The non-inferiority of hydromorphone compared with oxycodone for the treatment of cancer pain was confirmed in 183 Japanese cancer patients. Hydromorphone and oxycodone had similar efficacy and safety profiles.
Keywords: hydromorphone, oxycodone, cancer pain, palliative medicine, double-blind study
Abstract
Background
Hydromorphone is a standard opioid analgesic for cancer pain that, prior to this study, was not approved in Japan, where options for opioid switching are limited. We aimed to investigate the efficacy and safety of hydromorphone (DS-7113b) immediate-release tablets in opioid-naïve cancer patients with moderate to severe cancer pain.
Methods
Multicenter, active-controlled, randomized, double-blind, parallel-group, non-inferiority study of 183 cancer patients over 20 years of age at 50 clinical sites in Japan. Hydromorphone tablets or oxycodone hydrochloride powder was orally administered four times daily for 5 days. The initial doses of hydromorphone and oxycodone hydrochloride were 4 mg/day and 10 mg/day, respectively, and adjusted as necessary. Efficacy was evaluated as the intergroup difference (95% confidence interval [CI]) of the least squares mean by analysis of covariance, using the baseline visual analog scale (VAS) as a covariate for change in VAS score at treatment completion/discontinuation in the full analysis set.
Results
Non-inferiority of hydromorphone versus oxycodone was confirmed, with an intergroup difference (95% CI) in the least squares mean of −3.4 mm (−9.8 to 3.1 mm) for change in VAS scores, which was below the upper limit of the 95% CI at 10 mm, the non-inferiority limit determined during study planning. Adverse events occurred in 83.0% (73/88) of patients in the hydromorphone group and 77.4% (65/84) in the oxycodone group. The most frequently observed adverse events were somnolence, constipation, vomiting and nausea.
Conclusions
The efficacy and safety of hydromorphone tablets are equivalent to those of oxycodone immediate-release powder.
Introduction
Analgesia for cancer pain is based on the World Health Organization Guidelines for cancer pain relief published in 1986 (1). The World Health Organization’s ‘three-step ladder for cancer pain relief’ recommends the use of analgesics depending on the severity of pain, and the use of potent opioid analgesics is stipulated for moderate to severe pain (1). Potent opioid analgesics are the most effective for relieving cancer pain and can provide successful pain control (2). Morphine, oxycodone and fentanyl are currently used in Japan as the Step 3 opioid analgesics (3).
Hydromorphone is a selective μ-opioid receptor agonist analgesic that was synthesized in Germany in the 1920s and is currently used clinically in 45 countries and regions in the world (4). Hydromorphone is an alternative drug for morphine (5–8), but at the time this study commenced, it was not approved for use in Japan. Options for opioid switching are limited in Japan, so a request for the development of hydromorphone was submitted by the Japanese Society for Palliative Medicine.
We conducted a Phase 3, randomized, double-blind, comparative study to investigate the non-inferiority of hydromorphone tablets relative to oxycodone hydrochloride powder, and to investigate the efficacy of hydromorphone tablets in Japanese opioid-naïve patients with cancer pain.
Patients and methods
This study was conducted from 2013 to 2014 as a multicenter, active-controlled, randomized, double-blind, parallel-group comparison study, enrolling 183 patients at 50 sites. The participating centers and institutes are listed in the Supplemental File. The study was approved by the institutional review board of each study site and was conducted in compliance with ethical principles based on the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all patients prior to study participation.
Participants
The study participants were cancer patients over 20 years of age receiving non-opioid analgesics for pain relief who had not used opioid analgesics within 2 weeks of registration. At registration, the average pain within the last 24 h, as measured on the visual analog scale (VAS), was required to be ≥35 mm and Eastern Cooperative Oncology Group (ECOG) performance status (PS) was required to be ≤3. All patients were judged by the investigator to require treatment with potent opioid analgesics. Exclusion criteria included patients presenting with symptoms for which oxycodone or morphine are contraindicated or relatively contraindicated, patients receiving treatment with a monoamine oxidase inhibitor within 14 days of registration, patients participating in another clinical trial within 28 days of registration, and patients with a serious hepatic, renal or respiratory disorder of Common Terminology Criteria for Adverse Events Grade 3.
Study design
Patients were randomly allocated 1:1 to either hydromorphone immediate-release tablets (DS-7113b; Daiichi-Sankyo, Tokyo, Japan) or oxycodone immediate-release powder (OxiNorm® Powder; Shionogi, Osaka, Japan). The computer-generated block random allocation sequence was provided by Bell Medical Solutions Inc. (Tokyo, Japan) and was stratified according to history of opioid usage. A double-dummy method was employed for blinding, and patients received either hydromorphone plus placebo or a placebo tablet plus oxycodone hydrochloride powder orally four times daily for 5 days. This dosage frequency was selected based on the standard pharmacokinetic profile for immediate-release hydromorphone (i.e., onset of action in ~30 min and duration of action ~4 h) (9). The initial doses of hydromorphone and oxycodone hydrochloride were 4 mg/day and 10 mg/day, respectively. When a dose increase was deemed necessary during study drug administration, the dose could be increased up to the fourth dose (hydromorphone 16 mg/day, oxycodone 60 mg/day) by one step every 24 h (Table 1). Treatment was switched to appropriate analgesics after completion of study drug administration and after the post-study observation. The initial 4 mg/day dosage of hydromorphone was set based on 1–2 mg per dose as specified by the World Health Organization. The oxycodone hydrochloride powder dose used was that stipulated in the Japanese package insert for opioid-naïve patients.
Table 1.
Daily dose of the investigational product and single dose of the rescue medication at each investigational product dose
| Hydromorphone group | Oxycodone group | Morphine hydrochloride for rescue medicationa | |
|---|---|---|---|
| 1 | 4 mg/day | 10 mg/day | 5 mg |
| 2 | 8 mg/day | 20 mg/day | 5 mg |
| 3 | 12 mg/day | 40 mg/day | 10 mg |
| 4 | 16 mg/day | 60 mg/day | 15 mg |
aAt the time of this study, hydromorphone immediate-release tablets were not yet approved for use in Japan, so they could not be used. If oxycodone hydrochloride powder had been used as rescue medication, patients in the hydromorphone group might have used oxycodone, confounding the evaluation of which drug was causing any AEs. Therefore, oral morphine solution was chosen as the rescue medication for both groups.
Every day, from baseline to treatment completion (or discontinuation), patients evaluated their mean pain severity over the previous 24 h using the VAS and recorded their score in a diary. Oral morphine hydrochloride solution was used as rescue medication for both groups (Table 1). This is because, at the time of this study, hydromorphone immediate-release tablets were not yet approved for use in Japan, and so could not be used. If oxycodone hydrochloride powder had been used as rescue medication, patients in the hydromorphone group might have used oxycodone, confounding the evaluation of which drug was causing any AEs.
The following were prohibited throughout the study: coadministration of a monoamine oxidase inhibitor, opioid analgesic or narcotic antagonist; new administration of systemic non-opioid analgesics; supplementary analgesics; bisphosphonates; anti-RANKL antibody preparations; changes in dosage and administration; new initiation of radiotherapy, nerve block, percutaneous vertebroplasty, surgery, or cancer chemotherapy or immunotherapy. Magnesium oxide at 2 g/day and prochlorperazine maleate at 15 mg/day were administered to all patients to ensure adequate control of constipation and nausea/vomiting and to enable appropriate safety evaluations.
Outcomes
The primary efficacy endpoint was the change in VAS, a 100-mm rating scale commonly used in Japan to evaluate pain in clinical studies of opioid analgesics, from baseline to treatment completion or discontinuation. Mean pain over the previous 24 h was retrospectively evaluated by the participant on Days 1–6 as a secondary endpoint.
Changes in VAS and sleep, as an indicator of successful pain relief, were also evaluated on each evaluation day (Days 1–6) as a secondary endpoint. Sleep quality was evaluated at completion/discontinuation of treatment using a 4-point rating scale (0, very unsatisfactory or did not sleep at all; 1, markedly unsatisfactory; 2, slightly unsatisfactory; and 3, satisfactory).
Safety endpoints were adverse events, laboratory data, vital signs and 12-lead electrocardiogram (ECG).
Statistical analysis
The full analysis set (FAS) consisted of all patients with at least one measurement of the primary efficacy parameter, at least one dose of study medication, and no serious GCP violation, and was the primary analysis population for efficacy. The level of significance used for the hypothesis test was 5% (two-sided), and the confidence interval (CI) was 95% (two-sided). SAS System Release 9.2 (SAS Inc., Cary, NC, USA) was used for statistical analysis. Summary statistics were calculated for VAS scores at baseline, at treatment completion/discontinuation and for the change in VAS scores. For primary efficacy evaluation, analysis of covariance (ANCOVA) was conducted using the baseline VAS scores as a covariate to calculate the 95% CI (two-sided) for a difference in the least squares mean in the magnitude of change in VAS scores between the hydromorphone and oxycodone groups. The numeric pain rating scale, a method widely used in clinical settings, also employs an 11-step scale. Assuming that the change between each step is equivalent to 10 mm in the VAS, the limit of non-inferiority was determined to be 10 mm and was considered a clinically acceptable difference against the oxycodone group. A sample size of 180 patients (N = 90/arm) was determined to achieve a power of at least 80% to show non-inferiority, assuming the difference in VAS change from baseline between groups was 0 mm and the standard deviation for this parameter was 22.5 mm, referring to other clinical studies in opioid-naïve patients (data on file: available at http://www.pmda.go.jp/drugs/2010/P201000027/index.html [in Japanese]). Pvalues and least squares means for each group were calculated.
Summary statistics were calculated for VAS scores on each evaluation day (Days 1–6) and for changes in VAS scores between baseline and each evaluation day. A t-test was conducted between groups to calculate the 95% CI (two-sided) for a difference in means. A paired t-test was conducted on baseline VAS scores and VAS scores on each evaluation day (Days 2–6) to calculate the 95% CI (two-sided) for a difference in means.
Results
Patients and treatment exposure
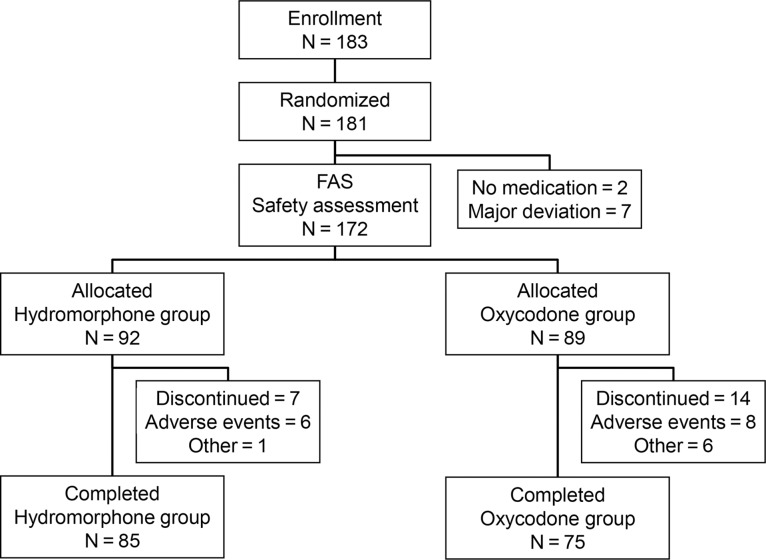
Figure 1 shows patient disposition. Of the 183 patients registered in this study, 181 were randomized: 92 to the hydromorphone group and 89 to the oxycodone group; of these, 85/92 and 75/89, respectively, completed the study. The main reason for study discontinuation was adverse events: 6/7 and 8/14 patients in the hydromorphone and oxycodone groups, respectively.
Figure 1.
Patient disposition. FAS, full-analysis set.
A total of 172 patients were included in the FAS. Two patients in the oxycodone group were excluded from the FAS because they discontinued the study before initiation of the study drug. Additionally, four patients in the hydromorphone group and three in the oxycodone group were excluded because of other major deviations. These patients were also excluded from the per-protocol set (PPS) and safety analysis population. Protocol deviation was observed in 10 and 18 patients in the hydromorphone and oxycodone groups, respectively; these patients were excluded from the PPS.
Table 2 shows demographics of the FAS. Male patients accounted for 61.4% (54/88) in the hydromorphone group, and 73.8% (62/84) in the oxycodone group. The proportion of patients with ECOG PS of 0 was higher in the hydromorphone than oxycodone group (33.0% [29/88] vs 20.2% [17/84]). All other characteristics were similar between the two groups.
Table 2.
Baseline demographics and clinical characteristics
| Hydromorphone group N = 88 n (%) | Oxycodone group N = 84 n (%) | Total N = 172 n (%) | |
|---|---|---|---|
| Age (years) | |||
| Mean | 67.7 | 66.8 | 67.3 |
| Standard deviation | 10.29 | 10.14 | 10.19 |
| Sex | |||
| Male | 54 (61.4) | 62 (73.8) | 116 (67.4) |
| Female | 34 (38.6) | 22 (26.2) | 56 (32.6) |
| Body weight (kg) | |||
| Mean | 54.06 | 55.98 | 55.00 |
| Standard deviation | 10.682 | 11.220 | 10.958 |
| Body mass index (kg/m2) | |||
| <25 kg/m2 | 73 (83.0) | 73 (86.9) | 146 (84.9) |
| ≥25 kg/m2 | 15 (17.0) | 11 (13.1) | 26 (15.1) |
| Underlying disease (tumor type) | |||
| Head/neck | 2 (2.3) | 0 (0.0) | 2 (1.2) |
| Lung | 30 (34.1) | 33 (39.3) | 63 (36.6) |
| Breast | 4 (4.5) | 0 (0.0) | 4 (2.3) |
| Gastrointestinal | 24 (27.3) | 26 (31.0) | 50 (29.1) |
| Hepatic-biliary-pancreatic | 13 (14.8) | 9 (10.7) | 22 (12.8) |
| Urogenital | 11 (12.5) | 9 (10.7) | 20 (11.6) |
| Others | 4 (4.5) | 7 (8.3) | 11 (6.4) |
| ECOG performance status | |||
| 0 | 29 (33.0) | 17 (20.2) | 46 (26.7) |
| 1 | 38 (43.2) | 41 (48.8) | 79 (45.9) |
| 2 | 16 (18.2) | 17 (20.2) | 33 (19.2) |
| 3 | 5 (5.7) | 9 (10.7) | 14 (8.1) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| VAS (mm) | |||
| Mean | 54.8 | 53.9 | 54.3 |
| Standard deviation | 15.44 | 12.09 | 13.88 |
VAS, visual analog scale; ECOG, Eastern Cooperative Oncology Group.
Mean (standard deviation [SD]) baseline VAS scores in the FAS were 54.8 (15.44) mm in the hydromorphone group and 53.9 (12.09) mm in the oxycodone group, with no notable difference between groups.
Most patients in both groups, specifically 75.0% (66/88) in the hydromorphone group and 71.4% (60/84) in the oxycodone group, completed or discontinued without their dose being altered, with no notable intergroup difference. A dose increase was required more than twice for two patients in the hydromorphone group and six patients in the oxycodone group.
There was no between-group difference in the use of rescue pain medication: the mean daily use of such medication was less than once for both groups on all evaluation days.
Efficacy
Table 3 shows the analysis of change in VAS scores for the FAS. The mean (SD) VAS score was 54.8 (15.44) mm at baseline and 24.7 (22.11) mm at completion/discontinuation of treatment in the hydromorphone group; corresponding values were 53.9 (12.09) mm and 27.9 (21.05) mm in the oxycodone group, thus showing a decrease from baseline in both groups.
Table 3.
Changes in visual analog scale scores in the full-analysis set
| Hydromorphone group N = 88 | Oxycodone group N = 84 | |
|---|---|---|
| VAS (baseline; mm) | ||
| Mean | 54.8 | 53.9 |
| Standard deviation | 15.44 | 12.09 |
| Minimum | 18 | 36 |
| Median | 50.0 | 51.0 |
| Maximum | 100 | 100 |
| VAS (at completion/discontinuation of treatment; mm) | ||
| Mean | 24.7 | 27.9 |
| Standard deviation | 22.11 | 21.05 |
| Minimum | 0 | 0 |
| Median | 18.0 | 26.0 |
| Maximum | 85 | 89 |
| VAS (change from baseline to completion/discontinuation of treatment; mm) | ||
| Mean | −30.0 | −26.0 |
| Standard deviation | 24.12 | 23.65 |
| Minimum | −85 | −100 |
| Median | −33.5 | −23.0 |
| Maximum | 31 | 26 |
| Least squares meana | −29.7 | −26.4 |
| Difference in least squares meana | −3.4 | |
| 95% confidence interval for the difference | [−9.8, 3.1] | |
| P value | 0.3057 | |
Analysis of covariance (explanatory variable: baseline VAS, groups).
ahydromorphone group − oxycodone group.
VAS, visual analog scale.
The magnitude of change in mean VAS score [SD] from baseline to completion/discontinuation of treatment was similar in the hydromorphone versus oxycodone group (−30.0 [24.12] vs −26.0 [3.65] mm). The intergroup difference (95% CI) in the least squares mean for change in VAS score at completion/discontinuation of treatment was −3.4 mm (−9.8 to 3.1 mm) using ANCOVA. Given that the upper limit of the 95% CI was <10 mm—the non-inferiority limit determined at the time of planning—non-inferiority of hydromorphone relative to oxycodone was suggested.
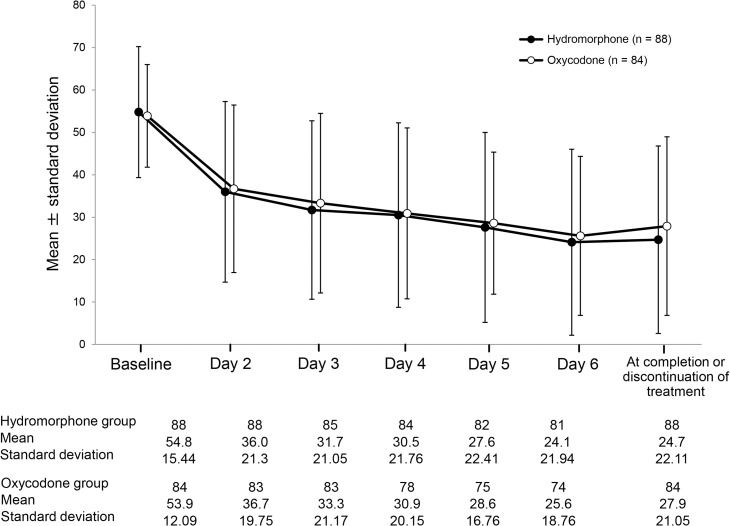
Figure 2 shows VAS scores on each evaluation day, a transition diagram (mean ± SD) for measured VAS scores, and the magnitude of change from baseline on each evaluation day. Mean VAS scores decreased on Day 2 in both groups and the mean level of change tended to increase with treatment progression. A paired t-test was conducted for VAS scores at baseline and for each evaluation day (Days 2–6). A significant difference was observed between baseline VAS scores and those on each evaluation day (paired t-test: P < 0.0001). There was no significant intergroup difference at each evaluation day.
Figure 2.
Changes in visual analog scale scores in the full-analysis set.
Many patients in both groups showed improvements in sleep at completion/discontinuation of treatment compared with baseline. A significant difference was observed in both the hydromorphone group (Wilcoxon signed rank test: P < 0.0001) and oxycodone group (P = 0.0012); thus, there was no significant intergroup difference.
Safety and tolerability
Table 4 shows adverse events that occurred with an incidence of ≥5%. Of the 181 randomized patients, 172 (88 in the hydromorphone group and 84 in the oxycodone group) were included in the safety analysis, after excluding two patients (both in the oxycodone group) who did not receive the study drug. Additionally, seven patients (four in the hydromorphone group and three in the oxycodone group) were excluded for other major deviations. The incidence of adverse events was 83.0% (73/88) in the hydromorphone group and 77.4% (65/84) in the oxycodone group, and no significant intergroup difference was observed. The most frequent adverse events (incidence of ≥10%) were somnolence, constipation, vomiting, nausea and diarrhea.
Table 4.
List of adverse events with incidence of ≥5%
| PTa | Hydromorphone group N = 88 n (%) | Oxycodone group N = 84 n (%) | Total N = 172 n (%) |
|---|---|---|---|
| Number of patients with adverse event | 73 (83.0) | 65 (77.4) | 138 (80.2) |
| Somnolence | 23 (26.1) | 21 (25.0) | 44 (25.6) |
| Constipation | 21 (23.9) | 19 (22.6) | 40 (23.3) |
| Vomiting | 17 (19.3) | 15 (17.9) | 32 (18.6) |
| Nausea | 14 (15.9) | 14 (16.7) | 28 (16.3) |
| Diarrhea | 12 (13.6) | 9 (10.7) | 21 (12.2) |
| Delirium | 2 (2.3) | 5 (6.0) | 7 (4.1) |
aMedDRA V.16.1 Preferred Term (PT).
The incidence of serious adverse events (including death) was 8.0% (7/88) in the hydromorphone group and 9.5% (8/84) in the oxycodone group, with no significant intergroup difference. Events judged to be causally related to the study drug occurred in four patients in the hydromorphone group (one event each of somnolence, aspiration pneumonia, toxic skin eruption and nausea/vomiting), and three patients in the oxycodone group (one event each of somnolence, aspiration pneumonia and abnormal hepatic function). All patients improved or recovered after discontinuing the study drug, or with appropriate medical intervention, except for the patients with aspiration pneumonia and abnormal hepatic function, both of whom died.
There were no pronounced changes in laboratory data or vital signs. Clinically problematic QT prolongation was not observed using 12-lead ECG.
Discussion
This was a double-blind, double-dummy comparative study to compare the efficacy of hydromorphone immediate-release tablets with oxycodone hydrochloride immediate-release powder. The results showed equivalent efficacy and safety between treatments. Our data demonstrate that hydromorphone immediate-release tablets can achieve clinically meaningful pain management in Japanese patients with cancer. Only opioid-naïve cancer patients were included to eliminate potential bias from previous opioid analgesia.
The dosing interval of hydromorphone immediate-release tablets is reported to be ~4 h (1,10–14), but in this double-dummy study, hydromorphone had to be administered four times daily to match the administration of oxycodone.
There was a significant improvement in sleep scores compared with baseline in both treatment groups, with no significant intergroup difference. The type and incidence of adverse events did not differ greatly from previous reports (15–19).
The results of this study suggest that the analgesic effect of hydromorphone immediate-release tablets given four times daily was comparable with that of oxycodone immediate-release powder, even though, typically, the standard regimen of hydromorphone is administered every 4 h (equivalent to six doses daily). With the latter schedule, or with administration of immediate-release morphine formulations, patients must be woken for drug administration at 4-hourly intervals; patients are therefore not permitted to sleep continuously for more than 4 h. As four-times-daily hydromorphone proved non-inferior to oxycodone in the present analysis, this hydromorphone schedule may have quality-of-life advantages over conventional opioid regimens. That is, the need to interrupt night-time sleep to administer conventional opioid medication can be avoided, as can double-dosing before bedtime and the potential for overdose-related adverse events during sleep. Because of these potential benefits, four-times-daily hydromorphone may be an appropriate treatment schedule for opioid-naïve patients.
Our study had some limitations. The analgesic effect was investigated only at relatively low doses because patients were opioid-naïve, so the efficacy and safety of four daily doses of hydromorphone need to be confirmed in patients who switch from other opioids, and in patients who require high-dose opioids. All patients in this study were required to take a laxative and antiemetics so that it was possible to compare between groups the onset of nausea/vomiting and constipation, which are adverse drug reactions often observed with opioids (20). The impact of prophylactic administration of laxatives and antiemetics on opioid safety has not been specifically investigated, and the incidence and severity of nausea/vomiting and constipation observed in this study cannot be compared with other studies that did not include prophylactic antiemetics and laxatives.
Conclusions
We verified the non-inferiority of hydromorphone given four times daily relative to oxycodone given four times daily in Japanese opioid-naïve cancer patients. There were no notable intergroup differences in the incidence of adverse events or serious adverse events. The efficacy and safety of hydromorphone tablets are, therefore, comparable to those of oxycodone immediate-release powder.
Supplementary Material
Acknowledgements
The authors wish to thank all of the investigators at the participating centers for their contributions to this study (this information is available in Supplementary Data File 1). The authors also thank Dr Keyra Martinez Dunn and David Murdoch of Edanz Medical Writing for providing editorial support during the development of this manuscript.
Supplementary data
Supplementary data are available at Japanese Journal of Clinical Oncology online.
Funding
This work was supported by Daiichi Sankyo company, which paid for all expenses related to preparation of this report and study implementation.
Conflict of interest statement
This study and the preparation of the manuscript were funded by Daiichi Sankyo Co., Ltd. Three of the authors are employees of Daiichi Sankyo Co., Ltd.; therefore, the sponsor had a role in the study design; in the collection, analysis, and interpretation of data; in writing of the report; and in the decision to submit the article for publication. Yoji Saito participated in this study as a medical specialist and Satoru Tsuneto and Etsuko Aruga functioned as safety assessment advisors. Satoshi Inoue, Hiroshi Takahashi, and Mitsutoshi Uemori are employees of Daiichi Sankyo Co., Ltd. Yoji Saito, Satoru Tsuneto and Etsuko Aruga have received personal fees from Daiichi Sankyo Co., Ltd.
Abbreviations
ANCOVA, analysis of covariance; CI, confidence interval; ECG, electrocardiogram; ECOG PS, Eastern Cooperative Oncology Group performance status; FAS, full analysis set; PPS, per-protocol set; SD, standard deviation; VAS, visual analog scale.
References
- 1. WHO Cancer Pain Relief: With a Guide to Opioid Availability. 2nd edn Geneva: World Health Organization, 1996; apps.who.int/iris/bitstream/10665/37896/1/9241544821.pdf (23 March 2018, date last accessed). [Google Scholar]
- 2. Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65–76. [DOI] [PubMed] [Google Scholar]
- 3. Hastie BA, Gilson AM, Maurer MA, Cleary JF. An examination of global and regional opioid consumption trends 1980–2011. J Pain Palliat Care Pharmacother 2014;28:259–75. [DOI] [PubMed] [Google Scholar]
- 4. International Narcotics Control Board Report of the International Narcotics Control Board for 2013. United Nations, New York: International Narcotics Control Board, 2014; www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2013/Part_4_tables_EFS.pdf (23 March 2018, date last accessed). [Google Scholar]
- 5. Hanks GW, Conno F, Cherny N, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer 2001;84:587–93. 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012;13:e58–68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 7. Ripamonti CI, Santini D, Maranzano E, et al. ESMO Guidelines Working Group: management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23:vii139–54. 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network Adult cancer pain. Version 2. National Comprehensive Cancer Network, 2012. www.nccn.org/professionals/physician_gls/pdf/pain.pdf (30 May 2016, date last accessed).
- 9. Murray A, Hagen NA. Hydromorphone. J Pain Symptom Manage 2005;29:S57–66. [DOI] [PubMed] [Google Scholar]
- 10. Fitzgibbon DR. Cancer pain: principles of management and pharmacotherapy In: Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica’s Management of Pain, 4e. Philadelphia: Lippincott, Williams and Wilkins, 2010;582–604. [Google Scholar]
- 11. Yaksh TL, Wallace MS. Opioids, analgesia and pain management In: Brunton L, Chabner B, Knollman B, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12 edn New York: McGraw-Hill, 2011;601–33. [Google Scholar]
- 12. Twycross R, Wilcock A, Howard P, editors. Palliative Care Formulary. 5th edn Nottingham: Palliativecare.com Ltd, 2014. [Google Scholar]
- 13. Twycross R. Pain relief In: Twycross R, Wilcock A, Toller CS, editors. Symptom Management in Advanced Cancer. 4th edn Nottingham: Palliativedrugs.com Ltd, 2009;13–60. [Google Scholar]
- 14. Fallon M, Cherny NI, Hanks G. Opioid analgesic therapy In: Smith HS, editor. Opioid Therapy in the 21st Century. 2nd edn New York: Oxford University Press, 2014;661–98. [Google Scholar]
- 15. Samolsky Dekel BG, Tomasi M, Vasarri A, et al. Opioid titration with sustained-release oxycodone and immediate-release morphine for moderate/severe cancer pain: a pilot assessment of the CoDem protocol. J Opioid Manag 2014;10:29–38. 10.5055/jom.2014.0189. [DOI] [PubMed] [Google Scholar]
- 16. Lazzari M, Greco MT, Marcassa C, et al. Efficacy and tolerability of oral oxycodone and oxycodone/naloxone combination in opioid-naïve cancer patients: a propensity analysis. Drug Des Devel Ther 2015;9:5863–72. 10.2147/DDDT.S92998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koizumi W, Toma H, Watanabe K, et al. Efficacy and tolerability of cancer pain management with controlled-release oxycodone tablets in opioid-naïve cancer pain patients, starting with 5 mg tablets. Jpn J Clin Oncol 2004;34:608–14. 10.1093/jjco/hyh104. [DOI] [PubMed] [Google Scholar]
- 18. Imanaka K, Tominaga Y, Etropolski M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr Med Res Opin 2013;29:1399–409. 10.1185/03007995.2013.831816. [DOI] [PubMed] [Google Scholar]
- 19. Mercadante S, Porzio G, Ferrera P, et al. Tapentadol in cancer pain management: a prospective open-label study. Curr Med Res Opin 2012;28:1775–9. 10.1185/03007995.2012.739151. [DOI] [PubMed] [Google Scholar]
- 20. Vignaroli E, Bennett MI, Nekolaichuk C, et al. Strategic pain management: the identification and development of the IAHPC opioid essential prescription package. J Palliat Med 2012;15:186–91. 10.1089/jpm.2011.0296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.