Abstract
Methylmercury (MeHg) is an environmental pollutant that affects primarily the central nervous system (CNS), causing neurological alterations. An early symptom of MeHg poisoning is the loss of body weight and appetite. Moreover, the CNS has an important role in controlling energy homeostasis. It is known that in the hypothalamus nutrient and hormonal signals converge to orchestrate control of body weight and food intake. In this study, we investigated if MeHg is able to induce changes in the expression of key hypothalamic neuropeptides that regulate energy homeostasis. Thus, hypothalamic neuronal mouse cell line GT 1-7 was treated with MeHg at different concentrations (0, 0.5, 1, and 5 µM). MeHg induced the expression of the anorexigenic neuropeptide pro-omiomelanocortin (Pomc) and the orexigenic peptide Agouti-related peptide (Agrp) in a concentration-dependent manner, suggesting deregulation of mechanisms that control body weight. To confirm these in vitro observations, 8-week-old C57BL/6J mice (males and females) were exposed to MeHg in drinking water, modeling the most prevalent exposure route to this metal. After 30-day exposure, no changes in body weight were detected. However, MeHg treated males showed a significant decrease in fat depots. Moreover, MeHg affected the expression of hypothalamic neuropeptides that control food intake and body weight in a gender- and dose-dependent manner. Thus, MeHg increases Pomc mRNA only in males in a dose-dependent way, and it does not have effects on the expression of Agrp mRNA. The present study shows, for first time, that MeHg is able to induce changes in hypothalamic neuropeptides that regulate energy homeostasis, favoring an anorexigenic/catabolic profile.
Keywords: methylmercury, hypothalamus, neuropeptides, control body weight, glucose homeostasis
Methylmercury (MeHg) is highly toxic environmental pollutant. Nowadays, contaminated fish from polluted areas are the primary source of MeHg exposure, secondary to biomethylation of inorganic mercury in the aquatic sediments to the organic form of this metal. This reaction is catalyzed by aquatic microorganisms and the resulting MeHg accumulates in the food chain, reaching the highest concentrations in carnivore predatory fish, such as swordfish, tuna, and shark. Its primary target is the central nervous system (CNS). Upon ingestion of MeHg, a large fraction (approximately 95%) is absorbed by the gastrointestinal tract and distributed ubiquitously (Syversen and Kaur, 2012). MeHg distributes to all brain regions by readily crossing the blood-brain barrier (Aschner and Clarkson, 1988).
In adults, MeHg poisoning has a long latency period (weeks to months) before the appearance of symptoms. The initial symptoms comprise weight loss, blurred vision and loss of sensation of the extremities of hands and feet and in the circumoral area (paresthesias), followed by constriction of visual field, loss of coordination in gait (ataxia), slurred speech (dysarthria), hearing loss, muscle weakness, tremor, and mental deterioration. Among these symptoms, the least studied is the weight loss. Loss of appetite and body weight are commonly reported in response to intoxication to a host of toxic agents. In vivo studies have also reported that MeHg poisoning causes weight loss in CD1 male mice and in Wistar rats orally administrated with 0.25–4 mg MeHg/kg during 60 days or 8 mg MeHg/kg body weight for 5 days, respectively (Berthoud et al., 1976; Magos, 1982). Indeed several studies have used body weight loss as an early sign of toxicity. For example, in Balb/C female mice treated with 0.01 mM MeHg in drinking water for 30 days and in Sprague-Dawley male rats gavaged with 0.001 g MeHg/kg body weight the loss of body weight was reported as a sign of MeHg toxicity (Li et al., 2014; Shi et al., 2011). Given MeHg’s propensity to act in multiple ways to affect the body weight, we reasoned it was timely to understand the basis for this effect.
Regulation of food intake, energy homeostasis and body weight is controlled by complex processes. Misbalance in these mechanisms leads to loss of body weight or obesity. Energy homeostasis, and the resulting changes in body weight, is controlled by the CNS, predominately by the hypothalamus (Morton et al., 2006; Schwartz et al., 2000). Nutrient and hormonal signals converge directly and indirectly on the hypothalamus, forming a network between peripheral tissues and the CNS, which controls the energy balance through complex and interconnected signal cascades (Hussain and Bloom, 2013). The arcuate nucleus (ARC) in the hypothalamus is one of the main nuclei regulating body weight. It is characterized by at least 2 distinct neuronal populations; one that secretes orexigenic/anabolic peptides, such as neuropeptide Y (NPY) and Agouti-related peptide (AgRP) (Shutter et al., 1997; Stanley et al., 1986) and the other, synthesizing anorexigenic/catabolic substances, such as pro-opimelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) (Krude et al., 1998; Lambert et al., 1998). Downstream of the ARC, other brain nuclei with a role in controlling of body weight, such as the paraventricular nucleus, are endowed with inducing satiety, and the lateral hypothalamus functions to initiate food intake (Elmquist et al., 1998). Thus, changes in these hypothalamic circuits are regulating body weight, and modulate energy intake (food intake) and energy expenditure (metabolism, thermogenesis, and physical activity).
Given that one of the primary symptoms of MeHg intoxication is loss of body weight, and since the brain has high affinity for MeHg, we hypothesized that MeHg may affect hypothalamic neurons, altering the expression of neuropeptides and receptors that control energy homeostasis and body weight. As the main source of MeHg exposure in humans is through consumption of contaminated fish, we opted to test our hypothesis in a low-dose MeHg exposure model.
MATERIALS AND METHODS
Cell culture
Hypothalamic neuronal GT1-7 cells (kindly provided by Dr Pamela Mellon, University of California, San Diego) (Mellon et al., 1990) (the sex of the donor was not determined), were cultured in Dulbecco’s modified Eagles’ medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (1% penicillin/streptomycin) and maintained at 37°C with 5% CO2.
Cells were subcultured into 6-well culture plates before experiments at 9 × 105 cells and treated with 0, 0.5, 1, or 5 µM of MeHg (II) chloride (MeHg) (Sigma-Aldrich, St Louis, MO, USA). Cells were harvested after 6 h for RNA analysis. Based on literature reports (Castoldi et al., 2001; Sarafian et al., 1994), we piloted several experimental conditions with sublethal concentrations and times of exposure. The results led us to treat in the in vitro study for 6 h for as the preferred time. The chosen concentrations are compatible with human studies and real-life exposure scenarios, and are expected to lead an accumulation of 0, 0.25, 5, and 25 µHg/g protein, respectively. These concentrations are expected to produce a mercury (Hg) accumulations at equivalent levels that had been reported to cause a sub-toxic threshold in mammalian CNS, which are estimated to be lower than 68.5 µHg/g in human populations (Aschner, 2012). Moreover, only 5 µM caused a significant decrease up to 20% in cell viability from 6 h of treatment (data not shown).
Mice
Animal experiments were performed in accordance with the guidelines of Albert Einstein College of Medicine Institute of Animal Studies, and approved by the local IACUC. The experiment was repeated twice in 8-week-old C57BL/6 J female (n = 5 per group for the first experiment and n = 5–6 for the second one) and males (n = 5 per group for the first experiment and n = 5–6 for the second one). As both experiments were performed under the same conditions and no significant differences were observed between then (Supplementary Figs. 1–3) the data have been combined. Mice were purchased from The Jackson Lab. All animals were kept under constant temperature at 21°C ± 2°C and under 12-h light-dark cycle with ad libitum access to regular chow diet (Research Diet, Inc, New Brunswick, NJ, USA) and water unless otherwise stated.
MeHg treatment
Mice were assigned to control or treatment groups (5 mice in each group in the first experiment and 5–6 mice group in the second experiment). In the first experiment animals were housed individually and in the second experiment, as no differences were detected in food intake in the first one, they were housed with a maximum 3 animals per cage. After 4 days of acclimatization the treatment started. The control group received drinking water and the treatment groups received drinking water with 0.5 or 5 ppm MeHg (II) chloride (MeHg) (Sigma-Aldrich, St Louis, MO, USA). Fresh MeHg solutions were prepared once a week. These MeHg doses are expected to lead to Hg accumulation of 0.25 and 25 µg Hg/g protein in brain, respectively, capturing the subtoxic threshold reported in mammalians (Aschner, 2012). Thus, it is described that adult mice exposed to 30 ppm MeHg in drinking water developed neurological changes with an accumulation of 12.9 µHg/g in brain cortex (Fujimura et al., 2009). In relation with human exposures, we expect our animals to accumulate less than the lethal doses observed in Iraq populations, which accumulated 68.5 µHg/g in brain (Aschner, 2012).
Body weight, food intake, water consumption, and body composition
In the first experiment mice were isolated individually and fed ad libitum with regular chow diet (Research Diets, Inc, New Brunswick, NJ, USA) and water (control or MeHg treated). After an acclimation period (4 days), body weight, food intake and water consumption were measured daily for 30 consecutive days. As no changes in food intake were observed, in the second experiment animals were housed with a maximum of 3 animals per cage, and as in the previous experiment after 4 days of acclimation, body weight, food intake and water consumption were recorded. In the second experiment, food intake and water consumption were corrected for the number of animals in the cage. No differences between experiments were observed.
In addition, liver, visceral (gonadal), and subcutaneous (dorsal area) white adipose tissue (WAT) and brown adipose tissue (BAT) were removed and weighed upon euthanasia of the animals.
Insulin tolerance test and oral glucose tolerance test
The glucose homeostasis status of the treated mice was evaluated by insulin tolerance test (ITT) and oral glucose tolerance test (OGTT) at days 18 and 25 of treatment, respectively. For ITT, mice were fasted 4 h before intraperitoneal injection with insulin (0.75 unit insulin/kg body weight). Serum glucose was measured from tail vein using an ACCU CHECK glucometer (Roche, Indianapolis, IN, USA) at 0, 15, 30, 45, 60, and 120 min after insulin injection. For OGTT, mice were orally administrated with glucose (2.5 g/kg body weight) after an 18 h fasting period. Serum glucose from the tail vein was measured with an ACCU CHECK glucometer (Roche, Indianapolis, IN, USA) at 0, 15, 30, 45, 60, 90, and 120 min.
Measurement of triglycerides and fatty acids content in liver
Analysis of liver triglyceride content was performed by liver saponification in ethanolic KOH as described in Jouihan (2012), except triglyceride content was measured (EnzyChrom Triglyceride Assay, BioAssay Systems, Hayward, CA, USA).
Analysis of fatty acids content was performed by liver homogenization in 5% isopropanol and 5% Triton-X-100 in water followed by filtration through a 0.45 µm polytetrafluoroethylene syringe filter. Fatty acid content was measured using EnzyChrom Free Fatty Acid Assay, BioAssay Systems, Hayward, CA, USA.
Measurement of metabolites and hormones in serum
Blood samples were obtained when mice were euthanized and centrifuged to extract serum. Commercial kit was used to measure serum levels of triglycerides and free fatty acid (EnzyChrom Triglyceride Assay, BioAssay Systems and EnzyChrom Free Fatty Acid Assay, BioAssay Systems, Hayward, CA, USA, respectively).
Insulin serum levels were also measure using a Rat/Mouse Insulin ELISA assay (Millipore, Ontario, Canada).
Cellular mercury content
Total cellular mercury content was quantified in the cortex by inductively coupled plasma mass spectrometry (ICP-MS; Agilent 8800 ICP-QQQ, Agilent Technologies Deutschland GmbH, Böbligen, Germany) (Lohren et al., 2015).
Quantitative real time PCR
For animal studies total RNA was isolated from hypothalami, livers and visceral adipose tissues using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA was isolated using chloroform (Fisher Scientific, Hampton, USA) and then precipitated with isopropyl alcohol (Millipore, Ontario, Canada).
For cell cultures, total RNA was isolated using the RNeasy Mini kit (Qiagen, Germantown, MD, USA) following the manufacturer’s instructions.
Isolated RNA was reverse transcribed with High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) following manufacturer’s instructions. Quantitative Real Time PCR was performed with iCycler Thermal Cycler (BioRad, Hercules, CA, USA) using the predesigned TaqMan probes describe in Table 1 which was selected from TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). Relative quantification of mRNA expression was normalized to the standard housekeeping gene Gapdh ID Mm99999915. Change in expression was determined with the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
TaqMan Probes for qPCR Analysis
| Gene | ID |
|---|---|
| Hypothalamic neuropeptides | |
| Orexigenic genes | |
| Agouti-related peptide (Agrp) | Mm00475829 |
| Neuropeptide y (Npy) | Mm01410146 |
| Promelanin-concentrating hormone (Pmch) | Mm01242886 |
| Anorexigenic genes | |
| CART prepropeptide (Cartpt) | Mm04210469 |
| Corticotrophin-released hormone (Crh) | Mm01293920 |
| Pro-omiomelanocortin (Pomc) | Mm00435874 |
| Others | |
| Gonadotropin releasing hormone 1 (Gnrh1) | Mn01315604 |
| Receptors and hormones | |
| Receptors | |
| Growth hormone secretagogue receptor 1a (Ghsr1a) | Mm00616415 |
| Insulin receptor (Ins) | Mm01211875 |
| Leptin receptor (Lepr) | Mm00440181 |
| Hormones | |
| Leptin (Lep) | Mm00434759 |
| Lipid metabolism | |
| Lipogenic genes | |
| Acetyl-Coenzyme A carboxylase alpha (Acaca) | Mm01304257 |
| Fatty acid synthase (Fasn) | Mm00662319 |
| Peroxisome proliferator-activated receptor gamma (Pparg) | Mm00440945 |
| Lipolytic genes | |
| Hepatic lipase (Lipc) | Mm01171487 |
| Lipoprotein lipase (Lpl) | Mm00434764 |
| Patatin-like phosphplipase domain containing 2 (Pnpla2) | Mm00503040 |
| β oxidation | |
| Carnitine palmitoyltransferase 1a (Cpt1a) | Mm01231183 |
| Transport | |
| Fatty acid translocase Cd36 | Mm00432403 |
| Antioxidation | |
| Nuclear factor, erythroid derived 2, like 2 (Nfe2l2) | Mm00477784 |
| Housekeeping gene | |
| Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) | Mm99999915 |
Statistical analysis
All data are presented as mean ± SEM. Statistical calculations were performed using the Statistical Package for Social Sciences software. The in vitro data on gene expression was analyzed by 1-way analysis of variance (ANOVA) to determine the effect of the treatment followed by Tukey post hoc test. The in vivo data on gene expression, triglycerides and fatty acid liver content, metabolits and insulin serum levels, and tissue weights were also analyzed by ANOVA to determine the effect of the doses within each sex separately, followed by Tukey post hoc test. Body weight changes, food intake, water consumption, OGTT and ITT were analyzed with a repeated measure ANOVA with dose and time as main factors. Significant differences were deemed to be at p < .05.
RESULTS
MeHg Induces Expression of Hypothalamic Factors in GT1-7 Cells
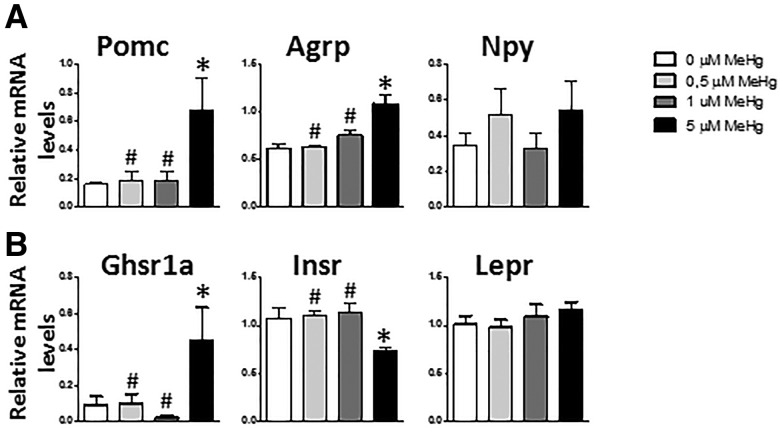
To ascertain if MeHg directly affects the regulation of energy balance, we analyzed its effect in hypothalamic neuronal mouse cell line GT1-7, focusing on the expression of neuropeptides that regulate energy balance. GT 1-7 cells are characterized by secreting gonadotropin releasing hormone (Mellon et al., 1990) (Supplementary Figure 4A), and after 6 h of treatment with MeHg none of the concentrations affected its expression (Supplementary Figure 4A). Moreover, after 6 h of treatment with 5 µM MeHg, Pomc, and Agrp mRNA was increased (F[3, 22] = 4.826, p = .014 and F[3, 24] = 15.132, p < .001, respectively) (Figure 1A) . In addition, the increase in POMC mRNA was also observed in cells treated with 5 µM for 1 h (Supplementary Figure 4B). Neither 0.5 nor 1 µM MeHg produced any effect in the expression of these neuropeptides. Npy mRNA expression was not affected by any of the MeHg concentrations.
Figure 1.
Gene expression in GT 1-7 cells after 6 h exposure to MeHg. Effects on the anorexigenic neuropeptide Pomc (n = 6–7), the orexigenic peptide Agrp (n = 7), and Npy (n = 7) (A) and the receptors, Ghsr1a (n = 6–7), Insr (n = 7), and Lepr (n = 7) (B). *p < .05 versus control (0 µM MeHg), #p < .05 versus high dose (5 µM MeHg).
Hypothalamic insulin receptor (Insr), leptin receptor (Lepr), and growth hormone secretagogue receptor 1a (Ghsr1a, which is the ghrelin receptor), are key receptors in the regulation of body weight. Here, we analyzed whether MeHg affected their expression. After 6 h of treatment with 5 µM MeHg significant decreases in Insr mRNA (F[3, 24] = 13.241, p = .007) and significant increases in Ghsr1a mRNA (F[3, 22] = 4.331, p = .015, post hoc p = .05 5 µM compare control) expression (Figure 1B) were noted. No changes in the expression of the leptin receptor were observed (Figure 1B).
MeHg Exposure via Drinking Water for 30 Days Produced Changes in Body Composition
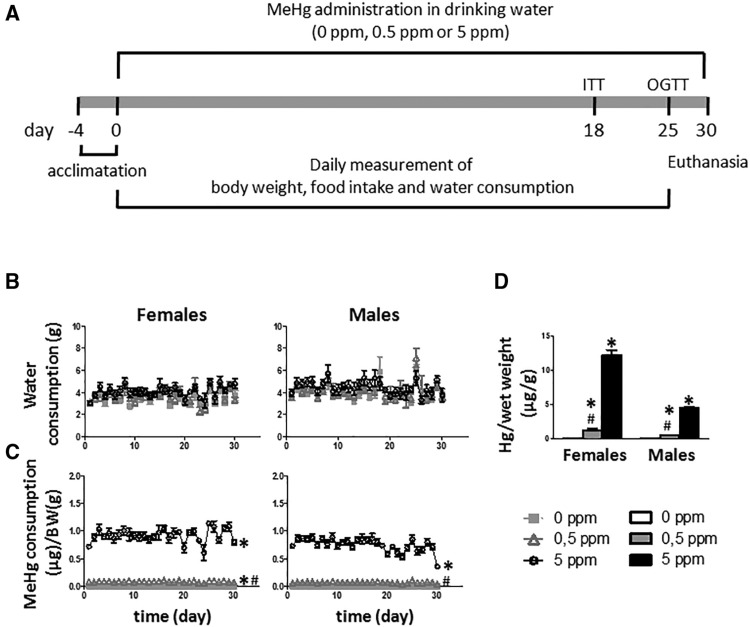
Given the alterations in hypothalamic factors that regulate energy balance in the above described cellular model, next, we sought to determine if similar changes are induced by MeHg in vivo. The control of energy balance is a complex process that implicates several tissues. Notably, the in vivo model allows us to complement the findings observed in vitro, and analyze the effects of MeHg on the crosstalk between the hypothalamus and peripheral tissues that play a role in the regulation of the energetic homeostasis, such as liver and adipose tissue. This latter interaction is precluded in studies with the cell line model. The experimental design is illustrated in Figure 2A. No difference in water consumption was noted between the treatment and control groups in both males and females (Figure 2B). As expected, the groups that received higher doses of MeHg (5 ppm) ingested more MeHg per gram body weight (F[2, 12] = 1444.81, p < .001 for females and F[2, 12] = 170.749, p < .001 for males) (Figure 2C). Tissue accumulation of MeHg was also assessed. Because of the small size of the hypothalamus it was only used for gene expression analysis and not metal accumulation determination. MeHg accumulation was measured in the cortex as it is one of the primary areas where MeHg accumulates (Glaser et al., 2010; Miyamoto et al., 2001). As expected, mice that ingested MeHg had a significant and dose-dependent increase in MeHg accumulation (F[2, 12] = 288.448, p < .001 for females and F[2, 12] = 79.791, p < .001 for males) (Figure 2D).
Figure 2.
A, General outline of the experiment. Eight-weeks old female and male mice were housed individually and acclimated for 4 days before treatment. Mice were exposed to MeHg in drinking water. After 18 days of exposure, an ITT was performed, and at day 25 an OGTT was done. Finally, 5 days later animals were killed and blood and tissues were collected. B, Water consumption during treatment in females (left) and males (right) (n = 10–11 animals per group). C, MeHg consumption in females (left) and males (right) (n = 10–11 animals per group). D, Mercury accumulation in cortex after 30 days of MeHg exposure (n = 5 animals per group). *p < .05 versus control (0 ppm MeHg), #p < .05 versus high dose (5 ppm MeHg).
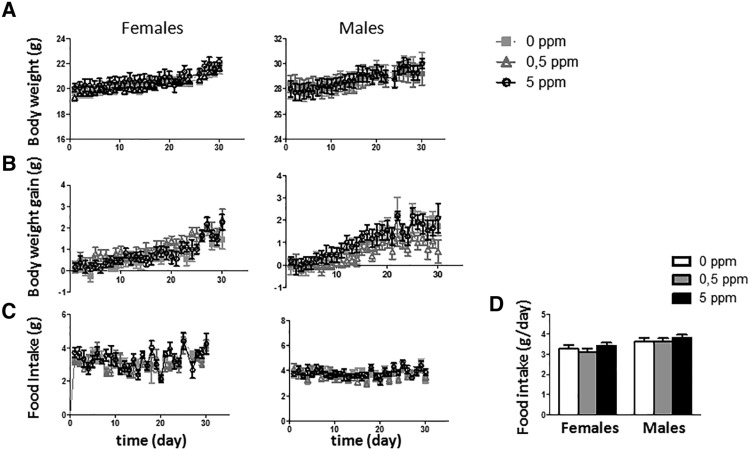
During the treatment period body weight and food intake were recorded daily. Neither of them was significantly affected by MeHg treatments (Figure 3).
Figure 3.
Effect of MeHg in drinking water on absolute body weight (A), body weight gain (B), and food intake (C) in females (left) and males (right) during the treatment. D, Food intake average by day.
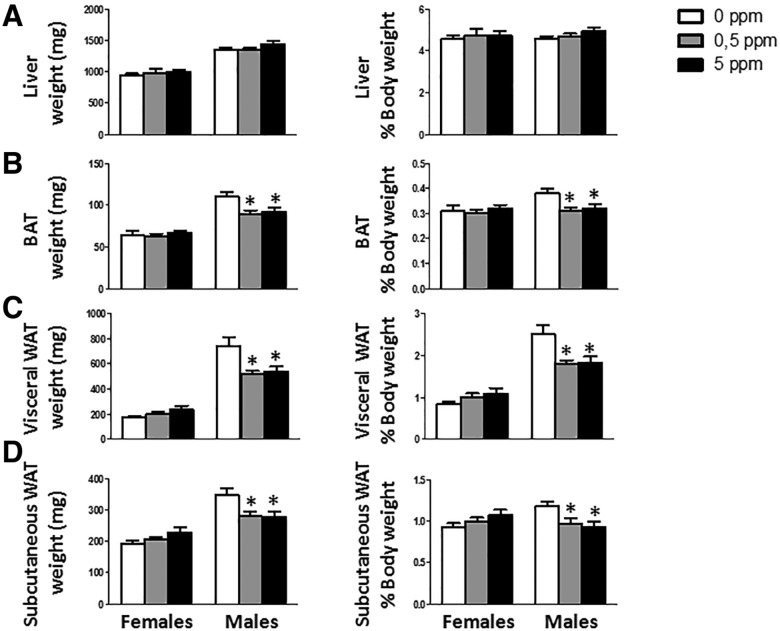
Upon euthanasia liver and fat depots (visceral and subcutaneous WAT and BAT) were dissected out and weighted. MeHg did not cause any change in liver weights in either females or males (Figure 4A). No effect of MeHg was detected on any of the fat depots analyzed in females (Figs. 4B–D). Although no significant changes in males body weight or body weight gain were detected (Figs. 3A and 3B), there were changes in several of the analyzed tissues. After 30 days, males treated with MeHg showed a decrease in fat depots sizes. Visceral WAT was significantly decreased (F[2, 29] = 5.725, p = .008 for absolute weight and F[2, 29] = 6.671, p = .004 referred as % of body weight) (Figure 4C). Both MeHg doses also reduced subcutaneous WAT weight (F[2, 27] = 4.536, p = .02 for absolute weight and F[2, 27] = 5.116, p = .013 referred as % of body weight), although the effect of the lower doses (0.5 ppm) was marginally significant (post hoc p = .052 and .05 for absolute weight and referred as % of body weight, respectively) (Figure 4D). BAT was also significantly decreased (F[2, 26] = 5.144, p = .013 for absolute weight and F[2, 26] = 4.289, p = .025 referred as % of body weight) (Figure 4B).
Figure 4.
Absolute and relative (% of body weight) weights of liver (A), BAT (B), visceral WAT (C) and subcutaneous WAT (D) (n = 8–11 animals per group). *p < .05 versus control (0 ppm MeHg).
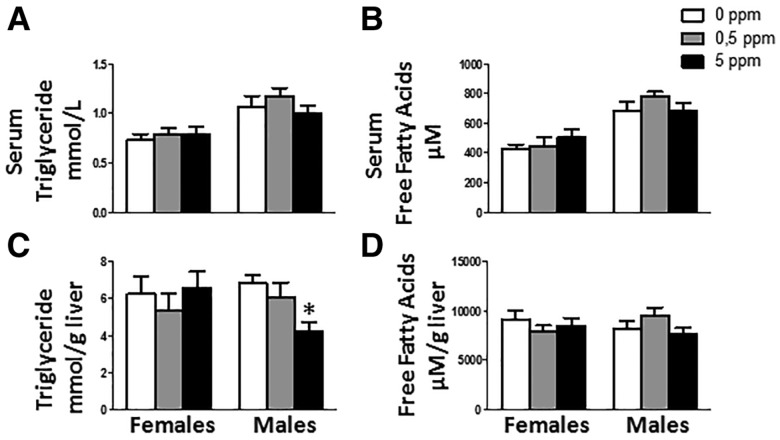
MeHg Exposure Decreases Triglyceride Content in Male Livers
Given that MeHg caused decreased fat depots (Figure 4), next, we assessed changes in triglyceride and fatty acid levels in serum. MeHg did not alter serum lipids both in females or males (Figs. 5A and 5B). In addition, hepatic triglyceride and fatty acid content was analyzed. Whereas no difference was found in females, males treated with the high dose of MeHg (5 ppm) showed a decrease in the content of hepatic triglycerides (F[2, 29] = 5.369, p = 0.01) (Figure 5C). No changes in fatty acid hepatic content were detected in males (Figure 5D).
Figure 5.
MeHg treatment effect on serum triglyceride (A) and serum free fatty acid (B) levels, and in hepatic content of triglycerides (C) and fatty acids (D) (n = 10–11 animals per group). *p < .05 versus control (0 ppm MeHg).
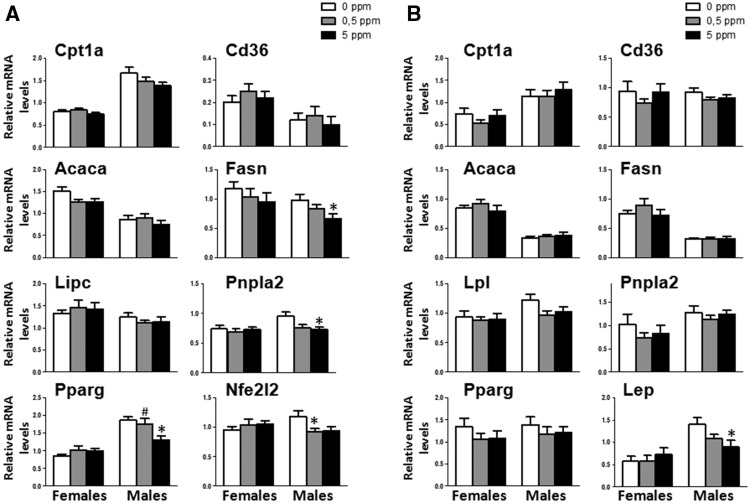
MeHg Exposure Induces a Deregulation of Genes That Control Hepatic Lipid Metabolism in Males
Expression levels of several genes associated with lipid metabolism was analyzed in adipose tissue (Figure 6B). This included adipogenic genes (peroxisome proliferator-activated receptor gamma [Pparg]), lipogenic genes (acetyl-Coenzyme A carboxylase alpha [Acaca], and fatty acid synthase Fasn), lipolytic genes (patatin-like phosphplipase domain containing 2 (Pnpla2, which codifies for the protein adipose triglyceride lipase [ATGL]) and lipoprotein lipase [Lpl]), genes that control β-oxidation (carnitine palmitoyltransferase 1a [Cpt1a]) and transport (Cd36, a fatty acid translocase). MeHg failed to significantly affect these genes in both females and males. In contrast, mRNA expression levels of leptin, a major adipokine that regulates food intake was unchanged in females, while in males MeHg significantly decreased its expression levels in a dose-dependent manner (F[2, 28] = 4.052, p = .028) (Figure 6B).
Figure 6.
A, Effect of MeHg on the expression of genes that control β-oxidation Cpt1a, transport Cd36, lipogenic genes Acaca, Fasn, and Pparg, lipolytic genes (Lipc and Pnpla2), and antioxidant gene nuclear Nfe2l2 in liver (n = 8–11). B, Effect of MeHg on the expression of genes Cpt1a, Cd36, Acaca, Fasn, Pparg, Lpl, Pnpla2, and leptin in visceral WAT (n = 10–11). *p < .05 versus control (0 ppm MeHg), #p < .05 versus high dose (5 ppm MeHg).
The expression of some of the above referenced genes was also carried out in liver. MeHg did not affect any of these genes in females. In contrast, in males treated with MeHg, the expression of Cpt1a, Cd36 and Acaca was indistinguishable from controls (Figure 6A). There were also no changes in the expression of hepatic lipase (Lipc), a lipolytic enzyme that, like LPL, hydrolyzes triglycerides and phospholipids present in circulating plasma proteins (Figure 6A). However, MeHg decreased Fasn and Pnpla2 mRNA in a dose-dependent manner (F[2, 28] = 3.547, p = .042 and F[2, 28] = 4.512, p = .02, respectively) (Figure 6A). Furthermore, only the high MeHg dose decreased PPARγ mRNA expression (F[2, 25] = 5.630, p = 0.01). The gene expression of nuclear factor, erythroid derived 2, like 2 (Nfe2l2), a regulator of induction of genes encoding antioxidant proteins and phase 2 detoxifying enzymes, was also evaluated in liver. Whereas MeHg treatment did not produce any change in females, in males treated with the lower MeHg dose, Nfe2l2 mRNA expression was significantly reduced (F[2, 29] = 3.471, p = .036, post hoc p = .05 for 0.5 ppm dose compare with control) (Figure 6A).
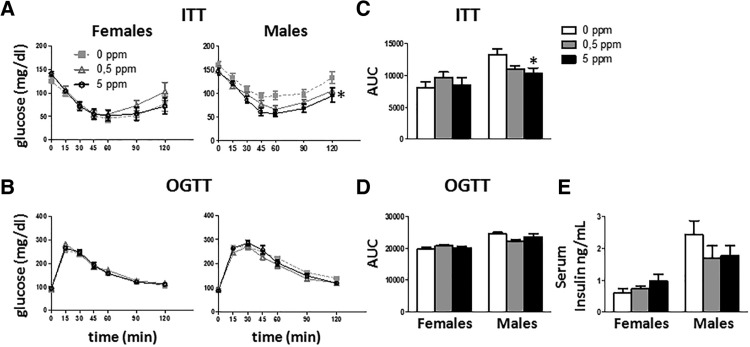
MeHg Exposure Has Minimal Effects in Glucose Homeostasis
As changes in body weight and fat content could affect the glucose homeostasis, ITT, and OGTT were performed on days 18 and 25, respectively. No effect of MeHg was detected in females (Figure 7), whereas males treated with MeHg showed greater dose-dependent insulin sensitivity (F[2, 23] = 5.073, p = .015) (Figure 7A). The analysis of the area under the curve (AUC) also showed that males treated with MeHg were more insulin sensitive when compared to females (F[2, 22] = 4.072, p = .031) (Figure 7C).
Figure 7.
ITT (A) and OGTT (B) in females (left) and males (right). For ITT mice were fasted for 4 h and then injected i.p. with 0.75 U/kg of insulin. For OGTT, mice were starved for 18 h followed by administration of an oral glucose dose (2 g/kg body weight). Tail blood glucose was measured at different times (n = 10–11 animals per group). C, AUC for ITT (n = 10–11 animals per group). D, AUC for OGTT (n = 10–11 animals per group). E) Insulin serum levels (n = 10–11 animals per group). *p < .05 versus control (0 ppm MeHg).
In addition, MeHg did not affect insulin serum levels in either females or males (Figure 7E).
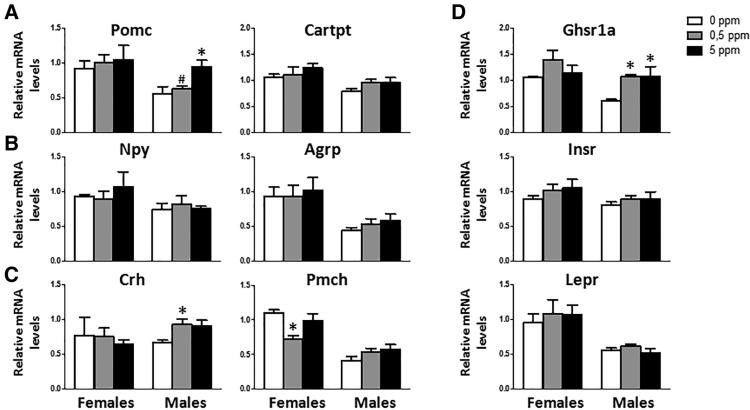
MeHg Exposure Induces an Anorexigenic Gene Expression Profile in Males
In the cellular model (GT1-7 cells culture) MeHg altered the expression of several hypothalamic neuropeptides that regulate energy homeostasis (Figure 1); thus in the animals exposed to MeHg in drinking water, hypothalamic expression of several of these genes was assessed. Males were more sensitive to MeHg than females (Figure 8). MeHg failed to affect the expression of the orexigenic peptides Agrp and Npy, or the expression of the CART prepropeptide (Cartpt), precursor of the anorexigenic peptide CART, in both males and females (Figs. 8A and 8B). However, Pomc mRNA levels (another anorexigenic neuropeptide) were significantly increased in males treated with 5 ppm MeHg (F[2, 8] = 7.433, p = .015) (Figure 8A), but not in females. Gene expression levels of corticotrophin-released hormone (Crh) and promelanin-concentrating hormone (Pmch) were also analyzed. In males the lower dose of MeHg (0.5 ppm) increased the expression of Crh (F[2, 9] = 6.389, p = .019) (Figure 8C), but no effect was observed at the higher dose. In females, although no effects were observed in Crh, a decrease in Pmch was noted upon treatment with 0.5 ppm MeHg (F[2, 9] = 5.376, p = .029) (Figure 8C). Males did not show any change in the expression of Pmch mRNA after MeHg exposure (Figure 8C).
Figure 8.
MeHg’s effect on hypothalamic mRNA expression of anorexigenic peptides Pomc and Cartpt (A); orexigenic neuropeptides Npy and Agrp (B), Crh and Pmch (C) and the receptors Ghsr1a, Insr, and Lepr (D) (n = 3–5 animals per group), *p at least < .05 versus control (0 ppm MeHg), #p < .05 versus high dose (5 ppm MeHg).
Gene expression of Ghsr1a, Insr, and Lepr were also studied in this model. MeHg did not alter gene expression levels of these receptors in females (Figure 8D). In males, MeHg only affected Gshr1a mRNA, with both doses (0.5 and 5 ppm) causing an increase on gene expression of this receptor (F[2, 7] = 6.685, p = .024) (Figure 8D).
DISCUSSION
Loss of body weight is one of the early symptoms observed after MeHg poisoning in adults (Berthoud et al., 1976; Ralston and Raymond, 2010; Schroeder and Mitchener, 1975). Body weight is, at least in part, regulated by the hypothalamus (Morton et al., 2006; Schwartz et al., 2000) via secretion of orexigenic/anabolic peptides, such as NPY and AgRP, and anorexigenic/catabolic substances, such as POMC and CART (Elmquist et al., 1998). Thus, here we tested hypothesis that MeHg affects the expression of these neuropeptides, underlying an important mechanism of its neurotoxicity.
To test this hypothesis, we treated for 6 h immortalized hypothalamic neuronal cell line GT 1-7 (GT 1-7 cells) (Mellon et al., 1990) with various concentrations (0, 0.5, 1, or 5 µM) of MeHg. MeHg increased the expression of Ghsr1a mRNA, the receptor for ghrelin, an orexigenic/anabolic hormone (Asakawa et al., 2003; Kamegai et al., 2001) and decreased insulin receptor mRNA expression, the latter signaling to decrease body weight (Bruning et al., 2000; Woods et al., 1979). This suggests that mechanisms to increase food intake and anabolism might be activated in response to MeHg to compensate for the decreased body weight. Surprisingly, the high MeHg concentration (5 µM) increased not only Pomc mRNA, but also Agrp mRNA expression. POMC and AgRP play opposing roles in regulating energy homeostasis and body weight in vivo, inhibiting and stimulating, respectively, the melanocortin 4-receptor (Bagnol et al., 1999). Frequently, these peptides are regulated in opposite directions, such as in fasting situations (Korner et al., 2001). Thus, the results obtained in vitro suggest that MeHg might affect the expression of these neuropeptides in other cells that produce them. In mammals, these neuropeptides are produced in different neuronal populations. MeHg might impair the regulation of body weight, by acting on one or more of the cellular populations that regulate this response in vivo.
As body weight control is a complex process regulated by CNS and implies the participation of other tissues and crosstalk between them; thus, we decided to study whether MeHg affects the regulation of body weight and energy homeostasis in vivo. Thus, to understand if these mechanisms are also altered in vivo, we treated male and female mice with 0, 0.5, or 5 ppm MeHg via drinking water. The treatment did not affect water consumption, neither in males nor females. Animals treated with MeHg in drinking water accumulated mercury in a dose-dependent manner. Although several authors reported that MeHg decreased body weight (Berthoud et al., 1976; de Freitas et al., 2009; Li et al., 2014; Suzuki, 1971), in our experiment, after 30 days of treatment no changes were detected in this parameter, neither in males nor females. These seemingly contradictory results might be explained by differences in the administration methods, doses and strain used in the various studies. It is also possible that the diet per se was effective in ameliorating the effect of MeHg. Berthoud et al. (1976) reported that when mice were fed a milk liquid diet, body weight decreased in the MeHg treated animals, but this effect was absent in mice of the same strain, sex, and age maintained on a chow diet (Berthoud et al., 1976). Moreover, a recovery in survival in chow-fed animals was noted, as well as upon a switch from liquid diet to a chow diet. Therefore, it is possible that we failed to detect changes in body weight because the diet itself has a protective effect. A protective element in the diet might be selenium, which is abundantly present in rodent chow (Kozul et al., 2008). Moreover, several studies have shown a protective role of selenium against MeHg toxicity, ameliorating body weight loss (Li et al., 2014; Ralston et al., 2008; Sakamoto et al., 2013; Stillings, 1974). It was also noted that mice strains have differential susceptibility to MeHg (Yasutake and Hirayama, 1988). Therefore, we may not discard the possibility that the C57BL/6J strain may be more resistant to MeHg toxicity, requiring longer exposures to detect changes in body weight. Analysis of the selenium content and a longer exposure period to MeHg might help in clarifying this point.
Despite the absence of changes in total body weight, there were several differences in tissue weights after 30 days of MeHg treatment. MeHg exerted sex-specific effects. Whereas female tissue weights were indistinguishable from controls, males treated with MeHg showed a decrease in fat depots (visceral and subcutaneous WAT) and BAT, absent changes in liver weights. The effect of mercury on adipose tissue was described previously in a high fat diet-induced obesity model by Kawakami and coworkers (Kawakami et al., 2012), where after subcutaneous administration of inorganic mercury the size of adipocytes decreased. As no changes in total body weight were observed, and there was a diminution in fat depot weights, changes must occur in other tissues to compensate for the decrease observed in adipose tissue. As no alterations in liver weights were detected, it is likely that MeHg induced a catabolic state that mobilizes the fats from adipose tissue to lean mass for its consumption.
Next, we posited that the decrease in fat depots size might affect lipid levels in serum. As the effect of MeHg in serum triglycerides is controversial, and seems to be dependent on experimental model, administration method and dose (Jin et al., 2009; Moreira et al., 2012; Silva de Paula et al., 2016), we analyzed triglycerides and free fatty acid levels in serum. MeHg had no significant effects neither on triglyceride nor free fatty acids serum levels in both females and males. Moreover, in males, hepatic triglyceride content was decreased, despite the fact that no changes in total liver tissue weight were observed. This decrease in triglyceride accumulation in the liver might reflect an increase in the secretion ratio or in fatty acid oxidation, or a decrease in lipid synthesis (Willecke et al., 2015), indicating that catabolic process are activated; hence, MeHg might be acting at a central level activating signaling pathways to increase the energy expenditure, as well as at peripheral levels to affect lipid clearance. To clarify this point, genes associated with lipid metabolism were analyzed. In liver, PPARϒ is involved in regulation of lipid homeostasis stimulating lipogenesis (Kersten, 2001). Its expression is elevated in murine models of hepatic steatosis (Gavrilova et al., 2003; Matsusue et al., 2008). In our model, after 30 days of MeHg exposure (5 ppm in drinking water) the expression of hepatic PPARϒ mRNA was significantly reduced in males, indicating a possible decrease in lipogenesis. Consistent with this observation, expression of Fasn mRNA, a gene that regulates lipogenesis, was also decreased in males treated with MeHg. Thus, the observed decrease in triglyceride hepatic content in males treated with high dose of MeHg might be due to decreased expression of PPARϒ and Fasn, reflective of a decrease in lipogenesis.
Fat accumulation is the result of a subtle balance between lipogenesis (fat synthesis) and lipolysis/fatty acid oxidation (fat breakdown) (Kersten, 2001; Saponaro et al., 2015). Consequently, the decrease in triglyceride hepatic content might be due a decrease in lipogenesis as well as changes in lipolysis and/or beta-oxidation of fatty acids. CPT1a is a key enzyme of beta-oxidation mediating the transport of fatty acids across the mitochondrial membrane for their beta-oxidation. After 30 days of MeHg exposure via drinking water, no significant effect on the expression of CPT1a mRNA was noted. However, we cannot rule out the possibility that changes in protein expression or activity are not reflected by the lack of change in mRNA levels. Additionally, we also measured Pnpla2 gene expression, which codifies for the protein ATGL, a key enzyme in lipolysis that catalyzes the first steps in triglyceride hydrolysis. Surprisingly, MeHg reduced the expression of Pnpla2 mRNA. This decrease might be explained by a direct effect or by an indirect effect of MeHg caused by decreased expression of PPARϒ. Cumulative evidence has suggested that PPARϒ not only regulates adipogenesis and lipogenesis, but also affects lipolysis, thus regulating the balance between fat mobilization and fat deposition, mainly in adipose tissues, but also in liver (Jin et al., 2014; Rodriguez-Cuenca et al., 2012). Combined, the changes observed in the gene expression and the accumulation of triglycerides suggest that MeHg deregulates lipid metabolism.
In addition, the expression of genes related to lipid metabolism was studied in visceral adipose tissue. A study in high fat diet obese mice treated with inorganic Hg suggested that decrease in adipose tissue weight after Hg treatment is the result of downregulation of Cd36 mRNA, which encodes a lipoprotein receptor/fatty acid transporter. Moreover, Cd36 deletion impairs fatty acids uptake in adipose tissue (Coburn et al., 2000). This effect is corroborated in our model, as we noted that adipose tissue weight was decreased by MeHg exposure. Accordingly, we analyzed if MeHg affected the expression of Cd36 mRNA. No significant effects were found in either females or males, suggesting that the fatty acid transport is not impaired by MeHg exposure. Next, we analyzed several lipogenic and lipolytic genes. No changes in mRNA expression of these genes were detected. As we failed to detect changes in genes related to lipid metabolism, next, we analyzed if MeHg affected adipogenesis. First, we analyzed PPARϒ, which in adipose tissue plays a key role in regulating adipogenesis. PPARϒ gene expression was not affected by MeHg. Surprisingly, MeHg had no effect on the expression of genes related to adipogenesis, transport or lipid metabolism, although males treated with MeHg showed decreased weight of fat depots. Despite the lack of changes in the expression of these genes, we may not rule out the possibility that MeHg treatment caused changes in protein expression or in the activity of the enzymes encoded by these genes. Thus, more studies are necessary to clarify this question.
Leptin is a hormone secreted mainly by adipocytes and its main role is to decrease food intake and increase energy expenditure via mechanisms mediated by binding to its receptor in hypothalamus (Zhang et al., 1994). Whereas MeHg treatment decreased the expression of leptin mRNA in a dose-dependent manner in males, it had no effect in females. It is known that leptin mRNA and serum levels directly correlate with adipose tissue weight (Lonnqvist et al., 1997). Thus, it is possible that the decrease in leptin mRNA expression might be the result of decreased fat depots mass caused by the increase in the MeHg-induced anorexigenic and catabolic pathways in the hypothalamus.
Furthermore, it is known that MeHg induces apoptosis and impairs insulin secretion in pancreatic β-cell-derived HIT—T15 and in isolated mouse pancreatic islets (Chen et al., 2006a). MeHg also increases glucose plasma levels and glucose intolerance in IRC male mice treated for 4 weeks with an orally administrated dose of 20 µg/kg/day during 4 weeks (Chen et al., 2006b). In our study, MeHg had minimal effects on glucose homeostasis. No effects on glucose tolerance after 25 days of exposure to MeHg were detected. Consistently, there were no changes in serum insulin levels. Moreover, males treated with MeHg were more insulin sensitive in a dose-dependent way. This increase in insulin sensitivity might be explained by the decrease in fat depots content. It is known that insulin sensitivity correlates with adipose tissue, thus, a decrease in fat depot accumulation improves insulin sensitivity (Goodpaster et al., 1999). A prolonged exposure to MeHg might be necessary to better characterize the detrimental effects of MeHg on the pancreas as previously observed by others (Chen et al., 2006a).
MeHg also affected the expression of hypothalamic factors that regulate body weight and energy balance. In males, after 30 days of exposure to MeHg in drinking water both the high (5 ppm) and low dose (0.5 ppm) produced changes that signal to decrease body weight, increasing the anorexigenic/catabolic peptides Pomc mRNA and Crh mRNA, respectively. In females, MeHg also activated mechanisms associated with decreased body weight. Whereas the high dose of MeHg did not have any effects, the low dose decreased the expression of Pmch mRNA, an orexigenic/anabolic neuropeptide. Furthermore, MeHg increased the expression of Ghsr1a mRNA only in males, suggesting mechanisms that compensate for the increased anorexigenic effect might be activated. However, MeHg might be acting also at the peripheral level, decreasing the circulating levels of ghrelin, and the increase in the expression of Ghsr1a mRNA in the hypothalamus might represent a compensatory response to maintain normal function. The analysis of ghrelin production in the stomach and ghrelin serum levels could help to clarify this point.
We show that MeHg is able to increase the expression of Pomc mRNA in vitro (GT 1-7 cell line) and in vivo (C57Bl/6J male mice) models. It is also established that MeHg potently increases ROS formation in multiple experimental models: in vivo studies showed ROS increase after intraperitoneal administration of MeHg in the cerebellum in both mice and rats; in vitro models, such as astrocytes from neonatal rat brains or in cellular cultures as GT 1-7 cells treated with MeHg corroborate increased ROS in a dose-dependent manner (Aschner et al., 2007; Sarafian et al., 1994). In the last decades several studies (Andrews et al., 2008; Drougard et al., 2015) have shown that ROS generation is not only a by-product of substrate oxidation, but rather, plays an important role in regulating neuronal responses. Hence, in the hypothalamus ROS production has a critical role in the regulation of the energy balance. Thus, during a positive energy balance scenario, glucose levels are increased. In POMC neurons, which use glucose as main source of fuel, glycolysis is activated and the production of free radicals increases, POMC neurons are firing at higher level and ROS accumulates in these cells (Andrews et al., 2008). In NPY/AgRP neurons, which use free fatty acids as main subtract, in the presence of glucose, glycolysis is activated and β-oxidation is inhibited, reducing NPY/AgRP neuronal activity. Conversely, during negative energy balance (like in fasting or before meals) ghrelin activate NPY/AgRP neurons increasing firing and buffering ROS production via UCP2 activation, whereas in POMC neurons ROS levels are reduced and the neurons remain inactive. Accordingly, it appears that the activation of NPY/AgRP neurons is mediated by a decrease in ROS production, whereas POMC neuron activation seems to be driven by ROS, inhibiting food intake and decreasing energy expenditure (Drougard et al., 2015). Therefore, MeHg might be activating POMC neurons via mechanism/s that involve ROS production.
After 30 days of MeHg exposure in drinking water, males were more affected by MeHg than females. The reasons for this sexual-dimorphic response are unclear. Several studies show differences in mercury susceptibility and accumulation between genders in different species (Bjorklund et al., 2007; Gimenez-Llort et al., 2001; Hirayama et al., 1987; Thomas et al., 1986). One reasonable explanation is sexual hormones. In castrated male mice exposed to MeHg the differences with females disappeared, whereas females exposed to MeHg increased urine mercury secretion when are injected with testosterone (Hirayama et al., 1987; Tanaka et al., 1992). In addition, it has been reported that estrogens have antioxidative properties, buffering ROS production (Malagutti et al., 2009). In our study males were more affected by MeHg than females even though they accumulate less mercury.
In conclusion, MeHg effects on body weight and metabolism might reflect peripheral as well as central alterations in the regulation of energy homeostasis. The present study demonstrates, for the first time, that MeHg is able to induce an anorexigenic/catabolic response in the hypothalamus in a dose- and sex-dependent manner.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institute of Environmental Health Sciences (NIEHS) (R01 ES10563, R01 ES020852).
Supplementary Material
REFERENCES
- Andrews Z. B., Liu Z. W., Walllingford N., Erion D. M., Borok E., Friedman J. M., Tschop M. H., Shanabrough M., Cline G., Shulman G. I. (2008). UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature 454, 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A., Inui A., Kaga T., Katsuura G., Fujimiya M., Fujino M. A., Kasuga M. (2003). Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52, 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. (2012). Considerations on methylmercury (MeHg) treatments in in vitro studies. Neurotoxicology 33, 512–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M., Clarkson T. W. (1988). Uptake of methylmercury in the rat brain: Effects of amino acids. Brain Res. 462, 31–39. [DOI] [PubMed] [Google Scholar]
- Aschner M., Syversen T., Souza D. O., Rocha J. B., Farina M. (2007). Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz. J. Med. Biol. Res. 40, 285–291. [DOI] [PubMed] [Google Scholar]
- Bagnol D., Lu X. Y., Kaelin C. B., Day H. E., Ollmann M., Gantz I., Akil H., Barsh G. S., Watson S. J. (1999). Anatomy of an endogenous antagonist: Relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. 19, RC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H. R., Garman R. H., Weiss B. (1976). Food intake, body weight, and brain histopathology in mice following chronic methylmercury treatment. Toxicol. Appl. Pharmacol. 36, 19–30. [DOI] [PubMed] [Google Scholar]
- Bjorklund O., Kahlstrom J., Salmi P., Ogren S. O., Vahter M., Chen J. F., Fredholm B. B., Dare E. (2007). The effects of methylmercury on motor activity are sex- and age-dependent, and modulated by genetic deletion of adenosine receptors and caffeine administration. Toxicology 241, 119–133. [DOI] [PubMed] [Google Scholar]
- Bruning J. C., Gautam D., Burks D. J., Gillette J., Schubert M., Orban P. C., Klein R., Krone W., Muller-Wieland D., Kahn C. R. (2000). Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125. [DOI] [PubMed] [Google Scholar]
- Castoldi A. F., Coccini T., Ceccatelli S., Manzo L. (2001). Neurotoxicity and molecular effects of methylmercury. Brain Res. Bull. 55, 197–203. [DOI] [PubMed] [Google Scholar]
- Coburn C. T., Knapp F. F. Jr., Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. (2000). Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529. [DOI] [PubMed] [Google Scholar]
- Chen Y. W., Huang C. F., Tsai K. S., Yang R. S., Yen C. C., Yang C. Y., Lin-Shiau S. Y., Liu S. H. (2006a). Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem. Res. Toxicol. 19, 1080–1085. [DOI] [PubMed] [Google Scholar]
- Chen Y. W., Huang C. F., Tsai K. S., Yang R. S., Yen C. C., Yang C. Y., Lin-Shiau S. Y., Liu S. H. (2006b). The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes 55, 1614–1624. [DOI] [PubMed] [Google Scholar]
- de Freitas A. S., Funck V. R., Rotta M. d S., Bohrer D., Mörschbächer V., Puntel R. L., Nogueira C. W., Farina M., Aschner M., Rocha J. B. T. (2009). Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Res. Bull. 79, 77–84. [DOI] [PubMed] [Google Scholar]
- Drougard A., Fournel A., Valet P., Knauf C. (2015). Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake. Front. Neurosci. 9, 56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist J. K., Maratos-Flier E., Saper C. B., Flier J. S. (1998). Unraveling the central nervous system pathways underlying responses to leptin. Nat. Neurosci. 1, 445–450. [DOI] [PubMed] [Google Scholar]
- Fujimura M., Usuki F., Sawada M., Takashima A. (2009). Methylmercury induces neuropathological changes with tau hyperphosphorylation mainly through the activation of the c-jun-N-terminal kinase pathway in the cerebral cortex, but not in the hippocampus of the mouse brain. Neurotoxicology 30, 1000–1007. [DOI] [PubMed] [Google Scholar]
- Gavrilova O., Haluzik M., Matsusue K., Cutson J. J., Johnson L., Dietz K. R., Nicol C. J., Vinson C., Gonzalez F. J., Reitman M. L. (2003). Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 278, 34268–34276. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L., Ahlbom E., Dare E., Vahter M., Ogren S., Ceccatelli S. (2001). Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: Age and gender-dependent effects. Environ. Toxicol. Pharmacol. 9, 61–70. [DOI] [PubMed] [Google Scholar]
- Glaser V., Nazari E. M., Muller Y. M., Feksa L., Wannmacher C. M., Rocha J. B., de Bem A. F., Farina M., Latini A. (2010). Effects of inorganic selenium administration in methylmercury-induced neurotoxicity in mouse cerebral cortex. Int. J. Dev. Neurosci. 28, 631–637. [DOI] [PubMed] [Google Scholar]
- Goodpaster B. H., Kelley D. E., Wing R. R., Meier A., Thaete F. L. (1999). Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48, 839–847. [DOI] [PubMed] [Google Scholar]
- Hirayama K., Yasutake A., Inoue M. (1987). Effect of sex hormones on the fate of methylmercury and on glutathione metabolism in mice. Biochem. Pharmacol. 36, 1919–1924. [DOI] [PubMed] [Google Scholar]
- Hussain S. S., Bloom S. R. (2013). The regulation of food intake by the gut-brain axis: Implications for obesity. Int J Obes. (Lond) 37, 625–633. [DOI] [PubMed] [Google Scholar]
- Jin D., Sun J., Huang J., He Y., Yu A., Yu X., Yang Z. (2014). TNF-alpha reduces g0s2 expression and stimulates lipolysis through PPAR-gamma inhibition in 3T3-L1 adipocytes. Cytokine 69, 196–205. [DOI] [PubMed] [Google Scholar]
- Jin X., Lok E., Caldwell D., Mueller R., Kapal K., Liston V., Kubow S., Chan H. M., Mehta R. (2009). Dietary fats altered nephrotoxicity profile of methylmercury in rats. J. Appl. Toxicol 29, 126–140. [DOI] [PubMed] [Google Scholar]
- Jouihan H. (2012). Measurament of liver triglyceride content. Bio-Protocol. 2, e223. [Google Scholar]
- Kamegai J., Tamura H., Shimizu T., Ishii S., Sugihara H., Wakabayashi I. (2001). Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 50, 2438–2443. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Hanao N., Nishiyama K., Kadota Y., Inoue M., Sato M., Suzuki S. (2012). Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol. Appl. Pharmacol. 258, 32–42. [DOI] [PubMed] [Google Scholar]
- Kersten S. (2001). Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J., Savontaus E., Chua S. C. Jr., Leibel R. L., Wardlaw S. L. (2001). Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J. Neuroendocrinol. 13, 959–966. [DOI] [PubMed] [Google Scholar]
- Kozul C. D., Nomikos A. P., Hampton T. H., Warnke L. A., Gosse J. A., Davey J. C., Thorpe J. E., Jackson B. P., Ihnat M. A., Hamilton J. W. (2008). Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem. Biol. Interact. 173, 129–140. [DOI] [PubMed] [Google Scholar]
- Krude H., Biebermann H., Luck W., Horn R., Brabant G., Gruters A. (1998). Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, 155–157. [DOI] [PubMed] [Google Scholar]
- Lambert P. D., Couceyro P. R., McGirr K. M., Vechia S. E. D., Smith Y., Kuhar M. J. (1998). CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse 29, 293–298. [DOI] [PubMed] [Google Scholar]
- Li X., Yin D., Yin J., Chen Q., Wang R. (2014). Dietary selenium protect against redox-mediated immune suppression induced by methylmercury exposure. Food Chem. Toxicol. 72, 169–177. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lohren H., Bornhorst J., Galla H.J., Schwerdtle T. (2015) The blood-cerebrospinal fluid barrier–first evidence for an active transport of organic mercury compounds out of the brain. Metallomics. 7, 1420–1430. [DOI] [PubMed] [Google Scholar]
- Lonnqvist F., Nordfors L., Jansson M., Thorne A., Schalling M., Arner P. (1997). Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J. Clin. Invest. 99, 2398–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magos L. (1982). Neurotoxicity, anorexia and the preferential choice of antidote in methylmercury intoxicated rats. Neurobehav Toxicol Teratol 4, 643–646. [PubMed] [Google Scholar]
- Malagutti K. S., da Silva A. P., Braga H. C., Mitozo P. A., Soares Dos Santos A. R., Dafre A. L., de Bem A. F., Farina M. (2009). 17beta-estradiol decreases methylmercury-induced neurotoxicity in male mice. Environ. Toxicol. Pharmacol. 27, 293–297. [DOI] [PubMed] [Google Scholar]
- Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S., Gonzalez F. J. (2008). Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 7, 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P. L., Windle J. J., Goldsmith P. C., Padula C. A., Roberts J. L., Weiner R. I. (1990). Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5, 1–10. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Nakanishi H., Moriguchi S., Fukuyama N., Eto K., Wakamiya J., Murao K., Arimura K., Osame M. (2001). Involvement of enhanced sensitivity of N-methyl-D-aspartate receptors in vulnerability of developing cortical neurons to methylmercury neurotoxicity. Brain Res. 901, 252–258. [DOI] [PubMed] [Google Scholar]
- Moreira E. L., de Oliveira J., Dutra M. F., Santos D. B., Goncalves C. A., Goldfeder E. M., de Bem A. F., Prediger R. D., Aschner M., Farina M. (2012). Does methylmercury-induced hypercholesterolemia play a causal role in its neurotoxicity and cardiovascular disease? Toxicol Sci. 130, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton G. J., Cummings D. E., Baskin D. G., Barsh G. S., Schwartz M. W. (2006). Central nervous system control of food intake and body weight. Nature 443, 289–295. [DOI] [PubMed] [Google Scholar]
- Ralston N. V., Ralston C. R., Blackwell J. L. 3rd, Raymond L. J. (2008). Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 29, 802–811. [DOI] [PubMed] [Google Scholar]
- Ralston N. V., Raymond L. J. (2010). Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 278, 112–123. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S., Carobbio S., Velagapudi V. R., Barbarroja N., Moreno-Navarrete J. M., Tinahones F. J., Fernandez-Real J. M., Oresic M., Vidal-Puig A. (2012). Peroxisome proliferator-activated receptor gamma-dependent regulation of lipolytic nodes and metabolic flexibility. Mol. Cell Biol. 32, 1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M., Yasutake A., Kakita A., Ryufuku M., Chan H. M., Yamamoto M., Oumi S., Kobayashi S., Watanabe C. (2013). Selenomethionine protects against neuronal degeneration by methylmercury in the developing rat cerebrum. Environ. Sci. Technol. 47, 2862–2868. [DOI] [PubMed] [Google Scholar]
- Saponaro C., Gaggini M., Carli F., Gastaldelli A. (2015). The subtle balance between lipolysis and lipogenesis: A critical point in metabolic homeostasis. Nutrients 7, 9453–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian T. A., Vartavarian L., Kane D. J., Bredesen D. E., Verity M. A. (1994). bcl-2 expression decreases methyl mercury-induced free-radical generation and cell killing in a neural cell line. Toxicol. Lett. 74, 149–155. [DOI] [PubMed] [Google Scholar]
- Schroeder H. A., Mitchener M. (1975). Life-term effects of mercury, methyl mercury, and nine other trace metals on mice. J Nutr 105, 452–458. [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Woods S. C., Porte D. Jr., Seeley R. J., Baskin D. G. (2000). Central nervous system control of food intake. Nature 404, 661–671. [DOI] [PubMed] [Google Scholar]
- Shi J. Z., Kang F., Wu Q., Lu Y. F., Liu J., Kang Y. J. (2011). Nephrotoxicity of mercuric chloride, methylmercury and cinnabar-containing Zhu-Sha-An-Shen-Wan in rats. Toxicol. Lett. 200, 194–200. [DOI] [PubMed] [Google Scholar]
- Shutter J. R., Graham M., Kinsey A. C., Scully S., Luthy R., Stark K. L. (1997). Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 11, 593–602. [DOI] [PubMed] [Google Scholar]
- Silva de Paula E., Carneiro M. F., Grotto D., Hernandes L. C., Antunes L. M., Barbosa F. Jr. (2016). Protective effects of niacin against methylmercury-induced genotoxicity and alterations in antioxidant status in rats. J. Toxicol. Environ. Health A 79, 174–183. [DOI] [PubMed] [Google Scholar]
- Stanley B. G., Kyrkouli S. E., Lampert S., Leibowitz S. F. (1986). Neuropeptide Y chronically injected into the hypothalamus: A powerful neurochemical inducer of hyperphagia and obesity. Peptides 7, 1189–1192. [DOI] [PubMed] [Google Scholar]
- Stillings B. R., Lagally H., Bauersfeld P., Soares J. (1974). Effect of cysteine, selenium and fish protein on the toxicity and metabolism of methylmercury in rats. Toxicol. Appl. Pharmacol. 30, 243–254. [Google Scholar]
- Suzuki T., Miyama T. M. (1971). Neurological symptoms and mercury concetration in the brain of mice fed with methylmercury salt. Industrial Health 9, 51–58. [Google Scholar]
- Syversen T., Kaur P. (2012). The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 26, 215–226. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Naganuma A., Miura N., Imura N. (1992). Role of testosterone in gamma-glutamyltranspeptidase-dependent renal methylmercury uptake in mice. Toxicol. Appl. Pharmacol. 112, 58–63. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Fisher H. L., Sumler M. R., Marcus A. H., Mushak P., Hall L. L. (1986). Sexual differences in the distribution and retention of organic and inorganic mercury in methyl mercury-treated rats. Environ. Res. 41, 219–234. [DOI] [PubMed] [Google Scholar]
- Willecke F., Scerbo D., Nagareddy P., Obunike J. C., Barrett T. J., Abdillahi M. L., Trent C. M., Huggins L. A., Fisher E. A., Drosatos K. et al. , (2015). Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arterioscler. Thromb. Vasc. Biol. 35, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. C., Lotter E. C., McKay L. D., Porte D. Jr. (1979). Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282, 503–505. [DOI] [PubMed] [Google Scholar]
- Yasutake A., Hirayama K. (1988). Sex and strain differences of susceptibility to methylmercury toxicity in mice. Toxicology 51, 47–55. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.