Abstract
Orbital floor fractures alone or in conjunction with other facial skeletal fractures are the most commonly encountered midfacial fractures. The technological advances in 3-dimensional (3D) printing allow the physical prototyping of 3D models, so creates an accurate representation of the patient's specific anatomy. A 56-year-old Caucasian man with severe hypoglobus and enophthalmos with an extensive blowout fracture was scheduled for reconstruction. First, 3D physical models were created based on the computed tomography scan datasets from patient. Then, this model was used as templates for preoperative trimming the implant. Surgical reconstruction with the aid of pre-shaped, customized prosthesis based on 3D anatomical model resulted in significant esthetic and clinical improvement. It is possible to build anatomical models on the basis of computed tomography scan datasets. It is relatively inexpensive and can be used in the repair of complex orbital floor fractures.
Keywords: Blowout fracture, computed tomography, orbital implants, three-dimensional printing
Introduction
Orbital floor fractures alone or in conjunction with other facial skeletal fractures are the most common midfacial fractures.[1]
The aim of surgical treatment of blowout fracture is the reconstruction of the injured area using a trimmed implant to maintain the shape and volume of orbit, restore the physiologic function and also address esthetic goals.[2]
The rapid prototyping (RP) is a process which directly generates physical objects with defined structure and shape on the basis of virtual mode data. The technological advances in 3D printing allow the physical prototyping of 3D models. With the aid of this technology, models are created that are an accurate representation of the patient's anatomy.[3]
In this study, we designed a pre-shaped, customized porous polyethylene plate (MEDPOR TM) prosthesis based on the 3D anatomical model with the source the computed tomography (CT) scans datasets to repair an orbital floor fracture.
Case Report
A 56-year-old Caucasian man was referred to our clinic 3 months after a crush injury to the right side of his face sustained in a motor vehicle accident. The delay was for stabilizing the general health condition of the patient.
On examination, severe hypoglobus, enophthalmos, and limitation of upgaze were observed [Figure 1]. Slit lamp examination was unremarkable.
Figure 1.
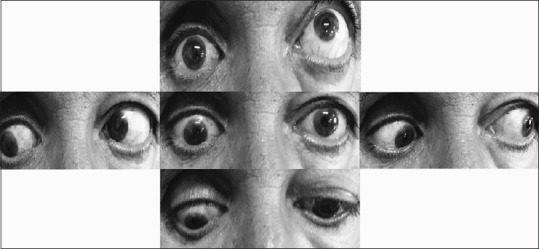
Hypoglobus, enophthalmos, and ocular movements preoperatively
On his initial examination, visual acuity with correction was 20/20 in both eyes. Hertel measurements were 18 mm right eye and 21 mm left eye. The remainder of the ophthalmic examination was normal.
The patient was scheduled for reconstruction and underwent CT (multislice 64). Magnified slices with 0.625 mm thickness were obtained using a soft tissue standard filter (a matrix of 512 × 512 pixels), and imaging data were stored as Digital Imaging and Communications in Medicine (DICOM) format. A fused deposition modeling RP system (Prusa i3 by RepRap Core Developer Prusa jr) and polylactic acid filament (diameter of 1.75 mm) as printing material were used to manufacture the presurgical model, with a nozzle diameter of 0.3 mm and printing temperature of 210° C. Layer heights were set to be 0.2 mm. To prepare data for feeding the printer, DICOM images were processed, and the desired area was stripped of soft tissue, by applying a Hounsfield unit range of 150–1000, using open-source “3D slicer” application (version 4.4.0). After cropping and keeping the area of interest, stereolithography (STL) files, which are a commonly used file format in the field of 3D printing, were exported. At this stage, mesh triangle complex in the produced STL files was refined and edited using “Autodesk Meshmixer” (version 11.0.544). G-code, a language used by the computer to communicate with the 3D printer and acts as an instruction to print layer by layer, was generated from the final STL file, using “ Slic3r” (version 1.2.9) on “Repetier-Host” software (version 1.5.4). After provision of the physical model [Figure 2a], the porous polyethylene plate (MEDPOR TM) was overlaid on the ledges of the defect and properly sized and trimmed ex vivo [Figure 2b–d]. It sent for sterilization before surgery.
Figure 2.
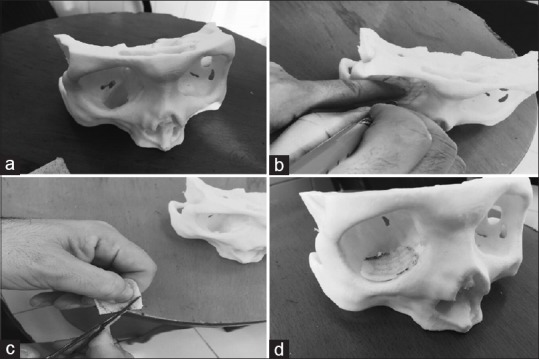
(a) Physical model constructed on the basis of Digital Imaging and Communications in Medicine dataset with a defect in the inferior orbital wall; (b and c) preoperative sizing and trimming of the implant; (d) solid model with the trimmed implant
The surgery was performed by transconjunctival approach without cantholysis. The preoperative orientation of the defect made finding and tracking the ledges of defect easier in a shorter time. The preprepared and trimmed implant was inserted over the defect. Passive movement of the globe was evaluated at the end of the surgery. There was no intraoperative complication.
At the 1st day visit, the hypoglobus was completely corrected. There was no enophthalmos. Diplopia was present only in upgaze. The improvement of symptoms continued postoperatively. Implant migration and infection did not occur. At the 1-month visit, diplopia in primary and reading position were abolished completely, and there was trivial diplopia only in extreme upgaze [Figure 3]. The esthetic outcome was pleasant.
Figure 3.
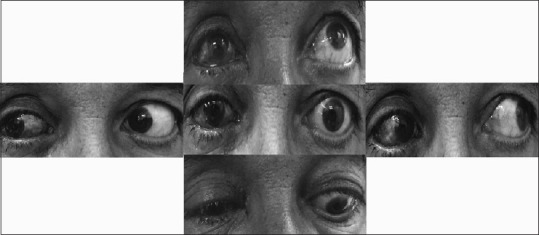
Ocular movements postoperatively
Discussion
With the advent of 3D and RP technology, they have been found applications in nearly every surgical field that needs patient-specific models.[4] With this technique, a customized and patient-specific medical template is accurately designed using a computer-aided design (CAD) technique.[5] RP uses 3D CAD data and could manufacture any 3D forms into physical objects.[6] A real object from a virtual model is generated in a CAD system.[7]
Our report is a simple and practical application of RP. In particular, there is no need for navigation-aided facilities. The unique aspect of this method in comparison with previous studies is that we directly trim and size the implant ex vivo on the 3D model [Figure 2]. We did not fabricate the implant by computer aided of mirroring reconstruction of 3D images as Oh et al. did in their study.[8] Advantages of this strategy are straightforward shaping and sizing of the implant. In addition, better preoperative understanding and orientation of orbital disruption, lower the number of attempts at positioning the implant in orbit. It decreases the trauma to periorbital tissues and effectively shortens the operation time. Its application is also beneficial for the novice surgeons who begin blowout fracture surgery.
There is a paucity of similar studies in the literature. In a similar study, Callahan et al. presented five cases of orbital reconstructions with the aid of 3D-printed implant templates.[9] First, the shape of an orbital defect was measured based on the intact contralateral orbit as a standard Tessellation language file, and then, the mock implant was printed. After sterilization, they used the mock implant intraoperatively as a stencil and shaped on the actual implant. They showed the practical application of relatively inexpensive 3D printing technology in the orbital reconstructions. Another application of this technology recently has been introduced by Alam et al.[10] is providing a custom-made ocular prosthesis in anophthalmic sockets with a highly encouraging outcome. Using CAD and rapid manufacturing technology and based on CT scan of wax model of the socket, a prototype model of an ocular prosthesis was fabricated. They showed that in comparison to the conventional prosthesis, the process of fabrication was shorter and it was more comfortable for the patient.
Our introduced method is an application of technological advances in 3D printing. It is a relatively inexpensive method without a need to special navigation setup [11] and with high accuracy. There is no need of relapsing the steep learning curve and on the other hand is optimal for beginner surgeons. Hence, this system appears to be well suited for orbital surgery by oculoplastic surgeons and improves clinical and esthetic outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Joseph JM, Glavas IP. Orbital fractures: A review. Clin Ophthalmol. 2011;5:95–100. doi: 10.2147/OPTH.S14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammer B, Prein J. Correction of post-traumatic orbital deformities: Operative techniques and review of 26 patients. J Craniomaxillofac Surg. 1995;23:81–90. doi: 10.1016/s1010-5182(05)80453-6. [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Zhang X. 3D printing: Print the future of ophthalmology. Invest Ophthalmol Vis Sci. 2014;55:5380–1. doi: 10.1167/iovs.14-15231. [DOI] [PubMed] [Google Scholar]
- 4.Hoang D, Perrault D, Stevanovic M, Ghiassi A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann Transl Med. 2016;4:456. doi: 10.21037/atm.2016.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan H, Fu J, Li X, Pei Y, Li X, Pei G, et al. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J Surg Oncol. 2015;13:308. doi: 10.1186/s12957-015-0723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoor P, Suomalainen A, Lindqvist C, Mesimäki K, Danielsson D, Westermark A, et al. Rapid prototyped patient specific implants for reconstruction of orbital wall defects. J Craniomaxillofac Surg. 2014;42:1644–9. doi: 10.1016/j.jcms.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Silva DN, Gerhardt de Oliveira M, Meurer E, Meurer MI, Lopes da Silva JV, Santa-Bárbara A, et al. Dimensional error in selective laser sintering and 3D-printing of models for craniomaxillary anatomy reconstruction. J Craniomaxillofac Surg. 2008;36:443–9. doi: 10.1016/j.jcms.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Oh TS, Jeong WS, Chang TJ, Koh KS, Choi JW. Customized orbital wall reconstruction using three-dimensionally printed rapid prototype model in patients with orbital wall fracture. J Craniofac Surg. 2016;27:2020–4. doi: 10.1097/SCS.0000000000003195. [DOI] [PubMed] [Google Scholar]
- 9.Callahan AB, Campbell AA, Petris C, Kazim M. Low-cost 3D printing orbital implant templates in secondary orbital reconstructions. Ophthal Plast Reconstr Surg. 2017;33:376–80. doi: 10.1097/IOP.0000000000000884. [DOI] [PubMed] [Google Scholar]
- 10.Alam MS, Sugavaneswaran M, Arumaikkannu G, Mukherjee B. An innovative method of ocular prosthesis fabrication by bio-CAD and rapid 3-D printing technology: A pilot study. Orbit. 2017;36:223–7. doi: 10.1080/01676830.2017.1287741. [DOI] [PubMed] [Google Scholar]
- 11.Herford AS, Miller M, Lauritano F, Cervino G, Signorino F, Maiorana C, et al. The use of virtual surgical planning and navigation in the treatment of orbital trauma. Chin J Traumatol. 2017;20:9–13. doi: 10.1016/j.cjtee.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


