Split-course stereotactic ablative radiotherapy (SABR) appeared to achieve favorable toxicity profiles and local control outcomes comparable with those of continuous SABR in the patients with oligometastases.
Keywords: oligometastasis, stereotactic ablative radiotherapy, split-course radiotherapy, treatment outcome, toxicity
Abstract
Background
There is growing interest in the use of stereotactic ablative radiotherapy (SABR) for oligometastases. However, extreme caution should be exercised in treating tumors closely located to organs at risk (OARs) with SABR. To reduce complications, we have applied split-course SABR to oligometastases closely located to OARs or to those being retreated with radiotherapy.
Methods
We retrospectively reviewed the records of patients with oligometastases who were treated with planned split-course SABR between January 2012 and December 2016.
Results
A total of 23 patients with 29 oligometastatic lesions were enrolled. The primary diagnoses were bone and soft tissue cancers in 13 lesions, liver cancers in 12 lesions, and colorectal cancers in four lesions. The median tumor volume was 78 cm3 (range, 4–1781 cm3). The lesions were treated with 1–3 fractions in the first stage of SABR (first SABR), and one or two fractions in the second stage of SABR (second SABR). The time interval between the two stages was about 4 weeks. A partial response was noted in 16 lesions (55%) after the first SABR, and practical reductions in the doses to OARs were observed in the second SABR compared with the first SABR. The 1-, 2- and 3-year local control rates were 92%, 65% and 43%, respectively. No Grade 4 or 5 toxicities were observed during or after treatment.
Conclusion
Split-course SABR appeared to be feasible for the treatment of oligometastases closely located to OARs.
Introduction
Oligometastasis is the state of metastatic relapse of cancer in sites distant from the primary tumors (1,2). Local treatment of the metastases with surgery or radiotherapy (RT) may improve patient survival. For several anatomical sites, surgical resection of metastases prolonged survival in selected patients (2,3). Likewise, RT may also play an important role in the local control of oligometastases. Stereotactic ablative radiotherapy (SABR), which delivers high-dose radiation in 1–5 fractions to the target tumor volume, is one of the emerging radiation treatment modalities. There is growing interest in the use of SABR for oligometastases, and it has previously been reported that SABR is effective in controlling oligometastases with local control rates of about 80% (4,5).
Owing to remarkable advances in tumor imaging, dosimetry, treatment planning and radiation delivery technology, SABR is able to accurately deliver high-dose hypofractionated radiation to target the tumor volume with rapid fall-off away from the target tumors. However, despite the highly sophisticated technical measures in SABR, exposure of surrounding normal tissues may not be completely avoided when treating tumors with SABR, particularly when the volume of the surrounding normal tissues is large and structurally complex. Importantly, the exposure of many critical normal tissues to radiation, even to a small volume, may lead to serious late clinical implications. Therefore, extreme caution should be exercised in treating tumors with SABR, particularly in the following situations: (a) the tumors are located adjacent to organs at risk (OARs) like the gastrointestinal (GI) tract or other ‘series’ type organs, such as the esophagus or neurologic tissue, and (b) the tumor sites were previously treated with RT. The extent of normal tissue toxicity in SABR depends on many factors, including both treatment and host factors. We have previously showed that, in patients treated for abdominopelvic malignancies, the incidence of severe intestinal toxicity was higher after treating the tumors with three fractions of SABR on three consecutive working days than after treating over 4–8 days including weekends (18 vs. 0%, P = 0.037) (6). Based on this experience, allowing a 2- or 3-day break between fractions became the standard SABR practice for treating abdominopelvic malignancy in our institute.
We have previously reported our clinical results, including local control, survival gain and complications of patients treated with SABR for oligometastases (6–13). As one of the strategies to reduce toxicity in conventional RT, especially for high risk patients unable to tolerate continuous-course definitive RT, split-course RT has been occasionally used, and was shown to be a well-tolerated and effective method (14–18). To reduce complications without compromising local control in the treatment of oligometastases with SABR, we applied split-course SABR in patients with a high risk of complications. This report describes our clinical observation of the safety and outcomes of split-course SABR of oligometastases.
Material and methods
Patient selection
We retrospectively reviewed the records of patients with oligometastases regardless of the primary site, who were treated with split-course SABR between January 2012 and December 2016 at Korea Institute of Radiological and Medical Sciences. Split-course SABR was used for treating patients who were identified to be at high risk with conventional SABR (Fig. 1). This study was approved by the Institutional Review Board.
Figure 1.
Clinical decision-making flow chart for treating oligometastasis with SABR. The standard split-course SABR of our center consists of two treatment courses. One to three fractions were delivered in the first SABR. Patients underwent a computed tomography (CT) scan ~4 weeks after the completion of the first SABR for the planning of the second SABR. One or two fractions were additionally delivered for the second SABR. SABR, stereotactic ablative radiotherapy.
Split-course SABR
The split-course SABR in our institute is comprised of two stages of treatment. In the first stage of SABR (first SABR), tumors were irradiated in 1–3 fractions. The patients were then examined with a set of planning computed tomography (CT) scans for the second stage of SABR (second SABR) ~4 weeks after the completion of the first SABR. The second SABR was planned based on the changes in the CT scans and one or two fractions of radiation were delivered. The second SABR was delivered about 4 weeks (range, 18–60 days) after the completion of the first SABR.
Briefly, SABR was performed as follows. Contrast-enhanced CT scans with 2.5-mm slice thickness were obtained while the patients were in the supine position with both arms raised above the head. SABR dose planning and delivery were carried out using volumetric modulated arc therapy (VMAT). The gross tumor volume (GTV) was defined as the visible lesion observed on CT scans. The planning target volume (PTV) included an expansion of the GTV by 2 mm antero-posteriorly and laterally, and 4 mm cranio-caudally. To cover at least 95% of the PTV, radiation doses were prescribed at an isodose line of 95–100%. Various fractionation schedules were used considering the expected morbidity based on target volume, patient performance and normal tissue constraints.
Evaluation and follow-up
We assessed volumetric/dosimetric changes in target volumes and OARs between the two stages of SABR. The changes in GTVs were evaluated on the first and second planning CT scans. The volumes of OARs that received 100%, 70% and 50% of the prescribed dose (Vx%) were assessed on each planning CT scans. The response of the tumors was classified according to the following criteria: responder group (>10% decrease in GTV) and non-responder group (<10% decrease or increase in GTV).
Disease progression was defined as an increase in tumor size observed on CT or an increase in uptake observed on positron emission tomography-computed tomography (PET-CT). Local failure was defined as disease progression in the GTV, and distant failure was evidenced by imaging findings consistent with metastatic disease.
Toxicities were retrospectively reviewed through the clinical notes, imaging studies and surgery records. Acute and late toxicities were evaluated using the Common Terminology Criteria of Adverse Events (CTCAE) version 4.0 and the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) radiation morbidity criteria, respectively.
Statistical analysis
Local control was calculated from the start date of the first SABR using the Kaplan–Meier method. Variables were evaluated from patient records for prognostic factor analysis. The Pearson chi-squared test was used to compare incidences between the two groups. All calculations were performed using the Statistical Package for the Social Sciences, version 13.0 (SPSS Inc., Chicago, IL, USA). P values <0.05 were considered significant.
Results
Patients and tumors
A total of 23 patients with 29 oligometastatic lesions were enrolled. The median follow-up duration was 20 months (range, 3–53 months). Tumor characteristics are summarized in Table 1. The primary diagnoses were bone and soft tissue cancers in 13 lesions, liver cancers in 12 lesions, and colorectal cancers in 4 lesions. Most lesions (n = 25) were located very close to OARs: 21, 3 and 1 lesions were close to the GI tract, spinal cord and heart, respectively. Of the 21 lesions close to the GI tract, 15 were close to small bowel, three to stomach, two to esophagus, one to colon, and one to rectum. This includes one lesion close to both stomach and esophagus. Two lesions located in the liver were previously treated with SABR. The median tumor volume was 78 cm3 (range, 4–1781 cm3). Treatment characteristics are summarized in Table 2.
Table 1.
Patient and tumor characteristics
| Characteristics | Number of lesions (n = 29) |
|---|---|
| Age (years) | 40–79 (median 65) |
| Sex | |
| Male/Female | 23/6 |
| ECOG performance status | |
| 0/1/2 | 7/19/3 |
| Primary site (Histology) | |
| Bone & soft tissue (Liposarcoma/Osteosarcoma/Leiomyosarcoma) | 13 (10/2/1) |
| Liver (Hepatocellular carcinoma/Adenocarcinoma) | 12 (11/1) |
| Colon & rectum (Adenocarcinoma) | 4 (4) |
| Treated site | |
| Abdomena | 20 |
| Spine | 5 |
| Othersb | 4 |
| Recurrent type | |
| Local recurrence at primary site | 6 |
| Distant metastasis | 23 |
| Reason for the split-course SABR | |
| Close to organs at risk (GI tract/Spinal cord/Heart) | 25 (21/3/1) |
| Re-irradiation | 4 |
aAbdomen includes sites in the abdomen, pelvis, and retroperitoneum.
bOthers includes sites in the mandible, chest wall, buttock and supraclavicular area.
ECOG, Eastern Cooperative Oncology Group; SABR, stereotactic ablative radiotherapy; GI, gastrointestinal.
Table 2.
Treatment characteristics
| Characteristics | Number of lesions |
|---|---|
| Initial GTV (cm3) | 4–1781 (median 78) |
| <50 | 10 |
| 50–100 | 9 |
| 100–500 | 8 |
| >500 | 4 |
| First SABR | |
| 10 Gy/1 fraction | 5 |
| 20–24 Gy/2 fractions | 10 |
| 24–39 Gy/3 fractions | 14 |
| Second SABR | |
| 8–12 Gy/1 fraction | 23 |
| 20–24 Gy/2 fractions | 6 |
| Interval between two courses of SABR (weeks) | 3–8 (median 4) |
| 3/4/5/6/7/8 | 9/10/6/2/1/1 |
GTV, gross tumor volume; SABR, stereotactic ablative radiotherapy.
Volumetric/dosimetric changes in target volumes and OARs
Figure 2A shows a waterfall plot of the percentage changes in the GTV after the first SABR. The mean tumor volume change was −19% (range, −72–18%). Sixteen lesions (55%) were in the responder group and 13 lesions (45%) were in the non-responder group. As shown in Fig. 2A, lesion No. 1 had the best response with a 72% reduction in the GTV (from 275 to 78 cm3). Lesion No. 29 had the worst response with an 18% increase in the GTV (from 3.8 to 4.5 cm3) (Fig. 2A). Higher proportions of lesions treated with a high dose in the first SABR (more than 20 Gy in 2 fractions) were in the responder group than those treated with a low dose in the first SABR (Table 3).
Figure 2.
Waterfall plot demonstrating the proportional volume change of GTVs and OARs. Volumetric/dosimetric changes in target volumes and OARs between two courses of SABR were assessed. Lesions were ranked by proportional volume change in the GTV. (A) Sixteen lesions (55%) were in the responder group, showing more than a 10% change in the GTV after the first SABR treatment, and 13 lesions (45%) were in the non-responder group. (B) Dosimetric changes of OARs in 16 lesions that showed a response after the first SABR were analyzed. Practical radiation dose reductions of OARs were observed in the second SABR compared with the first SABR in lesions that responded after the first SABR. GTV, gross tumor volume; OAR, organ at risk; SABR, stereotactic ablative radiotherapy; Vx%, volume of OARs that received x% of prescribed dose; GI, gastrointestinal tract; SC, spinal cord.
Table 3.
Response of tumor volume in each lesion according to different factors
| Characteristics | GTV change after first SABR | P | |
|---|---|---|---|
| Respondera | Non-responder | ||
| Histologic type | 0.128 | ||
| Hepatocellular carcinoma | 8 | 3 | |
| Liposarcoma | 3 | 7 | |
| Others | 5 | 3 | |
| Dose of first SABR | 0.005 | ||
| ≤20 Gy/2fx | 4 | 10 | |
| >20 Gy/2fx | 12 | 3 | |
| Interval | 0.684 | ||
| ≤4 weeks | 11 | 8 | |
| >4 weeks | 5 | 5 | |
| GTV | 0.774 | ||
| ≤100 cc | 9 | 8 | |
| >100 cc | 7 | 5 | |
GTV, gross tumor volume, SABR, stereotactic ablative radiotherapy.
aResponder group: reduction of proportional GTV change after first SABR is over 10%.
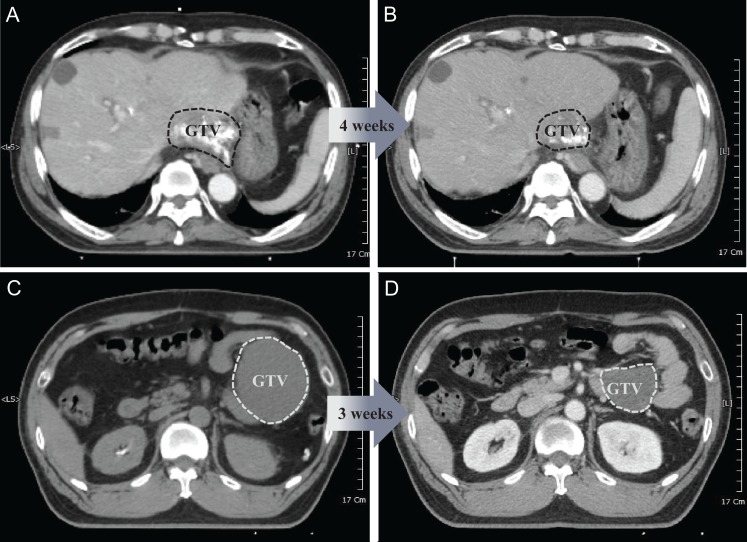
We analyzed the volumetric/dosimetric changes of OARs in the responder group. Practical reductions in the dose to OARs were observed in the second SABR compared with the first SABR when lesions responded after the first SABR. Figure 2B shows the percentage changes in Vx% of OARs between the first and second SABR. For example, lesion No. 1 showed a 72% reduction in the GTV (from 275 to 78 cm3). As a result, the V100% of the GI tract decreased by 85% (from 12 to 2 cm3) and the V70% of the GI tract decreased by 82% (from 155 to 29 cm3). Lesion No. 5 showed a 50% reduction in the GTV (from 55 to 27 cm3). As a result, the V100% of the GI tract decreased by 100% (from 2 to 0 cm3) and the V70% of the GI tract also decreased by 100% (from 13 to 0 cm3). Figure 3 shows the planning CTs for the first and second SABR for cases with an excellent response after the first SABR.
Figure 3.
Illustrated examples of tumors that showed an excellent response after the first SABR. (A) The first patient was a 66-year-old male with a history of hepatocellular carcinoma who was previously treated with radiofrequency ablation and transcatheter arterial chemoembolization and presented with recurrent disease in the liver. Pre-treatment CT imaging revealed a 6.5-cm mass abutting the stomach. The patient was subsequently treated with split-course SABR (39 Gy/3fx + 11 Gy/1fx boost) separated by a 4-week break. (B) After the first SABR, CT imaging demonstrated a dramatically reduced tumor size, and a safety margin of about 1 cm could be secured from the stomach in the second SABR. (C) The second patient was a 46-year-old male with a history of multiple recurrent liposarcoma, previously treated with several resections, who presented with recurrent disease in the mesentery. Pre-treatment CT imaging revealed an 8.5-cm mass abutting the small bowel. The patient was subsequently treated with split-course SABR (10 Gy/1fx + 10 Gy/1fx boost) separated by a 3-week break. (D) After the first SABR, CT imaging demonstrated a dramatically reduced tumor size, making it possible to reduce the irradiated volume of the small bowel in the second SABR. SABR, stereotactic ablative radiotherapy; CT, computed tomography.
Disease control
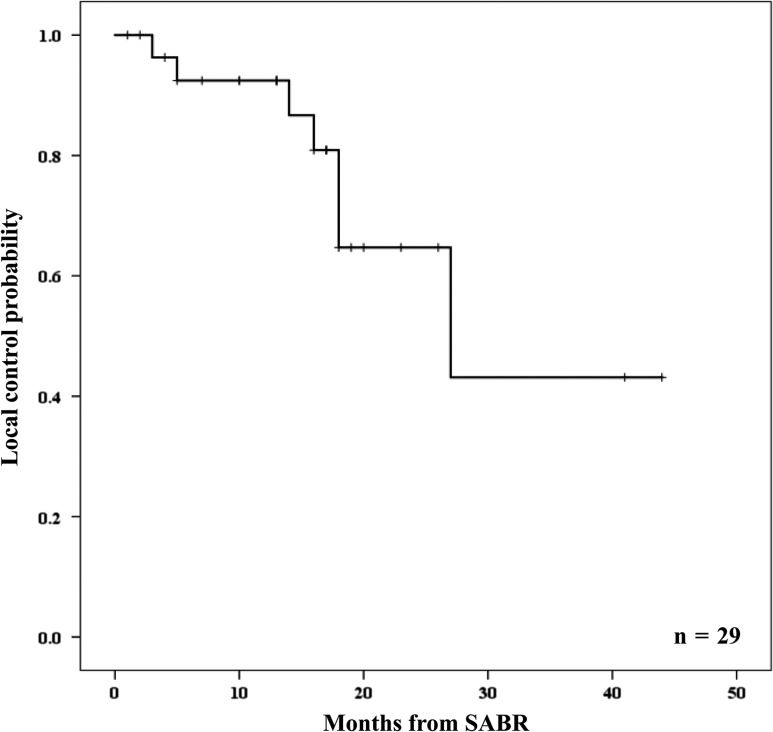
The 1-, 2- and 3-year local control rates were 92%, 65% and 43%, respectively (Fig. 4). There were no prognostic factors for local control. GTV, histology, total dose of SABR, and response after the first SABR did not affect the prognosis. Disease progression during follow-up occurred in 21 of 23 patients (91%). Distant failure was observed in 19 of 23 patients (83%).
Figure 4.
Local control probability of oligometastatases treated with split-course SABR. SABR, stereotactic ablative radiotherapy.
Toxicity
No Grade 4 or 5 toxicities were observed during or after the treatments. One patient, who had a recurrent mesenteric mass from duodenal cancer, required hospitalization 12 months after split-course SABR because of the development of a fistula of the transverse colon (Grade 3 late toxicity). The lesion was treated with fractionated RT of dose 48 Gy in 24 fractions plus a boost of 10 Gy in 1 fraction 8 months before the retreatment with split-course SABR. The split-course SABR was delivered with a first SABR of 30 Gy in 3 fractions and a second SABR of 10 Gy in 1 fraction to the mesenteric mass. The second SABR was delivered 30 days after the first SABR. Unfortunately, there was no reduction in the GTV after the first SABR.
Discussion
The purpose of splitting the course in conventional fractionated RT was to reduce the incidence of severe acute toxicity in normal tissues and to improve the tolerability of intensive therapy through the repair and repopulation of normal tissues. However, splitting the RT course is also associated with significant tumor repopulation during the pause in treatment. Indeed, some clinical results demonstrated negative outcomes of split-course RT for local tumor control of several types of tumors, including non-small cell lung cancer (NSCLC) and head and neck cancer (19–24). Therefore, split-course RT has been applied only for specific situations, such as radiosensitive tumors or patients with a high risk of complications. For example, RT of anal cancer, which is a radiosensitive tumor, usually induces severe acute complications of anoproctitis and perineal dermatitis. Treatment breaks in RT for anal cancer showed a reduction in the severity of acute complications (25,26). For patients with locally advanced head and neck cancers who were not candidates for standard continuous course RT because of a high risk of complications, split-course RT was applied and showed well-tolerated, safe treatment results with significant durable locoregional control (14). Gielda et al. (16) reported their experience of split-course RT with chemotherapy in Stage III NSCLC patients with pre-treatment weight loss and a poor performance status. These patients completed the planned treatment and showed a favorable outcome with a median survival of 22 months. It is presumed that a split course of conventional RT increased the tolerability to the treatment. Further, in most cases, it does not seem to compromise the survival benefit.
There have been a few reports of split-course SABR (27–29), mostly in treatment of the brain. It is widely accepted that the radiobiological factors that affect the efficacy and complication of multi-fractionated conventional RT are based on the four Rs (repair, repopulation, reoxygenation and reassortment). However, in SABR, radiobiological factors that affect the efficacy and complication of SABR are more complicated and have not been evaluated much compared with conventional RT. With regard to tumors, reoxygenation, repair of sublethal damage, reassortment of cell cycle distribution and repopulation may all occur during SABR, as in conventional RT. However, SABR using ablative doses causes additional severe vascular damage in the tumor and secondary tumor cell death occurs (30–32). Recently, it was proven that the ensuing degradation of tumor cells after SABR would then release very large amounts of tumor-specific antigens, thereby producing a much more intense anti-tumor immune response in SABR (33). Therefore, the conventional four Rs theory based on DNA damage of tumor cells may not play such a big role in split-course SABR as in conventional RT. As normal tissue can receive a low to high range of radiation doses during SABR, repair of sublethal damage and repopulation in normal cells may all occur during the interval between the first and second stages of SABR, and play a big role in normal tissue damage in SABR as they do in conventional RT. This present study shows that it is feasible to treat oligometastatic lesions closely located to OARs using split-course SABR with an interval of about 3–4 weeks without causing serious normal tissue complications. Previously, we analyzed patients whose tumors were located in a region near the GI tract in which the exposed doses were more than 20% of the prescribed doses of SABR, and reported that severe GI toxicities developed in 15% of the patients (34). In contrast, in the present study, severe toxicity (Grade 3) developed in only 1 case out of 29 lesions (3%) even though they had a high possibility of developing complications. A possible explanation would be the recovery of normal tissue during the approximate 3 weeks of respite from treatment. As an example, it has been shown that acute radiation enteropathy occurs due to damage in the intestinal mucosa, and that gradual regeneration of the villus epithelium takes place over a period of 1–3 weeks following radiation exposure (35,36)
Based on our clinical experiences, the rate of tumor regression after SABR seems to be rapid compared to that after conventional RT. Therefore, it is presumed that the reduction of the GTV would be induced before the beginning of repopulation of tumor in the split-course SABR. In a report of conventional fractionated RT, rapidly growing head and neck squamous cell carcinomas showed accelerated repopulation beginning about 4 weeks after the initiation of fractionated RT (37), leading to lower local control rates in split-course regimens than in continuous regimens of the same total dose (18,38). In contrast, prostate cancers are known to grow slowly, and thus a relatively long time interval between the split-courses may be used (39,40). Considering the timing of accelerated repopulation, it is thought that intervals in conventional RT should be spaced differently depending on the type of tumor. To our knowledge, no study on split-course SABR applied to tumors in the body has been reported. Only a few studies reported split-course SABR in brain metastases and 2–4 week interfraction intervals were applied between the two stages of SABR (27–29). Higuchi et al. treated 43 patients with large brain metastasis (>10 cm3) with 30 Gy in three stages of SABR delivered in 2-week interfraction intervals. Tumor volumes decreased by 18.8% after first SABR and 39.8% after second SABR. Overall tumor shrinkage was observed in 90.7% of the tumors (27). Angelov et al. also treated large brain metastases with split-course SABR with 4 week intervals and observed that the median tumor volume decreased by 33.3% at the time of second SABR (29). Similarly, during the time break of 3–4 weeks between the first and second SABR, we observed that the gross tumor volumes decreased significantly after the first SABR in 16 lesions (55%). The reduction in tumor volumes induced significant reduction in the radiation doses to OARs in the second SABR (Fig. 2B). Although 13 lesions did not show significant tumor reduction after the first SABR, most remained stable without significant repopulation of tumor cells during split-course SABR. In SABR using an ablative dose to the tumor, it is difficult to make a definite conclusion regarding the optimal interval because clinical experiences are still rare. Considering the present results, an interval of ~4 weeks in SABR is suggested for favorable regeneration of normal tissue without significant induction of repopulation. To arrive at a conclusion about the optimal interval for patients with a high risk of complications, variable Phase II or III protocols for split-course SABR based on expanded radiobiological knowledge should be developed.
In conclusion, split-course SABR appeared to achieve favorable toxicity profiles and local control outcomes comparable with those of continuous SABR in the patients with oligometastases. Tumor regression during an interval of 3–8 weeks allowed reduction of radiation dose to OARs. Further studies are necessary to determine the optimal interval in the SABR regimen to increase the radiobiological benefit as regeneration of normal tissue, and to prevent repopulation of tumor cell in cancer patients with a high risk of complications.
Acknowledgements
This study was supported by a grant of the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by Ministry of Science and ICT (MSIT), Republic of Korea. (No. 50541-2018).
Conflict of interest statement
None declared.
References
- 1. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- 2. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378–82. [DOI] [PubMed] [Google Scholar]
- 3. Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol 2006;16:120–30. [DOI] [PubMed] [Google Scholar]
- 4. Tree AC, Khoo VS, Eeles RA, et al. . Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28–37. [DOI] [PubMed] [Google Scholar]
- 5. Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012;17:1100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bae SH, Kim MS, Kim SY, et al. . Severe intestinal toxicity after stereotactic ablative radiotherapy for abdominopelvic malignancies. Int J Colorectal Dis 2013;28:1707–13. [DOI] [PubMed] [Google Scholar]
- 7. Choi CW, Cho CK, Yoo SY, et al. . Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys 2009;74:147–53. [DOI] [PubMed] [Google Scholar]
- 8. Kim MS, Yoo SY, Cho CK, et al. . Stereotactic body radiotherapy for isolated para-aortic lymph node recurrence after curative resection in gastric cancer. J Korean Med Sci 2009;24:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim MS, Yoo SY, Cho CK, et al. . Stereotactic body radiation therapy using three fractions for isolated lung recurrence from colorectal cancer. Oncology 2009;76:212–9. [DOI] [PubMed] [Google Scholar]
- 10. Kim MS, Cho CK, Yang KM, Lee DH, Moon SM, Shin YJ. Stereotactic body radiotherapy for isolated paraaortic lymph node recurrence from colorectal cancer. World J Gastroenterol 2009;15:6091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim MS, Choi C, Yoo S, et al. . Stereotactic body radiation therapy in patients with pelvic recurrence from rectal carcinoma. Jpn J Clin Oncol 2008;38:695–700. [DOI] [PubMed] [Google Scholar]
- 12. Cha YJ, Kim MS, Jang WI, et al. . Stereotactic body radiation therapy for liver oligo-recurrence and oligo-progression from various tumors. Radiat Oncol J 2017;35:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seo YS, Kim MS, Cho CK, et al. . Stereotactic body radiotherapy for oligometastases confined to the para-aortic region: clinical outcomes and the significance of radiotherapy field and dose. Cancer Invest 2015;33:180–7. [DOI] [PubMed] [Google Scholar]
- 14. Bledsoe TJ, Noble AR, Reddy CA, et al. . Split-Course Accelerated Hypofractionated Radiotherapy (SCAHRT): a safe and effective option for head and neck cancer in the elderly or infirm. Anticancer Res 2016;36:933–9. [PubMed] [Google Scholar]
- 15. Dubben HH, Krull A, Beck-Bornholdt HP. Split-course radiotherapy: where do we stand? Strahlenther Onkol 2001;177:227–39. [DOI] [PubMed] [Google Scholar]
- 16. Gielda BT, Marsh JC, Zusag TW, et al. . Split-course chemoradiotherapy for locally advanced non-small cell lung cancer: a single-institution experience of 144 patients. J Thorac Oncol 2011;6:1079–86. [DOI] [PubMed] [Google Scholar]
- 17. Overgaard J, Hjelm-Hansen M, Johansen LV, Andersen AP. Comparison of conventional and split-course radiotherapy as primary treatment in carcinoma of the larynx. Acta Oncol 1988;27:147–52. [DOI] [PubMed] [Google Scholar]
- 18. Parsons JT, Bova FJ, Million RR. A re-evaluation of split-course technique for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1980;6:1645–52. [DOI] [PubMed] [Google Scholar]
- 19. Fowler JF, Chappell R. Non-small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys 2000;46:516–7. [DOI] [PubMed] [Google Scholar]
- 20. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th edn Philadelphia: Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 21. Petereit DG, Sarkaria JN, Chappell R, et al. . The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys 1995;32:1301–7. [DOI] [PubMed] [Google Scholar]
- 22. Fu KK, Pajak TF, Trotti A, et al. . A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000;48:7–16. [DOI] [PubMed] [Google Scholar]
- 23. Cox JD, Pajak TF, Asbell S, et al. . Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys 1993;27:493–8. [DOI] [PubMed] [Google Scholar]
- 24. Overgaard J, Alsner J, Eriksen J, Horsman MR, Grau C. Importance of overall treatment time for the response to radiotherapy in patients with squamous cell carcinoma of the head and neck. Rays 2000;25:313–9. [PubMed] [Google Scholar]
- 25. Konski A, Garcia M Jr., John M, et al. . Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys 2008;72:114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ben-Josef E, Moughan J, Ajani JA, et al. . Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of Radiation Therapy Oncology Group trials 87-04 and 98-11. J Clin Oncol 2010;28:5061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higuchi Y, Serizawa T, Nagano O, et al. . Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 2009;74:1543–8. [DOI] [PubMed] [Google Scholar]
- 28. Hsieh CH, Chang HT, Lin SC, et al. . Toxic risk of stereotactic body radiotherapy and concurrent helical tomotherapy followed by erlotinib for non-small-cell lung cancer treatment – case report. BMC Cancer 2010;10:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Angelov L, Mohammadi AM, Bennett EE, et al. . Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases >/=2 cm. J Neurosurg 2017;22:1–17. [DOI] [PubMed] [Google Scholar]
- 30. Withers HR, Peters LJ. Biological Aspects of Radiation Therapy. 3rd edn Philadelphia: Lea & Febiger, 1980. [Google Scholar]
- 31. Steel GG. Growth Kinetics of Tumours. New York: Oxford University Press, 1977. [Google Scholar]
- 32. Kim MS, Kim W, Park IH, et al. . Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiat Oncol J 2015;33:265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bae SH, Kim MS, Cho CK, et al. . Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys 2012;84:e469–74. [DOI] [PubMed] [Google Scholar]
- 35. Devik F. Intestinal cell kinetics in irradiated mice. A quantitative investigation of the acute reaction to whole body roentgen irradiation. Acta Radiol Ther Phys Biol 1971;10:129–49. [DOI] [PubMed] [Google Scholar]
- 36. Poulakos L, Osborne JW. The kinetics of cellular recovery in locally x-irradiated rat ileum. Radiat Res 1973;53:402–13. [PubMed] [Google Scholar]
- 37. Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 1988;27:131–46. [DOI] [PubMed] [Google Scholar]
- 38. Parsons J. Time-Dose-Volume Relationships in Radiation Therapy. Philadelphia: Lippincott, 1984. [Google Scholar]
- 39. Pino y Torres JL, Lee DJ, Leibel SA, et al. . Local control and reduced complications in split course irradiation of prostatic cancer. Int J Radiat Oncol Biol Phys 1981;7:43–7. [DOI] [PubMed] [Google Scholar]
- 40. Parsons JT, Thar TL, Bova FJ, Million RR. An evaluation of split-course irradiation for pelvic malignancies. Int J Radiat Oncol Biol Phys 1980;6:175–81. [DOI] [PubMed] [Google Scholar]