an interval CRC was defined as a CRC diagnosed after a screening colonoscopy and before a scheduled colonoscopy. A high risk adenoma was defined as the presence of three or more adenomas, an adenoma larger than 10 mm, an adenoma with high-grade dysplasia, or an adenoma with a villous component.
Ethics
The research proposal was reviewed by the local ethics committee of our hospital (CPP IDF 2, CERHUPO 2014-04-15). Written informed consent was obtained from all patients entering the PRED-IdF network, for both prospective and retrospective data analysis.
Statistical analyses
Continuous variables are described using means and standard deviations (SDs). We compared means and proportions between groups using a Student’s t test or the Chi-square test (or Fisher’s exact test, if appropriate). All the statistical tests were two-sided, and p-values <0.05 were considered significant.
Results
Colonoscopies and patients inclusion
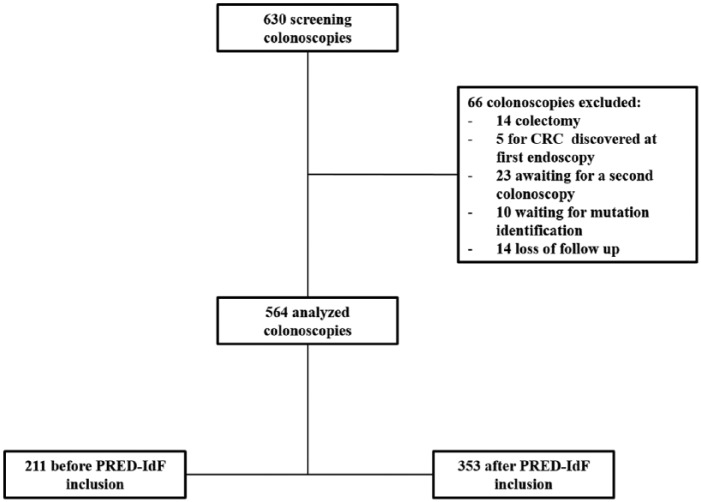
Between January 2010 and January 2016, 630 screening colonoscopies were collected in 192 consecutive LS patients. A total of 564 colonoscopies from 144 patients were analyzed: 353 prospective colonoscopies and 211 retrospectives (Figure 2). Retrospective colonoscopies before inclusion were performed between 2002 to 2012. Among these 144 patients, only 12 had no colonoscopy prior to network inclusion. Mean follow-up period after inclusion was 63.7 ± 31.53 (months), and mean number of colonoscopies per patient was 5 ± 1.4, including 1 ± 0.8 before inclusion and 3 ± 1.4 after. Colonoscopies were excluded on the basis of: total colectomy (n = 14), diagnostic of CRC at first screening colonoscopies (n = 5), awaiting follow-up colonoscopy (n = 23), awaiting mutation identification (n = 10) or a loss of follow-up contact (n = 14). No major adverse events after colonoscopy were reported in our cohort.
Figure 2.
Flowchart of the study population.
CRC, colorectal cancer; PRED-IdF, Prédisposition au Cancer Colorectal-Ile de France network.
PRED-IdF impact on colonoscopy quality
Baseline characteristics of the study population are presented in Table 1. The characteristics of patients at their first reported colonoscopy before and after inclusion were similar. Colonoscopies were performed with both standard and high definition endoscopes. Regarding patient compliance, 98/144 (68%) had optimal colonoscopies after inclusion versus 33/132 (25%) before (p < 0.0001). Optimal colonoscopies were more often performed after network inclusion 304/353 (86%) than before 87/211 (41%) (p < 0.0001).
Table 1.
Baseline characteristics of the populations at their first reported colonoscopies, before and after network inclusion.
p-values are for the comparison between after and before PRED-IdF inclusion.
Chi-square tests or Fisher’s exact tests were used for the comparison of categorical variables, and the Student’s t test was used for the comparison of nonparametric variables.
| Before inclusion | After inclusion | p-value | |
|---|---|---|---|
| General characteristics | N = 118 | N = 144 | |
| Age – mean ± SD (years) | 46 ± 13 | 51 ± 13 | <0.005 |
| Sex male — no. (%) | 38 (32) | 50 (34.7) | 0.7 |
| Personal history of CRC at inclusion — no. (%) | 36 (27) | 36 (25) | 0.7 |
| Personal history of adenoma at inclusion — no. (%) | 30 (31) | 47 (32.6) | 0.6 |
| Personal history of high risk adenoma at inclusion — no. (%) | 6 (5) | 12 (8) | 0.8 |
| Personal history of previous screening colonoscopy at inclusion — no. (%) | 71 (60) | 132 (92) | <0.005 |
| Number of previous screening colonoscopies at inclusion | − | 1 ± 0.8 | |
| Mutations — no. (%) | N = 118 | N = 144 | |
| MLH1 | 46 (39) | 56 (39) | 1 |
| MSH2 | 52 (45) | 64 (45) | 1 |
| MSH6 | 18 (15) | 22 (15) | 1 |
| PMS2 | 2 (1) | 2 (1) | 1 |
CRC, colorectal cancer; PRED-IdF, Prédisposition au Cancer Colorectal-Ile de France network; SD, standard deviation.
Analysis of quality criteria of colonoscopies showed that the optimal bowel preparation rate was 327/353 (92%) after inclusion versus 159/211 75.8% (p < 0.002) before inclusion. The completion rate of indigo carmine chromoendoscopy was 300/353 (85%) after inclusion versus 129/211 (61.1%) before inclusion (p < 0.0001). Moreover, when an optimal bowel preparation was obtained, chromoendoscopy was more often applied after inclusion with a completion rate of 300/325 (92.3%) versus 129/159 (81.1%) before (p < 0.0001). The mean delay between colonoscopies was 22 ± 9.4 months after inclusion versus 24 ± 11.5 before inclusion (p = 0.001). Reasons for nonoptimal colonoscopies were either an absence of indigo carmine chromoendoscopy 75/173 (43.3%), too long an interval between two colonoscopies 65/173 (37.6%) or both 33/173 (19.1%).
Cancer occurrence findings
A total of one CRC was diagnosed after inclusion compared with six before (p = 0.012). Remarkably, no interval CRC was diagnosed in both groups. The characteristics of all cancers are described in Table 2. Among these CRCs, six were diagnosed in patients presenting from nonoptimal colonoscopies: one without previous chromoendoscopy, one with a too long interval between colonoscopies and four for both reasons. Most cancers were proximal (71%) and diagnosed at an early stage (85.6%). The mean delay between CRC diagnosis and previous screening colonoscopy was 27 ± 28.2 months. No death due to CRC was reported in our population study.
Table 2.
Characteristics of the interval CRCs.
Delays are expressed in months.
The bowel preparation scale was assessed as follows:
– insufficient: when preparation was poor according to endoscopist evaluation or when the Boston underscore for one segment was <2.
– sufficient: when preparation was moderate or good according to endoscopist evaluation or when the Boston underscore for each segment was >2.
– excellent: when preparation was excellent according to endoscopist evaluation or when the Boston underscore for each segment was 3.
| Age (years) | Mutation | TNM stage | Localization | Delay since prior complete colonoscopy | Bowel preparation quality on prior colonoscopy | Delay since prior chromo colonoscopy | Adenoma detected in prior colonoscopy | PRED-IdF inclusion |
|---|---|---|---|---|---|---|---|---|
| 71 | hMSH6 | T2N0M0 | Transverse | 12 | Sufficient | 36 | No | Yes |
| 75 | hMSH2 | T1N0M0 | Left | 22 | Sufficient | 52 | No | No |
| 47 | hMSH2 | T2N0M0 | Right | 12 | Insufficient | 25 | No | No |
| 57 | hMLH1 | T1N0M0 | Right | 41 | Excellent | 101 | No | No |
| 28 | hMLH1 | T1N1M0 | Right | 30 | Sufficient | 30 | Yes | No |
| 50 | hMSH2 | T2N0M0 | Left | 27 | Sufficient | 27 | No | No |
| 30 | hMLH1 | T4N1M0 | Right | 15 | Insufficient | 27 | Yes | No |
CRC, colorectal cancer; PRED-IdF, Prédisposition au Cancer Colorectal-Ile de France network.
Lesion detection rates
A total of 658 polyps including 250 adenomas were detected. Lesion detection rates are presented in Table 3. There was no difference for ADR before and after inclusion in the PRED-IdF network, with an ADR of 28% versus 28.8% respectively (p = 0.932). Concerning high risk adenoma and detection of at least two adenomas, ADRs were increased after network inclusion but without reaching statistical significance. More flat adenomas were detected after inclusion with a specific ADR of 15.6% (p < 0.005). Notably, detection of adenomas were higher in colonoscopies performed on male patients (p = 0.001) and for patients above 40 years old (p = 0.011). No difference was detected for the PDR, with 170/353 (48.1%) after inclusion versus 89/211 (42.2%) before, (p > 0.05).
Table 3.
Colonoscopies findings, according to the inclusion status.
bp-values are for the comparison between after PRED-IdF and before PRED-IdF groups.
Chi-square tests or Fisher’s exact tests were used for the comparison of categorical variables, and the Student’s t test was used for the comparison of nonparametric variables.
| All colonoscopies |
After PRED-IdF inclusion |
Before PRED-IdF inclusion |
p-valuea | ||||
|---|---|---|---|---|---|---|---|
| (N = 564) |
(N = 353) |
(N = 211) |
|||||
| N | N | N | |||||
| Cancer — no. (%) | |||||||
| Interval CRC | 7 (0.12%) | 1 (0.28%) | 6 (2.8%) | 0.012 | |||
| Adenomas — no. (%) | |||||||
| At least one adenoma | 564 | 159 (28.20%) | 353 | 99 (28.00%) | 211 | 60 (28.840%) | 0.932 |
| Male adenoma | 211 | 78 (35.00%) | 128 | 44 (33.37%) | 83 | 34 (42.16%) | 0.381 |
| Female adenoma | 353 | 80 (22.66%) | 226 | 55 (17.82%) | 127 | 25 (19.68%) | 0.355 |
| Under 40 y-old ⩽ | 152 | 31 (20.39%) | 67 | 21 (31.34%) | 51 | 10 (19.42%) | 0.205 |
| Above 40 y-old > | 412 | 128 (31.06%) | 255 | 78 (30.58%) | 127 | 50 (39.37%) | 0.107 |
| High risk adenoma | 564 | 45 (7.97%) | 353 | 32 (9.06%) | 211 | 13 (6.16%) | 0.261 |
| Flat adenoma | 564 | 68 (12.05%) | 353 | 55 (15.58%) | 211 | 13 (6.16%) | <0.005 |
| Right-sided adenoma | 564 | 66 (11.91%) | 353 | 45 (12.74%) | 211 | 21 (9.95%) | 0.345 |
| At least two adenomas or more | 564 | 60 (10.63%) | 353 | 39 (11.04%) | 211 | 21 (9.95%) | 0.778 |
| Polyps | |||||||
| At least one polyp — no. (%) | 564 | 258 (45.74%) | 353 | 170 (48.15%) | 211 | 89 (42.18%) | 0.190 |
| Number of polyps — no. (%) | |||||||
| (0–5) | 564 | 231 (40.57%) | 353 | 153 (43.34%) | 211 | 78 (36.96%) | 0.156 |
| (6–10) | 564 | 23 (4.07) | 353 | 14 (3.96%) | 211 | 9 (3.96%) | 0.828 |
| >10 | 564 | 7 (1.24%) | 353 | 5 (1.41%) | 211 | 2 (0.94%) | 1 |
CRC, colorectal cancer; PRED-IdF, Prédisposition au Cancer Colorectal-Ile de France network.
Discussion
This monocentric French study involving 144 LS patients within a specialized network is one of the first to illustrate the potential beneficial impact of an optimized colonoscopic screening program based on colonoscopic quality and colonoscopy detection rate improvement. This will possibly lead to the prevention of CRC occurrence.
The PRED-IdF is a dedicated network to hereditary CRC and is not limited to LS. This network also involves familial adenoma polyposis, familial colorectal type X cancer, Peutz–Jeghers syndrome, serrated polyposis, Cowden syndrome and juvenile polyposis. Funded by the Institut National du Cancer (INCa), the feasibility and the deployment of our network was only possible thanks to institutional funding from the INCa. Indeed, in the first three opened centers, we observed an important impact in terms of activity and the need for personnel recruitment. Currently, more than 2700 patients are followed up in the seven centers, responsible for more than 4600 dedicated multidisciplinary meetings since the creation of this network. An economic evaluation of the medical impact of our network has to be assessed to address whether this platform is efficient and whether it could be nationally deployed.
In LS, colorectal cancer screening based on regular colonoscopies has shown its effectiveness in reducing CRC-related mortality. In 2007, Mecklin and colleagues evaluated a cohort of 420 LS patients with screening colonoscopies performed every 2 years. In this study, the mean follow up was 6.7 years and they detected 25 interval cancers, mostly at an early stage (80%) and a proximal localization (57%).3 Since 2007, three prospective studies were published involving between 109 to 1126 LS patients with various screening programs: colonoscopies performed every year to every 2 years and with a mean follow up ranging from 3.7 to 10 years.10,16,17
Comparatively, the PRED-IdF CRC screening algorithm is innovative because it adds two major criteria: indigo carmine chromoendoscopy achievement and adenoma detection. Indigo carmine chromoendoscopy consists in a topical application of the blue dye, indigo carmine, over the colonic mucosa using a dye spray catheter in order to improve mucosal contrast.11,18 Several studies have demonstrated the effectiveness in both standard and LS CRC screening programs, by improving ADR especially for flat and small adenomas.12,19,20 Recently, Rahmi and colleagues showed, in a multicenter prospective tandem colonoscopy study, that indigo carmine chromocolonoscopy significantly improved ADR compared with standard colonoscopy.13
Adenomas appear to be the most important precursor of CRC in LS. In sporadic CRC, progression through the adenoma–carcinoma sequence is believed to take a decade while in LS, this process is accelerated.4 Flat or small adenomas are more often diagnosed in LS and can be particularly prone to malignant transformation, even at a small size.21 In the literature, the rate of missing flat adenoma lesions can be up to 20%.3,22,23 Consequently, the PRED-IdF recommends reducing the interval between two screening colonoscopies to 1 year if an adenoma is detected at previous colonoscopy.
In a previous study, Stoffel and colleagues demonstrated that factors associated with an appropriate CRC surveillance program were a personal history of CRC, a first degree relative with CRC at age <50, and having undergone a genetic evaluation.7 In our cohort, all our patients had undergone a genetic evaluation, and more than 25% had a personal history of CRC. Furthermore, we showed that following inclusion in our network, the colonoscopic quality had remarkably increased with improved patient compliance (higher rate of adequate bowel preparation) and improved physician awareness and training (higher rate of chromoendoscopy). Even though our follow-up period was moderate, only five patients were lost during follow up representing 3.4% (5/144) of our study population. These results are encouraging and need to be confirmed through a longer follow-up period.
CDR criteria in our study excluded colonoscopies where CRC was detected at the first screening. Even when the ADR and PDR were similar, we found a significant difference for the CRC detection rate before and after implementation of our screening program. This result must be approached with pessimism due to potential related bias of our study population. Indeed, since we studied the same cohort before and after an intervention, where new patients were enrolled over time, this caused lead-time bias. This is reflected by the mean age of patients being significantly older and a higher rate of personal history of colorectal screening at inclusion. The historical effect is important in our cohort, thus leading to a more naïve cohort and an increased number of cancers detected before inclusion.
Characteristics of diagnosed CRC were similar to previous studies: mostly at early stage (80%) and localized in the right colon (50%).16,17 Remarkably, one of the CRCs detected before inclusion was diagnosed in the distal colon only after an optimal screening program. This patient was 50 years old and had a previous history of CRC. His previous screening colonoscopy was performed with a nonhigh definition (HD) colonoscope and was 4 months delayed according to the PRED-IdF guidelines. The most likely reason to explain this CRC is a missed adenoma at previous colonoscopy. Nowadays, HD endoscopes are recommended because they are known to significantly improve ADR and should be considered for all screening colonoscopies in the LS population.24
Another important factor for CRC occurrence after an optimal colonoscopy could be the development of CRC secondary to nonpolypoid pathways. In LS, pathways other than the conventional adenoma–carcinoma pathway have been described. Firstly, the serrated adenoma pathway is responsible for almost 30% of CRCs in the general population, and CRC arising from sessile serrated adenomas/polyps have been described in the LS population. Recently, Vleugels and colleagues showed that the detection rate of sessile serrated polyps/adenomas in LS patients was comparable with the standard population.25 Secondly, the colonic gut-associated lymphoid tissue (GALT) pathway can give rise to CRC; however, this occurs much less frequently comparatively with others. Indeed, in a recent small cohort study, Rubio and colleagues described 20 cases of CRCs that developed from GALT with two cases where CRC developed from patients with LS.26
Despite a significant difference between groups for the CDR, we found no difference in the ADR. These results may be explained by three reasons. Firstly, the historical effect results in the adenoma detection being higher in the beginning of the CRC screening. Following adenoma resections, the incidence would decline thus leading to a lower detection rate than expected. Secondly, the low mean age of our cohort is biased by the adenoma development rate which is lower in patients under the age of 40 years old even in LS. Accordingly, these data are supported by a lower ADR for patients under 40 years old of 33.5% (128/132) after inclusion, versus 24.2% (31/128) before; p = 0.06. Lastly, the small number of patients included in our study leads to difficulties in obtaining statistical power. Ideally, to show an increase of 5% of the ADR after inclusion, a total of 359 colonoscopies in each group would have been required (alpha error: 5%, beta error: 20%, power of the study: 90%).
In the standard population, the ADR is an important quality indicator for screening colonoscopies. Geographic differences in the epidemiology of CRC and its precursors makes establishing a universal threshold for the rate of adenoma detection impossible. Nevertheless, a minimal rate of 20% for adenoma detection in patients over 50 years old has been proposed, with a specific rate of 25% for men and of 15% for women.27,28 Such valuable data do not exist in the LS population. The two recent studies based on tandem colonoscopies comparing white light colonoscopy and indigo carmine chromoendoscopy described the ADR in the LS population. First, Lecomte and colleagues observed an ADR of 30.6% after the analysis of 36 colonoscopies.12 Second, Rahmi and colleagues confirmed this result with an ADR of 30.8% after the analysis of 78 colonoscopies.13 In our study, we observed a lower ADR of 28%. The minimal ADR needs to be assessed in LS through a large database study; however, this study still provides valuable insight into ADR detection in LS.
An important limitation of our study is its monocentric design. In our study, we evaluated the impact of a screening strategy developed within a network only at our institution, the Georges Pompidou European Hospital. Although screening recommendations are the same for all centers, we cannot exclude a center effect. As in other retrospective studies, selection bias can influence the data. Although complete information was obtained and verified, the accuracy of the data was dependent on the medical records. One other limitation is due to our study population. We used the number of colonoscopies rather than patients as our statistical unit. This was responsible for an important bias called the historical effect, with different follow-up times and different exposures to colonoscopic examination. Overall, one of our objectives was to evaluate the effectiveness of the PRED-IdF algorithm based only on colonoscopic characteristics, independently from patients. The optimal study would have been a randomized multicentric controlled study, comparing our screening strategy with the European guidelines screening strategy.
Conclusion
In this study, we demonstrated that the PRED-IdF CRC screening program in the LS population might improve colonoscopy screening quality, thus possibly increasing the preneoplastic lesion detection rate and decreasing CRC occurrence. Our results support the finding that in the LS population, the use of an optimized CRC screening program based on the delay between colonoscopies, chromoendoscopy achievement, and adenoma detection is needed. Long-term follow-up studies are necessary to investigate whether such a screening protocol can reduce CRC occurrence and therefore, reduce CRC-related mortality.
Acknowledgments
We would like to thank all patients followed up in our network who kindly authorized us to use their medical data and perform this study. We would like to thank all physicians and nurses working in the endoscopy department of the European Georges Pompidou Hospital, who helped us to perform all colonoscopies.
Footnotes
Funding: The PRED-IDF network is supported and funded by the INCa (French National Cancer Institute).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Guillaume Perrod  https://orcid.org/0000-0003-4984-0310
https://orcid.org/0000-0003-4984-0310
Contributor Information
Guillaume Perrod, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, FranceFaculté de médecine René Descartes, Paris, France.
Elia Samaha, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, France.
Gabriel Rahmi, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, France; Faculté de médecine René Descartes, Paris, France.
Sherine Khater, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, France.
Leila Abbes, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, France.
Camille Savale, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, France.
Geraldine Perkins, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’oncologie digestive, Paris, France.
Aziz Zaanan, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’oncologie digestive, Paris, France; Faculté de médecine René Descartes, Paris, France.
Gilles Chatellier, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Unité d’Epidémiologie et de Recherche Clinique, Paris, France; Faculté de médecine René Descartes, Paris, France.
Georgia Malamut, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, France; Faculté de médecine René Descartes, Paris, France.
Christophe Cellier, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’hépato-gastro-entérologie et d’endoscopie, Paris, France; Faculté de médecine René Descartes, Paris, France.
References
- 1. Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305: 2304–2310. [DOI] [PubMed] [Google Scholar]
- 2. Jenkins MA, Hayashi S, O’Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology 2007; 133: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mecklin J-P, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 4. Edelstein DL, Axilbund J, Baxter M, et al. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin Gastroenterol Hepatol 2011; 9: 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Järvinen HJ, Mecklin JP, Sistonen P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 1995; 108: 1405–1411. [DOI] [PubMed] [Google Scholar]
- 6. Vasen HFA, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013; 62: 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stoffel EM, Mercado RC, Kohlmann W, et al. Prevalence and predictors of appropriate colorectal cancer surveillance in Lynch syndrome. Am J Gastroenterol 2010; 105: 1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000; 118: 829–834. [DOI] [PubMed] [Google Scholar]
- 9. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003; 348: 919–932. [DOI] [PubMed] [Google Scholar]
- 10. Engel C, Rahner N, Schulmann K, et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010; 8: 174–182. [DOI] [PubMed] [Google Scholar]
- 11. Jaramillo E, Watanabe M, Slezak P, et al. Flat neoplastic lesions of the colon and rectum detected by high-resolution video endoscopy and chromoscopy. Gastrointest Endosc 1995; 42: 114–122. [DOI] [PubMed] [Google Scholar]
- 12. Lecomte T, Cellier C, Meatchi T, et al. Chromoendoscopic colonoscopy for detecting preneoplastic lesions in hereditary nonpolyposis colorectal cancer syndrome. Clin Gastroenterol Hepatol 2005; 3: 897–902. [DOI] [PubMed] [Google Scholar]
- 13. Rahmi G, Lecomte T, Malka D, et al. Impact of chromoscopy on adenoma detection in patients with Lynch syndrome: a prospective, multicenter, blinded, tandem colonoscopy study. Am J Gastroenterol 2015; 110: 288–298. [DOI] [PubMed] [Google Scholar]
- 14. Barret M, Camus M, Leblanc S, et al. Toward an easier indigocarmine chromoendoscopy. World J Gastrointest Endosc 2015; 7: 830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanduleanu S, le Clercq CMC, Dekker E, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. Epub ahead of print 5 September 2014. DOI: 10.1136/gutjnl-2014-307992. [DOI] [PubMed] [Google Scholar]
- 16. Vasen HF, Abdirahman M, Brohet R, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology 2010; 138: 2300–2306. [DOI] [PubMed] [Google Scholar]
- 17. Stuckless S, Green JS, Morgenstern M, et al. Impact of colonoscopic screening in male and female Lynch syndrome carriers with an MSH2 mutation. Clin Genet 2012; 82: 439–445. [DOI] [PubMed] [Google Scholar]
- 18. Rembacken BJ, Fujii T, Cairns A, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet 2000; 355: 1211–1214. [DOI] [PubMed] [Google Scholar]
- 19. Stoffel EM, Turgeon DK, Stockwell DH, et al. Chromoendoscopy detects more adenomas than colonoscopy using intensive inspection without dye spraying. Cancer Prev Res (Phila) 2008; 1: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pohl J, Schneider A, Vogell H, et al. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut 2011; 60: 485–490. [DOI] [PubMed] [Google Scholar]
- 21. Lynch HT, Smyrk T, Jass JR. Hereditary nonpolyposis colorectal cancer and colonic adenomas: aggressive adenomas? Semin Surg Oncol 1995; 11: 406–410. [DOI] [PubMed] [Google Scholar]
- 22. Stoffel EM, Turgeon DK, Stockwell DH, et al. Missed adenomas during colonoscopic surveillance in individuals with Lynch Syndrome (hereditary nonpolyposis colorectal cancer). Cancer Prev Res (Phila) 2008; 1: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006; 101: 343–350. [DOI] [PubMed] [Google Scholar]
- 24. Jrebi NY, Hefty M, Jalouta T, et al. High-definition colonoscopy increases adenoma detection rate. Surg Endosc. Epub ahead of print 10 June 2016. DOI: 10.1007/s00464-016-4986-7. [DOI] [PubMed] [Google Scholar]
- 25. Vleugels JLA, Sahin H, Hazewinkel Y, et al. Endoscopic detection rate of sessile serrated lesions in Lynch syndrome patients is comparable to an age- and gender-matched control population: case-control study with expert pathology review. Gastrointest Endosc. Epub ahead of print 9 December 2017. DOI: 10.1016/j.gie.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 26. Rubio CA, Puppa G, de Petris G, et al. The third pathway of colorectal carcinogenesis. J Clin Pathol 2018; 71: 7–11. [DOI] [PubMed] [Google Scholar]
- 27. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 28. Lieberman D, Nadel M, Smith RA, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc 2007; 65: 757–766. [DOI] [PubMed] [Google Scholar]