Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a ubiquitous environmental toxicant and endocrine disrupting chemical, but little is known about its effects on female reproduction. Thus, we tested the hypothesis that prenatal exposure to DEHP accelerates the onset of puberty, disrupts estrous cyclicity, disrupts birth outcomes, and reduces fertility in the F1, F2, and F3 generations of female mice. Pregnant CD-1 mice were orally dosed with corn oil (vehicle control) or DEHP (20 and 200 µg/kg/day and 500 and 750 mg/kg/day) from gestation day 10.5 until birth. F1 females were mated with untreated males to obtain the F2 generation. F2 females were mated with untreated males to produce the F3 generation. In all generations, the onset of puberty, estrous cyclicity, select birth outcomes, and fertility-related indices were evaluated. In the F1 generation, prenatal DEHP exposure (200 µg/kg/day) accelerated the onset of puberty, it (200 µg and 500 mg/kg/day) disrupted estrous cyclicity, and it (20 and 200 µg/kg/day) decreased fertility-related indices. In the F2 generation, ancestral DEHP exposure (500 mg/kg/day) accelerated the onset of puberty, it (20 and 200 µg/kg/day) disrupted estrous cyclicity, it (20 µg and 500 mg/kg/day) increased litter size, and it (500 mg/kg/day) decreased fertility-related indices. In the F3 generation, ancestral DEHP exposure (20, 200 µg, and 500 mg/kg/day) accelerated the onset of puberty, it (20 µg/kg/day) disrupted estrous cyclicity, and it (750 mg/kg/day) decreased female pup anogenital index. Collectively, these data indicate that prenatal DEHP exposure causes female reproductive problems in a multigenerational and transgenerational manner.
Keywords: fertility, DEHP, transgenerational, prenatal
Phthalate esters are a family of synthetic chemicals primarily used as plasticizers to confer flexibility, prevent plastics from becoming brittle, allow products to become transparent, and improve the longevity of products (ATSDR, 2002). Di(2-ethylhexyl) phthalate (DEHP) in particular is a synthetic, high-molecular weight, organic phthalate compound. On average, 300 million pounds of DEHP are produced annually in the United States (ATSDR, 2002). DEHP is incorporated in a multitude of products such as personal care products, medical equipment (e.g., blood and IV bags), car upholstery, food and beverage containers, and building materials (Hannon and Flaws, 2015). Because DEHP is noncovalently bound, it leaches from products and into the environment (Heudorf et al., 2007). Due to the frequent use of products containing DEHP, the high volume of DEHP production, and the ability of DEHP to leach from products, humans are repeatedly and continuously exposed to DEHP (Hannon and Flaws, 2015). The average range of human exposure to DEHP is between 3 and 30 µg/kg/day (Koch and Calafat, 2009), and studies show that 100% of human urine samples test positive for DEHP and its metabolites (Silva et al., 2017).
Phthalate exposure is higher in women than men, likely due to the presence of phthalates in personal care products and the higher use of personal care products among women compared with men (James-Todd et al., 2016). In women, DEHP can be found in developmental and reproductive tissues (Hannon and Flaws, 2015). Specifically, DEHP and its metabolites have been found in cord blood samples from newborns, breast milk from nursing mothers, and human ovarian follicular fluid. Further, DEHP and its metabolites are present in amniotic fluid from fetuses, demonstrating that DEHP has the ability to cross the placenta (Hannon and Flaws, 2015). This is concerning because a pregnant mother exposed to DEHP also risks exposure to her developing fetus.
Of concern, DEHP acts as an endocrine disrupting chemical (EDC) and the developing fetus and the female reproductive tract are particularly susceptible to EDCs. Previous studies have shown that prenatal exposure to DEHP adversely affects reproductive outcomes in rodents (Hannon et al., 2016; Niermann et al., 2015; Pocar et al., 2017). Specifically, our laboratory has shown that prenatal DEHP exposure (20 µg/kg/day) increased the time to pregnancy and it (750 mg/kg/day) increased the number of dead pups in the F1 generation of female mice (Niermann et al., 2015). Further, DEHP exposure (200 µg/kg/day) increased the male-to-female sex ratio in litters of the F1 generation (Niermann et al., 2015). Prenatal exposure to DEHP (200 µg, 500, and 750 mg/kg/day) also decreased the percentage of atretic follicles in the ovary and it (20 µg/kg/day, 500, and 750 mg/kg/day) increased serum estradiol levels at postnatal day (PND) 8 in the F1 generation (Rattan et al., 2018). In addition, another study has shown that maternal DEHP exposure accelerated the rate of follicular recruitment in the F1 generation, which could deplete the primordial follicle reserve and lead to early reproductive senescence (Pocar et al., 2017; Zhang et al., 2015).
Exposure to DEHP during development may have further implications for future generations (Culty et al., 2008; Davis et al., 1994; Gupta et al., 2010; Rattan et al., 2018). Studies have shown that DEHP exposure during embryonic development causes transgenerational inheritance of testicular disease in male mice (Doyle et al., 2013; Manikkam et al., 2013). Transgenerational effects occur when the effects of DEHP are inherited and passed through the germ-line (Skinner, 2008, 2016). Although few studies have investigated the transgenerational effects of DEHP exposure on female reproductive outcomes, one study has shown that maternal exposure to DEHP (0.05–5 mg/kg/day) accelerated folliculogenesis in the ovary in the F2 and F3 generations of mice (Pocar et al., 2017). In addition, the results from another study indicated that ancestral exposure to a phthalate mixture, which included DEHP, increased uterine weight, decreased anogenital distance (AGD), and induced fertility complications in a transgenerational manner in mice (Zhou et al., 2017a). Previously, our study has shown that ancestral exposure to DEHP decreased serum estradiol levels and disrupted serum progesterone levels in the F2 generation of female mice (Rattan et al., 2018).
Although previous studies show that prenatal exposure to DEHP has adverse effects on ovarian follicle numbers and hormone levels, they have not assessed the effects of prenatal DEHP exposure on the onset of puberty, estrous cyclicity, and fertility (Manikkam et al., 2013; Niermann et al., 2015; Pocar et al., 2017). Thus, we hypothesized that daily prenatal exposure to DEHP during the second half of pregnancy in the F0 generation adversely affects reproductive outcomes in female mice in a transgenerational manner. Specifically, we investigated whether prenatal DEHP exposure accelerates the onset of puberty, disrupts estrous cyclicity, decreases the number of pregnancies and litters, and adversely affects the birth outcomes of pups in the F1–F3 generations of female mice.
MATERIALS AND METHODS
Chemicals
DEHP (99% purity) was purchased from Sigma-Aldrich (St Louis, Missouri). Stock solutions of DEHP (0.022, 0.224, 560, and 840 mg/ml) were prepared by diluting DEHP in tocopherol-stripped corn oil (MP Biomedicals, Solon, Ohio). These stock solutions were diluted to create doses of 20 µg/kg/day, 200 µg/kg/day, 500 mg/kg/day, and 750 mg/kg/day of DEHP. DEHP concentrations were chosen based on previous studies and their environmental relevance (Doyle et al., 2013; Hannon et al., 2015a, b; Hannon et al., 2014, 2016; Niermann et al., 2015). Specifically, the 20 µg/kg/day dose of DEHP was selected because the U.S. Environmental Protection Agency established the chronic oral reference dose as 20 µg/kg/day of DEHP. The reference dose is an estimate of the daily oral exposure of DEHP in the general population that has a low risk of adverse effects during the lifetime (EPA, 1987). In addition, 20 µg/kg/day of DEHP falls within the estimated human exposure range based on urinary metabolite levels (Koch and Calafat, 2009). The 200 µg/kg/day dose of DEHP was used because it falls within the estimated occupational range of exposure (Hannon and Flaws, 2015). In addition, adult exposure to 200 µg/kg/day of DEHP has been shown to cause abnormal estrous cyclicity and accelerate primordial follicle recruitment in female CD-1 mice (Hannon et al., 2014). The 500 mg/kg/day dose of DEHP was selected because it has been shown to cause abnormalities in spermatogonial stem cells across multiple generations in male CD-1 mice (Doyle et al., 2013). The 750 mg/kg/day dose of DEHP was selected because adult exposure has been shown to cause abnormal estrous cyclicity and accelerate primordial follicle recruitment in adult female CD-1 mice (Hannon et al., 2014).
Animals and dosing paradigm
Adult female and male CD-1 mice (Charles River, Portage, Michigan and Raleigh, North Carolina) were housed at 25°C in conventional polysulfone, ventilated cages on 12:12 (L:D) cycles. The mice were fed Teklad Rodent Diet 8604 (Envigo, Indianapolis, Indiana) and provided highly purified water (reverse osmosis filtered water) in polysulfone water bottles ad libitum. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and abide by the guidelines set forth by the National Institute of Health for the Care and Use of Laboratory Animals.
At 8 weeks of age, female mice (F0) were mated with control male mice of the same age. The female mice were monitored twice a day for the presence of a copulatory vaginal sperm plug to confirm mating. Once a copulatory vaginal sperm plug was confirmed, the presence of which was considered gestational day (GD) 0.5, the females were removed, weighed, and individually housed. Subsequently, the mice were weighed twice a week to confirm successful pregnancy. From GD 10.5 until birth of the pups, 66 pregnant dams (F0 generation) were orally dosed (7–27 dams/treatment group) once a day with the vehicle control (tocopherol-stripped corn oil) or with DEHP (20 µg/kg/day, 200 µg/mg/day, 500 mg/kg/day, and 750 mg/kg/day) by placing a pipette tip with the dosing solution into the cheek pouch of the mouse. This dosing regimen was selected to mimic oral exposure to DEHP in humans (ATSDR, 2002; Hannon et al., 2016; Hannon et al., 2014). The doses were calculated and adjusted based on daily body weights, and delivered in 25–33 µl of tocopherol-stripped corn oil. The treatment window was chosen because it is a critical time period of ovarian development. Specifically, this is when primordial germ cells arrive at the gonad (Hirshfield, 1991; Pepling, 2006), and sex determination (Menke et al., 2003), global demethylation, and imprint erasure of primordial germ cells occur (Durcova-Hills and Capel, 2008).
Pregnant mice were allowed to deliver naturally and the day of birth was considered PND 0. Mice born from the F0 generation were labeled the F1 generation. Female mice from the F1 generation were mated with nontreated male CD-1 mice to produce the F2 generation. Females from the F2 generation were mated with nontreated male CD-1 mice to produce the F3 generation. No mice were mated with family members.
Onset of puberty
In all generations, female mice were weaned at PND 21. One female mouse from each litter was weighed and observed daily for the presence of a vaginal opening. Once a vaginal opening was observed, the age and weight of the females were recorded, and daily vaginal lavages were performed for 30 consecutive days to monitor the onset of estrus and estrous cyclicity.
Fertility tests
In all generations, female mice were subjected to daily vaginal lavages at 3, 6, and 9 months of age for 14 consecutive days to monitor estrous cyclicity. Following 14 days of consecutive vaginal lavages, female mice were weighed and paired with nontreated male CD-1 mice to test fertility. Female mice were monitored twice a day for the presence of a copulatory vaginal sperm plug to indicate successful mating. The latency to a copulatory vaginal sperm plug was recorded. All female mice were weighed twice a week to monitor weight gain associated with pregnancy regardless of a positive copulatory vaginal sperm plug. Once a copulatory vaginal sperm plug was observed, female mice were removed from the male cages and placed individually in a fresh cage, and weight gain was continuously monitored. Female mice were paired with nontreated CD-1 male mice for a maximum of 14 days for the fertility tests. If a vaginal sperm plug was not observed, female mice were housed individually and body weight gain was continuously monitored. Body weight gain was used to monitor pregnancy loss and/or maintenance of pregnancy. The number of total females, females with a copulatory plug, pregnant females, and females that delivered pups were recorded (Supplementary Table 1). The total number of females was defined as females paired with males. Plugged females were defined as either: (1) females that had a copulatory vaginal sperm plug or (2) females that did not have a copulatory vaginal sperm plug, but gained at least 4 g of weight. Pregnant females were defined as females that either: (1) had a copulatory vaginal sperm plug and gained at least 4 g of weight during their pregnancy, (2) did not have a copulatory plug, but gained at least 4 g of weight, or (3) did not have a copulatory plug, but produced a litter. Females that delivered pups were defined as dams that gave birth to either live or dead pups. These definitions were used to calculate the mating index, pregnancy rate, fertility rate, and gestational index based on the following equations used by previous studies (Tyl et al., 2008; Zhou et al., 2017a; Ziv-Gal et al., 2015):
Mating index = number of females with copulatory vaginal sperm plugs/number of total females × 100
Pregnancy rate = number of pregnant females/number of total females × 100
Fertility index = number of pregnant females/number of females with copulatory vaginal sperm plugs × 100
Gestational index = number of females who delivered/number of pregnant females × 100
Once a pregnant dam gave birth to pups, all pups were counted and sexed and live pups were weighed. At PND 21, male and female body weight and AGD were recorded. The anogenital index (AGI) was calculated as the AGD divided by the cubed root of body weight (Gallavan et al., 1999). Any pup deaths between PND 0 and PND 21 were recorded and reported as the percent of dead pups per litter.
Infertility during early, mid-, or late gestation was recorded for all females. Early gestation infertility was classified as no observed copulatory vaginal sperm plug, or an observed copulatory vaginal sperm plug, but no significant weight gain. Mid-gestation infertility was defined as an observed copulatory vaginal sperm plug, body weight gain around GD7 –12, but loss of the gained weight and a return to prepregnancy weight without birth of pups. Late gestation infertility was defined as a successful completion of pregnancy, but no live pups were born, suggesting infertility may be caused by late gestation issues (Wang et al., 2014).
Statistical analyses
Data were expressed as the mean ± SEM. In all generations, data from multiple female pups originating from the same litter were averaged and combined as n = 1, and data from at least 3 separate litters were used in the analyses. Data were analyzed by comparing treatment groups to control using SPSS software (SPSS Inc., Chicago, Illinois). Outliers were removed by the Grubb’s test using GraphPad outlier calculator software (GraphPad Software Inc., La Jolla, California). Two cohorts (2 groups of animals) were mated to produce the F3 generations. To test for cohort differences, data were tested using a general linear model univariate test. If there was an interaction between treatment and cohort in tests of between-subjects effects, then the data from the first cohort were analyzed. If no interaction effect between treatment and cohort occurred, then both cohorts were analyzed together. Data that were continuous were assessed for normal distribution by Shapiro-Wilk analysis. If data met assumptions of normal distribution and homogeneity of variance, data were analyzed by 1-way analysis of variance (ANOVA) followed by Dunnett (2-sided) post hoc comparisons. If data were presented as percentages, were not normally distributed, and/or did not meet homogeneity of variance assumptions, the independent sample Kruskal-Wallis H followed by Mann-Whitney U nonparametric tests were performed. Data that were nominal were analyzed using the 1-tailed Fisher’s Exact test to compare individual treatment groups to control. For all comparisons, statistical significance was determined by a p-value ≤ .05. In instances in which p-values were > .05, but < .1, data were considered to exhibit a trend towards significance.
RESULTS
Effects of DEHP Exposure on Pubertal Body Weights, Age of Vaginal Opening, and Age of First Estrus
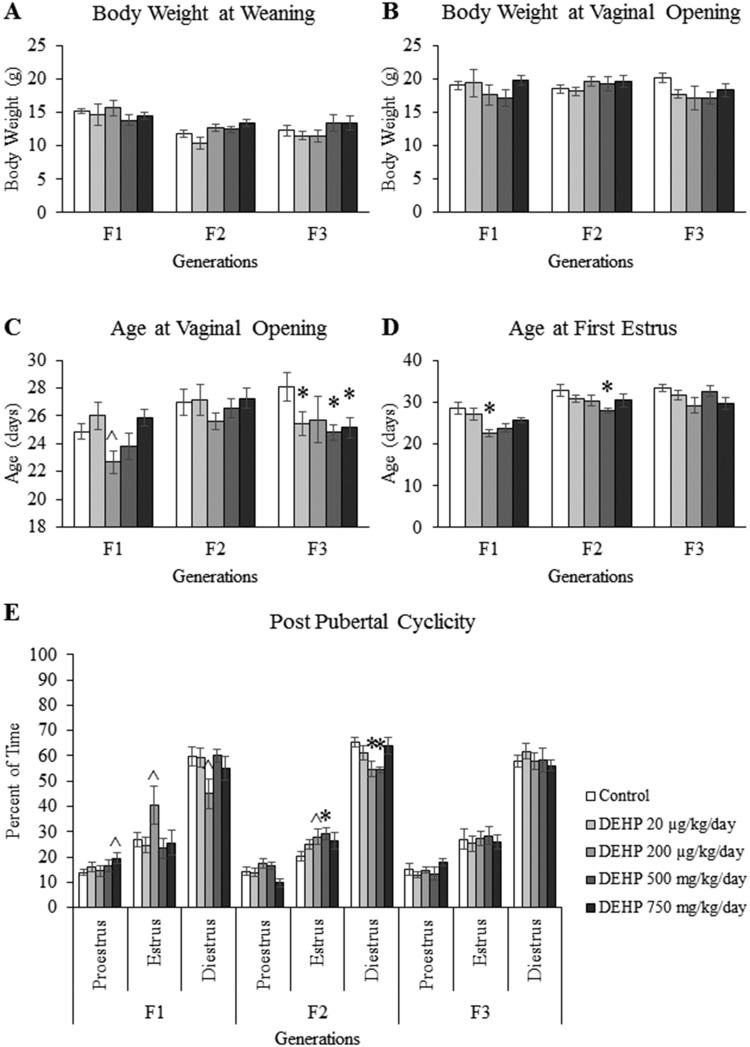
To monitor puberty, the body weight at weaning, body weight at vaginal opening, age at vaginal opening, and age at first estrus were recorded. Our results indicate that in the F1–F3 generations, prenatal and ancestral exposure to DEHP did not affect the body weight of female mice at weaning (PND 21) (Figure 1A) or the body weight of female mice at vaginal opening (Figure 1B).
Figure 1.
The effects of prenatal exposure to DEHP on pubertal outcomes in the F1–F3 generations. The body weight at weaning (A), body weight at vaginal opening (B), age at vaginal opening (C), age at first estrus (D), and post pubertal cyclicity (E) are shown for the F1–F3 generations. Graphs represent mean ± SEM from 5 to 16 dams per treatment group. *p ≤ .05 (significant difference compared with the control); .05 < ^p ≤ .078 (borderline difference compared with the control).
In the F1 generation, prenatal exposure to the 200 µg/kg/day dose of DEHP accelerated the age at vaginal opening, but this change was of borderline statistical significance compared with controls (Figure 1C, n = 5–16 dams/treatment group; p = .061). In contrast, exposure to the 200 µg/kg/day dose of DEHP significantly accelerated the age at first estrus compared with controls (Figure 1D, n = 6–15 dams/treatment group; p ≤ .05). In the F2 generation, ancestral exposure to DEHP did not significantly affect the age at vaginal opening (Figure 1C), but the 500 mg/kg/day dose of DEHP significantly accelerated the age at first estrus compared with controls (Figure 1D, n = 11–15 dams/treatment group; p ≤ .05). In the F3 generation, ancestral exposure to the 20 µg/kg/day and 500 and 750 mg/kg/day doses of DEHP significantly accelerated the age at vaginal opening compared with controls (Figure 1C, n = 7–11 dams/treatment group; p ≤ .05), but DEHP exposure did not affect the age at first estrus (Figure 1D).
Effects of DEHP Exposure on Postpubertal Cyclicity
To examine the effects of DEHP exposure on estrous cyclicity following the onset of puberty, estrous cyclicity was monitored for 30 consecutive days following vaginal opening. In the F1 generation, prenatal exposure to the 750 mg/kg/day dose of DEHP increased the percent of time in proestrus and the 200 µg/kg/day dose of DEHP increased the percent of time in estrus and decreased the percent of time in diestrus compared with controls, but the changes were borderline statistically significant (Figure 1E, n = 6–15 dams/treatment group; p = .068 for the increased percent of time in proestrus in the 750 mg/kg/day group vs control; p = .078 for the increased percent of time in estrus in the 200 µg/kg/day group vs control; p = .070 for the decreased percent of time in diestrus in the 200 µg/kg/day group vs control). In the F2 generation, ancestral exposure to the 200 µg/kg/day and 500 mg/kg/day doses of DEHP increased the percent of time in estrus and decreased the percent of time that the mice spent in diestrus compared with controls (Figure 1E, n = 11–15 dams/treatment group; p ≤ .05 for the percent of time in diestrus in the 200 µg/kg/day group vs control and for the percent of time in estrus and diestrus in the 500 mg/kg/day group vs control; p = .078 for the percent of time in estrus 200 µg/kg/day group vs control). In the F3 generation, ancestral DEHP exposure did not affect postpubertal estrous cyclicity (Figure 1E).
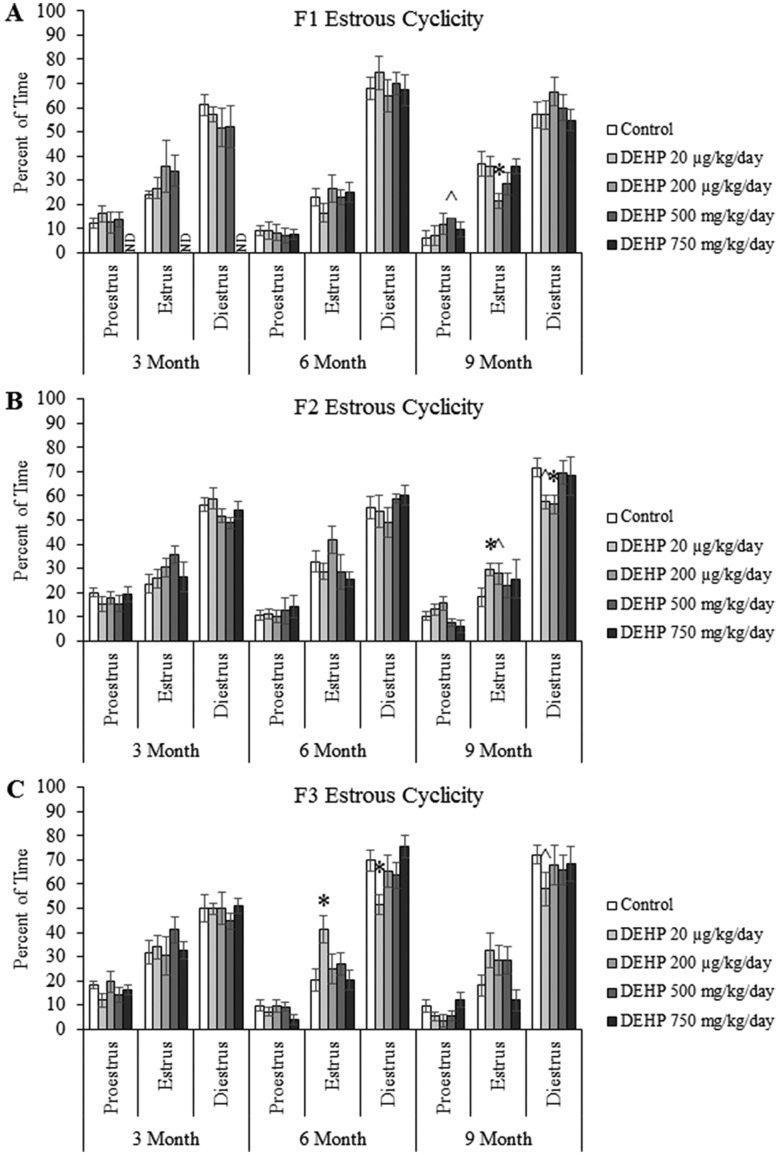
Effects of DEHP Exposure on Estrous Cyclicity
To examine the effects of DEHP exposure on estrous cyclicity during adult life, estrous cyclicity was monitored for 14 consecutive days at 3, 6, and 9 months of age. In the F1 generation, prenatal exposure to DEHP did not affect estrous cyclicity at 3 or 6 months of age, but the 500 mg/kg/day dose of DEHP borderline significantly increased the percent of time that mice spent in proestrus and the 200 µg/kg/day dose of DEHP significantly increased the percent of time that mice spent in estrus compared with controls at 9 months of age (Figure 2A, n = 4–7 dams/treatment group; p = 0.071 for the 500 mg/kg/day group vs control; p ≤ .05 for the 200 µg/kg/day group vs control). In the F2 generation, ancestral exposure to DEHP did not affect estrous cyclicity at 3 or 6 months of age. However, the 20 µg/kg/day dose of DEHP significantly increased the percent of time that the mice spent in estrus and the 200 µg/kg/day dose significantly decreased the amount of time that the mice spent in diestrus compared with controls at 9 months of age (Figure 2B, n = 10–15 dams/treatment group; p ≤ .05). Further, the 200 µg/kg/day dose of DEHP increased the time that the mice spent in estrus and the 20 µg/kg/day dose of DEHP decreased the time the mice spent in diestrus at 9 months of age, but these changes were borderline statistically significant (Figure 2B, n = 10–15 dams/treatment group; p = .086 for the 200 µg/kg/day group vs control; p = .066 for the 20 µg/kg/day group vs control). In the F3 generation, ancestral DEHP exposure did not affect estrous cyclicity at 3 months of age, but the 20 µg/kg/day dose of DEHP significantly increased the percent of time mice spent in estrus and decreased the percent of time that mice spent in diestrus compared with controls at 6 months of age (Figure 2C, n = 9–10 dams/treatment group; p ≤ .05). Further, the 20 µg/kg/day dose of DEHP decreased the time that the mice spent in diestrus at 9 months of age, but this was of borderline significance (Figure 2C, n = 9 dams/treatment group; p = .089).
Figure 2.
The effects of prenatal exposure to DEHP on estrous cyclicity are shown for the F1 generation (A), F2 generation (B), and F3 generation (C) at 3, 6, and 9 months of age. Graphs represent mean ± SEM from 2 to 15 dams per treatment group. *p ≤ .05 (significant difference compared with the control); .05 < ^p ≤ .086 (borderline difference compared with the control); ND, no data.
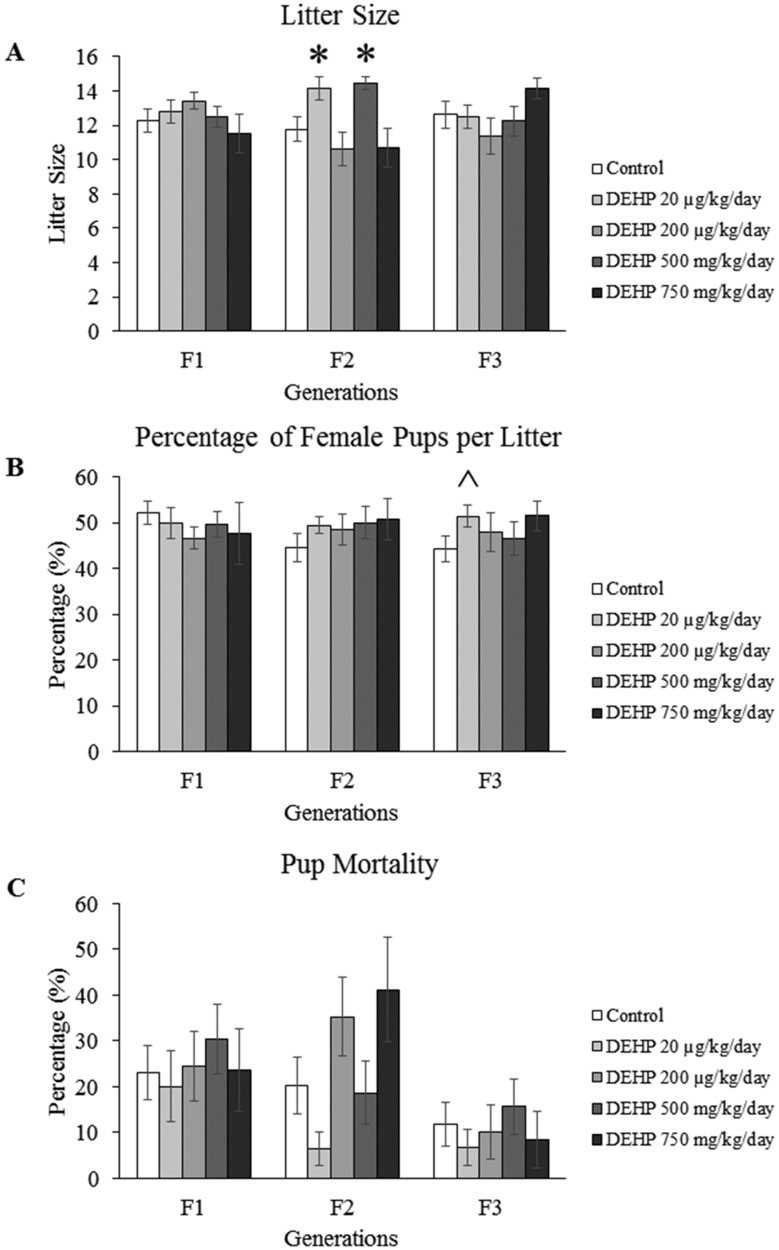
Effects of DEHP Exposure on Birth Outcomes
Select birth outcomes such as litter size, percentage of female pups, and mortality rates were monitored during the fertility tests. In the F1 and F3 generations, exposure to DEHP did not affect the litter size compared with controls. However, in the F2 generation, ancestral exposure to the 20 µg/kg/day and 500 mg/kg/day doses of DEHP increased the litter size compared with controls (Figure 3A, n = 7–26 dams/treatment group; p ≤ .05).
Figure 3.
The effects of prenatal exposure to DEHP on birth outcomes such as litter size (A), percentage of females per litter (B), and mortality rate (C) are shown for the F1–F3 generations. Graphs represent mean ± SEM from 7 to 44 dams per treatment group. *p ≤ .05 (significant difference compared with the control); ^p = .080 (borderline difference compared with the control).
In the F1 and F2 generations, prenatal and ancestral exposure to DEHP did not affect the percentage of female pups. However, in the F3 generation, ancestral exposure to the 20 µg/kg/day dose of DEHP increased the percentage of female pups per litter, but the increase only trended towards significance (Figure 3B, n = 24–26 dams/treatment group; p = .080). In addition, in the F1–F3 generations, exposure to DEHP did not affect the pup mortality rate compared with controls (Figure 3C).
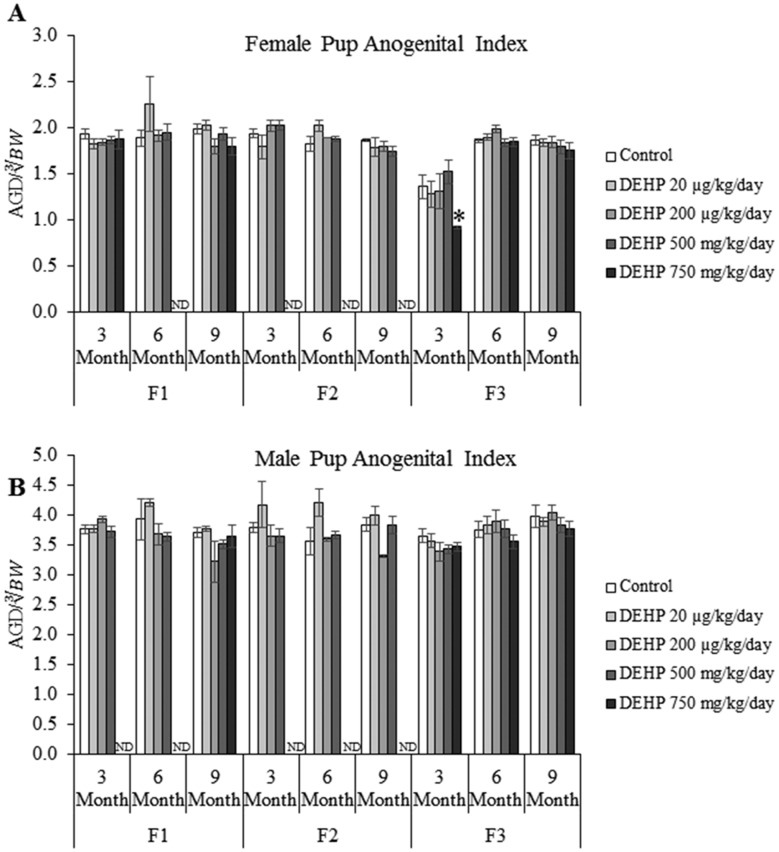
Effects of DEHP Exposure on AGI
In the F1 and F2 generations, prenatal and ancestral DEHP exposure did not affect female pup AGI compared with controls (Figure 4A). However, in the F3 generation, ancestral exposure to the 750 mg/kg/day dose of DEHP decreased the female pup AGI in the 3-month-old litter compared with controls (Figure 4A, n = 5–10 dams/treatment group; p ≤ .05), but it did not affect the female AGI in the 6- or 9-month-old litters. In the F1–F3 generations, prenatal and ancestral exposure to DEHP did not affect the male pup AGI compared with controls (Figure 4B).
Figure 4.
The effects of prenatal exposure to DEHP on female pup AGI (A) and male pup AGI (B) are shown for the F1–F3 generations at 3, 6, and 9 months of age. Graphs represent mean ± SEM from 2 to 13 dams per treatment group. *p ≤ .05 (significant difference compared with the control); ND, no data.
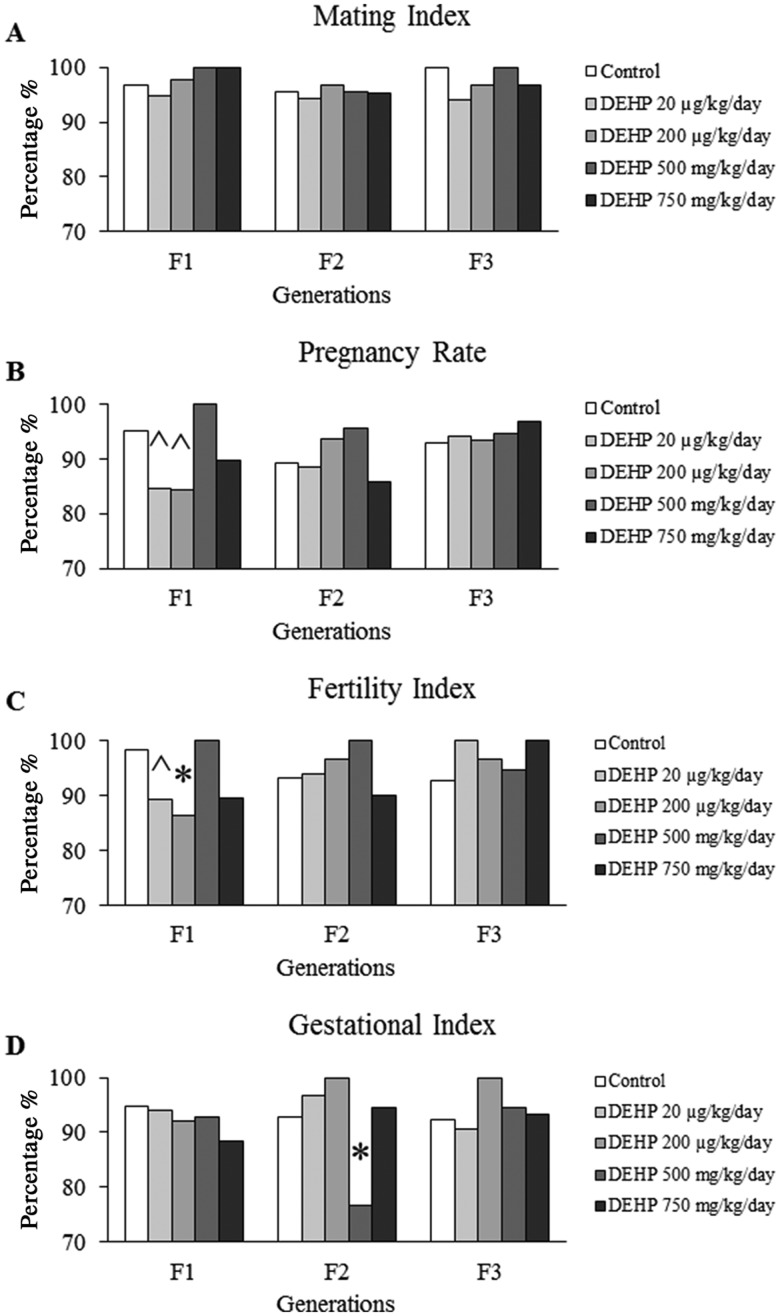
Effects of DEHP Exposure on Fertility-Related Indices
Exposure to DEHP did not affect the mating index in any generations. Specifically, the mating index ranged from 94% to 100% in all treatment groups in the F1–F3 generations (Figure 5A). In the F1 generation, prenatal exposure to the 20 and 200 µg/kg/day doses of DEHP decreased the pregnancy rate compared with controls, but the decrease only trended towards statistical significance (Figure 5B, n = 37–60 dams/treatment group; p = .082 and .069, respectfully). Ancestral DEHP exposure did not significantly affect the pregnancy rate in the F2 or F3 generations. In the F1 generation, prenatal exposure to the 20 and 200 µg/kg/day doses of DEHP decreased the fertility index compared with controls (Figure 5C, n = 33–58 dams/treatment group; p = .074 and p ≤ .05, respectfully). In contrast, ancestral exposure to DEHP did not affect the fertility index in the F2 or F3 generations. In the F1 and F3 generations, prenatal and ancestral exposure to DEHP did not affect the gestational index. However, in the F2 generation, ancestral exposure to the 500 mg/kg/day dose of DEHP significantly decreased the gestational index compared with controls (Figure 5D, n = 41–44 dams/treatment group; p ≤ .05).
Figure 5.
The effects of prenatal exposure to DEHP on the fertility-related indices the F1, F2, and F3 generations. The mating index (A), pregnancy rate (B), fertility index (C), and gestational index (D) are represented. Graphs represents mean ± SEM from 12 to 60 dams per treatment group. *p ≤ .05 (significant difference compared with the control); .05 < ^p ≤ .082 (borderline difference compared with the control).
Effects of DEHP Exposure on Types of Infertility
In the F1 generation, prenatal exposure to the 20 µg/kg/day dose of DEHP increased the number of dams with early gestation infertility and the 200 µg/kg/day dose of DEHP increased the number of dams with early and mid-gestation infertility compared with controls (Table 1). In the F2 generation, ancestral exposure to the 500 mg/kg/day dose of DEHP increased the number of dams with midgestation infertility compared with controls (Table 1). In the F3 generation, ancestral DEHP exposure did not appear to change the types of infertility compared with controls (Table 1).
Table 1.
The Number of Female Mice That Experience Infertility in Early, Mid-, or Late Gestation in the F1–F3 Generations
| Treatment |
||||||
|---|---|---|---|---|---|---|
| Generation | Type of Infertility | Control | DEHP 20 µg/kg/day | DEHP 200 µg/kg/day | DEHP 500 mg/kg/day | DEHP 750 mg/kg/day |
| F1 | Early gestation | 3 | 6 | 5 | 0 | 3 |
| Mid gestation | 3 | 3 | 5 | 3 | 2 | |
| Late gestation | 0 | 0 | 1 | 2 | 2 | |
| F2 | Early gestation | 6 | 3 | 2 | 1 | 2 |
| Mid gestation | 2 | 2 | 0 | 11 | 2 | |
| Late gestation | 0 | 0 | 0 | 0 | 2 | |
| F3 | Early gestation | 3 | 2 | 1 | 1 | 4 |
| Mid gestation | 2 | 1 | 1 | 3 | 1 | |
| Late gestation | 0 | 1 | 1 | 0 | 0 | |
DISCUSSION
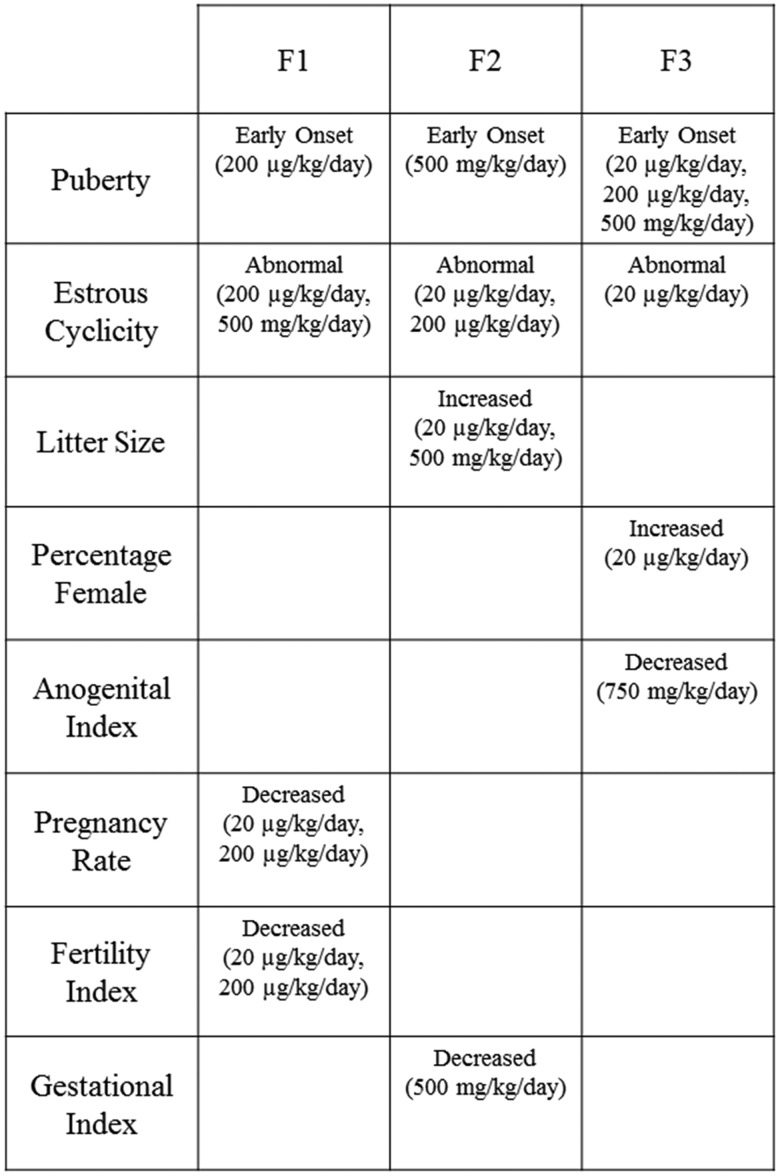
Our data indicate that prenatal and ancestral exposure to DEHP negatively impacts reproductive outcomes across the F1–F3 generations of mice (Figure 6). Specifically, DEHP exposure accelerated puberty, interfered with normal estrous cyclicity, increased litter size, decreased AGI in female offspring, and decreased pregnancy rate, fertility index, and gestational index in multigenerational and transgenerational manners (Figure 6).
Figure 6.
The effects of DEHP on female fertility at each dose are summarized for the F1–F3 generations. The figure summarizes the main finding from the study.
It is interesting that prenatal DEHP exposure did not produce a linear dose response. DEHP is a known EDC, and a characteristic of EDCs is that the effects are not proportional to the dose and therefore, often do not follow a linear relationship (Zoeller and Vandenberg, 2015). It is also important to note that the effects of DEHP exposure were not always the same in each generation. The different effects of DEHP exposure in each generation are likely due to the different developmental windows of exposure. During prenatal DEHP exposure, the F1 generation was exposed as the developing pup, the F2 generation was exposed as the developing ovaries within the F1 pup, and the F3 generation was not directly exposed to DEHP. Therefore, the effects of DEHP exposure in the F3 generation are considered transgenerational.
Our data showed that DEHP exposure accelerated the onset of puberty in all 3 generations. It is likely that the ability of prenatal and ancestral DEHP exposure to accelerate puberty is not through a mechanism involving body weight because our data indicated that prenatal and ancestral DEHP exposure did not affect body weight. Further, it is likely that prenatal and ancestral exposure to DEHP did not accelerate the onset of puberty through the alteration of serum sex steroid hormone levels. Previously, our laboratory demonstrated that prenatal and ancestral exposure to DEHP did not significantly affect serum estradiol levels at PND 21, an age earlier than the observed pubertal onset (Rattan et al., 2018). Although we did not detect differences in serum estradiol at PND 21, it is possible that serum estradiol levels at other time points impacted the onset of puberty.
One study demonstrated that ancestral exposure to a plasticizer mixture consisting of 750 mg/kg/day of DEHP delayed pubertal onset in the F1 generation and accelerated pubertal onset in the F3 generation in rats (Manikkam et al., 2013). However, our data differ from Manikkam et al. because that study showed that the mixture consisting of 750 mg/kg/day of DEHP delayed pubertal onset in the F1 generation, whereas we did not observe a delay in pubertal onset in the 750 mg/kg/day DEHP dose group in the F1 generation. The reasons for differences between our F1 generation data and those from Manikkam et al. are unclear, but they may be due to species differences between mice and rats or interactions between chemicals in the mixture that produced different effects than DEHP alone.
In addition, our data showed that prenatal and ancestral exposure to DEHP disrupted postpubertal cyclicity in the F1 generation and disrupted postpubertal cyclicity and adult estrous cyclicity in the F2 and F3 generations. Our results agree with previous studies showing that adult exposure to DEHP (20 µg/kg/day) increased the percentage of days that the mice spent in estrus (Hannon et al., 2016). Previously, our laboratory showed that ancestral exposure to DEHP (200 µg/kg/day and 500 and 750 mg/kg/day) decreased serum progesterone levels during the diestrus phase in the F2 generations (Rattan et al., 2018). Our current study showed that mice exposed to DEHP spent more time in diestrus, a time when basal levels of progesterone are normally low (Levine, 2015). Thus, these data are consistent with our previous data showing that ancestral DEHP exposure decreased serum progesterone levels. Further, rodents undergoing reproductive senescence will experience a state of persistent estrus followed by complete acyclicity, or a chronic state of diestrus (LeFevre and McClintock, 1988). Therefore, our data suggest that ancestral DEHP exposure may accelerate the onset of reproductive senescence in the F2 and F3 generations and that it can have lasting effects on estrous cyclicity in both a multigenerational and transgenerational manner.
Ancestral exposure to DEHP increased litter size in the F2 generation. These data were unexpected because previously we showed that ancestral exposure to DEHP did not affect the number of corpora lutea in mice (Rattan et al., 2018). Corpora lutea are used as indicators of ovulation, and no change in the number of corpora lutea suggests normal ovulation rates, and subsequently, normal litter sizes. However, this is the opposite of what was observed in the present study. Interestingly, previous literature has shown that direct exposure to DEHP (250 and 500 mg/kg/day) reduced the number and size of corpora lutea in mice (Guo et al., 2015). It is possible that direct exposure to DEHP and ancestral exposure to DEHP impacts ovulation differently. The different ovulation rates may explain the difference in the results in our study versus that of Guo et al. (2015). However, future studies should investigate the mechanism by which ancestral DEHP exposure increased litter size without affecting the number of corpora lutea.
Ancestral exposure to DEHP increased the female-to-male sex ratio in litters produced by the F3 generation. Although Niermann et al. (2015) showed that prenatal exposure to DEHP (750 mg/kg/day) increased the male to female sex ratio in litters produced by the F1 generation, we observed a different trend in the F3 generation. Although the increase in the percentage of female pups per litter was only borderline statistically significant (p = .080), it appears that our control in the F3 generation was much lower than the expected 50% of female pups. The lower percentage of females in the control group may have influenced the observed borderline increase in percentage of female pups per litter observed in the F3 generation. In addition, random variation may have influenced the increased female to male ratio.
Our data indicate that ancestral exposure to DEHP decreased female AGI at 3 months of age in the litters produced by the F3 generation, revealing a transgenerational effect of DEHP exposure on AGI. AGI is an indicator of androgen exposure to the fetus in utero. It is possible that androgen levels were decreased in the F3 mothers, therefore decreasing the AGI of the female pups, however future studies should test this hypothesis. Our findings are in contrast to another study that showed that maternal ancestral DEHP exposure (5 µg/kg/day) increased AGI in the F3 generation of female mice (Quinnies et al., 2017). It is likely that our results differ from that study because we used different doses of DEHP. DEHP exerts nonmonotonic dose responses, and therefore, different doses may affect the AGI differently. Further, the previous study observed a decrease in body weight in the 5 µg/kg/day pups, which likely drove the significant effects on AGI (Quinnies et al., 2017). However, in our study, we did not observe a significant difference in the body weights of the pups produced at 3 months of age in the F3 generation (data not shown).
The different fertility-related indices were calculated based on if a female mated, became pregnant, and gave birth. Our data indicate that DEHP exposure does not affect the mating index. Our data agree with previous studies that have shown that prenatal exposure to a phthalate mixture which included DEHP did not impact the mating index (Zhou et al., 2017a,b). In contrast, Quinnies et al. demonstrated that prenatal exposure to DEHP at 400 µg/kg/day caused females to be less interactive and potentially less interested in mating with males (Quinnies et al., 2017). A lack of interest in mating with males should produce a low mating index; however, we did not observe this in our study. It is possible that inbred strain (C57BL/6J) used in the Quinnies et al. study and outbred strain (CD-1) used in our study contribute to the observed differences in results (Brooks et al., 2005; Zarcone and Fowler, 2001).
Our data indicate that prenatal exposure to DEHP reduced the pregnancy rate in the F1 generation. The reduced pregnancy rate, but lack of an impact on mating index, indicates that DEHP exposure does not affect the ability of the mice to mate, but it affects the ability of the mice to become pregnant. Further, DEHP reduced the fertility index in the F1 generation, suggesting that DEHP decreases the number of females that became pregnant after mating. The exact mechanisms by which DEHP interferes with pregnancy and fertility are unknown. It is possible that DEHP exposure targets the ovary because DEHP exposure has been shown to decrease the ovarian reserve and increase atretic follicles in mice (Grande et al., 2007; Hannon et al., 2016; Pocar et al., 2017). Additionally, it is possible that DEHP exposure interferes with uterine functions as indicated by previous literature demonstrating that exposure to a phthalate mixture, including DEHP (20 and 200 µg/kg/day phthalate mixture), increases uterine weight in young mice (Zhou et al., 2017b). In our previous study, prenatal exposure to DEHP increased serum estradiol levels early in life (Rattan et al., 2018), and this increase persisted at 1 year of age in the F1 generation (Brehm et al., 2018). Although this increase in estradiol was not at the same age as the observed decreases in the pregnancy and fertility rates in the F1 generation, it is possible that estradiol levels were affected throughout life and impacted fertility-related indices because estradiol is critical for normal fertility.
Interestingly, ancestral DEHP exposure reduced the gestational index in the F2 generation. The data indicate that although the females successfully mated and became pregnant, they had difficulty carrying the pregnancy to term in the F2 generation. The exact mechanism by which ancestral DEHP exposure affects the maintenance of pregnancy is not known, but previous literature has shown that exposure to DEHP (250 and 500 mg/kg/day) during pregnancy inhibited vascularization of corpora lutea and increased corpora lutea regression, suggesting that it might increase the risk of miscarriage by suppressing luteal function (Guo et al., 2015). The potential for DEHP to cause miscarriages is supported by our data indicating that ancestral DEHP exposure increased mid-gestation infertility in the F2 generation. Further, the potential of DEHP to cause miscarriages is supported by a study showing that DEHP exposure (250 and 500 mg/kg/day) is teratogenic and has a lethal impact on the mouse fetus (Ungewitter et al., 2017). We have previously shown that ancestral exposure to DEHP decreased serum progesterone levels in young adult mice (Rattan et al., 2018) and at 1 year of age in the F2 generation (Brehm et al., 2018). Progesterone is important for thickening the lining of the uterus and maintaining a pregnancy (Mazur et al., 2015). Thus, DEHP-induced low levels of progesterone may contribute to the reduced gestational index observed in the F2 generation of our study.
In our study, the F0 dams were orally exposed to DEHP, but previous studies have shown that the bioactive metabolite, MEHP, is responsible for the toxic effects (Hannon et al., 2015a; Lovekamp-Swan and Davis, 2003). Therefore, it is likely that the bioactive metabolite, MEHP, affected the observed onset of puberty, estrous cyclicity, and fertility-related indices. In our study, we did not measure serum metabolite levels of DEHP in the F0 dams. However, future studies on the toxicokinetics of DEHP exposure are important and would be useful for comparison to human exposure levels.
Collectively, our results indicate that prenatal DEHP exposure induced some multigenerational and transgenerational effects on female reproductive outcomes. Multigenerational effects on female reproductive outcomes were observed in the onset of puberty, estrous cyclicity, litter size, and fertility-related indices. Transgenerational effects on female reproductive outcomes were observed in the onset of puberty, estrous cyclicity, and AGI. Future studies should investigate the underlying mechanisms of the DEHP-induced effects on reproductive outcomes in the F1–F3 generations. Given that DEHP is quickly metabolized and cleared from the body soon after birth (Koch et al., 2004; Koch and Calafat, 2009; Lee et al., 2017) and the effects are observed in the adults of the F1 generation and into the F2 and F3 generations, these observations suggest that DEHP exposure causes toxicity via epigenetic mechanisms. Thus, future studies should investigate whether epigenetic changes contribute to the decrease in reproductive outcomes observed in our study. Further, recently, the use of DEHP replacements has steadily increased over the years (Erythropel et al., 2014; Zota and Woodruff, 2014). Although phasing out DEHP would be ideal, few studies have investigated the effects of DEHP replacements on female reproduction. Therefore, we suggest that future studies investigate the effects of DEHP replacements on female reproduction before replacing DEHP with a chemical of unknown toxicity.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institutes of Health (P01 ES022848 and T32 ES007326); Environmental Protection Agency (RD 83459301).
Supplementary Material
ACKNOWLEDGMENT
We would like to thank all of the Flaws’ laboratory members for their assistance and support.
REFERENCES
- ATSDR (2002). Toxicological Profile for Di(2-ethylhexyl) Phthalate. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp9.pdf. Accessed February 23, 2018. [Google Scholar]
- Brehm E., Rattan S., Gao L., Flaws J. A. (2018). Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology 1592, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. P., Pask T., Jones L., Dunnett S. B. (2005). Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: Cognitive tests. Genes Brain Behav. 4, 307–317. [DOI] [PubMed] [Google Scholar]
- Culty M., Thuillier R., Li W., Wang Y., Martinez-Arguelles D. B., Benjamin C. G., Triantafilou K. M., Zirkin B. R., Papadopoulos V. (2008). In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol. Reprod. 78, 1018–1028. [DOI] [PubMed] [Google Scholar]
- Davis B. J., Maronpot R. R., Heindel J. J. (1994). Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol. Appl. Pharmacol. 128, 216–223. [DOI] [PubMed] [Google Scholar]
- Doyle T. J., Bowman J. L., Windell V. L., McLean D. J., Kim K. H. (2013). Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod. 88, 112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcova-Hills G., Capel B. (2008). Development of germ cells in the mouse. Curr. Top. Dev. Biol. 83, 185–212. [DOI] [PubMed] [Google Scholar]
- EPA, U. S. (1987). Di(2-ethylhexyl)phthalate (DEHP); CASRN 117-81-7. Available at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0014_summary.pdf#nameddest=rfd. Accessed February 23, 2018. [Google Scholar]
- Erythropel H. C., Maric M., Nicell J. A., Leask R. L., Yargeau V. (2014). Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 98, 9967–9981. [DOI] [PubMed] [Google Scholar]
- Gallavan R. H. Jr., Holson J. F., Stump D. G., Knapp J. F., Reynolds V. L. (1999). Interpreting the toxicologic significance of alterations in anogenital distance: Potential for confounding effects of progeny body weights. Reprod. Toxicol. 13, 383–390. [DOI] [PubMed] [Google Scholar]
- Grande S. W., Andrade A. J., Talsness C. E., Grote K., Golombiewski A., Sterner-Kock A., Chahoud I. (2007). A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Reproductive effects on adult female offspring rats. Toxicology 229, 114–122. [DOI] [PubMed] [Google Scholar]
- Guo M., Lai L., Zong T., Lin Y., Yang B., Zhang L., Li M., Kuang H. (2015). Exposure to di(2-ethylhexyl) phthalate inhibits luteal function via dysregulation of CD31 and prostaglandin F2alpha in pregnant mice. Reprod. Biol. Endocrinol. 13, 11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Singh J. M., Leslie T. C., Meachum S., Flaws J. A., Yao H. H. (2010). Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 242, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Flaws J. A. (2015a). Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol Reprod 925, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Gupta R. K., Flaws J. A. (2015b). Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 284, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Flaws J. A. (2015). The effects of phthalates on the ovary. Front. Endocrinol. (Lausanne) 6, 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Niermann S., Flaws J. A. (2016). Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol. Sci. 150, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Peretz J., Flaws J. A. (2014). Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol. Reprod. 90, 136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U., Mersch-Sundermann V., Angerer J. (2007). Phthalates: Toxicology and exposure. Int J Hyg Environ Health 210, 623–634. [DOI] [PubMed] [Google Scholar]
- Hirshfield A. N. (1991). Development of follicles in the mammalian ovary Int Rev Cytol 124, 43–101. [DOI] [PubMed] [Google Scholar]
- James-Todd T. M., Meeker J. D., Huang T., Hauser R., Ferguson K. K., Rich-Edwards J. W., McElrath T. F., Seely E. W. (2016). Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int. 96, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. M., Bolt H. M., Angerer J. (2004). Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 78, 123–130. [DOI] [PubMed] [Google Scholar]
- Koch H. M., Calafat A. M. (2009). Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Martinez-Arguelles D. B., Campioli E., Papadopoulos V. (2017). Fetal exposure to low levels of the plasticizer DEHP predisposes the adult male adrenal gland to endocrine disruption. Endocrinology 158, 304–318. [DOI] [PubMed] [Google Scholar]
- LeFevre J., McClintock M. K. (1988). Reproductive senescence in female rats: A longitudinal study of individual differences in estrous cycles and behavior. Biol. Reprod. 38, 780–789. [DOI] [PubMed] [Google Scholar]
- Levine J. E. (2015). Chapter 26 - neuroendocrine control of the ovarian cycle of the rat In Knobil and Neill’s Physiology of Reproduction, 4th ed., pp. 1199–1257. Academic Press, San Diego. [Google Scholar]
- Lovekamp-Swan T., Davis B. J. (2002). Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 111, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M. K. (2013). Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8, e55387.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur E. C., Large M. J., DeMayo F. J. (2015). Chapter 24 - human oviduct and endometrium: Changes over the menstrual cycle In Knobil and Neill’s Physiology of Reproduction, 4th ed, pp. 1077–1097. Academic Press, San Diego. [Google Scholar]
- Menke D. B., Koubova J., Page D. C. (2003). Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 262, 303–312. [DOI] [PubMed] [Google Scholar]
- Niermann S., Rattan S., Brehm E., Flaws J. A. (2015). Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod. Toxicol. 53, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling M. E. (2006). From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis 44, 622–632. [DOI] [PubMed] [Google Scholar]
- Pocar P., Fiandanese N., Berrini A., Secchi C., Borromeo V. (2017). Maternal exposure to di(2-ethylhexyl)phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol. Appl. Pharmacol. 322, 113–121. [DOI] [PubMed] [Google Scholar]
- Prevot V. (2015). Chapter 30 - puberty in mice and rats In Knobil and Neill's Physiology of Reproduction, 4th ed., pp. 1395–1439. Academic Press, San Diego. [Google Scholar]
- Quinnies K. M., Harris E. P., Snyder R. W., Sumner S. S., Rissman E. F. (2017). Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS One 12, e0171977.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Brehm E., Gao L., Niermann S., Flaws J. A. (2018). Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod. 981, 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J., Wong L. Y., Samandar E., Preau J. L., Calafat A. M., Ye X. (2017). Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Arch Toxicol. 9110, 3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K. (2016). Epigenetic transgenerational inheritance. Nat. Rev. Endocrinol. 12, 68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K. (2008). What is an epigenetic transgenerational phenotype? F3 or F2. Reprod. Toxicol. 25, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyl R. W., Myers C. B., Marr M. C., Sloan C. S., Castillo N. P., Veselica M. M., Seely J. C., Dimond S. S., Van Miller J. P., Shiotsuka R. N. et al. , (2008). Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 104, 362–384. [DOI] [PubMed] [Google Scholar]
- Ungewitter E., Rotgers E., Bantukul T., Kawakami Y., Kissling G. E., Yao H. H. (2017). From the cover: Teratogenic effects of in utero exposure to di-(2-ethylhexyl)-phthalate (DEHP) in B6: 129S4 mice. Toxicol. Sci. 157, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Hafner K. S., Flaws J. A. (2014). In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol. Appl. Pharmacol. 276, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone T. J., Fowler S. C. (2001). Digital measurement of operant disk press force maintained in CD-1, BALB/c, and C57BL/6 mice. Behav. Res. Methods Instrum. Comput. 33, 415–421. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Zhang T., Han Z., Liu J. C., Liu Y. P., Ma J. Y., Li L., Shen W. (2015). Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod. Fertil. Dev. 27, 1213.. [DOI] [PubMed] [Google Scholar]
- Zhou C., Gao L., Flaws J. A. (2017a). Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology 158, 1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Gao L., Flaws J. A. (2017b). Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol. Appl. Pharmacol. 318, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv-Gal A., Wang W., Zhou C., Flaws J. A. (2015). The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 284, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller R. T., Vandenberg L. N. (2015). Assessing dose-response relationships for endocrine disrupting chemicals (EDCs): A focus on non-monotonicity. Environ. Health 14, 42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A. R., Woodruff T. J. (2014). Changing trends in phthalate exposures: Zota and Woodruff respond. Environ. Health Perspect. 122, A264–A265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.