Abstract
Gastric bypass surgery leads to profound changes in the secretion of gut hormones with effects on metabolism, appetite, and food intake. Here, we discuss their contributions to the improvement in glucose tolerance and the weight loss that results from the operations. We find that the improved glucose tolerance is due the following events: a negative energy balance and resulting weight loss, which improve first hepatic and later peripheral insulin sensitivity, in combination with increased postprandial insulin secretion elicited particularly by exaggerated glucagon-like peptide-1 responses. The weight loss is due to loss of appetite resulting in reduced energy intake, and we find it probable that this process is driven by exaggerated secretion of appetite-regulating gut hormones including, but probably not limited to, glucagon-like peptide-1 and peptide-YY. The increased secretion is due to an accelerated exposure to and absorption of nutrients in the small intestine. This places the weight loss and the gut hormones in key positions with respect to the metabolic improvements after bypass surgery.
Keywords: GLP-1, PYY, Insulin resistance, Carbohydrate absorption, Protein absorption, Paracetamol absorption
The interest in the hormonal changes after bariatric surgery derives from 2 fundamental observations: (1) the weight loss appears to result from reductions in appetite and food intake, which suggests that the operation interferes with the normal regulation of appetite and food intake, and (2) the resolution of type 2 diabetes that occurs already a few days after surgery before any major weight loss has occurred [1], [2], suggesting that mechanisms independent of weight loss are involved.
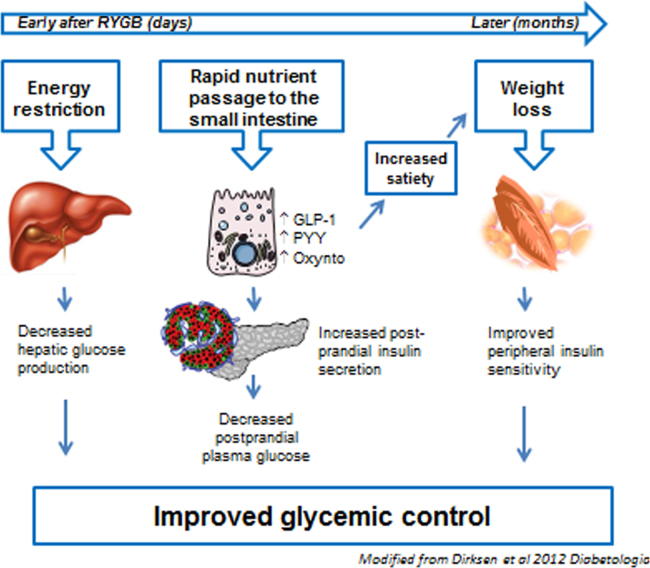
In our laboratory, the interest in the hormonal changes after bariatric surgery began with the observation that jejuno-ileal bypass in humans causes grossly elevated postprandial responses of “enteroglucagon.” Enteroglucagon is a collective designation for glicentin and oxyntomodulin, products of the glucagon precursor, proglucagon, which is also expressed in the L-cells of the gut and here is processed to these 2 peptides [3]. A few years later we discovered that proglucagon in the gut gives rise to 2 additional glucagon-like peptides (GLPs), GLP-1 and GLP-2 [4], with important effects on insulin secretion [5], food intake [6], and intestinal growth [7]. Consistent with our findings after jejuno-ileal bypass, we found that GLP-2 secretion is greatly upregulated by ileal transposition in rats [8]. The potential importance of these changes in hormone secretion for the weight changes was supported by studies by Näslund et al. [9], [10]. However, because of the powerful insulinotropic effects of GLP-1, it was relevant to examine secretion of this hormone in a group of patients undergoing Roux-en-Y gastric bypass (RYGB), who suffered from severe postoperative hypoglycemia [11]. Indeed, in these patients, we measured dramatically elevated postprandial levels of GLP-1, reaching between 300 and>500 pmol/L (i.e., 10–20 times higher normally observed except in people undergoing gastrectomies and those with postoperative dumping) [12]. Soon after, Le Roux et al. [13] published more comprehensive data from both human and animal studies showing exaggerated release of not only GLP-1 but also the additional L-cell product peptide-YY (PYY), which is also considered an appetite-regulating hormone [14]. In further studies, we observed that increases did not involve the other incretin hormone, glucose-dependent insulinotropic polypeptide (GIP), and that similar changes were not observed in patients undergoing gastric banding operations [15]. We also observed that hypoglycemia occurring after RYGB was clearly hyperinsulinemic and was associated with even higher GLP-1 responses and that glucagon responses were paradoxically increased (in spite of the hyperglycemia and the exaggerated GLP-1 responses, which would be expected to inhibit glucagon secretion) [16]. These findings suggested that the surgical rearrangement after RYGB, similar to that after jejuno-ileal bypass, was responsible for the dramatic change in gut hormone secretion and that diabetes resolution and perhaps also loss of appetite/weight loss might be consequences of these changes. Indeed, in a patient with insulin-treated type 2 diabetes who had a gastrostomy catheter inserted after the operation, we were able to study metabolite and hormone secretion on 2 consecutive days 5 weeks postoperatively, when the patient was completely well and was given identical meals either orally (via the bypass) or via the gastrostomy catheter (i.e., via the “original” pathway: stomach, duodenum, and upper small intestine) [17]. On the day of gastrostomy feeding, he had diabetes and “normal” GLP-1 and insulin values; on the oral day, he had normal glucose tolerance and large GLP-1 and insulin responses. On the background of these and other studies we formulated a hypothesis [18] regarding diabetes resolution and weight loss after RYGB, shown diagrammatically in Fig. 1 [19].
Fig. 1.
Timeline of important mechanisms in weight loss and diabetes resolution after RYGB.
Mechanisms explaining diabetes resolution after RYGB
Early improvements in insulin sensitivity
The most important early event is probably the dramatic change in hepatic insulin resistance, which occurs within a few days and reaches approximately 50% of preoperative values after just 1 week [2]. At this time, the values are equivalent to those obtained in matched, glucose-tolerant individuals. The decrease is evident whether determined by calculation of the homeostatic model assessment - insulin resistance (HOMA-IR) value from basal glucose and insulin levels or measured using clamp and glucose tracer methodology [20]. Concomitantly with the changes in hepatic insulin sensitivity, insulin clearance increases immediately after RYGB, which importantly influences peripheral insulin concentrations (i.e., evaluation of changes in insulin secretion must be based on C-peptide measurements). These improvements are likely due to the postoperative calorie restriction and the ensuing loss of liver fat as elegantly demonstrated using magnetic resonance imaging for liver fat [21], although the improvement in insulin sensitivity after surgery was actually larger than that observed after a very low calorie intervention [22]. As recently demonstrated, liver fat is an important determinant of HOMA-IR/hepatic insulin sensitivity as well as insulin clearance [23], [24].
The early changes in hepatic insulin sensitivity lead to a rapid lowering of basal glucose concentrations, which may contribute to removal of glucotoxic effect on pancreatic beta cells as illustrated by the rapid enhancement of first phase insulin secretion in patients with type 2 diabetes [25]. With time and the accompanying weight loss, glucose disposal in peripheral tissues (muscle and fat) shows continued improvement (in fact near-normalization), which is explained by an enhanced insulin sensitivity and signaling [20], [26], whereas glucose effectiveness seems to be unchanged after RYGB [25]. The changes undoubtedly contributes importantly to the improved glucose tolerance after RYGB.
Digestion and absorption of nutrients
As soon as the newly operated patient begins to ingest nutrients, another mechanism sets in. As is clear today, the creation of the gastric pouch and the gastroenteroanastomosis in no way represents a restriction; on the contrary, the entry of nutrients into the more distal small intestine via the Roux limb (the alimentary limb) is tremendously accelerated. This can be documented with scintigraphic methods [27] but is perhaps most clearly illustrated by paracetamol absorption rates, which are hugely accelerated after bypass so that maximum absorption values are observed immediately after food intake compared with approximately an hour after meal intake before the operation [28]. Not only is the more distal mucosa exposed to ingested nutrients at an increased rate, the absorption of the nutrients is similarly accelerated. This was clearly demonstrated in careful studies by Jacobsen et al. [29] using double tracer technology, in which not only endogenous glucose turnover was followed, but also the fate of ingested nutrients using specific labeling. In agreement with other studies, Jacobsen et al. [29] found a pronounced early postprandial increase in the plasma glucose concentration followed by a rapid decline to much lower levels after 1 to 2 hours, compared with preoperative values. The glucose production in the liver was strongly but similarly inhibited in both cases (and therefore had little influence), but the plasma glucose profile was completely explained by a combination of a massive rise in glucose rate of appearance resulting from absorption of the meal glucose and a similar rise in glucose disappearance, which was paralleled by a much greater rise in insulin concentrations. Importantly, none of these changes were due to urinary glucose losses, and the recovery of the ingested glucose was nearly complete, documenting that the fate of the ingested glucose could be completely accounted for [29].
Interestingly, using other tracers we also followed the fate of ingested proteins. Using tracers for phenylalanine and leucine (biosynthetically incorporated into the ingested casein proteins) we found a similarly accelerated absorption of the ingested proteins [30]. These experiments illustrate that proteins are extremely rapidly digested and absorbed after RYGB surgery and that the location of accelerated exposure and absorption occurs beyond the Roux limb. This is because, after the anastomosis with the biliopancreatic limb, it is only in the common limb that ingested proteins will meet the endoproteases from the pancreas and stomach. Clearly, without these proteases, the large proteins (in casu casein) of the meal could not be digested.
Stimulation of hormone secretion
Recent research into the mechanisms for release of the gut hormones has demonstrated that it is not exposure of the gut to the nutrients, but absorption of nutrients that constitutes the most powerful stimulus for secretion [31], [32], [33]. In the case of glucose, which is perhaps the most important stimulus for GLP-1 secretion after bypass [34], it is transported intracellularly by the sodium-glucose cotransporter-1 (SGLT-1) transporter and partly metabolized; both processes mediate a depolarization of the secretory L-cells, leading to calcium entry, which triggers basolateral secretion [31], [35]. Indeed, it can be demonstrated that secretion rates parallel glucose absorption rates at the cellular level [33]. It is therefore not surprising that a greatly exaggerated GLP-1 postprandial response is observed as soon as meal ingestion is resumed after surgery [36] (for review see Madsbad et al. [19]). Bile acids have also been suggested to play a role and may contribute, but the exaggerated response to mixed meal stimulation after RYGB does not seem to be explained by bile acid stimulation [37].
It has been speculated that adaptive mechanisms involving increased numbers of endocrine cells in both the alimentary and the common limb could contribute to the hypersecretion, but as demonstrated in the previously mentioned experiment with gastrostomy feeding [17], [38] and more recently in experiments with reversal of RYGB [39], the secretory changes after surgery are immediately reversed after rerouting of nutrients to the stomach and after reversal of RYGB. In addition, although there are signs of moderate changes in the density gut endocrine cells after the operation [40], they do not seem to be able to explain the dramatic changes in secretion.
The role of GLP-1
Thus, just a few days after the operation there is a dramatically increased secretion of several gut hormones, including the insulinotropic peptide GLP-1, occurring simultaneously with an exaggerated increase in plasma glucose. This combination is known to provide a powerful stimulus to the beta cells [41] and, as already mentioned, we do see a correspondingly elevated secretion of insulin in the patients. Is it possible to link the GLP-1 response in humans to the insulin secretion and the rapid postprandial glucose deposition? Administration of the potent GLP-1 receptor antagonist exendin 9-39 allows an investigation of this [42]. Jorgensen et al. [36] studied meal-induced responses in obese patients with type 2 diabetes scheduled for RYGB, both before and 1 week and 3 months after the operation with or without simultaneous infusions of large doses of the GLP-1 receptor antagonist. The GLP-1 receptor antagonist very significantly impaired insulin secretion (as determined by calculation of actual insulin secretion rates from C-peptide concentrations) before, and particularly after, the operation; as a result, glucose tolerance, which was normalized during the course of the 3 months, was also significantly impaired by the antagonist. Indeed, both insulin secretion and glucose tolerance reverted to the preoperative values by the antagonist. The mechanism of action of GLP-1 on the beta cell secretion consists in essence of its ability to increase beta cell sensitivity to glucose [43], and the improvement in this parameter was also completely reverted to postoperative values by the antagonist [36]. These studies clearly pointed to a very important role of the exaggerated GLP-1 response for the postprandial improvement in glucose tolerance after RYGB. Salehi et al. [44] presented similar results. These apparent effects of endogenous GLP-1 are in complete agreement with the powerful effects of GLP-1 receptor agonists on glucose regulation and weight in obese patients with type 2 diabetes (see, e.g., Marso et al. [45]). The importance of the other incretin hormone GIP for the improved glucose tolerance after RYGB has been debated, and both slightly increased [28], unchanged [2], [34], and slightly decreased [15] secretion has been reported, which speaks against a major role of GIP. Accordingly, enhancement of active GIP concentrations during administration of the dipeptidyl-peptidase-4 (DPP-4) inhibitor sitagliptin did not improve insulin secretion [46]. However, diminished glucotoxicity after RYGB could hypothetically restore the insulinotropic effect of GIP [47], [48] after surgery.
It has been suggested, particularly on the basis of the early reports of islet hyperfunction explaining hypoglycemia after RYGB [49], that an adaptive growth of the islets (e.g., stimulated by GLP-1) might explain some of the benefits of RYGB, but careful studies of islet function performed for up to 3 years after RYGB have shown that the intrinsic beta cell function, if anything, is slightly lowered with time, probably as an adaptation for improved insulin sensitivity, whereas the postprandial gastrointestinal stimulation factor persists [50]. Also, the early reversal of hypoglycemia after reversal of RYGB argues against islet growth as a mechanism for post-RYGB hypoglycemia [39].
RYGB and weight loss
As discussed earlier, negative energy balance and the subsequent weight loss play an important role for the improvement in glucose tolerance induced by RYGB, but what is the mechanism of the weight loss?
Again, gut hormones may play an important role. Numerous hypotheses for the weight loss mechanisms have been presented, the earliest including mechanical restriction of food intake and malabsorption of macronutrients, consistent with the nature of the surgical intervention. More recent studies conclude that neither is involved to an appreciable degree. First, malabsorption is generally absent [51] and, as discussed, both absorption kinetics and scintigraphy studies indicate that nutrient passage through the gastric pouch is facilitated rather than restricted [27], [28]. Postoperative changes in energy metabolism have also been discussed at great length, in particular inspired by rodent studies, which generally have shown increased energy expenditure after RYGB [52]. Meal-associated thermogenesis was also reported to increase after RYGB in humans (whereas total energy expenditure decreased in parallel with the weight loss [53]). However, meal-associated thermogenesis increased only when expressed in relation to total energy expenditure, which decreased while absolute meal-associated thermogenesis did not change, which is perhaps not too surprising. In general, as might be expected, energy metabolism seems to follow weight closely, as recently demonstrated in experiments with respiratory chambers where patients were randomized to RYGB or a similar calorie restriction [54]. It may be concluded, therefore, that the best explanation for the weight loss is reduced energy intake, and this has been documented both in laboratory studies (showing a 35% decreased ad libitum food intake [55]) and during free living [56]. A question then arises: Why does food intake decrease? One explanation is, of course, an increased frequency of the so-called dumping syndrome: postprandial nausea, dizziness, and pain probably generated by the rapid entry of hyperosmotic nutrients into the small intestine [57]. However, food intake also decreases independently of dumping and appears to be related to decreased appetite [54], [58], suggesting that differences in appetite regulation may be important.
This is where the appetite-regulating hormones enter the stage. A convincing demonstration of a possible role for the exaggerated release of gut hormones was provided by Le Roux et al. [59] who found increased food intake in RYGB patients in response to an injection of a somatostatin analogue that simultaneously, as expected, eliminated the gut hormone responses to meal intake. Similarly, looking at “good responders” versus “bad responders” (i.e., those showing large versus small weight losses) gut hormone responses were larger in “good responders” [58], [60]. But which gut hormones are responsible? The operation increases the secretion of many of the hormones, which have a documented role in appetite regulation, including cholecystokinin, GLP-1, and PYY [34], [59]. Other candidate inhibitors of appetite include oxyntomodulin and neurotensin, which are also increased [61], [62]. Ghrelin represents a special case: Ghrelin increases appetite and food intake, and its postprandial secretion decreases after RYGB [28]. Statistical analysis strongly suggests that the decrease may be involved in the early decrease in appetite [54]. However, after some time, ghrelin levels return to preoperative levels, whereas appetite and food intake remain lower [28]. The most potent of these appetite regulators may be PYY and GLP-1, which can both be demonstrated to inhibit food intake in overweight individuals. When administered together their inhibitory effects seem to be supra additive [63]. We [54] made an attempt to isolate the particular role of GLP-1 for inhibition of food intake after RYGB using the GLP-1 receptor antagonist exendin 9-39, which clearly resulted in increased food intake in obese patients with type 2 diabetes scheduled for RYGB, but surprisingly the (lower) food intake remained unchanged during antagonist administration after the operation. However, closer analysis of the hormonal changes occurring during exendin 9-39 administration revealed that not only did GLP-1 responses increase markedly, due to elimination of a well-known negative feedback regulatory mechanism [36], but the already greatly exaggerated postoperative PYY responses were similarly further increased by the antagonist. The experiment, therefore, was interpreted to indicate that an inhibitory influence of GLP-1 may very well have been removed with the antagonist, but at the same time an even larger PYY response occurs, which might in itself cause a further inhibition of food intake so that the net result is neutral. It was therefore decided to try to block both GLP-1 and PYY actions. Again, exendin 9-39 could be employed to block GLP-1 actions, but no antagonist of PYY action is available for human use. However, PYY is secreted as PYY 1-36, a peptide of 36 amino acids, which actually stimulates food intake via an interaction with Y1 and Y5 receptors [64]. Like GLP-1, PYY is metabolized rapidly after its release by the enzyme dipeptidyl peptidase-4 (DPP-4), and the product PYY 3-36 is the actual appetite-inhibiting hormone, interacting with Y2 receptors in the arcuate nucleus. DPP-4 inhibitors for human use are abundantly available. A crossover experiment comprising 4 interventions: placebo, exendin 9-39, sitagliptin (a DPP-4 inhibitor), and a combination of exendin 9-39 and sitagliptin was therefore carried out in RYGB-operated individuals. Analysis of the postprandial hormone responses revealed greatly exaggerated GLP-1 and PYY concentrations during placebo, and further increases during exendin 9-39 were observed as expected. The DPP-4 inhibitor caused even further increases in active GLP-1 concentrations but almost abolished the PYY 3-36 responses to food intake, and the combined administration of the inhibitors resulted in a 20% increase in food intake [55]. These results strongly support that the 2 hormones are involved in the inhibition of appetite and food intake after RYGB. It should be noted that the plasma concentrations of the 2 hormones observed after RYGB are actually greater than those that can be employed in studies of their effects on food intake in healthy individuals because of side effects [64], [65], indicating that the magnitude of their responses in the RYGB patients is consistent with a powerful effect on appetite and food intake. Clearly, these results do not exclude the possibility that other gut hormones may also play an important role.
It may be concluded that the particularly postprandial changes in gut hormone secretion postoperatively are likely to play important roles for not only insulin secretion but also for appetite and food intake. It is therefore attractive to mimic these responses to treat overeating, obesity, and diabetes. As discussed, the effects of GLP-1 on glucose and metabolism are already being exploited in the GLP-1–based therapies, and attempts are being made to develop therapies based on the actions of the other hormones [61] as well. Attempts to increase the secretion of the endogenous hormones using stimulators of their secretion will also be of particular interest due to the high local hormone concentrations with potential paracrine and neural actions that may be generated with this approach, in addition to the endocrine effects [66].
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
Footnotes
Supported by grants from the Danish Medical Research Council, the NovoNordisk Foundation, and by an advanced ERC Grant (no. 695069 to J.J.H.).
References
- 1.Buchwald H., Avidor Y., Braunwald E. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen N.B., Jacobsen S.H., Dirksen C. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–E131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 3.Holst J.J., Sorensen T.I., Andersen A.N. Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio. Scand J Gastroenterol. 1979;14(2):205–207. doi: 10.3109/00365527909179871. [DOI] [PubMed] [Google Scholar]
- 4.Orskov C., Holst J.J., Knuhtsen S., Baldissera F.G., Poulsen S.S., Nielsen O.V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986;119(4):1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- 5.Holst J.J., Orskov C., Nielsen O.V., Schwartz T.W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- 6.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drucker D.J., Erlich P., Asa S.L., Brubaker P.L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93(15):791–796. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thulesen J., Hartmann B., Kissow H. 2001. Intestinal growth adaptation and glucagon-like peptide 2 in rats with ileal–jejunal transposition or small bowel resection. Dig Dis Sci. 2001;46(2):379–388. doi: 10.1023/a:1005572832571. [DOI] [PubMed] [Google Scholar]
- 9.Naslund E., Gryback P., Hellstrom P.M. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord. 1997;21(5):387–392. doi: 10.1038/sj.ijo.0800418. [DOI] [PubMed] [Google Scholar]
- 10.Naslund E., Melin I., Gryback P. Reduced food intake after jejunoileal bypass: a possible association with prolonged gastric emptying and altered gut hormone patterns. Am J Clin Nutr. 1997;66(1):26–32. doi: 10.1093/ajcn/66.1.26. [DOI] [PubMed] [Google Scholar]
- 11.Patti M.E., McMahon G., Mun E.C. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 12.Miholic J., Orskov C., Holst J.J., Kotzerke J., Meyer H.J. Emptying of the gastric substitute, glucagon-like peptide-1 (GLP- 1), and reactive hypoglycemia after total gastrectomy. Dig Dis Sci. 1991;36(10):1361–1370. doi: 10.1007/BF01296800. [DOI] [PubMed] [Google Scholar]
- 13.Le Roux C.W., Aylwin S.J., Batterham R.L. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batterham R.L., Cowley M.A., Small C.J. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 15.Korner J., Bessler M., Inabnet W., Taveras C., Holst J.J. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3(6):597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare K.J., Vilsboll T., Asmar M., Deacon C.F., Knop F.K., Holst J.J. The glucagonostatic and insulinotropic effects of glucagon-like peptide-1 contribute equally to its glucose-lowering action. Diabetes. 2010;59(7):1765–1770. doi: 10.2337/db09-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirksen C., Hansen D.L., Madsbad S. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33(2):375–377. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst J.J. Postprandial insulin secretion after gastric bypass surgery: the role of glucagon-like peptide 1. Diabetes. 2011;60(9):2203–2205. doi: 10.2337/db11-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsbad S., Dirksen C., Holst J.J. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152–164. doi: 10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]
- 20.Bojsen-Moller K.N., Dirksen C., Jorgensen N.B. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014;63(5):1725–1737. doi: 10.2337/db13-1307. [DOI] [PubMed] [Google Scholar]
- 21.Steven S., Hollingsworth K.G., Small P.K. Calorie restriction and not glucagon-like peptide-1 explains the acute improvement in glucose control after gastric bypass in type 2 diabetes. Diabet Med. 2016;33(12):1723–1731. doi: 10.1111/dme.13257. [DOI] [PubMed] [Google Scholar]
- 22.Vetter M.L., Wadden T.A., Teff K.L. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes. 2015;64(2):434–446. doi: 10.2337/db14-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isokuortti E., Zhou Y., Peltonen M. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: a population-based and inter-laboratory study. Diabetologia. 2017;60(10):1873–1882. doi: 10.1007/s00125-017-4340-1. [DOI] [PubMed] [Google Scholar]
- 24.Kotronen A., Vehkavaara S., Seppala-Lindroos A., Bergholm R., Yki-Jarvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293(6):E1709–E1715. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 25.Martinussen C., Bojsen-Moller K.N., Dirksen C. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab. 2015;308(6):E535–E544. doi: 10.1152/ajpendo.00506.2014. [DOI] [PubMed] [Google Scholar]
- 26.Albers P.H., Bojsen-Moller K.N., Dirksen C. Enhanced insulin signaling in human skeletal muscle and adipose tissue following gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2015;309(5):R510–R524. doi: 10.1152/ajpregu.00228.2014. [DOI] [PubMed] [Google Scholar]
- 27.Dirksen C., Damgaard M., Bojsen-Moller K.N. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25(4):346. doi: 10.1111/nmo.12087. –e255. [DOI] [PubMed] [Google Scholar]
- 28.Falken Y., Hellstrom P.M., Holst J.J., Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96(7):2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen S.H., Bojsen-Moller K.N., Dirksen C. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013;56(10):2250–2254. doi: 10.1007/s00125-013-3003-0. [DOI] [PubMed] [Google Scholar]
- 30.Bojsen-Moller K.N., Jacobsen S.H., Dirksen C. Accelerated protein digestion and amino acid absorption after Roux-en-Y gastric bypass. Am J Clin Nutr. 2015;102(3):600–607. doi: 10.3945/ajcn.115.109298. [DOI] [PubMed] [Google Scholar]
- 31.Kuhre R.E., Frost C.R., Svendsen B., Holst J.J. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2015;64(2):370–382. doi: 10.2337/db14-0807. [DOI] [PubMed] [Google Scholar]
- 32.Gribble F.M., Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 33.Kuhre R.E., Christiansen C.B., Saltiel M.Y., Wewer Albrechtsen N.J., Holst J.J. On the relationship between glucose absorption and glucose-stimulated secretion of GLP-1, neurotensin, and PYY from different intestinal segments in the rat. Physiol Rep. 2017;5:(23). doi: 10.14814/phy2.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen S.H., Olesen S.C., Dirksen C. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22(7):1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 35.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorgensen N.B., Dirksen C., Bojsen-Moller K.N. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–3052. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen S., Svane M.S., Kuhre R.E. Chenodeoxycholic acid stimulates glucagon-like peptide-1 secretion in patients after Roux-en-Y gastric bypass. Physiol Rep. 2017;5(3):e13140. doi: 10.14814/phy2.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin T., Peck M., Holst J., Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95(4):1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 39.Svane M.S., Toft-Nielsen M.B., Kristiansen V.B. Nutrient re-routing and altered gut-islet cell crosstalk may explain early relief of severe postprandial hypoglycaemia after reversal of Roux-en-Y gastric bypass. Diabet Med. 2017;34(12):1783–1787. doi: 10.1111/dme.13443. [DOI] [PubMed] [Google Scholar]
- 40.Rhee N.A., Wahlgren C.D., Pedersen J. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia. 2015;58(10):2254–2258. doi: 10.1007/s00125-015-3696-3. [DOI] [PubMed] [Google Scholar]
- 41.Holst J.J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 42.Schirra J., Sturm K., Leicht P., Arnold R., Goke B., Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7- 36)amide in humans. J Clin Invest. 1998;101(7):1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kjems L.L., Holst J.J., Volund A., Madsbad S. The influence of GLP-1 on the glucose-induced insulin secretion: effects on beta cell sensitivity in type 2 diabetic patients and controls. Diabetes. 2003;52(2):380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 44.Salehi M., Gastaldelli A., D'Alessio D.A. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–680. doi: 10.1053/j.gastro.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marso S.P., Bain S.C., Consoli A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 46.Svane M.S., Bojsen-Moller K.N., Nielsen S. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab. 2016;310(7):E505–E514. doi: 10.1152/ajpendo.00471.2015. [DOI] [PubMed] [Google Scholar]
- 47.Hojberg P.V., Vilsboll T., Rabol R. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 48.Aaboe K., Knop F.K., Vilsboll T. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12(4):323–333. doi: 10.1111/j.1463-1326.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 49.Service G.J., Thompson G.B., Service F.J., Andrews J.C., Collazo-Clavell M.L., Lloyd R.V. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 50.Dirksen C., Eiken A., Bojsen-Moller K.N. No islet cell hyperfunction, but altered gut-islet regulation and postprandial hypoglycemia in glucose-tolerant patients 3 years after gastric bypass surgery. Obes Surg. 2016;26(9):2263–2267. doi: 10.1007/s11695-016-2197-x. [DOI] [PubMed] [Google Scholar]
- 51.Odstrcil E.A., Martinez J.G., Santa Ana C.A. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92(4):704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 52.Lutz T.A., Bueter M. The use of rat and mouse models in bariatric surgery experiments. Front Nutr. 2016;3:25. doi: 10.3389/fnut.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werling M., Fandriks L., Olbers T. Roux-en-Y gastric bypass surgery increases respiratory quotient and energy expenditure during food intake. PLoS One. 2015;10(6):e0129784. doi: 10.1371/journal.pone.0129784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt J.B., Pedersen S.D., Gregersen N.T. Effects of RYGB on energy expenditure, appetite and glycaemic control: a randomized controlled clinical trial. Int J Obes (Lond) 2016;40(2):281–290. doi: 10.1038/ijo.2015.162. [DOI] [PubMed] [Google Scholar]
- 55.Svane M.S., Jorgensen N.B., Bojsen-Moller K.N. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond) 2016;40(11):1699–1706. doi: 10.1038/ijo.2016.121. [DOI] [PubMed] [Google Scholar]
- 56.Laurenius A., Larsson I., Bueter M. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond) 2012;36(3):348–355. doi: 10.1038/ijo.2011.217. [DOI] [PubMed] [Google Scholar]
- 57.Laurenius A., Werling M., Le Roux C.W., Fandriks L., Olbers T. Dumping symptoms is triggered by fat as well as carbohydrates in patients operated with Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2017;13(7):1159–1164. doi: 10.1016/j.soard.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Dirksen C., Jorgensen N.B., Bojsen-Moller K.N. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond) 2013;37(11):1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 59.Le Roux C.W., Welbourn R., Werling M. Gut hormones as mediators of appetite and weight loss after roux-en-y gastric bypass. Ann Surg. 2007;246(5):780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 60.de Hollanda A., Casals G., Delgado S. Gastrointestinal hormones and weight loss maintenance following Roux-en-Y gastric bypass. J Clin Endocrinol Metab. 2015;100(12):4677–4684. doi: 10.1210/jc.2015-3065. [DOI] [PubMed] [Google Scholar]
- 61.Tan T., Behary P., Tharakan G. The effect of a subcutaneous infusion of GLP-1, OXM and PYY on energy intake and expenditure in obese volunteers. J Clin Endocrinol Metab. 2017;102(7):2364–2372. doi: 10.1210/jc.2017-00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wewer Albrechtsen N.J., Hornburg D., Albrechtsen R. Oxyntomodulin identified as a marker of type 2 diabetes and gastric bypass surgery by mass-spectrometry based profiling of human plasma. EBioMedicine. 2016;7:112–120. doi: 10.1016/j.ebiom.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt J.B., Gregersen N.T., Pedersen S.D. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am J Physiol Endocrinol Metab. 2014;306(11):E1248–E1256. doi: 10.1152/ajpendo.00569.2013. [DOI] [PubMed] [Google Scholar]
- 64.Sloth B., Holst J.J., Flint A., Gregersen N.T., Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292(4):E1062–E1068. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 65.Ritzel R., Orskov C., Holst J.J., Nauck M.A. Pharmacokinetic, insulinotropic, and glucagonostatic properties of GLP-1 [7-36 amide] after subcutaneous injection in healthy volunteers. Dose-response-relationships. Diabetologia. 1995;38(6):720–725. doi: 10.1007/BF00401846. [DOI] [PubMed] [Google Scholar]
- 66.Holst J.J., Gribble F., Horowitz M., Rayner C.K. Roles of the gut in glucose homeostasis. Diabetes Care. 2016;39(6):884–892. doi: 10.2337/dc16-0351. [DOI] [PubMed] [Google Scholar]