Abstract
Exposure to ozone (O3) induces lung injury, pulmonary inflammation, and alters lipid metabolism. During tissue inflammation, specialized pro-resolving lipid mediators (SPMs) facilitate the resolution of inflammation. SPMs regulate the pulmonary immune response during infection and allergic asthma; however, the role of SPMs in O3-induced pulmonary injury and inflammation is unknown. We hypothesize that O3 exposure induces pulmonary inflammation by reducing SPMs. To evaluate this, male C57Bl/6J mice were exposed to filtered air (FA) or 1 ppm O3 for 3 h and necropsied 24 h after exposure. Pulmonary injury/inflammation was determined by bronchoalveolar lavage (BAL) differentials, protein, and lung tissue cytokine expression. SPMs were quantified by liquid chromatography tandem mass spectrometry and SPM receptors leukotriene B4 receptor 1 (BLT-1), formyl peptide receptor 2 (ALX/FPR2), chemokine-like receptor 1 (ChemR23), and SPM-generating enzyme (5-LOX and 12/15-LOX) expression were measured by real time PCR. 24 h post-O3 exposure, BAL PMNs and protein content were significantly increased compared to FA controls. O3-induced lung inflammation was associated with significant decreases in pulmonary SPM precursors (14-HDHA, 17-HDHA), the SPM PDX, and in pulmonary ALX/FPR2, ChemR23, and 12/15-LOX expression. Exogenous administration of 14-HDHA, 17-HDHA, and PDX 1 h prior to O3 exposure rescued pulmonary SPM precursors/SPMs, decreased proinflammatory cytokine and chemokine expression, and decreased BAL macrophages and PMNs. Taken together, these data indicate that O3-mediated SPM reductions may drive O3-induced pulmonary inflammation.
Keywords: specialized pro-resolving mediators, ozone, pulmonary inflammation
Despite increasing regulations, air pollution remains a serious environmental health concern globally leading to 9 million premature deaths each year (Landrigan et al., 2018). Ozone (O3) is a central component of air pollution that is associated with >3000 deaths annually in the US (Hubbell et al., 2005). Acute increases in ambient O3 levels are associated with cardiopulmonary complications, including decreased pulmonary function, acute lung injury, and exacerbation of pre-existing pulmonary and cardiovascular diseases (Basu, 2009; Liu et al., 2009; Srebot et al., 2009). These detrimental health effects occur in part through O3-induced pulmonary inflammation and injury as defined by epithelial cell damage, inflammatory cytokine/chemokine release, suppression of alveolar macrophage (Mφ) phagocytosis, and inflammatory cell influx into the airspace (Hollingsworth et al., 2007; Pendino et al., 1995; Tighe et al., 2011). Currently, the mechanisms triggering O3-induced inflammatory responses are ill-defined and there are no effective methods to prevent O3-induced pulmonary inflammation and injury.
Tissue inflammation is an active, dynamic process mediated by temporally specific signaling mediators, both pro-inflammatory and pro-resolving. During resolution of inflammation, pulmonary lipid metabolism shifts to the production of specialized pro-resolving mediators (SPMs) (Levy et al., 2001). If this shift does not occur, inflammation persists and can contribute to chronic inflammation and lung disease (Basil and Levy, 2016). Therefore, understanding these components offers the opportunity to tune inflammatory responses. Pro-inflammatory lipid mediators such as prostaglandins (PGs) and leukotrienes (LTs) are increased in the lungs of O3-exposed rodents and humans (Devlin et al., 1991; Koren et al., 1989) but the effects of O3 on pro-resolving lipid mediator generation, such as SPMs, are unknown. Furthermore, it is unknown if a primary factor driving O3-induced inflammation is dysregulation of SPM production and signaling.
SPMs are a family of endogenously produced bioactive lipid mediators including resolvins, protectins, and maresins that are synthesized from eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (AA). DHA is metabolized through 3 pathways that lead to DHA-derived lipid mediators. Through the COX-2 pathway, 17R-hydroperoxy (Hp)DHA is metabolized by peroxidase into the SPM precursor 17R-hydroxy DHA (17-HDHA). 17-HDHA is further metabolized via 5-lipoxygenase (LOX) to produce D-series resolvins. Through the 15-LOX pathway, DHA is metabolized to 17S-HpDHA which leads to the production of the SPM 10(R), 17(S)-DiHDoHE (PD1) (Serhan and Petasis, 2011). Finally, DHA is metabolized into the SPM precursor 14-HDHA which leads to downstream maresin production by the 12-LOX pathway (Kuda, 2017). Dietary DHA supplementation increases systemic DHA-derived lipid mediators including SPM precursors 14-HDHA, 17-HDHA and the SPM PD1 (Guesdon et al., 2018; Levy and Serhan, 2014; Skarke et al., 2015).
SPMs regulate pro-resolving molecular pathways via the G-protein coupled receptors leukotriene B4 receptor 1 (BLT-1), formyl peptide receptor 2 (ALX/FPR2), and chemokine-like receptor 1 (ChemR23) (Serhan and Chiang, 2013). ChemR23 and ALX/FPR2 signaling enhances Mφ phagocytosis of apoptotic cells, decreases pro-inflammatory cytokine production, and increases anti-inflammatory cytokine production (Serhan and Chiang, 2013). The loss of these functions during inflammation following O3 exposure (Cabello et al., 2015; Hotchkiss et al., 1989; Pendino et al., 1995) suggests that the loss of SPMs could be a possible mechanism of O3-induced pulmonary inflammation and injury.
This study evaluated the role of SPM precursors and SPMs in O3-induced pulmonary inflammation and determined if DHA-derived lipid mediator administration prior to O3 exposure mitigates O3-induced immune responses. These data presented herein indicate that O3 exposure leads to a decrease in DHA-derived lipid mediators, pulmonary SPM receptor and enzyme expression, and augmented pulmonary inflammation and injury. To our knowledge, these findings are the first to implicate SPM production and signaling in the adverse health effects associated with O3 exposure.
MATERIALS AND METHODS
Reagents
14(S)-HDHA, 17(R)-HDHA, and 10(S), 17(S)-DiHDoHE (PDX) were purchased in ethanol from Cayman Chemicals (Ann Arbor, MI). 17(R) HDHA is metabolized to 17R-RvD1 which has a longer half-life in vivo and is less susceptible to rapid inactivation by endogenous 15-prostaglandin dehydrogenase/eicosanoid oxidoreductase compared to 17(S) HDHA derived 17S-RvD1 (Hsiao et al., 2013). Each lipid mediator was diluted to 1000 ng/250 µl in PBS for each intraperitoneal (i.p.) injection. This dose of SPMs/SPM precursors was used in previous pulmonary studies that demonstrated reduced inflammation (Aoki et al., 2008; Hsiao et al., 2015; Kosaraju et al., 2017). 17-HDHA and 14-HDHA are metabolized to resolvins and maresins, respectively. Each SPM precursor/SPM injection was given separately to each mouse, with a total of 3 i.p. injections per mouse. Three i.p. injections were used because dietary DHA consumption increases 14-HDHA, 17-HDHA, and PDX which all have pre-established resolving properties in pulmonary Mφs and neutrophils (Guesdon et al., 2018; Kosaraju et al., 2017; Levy and Serhan, 2014). Stringent precautions were taken to prevent oxidation of DHA-derived lipid mediators as previously described (Kosaraju et al., 2017).
Mice
C57BL/6J male mice (8–12 weeks and 22–27 g) were obtained from Jackson Laboratories (Bar Harbor, ME). All experiments were performed in accordance with the Animal Welfare Act and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals after review by the Animal Care and Use Committee of East Carolina University.
In vivo exposures
Mice were placed in stainless steel wire exposure chambers inside metal Hinner’s chamber and exposed to filtered air (FA) or ozone (O3) for 3 h at a dose of 1 ppm as previously described (Kummarapurugu et al., 2013). The 1 ppm O3 exposure mimics the human exposure of 200 ppb and is based on prior data and published deposition fractions between rodents and humans (Hatch et al., 1994; Wiester et al., 1988). O3 was generated in the chamber by directing 100% oxygen through an ultraviolet light generator using a Teledyne T703 O3 calibrator (Teledyne API, San Diego, CA) and then mixed with a FA supply. Temperature and humidity of air in the chamber were monitored continuously, as was the O3 concentration with a Teledyne T400 ultraviolet light photometer (Teledyne API). For some experiments, mice were administered 3 i.p. injections with each containing 1 of 3 SPM/SPM precursors (14-HDHA, 17-HDHA, or PDX) as described above, 1 h prior to O3 or FA exposure. Each injection of 14-HDHA, 17-HDHA, or PDX was diluted in PBS. Vehicle control i.p. injections of ethanol were given at a 1:25 dilution in PBS. Although injections are mixtures of SPM precursors (14-HDHA, 17-HDHA) and the SPM PDX, all graphs will refer to these treatment groups as “FA + SPM” or “O3+SPM” for simplicity. Mice were euthanized 24 h post-O3 exposure with i.p. injections of ketamine/xylazine (90 mg/kg/10 mg/kg). Immediately following euthanasia, blood was collected from the right ventricle with a 25-gauge needle into an EDTA tube and complete blood counts (CBCs) were determined. CBCs were determined by Beckman Coulter AcT Diff hematology analyzer and differentials were determined with blood smears. Spleens and lungs were removed and immediately snap frozen in liquid nitrogen and analyzed as a marker of systemic changes in SPM and SPM precursors.
Bronchoalveolar lavage fluid (BALF) collection and analysis
BALF was collected immediately following sacrifice. The right lung lobes were lavaged 3 times with 3 volumes of phosphate buffered saline (PBS) (Gibco Life Technologies, Grand Island, NY). The lavage volume was based on bodyweight (26.25 ml/kg body weight) as previously described (Draper et al., 2010; Wang et al., 2011). The resulting lavage was centrifuged (1800 RPM, 6 min, 4°C) and an aliquot of the supernatant was removed for protein analysis using a Pierce BCA Protein-Assay Kit (Thermo Scientific, Hercules, CA). The cell pellets were suspended in 1 ml of red blood cell ammonium chloride potassium (ACK) lysis buffer, vortexed, and incubated for 1 min. To stop the reaction, 4 ml of 1×PBS was added. The cells were then centrifuged again at 1800 RPM for 6 min, aspirated, and 1 ml of 10% fetal bovine serum (FBS) was added to the cells. Total cell counts in the lavage fluid of the right lobe were obtained by manually counting with a hemocytometer (Hausser Scientific, Horsham, PA). Each sample (120 μl) was centrifuged onto slides using a Cytospin 4 (ThermoFisher, Waltham, MA) and subsequently stained with Diff Quik solution (ThermoFisher) for differential cell counts, with at least 200 cells counted from each slide.
Lipid mediator sample preparation
All standards and internal standards used for reverse phase HPLC tandem mass spectrometry (LC-MS/MS) analysis of AA, DHA, and linoleic acid derived lipid mediators were purchased from Cayman Chemical (Ann Arbor, Michigan). All HPLC solvents and extraction solvents were HPLC grade or better.
Tissue samples were homogenized, and lipid mediators were isolated with modifications from a prior protocol (Yang et al., 2011). Tissue samples were pre-weighed and transferred into a dry ice chilled, pre-weighed TissueLyser tube (Qiagen, Hilden, Germany) with a 5 mm stainless steel ball. 1.0 ml of −20°C chilled methanol and 10 µl of internal standard solution (10 pg/µl or 100 pg total/each of 8-iso-PGF2a-d4, LTB4-d4, PGE2-d4, RvD1-d5, and RvD2-d5 in ethanol) was added and the samples were homogenized at 50 hz for 2–15 min depending on the tissue type. The sample was then centrifuged at 14 000 RPM for 10 min at 4°C. The supernatant was removed and transferred to a new 1.5 ml microfuge tube and dried in a speed vac until completely dry. The dried sample was then reconstituted with 1.0 ml of 10% methanol. The samples were then loaded on a Strata-X 33 µm 30 mg/1 ml solid phase extraction (SPE) column (Phenomenex, Torrance, California) preconditioned with 1.0 ml of methanol followed by 1.0 ml of water. The SPE column was then washed with 10% methanol and then eluted directly into a reduced surface activity/maximum recovery glass autosampler vial with 1.0 ml of methyl formate. The methyl formate was evaporated completely from the vial with a stream of nitrogen and then the SPE cartridge was eluted with 1.0 ml of methanol directly into the same autosampler vial. The methanol was evaporated to dryness with a stream of nitrogen and then the sample was reconstituted with 20 µl of ethanol. The samples are analyzed immediately or frozen at −70°C until analysis.
Liquid chromatography-mass spectrometry
Quantitation of lipid mediators was performed using LC-MS/MS. The HPLC system consisted of an Agilent 1260 autosampler (Agilent Technologies, Santa Clara, CA), an Agilent 1260 binary loading pump (pump 1), an Agilent 1260 binary analytical pump (pump 2) and a 6-port switching valve. Pump 1 buffers consisted of 0.1% formic acid in water (solvent A) and 9:1 v:v acetonitrile: water with 0.1% formic acid (solvent B). Pump 2 buffers consisted of 0.01% formic acid in water (solvent C) and 1:1 v:v acetonitrile: isopropanol (solvent D).
Extracted sample (10 µl) was injected onto an Agilent SB-C18 2.1X5 mm 1.8 µm trapping column using pump 1 at 2 ml/min for 0.5 min with a solvent composition of 97% solvent A: 3% solvent B. At 0.51 min the switching valve changed the flow to the trapping column from pump 1 to pump 2. The flow was reversed, and the trapped lipid mediators were eluted onto an Agilent Eclipse Plus C-18 2.1X150 mm 1.8 µm analytical column using the following gradient at a flow rate of 0.3 ml/min: hold at 75% solvent A: 25% solvent D from 0 to 0.5 min, then a linear gradient from 25 to 75% D over 20 min followed by an increase from 75 to 100% D from 20 to 21 min, then holding at 100% D for 2 min. During the analytical gradient pump 1 washed the injection loop with 100% B for 22.5 min at 0.2 ml/min. Both the trapping column and the analytical column were re-equilibrated at starting conditions for 5 min before the next injection.
Mass spectrometric analysis was performed on an Agilent 6490 triple quadrupole mass spectrometer (Agilent, Santa Clara, CA) in negative ionization mode. The drying gas was 250°C at a flow rate of 15 ml/min. The sheath gas was 350°C at 12 ml/min, the nebulizer pressure was 35 psi andthe capillary voltage was 3500 V. Data for lipid mediators was acquired in dynamic MRM mode using experimentally optimized collision energies obtained by flow injection analysis of authentic standards (Table 1). Calibration standards for each lipid mediator were analyzed over a range of concentrations from 0.25 to 5000 pg on the column. Calibration curves for each lipid mediator were constructed using Agilent Masshunter Quantitative Analysis software. Samples were quantitated using the calibration curves to obtain the on-column concentration, followed by multiplication of the results by the appropriate dilution factor to obtain the concentration in pg/mg of tissue.
Table 1.
MRM Parameters, Retention Times and Associated Internal Standards for Lipid Mediators by LC-MS/MS
| Compound | MRM | CE | RT | Internal Standard |
|---|---|---|---|---|
| Resolvin D2-d5 (IS) | 380.2 → 141.0 | 13 | 9.30 | NA |
| Resolvin D2 | 375.2 → 141.0 | 13 | 9.35 | Resolvin D2-d5 |
| Resolvin D1 | 375.2 → 141.0 | 13 | 10.13 | Resolvin D2-d5 |
| 10(S), 17(S)-DiHDoHE (PDX) | 359.2 → 153.0 | 13 | 12.95 | LTB4-d4 |
| 7S Maresin-1 | 359.2 → 341.2 | 9 | 12.95 | LTB4-d4 |
| 7R Maresin-1 | 359.2 → 250.0 | 17 | 13.02 | LTB4-d4 |
| 17(S)-HDHA | 343.2 → 281.2 | 9 | 17.40 | 9(S)-HODE-d4 |
| 14(S)-HDHA | 343.2 → 205.0 | 9 | 17.73 | 9(S)-HODE-d4 |
Due to a lack of commercially available standard for protectin D1 (PD1), only PDX is quantified.
Fatty acid analysis in lung tissue
Lipids were organically extracted using the Folch Method as previously described (Folch et al., 1957). Lung samples were minced using forceps and a scalpel and returned to a chloroform solution in each tube. Samples were sonicated 15 min, centrifuged in the same tube or transferred to smaller tubes, and centrifuged for 15 min. The organic phase was recovered with a glass Pasteur pipette and transferred to a borosilicate tube. The organic phase was evaporated in the Savant Speed Vac Concentrator (ThermFisher) with refrigerated condensation trap for 30 min. The materials left after the evaporation process were then stored in the freezer at −80°C. To begin the transmethylation process, lipid extracts were removed from the freezer and warmed to room temperature. Each extract was reconstituted with methanolic KOH solution, vortexed, and transferred to a 15 ml tube. The 15 ml tube was then topped with nitrogen, capped tightly, and saponified for 15 min at 80˚C. Samples were transferred to room temperature, methylated by adding boron trifluoride, topped with nitrogen and capped. The samples were warmed in an 80˚C water bath for 10 min and cooled to room temperature. Saturated sodium chloride solution was added to each tube and vortexed. Each sample was extracted 3× with hexane and BHT dried on speed vac. Samples were suspended in toluene for GC analyses (Basile et al., 2011). Fatty acid analysis is presented as a percent of total fatty acids. Craft Technologies (Wilson, NC) performed the extraction and fatty acid analyses of lung samples.
SPM receptors, lipoxygenase enzyme, and cytokine/chemokine expression in lung tissue
The left lungs of mice were flash frozen and total RNA was isolated using a Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). Concentrations of RNA for each sample were determined using the NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE). RNA was reverse transcribed using a high capacity RNA to cDNA kit (ThermoFisher) and qPCR was performed with a Taqman assay kit (Invitrogen, Waltham, MA). Genes were amplified with Applied Biosytem’s Veriti 96-well Therma Cycler (Life Technologies, Carlsbad, CA) and an Applied Biosystems StepOnePlus real time polymerase chain reaction (RT-PCR) (Life Technologies). In a one-step reaction, iScript One-Step RT-PCR kit was utilized with Syber Green reagents (Biorad, Hercules, CA) and custom-made by Invitrogen primers. Genes were amplified and detected using a Bio-Rad iCycler to obtain cycle threshold (Ct) values for target and internal reference cDNA levels. Fold changes in expression for mRNA quantities were calculated using the 2−ΔΔCt method and Ct values. Samples were normalized to GAPDH and β-actin or to 18S as previously described (Sullivan et al., 2017). See Table 2 for Invitrogen primers (Waltham, MA) and Table 3 for Taqman primer (Applied Biosystems, Foster City, CA) information.
Table 2.
Primer Sequences for SPM Receptors and Lipoxygenase Enzymes
| Primer | Sequence (Forward, Reverse) |
|---|---|
| β-actin | 5′-TCCGATGCCCTGAGGCTCTTTTC-3′ |
| 5′-CTTGCTGATCCACATCTGCTGGAA-3′ | |
| GAPDH | 5′-GGTGTGAACGGATTTGGCCGTATT-3′ |
| 5′-GTCGTTGATGGCAACAATCTCCAC-3′ | |
| BLT1 | 5′-TGACTTAGCTGACAGACCTAGA-3′ |
| 5′-AGGATGCTCCACACTACAAAG-3′ | |
| ChemR23 | 5′-CCACTACCAGAACAACTGAGAA-3′ |
| 5′-CTCCTCCAAGTCCACAAAGTAG-3′ | |
| ALX/FPR2 | 5′-CACAGGAACCGAAGAGTGTAAG-3′ |
| 5′-GGAATCCAGCTACCCAAATCA-3′ | |
| Alox5 | 5′-CTGCTGGACAAGGCATTCTA-3′ |
| 5′-TCCATCCCTCAGGACAATCT-3′ | |
| 12/15lox | 5′-CTCTCAAGGCCTGTTCAGGA-3′ |
| 5′'-GTCCATTGTCCCCAGAACCT-3′ |
Primer sequences custom made from Invitrogen for analysis of SPM receptors, LOX enzymes, and housekeeping genes for whole lung homogenate and quantitative RT-PCR.
Table 3.
Primer Sequences for Mouse Cytokines and Chemokines
| Taqman Primers | |
|---|---|
| Primer | Assay ID |
| Euk 18s rRNA | Hs99999901_s1 |
| IL-6 | Mm00446190_m1 |
| CXCL1 | Mm04207460_m1 |
| CXCL2 | Mm00436450_m1 |
| CCL3 | Mm00441259_g1 |
| TNF-α | Mm00443258_m1 |
| IL-1β | Mm00434228_m1 |
Primer sequences obtained from Taqman for quantitative PCR of chemokines and cytokine gene expression in whole lung homogenate.
Histopathology
The left lung perfused with 10% neutral buffered formalin fixative and allowed to fix for 24 h prior to processing. Lungs were processed and embedded in paraffin, and 5 µM sections were mounted on slides. Sections were stained with hematoxylin and eosin to detect inflammatory and morphological changes.
Statistical analysis
Data were pooled from 2 initial exposure studies/experiments to compare O3 and FA exposure. For the SPM/vehicle administration prior to O3 or FA exposure, data was pooled from 3 exposure studies/experiments. Data are expressed as means ± SEM. Data were analyzed using parametric or nonparametric one-way ANOVA (Kruskal-Wallis test) followed by comparison using a Dunn’s multiple comparisons test to correct for multiple comparisons using statistical hypothesis testing in GraphPad Prism 7.0 (San Diego, CA). Where deemed appropriate, unpaired nonparametric t-test (Mann-Whitney tests) were utilized to analyze data. A value of p < .05 was significant.
RESULTS
Acute O3 Exposure Causes Pulmonary Inflammation and Injury
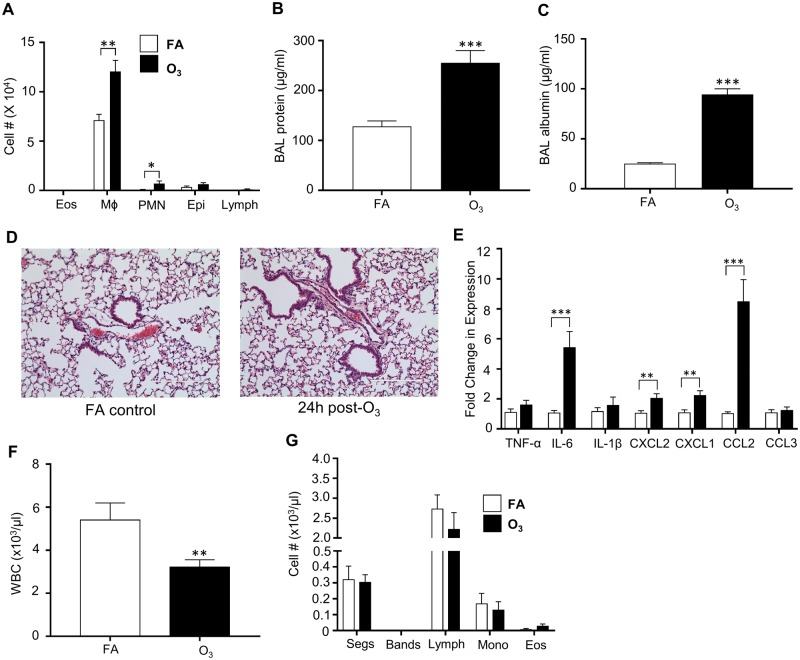
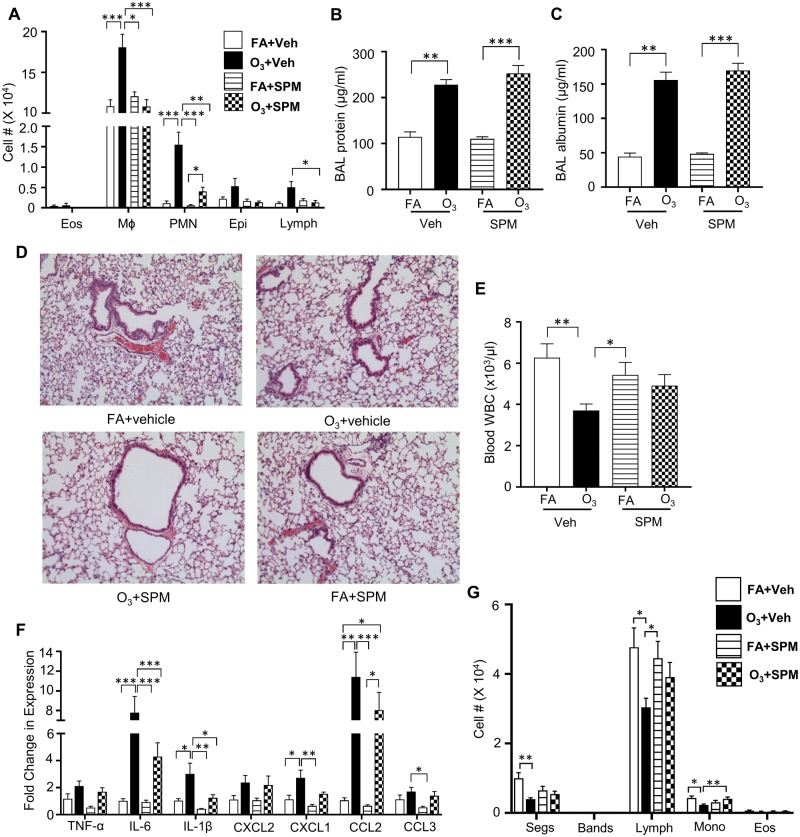
O3 exposure causes pulmonary inflammation as measured by increased airspace immune cells (neutrophils-(PMNs) and Mφs), cytokine/chemokine (IL-6, IL-1β, and TNF-α) release/expression, and microvascular injury (BAL total protein and albumin) (Arsalane et al., 1995; Cabello et al., 2015; Hotchkiss et al., 1989; Pendino et al., 1995). Consistent with prior work (Kummarapurugu et al., 2013; Watkinson et al., 2001), our study indicated that O3 induces pulmonary inflammation at 24 h following acute O3 exposure (1 ppm for 3 h). O3-exposed mice had increases in BAL Mφs and PMNs (Figure 1A). There were no significant increases in BAL eosinophils (eos), epithelial cells (epi) or lymphocytes (lymph) cells. Furthermore, BAL total protein and albumin concentrations were significantly increased (Figs. 1B and 1C). Hematoxylin and eosin staining of FA and O3-exposed lung tissue sections indicate minimal pulmonary pathology with acute 1 ppm O3 exposures (Figure 1D), similar to other studies (Bouthillier et al., 1998; Elkhidir et al., 2016). Enhanced expression of pro-inflammatory cytokines IL-6, neutrophil chemoattractant CXCL1 (also known as KC), CXCL2 (or Mφ inflammatory protein; MIP2), and monocyte chemoattractant CCL2 (also known as MCP1) was also observed (Figure 1E).
Figure 1.
O3 exposure increases pulmonary inflammation and injury while decreasing circulating immune cells. Inflammatory response to O3 exposure in lung tissue. Mice were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied 24 h following exposure. Bronchoalveolar lavage (BAL) was analyzed for A, cell differentials (n = 10 per group). B, total protein (n = 10 per group), and C, increases in BAL albumin concentrations (n = 3–5 per group). Lung tissue was stained with D, hematoxylin and eosin to examine pulmonary damage. E, Whole lung homogenate was analyzed for gene expression of cyto/chemokines which was normalized to 18S (n = 5 per group). Mice were also analyzed for F, total white blood cell (WBC) counts (n = 10 per group) and G, cell differentials (n = 5 per group). *p < .05; **p < .01; ***p < .001.
O3 Exposure Decreases Circulating WBCs
In addition to pulmonary effects, O3 alters systemic mediators such as circulating lipid species (Miller et al., 2016b; Watkinson et al., 2001). To determine the effect of O3 on circulating immune cell composition, white blood cell counts (WBCs) were performed. At 24 h post-exposure, O3-exposed mice exhibited decreased total WBCs (Figure 1F). Differential cell counts identified no statistical significance in cellular differentials (Figure 1G).
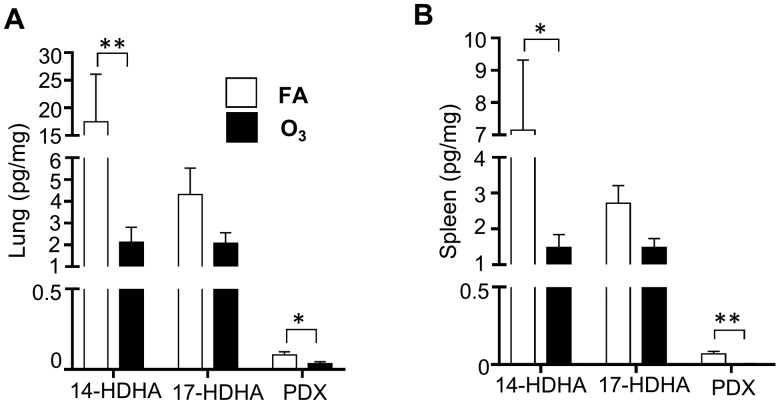
O3 Exposure Decreases Pulmonary and Splenic SPMs
Increased inflammation has been associated with decreased SPM production (Miyata et al., 2013) but it is unclear if O3 alters pulmonary SPM concentrations. Using reverse phase HPLC tandem mass spectrometry, SPMs and their precursors were measured in the lung and spleen. Spleen tissue contains immune cells that migrate to injured tissues and changes in splenic immune cell composition can indicate systemic inflammation (Swirski et al., 2009). In the lungs and spleens of FA and O3 exposed mice, SPMs including resolvin D1, resolvin D2, 7S maresin-1, lipoxin A4, and 7R maresin-1, were below the limit of detection. However, several DHA-derived lipid mediators were measurable including 14-HDHA, 17-HDHA, and PDX. 10(R), 17(S)-DiHDoHE (PD1) is commercially unavailable due to its instability, and its isomer, PDX, was used in this study. Following O3 exposure, lung 14-HDHA and PDX concentrations were significantly decreased and 17-HDHA levels trended lower, but did not reach statistical significance (p = .09; Figure 2A). To determine if SPM concentrations were altered outside the lungs, splenic SPM content was measured. There was a significant decrease in splenic 14-HDHA and PDX levels (Figure 2B). These data demonstrate that O3 exposure results in decreased SPM precursors/SPMs in both pulmonary and splenic tissues.
Figure 2.
O3 exposure reduces SPM production in the lung and spleen tissue. 24 h following O3 or filtered air (FA) exposure, A, lungs and B, spleens were analyzed for specialized pro-resolving mediators (SPMs). *p < .05; **p < .01; n = 6 per group.
O3 Does Not Alter DHA Fatty Acid Levels
Given the reduction in 14-HDHA, 17-HDHA, and PDX, we were interested in determining if this was associated with a decline in the fatty acid DHA. When phospholipid fatty acids are cleaved from the cell membrane, they can be metabolized to DHA-derived lipid mediators 14-HDHA, 17-HDHA, and PDX. Whole lung homogenates were analyzed to determine if O3-induced decreases of 14-HDHA, 17-HDHA, and PDX levels were due to reduced pulmonary DHA levels. Lung concentrations of DHA (22: 6-n3), remained unchanged with O3-exposure (Table 4); however, 5 other fatty acids (γ linoleate, 11,14,17-eisosatrienoate, erucate, lignocerate, nervonate) decreased following O3-exposure. This may indicate that other fatty acids in the lungs are altered by O3-exposure.
Table 4.
Fatty Acid Analysis of Lung Tissue
| Filtered Air |
24h O3 |
||||
|---|---|---|---|---|---|
| Fatty Acid | Mean | SEM | Mean | SEM | p value |
| 14:0 | 1.38 | 0.02 | 1.38 | 0.05 | .96 |
| 14:1 | <LOD | <LOD | 0.03 | 0.00 | N/A |
| 16:0 | 35.48 | 0.52 | 36.20 | 1.35 | .73 |
| 16:1-n7 | 2.12 | 0.09 | 2.64 | 0.24 | .20 |
| 18:0 | 11.73 | 0.33 | 11.36 | 0.75 | .75 |
| 18:1-n7 | 1.60 | 0.05 | 1.53 | 0.02 | .15 |
| 18:1-n9 | 13.86 | 0.70 | 15.73 | 1.80 | .51 |
| 18: 2-n6 | 13.41 | 0.47 | 13.86 | 1.51 | .85 |
| 18:3-n6 | 0.17 | 0.01 | 0.11 | 0.01 | <.01** |
| 18:3-n3 | 0.42 | 0.05 | 0.48 | 0.09 | .70 |
| 20:0 | 0.16 | 0.01 | 0.15 | 0.00 | .21 |
| 20:1-n9 | 0.65 | 0.03 | 0.52 | 0.04 | .052 |
| 20:2-n6 | 0.57 | 0.03 | 0.53 | 0.03 | .34 |
| 20:4-n6 | 6.58 | 0.07 | 6.45 | 0.66 | .89 |
| 20:3-n3 | 0.05 | 0.00 | 0.04 | 0.00 | <.01** |
| 20:5-n3 | 0.51 | 0.02 | 0.41 | 0.04 | .16 |
| 22:0 | 0.15 | 0.01 | 0.13 | 0.01 | .18 |
| 22:1-n9 | 0.70 | 0.02 | 0.23 | 0.02 | <.001*** |
| 22:5-n6 | 2.17 | 0.05 | 1.78 | 0.16 | .14 |
| 24:0 | 0.15 | 0.02 | 0.30 | 0.04 | <.05* |
| 22:6-n3 | 7.18 | 0.19 | 6.06 | 0.60 | .25 |
| 24:1-n9 | 0.16 | 0.01 | 0.12 | 0.01 | <.05* |
Mice exposed to filtered air or 1 ppm O3 for 3 h were necropsied 24 h following exposure. Whole lung tissue homogenate was analyzed for fatty acid content as a percentage (%) of the total lung. *p < .05; **p < .01, ***p < .001; n = 3–6 per group.
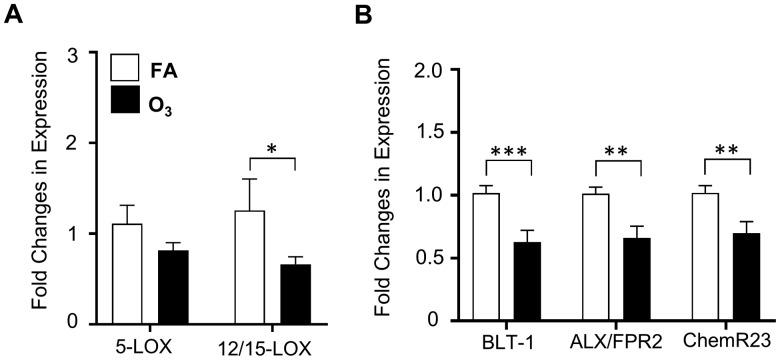
Pulmonary SPM Receptor and Lipoxygenase Expression
Decreases in SPM precursors and SPMs in the lung and spleen following O3 exposure suggested potential modifications to SPM synthetic enzyme or receptor expression. Lung tissue was analyzed by RT-PCR for O3-induced alterations in the expression of enzymes known to synthesize and drive SPM production, namely 5-LOX and 12/15-LOX. LOX enzymes metabolize fatty acids including linoleic acid, AA, EPA, and DHA into lipid mediators such as SPMs (Serhan and Chiang, 2013). Following O3 exposure, lung tissue 12/15-LOX expression was decreased (Figure 3A). This corresponded with O3-induced decreases in SPM levels, suggesting that the loss of synthetic LOX expression/function (12/15-LOX) could be limiting SPM synthesis. To determine if there were concomitant effects on SPM receptor expression (BLT-1, ALX/FPR2, and ChemR23), pulmonary tissue was analyzed by RT-PCR. O3 exposure decreased pulmonary SPM receptor expression (Figure 3B). These data support that in addition to a reduction in SPM synthesis, there is a subsequent reduction in SPM receptor gene expression following O3 exposure.
Figure 3.
O3 exposure decreases pulmonary SPM receptor and lipoxygenase expression. 24 h following a 3 h exposure to 1 ppm O3 or filtered air (FA), lung tissue was harvested and analyzed for A, 5-LOX and 12/15-LOX expression using quantitative RT-PCR and B, for SPM receptors BLT-1, ALX/FPR2, and ChemR23. Gene expression was normalized to GADPH and β-actin expression. *p < .05; **p < .01; ***p < .001; n = 11–12 per group.
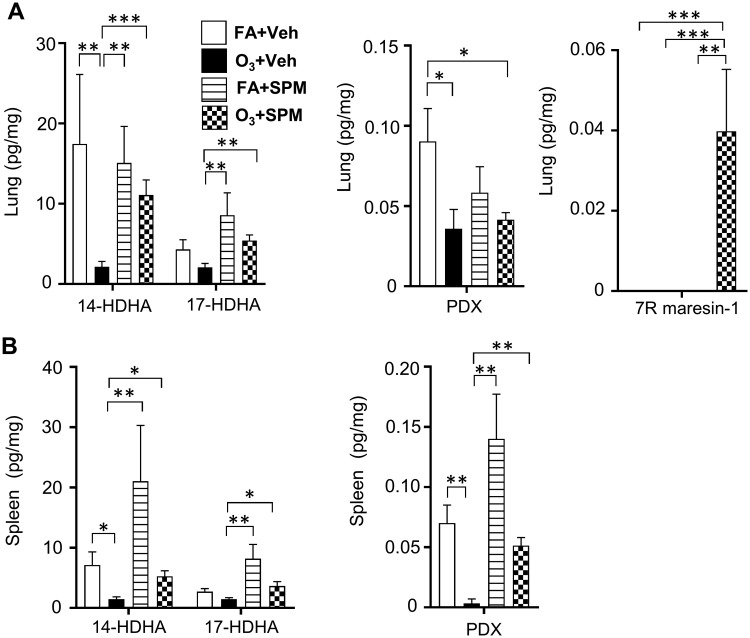
SPM Precursor/SPM Administration Prior to O3 Exposure Prevents Decreases in Pulmonary and Spleen SPM Levels
To determine if SPM levels could be preserved in the lungs and the spleen, mice were supplemented with 14-HDHA, 17-HDHA, and PDX 1 h prior to O3 exposure. Resolvin D1, resolvin D2, and lipoxin A4, were below the level of detection in the lungs and spleens of all treatment groups. In FA exposed mice, SPM/SPM precursor administration resulted in no changes in pulmonary SPM production. In O3 treated mice, SPM/SPM precursor administration increased 14-HDHA and 17-HDHA levels (Figure 4A). However, SPM precursor/SPM administration in O3 treated mice did not change PDX levels (Figure 4A). Interestingly, O3+SPM treated mice had an increase in 7 R maresin-1 (Figure 4A). 7 R maresin-1 was not detectable in the lung or spleen in any other treatment group. This suggests that 7 R maresin-1 was only produced in response to O3 following SPM precursor supplementation.
Figure 4.
SPM precursor/SPM administration prior to O3 exposure increases pulmonary 14-HDHA and 17-HDHA levels. 1 h prior to filtered air (FA) or 1 ppm O3 exposure (3 h) mice were i.p. injected with 14-HDHA, 17-HDHA, and PDX. 24 h following exposure, A, lung and B, spleen tissue was harvested for specialized pro-resolving mediator (SPM) analysis via LC-MS/MS. *p < .05; **p < .01; n = 5–6 per group.
In the spleens of FA exposed mice, SPM precursor/SPM administration trended towards increasing 14-HDHA, 17-HDHA (Figure 4B), and PDX levels (Figure 4B). In O3-exposed mice, SPM precursor/SPM administration maintained SPM levels comparable to FA and vehicle exposed mice. Taken together, these data indicate that SPM precursor/SPM administration may be able to increase basal levels of SPMs in the lung and spleen following O3 exposure.
Administration of SPM Precursors/SPM Improves Pulmonary Inflammation
Previous studies demonstrate that SPM precursor/SPM administration decreased pulmonary inflammation and restored immune cell function in models of allergic asthma and bacterial pneumonia (Aoki et al., 2008; Seki et al., 2010). To determine if SPM precursor/SPM administration prevents O3-induced pulmonary and systemic inflammation, mice were supplemented with DHA-derived lipid mediators; exposed to FA or O3; Make sure semi colon is regular size and not subscript and 24 h post-exposure, cellular inflammation, injury and cytokine gene expression was measured. SPM/SPM precursors administered to O3 exposed mice had decreased BAL Mφ and PMN counts relative to O3 exposed vehicle controls (Figure 5A). SPM precursor/SPM administration did not lower BALF protein concentrations or albumin concentrations suggesting that SPM precursor/SPM administration did not prevent O3-induced microvascular injury (Figs. 5B and 5C). Hematoxylin and eosin staining in mice pre-exposed with vehicle or SPM precursor/SPMs followed by FA or O3 exposure had minimal pulmonary pathology with acute 1 ppm O3 exposures (Figure 5D). In O3 exposed mice, SPM precursor/SPM administration significantly decreased the gene expression of pro-inflammatory cytokines IL-6 and IL-1β. CXCL1 trended lower but did not reach statistical significance (p = 0.09; Figure 5F). Together, this indicates that SPM precursor/SPM administration reduces inflammation by decreasing BAL PMN and Mφ cell counts, and decreasing pro-inflammatory cytokine gene expression.
Figure 5.
SPM precursor/SPM administration prior to O3 exposure decreases pulmonary inflammation but not markers of injury while increasing circulating immune cells. Mice were i.p. injected with 14-HDHA, 17-HDHA, and PDX 1 h prior to a 3 h exposure to 1 ppm O3 or filtered air (FA). 24 h following exposure, bronchoalveolar lavage (BAL) was performed and analyzed for A, cell differentials (n = 10–11 per group), B, total protein (n = 10–11 per group), and C, albumin concentrations (n = 5–6 per group). Lung tissue was stained with D, hematoxylin and eosin to examine pulmonary damage. Mice were analyzed for E, total white blood cell (WBC) counts and F, whole lung homogenate was analyzed for cyto/chemokines normalized to 18S using quantitative RT-PCR (n = 5 per group). Complete blood counts were used to determine G, cell differentials (n = 10–11 per group). *p < .05; **p < .01; ***p < .001.
SPM Precursor/SPM Administration Improves Systemic Immune Suppression
To determine if SPM precursor/SPM administration decreased systemic inflammation in addition to pulmonary inflammation, WBC counts were assessed. In FA exposed mice supplemented with SPM/SPM precursors, WBC levels remained unchanged, whereas SPM precursor/SPM administration prior to O3 exposure increased WBC counts (p = .053; Figure 5E). Compared to O3+vehicle exposed mice, O3+ SPM precursor/SPM administered mice had significantly increased circulating monocytes (Figure 5G). O3+ SPM precursor/SPM administration also increased lymphocyte counts, although this did not reach statistical significance (p = .06). Overall, the data suggests that SPM administration prior to O3 exposure prevents systemic immune suppression.
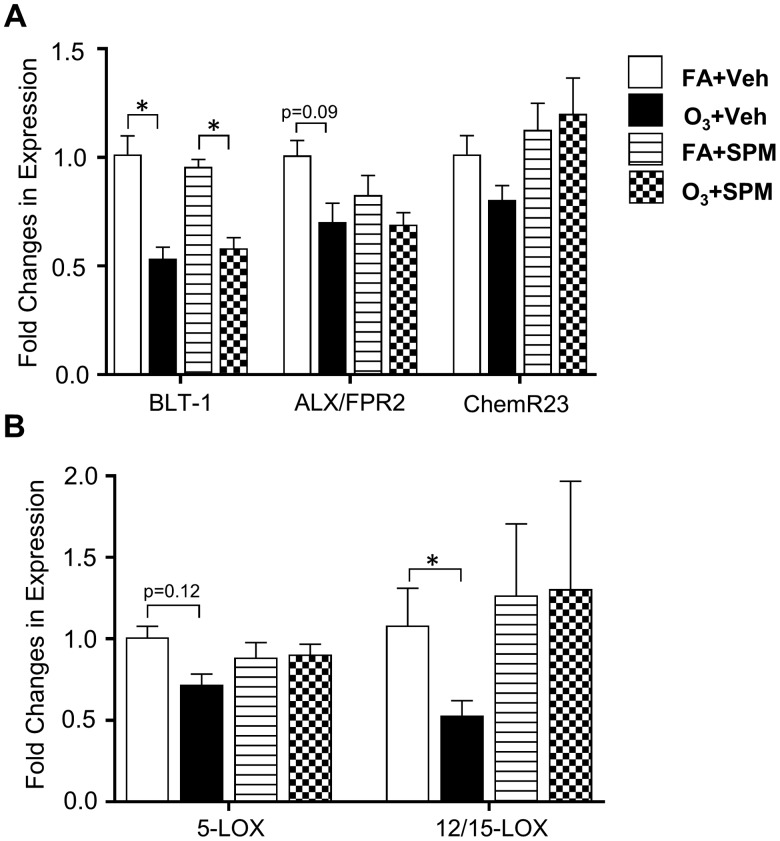
Pulmonary Lipoxygenase Enzyme Expression and SPM Receptors With SPM Precursor/SPM Administration
O3 exposure significantly decreased BLT-1 receptor gene expression. ALX/FPR2 receptor gene expression was decreased although not significantly (p = .09). Although O3 exposure did not significantly reduce ChemR23 receptor gene expression, there was a trend towards an increase (p = .14) in ChemR23 expression with SPM precursor/SPM administration (Figure 6A). However, there were no changes in BLT-1 and ALX/FPR2 expression with DHA-derived lipid mediator administration. There was a non-significant decrease in 5-LOX enzyme gene expression (p = .12) with O3-exposure (Figure 6B). In O3-exposed mice, there was a significant decrease in 12/15 LOX enzyme gene expression that was rescued with SPM precursor/SPM administration. Therefore, SPM precursor/SPM administration rescues O3 induced reductions in 12/15-LOX expression and increased production of SPMs, including 7R maresin-1.
Figure 6.
SPM precursor/SPM administration prior to O3 exposure increases pulmonary ChemR23 and 12/15-LOX expression. Mice were i.p. injected with 14-HDHA, 17-HDHA, and PDX 1 h prior to a 3 h exposure to 1 ppm O3 or filtered air (FA). Whole lung homogenates were analyzed for A, SPM receptors BLT-1, ALX/FPR2, and ChemR23 and B, lipoxygenase enzymes 5-LOX and 12/15-LOX. All gene expression was normalized to GAPDH and β-actin following quantitative RT-PCR (n = 5–6 per group). *p < .05.
DISCUSSION
O3 exposure causes pulmonary and systemic inflammation but the mechanisms leading to its resolution remain poorly defined. SPMs are known to promote resolution of inflammation in models such as cigarette-induced pulmonary inflammation, allergic asthma, and pneumonia (Aoki et al., 2008; Hsiao et al., 2013; Seki et al., 2010). However, the role of SPMs in O3-induced inflammatory responses are unknown. In this study, we demonstrated that: (1) O3-exposure increased pulmonary inflammation, and decreased pulmonary and splenic SPM levels, (2) O3-mediated SPM reductions were associated with decreased SPM generating enzymes (12/15-LOX) and SPM receptors, and (3) SPM precursor/SPM administration prevents O3-induced airspace neutrophilia/Mφs and the induction of pulmonary cyto/chemokine expression. These findings indicate that O3-induced inflammation may be perpetuated by reductions in SPMs and that SPM precursor/SPM administration prior to O3 exposure may prevent O3-induced cellular inflammation and cyto/chemokine expression.
Ambient O3 is a significant public health burden contributing to pulmonary inflammation, systemic inflammation, and exacerbation of pre-existing cardiopulmonary diseases. O3 does not directly affect lung structural cells due to its inability to transit the epithelial lining fluid (Postlethwait et al., 1998; Pryor, 1992) but rather generates a mixture of secondary and tertiary reactants such as oxidized phospholipids. The O3 generated oxidized phospholipids can cause direct lung injury and inflammation (MacNee, 2001). However, there are other bioactive lipids in the lungs besides oxidized phospholipids that may be reacting with O3. Miller et al. (2016a,b) demonstrated that O3 exposure alters peripheral lipid metabolism in both humans and rodents; however, they did not assess how O3 altered pulmonary lipid metabolites, specifically SPMs. We demonstrated that O3 exposure decreased 14-HDHA, 17-HDHA, and PDX with an associated reduction in SPM synthetic enzymes and signaling receptor expression. In the present study, we did not examine if this O3-induced reduction was due to decreased SPM production or increased SPM degradation. Prior work identified that pro-inflammatory lipid metabolites (prostaglandins and leukotrienes) are elevated in the lung after O3 exposure (Coffey et al., 1996; Schlesinger et al., 1990) but no data exists about pro-resolution lipids. Our data suggests that O3 exposure alters lipid metabolism resulting in SPM precursor or PDX reductions. Altered lipid mediator class switching has been described in the context of inflammatory conditions including sepsis, pneumonia, and atherosclerosis (Serhan, 2014). Together, this suggests that decreased SPM production and increased pro-inflammatory lipid mediator production may contribute to pulmonary inflammation after O3 exposure. We acknowledge that there are limitations to the present study including that SPM levels were only examined during peak O3-induced inflammation in the lung and that pulmonary injury/inflammation were assessed at only 1 timepoint post SPM administration. Future studies will determine SPM/SPM precursors at differing time points post-inflammation to determine if SPM suppression 24 h following O3-exposure leads to delayed resolution of injury. Additionally, given that our data indicate that SPM administration decreases cellular recruitment and pulmonary cyto/chemokine expression but does not alter total BAL protein and lung histology, future studies will determine if SPM precursors are important in airspace cellular recruitment after O3.
SPMs are a product of the metabolism of precursor fatty acids such as DHA, AA, and linoleic acid by 5-LOX and 12/15-LOX enzymes. Since no differences were observed in these fatty acid levels in the lung after O3 exposure, we investigated whether the reductions in 14-HDHA, 17-HDHA, and PDX were due to the loss of LOX enzyme expression. At 24 h following O3 exposure, pulmonary 12/15-LOX expression was reduced. The downregulation of LOX expression could explain the decreases in SPMs following O3 exposure. Our data contradict other reports which suggest that LOX expression is increased following O3 (Coffey et al., 1996) or O3-related DAMP exposure (oxidized phospholipids) (Zemski Berry and Murphy, 2016). A possible explanation for this discrepancy includes the use of different tissue samples (rodent lung tissue vs. human alveolar Mφ or epithelial cells) and/or exposure models (in vivo vs. in vitro). Another consideration could be the timing of the analysis. Pulmonary 5-LOX and 12/15-LOX expression were only examined at 1-time point (24 h) and 1 dose (3 h at 1 ppm) following O3. Thus, environmental exposures, such as O3, may decrease LOX enzyme expression which increases pulmonary inflammation whereas inhibition of LOX in chronic lung diseases dampens pulmonary inflammation. Future studies will consider if the gene, protein expression, and activity of pulmonary LOX during environmental exposures are associated with alterations in the production of endogenous SPMs.
Pulmonary and splenic reductions in SPM precursors and SPMs also corresponded with decreased SPM receptor expression (BLT-1, ALX/FPR2, and ChemR23) in the lungs. To our knowledge, these are the first studies to discover that O3 exposure reduces pulmonary gene expression of SPM receptors. The complete mechanism of this reduction was not elucidated in the present study; however, the correlation of reduced SPM receptor expression with decreased SPM production suggests that SPMs may stimulate their receptor expression. BLT-1 and ALX/FPR2 are predominantly expressed on PMNs (Barnes, 2004) while ChemR23 is principally expressed on immature mDCs, pDCs, NK cells, and Mφs (Luangsay et al., 2009; Parolini et al., 2007). Signaling through these SPM receptors promotes tissue resolution by increasing phagocytosis and decreasing pro-inflammatory cytokine production (Spite et al., 2014). Overexpression of BLT-1 has been shown to amplify PMN recruitment in murine models of acute skin inflammation, peritonitis, and reperfusion-initiated second organ injury (Chiang et al., 1999). Activation of ChemR23 by resolvins protects against pulmonary inflammation following diesel exhaust particles, cigarette smoke, or house dust mite exposure (Ohira et al., 2010; Provoost et al., 2016). Therefore, downregulation of these SPM receptors at 24 h following O3 exposure could be contributing to the observed pulmonary inflammation. Supporting this, SPM precursor/SPM administration prevented a decrease in pulmonary ChemR23 gene expression, which was associated with a decrease in O3-induced airspace infiltrates (BAL PMNs and Mφs) and pulmonary expression of cyto/chemokines (IL-6, IL-1β, and CXCL1). Interestingly, ChemR23 expression is restricted to monocyte/Mφ lineages in the lung (Arita et al., 2007). This suggests a possible interaction between SPM precursor/SPM administration and pulmonary monocytes/Mφs.
We were unable to detect many of the endpoint SPMs (i.e. resolvins, protectins) possibly due to rapid metabolism but were able to detect 7R-maresin-1 following SPM administration. This increase may be due to the increase in bioavailability of 14-HDHA and the resulting metabolism of 14-HDHA to 7R maresin-1 which is known to have pro-resolving properties in Mφs. Although detectable, 7R-maresin-1 values were low (∼0.07 pg/mg) and their biological significance unknown. In previous studies, maresins have decreased pulmonary inflammation in acute respiratory syndrome and in environmental dust exposure (Abdulnour et al., 2014; Nordgren et al., 2015).
In conclusion, O3 exposure increases pulmonary inflammation, suppresses the systemic immune response, and decreases SPM levels in the lung and spleen, which may contribute to O3-induced pulmonary inflammation. SPM precursors 14-HDHA, 17-HDHA, and the SPM PDX are synthesized from DHA by 5-LOX and 12/15-LOX which were lowered following O3 exposure. Furthermore, decreases in SPM levels led to downregulation in SPM receptor gene expression. Supplementing with 14-HDHA, 17-HDHA, and PDX prevents cellular inflammation, expression of certain pro-inflammatory cyto/chemokines, and prevents suppression of the systemic immune response. Additionally, the changes in SPMs do not appear to be driven by changes in the overall DHA concentrations in the lungs. Overall, a decrease in SPMs and/or SPM signaling may be the driving force of O3-induced inflammation. SPM precursor administration and/or dietary DHA supplementation to increase SPM levels may be a novel therapeutic to prevent O3-induced inflammation and more studies should be performed to identify the mechanism of how O3-induced lung damage can alter pulmonary SPM metabolism.
FUNDING
This work was supported by the Health Effects Institute Walter A. Rosenblith Award (to K.M.G.). Research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers. Additional funding was provided by the National Center for Complementary and Integrative Health [grant number NIH R01AT008375 (S.R.S.)], North Carolina State University’s Center for Human Health and the Environment through the National Institute of Environmental Health Science [grant number NIH P30ES025128] and by a National Institute of Environmental Health Sciences grant [grant number NIH R01ES027574 (R.M.T.)].
ACKNOWLEDGMENTS
We would like to thank Anita Coburn and Courtney Silliman for assistance with CBCs and Joani Zary Oswald for her assistance with lung histology. We would also like to thank Drs. William Guesdon and Edward Ross Pennington for their assistance with the SPM protocol and nitrogen purging, respectively.
REFERENCES
- Abdulnour R. E., Dalli J., Colby J. K., Krishnamoorthy N., Timmons J. Y., Tan S. H., Colas R. A., Petasis N. A., Serhan C. N., Levy B. D. (2014). Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci U.S.A. 111, 16526–16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H., Hisada T., Ishizuka T., Utsugi M., Kawata T., Shimizu Y., Okajima F., Dobashi K., Mori M. (2008). Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 367, 509–515. [DOI] [PubMed] [Google Scholar]
- Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., Serhan C. N. (2007). Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178, 3912–3917. [DOI] [PubMed] [Google Scholar]
- Arsalane K., Gosset P., Vanhee D., Voisin C., Hamid Q., Tonnel A. B., Wallaert B. (1995). Ozone stimulates synthesis of inflammatory cytokines by alveolar macrophages in vitro. Am. J. Respir. Cell Mol. Biol. 13, 60–68. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. (2004). Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 56, 515–548. [DOI] [PubMed] [Google Scholar]
- Basil M. C., Levy B. D. (2016). Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 16, 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile F., Sibray T., Belisle J. T., Bowen R. A. (2011). Analysis of lipids from crude lung tissue extracts by desorption electrospray ionization mass spectrometry and pattern recognition. Anal. Biochem. 408, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R. (2009). High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ. Health 8, 40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthillier L., Vincent R., Goegan P., Adamson I. Y., Bjarnason S., Stewart M., Guenette J., Potvin M., Kumarathasan P. (1998). Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1. Am. J. Pathol. 153, 1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello N., Mishra V., Sinha U., DiAngelo S. L., Chroneos Z. C., Ekpa N. A., Cooper T. K., Caruso C. R., Silveyra P. (2015). Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L1150–L1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N., Gronert K., Clish C. B., O’Brien J. A., Freeman M. W., Serhan C. N. (1999). Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J. Clin. Invest. 104, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey M. J., Wheeler C. S., Gross K. B., Eschenbacher W. L., Sporn P. H., Peters-Golden M. (1996). Increased 5-lipoxygenase metabolism in the lungs of human subjects exposed to ozone. Toxicology 114, 187–197. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Mann R., Becker S., House D. E., Schreinemachers D., Koren H. S. (1991). Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am. J. Respir. Cell Mol. Biol. 4, 72–81. [DOI] [PubMed] [Google Scholar]
- Draper D. W., Madenspacher J. H., Dixon D., King D. H., Remaley A. T., Fessler M. B. (2010). ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am. J. Respir. Crit. Care Med. 182, 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhidir H. S., Richards J. B., Cromar K. R., Bell C. S., Price R. E., Atkins C. L., Spencer C. Y., Malik F., Alexander A. L., Cockerill K. J. et al. , (2016). Plasminogen activator inhibitor-1 does not contribute to the pulmonary pathology induced by acute exposure to ozone. Physiol. Rep. 4, e12983.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- Guesdon W., Kosaraju R., Brophy P., Clark A., Dillingham S., Aziz S., Moyer F., Willson K., Dick J. R., Patil S. P. et al. , (2018). Effects of fish oils on ex vivo B-cell responses of obese subjects upon BCR/TLR stimulation: a pilot study. J. Nutr. Biochem. 53, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Slade R., Harris L. P., McDonnell W. F., Devlin R. B., Koren H. S., Costa D. L., McKee J. (1994). Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 150, 676–683. [DOI] [PubMed] [Google Scholar]
- Hollingsworth J. W., Maruoka S., Li Z., Potts E. N., Brass D. M., Garantziotis S., Fong A., Foster W. M., Schwartz D. A. (2007). Ambient ozone primes pulmonary innate immunity in mice. J. Immunol. 179, 4367–4375. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J. A., Harkema J. R., Kirkpatrick D. T., Henderson R. F. (1989). Response of rat alveolar macrophages to ozone: quantitative assessment of population size, morphology, and proliferation following acute exposure. Exp. Lung Res. 15, 1–16. [DOI] [PubMed] [Google Scholar]
- Hsiao H. M., Sapinoro R. E., Thatcher T. H., Croasdell A., Levy E. P., Fulton R. A., Olsen K. C., Pollock S. J., Serhan C. N., Phipps R. P. et al. , (2013). A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One 8, e58258.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H. M., Thatcher T. H., Colas R. A., Serhan C. N., Phipps R. P., Sime P. J. (2015). Resolvin D1 reduces emphysema and chronic inflammation. Am. J. Pathol. 185, 3189–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell B. J., Hallberg A., McCubbin D. R., Post E. (2005). Health-related benefits of attaining the 8-hr ozone standard. Environ. Health Perspect. 113, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Devlin R. B., Graham D. E., Mann R., McGee M. P., Horstman D. H., Kozumbo W. J., Becker S., House D. E., McDonnell W. F. et al. , (1989). Ozone-induced inflammation in the lower airways of human subjects. Am. Rev. Respir. Dis. 139, 407–415. [DOI] [PubMed] [Google Scholar]
- Kosaraju R., Guesdon W., Crouch M. J., Teague H. L., Sullivan E. M., Karlsson E. A., Schultz-Cherry S., Gowdy K., Bridges L. C., Reese L. R. et al. , (2017). B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J. Immunol. 198, 4738–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuda O. (2017). Bioactive metabolites of docosahexaenoic acid. Biochimie 136, 12–20. [DOI] [PubMed] [Google Scholar]
- Kummarapurugu A. B., Fischer B. M., Zheng S., Milne G. L., Ghio A. J., Potts-Kant E. N., Foster W. M., Soderblom E. J., Dubois L. G., Moseley M. A. et al. , (2013). NADPH: quinone oxidoreductase 1 regulates host susceptibility to ozone via isoprostane generation. J. Biol. Chem. 288, 4681–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan P. J., Fuller R., Acosta N. J. R., Adeyi O., Arnold R., Basu N. N., Balde A. B., Bertollini R., Bose-O'Reilly S., Boufford J. I. et al. , (2018). The Lancet Commission on pollution and health. Lancet. 391, 462–512. [DOI] [PubMed] [Google Scholar]
- Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001). Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619. [DOI] [PubMed] [Google Scholar]
- Levy B. D., Serhan C. N. (2014). Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 76, 467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Poon R., Chen L., Frescura A. M., Montuschi P., Ciabattoni G., Wheeler A., Dales R. (2009). Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ. Health Perspect. 117, 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luangsay S., Wittamer V., Bondue B., De Henau O., Rouger L., Brait M., Franssen J. D., de Nadai P., Huaux F., Parmentier M. (2009). Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 183, 6489–6499. [DOI] [PubMed] [Google Scholar]
- MacNee W. (2001). Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 429, 195–207. [DOI] [PubMed] [Google Scholar]
- Miller D. B., Ghio A. J., Karoly E. D., Bell L. N., Snow S. J., Madden M. C., Soukup J., Cascio W. E., Gilmour M. I., Kodavanti U. P. (2016a). Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am. J. Respir. Crit. Care Med. 193, 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. B., Snow S. J., Henriquez A., Schladweiler M. C., Ledbetter A. D., Richards J. E., Andrews D. L., Kodavanti U. P. (2016b). Systemic metabolic derangement, pulmonary effects, and insulin insufficiency following subchronic ozone exposure in rats. Toxicol. Appl. Pharmacol. 306, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata J., Fukunaga K., Iwamoto R., Isobe Y., Niimi K., Takamiya R., Takihara T., Tomomatsu K., Suzuki Y., Oguma T. et al. , (2013). Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 131, 353.. [DOI] [PubMed] [Google Scholar]
- Nordgren T. M., Bauer C. D., Heires A. J., Poole J. A., Wyatt T. A., West W. W., Romberger D. J. (2015). Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl. Res. 166, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T., Arita M., Omori K., Recchiuti A., Van Dyke T. E., Serhan C. N. (2010). Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 285, 3451–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini S., Santoro A., Marcenaro E., Luini W., Massardi L., Facchetti F., Communi D., Parmentier M., Majorana A., Sironi M. et al. , (2007). The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 109, 3625–3632. [DOI] [PubMed] [Google Scholar]
- Pendino K. J., Meidhof T. M., Heck D. E., Laskin J. D., Laskin D. L. (1995). Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am. J. Respir. Cell Mol. Biol. 13, 125–132. [DOI] [PubMed] [Google Scholar]
- Postlethwait E. M., Cueto R., Velsor L. W., Pryor W. A. (1998). O3-induced formation of bioactive lipids: estimated surface concentrations and lining layer effects. Am. J. Physiol. 274, L1006–L1016. [DOI] [PubMed] [Google Scholar]
- Provoost S., De Grove K. C., Fraser G. L., Lannoy V. J., Tournoy K. G., Brusselle G. G., Maes T., Joos G. F. (2016). Pro- and anti-inflammatory role of ChemR23 signaling in pollutant-induced inflammatory lung responses. J. Immunol. 196, 1882–1890. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. (1992). How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts? Free Radic. Biol. Med. 12, 83–88. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. B., Driscoll K. E., Gunnison A. F., Zelikoff J. T. (1990). Pulmonary arachidonic acid metabolism following acute exposures to ozone and nitrogen dioxide. J. Toxicol. Environ. Health 31, 275–290. [DOI] [PubMed] [Google Scholar]
- Seki H., Fukunaga K., Arita M., Arai H., Nakanishi H., Taguchi R., Miyasho T., Takamiya R., Asano K., Ishizaka A. et al. , (2010). The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 184, 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N. (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Chiang N. (2013). Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol. 13, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Petasis N. A. (2011). Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarke C., Alamuddin N., Lawson J. A., Li X., Ferguson J. F., Reilly M. P., FitzGerald G. A. (2015). Bioactive products formed in humans from fish oils. J. Lipid Res. 56, 1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M., Claria J., Serhan C. N. (2014). Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19, 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebot V., Gianicolo E. A., Rainaldi G., Trivella M. G., Sicari R. (2009). Ozone and cardiovascular injury. Cardiovasc. Ultrasound 7, 30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. M., Fix A., Crouch M. J., Sparagna G. C., Zeczycki T. N., Brown D. A., Shaikh S. R. (2017). Murine diet-induced obesity remodels cardiac and liver mitochondrial phospholipid acyl chains with differential effects on respiratory enzyme activity. J. Nutr. Biochem. 45, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J. L., Kohler R. H., Chudnovskiy A., Waterman P. et al. , (2009). Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe R. M., Li Z., Potts E. N., Frush S., Liu N., Gunn M. D., Foster W. M., Noble P. W., Hollingsworth J. W. (2011). Ozone inhalation promotes CX3CR1-dependent maturation of resident lung macrophages that limit oxidative stress and inflammation. J. Immunol. 187, 4800–4808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Wang X., Katwa P., Podila R., Chen P., Ke P. C., Rao A. M., Walters D. M., Wingard C. J., Brown J. M. (2011). Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part Fibre Toxicol. 8, 24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson W. P., Campen M. J., Nolan J. P., Costa D. L. (2001). Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environ. Health Perspect. 109, 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiester M. J., Tepper J. S., King M. E., Menache M. G., Costa D. L. (1988). Comparative study of ozone (O3) uptake in three strains of rats and in the guinea pig. Toxicol. Appl. Pharmacol. 96, 140–146. [DOI] [PubMed] [Google Scholar]
- Yang R., Chiang N., Oh S. F., Serhan C. N. Curr Protoc Immunol, November 2011, CHAPTER: Unit-14.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemski Berry K. A., Murphy R. C. (2016). Phospholipid ozonation products activate the 5-lipoxygenase pathway in macrophages. Chem. Res. Toxicol. 29, 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]