Abstract
Chemicals used in unconventional oil and gas (UOG) operations have the potential to cause adverse biological effects, but this has not been thoroughly evaluated. A notable knowledge gap is their impact on development and function of the immune system. Herein, we report an investigation of whether developmental exposure to a mixture of chemicals associated with UOG operations affects the development and function of the immune system. We used a previously characterized mixture of 23 chemicals associated with UOG, and which was demonstrated to affect reproductive and developmental endpoints in mice. C57Bl/6 mice were maintained throughout pregnancy and during lactation on water containing two concentrations of this 23-chemical mixture, and the immune system of male and female adult offspring was assessed. We comprehensively examined the cellularity of primary and secondary immune organs, and used three different disease models to probe potential immune effects: house dust mite-induced allergic airway disease, influenza A virus infection, and experimental autoimmune encephalomyelitis (EAE). In all three disease models, developmental exposure altered frequencies of certain T cell sub-populations in female, but not male, offspring. Additionally, in the EAE model disease onset occurred earlier and was more severe in females. Our findings indicate that developmental exposure to this mixture had persistent immunological effects that differed by sex, and exacerbated responses in an experimental model of autoimmune encephalitis. These observations suggest that developmental exposure to complex mixtures of water contaminants, such as those derived from UOG operations, could contribute to immune dysregulation and disease later in life.
Keywords: water pollutants, immunotoxicity, hydrofracking, influenza, autoimmune, allergy
Unconventional oil and gas (UOG) extraction combines hydraulic fracturing with horizontal drilling, and has unlocked oil and gas reserves that, until recently, were inaccessible. In this process, millions of gallons of water containing proprietary mixtures of chemicals are injected underground under very high pressure. This fractures the shale or coal bed layer, releasing trapped natural gas and oil (Vengosh et al., 2014; Wiseman, 2009). Over 1000 chemicals have been reported by the industry to be utilized in UOG operations, and over 200 of these have been independently measured in UOG wastewater, as well as surface and groundwater in UOG drilling-dense regions (Elsner and Hoelzer, 2016; United States Environmental Protection Agency, 2015; Vengosh et al., 2014; Waxman et al., 2011; Webb et al., 2014). Little is known about the potential health effects of exposure to water that is inadvertently contaminated with chemicals used in UOG. The scant nature of information hampers the ability to make informed decisions in order to reduce potential effects on human health and prevent unintentional deleterious impacts on complex ecosystems that sustain local economies and our natural environment.
Although research is limited, several epidemiological studies have reported adverse health metrics associated with proximity to UOG activity. A recent systematic review critically evaluated the levels of confidence and evidence for impacts of UOG operations on human reproduction, and found moderate evidence for an increased risk of preterm birth, miscarriage, birth defects, decreased semen quality, and prostate cancer (Balise et al., 2016). Other studies of health outcomes associated with exposures defined by proximity to UOG activities have reported both positive and null associations for preterm birth, low birth weight, and small for gestational age births (Casey et al., 2016; Stacy et al., 2015). An evaluation of 124 842 birth records in Colorado revealed an association between maternal residential proximity to gas development operations and congenital heart and neural tube birth defects among infants (McKenzie et al., 2014). In addition to developmental health outcomes, a positive correlation between residential proximity to oil and gas wells and acute lymphocytic leukemia, but not non-Hodgkin lymphoma, was reported in a case-control study of children and young adults (McKenzie et al., 2017). Associations between UOG operations and asthma exacerbations (Rasmussen et al., 2016), and increases in self-reported upper respiratory symptoms have also been reported (McKenzie et al., 2014; Rabinowitz et al., 2015). Collectively, these reports suggest human health impacts, although there remains uncertainty about potential adverse health effects of UOG operations. Factors that contribute to this uncertainty include: (1) limited information about which diseases to study in humans (or animals) living in regions with UOG activity; (2) the need to identify specific water contaminants and estimate exposures that might result from UOG activities; and (3) lack of research on the effects of chemicals associated with UOG operations using validated experimental systems that model common human diseases.
One category of compounds that have been identified in water near sites with active UOG operations are endocrine disrupting chemicals (EDCs). EDCs are broadly defined as exogenous compounds that singly, or as mixtures, mimic or interfere with the normal actions of hormones (Kassotis et al., 2016 b; Maqbool et al., 2016; Vandenberg et al., 2012; Zoeller et al., 2012). A combination of in vitro and in vivo approaches recently revealed endocrine activity of 23 chemicals used in UOG extraction, and demonstrated antagonism of the estrogen, androgen, progesterone, glucocorticoid, and thyroid receptors in vitro (Kassotis et al., 2014, 2015). Maternal exposure of mice to an equimass mixture of these 23 chemicals negatively affected development of male and female reproductive organs, and reproductive parameters such as hormone concentrations, sperm quality, and ovarian follicle development in C57Bl/6 offspring (Kassotis et al., 2014, 2015, 2016a. EDCs can also affect other physiological systems, including the immune system (Boule and Lawrence, 2016; Kassotis et al., 2016b; Kuo et al., 2012; Maqbool et al., 2016; Vandenberg et al., 2012), and early life exposure to several EDCs cause persistent alterations in immune function (Boule and Lawerence, 2016). Yet, little is known about the effects of developmental exposure to chemicals associated with UOG on the development or function of the mammalian immune system.
In rodents as in humans, immune system ontogeny begins in the womb, but continues after birth (Ciau-Uitz et al., 2014). The immune system is critical for maintaining host defense against pathogens, whereas simultaneously self-regulating to avoid immune-mediated tissue damage, autoimmune diseases, and allergic reactions. This is orchestrated by complex and tightly regulated interactions involving many types of immune cells, all of which arise from hematopoietic stem cells (HSCs) and lineage-committed progenitors. Imbalances in immune function can result in diminished ability to fight infections, or can manifest increased hypersensitivities and autoimmune diseases. To establish whether developmental exposure to chemicals associated with UOG could affect the immune system of adult offspring, we studied the same chemical mixture that was previously shown to alter the reproductive organs of male and female mice (Kassotis et al., 2015, 2016a). We characterized the impact of early life exposure to this mixture on the development of primary and secondary immune organs, and compared the effects between male and female offspring. To determine whether this exposure affects functional properties of the adaptive immune system, we focused on T cell responses. CD8+ T cells are essential for clearing intracellular infections, including many caused by viruses (Tscharke et al., 2015). CD4+ T cells are important for defenses against extracellular pathogens, and drive pathogenesis of immune-mediated diseases, such as allergic airway inflammation and autoimmune diseases (Sun and Zhang, 2014). Depending on the signals they receive during activation, CD4+ T cells differentiate into conventional subsets, including Th1 cells, Th2 cells, and Th17 cells, (Hirahara and Nakayama, 2016; Yamane and Paul, 2013). Another sub-type of CD4+ T cells, called regulatory T cells or Tregs, play a critical role in dampening immune responses (Josefowicz et al., 2012). Changes to T cell populations provide a metric of adaptive immune function. Therefore, we determined the consequences of developmental exposure to a mixture of chemicals associated with UOG on male and female offspring using three established models of distinct human diseases that require T cells: allergic airway disease (induced using house dust mite [HDM] extract), infectious disease (influenza A virus, IAV), and autoimmune disease (experimental autoimmune encephalomyelitis, EAE).
MATERIALS AND METHODS
Chemical mixture preparation
Twenty-three chemicals (≥97% purity, Sigma Aldrich) were selected based on developmental effects on other physiological systems, and prior demonstration of endocrine activity via estrogen, progesterone, glucocorticoid, and/or thyroid receptors (Kassotis et al., 2015, 2016a. They are also among chemicals used in UOG operations and detected in surface and groundwater in UOG drilling-dense regions (Colborn et al., 2011; United States Environmental Protection Agency, 2015; Waxman et al., 2011). Stock solutions of chemicals were prepared in 100% ethanol (ThermoFisher Scientific, Waltham, MA), stored at −20°C, and used in experiments within 6 months of preparation.
Mice and developmental exposure
Adult (6-week old) male and female C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in pre-washed polysulfone microisolator cages under specific pathogen-free conditions on a 12 h light/dark cycle. Mice received a standard chow diet (LabDiet 5010, St. Louis, MO), and glass water bottles containing reverse osmosis-purified water were used. Mice were randomly paired, and checked daily for a vaginal plug, indicating pregnancy. On gestational day (GD) 0, dams were separated from sires and randomly placed into one of three groups: control, 0.1 µg/ml, or 1.0 µg/ml of the 23-chemical mixture (Figs. 1A and 1B). Specifically, the dams’ drinking water was spiked with an equimass mixture of 23 chemicals (Figure 1A) at a final concentration of 0.1 or 1.0 µg/ml of each constituent chemical (exposed groups), or with 0.2% ethanol (control group). These concentrations in the drinking water result in an estimated 30 and 300 µg/kg body weight/day to the dam, respectively (Kassotis et al., 2015, 2016a). The doses were chosen based on estimates of environmentally relevant oral exposures, such that the two concentrations are similar to levels detected in surface and groundwater in UOG production regions (Cozzarelli et al., 2017; DiGiulio and Jackson, 2016; Gross et al., 2013; Orem et al., 2017; United States Environmental Protection Agency, 2015). Dams remained on treated water from GD0 until pup weaning at postnatal day (PND) 21. Water consumption was monitored daily. The water and water bottles were changed weekly, with freshly prepared dilutions (Kassotis et al., 2015, 2016a). This reduces potential degradation or loss of VOCs to the bottle head-space, diminishing fluctuations in the concentration over time.
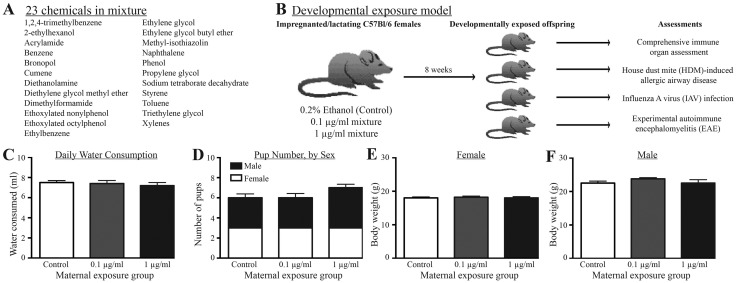
Figure 1.
Experimental design and average values for maternal and litter parameters. A, The mixture of 23 chemicals added, in equimass proportions, to the drinking water. B, Pregnant C57Bl/6 dams were placed on control water (0.2% ethanol) or water containing 0.1 or 1 μg/ml of the mixture on day of pregnancy (GD0), through weaning (PND21). There were 10 dams in each group. At maturity (6–8 weeks), offspring of separate dams from each treatment group were randomly assigned to one of four assessment groups for the indicated assessments: immune organ cellularity, HDM-induced airway disease, IAV infection, or EAE. Within each immunological assessment, there were at least 8 nonsibling males and 8 nonsibling females from each developmental exposure group. C, Average daily water consumption by dams. D, Average number of male and female offspring per litter according to treatment group. E, F, Average body weight of adult (8-week) female and male offspring by exposure group. Error bars represent SEM.
After weaning, pups were maintained on unspiked water until sacrifice. Time to parturition, pup number, sex, and body weight were recorded. No culling of litters was performed, and littermates were housed in same-sex groups. Other than determining sex, offspring were randomly assigned to each immunological assessment (Figure 1B). Eight to ten age-matched males, and eight to ten age-matched females from each of the three developmental exposure groups were used in the immunological assessments. Within each exposure group, offspring of the same sex were from different dams, and the age range of the offspring was uniformly distributed across the groups (6–10 weeks of age). All experiments were initiated in the morning.
All animal treatments and work with infectious agents were conducted with prior approval of Institutional Animal Care and Use Committee and Institutional Biosafety Committee of the University of Rochester. The University has accreditation through the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animals were treated humanely and with due consideration to alleviation of any distress and discomfort. All guidelines from the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals were followed in handling of vertebrate animals.
Collection and preparation of cells
Cells from mediastinal, peripheral (inguinal, axillary, and brachial), and cervical lymph nodes, thymus, spleen, or bone marrow were collected and processed into a single suspension as described previously (Reilly et al., 2015; Vorderstrasse et al., 2006). Erythrocytes were removed using an ammonium chloride lysing solution. The number of viable cells in each sample was determined using TC10 automated cell counter (Bio-Rad, Hercules, CA) or a hemocytometer and Trypan blue exclusion.
Analytical flow cytometry
Flow cytometry was used to identify and enumerate specific cell populations from offspring that were immunologically naïve, and from offspring that were used in the HDM, IAV, or EAE models. Isolated cells were incubated with previously determined optimal concentrations of fluorochrome-conjugated antibodies. Nonspecific staining was blocked by incubating cells with an anti-mouse CD16/32 mAb. For the work reported herein, the following antibodies against cell-surface antigens were used: CD3ε (clone 145-211), CD4 (clone RM4-5), CD8α (clone 53-6-7), CD11c (clone N418), CD11b (clone M1/70), CD19 (clone 1D3), CD25 (clone PC61.5), CD34 (clone RAM34), CD44 (clone IM7), CD62L (clone MEL-14), CD103 (clone 2E7), CD105 (clone ID4B), CD117 (cKit; clone 2B8), CD127 (clone A7R34), CD150 (clone TC15-12F12.2), F4/80 (clone BM8), gamma delta (γδ) TCR [clone GL3], Gr-1 (clone RB6-8C5), I-Ab (clone M5/114.15.2), NK1.1 (clone PK136), Sca-1 (clone D7), or a lineage antibody cocktail (CD3, CD11b, CD45R, Ly-G6 [Gr1], and Ter119). To identify virus-specific CD8+ T cells, allophycocyanin (APC)-labeled major histocompatibility (MHC) class I tetramers containing an immunodominant peptide epitope of HKx31 (nucleoprotein, DbNP366–375) were used. To identify CD4+ T cell subsets, after labeling with antibodies against CD4 and CD25, cells were fixed and permeabilized (Foxp3 Staining Kit, eBioscience, San Diego CA), and incubated with fluorochrome-conjugated antibodies against Foxp3 (clone FJK-16S), GATA3 (clone L50-823), RORγt (clone Q31-378), and TBet (clone 4IBO), as described previously (Boule et al., 2014). All antibodies were purchased from eBioscience (San Diego, CA) or BD Biosciences (San Jose, CA). Fluorescence minus one (FMO) controls were used to determine non-specific fluorescence and define gating parameters. Data were collected using an LSRII flow cytometer (BD Biosciences, San Jose, CA), and analyzed using the FlowJo software program (TreeStar, Ashland, OR).
House dust mite extract (HDM)-induced allergic airway disease
HDM (Dermatophagoides pteronyssinus) extract (lot #262538, Greer Laboratories, Lenoir, NC) was diluted in sterile PBS. Adult (6–8 weeks of age) female and male offspring from each developmental exposure group were sensitized and challenged by daily administration of 3 μg HDM intranasally (i.n.) for 10 days, which induces CD4+ T cell-dependent allergic airway disease (Knowlden et al., 2016). Forty-eight hours after the last HDM challenge, mice were euthanized, and T cells in the lung-draining mediastinal lymph nodes (MLN) were examined using flow cytometry. Also, bronchoalveolar lavage (BAL) was performed by instilling 0.75 mL PBS twice into the lungs using a Teflon cannula to collect immune cells in airways. BAL and MLN were collected from the same mice, and the BAL was collected first. Differential cell counts of BAL cells were performed after cytocentrifugation onto coded slides, and staining with Hema3 Staining Set (Fisher Scientific, Waltham, MA).
Influenza A virus infection
Male and female offspring (8–10 weeks of age) from each developmental exposure group were anesthetized by intraperitoneal (i.p.) injection of avertin (2,2,2-tribromoethanol), and infected (i.n.) with 120 hemagglutinating units (HAU) of IAV (HKx31; H3N2) diluted in sterile, endotoxin-tested PBS. The virus was prepared and titered as described previously (Warren et al., 2000). Morbidity and mortality were monitored daily, starting on the day of infection, and T cells were examined on the 9th day after infection using flow cytometry.
Induction of experimental autoimmune encephalomyelitis(EAE)
To determine whether developmental exposure to chemicals associated with UOG effects a T cell-dependent disease that mirrors aspects of a human autoimmune disease, we used EAE, which models multiple sclerosis (Mendel et al., 1995). Developmentally exposed adult offspring were immunized with an emulsion of myelin oligodendrocyte glycoprotein (MOG35-55), and disease progression was monitored every other day over a 6-week period (Robinson et al., 2014). Adult offspring (6–10 weeks of age) were immunized by subcutaneous injection with an emulsion of the MOG35–55 peptide (200 μg/mouse; AnaSpec, Freemont, CA) and complete Freund's adjuvant (4 mg/ml M. Tuberculosis; Becton Dickinson, Franklin Lakes, NJ) at day 0 (Stromnes and Goverman, 2006). Two doses of pertussis toxin (400 ng/mouse; List Biologicals, Campbell, CA) were given intraperitoneally: one on day 0 and the other on day 2 (Stromnes and Goverman, 2006). To identify and enumerate T cell subsets in the cervical lymph nodes 8–10 female mice and 8–10 male mice from each exposure group were sacrificed on days 7, 21, or 42 after immunization. There were no selection criteria used to determine which mice were sacrificed at a particular point in time after disease was initiated. Using 10 female and 10 male offspring per exposure group, disease progression was monitored and scored every other day for 42 days. Disease symptoms were scored using an established system: 0 = normal mouse, 1 = limp tail, 2 = limp tail and hind limb weakness, 3 = partial hind limb paralysis, 4 = complete hind limp paralysis, 5 = moribund (Stromnes and Goverman, 2006). During disease scoring, information on which exposure group the mice were in was not available (ie, disease scoring was performed in a blinded manner). At each point in time relative to administration of MOG peptide, offspring of the same sex were from a different treated dam.
Statistical analyses
The dam is defined as the statistical unit for all experiments. All offspring in each treatment group and at each point in time relative to immunological assessment were from a different treated dam. Data were analyzed using JMP software (SAS, Cary, NC). Differences between exposure group, sex, and, where applicable, time relative to immune challenge were evaluated using a two-way analysis of variance (ANOVA). Analyses included comparisons within sex across exposure groups, and across sex and exposure groups, using Tukey post-hoc tests, whereas comparisons within sex were analyzed using a Dunnett’s post-hoc test, with offspring of vehicle dams as the control group. Survival after infection was analyzed using a Mantel-Cox test. The onset of symptoms in mice with EAE was analyzed using a Kaplan Meier curve, and comparisons between treatment groups were performed using a Wilcoxon test. Differences were considered statistically significant when p-values were less than or equal to .05. Error bars on all graphs represent the standard error of the mean (SEM).
RESULTS
Developmental Exposure and Immune System Ontogeny
To determine whether developmental exposure to a mixture of water contaminants that have been associated with UOG has immunological consequences, we exposed pregnant dams to water containing an equimass mixture of 23 chemicals (Figs. 1A and 1B) at two concentrations (0.1 and 1 μg/ml), or to water spiked with the vehicle control (0.2% ethanol). Dams remained on the treated water until their pups were weaned. There was no difference in daily water consumption across the treatment groups (Figure 1C), and dam body weights were not different across the groups (Supplementary Table 1). Consistent with prior reports using this mixture (Kassotis et al., 2015), there was no difference in pregnancy success or the time to parturition (Supplementary Table 1), number of pups per litter, or sex ratio of pups among treatment groups (Figure 1D). At 8 weeks of age, the body weight of female and male offspring exposed to 0.1 or 1 μg/ml of the chemical mixture during development was not different than weights of sex- and age-matched offspring of vehicle control-treated dams (Figs. 1E and 1F).
The cellularity of primary and secondary lymphoid organs of male and female offspring that were developmentally exposed to this mixture was compared with age and sex-matched offspring of dams given vehicle control water. Within the same sex, there were no statistically significant differences in the total number of cells recovered from the thymus, spleen, or lymph nodes across the three treatment groups (Table 1). However, there were several differences in the percentage or number of several immune progenitor or lineage committed cell populations in the bone marrow, thymus, spleen, or lymph nodes (Tables 1 and 2). For example, in female offspring of dams given water containing the higher concentration of UOG mixture (1 µg/ml), there were one-third fewer bone marrow cells (Table 1). Yet, the percentage and number of HSCs in the bone marrow of female offspring was significantly elevated in these offspring. The mean percentage and number of HSCs in female offspring of dams given 0.1 µg/ml of the mixture was almost twice that female offspring of control dams, but these differences were not statistically significant. As another example, the percentage of granulocyte monocyte precursors (GMPs) was 1.7- to 1.9-fold higher than control in female offspring of dams exposed to the mixture (Table 2).
Table 1.
The Number of Immune Cells in Immunologically Naïve Female and Male Offspring
| Female 0.1 µg/ml versus Control |
Male 0.1 µg/ml versus Control |
F versus M |
Female 1 µg/ml versus Control |
Male 1 µg/ml versus Control |
F versus M |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 µg/ml | p-Valuea | Control | 0.1 µg/ml | p-Valuea | p-Valueb | 1 µg/ml | p-Valuea | 1 µg/ml | p-Valuea | p-Valueb | |
| Bone marrow | ||||||||||||
| Cell count | 12433333 ± 742930 | 11148333 ± 1153077 | .53 | 11850000 ± 640638 | 14412500 ± 781658 | .07 | .15 | 8305000 ± 150000 | .05* | 14050000 ± 819807 | .12 | .01* |
| HSC | 81.7 ± 30.3 | 123 ± 26 | .56 | 181 ± 47 | 233 ± 41 | .63 | .10 | 277 ± 40 | .02* | 232 ± 44 | .64 | .56 |
| LSK | 1681.2 ± 114.3 | 1681 ± 651 | .99 | 1617 ± 154 | 1746 ± 190 | .24 | .84 | 1249 ± 189 | .76 | 1993 ± 148 | .81 | .04* |
| LK | 37727 ± 3013 | 38353 ± 9465 | .99 | 40321 ± 1143 | 50790 ± 5188 | .09 | .27 | 33628 ± 192 | .89 | 50129 ± 2483 | .12 | .01* |
| MPP | 1441.3 ± 111.7 | 1335 ± 565 | .97 | 1253 ± 91 | 1207 ± 214 | .97 | .82 | 745 ± 185 | .45 | 1560 ± 146 | .33 | .03* |
| Lineage - | 334137 ± 40143 | 284293 ± 89951 | .81 | 313340 ± 23271 | 364988 ± 42847 | .48 | .41 | 305411 ± 16724 | .94 | 404781 ± 32400 | .15 | .12 |
| CLP | 4561 ± 1431 | 4516 ± 2171 | .99 | 4639 ± 446 | 4691 ± 778 | .99 | .93 | 156 ± 38 | .25 | 4616 ± 163 | .99 | <.0001* |
| GMP | 3229.2 ± 555.1 | 4890 ± 444 | .10 | 4417 ± 499 | 5646 ± 685 | .23 | .44 | 3727 ± 514 | .76 | 5258 ± 349 | .46 | .07 |
| Pre-GM | 8716.2 ± 691.5 | 8785 ± 2383 | .99 | 11272 ± 316 | 14981 ± 1787 | .09 | .09 | 5484 ± 265 | .39 | 14625 ± 931 | .13 | .003* |
| Pre-MegE | 15717 ± 1315 | 14403 ± 3406 | .89 | 14148 ± 331 | 17659 ± 1437 | .10 | .37 | 14119 ± 255 | .88 | 16469 ± 1344 | .31 | .31 |
| Thymus | ||||||||||||
| Cell count | 134700000 ± 15085534 | 7992833± 38156620 | .31 | 86075000 ± 6312702 | 84687500 ± 4441583 | .97 | .89 | 198750000 ± 33250000 | .29 | 77562500 ± 1852406 | .36 | .004* |
| DN1 | 1029398 ± 128817 | 629804 ± 229799 | .22 | 652118 ± 46528 | 633010 ± 37150 | .95 | .99 | 993675 ± 50325 | .99 | 593431 ± 62092 | .63 | .02* |
| DN2 | 154645 ± 17105 | 123131 ± 56585 | .78 | 82867 ± 4305 | 80256 ± 4563 | .95 | .41 | 150895 ± 41665 | .99 | 81100 ± 9931 | .98 | .08 |
| DN3 | 1684308 ± 190926 | 1055676 ± 502342 | .36 | 979296 ± 62343 | 968785 ± 21799 | .97 | .84 | 1995775 ± 324225 | .79 | 933129 ± 14914 | .63 | .01* |
| DN4 | 929283 ± 44022 | 1039598 ± 175690 | .81 | 927516 ± 118930 | 867171 ± 69782 | .88 | .35 | 1045175 ± 300425 | .83 | 812128 ± 111851 | .65 | .40 |
| DP | 64765250 ± 4519003 | 4214308± 21474537 | .47 | 43490400 ± 3354243 | 43503338 ± 5616368 | 1 | .95 | 79919750 ± 22160250 | .75 | 41521763 ± 2323515 | .92 | .05 |
| DP CD3+ | 37535 ± 4244 | 35116 ± 18488 | .98 | 94271 ± 24827 | 66288 ± 9805 | .45 | .17 | 35117.5 ± 13603 | .99 | 90209 ± 14613 | .98 | .08 |
| TCRβ+ | 6829968 ± 476592 | 6791068 ± 2675537 | .99 | 6917440 ± 462168 | 5960451 ± 41223 | .34 | .73 | 7544450 ± 1619550 | .95 | 5804218 ± 724325 | .25 | .30 |
| CD4+ | 5563755 ± 415844 | 4480870 ± 1974304 | .79 | 4891533 ± 349205 | 4651036 ± 137026 | .82 | .92 | 7347550 ± 1886050 | .62 | 4259121 ± 401819 | .31 | .08 |
| CD8+ | 680505 ± 55178 | 930392 ± 96601 | .16 | 885978 ± 206229 | 751826 ± 106954 | .75 | .29 | 533375 ± 185825 | .53 | 723828 ± 105159 | .67 | .38 |
| TCRγδ+ | 483158 ± 45652 | 313629 ± 138319 | .33 | 378986 ± 27842 | 377249 ± 23029 | .99 | .62 | 526650 ± 30150 | .93 | 395438 ± 69299 | .95 | .28 |
| Treg | 126705 ± 11419 | 120334 ± 25422 | .97 | 109801 ± 9453 | 103152 ± 7950 | .80 | .49 | 151058 ± 36863 | .69 | 88345 ± 7749 | .18 | .07 |
| Spleen | ||||||||||||
| Cell count | 86737500 ± 1371298 | 8221666± 31853497 | .98 | 8998750± 10669707 | 107612500 ± 8841907 | .32 | .06 | 76000000 ± 19500000 | .93 | 117512500 ± 6614390 | .10 | .42 |
| CD19+ | 42901875 ± 8244859 | 4150248± 17869820 | .99 | 4446851± 10596693 | 57843813 ± 5629245 | .38 | .36 | 42249750 ± 1 3999750 | .99 | 60730938 ± 9521355 | .26 | .17 |
| CD4+ | 9179363 ± 1756623 | 10686967 ± 4435512 | .91 | 11378904 ± 2552025 | 14578738 ± 865956 | .33 | .36 | 7227550 ± 1577550 | .88 | 15235463 ± 969390 | .22 | .01* |
| CD8+ | 6755044 ± 1108823 | 7373400 ± 2819035 | .96 | 7579081 ± 1212533 | 9894250 ± 747637 | .18 | .36 | 4506000 ± 1042550 | .68 | 9921128 ± 642899 | .17 | .01* |
| NK1.1+ | 1864925 ± 286698 | 1721660 ± 705751 | .97 | 2457671 ± 244963 | 2309921 ± 295240 | .86 | .43 | 1148275 ± 255575 | .55 | 2769471 ± 80402 | .54 | .001* |
| TCRγδ+ | 1404270 ± 224021 | 1332622 ± 564340 | .99 | 1378785 ± 208629 | 1592971 ± 114013 | .49 | .62 | 1039450 ± 288000 | .77 | 1663444 ± 65032 | .31 | .04* |
| Treg | 492888 ± 134031 | 572553 ± 240016 | .93 | 758630 ± 230462 | 933366 ± 142882 | .67 | .23 | 441275 ± 45775 | .97 | 995236 ± 64601 | .5 | .01* |
| F4/80+ | 2736355 ± 362139 | 2959867 ± 701954 | .94 | 2526675 ± 389486 | 3384780 ± 384762 | .22 | .59 | 3851600 ± 761050 | .38 | 3708730 ± 308728 | .08 | .84 |
| Gr-1+ | 4945213 ± 691402 | 5847227 ± 1601634 | .79 | 5473478 ± 1065955 | 7106785 ± 546481 | .26 | .44 | 5581725 ± 960025 | .91 | 7565911 ± 490051 | .14 | .10 |
| Gr-1+CD11b+ | 449216 ± 65317 | 889747 ± 275714 | .21 | 374870 ± 33306 | 469855 ± 33816 | .14 | .13 | 1457325 ± 242575 | .02* | 542254 ± 34726 | .01* | .004* |
| CD11c+MHCIIhi | 1196778 ± 203674 | 1127800 ± 425883 | .98 | 1103168 ± 222118 | 1405939 ± 137023 | .37 | .51 | 915300 ± 163850 | .78 | 1501859 ± 119692 | .21 | .04* |
| CD11b+DCs | 135978 ± 38691 | 146450 ± 46234 | .98 | 140680 ± 22079 | 180088 ± 4949 | .17 | .43 | 80218 ± 12418 | .61 | 196008 ± 13632 | .54 | .01* |
| CD103+DCs | 21814 ± 5846 | 29950 ± 12584 | .77 | 27152 ± 7706 | 27782 ± 9831 | .99 | .90 | 20668 ± 12758 | .99 | 40007 ± 9565 | .53 | .30 |
| Lymph nodes | ||||||||||||
| Cell count | 906250 ± 356587 | 1246333 ± 230860 | .88 | 1205250 ± 142966 | 1281250 ± 248510 | .95 | .92 | 1750500 ± 1279500 | .57 | 665000 ± 86920 | .12 | .24 |
| NK1.1+ | 339467 ± 152821 | 376573 ± 163194 | .99 | 324370 ± 20897 | 418592 ± 103462 | .48 | .81 | 726215 ± 510026 | .49 | 194269 ± 20803 | .28 | .18 |
| CD19+ | 184994 ± 89183 | 206919 ± 78508 | .99 | 195106 ± 16840 | 235362 ± 90192 | .82 | .83 | 428606 ± 319805 | .45 | 102250 ± 17897 | .41 | .17 |
| TCRγδ+ | 131612 ± 57008 | 166186 ± 59364 | .95 | 143302 ± 13848 | 195615 ± 78622 | .66 | .37 | 293009 ± 219062 | .46 | 74033 ± 13529 | .49 | .24 |
| CD4+ | 3744 ± 1433 | 4361 ± 1424 | .98 | 4831 ± 845 | 4884 ± 1376 | .99 | .83 | 8238 ± 6306 | .45 | 1909 ± 206 | .09 | .17 |
| CD8+ | 18441 ± 10365 | 22114 ± 7900 | .95 | 29839 ± 11946 | 14580 ± 3132 | .27 | .79 | 18404 ± 13411 | 1 | 6903 ± 997 | .08 | .18 |
| Treg | 13911 ± 6572 | 13545 ± 4531 | .99 | 16110 ± 3418 | 22151 ± 10075 | .73 | .52 | 34468 ± 26132 | .39 | 8232 ± 1871 | .59 | .18 |
| F4/80+ | 6276 ± 2376 | 11119 ± 2556 | .55 | 6332 ± 439 | 7071 ± 773 | .61 | .14 | 10887 ± 8202 | .65 | 3314 ± 540 | .01* | .21 |
| Gr-1+ | 51600 ± 20201 | 74254 ± 23100 | .91 | 62885 ± 6323 | 82352 ± 29324 | .66 | .85 | 163213 ± 117668 | .25 | 31898 ± 5218 | .39 | .14 |
| Gr-1+CD11b+ | 624 ± 266 | 1992 ± 1385 | .45 | 524 ± 85 | 613 ± 196 | .84 | .30 | 1766 ± 871 | .63 | 251 ± 33 | .26 | .04* |
| CD11c+MHCIIhi | 7617 ± 3016 | 11403 ± 3023 | .79 | 7074 ± 193 | 9223 ± 2031 | .38 | .56 | 15758 ± 10906 | .47 | 4117 ± 376 | .19 | .16 |
| CD11b+DCs | 1933 ± 679 | 4089 ± 1484 | .35 | 1610 ± 292 | 2094 ± 144 | .18 | .17 | 2521± 1721 | .93 | 1120 ± 68 | .17 | .26 |
| CD103+DCs | 1729 ± 749 | 2358 ± 1345 | .91 | 1540 ± 177 | 1925 ± 613 | .69 | .76 | 3302± 2455 | .66 | 718 ± 85 | .25 | .16 |
The table depicts the mean number (±SEM) of the indicated cell population in primary and secondary immune organs from immunologically naïve male and female offspring at maturity (6 weeks of age). There were 4–8 mice of each sex per group, and same-sex offspring within each group were from different dams. Bone marrow, thymus, and peripheral lymph nodes (PLN) were isolated and cell populations identified using multiparametric analytical flow cytometry. Cells enumerated in the bone marrow include: HSC (Lineage[Lin])negSca-1+cKit+CD105hiCD150+); LSK cells (LinnegSca-1+Kit+ cells); LK cells (LinnegKit+ cells); multipotent progenitors (MPP; LineagenegSca-1+cKit+CD105intCD150neg); common lymphocyte progenitors (CLP; LineagenegSca-1+cKit+CD105hiCD150+CD127+); granulocyte monocyte progenitors (GMP; LinnegKit+CD16/32+CD150neg); pre-granulocyte/monocyte cells (pre-GM; LinnegKit+CD16/32negCD150negCD105int); pre-megakaryocyte/erythrocyte cells (pre-MegE; LinnegKit+CD16/32negCD150+CD105hi). In the thymus, cells were defined as double negative: DN1 (CD44+CD25negCD4negCD8neg); DN2 (CD44+CD25+CD4negCD8neg); DN3 (CD44negCD25+CD4negCD8neg); DN4 (CD44negCD25negCD4negCD8neg); double positive (DP; CD3+CD4+CD8+); CD8 SP (CD3+CD4negCD8+); CD4 SP (CD3+CD4+CD8neg); Treg (Foxp3+CD25+CD4+CD8negCD3+); γδT cells (γδTCR+CD3+CD4-CD8-). In the lymph node, cells were defined as: CD8+ T cells (CD3+CD8+); CD4+ T cells (CD3+CD4+); Treg (Foxp3+CD25+CD4+); neutrophils (Gr-1+); macrophages (F4/80+); dendritic cells (DCs; CD11c+MHCIIhi), CD11b+DCs (CD11b+CD103−CD11c+MHCIIhi cells) and CD103+DCs (CD11b−CD103+CD11c+MHCIIhi cells); γδT cells (γδTCR+CD3+CD4−CD8−); NK cells (CD3negNK1.1+); B cells (CD3negCD19+).
The p-value for mixture-exposed group compared with control group within the indicated sex.
The p-value for female versus male offspring from dams given indicated concentration of the mixture.
Demarcates p ≤.05.
Table 2.
The Percentage of Immune Cells in Immunologically Naïve Offspring
| Female 0.1 µg/ml versus Control |
Male 0.1 µg/ml versus Control |
F versus M |
Female 1 µg/ml versus Control |
Male 1 µg/ml versus Control |
F versus M |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.1 µg/ml | p-Valuea | Control | 0.1 µg/ml | p-Valuea | p-Valueb | 1 µg/ml | p-Valuea | 1 µg/ml | p-Valuea | p-Valueb | |
| Bone marrow | ||||||||||||
| HSC | 0.0007 ± 0.0002 | 0.0011 ± 0.0002 | .51 | 0.0015 ± 0.0004 | 0.0016 ± 0.0002 | .96 | .07 | 0.003 ± 0.001 | .003* | 0.0017 ± 0.0003 | .91 | .49 |
| LSK | 0.014 ± 0.001 | 0.014 ± 0.005 | .99 | 0.014 ± 0.002 | 0.012 ± 0.001 | .69 | .98 | 0.015 ± 0.002 | .96 | 0.015 ± 0.002 | .92 | .97 |
| LK | 0.30 ± 0.02 | 0.33 ± 0.06 | .82 | 0.34± 0.02 | 0.35 ± 0.03 | .95 | .37 | 0.41 ± 0.01 | .26 | 0.36 ± 0.03 | .85 | .53 |
| MPP | 0.0117 ± 0.0001 | 0.011 ± 0.004 | .98 | 0.011 ± 0.001 | 0.008 ± 0.001 | .42 | .68 | 0.009 ± 0.002 | .79 | 0.011 ± 0.002 | .89 | .61 |
| Lineage - | 2.7 ± 0.17 | 2.4 ± 0.64 | .90 | 2.7 ± 0.28 | 2.5 ± 0.24 | .84 | .55 | 3.68 ± 0.13 | .31 | 2.9 ± 0.12 | .76 | .15 |
| CLP | 0.04 ± 0.01 | 0.04 ± 0.02 | .99 | 0.039 ± 0.004 | 0.03 ± 0.01 | .59 | .77 | 0.002 ± 0.001 | .25 | 0.033 ± 0.003 | .58 | .01* |
| GMP | 0.026 ± 0.004 | 0.044 ± 0.001 | .03* | 0.04 ± 0.01 | 0.04 ± 0.01 | .96 | 1.0 | 0.05 ± 0.01 | .04* | 0.038 ± 0.002 | .99 | .30 |
| Pre-GM | 0.070 ± 0.003 | 0.08 ± 0.02 | .88 | 0.09 ± 0.01 | 0.10 ± 0.01 | .80 | .09 | 0.066 ± 0.002 | .95 | 0.11 ± 0.01 | .69 | .08 |
| Pre-MegE | 0.13 ± 0.01 | 0.13 ± 0.02 | .99 | 0.120 ± 0.004 | 0.12 ± 0.01 | .95 | .55 | 0.1700 ± 0.00 | .19 | 0.12± 0.01 | .95 | .03* |
| Thymus | ||||||||||||
| DN1 | 0.76 ± 0.02 | 1.6 ± 0.86 | .41 | 0.77 ± 0.09 | 0.76 ± 0.07 | .98 | .30 | 0.51 ± 0.06 | .93 | 0.77 ± 0.08 | .99 | .07 |
| DN2 | 0.12 ± 0.01 | 0.17 ± 0.02 | .01* | 0.09 ± 0.01 | 0.09 ± 0.01 | .97 | .003* | 0.07 ± 0.01 | .06 | 0.11 ± 0.01 | .86 | .47 |
| DN3 | 1.25 ± 0.062 | 1.3 ± 0.09 | .99 | 1.14 ± 0.039 | 1.15 ± 0.040 | .99 | .33 | 1.01 ± 0.005 | .06 | 1.21 ± 0.033 | .43 | .18 |
| DN4 | 0.72 ± 0.09 | 5.9 ± 4.9 | .36 | 1.1 ± 0.21 | 1.04 ± 0.139 | .95 | .29 | 0.52 ± 0.07 | .99 | 1.04 ± 0.144 | .95 | .06 |
| DP | 49.2 ± 3.67 | 37.6 ± 16.1 | .62 | 50.5 ± 1.62 | 50.9 ± 4.31 | .99 | .44 | 39.5 ± 4.55 | .76 | 53.7 ± 3.35 | .73 | .08 |
| DP CD3+ | 0.03 ± 0.01 | 0.03 ± 0.01 | .85 | 0.12 ± 0.04 | 0.08 ± 0.01 | .53 | .06 | 0.017 ± 0.004 | .49 | 0.12 ± 0.02 | .99 | .01* |
| TCRβ+ | 5.3 ± 0.67 | 14.6 ± 6.4 | .19 | 8.2 ± 0.98 | 7.1 ± 0.43 | .54 | .22 | 3.8 ± 0.19 | .95 | 7.5 ± 0.86 | .73 | .04* |
| CD4+ | 4.2 ± 0.27 | 7.8 ± 2.4 | .17 | 5.7 ± 0.48 | 5.6 ± 0.39 | .94 | .32 | 3.6 ± 0.34 | .95 | 5.5 ± 0.51 | .9 | .07 |
| CD8+ | 0.54 ± 0.09 | 0.26 ± 0.05 | .36 | 1.1 ± 0.33 | 0.91 ± 0.18 | .83 | .30 | 0.26 ± 0.05 | .99 | 0.93 ± 0.14 | .86 | .02* |
| TCRγδ+ | 0.36 ± 0.02 | 0.55 ± 0.17 | .34 | 0.45 ± 0.05 | 0.45 ± 0.05 | .99 | .55 | 0.27 ± 0.03 | .78 | 0.51 ± 0.08 | .73 | .04* |
| Treg | 0.095 ± 0.004 | 0.63 ± 0.51 | .37 | 0.13 ± 0.02 | 0.12 ± 0.02 | .94 | .29 | 0.08 ± 0.01 | .99 | 0.11 ± 0.01 | .66 | .06 |
| Spleen | ||||||||||||
| CD19+ | 48.3 ± 3.16 | 49.1 ± 3.45 | .98 | 47.2 ± 8.41 | 53.6 ± 1.19 | .59 | .22 | 54.5 ± 4.45 | .47 | 51.5 ± 1.61 | .77 | .47 |
| CD4+ | 10.8 ± 1.51 | 12.3 ± 0.984 | .66 | 12.2 ± 1.69 | 13.7 ± 0.492 | .51 | .23 | 9.6 ± 0.39 | .81 | 12.9 ± 0.17 | .81 | .001* |
| CD8+ | 7.8 ± 0.39 | 9.03 ± 0.087 | .06 | 8.3 ± 0.47 | 9.2 ± 0.19 | .09 | .49 | 5.9 ± 0.16 | .02* | 8.4 ± 0.09 | .92 | .0001* |
| NK1.1+ | 2.2 ± 0.18 | 1.9 ± 0.24 | .62 | 2.8 ± 0.44 | 2.1 ± 0.14 | .16 | .55 | 1.5 ± 0.06 | .13 | 2.4 ± 0.09 | .41 | .004* |
| TCRγδ+ | 1.6 ± 0.07 | 1.5 ± 0.11 | .72 | 1.5 ± 0.06 | 1.49 ± 0.025 | .92 | .62 | 1.36 ± 0.030 | .16 | 1.4 ± 0.08 | .49 | .61 |
| Treg | 0.59 ± 0.14 | 0.71 ± 0.06 | .69 | 0.78 ± 0.20 | 0.86 ± 0.07 | .89 | .21 | 0.61 ± 0.09 | .99 | 0.85 ± 0.04 | .91 | .04* |
| F4/80+ | 3.3 ± 0.36 | 4.9 ± 1.9 | .49 | 2.8 ± 0.23 | 3.1 ± 0.13 | .29 | .30 | 5.2 ± 0.32 | .50 | 3.1 ± 0.11 | .25 | .002* |
| Gr-1+ | 5.8 ± 0.31 | 8.8 ± 2.2 | .24 | 5.9 ± 0.61 | 6.6 ± 0.20 | .33 | .31 | 7.5 ± 0.67 | .63 | 6.4 ± 0.07 | .52 | .06 |
| Gr-1+CD11b+ | 0.53 ± 0.05 | 2.56 ± 2.01 | .38 | 0.42 ± 0.02 | 0.44 ± 0.02 | .82 | .26 | 1.9 ± 0.19 | .66 | 0.46 ± 0.03 | .41 | .0002* |
| CD11c+MHCIIhi | 1.37 ± 0.044 | 1.38 ± 0.035 | .98 | 1.2 ± 0.19 | 1.3 ± 0.07 | .77 | .42 | 1.2 ± 0.10 | .23 | 1.3 ± 0.09 | .86 | .74 |
| CD11b+DCs | 0.15 ± 0.03 | 0.20 ± 0.05 | .39 | 0.16 ± 0.02 | 0.17 ± 0.01 | .63 | .32 | 0.11 ± 0.01 | .51 | 0.17 ± 0.01 | .72 | .03* |
| CD103+DCs | 0.025 ± 0.004 | 0.04 ± 0.01 | .24 | 0.03 ± 0.01 | 0.02 ± 0.01 | .81 | .22 | 0.02 ± 0.01 | 0.99 | 0.03 ± 0.01 | .97 | .51 |
| Lymph nodes | ||||||||||||
| NK1.1+ | 0.44 ± 0.05 | 0.34 ± 0.06 | .38 | 0.41 ± 0.07 | 0.36 ± 0.05 | .51 | .75 | 0.45 ± 0.04 | .99 | 0.29 ± 0.03 | .86 | .04* |
| CD19+ | 34.80 ± 6.010 | 28.6 ± 9.17 | .77 | 27.4 ± 1.69 | 32.3 ± 5.24 | .99 | .73 | 43.4 ± 2.55 | .68 | 29.6 ± 1.56 | .89 | .01* |
| TCRγδ+ | 1.8 ± 0.31 | 1.7 ± 0.29 | .96 | 2.3 ± 0.70 | 1.1 ± 0.04 | .90 | .08 | 1.06 ± 0.005 | .28 | 1.04 ± 0.068 | .85 | .87 |
| CD4+ | 19.7 ± 2.56 | 16.3 ± 5.48 | .76 | 16.5 ± 1.43 | 16.6 ± 3.62 | .79 | .97 | 23.9 ± 0.800 | .70 | 15.2 ± 1.36 | .27 | .01* |
| CD8+ | 14.6 ± 1.51 | 13.2 ± 4.18 | .91 | 12.4 ± 1.98 | 13.6 ± 3.16 | .13 | .94 | 16.3 ± 0.600 | .88 | 10.9 ± 0.959 | .11 | .02* |
| Treg | 1.5 ± 0.13 | 1.1 ± 0.38 | .49 | 1.4 ± 0.37 | 1.5 ± 0.45 | .99 | .57 | 1.9 ± 0.12 | .46 | 1.2 ± 0.18 | .89 | .07 |
| F4/80+ | 0.72 ± 0.11 | 0.92 ± 0.23 | .60 | 0.55 ± 0.07 | 0.55 ± 0.07 | .86 | .20 | 0.60 ± 0.03 | .85 | 0.51 ± 0.06 | .89 | .36 |
| Gr-1+ | 5.9 ± 0.68 | 5.9 ± 1.4 | .99 | 5.4 ± 0.72 | 5.8 ± 1.1 | .91 | .95 | 9.5 ± 0.20 | .09 | 4.7 ± 0.32 | .8 | .001* |
| Gr-1+CD11b+ | 0.07 ± 0.01 | 0.18 ± 0.13 | .55 | 0.05 ± 0.01 | 0.05 ± 0.01 | .99 | .29 | 0.14 ± 0.05 | .81 | 0.038 ± 0.003 | .77 | .03* |
| CD11c+MHCIIhi | 0.85 ± 0.18 | 0.89 ± 0.07 | .97 | 0.61 ± 0.06 | 0.71 ± 0.08 | .41 | .16 | 0.96 ± 0.08 | .86 | 0.63 ± 0.04 | .94 | .02* |
| CD11b+DCs | 0.23 ± 0.04 | 0.34 ± 0.14 | .57 | 0.14 ± 0.03 | 0.18 ± 0.03 | .53 | .26 | 0.16 ± 0.02 | .83 | 0.18 ± 0.03 | .53 | .62 |
| CD103+DCs | 0.19 ± 0.05 | 0.17 ± 0.07 | .95 | 0.13 ± 0.02 | 0.14 ± 0.03 | .95 | .67 | 0.19 ± 0.01 | .99 | 0.11 ± 0.01 | .69 | .01* |
The mean percentage (±SEM) of the indicated cell types in primary and secondary immune organs from female and male offspring in each treatment group. Cells were collected from the bone marrow, thymus, and lymph nodes of immunologically naïve offspring. There were 4–8 offspring of each sex in each exposure group, and same-sex offspring within each exposure group were from different dams. Cell populations in the bone marrow include: HSC (Lineage[Lin]negSca-1+cKit+CD105hiCD150+); LSK cells (LinnegSca-1+Kit+ cells); LK cells (LinnegKit+ cells); multipotent progenitors (MPP; LineagenegSca-1+cKit+CD105intCD150neg); common lymphocyte progenitors (CLP; LineagenegSca-1+cKit+CD105hiCD150+CD127+); granulocyte monocyte progenitors (GMP; LinnegKit+CD16/32+CD150neg); pre-granulocyte/monocyte cells (pre-GM; LinnegKit+CD16/32negCD150negCD105int); pre-megakaryocyte/erythrocyte cells (pre-MegE; LinnegKit+CD16/32negCD150+CD105hi). In the thymus, cells were defined as double negative: DN1 (CD44+CD25negCD4negCD8neg); DN2 (CD44+CD25+CD4negCD8neg); DN3 (CD44negCD25+CD4negCD8neg); DN4 (CD44negCD25negCD4negCD8neg); double positive (DP; CD3+CD4+CD8+); CD8 SP (CD3+CD4negCD8+); CD4 SP (CD3+CD4+CD8neg); Tregs (Foxp3+CD25+CD4+CD8negCD3+); γδT cells (γδTCR+CD3+CD4-CD8-). In the lymph node, cells were defined as: CD8+ T cells (CD3+CD8+); CD4+ T cells (CD3+CD4+); Tregs (Foxp3+CD25+CD4+); T cells (γδTCR+CD3+CD4-CD8-); neutrophils (Gr-1+); macrophages (F4/80+); dendritic cells (DCs; CD11c+MHCIIhi), CD11b+DCs (CD11b+CD103-CD11c+MHCIIhi cells), and CD103+DCs (CD11b-CD103+CD11c+MHCIIhi cells); NK cells (CD3negNK1.1+); B cells (CD3negCD19+).
The p-value for mixture-exposed group compared with control group within the indicated sex.
The p-value for female versus male offspring from dams treated with the indicated concentration of the mixture.
Denotes p ≤.05.
Among male offspring, neither GMPs nor other leukocyte lineages in the bone marrow or thymus were different from control in the offspring of dams exposed to either concentration of the mixture (Tables 1 and 2). In peripheral immune organs, there were modest shifts in leukocytes, such as a diminution in the percentage of CD8+ T cells in spleens of female offspring of dams given 1 µg/ml (Table 2), and an increase in the number of Gr1+CD11b+ myeloid cells in the spleen of both male and female offspring of dams treated with the higher concentration (Table 1). In addition to differences in cellularity associated with developmental exposure within sex, there were some differences between male and female offspring with regard to the percentage and number of lineage progenitors and lineage committed cell types in the bone marrow and thymus (eg, LK, pre-GM, DN, DP, CD4 SP, Treg).
HDM-Induced Allergic Airway Disease
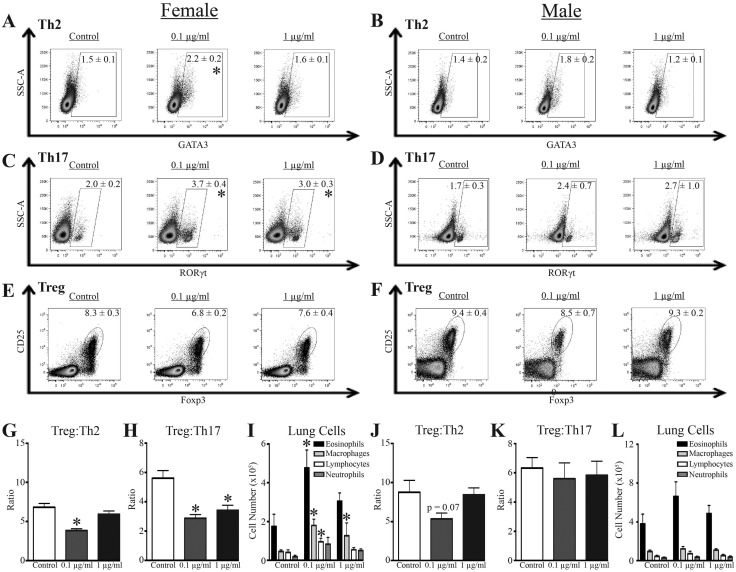
Offspring were sensitized and challenged with HDM, which induces CD4+ T cell-dependent allergic airway disease (Knowlden et al., 2016). Following HDM challenge, there were no differences in the number of CD4+ T cells in the MLN of male or female offspring from all three exposure groups (Supplementary Table 2). However, female offspring of dams given 0.1 μg/mL of the mixture had a statistically significant decrease in the percentage of CD4+ T cells. Moreover, additional differences associated with developmental exposure were revealed when subpopulations of CD4+ T cells were further examined. Two major subsets of helper CD4+ T cells that drive allergic immune responses and contribute to pathology are Th2 cells and Th17 cells (Hirahara and Nakayama, 2016; Vroman et al., 2015). Also, Tregs control the magnitude and duration of the response (Langier et al., 2012). Female mice that were developmentally exposed to 0.1 μg/ml of the mixture, but not 1 μg/ml of the mixture, had a significant increase in the percentage of Th2 cells compared with HDM challenged female offspring of control dams (Figure 2A;Supplementary Table 3). Female offspring exposed to either 0.1 or 1 μg/ml of the chemical mixture also had a greater frequency of Th17 cells (Figure 2C;Supplementary Table 3). Although the frequency of Tregs in female mice that were developmentally exposed to the chemical mixture was not significantly different from control offspring (Figure 2E;Supplementary Tables 2 and 3), the relative proportion of Tregs to Th2 and Th17 cells was diminished (Figs. 2G and 2H). Specifically, the ratio of Treg: Th2 cells was reduced by exposure to 0.1 μg/ml of the chemical mixture, whereas the Treg: Th17 cell ratio was reduced by both doses of the mixture in female offspring (Figs. 2G and 2H). Female offspring that were developmentally exposed to both concentrations of the mixture had a significant increase in airway macrophages (Figure 2I), and offspring of dams given 0.1 μg/ml also exhibited increased eosinophils and lymphocytes in the airways after HDM challenge, compared with offspring of control dams (Figure 2I).
Figure 2.
Immunological effects of developmental exposure in a model of allergic airway disease. At maturity (6–8 weeks of age), 9–10 female and 9–10 male offspring from each developmental exposure group were sensitized and challenged with HDM. Within each group, offspring of the same sex were from different dams. A–F, Representative dot plots from flow cytometric analyses of CD4+ T cell subsets from MLN 48 h after HDM challenge, and mean percentages ± SEM are depicted according to sex and treatment group for Th2 cells (A–B, GATA3+CD4+ T cells), Th17 cells (C–D, RORγt+CD4+ T cells), and Tregs (E–F, Foxp3+CD25+CD4+ T cells). All dot plots are gated on CD4+ T cells. G–H, Mean ratio (±SEM) of Treg:Th2 cells and Treg:Th17 cells in MLN from female offspring. I, Mean number (±SEM) of eosinophils, macrophages, lymphocytes, and neutrophils in BAL from female offspring. J–K, Mean ratio (±SEM) of Treg:Th2 cells and Treg:Th17 cells in MLN from male offspring. L, Mean number (±SEM) of eosinophils, macrophages, lymphocytes, and neutrophils in BAL from male offspring. An * represents a p-value ≤ .05 compared with same sex control.
In response to HDM sensitization and challenge, the male offspring did not exhibit statistically significant differences in the frequency of Th2 cells, Th17 cells, or Tregs as a result of developmental exposure to this mixture (Figs. 2B, 2D, and 2F). Likewise, male offspring did not present a significant change in Treg: conventional CD4+ T cell ratios, although the ratio of Treg:Th2 cells in male offspring exposed to 0.1 μg/m was slightly lower than that of control male offspring (Figs. 2J and 2K). Also in contrast to the female offspring, developmental exposure to this mixture did not change the number of immune cells in airways of male offspring (Figure 2L).
IAV Infection
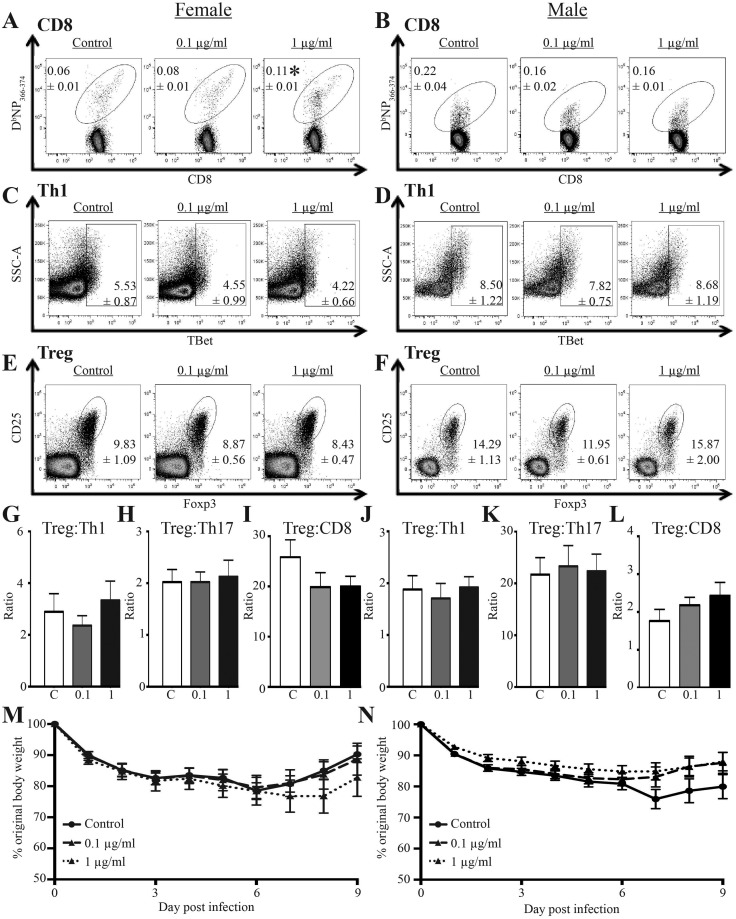
To assess whether developmental exposure changes T cell responses to infection, we administered an IAV challenge that causes mild infection. A sub-lethal infection was selected for two reasons: (1) it better mirrors human IAV infections, because the global burden associated with IAV stems less from mortality and more from the consequences of infection-related illness, which range from mild to severe; and (2) it permits measurement of the peak T cell response to infection, which occurs at about day 9 (Boule et al., 2014; Lawrence et al., 2006). The magnitude of key T cell responses generally predicts the overall outcome of the disease (Hayden et al., 1998; Kaiser et al., 2001). Within the same sex, there were no significant differences in the number or percentage of total CD4+ or CD8+ T cells 9 days after infection based on developmental exposure (Supplementary Tables 4 and 5). Yet, the percentage of viral nucleoprotein (NP)-specific CD8+ T cells was significantly elevated in female mice that were developmentally exposed to 1 μg/ml (but not 0.1 µg/ml) of the chemical mixture, compared with female offspring of control dams (Figure 3A). There was also a 1.7-fold increase in the number and percentage of CD44hiCD62LloCD8+ T cells (cytotoxic T lymphocytes, CTL) in female offspring exposed to 1 µg/mL of the mixture; however, this was not statistically significant from CTL frequencies among female offspring of control dams (Supplementary Tables 4 and 5). When the percentage and number of virus-specific CD8+ T cells were compared by sex, there was a significant difference between the frequency of NP+CD8+ T cells in female and male mice from control dams. However, among male offspring in the three exposure groups, there were no significant differences in the percentage (Figure 3B) or number of virus-specific CD8+ T cells (Supplementary Table 4), or in the percentage and number of CTL after IAV infection (Supplementary Tables 4 and 5).
Figure 3.
Effects of developmental exposure to chemicals associated with UOG on T cells and body weight change after viral infection. At maturity (6–8 weeks of age), 9–10 female and 9–10 male offspring from each exposure group infected with IAV. Within each group, offspring of the same sex were from different dams. A–F, Representative dot plots depict flow cytometric analyses of T cell subsets from MLN 9 days after infection: IAV NP-specific CD8+ T cells (A–B, DbNP366-375+CD8+ T cells, gated on CD3+CD8+ cells), Th1 cells (C–D, TBet+CD4+ cells, gated on CD3+CD4+ cells), and Tregs (E–F, Foxp3+CD25+CD4+ cells; gated on CD3+CD4+ cells). The mean percentages (±SEM) of the indicated T cell sub-types in each exposure group and for both sexes are denoted on the plots. G–L, The mean ratios (±SEM) for Treg:Th1 cells, Treg:Th17 cells, and Treg:NP-specific CD8+ T cells for each exposure group and separated by sex. M–N, Mean (±SEM) body weight change following infection for female (M) and male (N) offspring. An * represents a p-value ≤ .05 compared with sex matched offspring of control dams.
CD4+ T cells foster the development of a more robust CD8+ T cell response to IAV (Kohlmeier and Woodland, 2009; Strutt et al., 2013; Swain et al., 2012). Two critical effector CD4+ T cell populations in acute primary IAV infection are Th1 cells and Tregs (Strutt et al., 2013; Swain et al., 2012). These CD4+ T cell subsets were enumerated at day 9-post infection in female and male offspring. Compared with offspring of control dams, neither the percentage (Figs. 3C and 3D) nor the number (Supplementary Table 4) of Th1 cells were significantly altered by developmental exposure to either concentration of this mixture in male or female offspring. Similarly, the frequency of Tregs was not affected by developmental exposure to this mixture (Figs. 3E and 3F), nor were there statistically significant differences in the Treg:Th1, Treg:Th17, or Treg:NP+CD8+ cell ratios in male or female developmentally exposed offspring (Figs. 3G–L). Also, there was no statistically significant diminution in morbidity among female and male offspring that were developmentally exposed to the 23-chemical mixture and age-matched offspring of control dams (Figs. 3M and 3N). Similarly, there were no statistically significant differences in survival among infected offspring from the three developmental exposure groups (Supplementary Figure 1).
Experimental Autoimmune Encephalomyelitis (EAE)
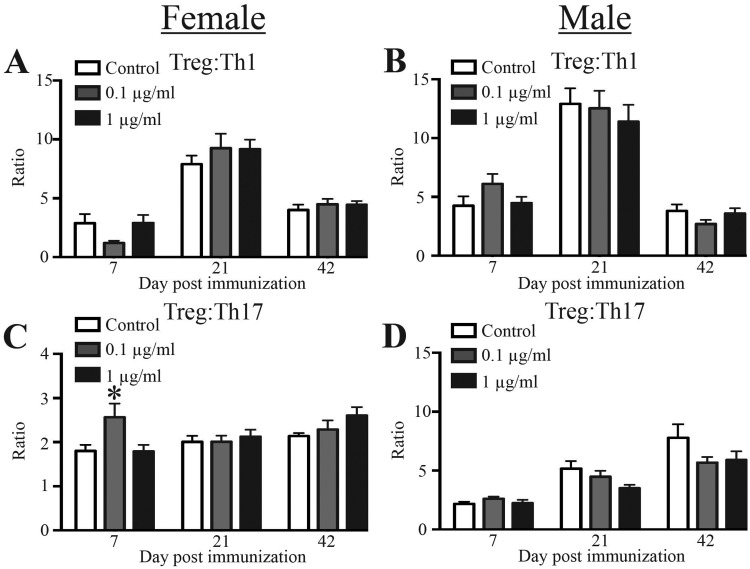
To determine whether developmental exposure to UOG chemical mixture affects a T cell-dependent disease that mirrors aspects of a human autoimmune disease, we used EAE, which models multiple sclerosis (Mendel et al., 1995). The main conventional helper T cells that drive immunopathology during EAE are Th17 and Th1 cells; whereas, Tregs are important in dampening immunopathology caused by these conventional Th subsets (Fletcher et al., 2010). In particular, during EAE, the relative proportion of these two T helper subsets and Tregs influence disease progression. The ratio of Tregs to conventional Th1 and Th17 cells in female (Figs. 4A and 4C) and male (Figs. 4B and 4D) offspring of dams treated with the mixture were similar to ratios in offspring of control dams. In female mice developmentally exposed to 0.1 μg/ml of the chemical mixture, there were more Th1 cells compared with offspring of control dams, and an overall decrease in the Treg: Th1 ratio (Figure 4A;Supplementary Table 6). In female offspring exposed to the lower dose, there was an increase in the Treg: Th17 ratio, reflecting that there were fewer Th17 cells compared with Tregs (Figure 4C, and Supplementary Table 6). Male developmentally exposed offspring had no significant differences in the ratio of Treg:conventional CD4+ T cells, compared with control-exposed mice at all time points (Figs. 4B and 4D). Thus, developmental exposure to chemicals associated with UOG may elicit transient, subtle shifts in CD4+ T cell sub-populations during the early onset of EAE that are more prominent in female offspring.
Figure 4.
CD4+ T cell subset proportions during EAE disease progression. Twenty-six adult (6–8 weeks of age) female and male offspring from each exposure group were immunized with a CFA/MOG35–55 emulsion to induce EAE. To enumerate CD4+ T cells in cervical lymph nodes, 8–10 female and 8–10 male mice from each group were sacrificed 7, 21, or 42 days after immunization. Mice were randomly assigned to each time point. A–D, The bar graphs depict the mean Treg:Th1 ratios (A–B) and mean Treg:Th17 ratios (±SEM) (C–D). Same sex offspring at each timepoint are from different dams. An * represents a p-value ≤ .05 compared with same sex control on the same day post immunization.
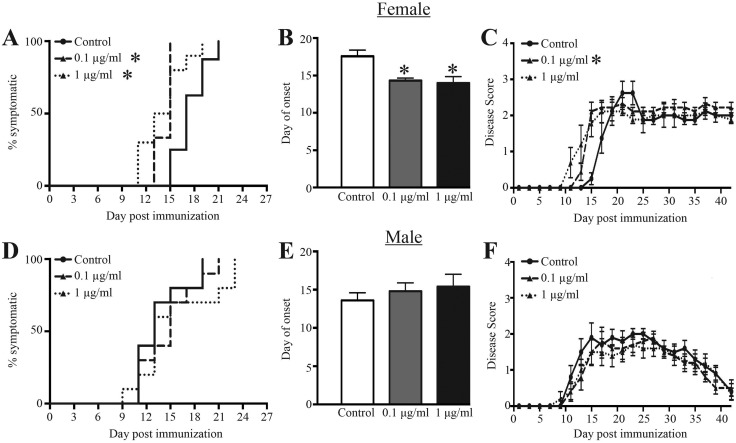
In contrast, there were more pronounced differences in disease severity, time of onset, and progression in female offspring exposed maternally to the chemical mixture. Compared with the female offspring of control dams, disease onset was expedited in female offspring exposed to 0.1 and 1 μg/ml of the chemical mixture during development (Figure 5A). On an average, disease onset in female offspring of dams treated with either dose of the chemical mixture occurred about 3–4 days earlier than in their untreated counterparts (Figure 5B). Female offspring that were developmentally exposed to 0.1 μg/ml, but not 1 μg/ml, also had significantly higher disease scores over time compared with control offspring (Figure 5C). Developmentally exposed male offspring, on the other hand, showed no significant difference in the onset, progression, or severity of disease symptoms when compared with control offspring (Figs. 5D–F).
Figure 5.
EAE symptom onset and severity following immunization with MOG peptide. Ten adult (6–8 weeks of age) female and male offspring from each exposure group were immunized with a CFA/MOG35–55 emulsion to induce EAE. Disease progression was monitored and scored every other day for 42 days. A, D, Kaplan Meier plots show the day of disease onset (disease score ≥1) in female (A) and male (D) offspring. B, E, The bar graphs depict the average day of onset for female (B) and male (E) developmentally exposed offspring. C, F, The average EAE disease score (±SEM) according to treatment group and day post immunization in female (C) and male (F) offspring. Disease scores were 0 = normal mouse, 1 = limp tail, 2 = limp tail and hind limb weakness, 3 = partial hind limb paralysis, 4 = complete hind limp paralysis, 5 = moribund. An * represents a p-value ≤ .05 compared with same sex control offspring.
DISCUSSION
Contamination of water supplies is a major global environmental health concern. In particular, threats to water quality due to anthropogenic activities and pollution by chemicals are emergent concerns. However, causality between adverse health outcomes and chemical contaminants in water is challenging to demonstrate. Reasons for this are multifaceted but include that the negative health impacts of chemical exposures often occur in a delayed manner, water testing is inconsistent between regions, and the list of chemicals tested is often incomplete. Further limitations in our ability to understand causal relationships stem from numerous gaps in knowledge of what cell types and physiological functions are perturbed by developmental exposures to water contaminants. The work reported herein establishes that developmental exposure to a mixture of chemicals used in UOG operations leads to several changes in the cellular composition of the mammalian immune system, and affects T cell composition and function in different disease models. Notably, developmental exposure expedited and exacerbated EAE disease symptoms in female but not male offspring. These results suggest that developmental exposure to chemicals associated with UOG operations has the potential to cause long-lasting, and possibly sex-biased effects on the immune system.
There is scant information on potential developmental immunotoxicity for most of the compounds in this mixture. Moreover, for many constituents of this mixture, there are either no data or the existing evidence of possible immune effects is lean. Nonetheless, there is some evidence that exposure to several components in this mixture, either singly or in smaller groupings, affects the immune system. In particular, benzene and styrene are considered strongly or moderately toxic to the mammalian immune system, respectively (Veraldi et al., 2006). Although the immunotoxicity of benzene has been known for decades, the association of benzene exposure with leukemogenesis and other cancers reflect the best-known aspects of its immunotoxicity (Wang et al., 2012). There are also data demonstrating that the immune system is a target organ of the combination of benzene, toluene, ethylbenzene, and xylenes (BTEX; Bahadar et al., 2014; Bolden et al., 2015). Yet, much of these data focus on cancer, leaving the noncarcinogenic effects of BTEX less well characterized, including its potential developmental immunotoxicity. A recent study reported that inhalation exposure of male mice to a different combination of volatile organics, a mixture of formaldehyde, benzene, toluene, and xylene, decreased the number of T cells in peripheral immune organs (Wang et al., 2016). Other studies have shown that direct exposure to other volatile organics, including ethylbenzene, is associated with changes in lymphocyte populations and multiple chemical sensitivity (Baines et al., 2004). Thus, although information on immunological effects of some constituents of the 23-chemical mixture is limited or nonexistent, there is evidence that several chemicals within this mixture likely affect the developing immune system.
Among the outcomes that were affected by developmental exposure to this mixture, one of the most evident changes was the advanced time of onset and severity of EAE, particularly in female offspring. This observation, and other data from these mice, suggest that there may be some sex-biased differences. Although a systematic and complete understanding of sex-specific differences in immune responses has not yet been achieved, the endocrine system influences the immune system (Gabriel and Arck, 2014; Oertelt-Prigione, 2012). There is extensive evidence that the immune responses of males and females are inherently different, and that sex affects the timing, magnitude or penetrance of many diseases, including allergic inflammation/asthma, the response to respiratory infections and autoimmune diseases (McClelland and Smith, 2011; Ngo et al., 2014). For example, differences in the frequency of T cells in male compared with female mice infected with IAV have been described previously (Gabriel and Arck, 2014; Oertelt-Prigione, 2012). Furthermore, there is mounting evidence that EDCs influence the immune system during development, and thereby contribute to disease at later stages in life (Kopras et al., 2014; Schug et al., 2011). For instance, developmental exposure to atrazine, bisphenol A, cadmium, and perfluorooctane sulfonate (separately) leads to sex-based differences in myriad immune system metrics in the offspring (Bauer et al., 2012; Bodin et al., 2014; Boule et al., 2015a,b; Boule and Lawrence, 2016; Hanson et al., 2012; Keil et al., 2008; Ng et al., 2006; O'Brien et al., 2014; Rooney et al., 2003). Conversely, there are examples in which developmental exposure has similar effects on the immune system of both male and female offspring (Mustafa et al., 2011; Roy et al., 2012; Vorderstrasse et al., 2006). There are also cases in which the same agent gives different results across model systems. For example, in two studies using the same mouse strain, maternal dosing design, and dosage, developmental exposure to BPA showed evidence of sex-biased differences in a mouse model of allergic airway diseases, but not in offspring infected with IAV (Bauer et al., 2012; Roy et al., 2012). Collectively, these studies illustrate that the relative sensitivity of one sex or the other to perturbation by a developmental immunotoxicant is multifactorial and includes aspects of the antigenic challenge or injury. Therefore, although the findings of this current study suggest that females may be more sensitive to early life exposure to this mixture, it is premature to conclude firmly that one sex is overall more sensitive to developmental exposure to chemicals associated with UOG.
In addition to differences in which offspring of different sexes exhibited different outcomes, some immune changes showed evidence of dose-responsiveness; however, other effects of developmental exposure were observed at the lower, but not the higher maternal dose. Examples of immune endpoints that were affected in both the lower and higher dose groups include the Treg:Th17 cell ratio in HDM challenge and the timing of onset in EAE. Yet, other findings show similarities with prior reports of nonmonotonic dose-response relationships in some consequences of exposure to EDCs with offspring of dams given the lower mixture concentration exhibiting a greater effect than higher dose group (Bodin et al., 2013; Vandenberg et al., 2012). Moreover, not all effects showed evidence of nonmonotonicity. For example, EAE disease onset was faster in female offspring of dams exposed to both concentrations of the mixture compared with female controls, whereas the overall disease severity scores were only significantly higher than control in female offspring exposed to the lower concentration. This may reflect dose-dependent differences in target tissues. For example, we measured CD4+ T cell populations in peripheral lymph nodes. Cellular changes at the site of the immune response (eg, the CNS) may be different from responses detected in lymphoid tissues. It is also possible that the complex interplay of signaling from CNS-resident cells and CNS-infiltrating immune cells underlies disease onset rate and disease severity, and these events have differential dose sensitivities (Alvarez et al., 2015; Kroner et al., 2009; Lee et al., 2012; Polfliet et al., 2002). Other studies that have examined exposure to some constituents of the mixture suggest that there are direct, dose-dependent alterations in the endocrine and neurological systems, which could trigger or synergize with altered immune function (Bahadar et al., 2015; Kajta and Wojtowicz, 2013). Consequently, developmental exposure to chemicals associated with UOG that have known endocrine-disrupting characteristics, such as the representative mixture used here, may cause persistent changes in the interplay between the immune, nervous, and endocrine systems.
Another finding was the association of developmental exposure to this mixture and changes in T cells after an immune challenge. Developmental exposure significantly shifted the proportion of specific T cell subsets in female offspring in the HDM model, and to a lesser extent in offspring challenged with IAV. This suggests that developmental exposure to this mixture may not affect T cells globally, but impinges on the pathways that are important during T cell responses to challenge. For example, the balance of regulatory and effector T cell subsets is an important indicator of the progression and severity of diseases, including allergic asthma and infections (Chapman and Georas, 2014). Although no studies to date have examined whether living near or working at UOG operations is associated with enhanced respiratory infections, a recent report linked proximity to UOG operations and increased asthma exacerbations (Rasmussen et al., 2016). Thus, examining human T cell subset distribution and responsive capacity may accelerate research in exposed populations in order to define associations between proximity to UOG operations, water contaminants, and altered immune function later in life. Further support for using differentiation of peripheral T cells to evaluate potential immunotoxicants comes from studies of other developmental exposures (reviewed in Boule and Lawrence, 2016). For example, in B6C3F1 mice, maternal exposure to cigarette smoke, another complex mixture, modulates T cell proliferative capacity and dampens their ability to kill tumor cells (Ng et al., 2006; Ng and Zelikoff, 2008). Yet, in other studies the magnitude and direction of change depends upon the anatomical site examined, such as in studies reporting alterations in the proportion of Tregs following developmental exposure to cadmium (Hanson et al., 2012) and dioxin (Boule et al., 2014, 2015b). Thus, although T cells are commonly affected by developmental immunotoxicants, the consequences measured later in life are highly dependent upon context, including anatomical site, timing, and the profile of T cells that respond to a particular antigenic challenge.
Although we report that developmental exposure to chemicals used in UOG operation has significant effects on the immune system, there are some limitations to our study. For instance, we deliberately selected a dose and strain of IAV that causes mild infection so that mice would survive, clear the virus, and T cell responses could be examined as the infection was resolved. The lack of significant change in morbidity and mortality following IAV infection suggests that developmental exposure to water containing this mixture of 23 chemicals did not overtly compromise aspects of immune function crucial for surviving mild acute respiratory viral infection. Further evaluation using more pathogenic strains of IAV, and other types of viruses, is needed. Also, in all three disease models, we defined CD4+ T cell subsets by the expression of lineage-specific transcription factors (Yamane and Paul, 2013), but future studies will be needed to determine exactly how T cell effector function was affected (eg, production of cytokines or other mediators). Also, in assessing immune responses, there are many other cell types that could be examined. It seems possible that the function of additional immune cell types could be altered by developmental exposure to this mixture, which could be productive areas of future research. Finally, environmental exposures may encompass maternal and direct exposures after birth (Boverhof et al., 2014; Dietert and Zelikoff, 2008). The goal of the present study was to characterize whether developmental exposure changed how the immune system responds later in life; hence, only vertical exposure to this mixture was used. Thus, it remains possible that these chemicals cause immune effects that are repaired during the gap between developmental exposure and immune assessment. For example, the immunomodulatory actions of other agents, such as irradiation and anticancer drugs, are not only immediate, but also cause changes to stem and progenitor cells, which are revealed only later on (Bracci et al., 2014; Johnston et al., 2013; Kusunoki and Hayashi, 2008; Li and Slayton, 2013). Despite this possibility, our studies are consistent with the idea that the developmental period constitutes a time during which the immune system is sensitive to modulation by environmental factors (Dietert, 2005; Dietert and Zelikoff, 2008; Luebke et al., 2006; Winans et al., 2011).
In summary, we report a study of developmental exposure to a mixture of chemicals associated with UOG operations on immune system development and function using three broad types of disease models: infection, allergic, and autoimmune. The major finding is that maternal exposure to this mixture durably affects the immune system of the offspring. Some of the observed changes were subtle, such as alterations in the number or percentage of certain cell types, whereas other changes were more manifest, such as advancement in the onset and severity of disease. Also, some alterations appeared to be more evident in the female offspring. Collectively, our findings suggest that developmental exposure to chemicals associated with UOG likely causes long-lasting changes in the mouse immune system.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Cornelius Green and Victoria Balise for preparing the chemical mixture used in all of these studies. We are also grateful to Dr Timothy Bushnell and the outstanding team at the University of Rochester Flow Cytometry Core. The authors declare they have no actual or potential competing financial interests.
FUNDING
This work was supported by a University of Rochester Provost’s Office Research Award, the National Institutes of Health [R01ES023260, R01ES004862, T32ES07026, P30ES01247, and R24AI-059830], and the Morris Foundation [D14ZO-084].
REFERENCES
- Alvarez J. I., Saint-Laurent O., Godschalk A., Terouz S., Briels C., Larouche S., Bourbonniere L., Larochelle C., Prat A. (2015). Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol. Dis. 74, 14–24. [DOI] [PubMed] [Google Scholar]
- Bahadar H., Abdollahi M., Maqbool F., Baeeri M., Niaz K. (2015). Mechanistic overview of immune modulatory effects of environmental toxicants. Inflamm. Allergy Drug Targets 13, 382–386. [DOI] [PubMed] [Google Scholar]
- Bahadar H., Mostafalou S., Abdollahi M. (2014). Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol. Appl. Pharmacol. 276, 83–94. [DOI] [PubMed] [Google Scholar]
- Baines C. J., McKeown-Eyssen G. E., Riley N., Cole D. E., Marshall L., Loescher B., Jazmaji V. (2004). Case-control study of multiple chemical sensitivity, comparing haematology, biochemistry, vitamins and serum volatile organic compound measures. Occup. Med. (Lond.) 54, 408–418. [DOI] [PubMed] [Google Scholar]
- Balise V. D., Meng C. X., Cornelius-Green J. N., Kassotis C. D., Kennedy R., Nagel S. C. (2016). Systematic review of the association between oil and natural gas extraction processes and human reproduction. Fertil. Steril. 106, 795–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S. M., Roy A., Emo J., Chapman T. J., Georas S. N., Lawrence B. P. (2012). The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood. Toxicol. Sci. 130, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin J., Bolling A. K., Becher R., Kuper F., Lovik M., Nygaard U. C. (2014). Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol. Sci. 137, 311–323. [DOI] [PubMed] [Google Scholar]
- Bodin J., Bolling A. K., Samuelsen M., Becher R., Lovik M., Nygaard U. C. (2013). Long-term bisphenol A exposure accelerates insulitis development in diabetes-prone NOD mice. Immunopharmacol. Immunotoxicol. 35, 349–358. [DOI] [PubMed] [Google Scholar]
- Bolden A. L., Kwiatkowski C. F., Colborn T. (2015). New look at BTEX: Are ambient levels a problem? Environ. Sci. Technol. 49, 5261–5276. [DOI] [PubMed] [Google Scholar]
- Boule L. A., Burke C. G., Fenton B. M., Thevenet-Morrison K., Jusko T. A., Lawrence B. P. (2015a). Developmental activation of the AHR increases effector CD4+ T Cells and exacerbates symptoms in autoimmune disease-prone Gnaq+/- mice. Toxicol. Sci. 148, 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule L. A., Lawrence B. P. (2016). Influence of early life environmental exposures on immune function across the lifespan. In Environmental Influences on the Immune System (C. Esser, Ed.), doi: 10.1007/978-3-7091-1890-0, pp. 21–54. Heidelberg, Germany: Springer.
- Boule L. A., Winans B., Lambert K., Vorderstrasse B. A., Topham D. J., Pavelka M. S. Jr, Lawrence B. P. (2015b). Activation of the aryl hydrocarbon receptor during development enhances the pulmonary CD4+ T cell response to viral infection. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L305–L313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule L. A., Winans B., Lawrence B. P. (2014). Effects of developmental activation of the AhR on CD4+ T-cell responses to influenza virus infection in adult mice. Environ. Health Perspect. 122, 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boverhof D. R., Ladics G., Luebke B., Botham J., Corsini E., Evans E., Germolec D., Holsapple M., Loveless S. E., Lu H. et al. , (2014). Approaches and considerations for the assessment of immunotoxicity for environmental chemicals: A workshop summary. Regul. Toxicol. Pharmacol. 68, 96–107. [DOI] [PubMed] [Google Scholar]
- Bracci L., Schiavoni G., Sistigu A., Belardelli F. (2014). Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 21, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. A., Savitz D. A., Rasmussen S. G., Ogburn E. L., Pollak J., Mercer D. G., Schwartz B. S. (2016). Unconventional natural gas development and birth outcomes in Pennsylvania, USA. Epidemiology 27, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T. J., Georas S. N. (2014). Regulatory tone and mucosal immunity in asthma. Int. Immunopharmacol. 23, 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciau-Uitz A., Monteiro R., Kirmizitas A., Patient R. (2014). Developmental hematopoiesis: Ontogeny, genetic programming and conservation. Exp. Hematol. 42, 669–683. [DOI] [PubMed] [Google Scholar]
- Colborn T., Kwiatkowski C., Schultz K., Bachran M. (2011). Natural gas operations from a public health perspective. Int. J. Human Ecol. Risk Assess. 17, 1039–1056. [Google Scholar]
- Cozzarelli I. M., Skalak K. J., Kent D. B., Engle M. A., Benthem A., Mumford A. C., Haase K., Farag A., Harper D., Nagel S. C. et al. , (2017). Environmental signatures and effects of an oil and gas wastewater spill in the Williston Basin, North Dakota. Sci. Total Environ. 579, 1781–1793. [DOI] [PubMed] [Google Scholar]
- Dietert R. R. (2005). New developments in the assessment of developmental immunotoxicology. J. Immunotoxicol. 2, 185–189. [DOI] [PubMed] [Google Scholar]
- Dietert R. R., Zelikoff J. T. (2008). Early-life environment, developmental immunotoxicology, and the risk of pediatric allergic disease including asthma. Birth Defects Res. B Dev. Reprod. Toxicol. 83, 547–560. [DOI] [PubMed] [Google Scholar]
- DiGiulio D. C., Jackson R. B. (2016). Impact to underground sources of drinking water and domestic wells from production well stimulation and completion practices in the pavillion, wyoming, field. Environ. Sci. Technol. 50, 4524–4536. [DOI] [PubMed] [Google Scholar]
- Elsner M., Hoelzer K. (2016). Quantitative survey and structural classification of hydraulic fracturing chemicals reported in unconventional gas production. Environ. Sci. Technol. 50, 3290–3314. [DOI] [PubMed] [Google Scholar]
- Fletcher J. M., Lalor S. J., Sweeney C. M., Tubridy N., Mills K. H. (2010). T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel G., Arck P. C. (2014). Sex, immunity and influenza. J. Infect. Dis. 209(Suppl 3), S93–S99. [DOI] [PubMed] [Google Scholar]
- Gross S. A., Avens H. J., Banducci A. M., Sahmel J., Panko J. M., Tvermoes B. E. (2013). Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J. Air Waste Manag. Assoc. 63, 424–432. [DOI] [PubMed] [Google Scholar]
- Hanson M. L., Holaskova I., Elliott M., Brundage K. M., Schafer R., Barnett J. B. (2012). Prenatal cadmium exposure alters postnatal immune cell development and function. Toxicol. Appl. Pharmacol. 261, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G., Fritz R., Lobo M. C., Alvord W., Strober W., Straus S. E. (1998). Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J. Clin. Invest. 101, 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K., Nakayama T. (2016). CD4+ T cell subsets in inflammatory diseases: Beyond the Th1/Th2 paradigm. Int. Immunol. 28, 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C. J., Manning C. M., Rangel-Moreno J., Randall T. D., Hernady E., Finkelstein J. N., Williams J. P. (2013). Neonatal irradiation sensitizes mice to delayed pulmonary challenge. Radiat. Res. 179, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz S. Z., Lu L. F., Rudensky A. Y. (2012). Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser L., Fritz R. S., Straus S. E., Gubareva L., Hayden F. G. (2001). Symptom pathogenesis during acute influenza: Interleukin-6 and other cytokine responses. J. Med. Virol. 64, 262–268. [DOI] [PubMed] [Google Scholar]
- Kajta M., Wojtowicz A. K. (2013). Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 65, 1632–1639. [DOI] [PubMed] [Google Scholar]
- Kassotis C. D., Bromfield J. J., Klemp K. C., Meng C. X., Wolfe A., Zoeller R. T., Balise V. D., Isiguzo C. J., Tillitt D. E., Nagel S. C. (2016a). Adverse reproductive and developmental health outcomes following prenatal exposure to a hydraulic fracturing chemical mixture in female C57Bl/6 mice. Endocrinology 157, 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis C. D., Klemp K. C., Vu D. C., Lin C. H., Meng C. X., Besch-Williford C. L., Pinatti L., Zoeller R. T., Drobnis E. Z., Balise V. D. et al. , (2015). Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 156, 4458–4473. [DOI] [PubMed] [Google Scholar]
- Kassotis C. D., Tillitt D. E., Davis J. W., Hormann A. M., Nagel S. C. (2014). Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology 155, 897–907. [DOI] [PubMed] [Google Scholar]
- Kassotis C. D., Tillitt D. E., Lin C. H., McElroy J. A., Nagel S. C. (2016b). Endocrine-disrupting chemicals and oil and natural gas operations: Potential environmental contamination and recommendations to assess complex environmental mixtures. Environ. Health Perspect. 124, 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil D. E., Mehlmann T., Butterworth L., Peden-Adams M. M. (2008). Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol. Sci. 103, 77–85. [DOI] [PubMed] [Google Scholar]
- Knowlden S. A., Hillman S. E., Chapman T. J., Patil R., Miller D. D., Tigyi G., Georas S. N. (2016). Novel inhibitory effect of a lysophosphatidic acid 2 agonist on allergen-driven airway inflammation. Am. J. Respir. Cell Mol. Biol. 54, 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier J. E., Woodland D. L. (2009). Immunity to respiratory viruses. Annu. Rev. Immunol. 27, 61–82. [DOI] [PubMed] [Google Scholar]
- Kopras E., Potluri V., Bermudez M. L., Williams K., Belcher S., Kasper S. (2014). Actions of endocrine-disrupting chemicals on stem/progenitor cells during development and disease. Endocr.Relat. Cancer 21, T1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A., Schwab N., Ip C. W., Ortler S., Gobel K., Nave K. A., Maurer M., Martini R., Wiendl H. (2009). Accelerated course of experimental autoimmune encephalomyelitis in PD-1-deficient central nervous system myelin mutants. Am. J. Pathol. 174, 2290–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. H., Yang S. N., Kuo P. L., Hung C. H. (2012). Immunomodulatory effects of environmental endocrine disrupting chemicals. Kaohsiung J. Med. Sci. 28, S37–S42. [DOI] [PubMed] [Google Scholar]
- Kusunoki Y., Hayashi T. (2008). Long-lasting alterations of the immune system by ionizing radiation exposure: Implications for disease development among atomic bomb survivors. Int. J. Radiat. Biol. 84, 1–14. [DOI] [PubMed] [Google Scholar]
- Langier S., Sade K., Kivity S. (2012). Regulatory T cells in allergic asthma. Isr. Med. Assoc. J. 14, 180–183. [PubMed] [Google Scholar]
- Lawrence B. P., Roberts A. D., Neumiller J. J., Cundiff J. A., Woodland D. L. (2006). Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung. J. Immunol. 177, 5819–5828. [DOI] [PubMed] [Google Scholar]
- Lee E., Chanamara S., Pleasure D., Soulika A. M. (2012). IFN-gamma signaling in the central nervous system controls the course of experimental autoimmune encephalomyelitis independently of the localization and composition of inflammatory foci. J. Neuroinflam. 9, 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Slayton W. B. (2013). Molecular mechanisms of platelet and stem cell rebound after 5-fluorouracil treatment. Exp. Hematol. 41, 635–645 e3. [DOI] [PubMed] [Google Scholar]
- Luebke R. W., Chen D. H., Dietert R., Yang Y., King M., Luster M. I., Immunotoxicology W. (2006). The comparative immunotoxicity of five selected compounds following developmental or adult exposure. J. Toxicol. Environ. Health B Crit. Rev. 9, 1–26. [DOI] [PubMed] [Google Scholar]
- Maqbool F., Mostafalou S., Bahadar H., Abdollahi M. (2016). Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 145, 265–273. [DOI] [PubMed] [Google Scholar]
- McClelland E. E., Smith J. M. (2011). Gender specific differences in the immune response to infection. Arch. Immunol. Ther. Exp. (Warsz.) 59, 203–213. [DOI] [PubMed] [Google Scholar]
- McKenzie L. M., Allshouse W. B., Byers T. E., Bedrick E. J., Serdar B., Adgate J. L. (2017). Childhood hematologic cancer and residential proximity to oil and gas development. PLoS One 12, e0170423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie L. M., Guo R., Witter R. Z., Savitz D. A., Newman L. S., Adgate J. L. (2014). Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ. Health Perspect. 122, 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel I., Kerlero de Rosbo N., Ben-Nun A. (1995). A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: Fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur. J. Immunol. 25, 1951–1959. [DOI] [PubMed] [Google Scholar]
- Mustafa A., Holladay S., Witonsky S., Zimmerman K., Manari A., Countermarsh S., Karpuzoglu E., Gogal R. (2011). Prenatal TCDD causes persistent modulation of the postnatal immune response, and exacerbates inflammatory disease, in 36-week-old lupus-like autoimmune SNF1 mice. Birth Defects Res. B Dev. Reprod. Toxicol. 92, 82–94. [DOI] [PubMed] [Google Scholar]
- Ng S. P., Silverstone A. E., Lai Z. W., Zelikoff J. T. (2006). Effects of prenatal exposure to cigarette smoke on offspring tumor susceptibility and associated immune mechanisms. Toxicol. Sci. 89, 135–144. [DOI] [PubMed] [Google Scholar]
- Ng S. P., Zelikoff J. T. (2008). The effects of prenatal exposure of mice to cigarette smoke on offspring immune parameters. J. Toxicol. Environ. Health A 71, 445–453. [DOI] [PubMed] [Google Scholar]
- Ngo S. T., Steyn F. J., McCombe P. A. (2014). Gender differences in autoimmune disease. Front. Neuroendocrinol. 35, 347–369. [DOI] [PubMed] [Google Scholar]
- O'Brien E., Bergin I. L., Dolinoy D. C., Zaslona Z., Little R. J., Tao Y., Peters-Golden M., Mancuso P. (2014). Perinatal bisphenol A exposure beginning before gestation enhances allergen sensitization, but not pulmonary inflammation, in adult mice. J. Dev. Orig. Health Dis. 5, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertelt-Prigione S. (2012). The influence of sex and gender on the immune response. Autoimmun. Rev. 11, A479–A485. [DOI] [PubMed] [Google Scholar]
- Orem W., Varonka M., Crosby L., Haase K., Loftin K., Hladik M., Akob D. M., Tatu C., Mumford A., Jaeschke J. et al. , (2017). Organic geochemistry and toxicology of a stream impacted by unconventional oil and gas wastewater disposal operations. Appl. Geochem. 80, 155–167. [Google Scholar]
- Polfliet M. M., van de Veerdonk F., Dopp E. A., van Kesteren-Hendrikx E. M., van Rooijen N., Dijkstra C. D., van den Berg T. K. (2002). The role of perivascular and meningeal macrophages in experimental allergic encephalomyelitis. J. Neuroimmunol. 122, 1–8. [DOI] [PubMed] [Google Scholar]
- Rabinowitz P. M., Slizovskiy I. B., Lamers V., Trufan S. J., Holford T. R., Dziura J. D., Peduzzi P. N., Kane M. J., Reif J. S., Weiss T. R. et al. , (2015). Proximity to natural gas wells and reported health status: Results of a household survey in Washington County, Pennsylvania. Environ. Health Perspect. 123, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S. G., Ogburn E. L., McCormack M., Casey J. A., Bandeen-Roche K., Mercer D. G., Schwartz B. S. (2016). Association between unconventional natural gas development in the marcellus shale and asthma exacerbations. JAMA Intern. Med. 176, 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly E. C., Martin K. C., Jin G. B., Yee M., O'Reilly M. A., Lawrence B. P. (2015). Neonatal hyperoxia leads to persistent alterations in NK responses to influenza A virus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 308, L76–L85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., Harp C. T., Noronha A., Miller S. D. (2014). The experimental autoimmune encephalomyelitis (EAE) model of MS: Utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 122, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney A. A., Matulka R. A., Luebke R. W. (2003). Developmental atrazine exposure suppresses immune function in male, but not female Sprague-Dawley rats. Toxicol. Sci. 76, 366–375. [DOI] [PubMed] [Google Scholar]
- Roy A., Bauer S. M., Lawrence B. P. (2012). Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS One 7, e38448.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug T. T., Janesick A., Blumberg B., Heindel J. J. (2011). Endocrine disrupting chemicals and disease susceptibility. J. Steroid. Biochem. Mol. Biol. 127, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy S. L., Brink L. L., Larkin J. C., Sadovsky Y., Goldstein B. D., Pitt B. R., Talbott E. O. (2015). Perinatal outcomes and unconventional natural gas operations in Southwest Pennsylvania. PLoS One 10, e0126425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes I. M., Goverman J. M. (2006). Active induction of experimental allergic encephalomyelitis. Nat. Protocol. 1, 1810–1819. [DOI] [PubMed] [Google Scholar]
- Strutt T. M., McKinstry K. K., Marshall N. B., Vong A. M., Dutton R. W., Swain S. L. (2013). Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol. Rev. 255, 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Zhang Y. (2014). Overview of orchestration of CD4+ T cell subsets in immune responses. Adv. Exp. Med. Biol. 841, 1–13. [DOI] [PubMed] [Google Scholar]
- Swain S. L., McKinstry K. K., Strutt T. M. (2012). Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 12, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharke D. C., Croft N. P., Doherty P. C., La Gruta N. L. (2015). Sizing up the key determinants of the CD8(+) T cell response. Nat. Rev. Immunol. 15, 705–716. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. (2015). Assessment of the Potential Impacts of Hydraulic Fracturing for Oil and Gas on Drinking Water Resources. Available at: http://ofmpub.epa.gov/eims/eimscomm.getfile? p_download_id=523539. Accessed October 9, 2017. [DOI] [PubMed]
- Vandenberg L. N., Colborn T., Hayes T. B., Heindel J. J., Jacobs D. R. Jr., Lee D. H., Shioda T., Soto A. M., vom Saal F. S., Welshons W. V. et al. , (2012). Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengosh A., Jackson R. B., Warner N., Darrah T. H., Kondash A. (2014). A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 48, 8334–8348. [DOI] [PubMed] [Google Scholar]
- Veraldi A., Costantini A. S., Bolejack V., Miligi L., Vineis P., van Loveren H. (2006). Immunotoxic effects of chemicals: A matrix for occupational and environmental epidemiological studies. Am. J. Ind. Med. 49, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse B. A., Cundiff J. A., Lawrence B. P. (2006). A dose-response study of the effects of prenatal and lactational exposure to TCDD on the immune response to influenza a virus. J. Toxicol. Environ. Health A 69, 445–463. [DOI] [PubMed] [Google Scholar]
- Vroman H., van den Blink B., Kool M. (2015). Mode of dendritic cell activation: The decisive hand in Th2/Th17 cell differentiation. Implications in asthma severity? Immunobiology 220, 254–261. [DOI] [PubMed] [Google Scholar]
- Wang F., Liu F., Liu H., Chen W., Si X., Ma X. (2016). Effects of immunological and hematological parameter in mice exposed to mixture of volatile organic compounds. Inhal. Toxicol. 28, 164–169. [DOI] [PubMed] [Google Scholar]
- Wang L., He X., Bi Y., Ma Q. (2012). Stem cell and benzene-induced malignancy and hematotoxicity. Chem. Res. Toxicol. 25, 1303–1315. [DOI] [PubMed] [Google Scholar]
- Warren T. K., Mitchell K. A., Lawrence B. P. (2000). Exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol. Sci. 56, 114–123. [DOI] [PubMed] [Google Scholar]
- Waxman H. A., Markey E. J., DeGette D. (2011). Chemicals Used in Hydraulic Fracturing. Available at: http://www.conservation.ca.gov/dog/general_information/Documents/Hydraulic Fracturing Report 4 18 11.pdf. Accessed July 13, 2016.
- Webb E., Bushkin-Bedient S., Cheng A., Kassotis C. D., Balise V., Nagel S. C. (2014). Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev. Environ. Health 29, 307–318. [DOI] [PubMed] [Google Scholar]
- Winans B., Humble M. C., Lawrence B. P. (2011). Environmental toxicants and the developing immune system: A missing link in the global battle against infectious disease? Reprod. Toxicol. 31, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman H. (2009). Untested Waters: The Rise of Hydraulic Fracturing in Oil and Gas Production and the Need to Revisit Regulation. 115.
- Yamane H., Paul W. E. (2013). Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol. Rev. 252, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller R. T., Brown T. R., Doan L. L., Gore A. C., Skakkebaek N. E., Soto A. M., Woodruff T. J., Vom Saal F. S. (2012). Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 153, 4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.