Abstract
Background
Two previous studies of SB742457, a 5-hydroxytryptamine (5-HT6) receptor antagonist, suggested the efficacy of improvements in cognition and global outcome in Alzheimer's disease (AD).
Methods
Two randomized, placebo-controlled trials investigated SB742457 15 and 35 mg daily in subjects with mild-to-moderate AD (Mini-Mental Health State Examination [MMSE] 10–26). Study 1 (n = 576) investigated SB742457 and donepezil (5–10 mg daily) as monotherapy for 6 months. Study 2 (n = 684) investigated SB742457 in subjects who were maintained on donepezil. Coprimary endpoints at 24 weeks assessed cognition (AD Assessment Scale-Cognitive Subscale [ADAS-Cog]) and global outcome (Study 1: Clinician Interview-Based Impression of Change Plus Caregiver Input [CIBIC+]; Study 2: Clinical Dementia Rating-Sum of Boxes [CDR-SB]). Safety was assessed throughout.
Results
Both studies failed to achieve formal statistical significance for their primary objectives. Study 1: SB742457 monotherapy was not statistically significantly different from placebo on any endpoint. Donepezil improved CIBIC+ but not ADAS-Cog. Study 2: SB742457 35 mg showed statistically significant differences relative to placebo for ADAS-cog (weeks 12, 24, and 48, but not week 36), ADCS-ADL (weeks 12–36, but not week 48), and CDR-SB (week 12 only).
Conclusion
Neither study met the overall criteria for success, but as an adjunct to donepezil, SB742457 was associated with sustained improvements for up to 48 weeks in cognition and ADL, compared with donepezil alone.
Clinical Trial Registration: Clinicaltrials.gov: Study 1 NCT00708552; Study 2 NCT00710684.
Keywords: 5-HT6 antagonist, Alzheimer's disease, ADAS-Cog, CIBIC+, CDR-SB, Donepezil
1. Background
Serotonin (5-hydroxytryptamine [5-HT]) plays a role in cognition and behavior, therefore, pharmacological agents that impact 5-HT pathways are of interest in Alzheimer's disease (AD). In particular, 5-HT6 receptors are found almost exclusively within the central nervous system in regions associated with cognition and behavior, including the striatum, nucleus accumbens, and to a lesser extent the hippocampus and cerebral cortex [1].
SB742457 is a potent and selective 5-HT6 receptor antagonist shown to reverse scopolamine-induced deficits in a rodent test of novel object recognition, and to enhance performance of a water maze task by aged rats [1]. Other 5-HT6 receptor antagonists have also shown activity in these tests and in similar models [2], [3], [4]. Results from two previous Phase II studies of SB742457 as monotherapy, suggested some improvements in cognition and global function in patients with mild-to-moderate AD [5], [6]. Post hoc analyses suggested that effects on cognition, measured using the AD Assessment Scale-cognitive subscale (ADAS-Cog), may be more evident in patients with moderate AD than in those with mild AD.
5-HT6 antagonists have modulatory effects on cholinergic, glutamatergic, and monoaminergic systems [7], [8], [9], [10], [11]. Although the relative contributions of these neurotransmitter effects on cognition is unknown, this action is clearly distinct from that of acetylcholinesterase inhibitors (AChEIs) [3], [4], which are approved for the symptomatic management of mild-to-moderate AD [12]. An adjunctive therapy that acts via a different neurotransmitter system to AChEIs could offer enhanced cognitive benefits. There are currently no treatments approved for adjunct therapy to AChEIs in mild-to-moderate AD, but preliminary data suggest that there are additive effects with AChEIs and 5-HT6 antagonists in preclinical cognition models [13].
Here, the results of two Phase II studies in patients with probable mild-to-moderate AD are presented:
-
•
Study 1 was performed to investigate the efficacy and safety of SB742457 15 and 35 mg when used as monotherapy for 24 weeks, compared with placebo. Donepezil was also included as an active control.
-
•
Study 2 examined the same doses of SB742457 as an adjunct treatment to donepezil therapy over 48 weeks, compared with placebo.
2. Methods
2.1. Study details
The study design and methodology are summarized later. Additional details are provided in the Technical Appendix. Study 1 (GlaxoSmithKline study: AZ3110865; ClinicalTrials.gov identifier: NCT00708552) and Study 2 (GlaxoSmithKline study: AZ3110866; ClinicalTrials.gov identifier: NCT00710684) were multicenter, double-blind, randomized, placebo-controlled, parallel-group trials.
Study 1 was conducted from July 2008 to March 2010 in 68 centers across 11 countries (Bulgaria, Chile, Czech Republic, Estonia, Germany, Greece, Korea, Mexico, Poland, Russia, and South Africa). Study 2 was conducted from July 2008 to November 2010 in 97 centers across nine countries (Argentina, Australia, Canada, Chile, Czech Republic, Germany, Italy, Spain, and USA).
The studies were conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was reviewed and approved by properly constituted ethics committees (national, regional, or investigational) or institutional review boards at each participating institution.
2.2. Research participants
Eligible participants were aged 50 to 85 years inclusive; met the criteria for probable AD in accordance with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria [14] and National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association criteria [15]; had a stable Mini-Mental Status Examination (MMSE) [16] score of 10 to 26 before randomization (with ≤3-point variation from screening to the end of the run-in period); had a Hachinski Ischemia score [17] of ≤4 at screening; and had a regular caregiver to assess cognitive function, activities of daily living (ADL), and behavior. In Study 2, participants were also required to have ≥6 months' history of donepezil therapy, with 2 months at a stable dose and no intent to change during the study.
Exclusion criteria included: significant psychiatric illness; history/evidence of another cause of dementia; history of seizures; known history of drug-related photosensitivity; abnormal findings precluding participation; or use of AChEIs (with the exception of donepezil in Study 2), memantine, selegiline, monoamine oxidase inhibitors, conventional antipsychotics, an investigational drug, or treatment with potential for interaction with SB742457. Antidepressants (other than monoamine oxidase inhibitors), thyroid hormones, atypical antipsychotics, benzodiazepines, and other sedatives/hypnotics were permitted conditionally if prescribed at a stable dose for ≥2 months before entry.
2.3. Study procedures
In both studies, research participants entered a 2-week screening period and a 4-week single-blind placebo run-in period.
-
•
In Study 1, participants then entered a 24-week treatment phase, receiving SB742457 (15 or 35 mg), donepezil (5–10 mg [an active control to assess assay sensitivity]), or placebo.
-
•
In Study 2, participants taking stable donepezil therapy initially entered a 24-week treatment phase, receiving SB742457 (15 or 35 mg) or placebo. At the end of the 24-week treatment period, participants were offered the opportunity to continue the treatment phase from 24 to 48 weeks, on their existing medication.
In both studies a follow-up visit was performed 2 weeks after the last treatment dose.
2.4. Randomization and masking
In both studies, SB742457 35 and 15 mg and matching placebo were provided by GlaxoSmithKline as tablets, and were indistinguishable in appearance, smell, and taste. For Study 1, donepezil was externally sourced by GlaxoSmithKline and encapsulated to provide 5 mg, 10 mg, and matching placebo capsules.
In Study 1, research participants were randomized in a 1:1:1:1 ratio, in random permuted blocks of 8, to SB742457 (15 or 35 mg), donepezil 5–10 mg, or placebo. Supplies were double-dummy; subjects received one tablet of SB742457 or matching placebo and also one capsule of donepezil or matching placebo each evening.
In Study 2, randomization was in blocks of 6, in a 1:1:1 ratio to SB742457 (15 or 35 mg) or placebo, in addition to their ongoing background donepezil therapy. The randomization schedule was generated by GlaxoSmithKline and implemented using an interactive voice-response system. Randomization in both studies was stratified according to baseline MMSE score (MMSE 10–15 ≤30% subjects; MMSE 16–20 ≤60% subjects; and MMSE 21–26 ≤30% subjects) to ensure a balance of subjects across the mild-to-moderate range.
2.5. Outcomes and assessments
Efficacy assessments were conducted at screening and then at the end of the run-in period to provide a baseline assessment and then at weeks 12 and 24 in both studies, with further assessments at weeks 36 and 48 in Study 2. Change from baseline in ADAS-Cog at week 24 was a coprimary endpoint in both studies. The Clinician Interview-Based Impression of Change plus Caregiver Input (CIBIC+) score [18] at week 24 was the coprimary global endpoint in Study 1, and the Clinical Dementia Rating-Sum of Boxes (CDR-SB) [19] at week 24 was the coprimary global endpoint in Study 2. In a post hoc analysis the primary endpoints were also assessed across two subgroups according to the baseline MMSE score (mild, MMSE 20–26; moderate, MMSE 10–19).
Secondary endpoints for both studies included the AD Co-operative Study-Activities of Daily Living Scale (ADCS-ADL) [20], the Repeatable Battery for Assessment of Neuropsychological Status (RBANS) [21], and the MMSE [16]. The Cornell Scale for Depression in Dementia (CSDD) [22] was an additional secondary endpoint in Study 1.
Standard safety assessments were performed at all visits, including physical examinations, pregnancy tests (if applicable), and assessments of adverse events (AEs), serious AEs (SAEs), vital signs, and electrocardiograms (ECGs). Routine laboratory assessments of clinical chemistry, hematology, and urinalysis parameters were also performed. As Study 2 was the first to coadminister SB742457 and donepezil, safety and pharmacokinetic data were reviewed by an Independent Data Monitoring Committee (IDMC) to evaluate potential interactions.
Pharmacokinetic parameters assessed in Study 2 included steady-state concentrations and area under the curve. Potential pharmacodynamic interactions between SB742457 and donepezil were assessed in a subgroup of subjects based on red blood cell acetylcholinesterase activity.
2.6. Statistical analysis
The studies were powered to detect a standardized effect size (treatment difference/standard deviation [SD]) of 0.42 for Study 1 and 0.33 for Study 2, with 90% power and a 0.05 significance level, under an assumed SD. This relates to a difference of 2.5 and 2 points in change from baseline in ADAS-Cog for Study 1 and 2, respectively (under an assumed SD of 6), and a difference of 0.47 for CIBIC+ (under an assumed SD of 1.12) for Study 1 and a difference in change from baseline in CDR-SB of 0.6 for Study 2 (under an assumed SD of 1.8). This required 122 and 190 subjects per treatment group for Study 1 and 2, respectively. Assuming a postrandomization drop-out rate of 15%, 576 subjects were planned for randomization in Study 1 and 672 subjects were planned for Study 2.
The two coprimary endpoints in each study needed to achieve a significance level of 0.05 at the same dose, to maintain an overall 5% significance level. A hierarchy was followed to account for the multiplicity of doses, with SB742457 35 mg being the primary comparison. Formal inferential testing was only performed for SB742457 15 mg if the first comparison, on both coprimary endpoints was significant. Primary inference was conducted using a mixed model for repeated measures analysis using restricted maximum likelihood estimation [23]. Other P values presented have been interpreted in light of the testing hierarchy; the analyses of all secondary endpoints were considered exploratory and were not interpreted inferentially.
The intent-to-treat (ITT) population used in efficacy analyses comprised all randomized subjects who took ≥1 dose of study medication and who had ≥1 postbaseline efficacy assessment. The safety population consisted of all randomized subjects who took ≥1 dose of study medication.
3. Results
3.1. Subject disposition and demographics
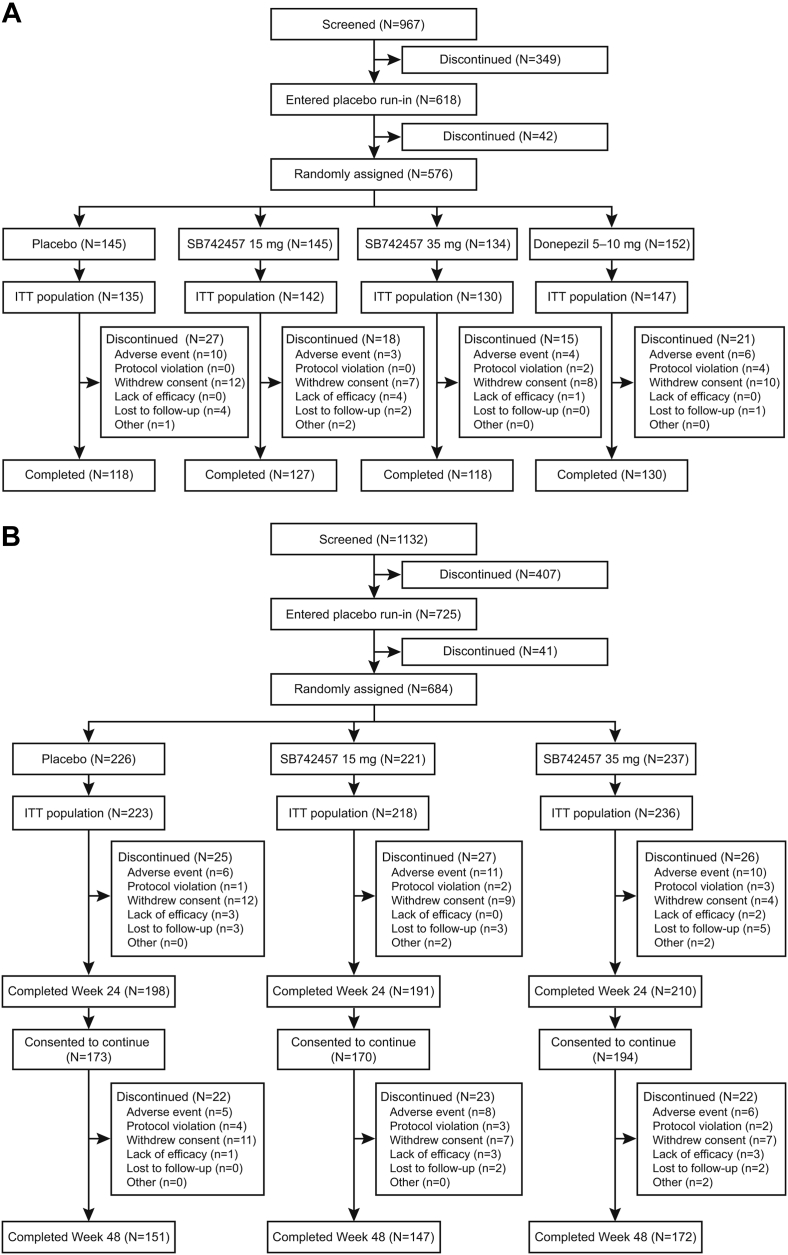
The screening, randomization, and completion rate of subjects in each study are outlined in Fig. 1. In Study 1, 967 subjects were screened, 576 were randomized, and 493 (86%) completed the 24-week trial. In Study 2, 1132 subjects were screened and 684 were randomized. Of the 599 (88%) subjects who completed the 24-week treatment period, 537 consented to continue treatment to week 48. There were 67 discontinuations during the second 6 months of the trial and 470 (69%) subjects completed the 48-week study. The IDMC found no reason to stop or alter the conduct or design of the study.
Fig. 1.
Flow of subjects through Study 1 (A) and Study 2 (B). ITT, intent-to-treat.
The most common reasons for withdrawal across the studies were AEs and withdrawn consent (Fig. 1).
Demographic and baseline characteristics were similar across all treatment groups (Table 1). Most subjects in each study (>90%) were white and approximately 60% were female. The mean baseline MMSE score of 18 in each study reflected the stratified study designs and was consistent with the subject populations having mild-to-moderate AD.
Table 1.
Summary of baseline demographic, AD characteristics, and efficacy assessment scores
| Population | Study 1 |
Study 2 |
|||||
|---|---|---|---|---|---|---|---|
| Placebo (N = 135) | SB742457 15 mg (N = 142) | SB742457 35 mg (N = 130) | Donepezil 5–10 mg (N = 147) | Placebo + donepezil (N = 223) | SB742457 15 mg + donepezil (N = 218) | SB742457 35 mg + donepezil (N = 236) | |
| Gender, % male:female | 36:64 | 32:68 | 42:58 | 35:65 | 42:58 | 46:54 | 37:63 |
| Age, yrs | 73.3 (6.80) | 72.4 (8.12) | 72.5 (7.38) | 71.1 (7.49) | 73.1 (7.49) | 74.2 (6.82) | 73.8 (6.92) |
| BMI, kg/m2 | 26.2 (4.04) | 26.3 (4.44) | 26.3 (3.97) | 26.1 (4.10) | 26.2 (4.44) | 26.5 (4.57) | 26.2 (4.33) |
| Education, yrs | 11.5 (3.66) | 11.0 (3.73) | 10.7 (3.43) | 11.3 (3.56) | 10.3 (4.0) | 10.0 (3.8) | 10.4 (4.0) |
| Family history of AD: yes, n (%) | 30 (22) | 28 (20) | 24 (18) | 29 (20) | 72 (32) | 59 (27) | 63 (27) |
| Median time since first diagnosis, yrs | 1.1 | 1.1 | 0.7 | 0.9 | 2.1 | 1.7 | 1.9 |
| MMSE | 18.2 (3.88) | 18.7 (3.68) | 18.6 (3.64) | 18.7 (3.75) | 18.4 (3.94) | 18.8 (3.71) | 18.7 (3.87) |
| Median MMSE (range) | 18 (10–26) | 19 (10–26) | 19 (11–26) | 19 (10–26) | 19 (10–26) | 19 (10–26) | 19 (10–26) |
| ADAS-Cog | 29.1 (12.43) | 27.9 (10.72) | 28.2 (10.80) | 26.2 (11.41) | 27.1 (11.29) | 26.5 (10.11) | 27.1 (10.76) |
| CDR-SB | – | – | – | – | 7.11 (3.514) | 6.94 (3.382) | 7.07 (3.433) |
| RBANS total | 94.3 (38.67) | 98.0 (36.98) | 97.2 (38.79) | 100 (41.28) | 92.1 (37.51) | 98.0 (36.00) | 96.3 (36.33) |
| ADCS-ADL total | 51.5 (17.78) | 52.4 (16.31) | 52.2 (15.07) | 53.1 (15.88) | 52.1 (16.36) | 51.4 (16.37) | 51.5 (16.30) |
Abbreviations: AD, Alzheimer's disease; BMI, body mass index; MMSE, Mini-Mental Status Examination; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; ADCS-ADL, Alzheimer's Disease Co-operative Study-Activities of Daily Living Scale; CDR-SB, Clinical Dementia Rating-Sum of Boxes; RBANS, Repeatable Battery for Assessment of Neuropsychological Status; SD, standard deviation.
NOTE. Data presented as mean (SD) unless otherwise stated.
3.2. Efficacy assessments
3.2.1. Study 1 (monotherapy)
At week 24, none of the treatment groups (SB742457 35 or 15 mg, or donepezil) produced a statistically significant difference on ADAS-Cog versus placebo (Table 2). For CIBIC+, there was no significant difference between placebo and either dose of SB742457, but donepezil was associated with a significant improvement versus placebo (P = .049). The observed SD values were found to compare well with the protocol assumptions (see Supplementary Materials for further details). No statistically significant baseline MMSE treatment interactions were observed (Table 3).
Table 2.
Summary of repeated measures analysis of change from baseline in primary and secondary endpoints in Study 1 and Study 2
| Assessment | Study 1 |
Study 2 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 12 |
Week 24 |
Week 24† |
Week 48‡ |
||||||||||||
| n |
Adjusted mean (SE) |
Difference vs placebo (95% CI) |
n |
Adjusted mean (SE) |
Difference vs placebo (95% CI) |
P-value∗ |
n |
Adjusted mean (SE) |
Difference vs placebo (95% CI) |
P-value∗ |
n |
Adjusted mean (SE) |
Difference vs placebo (95% CI) |
||
| Secondary endpoints | Coprimary endpoints | Coprimary endpoints | Secondary endpoints | ||||||||||||
| ADAS-Cog | |||||||||||||||
| Placebo§ | 124 | −0.1 (0.45) | 116 | −0.3 (0.56) | 193 | 1.2 (0.45) | 145 | 3.4 (0.52) | |||||||
| SB742457 15 mg§ | 133 | 0.0 (0.46) | 0.1 (−1.1, 1.4) | 124 | 0.8 (0.58) | 1.1 (−0.4, 2.7) | .159 | 184 | 0.5 (0.44) | −0.7 (−1.9, 0.5) | .279 | 142 | 3.4 (0.60) | −0.1 (−1.6, 1.5) | |
| SB742457 35 mg§ | 126 | −0.2 (0.45) | −0.1 (−1.4, 1.1) | 115 | 0.4 (0.62) | 0.7 (−0.9, 2.3) | .410 | 200 | −0.4 (0.41) | −1.5 (−2.7, −0.3) | .012 | 170 | 1.8 (0.50) | −1.6 (−3.1, −0.2) | |
| Donepezil 5–10 mg | 137 | −0.6 (0.40) | −0.5 (−1.6, 0.7) | 123 | −0.5 (0.45) | −0.2 (−1.6, 1.2) | .821 | – | – | – | – | – | – | – | |
| CIBIC+ (Study 1)/CDR-SB (Study 2) | |||||||||||||||
| Placebo§ | 126 | 3.9 (0.08) | 118 | 4.0 (0.11) | 191 | 0.9 (0.13) | 146 | 1.6 (0.16) | |||||||
| SB742457 15 mg§ | 133 | 4.0 (0.08) | 0.1 (−01, 0.3) | 125 | 4.2 (0.10) | 0.2 (−0.1, 0.5) | .254 | 184 | 0.8 (0.13) | −0.1 (−0.4, 0.3) | .711 | 142 | 1.9 (0.20) | 0.3 (−0.2, 0.8) | |
| SB742457 35 mg§ | 126 | 3.9 (0.09) | 0.0 (−0.2, 0.2) | 117 | 3.9 (0.10) | −0.1 (−0.4, 0.2) | .394 | 200 | 0.7 (0.11) | −0.1 (−0.5, 0.2) | .462 | 170 | 1.5 (0.16) | −0.1 (−0.5, 0.4) | |
| Donepezil 5–10 mg | 139 | 3.7 (0.08) | −0.2 (−0.4, 0.0) | 127 | 3.7 (0.10) | −0.3 (−0.6, 0.0) | .049 | – | – | – | – | – | – | – | |
| Secondary endpoints |
Secondary endpoints |
||||||||||||||
| ADCS-ADL total | |||||||||||||||
| Placebo§ | 126 | −0.4 (0.59) | 118 | −1.0 (0.71) | 191 | −3.2 (0.65) | 147 | −5.5 (0.85) | |||||||
| SB742457 15 mg§ | 133 | −1.1 (0.65) | −0.7 (−2.4, 1.0) | 123 | −1.4 (0.68) | −0.3 (−2.3, 1.6) | 184 | −1.9 (0.62) | 1.3 (−0.4, 3.1) | 144 | −5.0 (0.87) | 0.5 (−1.9, 2.9) | |||
| SB742457 35 mg§ | 124 | −0.1 (0.64) | 0.3 (−1.4, 2.0) | 114 | −1.1 (0.81) | −0.1 (−2.2, 2.0) | 202 | −1.3 (0.61) | 1.9 (0.2, 3.6) | 171 | −3.5 (0.76) | 1.9 (−0.3, 4.2) | |||
| Donepezil 5–10 mg | 139 | 0.2 (0.62) | 0.6 (−1.1, 2.2) | 128 | −1.2 (0.82) | −0.1 (−2.3, 2.0) | – | – | – | – | – | ||||
| RBANS | |||||||||||||||
| Placebo§ | 125 | −6.7 (1.16) | 116 | −2.3 (1.44) | 190 | −3.6 (1.18) | 143 | −7.3 (1.36) | |||||||
| SB742457 15 mg§ | 134 | −8.7 (1.24) | −1.9 (−5.2, 1.4) | 125 | −4.0 (1.42) | −1.6 (−5.6, 2.3) | 176 | −5.9 (1.29) | −2.4 (−5.8, 1.1) | 136 | −9.4 (1.45) | −2.1 (−6.0, 1.8) | |||
| SB742457 35 mg§ | 125 | −5.9 (1.11) | 0.9 (−2.2, 4.0) | 115 | −4.4 (1.49) | −2.1 (−6.1, 1.9) | 193 | −4.0 (1.09) | −0.5 (−3.6, 2.7) | 164 | −4.7 (1.25) | 2.6 (−1.0, 6.2) | |||
| Donepezil 5–10 mg | 138 | −3.4 (1.06) | 3.4 (0.3, 6.4) | 127 | −0.3 (1.21) | 2.0 (−1.7, 5.7) | – | – | – | – | – | – | |||
| MMSE | |||||||||||||||
| Placebo§ | Not assessed | 118 | −0.3 (0.29) | 195 | −0.4 (0.21) | 149 | −1.1 (0.28) | ||||||||
| SB742457 15 mg§ | 124 | −0.3 (0.28) | 0.0 (−0.8, 0.8) | 187 | −0.3 (0.23) | 0.0 (−0.6, 0.6) | 145 | −1.3 (0.33) | −0.1 (−1.0, 0.7) | ||||||
| SB742457 35 mg§ | 117 | −0.1 (0.29) | 0.3 (−0.5, 1.1) | 202 | −0.0 (0.21) | 0.3 (−0.2, 0.9) | 172 | −0.7 (0.27) | 0.4 (−0.3, 1.2) | ||||||
| Donepezil 5–10 mg | 128 | 0.5 (0.27) | 0.8 (0.0, 1.6) | – | – | – | – | – | – | ||||||
| CSDD | |||||||||||||||
| Placebo§ | Not assessed | 118 | 0.0 (0.26) | Not assessed | |||||||||||
| SB742457 15 mg§ | 125 | −0.1 (0.25) | −0.2 (−0.9, 0.5) | ||||||||||||
| SB742457 35 mg§ | 117 | 0.3 (0.26) | 0.2 (−0.5, 0.9) | ||||||||||||
| Donepezil 5–10 mg | 128 | 0.3 (0.25) | 0.3 (−0.4, 1.0) | ||||||||||||
Abbreviations: SE, standard error; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; CIBIC+, Clinician Interview-Based Impression of Change Plus Caregiver Input; ADCS-ADL, Alzheimer's Disease Co-operative Study-Activities of Daily Living Scale; CI, confidence interval; CDR-SB, Clinical Dementia Rating-Sum of Boxes; CI, confidence interval; CSDD, Cornell Scale for Depression in Dementia; MMSE, Mini-Mental Status Examination; RBANS, Repeatable Battery for Assessment of Neuropsychological Status.
NOTE. ADAS-Cog and CDR-SB: a negative difference represents benefit over placebo.
Only P values for the primary endpoints are shown.
From the week 24 analysis data set (includes all data from the primary analysis, up to and including the week 24 visit).
From the week 48 analysis data set (includes all data from the analysis of the complete and final data set, up to and including the week 48 visit).
In Study 2, SB742457 and placebo were administered as an adjunct to stable donepezil therapy.
Table 3.
Summary of repeated measures analysis of change from baseline in ADAS-Cog total score, CIBIC+, and CDR-SB in mild and moderate groups
| Assessment | Study 1 |
Study 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 24 |
Week 24∗ |
Week 48† |
|||||||
| n |
Adjusted mean (SE) |
Difference vs placebo (95% CI) |
n |
Adjusted mean (SE) |
Difference vs placebo (95% CI) |
n |
Adjusted mean (SE) |
Difference vs placebo (95% CI) |
|
| cDAS-Cog | cDAS-Cog | ||||||||
| Mild (MMSE 20–26) | |||||||||
| Placebo‡ | 50 | −1.2 (0.80) | 89 | 1.2 (0.66) | 66 | 1.9 (0.70) | |||
| SB742457 15 mg‡ | 55 | −0.2 (0.77) | 1.0 (−1.2, 3.1) | 78 | −0.1 (0.56) | −1.3 (−3.0, 0.4) | 64 | 1.9 (0.85) | 0.0 (−2.1, 2.2) |
| SB742457 35 mg‡ | 50 | −1.0 (0.97) | 0.2 (−2.3, 2.6) | 102 | −1.2 (0.45) | −2.4 (−4.0, −0.8)∗ | 89 | 0.5 (0.60) | −1.3 (−3.1, 0.5) |
| Donepezil 5–10 mg | 56 | −1.4 (0.66) | −0.3 (−2.3, 1.7) | – | – | – | – | – | – |
| Moderate (MMSE 10–19) | |||||||||
| Placebo‡ | 66 | 0.3 (0.78) | 104 | 1.2 (0.61) | 79 | 4.8 (0.77) | |||
| SB742457 15 mg‡ | 69 | 1.7 (0.86) | 1.4 (−0.9, 3.7) | 106 | 1.2 (0.63) | 0.1 (−1.7, 1.8) | 78 | 4.9 (0.86) | 0.1 (−2.1, 2.4) |
| SB742457 35 mg‡ | 65 | 1.7 (0.78) | 1.4 (−0.8, 3.5) | 98 | 0.5 (0.69) | −0.7 (−2.5, 1.1) | 81 | 3.0 (0.80) | −1.8 (−4.0, 0.4) |
| Donepezil 5–10 mg | 67 | 0.3 (0.64) | 0.0 (−2.0, 2.0) | – | – | – | – | – | – |
| CIBIC+ |
CDR-SB |
||||||||
| Mild (MMSE 20–26) | |||||||||
| Placebo‡ | 50 | 3.6 (0.14) | 88 | 0.4 (0.16) | 63 | 0.9 (0.19) | |||
| SB742457 15 mg‡ | 56 | 3.9 (0.13) | 0.3 (−0.1, 0.6) | 76 | 0.5 (0.17) | 0.1 (−0.3, 0.6) | 64 | 1.4 (0.30) | 0.5 (−0.2, 1.2) |
| SB742457 35 mg‡ | 50 | 3.6 (0.16) | −0.1 (−0.5, 0.3) | 100 | 0.3 (0.13) | 0.0 (−0.4, 0.3) | 88 | 1.0 (0.21) | 0.1 (−0.5, 0.6) |
| Donepezil 5–10 mg | 56 | 3.4 (0.16) | −0.2 (−0.7, 0.2) | – | – | – | – | – | – |
| Moderate (MMSE 10–19) | |||||||||
| Placebo‡ | 68 | 4.3 (0.16) | 103 | 1.2 (0.21) | 83 | 2.1 (0.24) | |||
| SB742457 15 mg‡ | 69 | 4.4 (0.14) | 0.1 (−0.3, 0.6) | 108 | 1.0 (0.19) | −0.3 (−0.8, 0.3) | 78 | 2.3 (0.27) | 0.2 (−0.5, 0.9) |
| SB742457 35 mg‡ | 67 | 4.2 (0.13) | −0.1 (−0.5, 0.3) | 100 | 1.0 (0.18) | −0.2 (−0.7, 0.3) | 82 | 1.9 (0.24) | −0.2 (−0.9, 0.5) |
| Donepezil 5–10 mg | 71 | 4.0 (0.13) | −0.3 (−0.7, 0.1) | – | – | – | – | – | – |
Abbreviations: SE, standard error; CI, confidence interval; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; MMSE, Mini-Mental Status Examination; CDR-SB, Clinical Dementia Rating-Sum of Boxes; CIBIC+, Clinician Interview-Based Impression of Change Plus Caregiver Input.
ADAS-Cog, CIBIC+, and CDR-SB: a negative difference represents benefit over placebo. *P < .01.
From the week 24 analysis data set (includes all data from the primary analysis, up to and including the week 24 visit).
From the week 48 analysis data set (includes all data from the analysis of the complete and final data set, up to and including the week 48 visit).
In Study 2, SB742457 and placebo were administered as an adjunct to stable donepezil therapy.
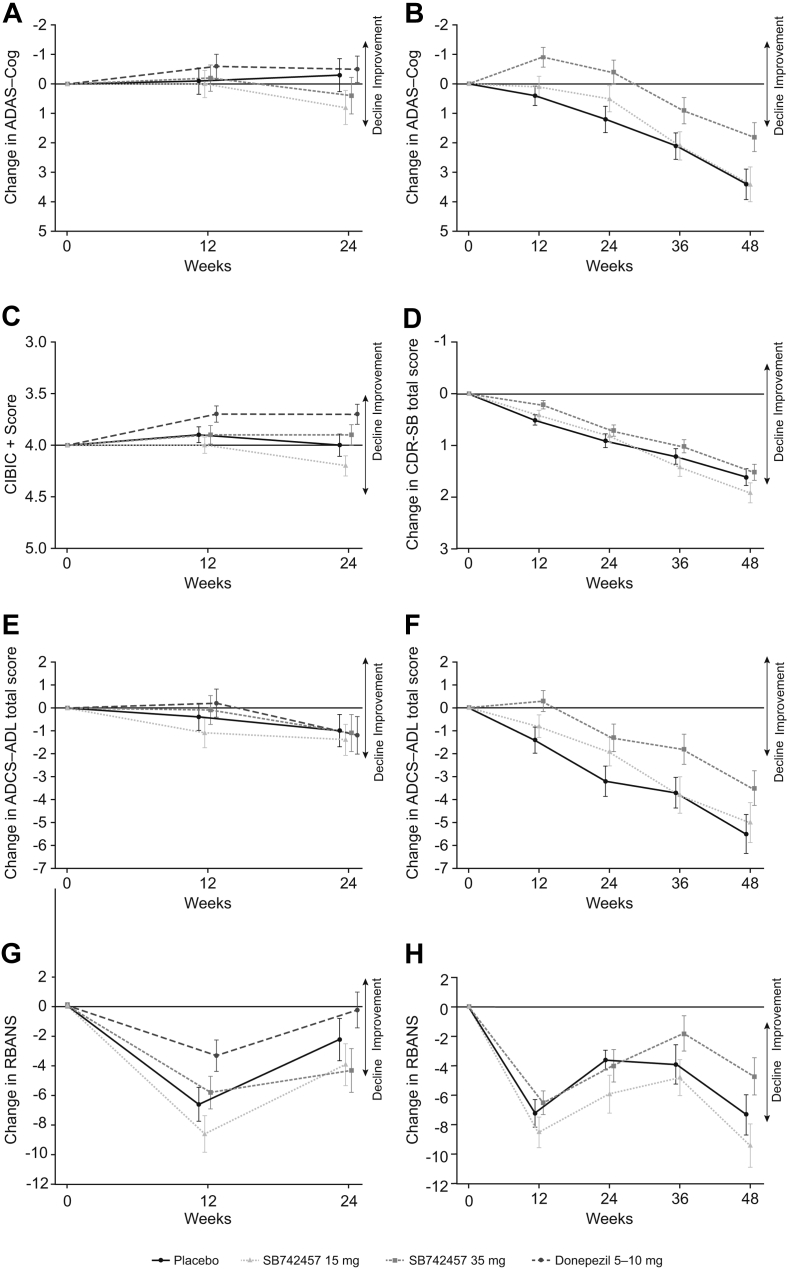
There were also no statistically significant differences between SB742457 and placebo on the secondary endpoints in the study, namely ADCS-ADL, RBANS, MMSE, or CSDD (Table 2; Fig. 2). Donepezil was associated with a significant improvement in MMSE versus placebo (P = .044) but not for other endpoints.
Fig. 2.
Results from analysis of change from baseline using a MMRM model in the assessment of treatment effect of SB742457 on the ADAS-Cog (A, B), CIBIC+ (C), CDR-SB (D), ADCS-ADL (E, F), and RBANS (G, H) in the ITT population of Study 1 (panels A, C, E, G) and 2 (panels B, D, F, H). Data presented as adjusted mean ± 95% CI. Abbreviations: MMRM, mixed model for repeated measures; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; CDR-SB, Clinical Dementia Rating-Sum of Boxes; CI, confidence interval; CIBIC+, Clinician Interview-Based Impression of Change Plus Caregiver Input; ADCS-ADL, Alzheimer's Disease Co-operative Study-Activities of Daily Living Scale; RBANS, Repeatable Battery for Assessment of Neuropsychological Status; ITT, intent-to-treat.
3.2.2. Study 2 (adjunct to donepezil)
The study demonstrated a statistically significant improvement in ADAS-Cog for SB742457 35 mg versus placebo at week 24 (P = .012; Table 2). The effects at weeks 36 and 48 were consistent with the primary analyses at week 24 (Fig. 2), with a difference of 1.6 points (P = .024) versus placebo at week 48. For CDR-SB, there was no statistically significant difference between SB742457 35 mg and placebo at week 24 or week 48 (Table 2). Overall, the observed SD values compared well with the protocol assumptions (see Supplementary Materials for further details). However, the study failed to meet the requirement for statistically significant efficacy on both coprimary endpoints for SB742457 35 mg, and, in accordance with the prespecified testing hierarchy, inferential testing for SB742457 15 mg was not performed.
An increase in ADCS-ADL was observed with SB742457 35 mg at week 12, and at week 24 the improvement was statistically significant versus placebo (P = .033; Table 2). This improvement was maintained through to week 48, although at week 48 the difference was not statistically significant (P = .088) (Fig. 2). Significant differences versus placebo were not observed for RBANS or MMSE (Table 2; Fig. 2). No statistically significant baseline MMSE treatment interactions were observed for the full ITT population, at either week 24 or week 48 (Table 3).
3.3. Safety and tolerability
On-treatment AEs were reported by 33% of subjects in Study 1. The cumulative incidence of on-treatment AEs in Study 2 was 60% at week 48. Within each study, the proportion of subjects reporting any on-treatment AE was similar across the treatment groups (Table 4, Table 5). Most AEs in both studies were of mild-to-moderate intensity. Headache, nasopharyngitis, and diarrhea were among the top five most common AEs for both studies (Table 4, Table 5).
Table 4.
On-treatment adverse events in ≥2% of subjects in any treatment arm (Study 1)
| Preferred term | Number of subjects, n (%) |
||||
|---|---|---|---|---|---|
| SB742457, 35 mg (N = 133) | SB742457, 15 mg (N = 145) | Donepezil, 5–10 mg (N = 151) | Placebo (N = 145) | Total, N = 574 | |
| Any AE | 39 (29) | 42 (29) | 65 (43) | 45 (31) | 191 (33) |
| Headache | 2 (2) | 5 (3) | 5 (3) | 6 (4) | 18 (3) |
| Nasopharyngitis | 3 (2) | 3 (2) | 4 (3) | 3 (2) | 13 (2) |
| Dizziness | 2 (2) | 3 (2) | 5 (3) | 1 (<1) | 11 (2) |
| Influenza | 3 (2) | 2 (1) | 4 (3) | 2 (1) | 11 (2) |
| Diarrhea | 2 (2) | 0 | 3 (2) | 5 (3) | 10 (2) |
| Nausea | 1 (<1) | 2 (1) | 5 (3) | 2 (1) | 10 (2) |
| Urinary tract infection | 3 (2) | 4 (3) | 3 (2) | 0 | 10 (2) |
| Insomnia | 1 (<1) | 2 (1) | 5 (3) | 0 | 8 (1) |
| Back pain | 0 | 1 (<1) | 3 (2) | 3 (2) | 7 (1) |
| Bronchitis | 1 (<1) | 3 (2) | 1 (<1) | 2 (1) | 7 (1) |
| Hyperglycemia | 2 (2) | 3 (2) | 1 (<1) | 1 (<1) | 7 (1) |
| Fall | 2 (2) | 0 | 1 (<1) | 3 (2) | 6 (1) |
| Hypertension | 1 (<1) | 1 (<1) | 4 (3) | 0 | 6 (1) |
| Agitation | 2 (2) | 1 (<1) | 0 | 0 | 3 (<1) |
| Nightmare | 0 | 0 | 3 (2) | 0 | 3 (<1) |
| Hypercholesterolemia | 2 (2) | 0 | 0 | 0 | 2 (<1) |
Abbreviation: AE, adverse event.
Table 5.
On-treatment adverse events in ≥2% of subjects in any treatment arm (Study 2, week 48 analysis)
| Number of subjects, n (%) |
||||
|---|---|---|---|---|
| SB742457 35 mg + donepezil (N = 236) | SB742457 15 mg + donepezil (N = 221) | Placebo + donepezil (N = 225) | Total (N = 682) | |
| Any AE | 146 (62) | 137 (62) | 125 (56) | 408 (60) |
| Nasopharyngitis | 18 (8) | 19 (9) | 17 (8) | 54 (8) |
| Urinary tract infection | 13 (6) | 11 (5) | 16 (7) | 40 (6) |
| Diarrhea | 11 (5) | 12 (5) | 9 (4) | 32 (5) |
| Fall | 5 (2) | 4 (2) | 13 (6) | 22 (3) |
| Headache | 7 (3) | 8 (4) | 6 (3) | 21 (3) |
| Hypertension | 7 (3) | 5 (2) | 7 (3) | 19 (3) |
| Bronchitis | 9 (4) | 4 (2) | 3 (1) | 16 (2) |
| Nausea | 4 (2) | 4 (2) | 8 (4) | 16 (2) |
| Dizziness | 3 (1) | 5 (2) | 7 (3) | 15 (2) |
| Cough | 8 (3) | 4 (2) | 2 (<1) | 14 (2) |
| Back pain | 4 (2) | 4 (2) | 5 (2) | 13 (2) |
| Pneumonia | 7 (3) | 1 (<1) | 4 (2) | 12 (2) |
| Contusion | 6 (3) | 1 (<1) | 3 (1) | 10 (1) |
| Insomnia | 6 (3) | 2 (<1) | 2 (<1) | 10 (1) |
| Edema peripheral | 1 (<1) | 6 (3) | 3 (1) | 10 (1) |
| Upper respiratory tract infection | 1 (<1) | 4 (2) | 5 (2) | 10 (1) |
| Vomiting | 2 (<1) | 5 (2) | 2 (<1) | 9 (1) |
Abbreviation: AE, adverse event.
The incidence of drug-related AEs, AEs leading to withdrawal, and SAEs was low and similar across the treatment groups in each study. The incidence of drug-related AEs was 6%–15% in Study 1 and 7%–13% in Study 2, whereas the incidence of AEs leading to withdrawal was 2%–6% in Study 1 and 7%–9% in Study 2. SAEs occurred at an incidence of 2%–7% across treatment groups in Study 1, and 8%–12% in Study 2 (Supplementary Table 1).
Three deaths were reported during Study 1 (SB742457 35 mg, donepezil, and placebo, all n = 1 [<1%]), and 10 deaths were reported in Study 2 (SB742457 35 mg, n = 4 [2%]; SB742457 15 mg, n = 5 [2%]; placebo, n = 1 [<1%]; see Supplementary Table 2). Two of the 10 deaths in Study 2 were reported in the week 24 analyses, and eight were reported between weeks 24 and 48; the only causes of death in more than one subject were pneumonia and cerebrovascular accident. None of the deaths in either study were considered by the study investigator to be drug related.
Mean changes in hematology, clinical chemistry, urinalysis, vital signs, and ECG parameters were generally small, not clinically relevant, and were comparable across treatment groups in both studies.
3.4. Pharmacokinetics and pharmacodynamics
Plasma exposures of SB742457 (minimum steady state concentration; area under the curve over the dosing interval at steady state) and donepezil (average concentration at steady state) were similar between the studies (Supplementary Table 3). Red blood cell acetylcholinesterase activities were similar before and after randomized adjunctive therapy for each treatment group in Study 2. Week 3 to baseline ratios (90% confidence intervals) were 1.01 (0.98, 1.05), 1.00 (0.97, 1.04), and 1.00 (0.97, 1.03) for placebo, SB742457 15 mg, and SB742457 35 mg, respectively.
4. Discussion
Two randomized, double-blind, placebo-controlled, Phase II trials of SB742457, as monotherapy and as add-on to donepezil, were conducted in subjects with mild-to-moderate AD. As monotherapy, SB742457 did not show a benefit on coprimary endpoints of cognition and global function. However, SB742457 35 mg added to donepezil therapy produced a significant difference in ADAS-Cog versus placebo in Study 2, and the magnitude of the effect was maintained throughout the 48-week treatment period. Significant improvements in function were also observed over the same time course, as measured by the ADCS-ADL, with SB742457 35 mg. However, the lack of effect on the other coprimary endpoint (CDR-SB) meant that the study failed to meet the overall criteria for success. Although there is no consensus regarding the definition of a clinically relevant benefit for an adjunctive therapy in patients with mild-to-moderate AD, the sustained magnitude of effect over 48 weeks in both cognitive and functional parameters suggests an effect of potential clinical value. The results for the coprimary endpoints were found to be unaffected by baseline MMSE scores.
Preliminary preclinical data using cognitive models and microdialysis suggest that there is a positive interaction between AChEIs and 5-HT6 antagonists, which may be related to additive effects on the cholinergic system (GlaxoSmithKline, unpublished data). 5-HT6 antagonists have also been shown to increase the release of other neurotransmitters such as glutamate and monoamines [7], [8], [9], [10]. These mechanisms may explain the effects over and above those offered by cholinesterase inhibition, but further studies are needed to characterize any potential additive or synergistic interactions between 5-HT6 antagonists and AChEIs. As such, it was decided to conduct two studies in parallel to investigate both the monotherapy effects and the add-on effects. In addition to Study 1 reported here, two other studies have assessed SB742457 as monotherapy in AD [5], [6]. Overall, the three studies have produced inconsistent results, which suggest that SB742457 is unlikely to be useful as monotherapy. These observations are consistent with other recent studies of monotherapy in AD, which have also proved unsuccessful [24], [25]. As donepezil did not demonstrate the expected level of efficacy, and no effect could be detected on the ADAS-Cog, there may also have been problems with assay sensitivity in Study 1. It is therefore possible that short-term monotherapy studies in drug-naïve patients are no longer suitable for the study of new treatments, especially given the wider availability of AChEIs and memantine.
Other factors that can influence aspects of data variability in large clinical trials include the inconsistent application of entry criteria across study sites, differences in clinical practice and clinical trial experience across sites, and the language and culture of participating countries. Furthermore, the number and order of assessments and their impact on patient fatigue, the range and sensitivity of scales, and the reliability and consistency of caregiver-reported outcomes have been identified as important areas for consideration in the design of randomized clinical trials in AD [26], [27], [28]. The two studies reported here were designed to mirror each other as far as possible in study design, and subject demographic profiles were generally similar across the studies. As such, a clear factor of study design or population does not account for the additive effects observed in Study 2, and it is difficult to establish whether any combination of factors could provide an explanation. Although a number of factors could potentially have introduced unwanted variability, there was sufficient power to detect an effect in each study, and the observed SDs in both studies compared well with the protocol assumptions. A recently reported phase 2 study (LADDER) assessing the safety and efficacy of another 5-HT6 antagonist (idalopirdine, Lu AE58054) also demonstrated an improvement in cognition compared with placebo at 24 weeks in subjects maintained on donepezil [29]. The LADDER study did not demonstrate statistically significant effects on secondary outcomes of activities of daily living or global clinical measures where the sample size was smaller than in Study 2 reported here.
A key difference between the studies that could potentially account for the study outcomes is differences in subject progression in the placebo arms compared with the active treatment arms. In Study 2, clear progression was observed on the clinical scales in the placebo group, whereas almost no progression was observed in Study 1, or in the two previous studies of SB742457 as monotherapy [5], [6]. This might suggest that an adjunct population has a more predictable and perhaps more rapid progression (in view of a more established disease course). This might enable a clearer outcome in clinical studies, compared with untreated subjects with a more recent diagnosis as in Study 1, where the median time to diagnosis was approximately 1 year less than in Study 2. Furthermore, subjects with the same MMSE score in Study 1 and Study 2 may not be at the same stage of disease as the subject's true MMSE score in Study 2 is inflated by the action of donepezil. Therefore, although the mean MMSE scores were similar across the two studies, this may hide the fact that subjects in Study 2 were at a more advanced stage of disease. Further adjunct studies would be required to confirm these hypotheses. The results reported here have important implications for the design of future studies investigating symptomatic or disease-modifying therapies in AD, and trials should ensure that the subject population will progress during the course of the study.
SB742457 was generally well tolerated as monotherapy or when added to existing donepezil therapy. The incidence of AEs was similar across treatment groups in each study and overall, the most common AEs were nasopharyngitis, urinary tract infection, headache, and diarrhea. The proportion of subjects experiencing a SAE, or AE leading to withdrawal was similar across treatment groups in Study 1 and slightly higher for subjects receiving SB742457 compared with placebo in Study 2. There did not appear to be any additional AE findings of note with adjunctive SB742457 treatment beyond those observed in previous monotherapy studies [5], [6]. There were four deaths in Study 1 (monotherapy); one each in the placebo, SB742457 35 mg, and donepezil treatment groups, and one in a nonrandomized subject. In Study 2, the number of deaths was higher for SB742457 when added to donepezil compared with placebo. Cerebrovascular accident/cerebral hemorrhage was the only cause of death in more than one subject treated with SB742457. Because of the small number of deaths in each treatment group, it was not possible to determine a temporal or dose relationship. None of the deaths in either study were considered by the Investigator to be drug-related. Mean changes in laboratory parameters, ECGs, and vital signs were generally small and comparable in all treatment groups in each study.
Pharmacokinetic analyses did not suggest any influence of SB742457 on donepezil concentrations, or of donepezil on SB742457 concentrations, with reported values in line with expectations. There was also no peripheral pharmacodynamic interaction in terms of effects on red blood cell acetylcholinesterase activity. The effects observed with SB742457 in Study 2 are therefore unlikely to be explained by an interaction of SB742457 with the metabolism or direct mechanism of action of donepezil.
In conclusion, neither study met the overall criteria for success, but as an adjunct to donepezil, SB742457 was associated with sustained improvements for up to 48 weeks in cognition and ADL, compared with donepezil alone.
Research in context.
-
1.
Systematic review: Study 1 sought to confirm findings from earlier studies regarding the potential of SB742457 as monotherapy in mild to moderate Alzheimer’s disease (AD). A donepezil arm was included for assay sensitivity to enable benchmarking to other historical studies.
Study 2 was the first adjunctive trial to investigate the efficacy and safety of SB742457 when added to stable donepezil therapy in subjects with mild to moderate AD.
-
2.
Interpretation: Study 1 failed to detect any efficacy for SB742457 as monotherapy and detected only modest efficacy for donepezil, indicating minimal assay sensitivity. Both SB742457 treatment arms demonstrated less efficacy than in previous studies.
In Study 2, SB742457 35 mg, when added to stable donepezil, improved cognition and functional status compared with donepezil treatment alone and had an acceptable safety profile.
The current results taken together with the earlier SB742457457 monotherapy studies suggest that SB742457 has limited utility as monotherapy but may have utility as an adjunct therapy.
-
3.
Future directions: Further research with SB742457 should seek to explore and confirm its potential as an adjunct to existing symptomatic treatments.
Acknowledgments
The authors thank the study investigators and staff at each center, and the subjects and caregivers who participated in the study.
GlaxoSmithKline, Research Triangle Park, NC, USA, provided funding for design and conduct of this study, the collection, management, analysis, and interpretation of the data, and coordinated the preparation and review of the manuscript. CW, JA, GM-E, MZ-H, CB, JD, DB, and MG are employees of GlaxoSmithKline and receive payment and stock remuneration as part of that employment; Martin T. Lowy, PhD (an employee of GlaxoSmithKline) and Natasha Thomas (of Fishawack Indicia Ltd, funded by GlaxoSmithKline) provided writing and editorial assistance. The authors thank Andrew Miskell (an employee of GlaxoSmithKline) for assistance on the figures.
Conflicts of interest: CW, JA, GM-E, MZ-H, CB, JD, and DB are employees of GlaxoSmithKline and receive payment and stock remuneration as part of that employment, but have no other financial interests or conflicts of interest. MG was an employee of GlaxoSmithKline at the time of study. AK, BS, JPS, OZ, and MF were investigators involved in the studies, which were sponsored by GlaxoSmithKline.
Study investigators: SB742457 Investigators; Study 1:
Bulgaria: Nadejda Deleva, Ivailo Petrov, Lachezar Traykov, Stefka Yancheva; Chile: Sergio Gloger, Manuel Lavados Montes, Luis Risco Neira, Gustav Rohde; Czech Republic: Ilona Divacka, Petr Kanovsky, Bronislav Kobeda, Ivan Rektor, Zdenek Solle, Eva Topinkova; Estonia: Katrin Gross-Paju, Ulla Linnamagi, Maarja Taal; Germany: Veit Ulrich Becker, Arnfin Bergmann, Ralf Bitter, Peter Donat, Hermann-Josef Gertz, Klaus Hager, Heinz Herbst, Alexander Kurz, Thomas Leyhe, Andreas Mahler, Wolfgang Mattern, Gereon Nelles, Andrej Pauls, Joerg Peltz, Gerd Reifschneider, Ruediger Schellenberg, Eugen Schlegel, Irma Schoell, Elmar Schumacher, Karl-Otto Sigel, Rhena Sloksnat, Klaus Christian Steinwachs, Hans-Peter Wunderlich; Greece: Antonios Kodounis, Dimosthenis Mitsikostas, Magdalini Tsolaki, Vassilios Vagenas, Dimitrios Vassilopoulos; Korea: Jee Hyang Jeong, Seung Hyun Kim, Jae-Hong Lee, Kyung Won Park, Sang Won Seo; Mexico: Ivonne Becerra-Laparra, Juan Corral-Garcia, Santiago-Paulino Ramirez-Diaz, Ricardo Salinas-Martínez, Mario Alberto Valdes Davila, Eduardo Vázquez de la Mora; Poland: Aleksander Araszkiewicz, Jan Ilkowski, Robert Kucharski, Hubert Kwiecinski, Danuta Pruchnik-Wolinska, Dorota Ussorowska; Russian Federation: Svetlana Gavrilova, Alla Guekht, Victor Kontsevoy, Sofia Sluchevskaya; South Africa: Gert Bosch, Judy Green, Stanley Lipschitz, David Lurie, Felix Potocnik, Juan Schrönen.
SB742457 Investigators; Study 2:
Argentina: Eduardo Amado Cattaneo, Gerardo Garcia Bonetto, Stella Maris Diamanti, Arturo Famulari, Maria Laura Garau, Edgardo Gabriel Reich, Gustavo, Saredo, Fernando Taragano; Australia: Nicola Lautenschlager, David Ames, Karyn Leah Boundy, Gerard Byrne, Roger Clarnette, Raymond Schwartz, Mark Yates, Henry Zeimer; Canada: Sharon Cohen, Marcel Germain, Jennifer Ingram, Mark Johnston, Elizabeth MacDonald, Giovanni Marotta, Tilak Mendis, Ziad Nasreddine, Emmanuelle Pourcher, Alain Robillard, Daniel Tessier; Chile: Sergio Gloger, Gustav Rohde, Manuel Lavados Montes, Luis Risco Neira; Czech Republic: Vaclav Dostal, Petr Kanovsky, Bronislav Kobeda, Helena Matlakova, Vit Matousek, Jiri Novak, Robert Rusina, Zdenek Solle; Germany: Walter Albrecht, Rainer Bachus, Katrin Bachus-Banaschak, Stephan Behrens, Heike Benes, Reinhard Ehret, Rolf Horn, Reinhard Huentemann, Hans Kluenemann, Alexander Kurz, Thomas Leyhe, Andreas Mahler, Wolfgang Molt, Christine Paschen, Ali Safavi, Irma Schoell, Karl-Otto Sigel, Konstanze Tinschert, Elke Wollenhaupt, Hans-Peter Wunderlich, Christine von Arnim; Italy: Piero Barbanti, Ubaldo Bonuccelli, Mariano Califano, Emanuele Cassetta, Massimo Franceschi, Giuseppe Gambina, Alfonso Iudice, Massimiliano Massaia, Patrizia Mecocci, Alessandra Monge, Marco Onofrj, Carla.Pettenati, Franco.Rengo, Elio Scarpini, Orazio Zanetti; Spain: Carmen Antúnez Almagro, Merce Boada, Purificación Cacabelos Pérez, Manuel Fernández Martínez, Miguel Goñi, Jose Miguel Lainez, José Félix Martí-Massó, María Dolores Martínez Lozano, Jordi Peña-Casanova, Ramón Reñé Ramirez, José Aurelio Vivancos Mora, Camino Sevilla Gomez, Elena Toribio Díaz, Jaime Morera Guitart; United States: Corey Anderson, Lisa Catapano-Friedman, Jerome Goldstein, Mark Hernandez, Richard Hubbard, Travis Jackson, Smita Kittur, David Margolin, Lora McGill, Ralph Richter, Beth Safirstein, Douglas Scharre, Joshua Shua-Haim, Jaron Winston.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2015.04.001.
Supplementary data
References
- 1.Upton N., Chuang T.T., Hunter A.J., Virley D.J. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease. Neurotherapeutics. 2008;5:458–469. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Codony X., Vela J.M., Ramirez M.J. 5-HT(6) receptor and cognition. Curr Opin Pharmacol. 2011;11:94–100. doi: 10.1016/j.coph.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Rosse G., Schaffhauser H. 5-HT6 receptor antagonists as potential therapeutics for cognitive impairment. Curr Top Med Chem. 2010;10:207–221. doi: 10.2174/156802610790411036. [DOI] [PubMed] [Google Scholar]
- 4.Hirst W.D., Stean T.O., Rogers T.C., Sunter D., Pugh P., Moss S.F. SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol. 2006;553:109–119. doi: 10.1016/j.ejphar.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Maher-Edwards G., Dixon R., Hunter J., Gold M., Hopton G., Jacobs G. SB-742457 and donepezil in Alzheimer disease: a randomized, placebo-controlled study. Int J Geriatr Psychiatry. 2011;26:536–544. doi: 10.1002/gps.2562. [DOI] [PubMed] [Google Scholar]
- 6.Maher-Edwards G., Zvartau-Hind M., Hunter A.J., Gold M., Hopton G., Jacobs G. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer's disease. Curr Alzheimer Res. 2010;7:374–385. doi: 10.2174/156720510791383831. [DOI] [PubMed] [Google Scholar]
- 7.Dawson L.A., Nguyen H.Q., Li P. The 5-HT(6) receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacology. 2001;25:662–668. doi: 10.1016/S0893-133X(01)00265-2. [DOI] [PubMed] [Google Scholar]
- 8.Lacroix L.P., Dawson L.A., Hagan J.J., Heidbreder C.A. 5-HT6 receptor antagonist SB-271046 enhances extracellular levels of monoamines in the rat medial prefrontal cortex. Synapse. 2004;51:158–164. doi: 10.1002/syn.10288. [DOI] [PubMed] [Google Scholar]
- 9.Marcos B., Chuang T.T., Gil-Bea F.J., Ramirez M.J. Effects of 5-HT6 receptor antagonism and cholinesterase inhibition in models of cognitive impairment in the rat. Br J Pharmacol. 2008;155:434–440. doi: 10.1038/bjp.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcos B., Gil-Bea F.J., Hirst W.D., Garcia-Alloza M., Ramirez M.J. Lack of localization of 5-HT6 receptors on cholinergic neurons: implication of multiple neurotransmitter systems in 5-HT6 receptor-mediated acetylcholine release. Eur J Neurosci. 2006;24:1299–1306. doi: 10.1111/j.1460-9568.2006.05003.x. [DOI] [PubMed] [Google Scholar]
- 11.Riemer C., Borroni E., Levet-Trafit B., Martin J.R., Poli S., Porter R.H. Influence of the 5-HT6 receptor on acetylcholine release in the cortex: pharmacological characterization of 4-(2-Bromo-6-pyrrolidin-1-ylpyridine-4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J Med Chem. 2003;46:1273–1276. doi: 10.1021/jm021085c. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Clinical Excellence . 2006. Dementia: supporting people with dementia and their carers in health and social care (NICE clinical guideline 42)http://www.nice.org.uk/guidance/cg42 (modifed 2012). Available at: Accessed on August 2014. [Google Scholar]
- 13.de Bruin N.M., Prickaerts J., van Loevezijn A., Venhorst J., de Groote L., Houba P. Two novel 5-HT6 receptor antagonists ameliorate scopolamine-induced memory deficits in the object recognition and object location tasks in Wistar rats. Neurobiol Learn Mem. 2011;96:392–402. doi: 10.1016/j.nlm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 15.McKhann G., Drachman D., Folstein M., Katzman R., Drice D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Rosen W.G., Terry R.D., Fuld P.A., Katzman R., Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 18.Schneider L.S., Olin J.T., Doody R.S., Clark C.M., Morris J.C., Reisberg B. Validity and reliability of the Alzheimer's Disease Cooperative Study—clinical global impression of change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 19.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 21.Randolph C. 1st ed. The Psychologcal Corporation: Harcourt Brace and Company; New York: 1998. Repeatable battery for the assessment of neuropsychological status. [Google Scholar]
- 22.Alexopoulos G.S., Abrams R.C., Young R.C., Shamoian C.A. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 23.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- 24.Jones R.W. Dimebon disappointment. Alzheimers Res Ther. 2010;2:25. doi: 10.1186/alzrt49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panisset M., Gauthier S., Moessler H., Windisch M., Cerebrolysin Study Group Cerebrolysin in Alzheimer's disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent. J Neural Transm. 2002;109:1089–1104. doi: 10.1007/s007020200092. [DOI] [PubMed] [Google Scholar]
- 26.Becker R.E., Greig N.H., Giacobini E. Why do so many drugs for Alzheimer's disease fail in development? Time for new methods and new practices? J Alzheimers Dis. 2008;15:303–325. doi: 10.3233/jad-2008-15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold M. Study design factors and patient demographics and their effect on the decline of placebo-treated subjects in randomized clinical trials in Alzheimer's disease. J Clin Psychiatry. 2007;68:430–438. doi: 10.4088/jcp.v68n0313. [DOI] [PubMed] [Google Scholar]
- 28.Irizarry M.C., Webb D.J., Bains C., Barrett S.J., Lai R.Y., Laroche J.P. Predictors of placebo group decline in the Alzheimer's disease Assessment Scale-cognitive subscale (ADAS-Cog) in 24 week clinical trials of Alzheimer's disease. J Alzheimer Dis. 2008;14:301–311. doi: 10.3233/jad-2008-14304. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson D., Windfeld K., Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer's disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13:1092–1099. doi: 10.1016/S1474-4422(14)70198-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.