Abstract
AIM
To perform a systematic review and meta-analysis on donor-to-recipient gender mismatch as a risk factor for post-transplant graft loss.
METHODS
A systematic literature search was performed using PubMed, Cochrane Library database and EMBASE. The primary outcome was graft loss after liver transplantation. Odds ratios and 95% confidence intervals were calculated to compare the pooled data between groups with different donor-to-recipient gender matches. Three analyses were done considering (1) gender mismatches (F-M and M-F) vs matches (M-M and F-F); (2) Female-to-Male mismatch vs other matches; and (3) Male-to-Female mismatch vs other matches.
RESULTS
A total of 7 articles were analysed. Gender mismatch (M-F and F-M) was associated with a significant increase of graft loss respect to match (M-M and F-F) (OR: 1.30; 95%CI: 1.13-1.50; P < 0.001). When F-M mismatch was specifically investigated, it confirmed its detrimental role in terms of graft survival (OR: 1.83; 95%CI: 1.20-2.80; P = 0.005). M-F mismatch failed to present a significant role (OR: 1.09; 95%CI: 0.73-1.62; P = 0.68).
CONCLUSION
Gender mismatch is a risk factor for poor graft survival after liver transplantation. Female-to-male mismatch represents the worst combination. More studies are needed with the intent to better clarify the reasons for these results.
Keywords: Graft survival, Female-to-male mismatch, Liver transplantation, Donor-to-recipient match, Gender
Core tip: Limited data exist on the role of donor-to-recipient gender mismatch after liver transplantation. This is the first systematic review and meta-analysis specifically investigating the role of gender match in the setting of liver transplant. Female-to-male mismatch was a risk factor for graft loss, with a 83-fold increased risk.
INTRODUCTION
Liver transplantation (LT) represents the gold-standard therapy for the treatment of more than fifty liver disorders, consenting to obtain excellent results in terms of survival rates even in case of dreadful pathologies[1]. However, LT represents a scarce resource. As a consequence, a careful matching between donor and recipient should be done, with the main intent to optimize the results in terms of post-LT survivals[2]. Gender match seems to represent one of the aspects influencing outcomes after LT, although this association is largely controversial. Monocentric studies showed a correlation between donor gender and graft loss, mainly in case of female donor-to-male recipient (F-M) mismatch[3,4]. On the opposite, a large international study based on 16410 LT subjects did not find any correlation[5].
Recently, several scores aimed at identifying the quality of donors have been developed, with the main intent to optimize the donor-to-recipient matching and to predict post-transplant outcomes[6,7]. However, no one of them showed donor gender as a risk factor for poor graft survival, thus raising the question of whether donor-recipient gender mismatch truly impacts on survival rates.
The main aim of the present study is to report a systematic review of the literature and a meta-analysis focused on investigating the role of donor-to-recipient gender match in the setting of liver transplantation as a potential predictor of graft loss.
MATERIALS AND METHODS
Search strategy
A systematic search was done in relation to relevant studies focusing on the role of gender match in organ donation for LT. The search strategy was done in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) guidelines, as well as PRISMA for abstracts[8]. A search of the electronic databases MEDLINE-PubMed, Cochrane Library and EMBASE was conducted using the following research terms: (gender[tw] OR sex[tw]) AND (discordance[tw] OR mismatch[tw] OR match[tw]) AND (liver transplant*[tw]).
Text word [tw] was preferred respect to MeSH words with the intent to identify In Process citations. Studies published before March 15, 2018, were taken into consideration.
Screening process
The present qualitative systematic review included a priori search criteria of journal articles among adult (age ≥ 18 years) human patients. Studies were limited to the English language.
Exclusion criteria were: (1) Papers lacking sufficient statistical details; (2) review articles; (3) nonclinical studies; (4) expert opinions; (5) letters; (6) conference summaries; and (7) case reports.
Study selection
Two reviewers (QL and FG) independently screened the identified studies and their extracted data. In case of disagreement, the paper was discussed by all the authors.
Quality assessment
Selected studies were reviewed based on the representativeness of the study population, comparability of cohorts, adequate assessment of outcomes, sufficient length of follow-up, adequacy of follow-up, and source of study funding. The quality of the papers was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS): Studies with scores > 6 were defined as high-quality studies[9].
NOS details of each selected study were reported in Table 1. The characteristics coming from each study were collected in Table 2. The following features were collected: Author, year of publication, number of transplanted cases, investigated follow-up period of the study, number of cases for each donor-to-recipient gender combination (M-M, F-F, M-F, and F-M), graft survival for each group reported at the last follow-up and patient survival for each group reported at the last follow-up.
Table 1.
Quality of studies evaluated by the modified Newcastle-Ottawa scale
| Ref |
Selection |
Comparability |
Outcome |
||||||
| Case definition | Representativeness | Selection of controls | Definition of controls | Comparable for therapy | Comparable for etiology | Assessment of outcomes | Integrity of follow-up | Quality score | |
| Kahn et al[17] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★★ |
| Marino et al[18] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★★ |
| Grande et al[19] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★★ |
| Berrevoet et al[20] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★★ |
| Brooks et al[21] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★★ |
| Croome et al[22] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★★ |
| Grat et al[23] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | ★★★★★★★★★ |
Table 2.
Demographic and clinical aspects of the selected studies
| Ref. | Year | n | FU (mo) |
Number for group |
Graft survival (%) |
Patient survival (%) |
|||||||||
| M-M | F-F | M-F | F-M | M-M | F-F | M-F | F-M | M-M | F-F | M-F | F-M | ||||
| Kahn et al[17] | 1993 | 883 | 2 | 350 | 121 | 312 | 50 | 72 | 64 | 72 | 40 | 85 | 83 | 83 | 62 |
| Marino et al[18] | 1995 | 462 | 24 | 201 | 71 | 92 | 98 | 72 | 64 | 78 | 55 | 77 | 82 | 82 | 66 |
| Grande et al[19] | 1997 | 423 | 60 | 189 | 64 | 69 | 101 | 52 | 64 | 59 | 71 | NA | NA | NA | NA |
| Berrevoet[20] | 1997 | 105 | 6 | 40 | 12 | 32 | 21 | 65 | 67 | 66 | 71 | 78 | 100 | 81 | 86 |
| Brooks et al[21] | 1997 | 994 | 24 | 392 | 219 | 247 | 126 | 74 | 76 | 76 | 56 | NA | NA | NA | NA |
| Croome et al[22] | 2013 | 1042 | 120 | 412 | 217 | 249 | 164 | 75 | 65 | 76 | 59 | NA | NA | NA | NA |
| Grat et al[23] | 2015 | 76 | 120 | - | 29 | 47 | - | - | 75 | 73 | - | NA | NA | NA | NA |
FU: Follow-up; M: Male; F: Female; NA: Not available.
Statistical analysis
Follow up period strongly varied from 2 to 120 mo in the investigated studies: graft survival rates were estimated at their last available value. Summary measures were extracted from each study and used to generate a pooled odds ratio (OR). Higgins I2 statistic was used to assess heterogeneity. Higgins I2 statistic values of 0-25%, 25%-50%, and > 50% were considered as indicative of homogeneity, moderate heterogeneity, and high heterogeneity, respectively. When Higgins I2 statistic value was < 25%, a fixed-effects model was used. Conversely, if Higgins I2 statistic value overpassed this threshold, a random-effects model was adopted. OR was considered statistically significant when the P value < 0.05; OR and 95%CI > 1 revealed a higher risk of graft loss, whereas a result < 1 had the opposite meaning. The analysis was performed using OpenMEE software (http://www.cebm.brown.edu/openmee/index.html).
RESULTS
The selection process of the articles is explained in Figure 1.
Figure 1.
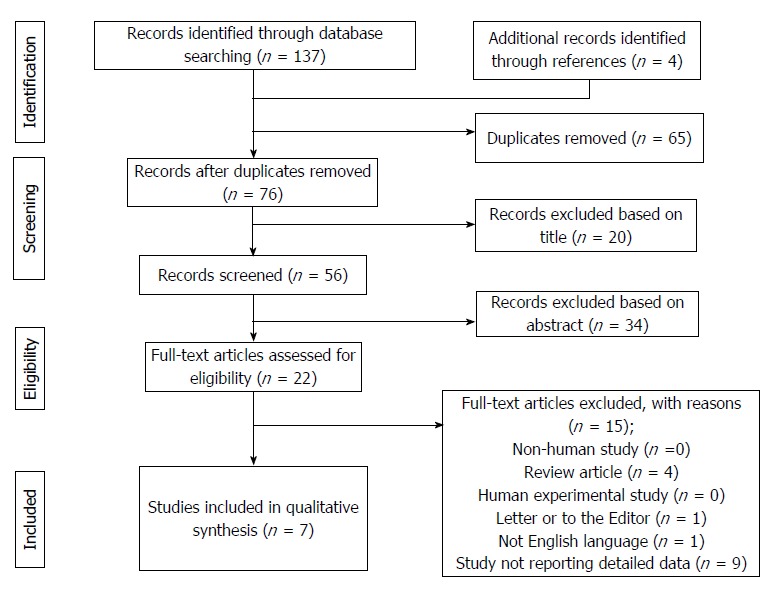
PRISMA flowchart of the literature search and study selection.
As for the selection process according to the PRISMA guidelines, the various examined databases provided a total of 137 articles to screen. Four more articles were added after manual research. After removal of 65 duplicates, 76 articles were available for the screening. According to the title and the abstract, 54 articles were removed. Of the remaining 22 papers, 15 were not considered eligible after full-text evaluation. Unfortunately, in 9 articles specifically investigating the role of gender matching in LT, not enough statistical information was available, thus determining their removal from further analyses[4-5,10-16].
Eventually, 7 articles were identified, with a total of 3935 investigated cases (Table 2)[17-23].
As for the quality of the reported studies, all the investigated articles were retrospective cohort studies all presenting the excellent NOS value of eight, thus reporting the overall high quality of the studies focused on this topic (Table 1).
Three studies were from European countries, three others were from the United States and one from Canada. Five of the reported studies were published before the year 2000. The number of reported cases ranged from 76 to 1042 subjects. Six studies reported all the possible combinations of gender match, while one study only reported M-F and F-F subjects[24-26]. Only looking at the six studies reporting all the possible combinations, M-M cases ranged from 38% to 45% of cases, F-F from 11% to 21%, M-F from 16% to 37% and F-M from 6% to 24%. Globally, M-M cases were 1584, F-F subjects were 743, M-F 1048 and F-M 560. Gender-matched cases (M-M and F-F) were 2327 (59%), whilst mismatched cases (M-F and F-M) were 1608 (41%).
Graft survival was reported in all the studies, although variable follow-up periods were used across the analysed series. In detail, M-M patients reported a graft survival ranging 52%-75%, F-F subjects 64%-75%, M-F cases 59%-78% and F-M individuals 40%-71%.
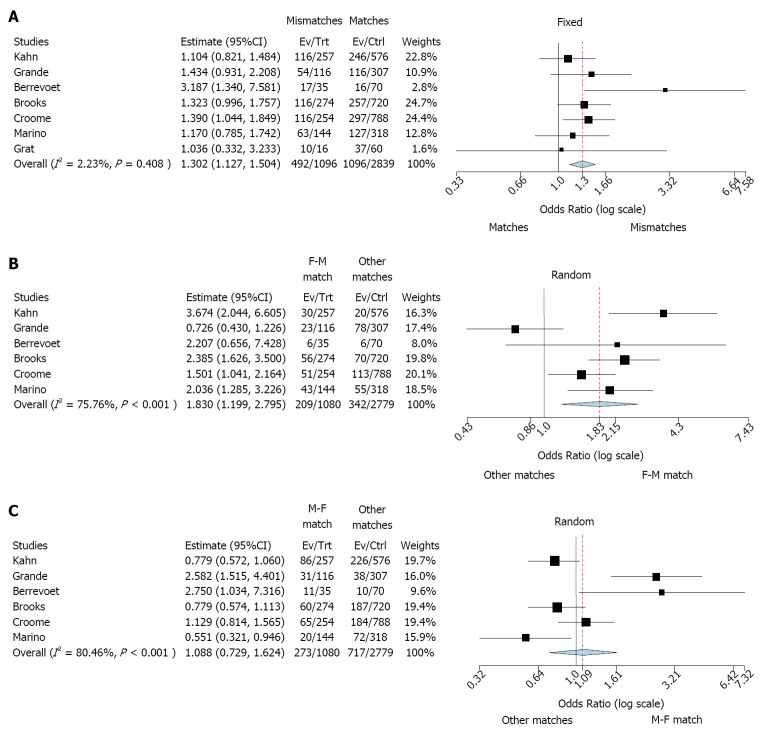
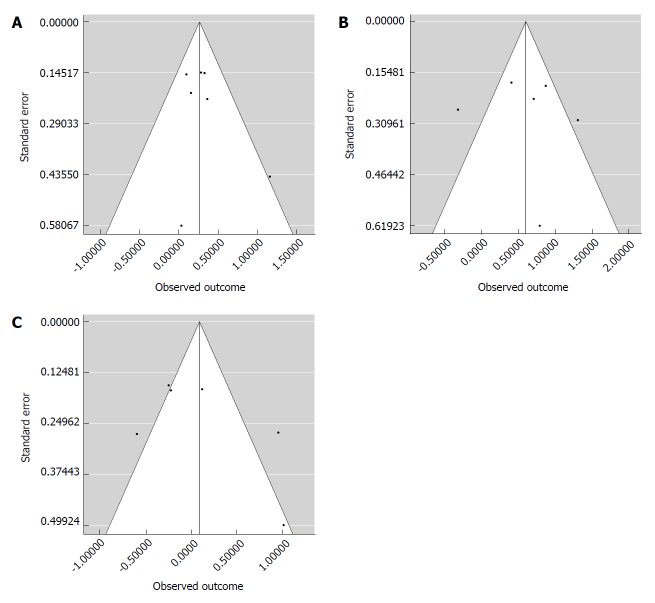
Three different meta-analyses were performed. First, a fixed-effects model was realized comparing matched (M-M and F-F) and mismatched (F-M and M-F) cases. We observed a higher risk for graft loss in mismatched cases (OR: 1.13; 95%CI: 1.30-1.50; P < 0.001) (Figure 2A). Higgins I2 statistic presented a value = 2.2% (P = 0.41), showing homogeneity among the examined studies; funnel plot also did not show publication biases (Figure 3A).
Figure 2.
Forest plot result. A: Forest plot of odds ratios and 95% confidence intervals for the association between any donor-to-recipient mismatch (F-M and M-F) and graft survival in patients undergoing liver transplantation. Weights are from binary fixed-effect analysis; B: Forest plot of odds ratios and 95% confidence intervals for the association between donor-to-recipient F-M mismatch and graft survival in patients undergoing liver transplantation. Weights are from binary random-effect analysis; C: Forest plot of odds ratios and 95% confidence intervals for the association between donor-to-recipient M-F mismatch and graft survival in patients undergoing liver transplantation. Weights are from binary random-effect analysis.
Figure 3.
Funnel plots of the patients undergoing liver transplantation. A: Funnel plot of the seven studies investigating the association between any donor-to-recipient mismatch (F-M and M-F) and graft survival in patients undergoing liver transplantation; B: Funnel plot of the six studies investigating the association between donor-to-recipient F-M mismatch and graft survival in patients undergoing liver transplantation; C: Funnel plot of the six studies investigating the association between donor-to-recipient M-F mismatch and graft survival in patients undergoing liver transplantation.
Then, starting from this evidence, two separated random-effects models were done investigating the specific role of F-M and M-F mismatches, respectively. When F-M mismatch was compared with the other three combinations, we reported a higher risk for graft loss in mismatched cases (OR: 1.83; 95%CI: 1.20-2.80; P = 0.005) (Figure 2B).
Higgins I2 statistic presented a value = 75.8% (P < 0.001), showing a great heterogeneity among the examined studies; funnel plot showed the presence of publication biases (Figure 3B).
Lastly, when M-F mismatch was compared with the other three combinations, we did not report any increased risk for graft loss in mismatched cases (OR: 1.09; 95%CI: 0.73-1.62; P = 0.68) (Figure 2C). Higgins I2 statistic presented a value = 80.5% (P < 0.001), showing a great heterogeneity among the examined studies; funnel plot showed the presence of publication biases (Figure 3B).
DISCUSSION
The results reported in the present meta-analysis suggest a detrimental role of the F-M mismatch in terms of graft survival. On the opposite, the M-F mismatch was not related to any negative course. These results may be connected with several possible explanations. For example, different donor female and male hormones should play a role in this phenomenon[11]. Some studies showed that a connection exists between estrogens and protection to ischemic injury: in other terms, when a female liver is removed from its homeostasis, the ischemic damage is major respect to a male one[24]. Estrogens also participate in favoring cholangiocyte proliferation and, consequently, the post-ischemic biliary repair[25].
Another possible explanation should be related to the differences in size among human females and males. Given that women are statistically smaller than men, and thus, by extension, have smaller livers, we should also postulate that an F-M mismatch may be connected with a greater risk for initial poor graft due to a small-for-size syndrome, a higher rate of complex vascular and biliary reconstruction due to the size discrepancy and, ultimately, longer warm ischemia times during the transplant[26]. Similar considerations should be done when other surrogates of size match have been investigated: for example, the American Donor Risk Index failed to demonstrate an effect of gender as a risk factor for graft failure, but the variable “height” was present, clearly demonstrating that a discrepancy in terms of donor-to-recipient size is an important risk factor[6].
It is interesting to note that the evidence that F-M mismatch is related to poor results has been reported in several experiences worldwide. A study from Japan showed that F-M mismatch related to a greater risk for patient death in a specific living-donor LT setting (OR: 2.10; 95%CI: 1.24-3.57; P = 0.006)[14]. A study from Germany based on 2144 LT cases showed that 1-, 5-, 10- and 15-year graft survival rates progressively decremented starting from the M-F combination (84%, 76%, 68% and 61%) to the F-F match (83%, 76%, 64% and 56%), the M-M match (85%, 72%, 63% and 53%) and, lastly, the F-M mismatch (80%, 66%, 56% and 49%) (P = 0.003)[15].
However, some caution should be taken into account in definitively considering F-M mismatch as a risk factor for graft loss. It is, in fact, important to underline that several confounders should influence the results observed in our study. An interesting study from the United States investigated a large multicentric population of 28222 LT recipients, showing that female donors were different respect to male ones for several risk factors of poor post-LT course, like age (median: 47 years vs 39 years), height (165 cm vs 178 cm), and cerebrovascular accident as cause of death (59% vs 35%) (P < 0.001): F-M mismatch was associated with a 17% increased risk of graft loss respect to an M-M match (95%CI: 1.11-1.24; P < 0.001), whereas M-F mismatch was not (HR = 1.02; 95%CI: 0.96-1.09; P = 0.46)[12]. These results are absolutely in line with the results observed in the present meta-analysis. However, when F-M mismatch was adjusted for significant recipient- and donor-related risk factors, its association with graft loss disappeared (HR = 0.95; 95%CI: 0.89-1.02; P = 0.18)[12].
The present study presents some shortcomings. The observed results should suggest the necessity of a meta-regression for minimizing the effect of potential confounders (donor age, donor ethnicity, ischemia time duration, and the presence of donor co-morbidities). Unfortunately, it was impossible to perform such an analysis according to the data obtainable from the selected studies. Funnel plots confirmed the presence of study biases, further suggesting the idea that some confounders may participate in altering the results of the meta-analysis. Another possible shortcoming of the present study is connected with the great heterogeneity observed among the studies in terms of the follow-up period. We can only assume that, although some studies presented a very short period of observation (only 60 d in one case[17]), such a period was able to capture a significant number of events: it is, in fact, clear that the first post-LT months typically represent the period in which the higher rate of graft loss is observed. Lastly, some studies were performed in the early nineties, thus reporting the early results of some LT centers. However, we should report that the negative role of F-M mismatch was observed also in more recent studies[22,23].
In summary, female to male donor-recipient mismatch represents a risk factor for graft loss after liver transplantation, with an 83-fold increased risk of graft failure. Several mechanisms should be postulated: Hormones, a major vulnerability to ischemic damages or size discrepancies have been advanced as possible explanations. However, some confounders should be taken into account. As a consequence, further large studies trying to design well-calibrated studies are needed, with the intent to definitively clarify the potential detrimental role of gender mismatch in the setting of liver transplantation.
ARTICLE HIGHLIGHTS
Research background
Donor-to-recipient gender match has been described as a possible risk factor for post-liver transplant outcomes, mainly when a female-to-male mismatch is done. However, no definitive data exist on this aspect, with only some, mainly monocentric, studies showing somewhat contrasting results. The impact of a meta-analysis on this aspect should be great, mainly in function of the opportunity to clarify a capital element of the organ allocation process in the setting of liver transplantation.
Research motivation
The main aim of the present study is to clarify the role of donor-to-recipient gender mismatching in the setting of liver transplantation. The problem connected to this research is that no definitive clarity exists on the possible risks connected with the use of female donors for transplanting male recipients, although several studies raised on some concerns about this specific matching. The possibility to better clarify this aspect is connected with a safer opportunity to allocate organ during liver transplantation, thus improving the postoperative outcomes of subjects undergoing this type of transplant.
Research objectives
The main objective of the study was to better clarify the role of donor-to-recipient gender mismatch as a possible real risk factor for post-liver transplant graft and patient survival, or if its negative role was caused by several other confounding aspects in the allocation process.
Research methods
Three separate meta-analyses were realized after the systematic collection of all the articles available on English literature focused on the specific argument of donor-to-recipient gender match. First, a meta-analysis focused on the comparison between matched and mismatched cases was done. After this, two separate analyses were done specifically looking at the F-M and M-F mismatches.
Research results
According to the observed results, donor-to-recipient gender mismatch represented a risk factor for post-transplant outcomes, with a 30-fold increased risk for graft loss. When F-M mismatch was specifically investigated, an 83-fold increased risk for graft loss was reported, while such a risk was not present when an M-F mismatch was investigated. Despite the results confirmed the negative role of an F-M mismatch, open questions remained on its effective role, mainly in light of the presence of possible confounding factors potentially justifying these poorer results (i.e., donor and recipient age, recipient disease severity and cause, donor ethnicity, ischemia time duration, and the presence of donor co-morbidities).
Research conclusions
Gender mismatch is a risk factor for poor graft survival after liver transplantation. Female-to-male mismatch represents the worst combination. A particular caution should be taken into account when this combination is present, thus improving the elements to consider during the organ allocation process.
Research perspectives
New studies are needed in this specific setting, with the intent to better clarify the reasons for the poor graft survivals observed in presence of a donor-to-recipient F-M gender mismatch. These studies mainly need to explore the possible confounders potentially being the cause for the reported results.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
First decision: March 30, 2018
Peer-review started: March 22, 2018
Article in press: May 18, 2018
P- Reviewer: Kute VB, Sugawara Y S- Editor: Wang XJ L- Editor: A E- Editor: Wang C
Contributor Information
Quirino Lai, Hepato-bilio-pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Rome 00161, Italy. lai.quirino@libero.it.
Francesco Giovanardi, Hepato-bilio-pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Rome 00161, Italy.
Fabio Melandro, Hepato-bilio-pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Rome 00161, Italy.
Zoe Larghi Laureiro, Hepato-bilio-pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Rome 00161, Italy.
Manuela Merli, Division of Gastroenterology, Department of Clinical Medicine, Sapienza University of Rome, Rome 00161, Italy.
Barbara Lattanzi, Division of Gastroenterology, Department of Clinical Medicine, Sapienza University of Rome, Rome 00161, Italy.
Redan Hassan, Hepato-bilio-pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Rome 00161, Italy.
Massimo Rossi, Hepato-bilio-pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Rome 00161, Italy.
Gianluca Mennini, Hepato-bilio-pancreatic and Liver Transplant Unit, Department of Surgery, Sapienza University of Rome, Rome 00161, Italy.
References
- 1.Starzl TE. The long reach of liver transplantation. Nat Med. 2012;18:1489–1492. doi: 10.1038/nm.2927. [DOI] [PubMed] [Google Scholar]
- 2.Avolio AW, Cillo U, Salizzoni M, De Carlis L, Colledan M, Gerunda GE, Mazzaferro V, Tisone G, Romagnoli R, Caccamo L, Rossi M, Vitale A, Cucchetti A, Lupo L, Gruttadauria S, Nicolotti N, Burra P, Gasbarrini A, Agnes S; Donor-to-Recipient Italian Liver Transplant (D2R-ILTx) Study Group. Balancing donor and recipient risk factors in liver transplantation: the value of D-MELD with particular reference to HCV recipients. Am J Transplant. 2011;11:2724–2736. doi: 10.1111/j.1600-6143.2011.03732.x. [DOI] [PubMed] [Google Scholar]
- 3.Velidedeoglu E, Mange KC, Frank A, Abt P, Desai NM, Markmann JW, Reddy R, Markmann JF. Factors differentially correlated with the outcome of liver transplantation in hcv+ and HCV- recipients. Transplantation. 2004;77:1834–1842. doi: 10.1097/01.tp.0000130468.36131.0d. [DOI] [PubMed] [Google Scholar]
- 4.Rustgi VK, Marino G, Halpern MT, Johnson LB, Umana WO, Tolleris C. Role of gender and race mismatch and graft failure in patients undergoing liver transplantation. Liver Transpl. 2002;8:514–518. doi: 10.1053/jlts.2002.33457. [DOI] [PubMed] [Google Scholar]
- 5.Zeier M, Döhler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13:2570–2576. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 6.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 7.Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, Porte RJ, Rogiers X, Ringers J; European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC) The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789–2796. doi: 10.1111/j.1600-6143.2012.04195.x. [DOI] [PubMed] [Google Scholar]
- 8.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Candinas D, Gunson BK, Nightingale P, Hubscher S, McMaster P, Neuberger JM. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet. 1995;346:1117–1121. doi: 10.1016/s0140-6736(95)91797-7. [DOI] [PubMed] [Google Scholar]
- 11.Lehner F, Becker T, Klempnauer J, Borlak J. Gender-incompatible liver transplantation is not a risk factor for patient survival. Liver Int. 2009;29:196–202. doi: 10.1111/j.1478-3231.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 12.Lai JC, Feng S, Roberts JP, Terrault NA. Gender differences in liver donor quality are predictive of graft loss. Am J Transplant. 2011;11:296–302. doi: 10.1111/j.1600-6143.2010.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Wen TF, Yan LN, Li B, Yang JY, Wang WT, Xu MQ, Wei YG. Predictors of patient survival following living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:248–253. doi: 10.1016/s1499-3872(11)60041-6. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizumi T, Shirabe K, Taketomi A, Uchiyama H, Harada N, Ijichi H, Yoshimatsu M, Ikegami T, Soejima Y, Maehara Y. Risk factors that increase mortality after living donor liver transplantation. Transplantation. 2012;93:93–98. doi: 10.1097/TP.0b013e318238dacd. [DOI] [PubMed] [Google Scholar]
- 15.Schoening WN, Helbig M, Buescher N, Andreou A, Bahra M, Schmitz V, Pascher A, Pratschke J, Seehofer D. Gender Matches in Liver Transplant Allocation: Matched and Mismatched Male-Female Donor-Recipient Combinations; Long-term Follow-up of More Than 2000 Patients at a Single Center. Exp Clin Transplant. 2016;14:184–190. [PubMed] [Google Scholar]
- 16.Zhang Y. Impact of Donor Recipient Gender and Race Mismatch on Graft Outcomes in Patients With End-Stage Liver Disease Undergoing Liver Transplantation. Prog Transplant. 2017;27:39–47. doi: 10.1177/1526924816679839. [DOI] [PubMed] [Google Scholar]
- 17.Kahn D, Gavaler JS, Makowka L, van Thiel DH. Gender of donor influences outcome after orthotopic liver transplantation in adults. Dig Dis Sci. 1993;38:1485–1488. doi: 10.1007/BF01308608. [DOI] [PubMed] [Google Scholar]
- 18.Marino IR, Doyle HR, Aldrighetti L, Doria C, McMichael J, Gayowski T, Fung JJ, Tzakis AG, Starzl TE. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22:1754–1762. [PMC free article] [PubMed] [Google Scholar]
- 19.Grande L, Rull A, Rimola A, Manyalic M, Cabrer C, Garcia-Valdecasas JC, Navasa M, Fuster J, Lacy AM, González FX, et al. Impact of donor gender on graft survival after liver transplantation. Transplant Proc. 1997;29:3373–3374. doi: 10.1016/s0041-1345(97)00945-7. [DOI] [PubMed] [Google Scholar]
- 20.Berrevoet F, Hesse UJ, de Laere S, Jacobs B, Pattyn P, de Hemptinne B. Impact of donor and recipient gender on liver transplantation. Transplant Proc. 1997;29:3431–3432. doi: 10.1016/s0041-1345(97)00966-4. [DOI] [PubMed] [Google Scholar]
- 21.Brooks BK, Levy MF, Jennings LW, Abbasoglu O, Vodapally M, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB. Influence of donor and recipient gender on the outcome of liver transplantation. Transplant Proc. 1997;29:475–476. doi: 10.1016/s0041-1345(96)00212-6. [DOI] [PubMed] [Google Scholar]
- 22.Croome KP, Segal D, Hernandez-Alejandro R, Adams PC, Thomson A, Chandok N. Female donor to male recipient gender discordance results in inferior graft survival: a prospective study of 1,042 liver transplants. J Hepatobiliary Pancreat Sci. 2014;21:269–274. doi: 10.1002/jhbp.40. [DOI] [PubMed] [Google Scholar]
- 23.Grąt M, Lewandowski Z, Patkowski W, Wronka KM, Grąt K, Krasnodębski M, Ligocka J, Zborowska H, Krawczyk M. Relevance of male-to-female sex mismatch in liver transplantation for primary biliary cirrhosis. Ann Transplant. 2015;20:116–123. doi: 10.12659/AOT.892394. [DOI] [PubMed] [Google Scholar]
- 24.Francavilla A, di Leo A, Eagon PK, Wu SQ, Ove P, van Thiel DH, Starzl TE. Regenerating rat liver: correlations between estrogen receptor localization and deoxyribonucleic acid synthesis. Gastroenterology. 1984;86:552–557. [PMC free article] [PubMed] [Google Scholar]
- 25.Alvaro D, Mancino MG, Onori P, Franchitto A, Alpini G, Francis H, Glaser S, Gaudio E. Estrogens and the pathophysiology of the biliary tree. World J Gastroenterol. 2006;12:3537–3545. doi: 10.3748/wjg.v12.i22.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshizumi T, Taketomi A, Uchiyama H, Harada N, Kayashima H, Yamashita Y, Soejima Y, Shimada M, Maehara Y. Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation. Liver Transpl. 2008;14:1007–1013. doi: 10.1002/lt.21462. [DOI] [PubMed] [Google Scholar]