Abstract
Introduction
The safety and efficacy of the novel α7 nicotinic acetylcholine receptor agonist ABT-126 were investigated in subjects with mild-to-moderate Alzheimer's dementia (AD).
Methods
Subjects not currently receiving acetylcholinesterase inhibitors were randomized to ABT-126 (5 or 25 mg once daily), donepezil 10 mg once daily, or placebo for 12 weeks. The primary efficacy end point was the change from baseline to final evaluation in the 11-item Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog) total score.
Results
A total of 274 subjects were randomized. Although the study did not meet its primary end point, trends toward improvement were seen with ABT-126 25 mg (least squares mean [standard error] difference from placebo −1.19 [0.90]; one-sided P = .095) and donepezil (−1.43 [0.90]; one-sided P = .057) on the 11-item ADAS-Cog total score change from baseline to the final evaluation. ABT-126 5 mg was numerically similar to placebo. An exposure-response analysis indicated a statistically significant relationship between ABT-126 exposure and the change from baseline in ADAS-Cog, with no evidence of a plateau. No clinically meaningful differences in safety were observed among treatment groups.
Discussion
Although the ABT-126 25 mg dose did not demonstrate statistically significant improvement, results of the exposure-response analysis suggest that higher doses may produce better efficacy, and the safety profile of ABT-126 in this study supports additional studies with higher doses in subjects with mild-to-moderate AD.
Clinical trial register number
Keywords: Clinical trials randomized controlled (CONSORT agreement), Alzheimer's disease, Assessment of cognitive disorders/dementia, Alzheimer's dementia/drug therapy, ABT-126
1. Background
α-7 nicotinic acetylcholine receptors (nAChRs) are acetylcholine-gated cation channels localized pre- and post-synaptically in areas of the brain critical to the synaptic plasticity associated with learning and memory [1]. α-7 nAChRs modulate the release of multiple neurotransmitters such as dopamine, glutamate, and acetylcholine [2] and activate intracellular signaling cascades such as cyclic-adenosine monophosphate response element binding and extracellular signal-regulated kinases. The mechanism of action for the α7 nAChR agonists is distinct from that of acetylcholinesterase inhibitors (AChEIs) that inhibit the enzymatic breakdown of acetylcholine, suggesting that they will provide a different efficacy and safety profile [3].
Growing evidence indicates that α7 nAChR agonists may improve cognition in Alzheimer's dementia (AD). Positive effects with α7 nAChR agonists and partial agonists have been observed in preclinical models of cognition [4], [5], [6], [7], [8]. Procognitive effects have also been reported with the partial α7 agonist EVP-6124 (encenicline) as a stand-alone treatment for AD or as an add-on treatment to AChEIs [9]. An initial study with another α7-nAChR partial agonist MEM3454/RG3487 also showed procognitive effects in subjects with AD [10] but the results were not replicated in a subsequent larger phase 2 add-on study [11].
ABT-126 is a potent and selective α7 nAChR agonist demonstrating efficacy in animal models across multiple cognitive domains relevant to AD, including social recognition memory, memory consolidation, inhibitory avoidance, and working memory [12]. Data from phase 1 studies of healthy adults, elderly subjects, and subjects with schizophrenia indicated that ABT-126 was generally well tolerated. This phase 2 proof-of-concept study was designed to evaluate the efficacy and safety of two doses of ABT-126 compared with placebo and donepezil for the symptomatic treatment of mild-to-moderate AD.
2. Methods
2.1. Study subject recruitment
This phase 2, double-blind, parallel, randomized, placebo- and active-controlled study was conducted at 27 sites in the United States, Bulgaria, Czech Republic, Slovakia, United Kingdom, and South Africa.
Eligible male and female subjects were aged 55–90 years, met National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria for probable AD [13], had reliable caregivers, a Mini-Mental Status Examination (MMSE) [14] score from 10 to 24, a Cornell Scale for Depression in Dementia (CSDD) [15] score ≤10, and a Modified Hachinski Ischemic Scale [16] score of ≤4 at the initial screening visit. Subjects were in general good health with no evidence of an alternative etiology for dementia on a computed tomography or magnetic resonance imaging performed within the previous 36 months. Subjects were excluded if they were currently taking or had taken an AD medication within 60 days before the first screening visit or if they were receiving cognitive therapy for AD. Concomitant pharmacologic treatments for AD were prohibited during the study. Vitamins and herbal supplements (with the exception of huperzine), antidepressants, low-dose anxiolytics, hypnotics, atypical antipsychotics, and pain and urinary incontinence medications were allowed provided treatment started >30 days before screening and were anticipated to remain stable. Subjects who had stopped donepezil therapy due to intolerance or a lack of efficacy after an adequate trial were also excluded.
2.2. Standard protocol approvals, registrations, and patients consents
The protocol, amendments, and informed consent forms were approved by an independent ethics committee or institutional review board. Written informed consent was obtained from each participant (or their representative) and their caregivers before screening procedures.
2.3. Study design
The study began with a screening period of up to 28 days that included two screening visits and a baseline visit (day −1). Subjects were randomized 1:1:1:1 to 12 weeks of treatment with placebo, ABT-126 5 mg, ABT-126 25 mg, or donepezil 10 mg once daily. Donepezil was initiated at 5 mg once daily for 4 weeks followed by 10 mg for 8 weeks. ABT-126 5 and 25 mg were expected to result in plasma concentrations within and above the predicted efficacy range from preclinical studies, respectively, for 24 hours. Study drug was identical in appearance to preserve the blind. Randomization occurred via an interactive voice response and interactive Web-based system using a randomization code provided by the sponsor, stratified by study site. Visits were at weeks 2, 4, 8, 12/early discontinuation, and follow-up (approximately 2 weeks after the last dose). Safety telephone assessments occurred at weeks 6 and 10 and 30 days after the last dose.
An independent safety data monitoring committee reviewed unblinded safety data during the study and made recommendations regarding trial conduct according to a predefined charter. Six interim efficacy evaluations were conducted according to a prespecified algorithm by an unblinded external statistician and an unblinded sponsor statistician not involved in other aspects of the study. These analyses provided a mechanism to cease enrollment to an inefficacious dose of ABT-126 or to terminate the entire study early due to futility of both ABT-126 dose groups. Evaluations were conducted when approximately 100, 120, 140, 160, 180, and 200 subjects had completed or discontinued from the study. If a dose demonstrated a predictive probability of .10 that the final P value of the ABT-126 dose versus placebo would be ≤.05 for three consecutive interim evaluations or the predictive probabilities showed a decreasing trend for three consecutive evaluations with the last evaluation reaching the threshold of .10, then no additional subjects would have been enrolled in that dose group. The sites were not informed of the timing of the conduct or the outcome of these evaluations.
2.4. Assessments
The primary efficacy measure was the 11-item Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog). Secondary efficacy measures included total scores of the 13-item ADAS-Cog [17], [18], the MMSE, the Clinician Interview-Based Impression of Severity (CIBIS, baseline only), the Clinician Interview-Based Impression of Change-plus (CIBIC-plus) [19], the Neuropsychiatric Inventory (10- and 12-item) [20], [21], and the Alzheimer's Disease Cooperative Study Activities of Daily Living (ADSC-ADL) [22] scale and the subject and caregiver scores of the Quality of Life - Alzheimer's Disease [23] scale.
Safety was assessed via adverse events (AEs), vital signs, electrocardiograms (ECGs), physical examinations, brief neurologic examinations, brief psychiatric assessments, clinical laboratory assessments, the CSDD, and the Physician Withdrawal Checklist (PWC-20) [24]. One blood sample for pharmacokinetic analysis was collected at weeks 2, 4, 8, and 12.
2.5. Statistical methods
With approximately 65 subjects per treatment group (260 total) and assumed pooled standard deviation (SD) of six, the study was designed to have 80% power to detect a treatment difference of −3.08 change from baseline to final observation on the 11-item ADAS-Cog total score between an ABT-126 dose group and placebo at a one-sided significance level of .05 (assumed pooled SD of six based on AChEIs' effect in studies of 12–24 weeks duration) [25]. The study was powered to detect an effect size of approximately 0.5, which is approximately 30% greater than the average effect size of AChEIs because newer medications for AD need to demonstrate larger effects than AChEIs to provide a useful alternative therapy to patients. Because of the exploratory nature of the study, a one-sided test was used for all efficacy analyses to quantify statistical significance when an ABT-126 dose group demonstrated greater numerical improvement compared with placebo. Therefore, all reported P values for efficacy variables are one-sided. There was no adjustment for multiple comparisons of two ABT-126 treatment groups. With the exception of the 13-item ADAS-Cog outcome, this proof-of-concept study was not powered to detect significant differences on any other efficacy variables, which generally have smaller effect sizes compared with the ADAS-Cog 11-item total score. For the interim analyses, futility criteria were based on Bayesian predictive probability as described previously. There was no hypothesis testing at the interim analyses, and the significance level for statistical testing at the final analysis remained at .05.
Efficacy and safety populations included all subjects who took ≥one dose of study drug. The primary efficacy variable was the change from baseline to the final evaluation in the 11-item ADAS-Cog total score. The primary efficacy analysis was conducted using an analysis of covariance (ANCOVA) model with treatment and study site as the main effects and baseline score as the covariate. The difference between an ABT-126 dose group and placebo and the difference between donepezil and placebo were tested at a one-sided significance level of .05. Type III sum-of-squares was used to generate the least squares (LS) means of treatment-group differences. As a sensitivity analysis, treatment-group differences for the change from baseline to weeks 4, 8, and 12 on the 11-item ADAS-Cog total score were assessed using a mixed-effects, maximum likelihood, repeated measures (MMRM) analysis. The model included fixed effects of treatment, study site, visit, and treatment-by-visit interaction, with baseline score as a covariate, and the baseline-by-visit interaction.
All secondary efficacy variables except the CIBIC-plus were analyzed by the ANCOVA model as described for the primary efficacy analysis to evaluate treatment group differences in change from baseline to final. The change from baseline to each postbaseline assessment in the 13-item ADAS-Cog, MMSE, NPI (10- and 12-item), and ADSC-ADL total scores were also analyzed using the aforementioned MMRM analysis model.
The final observation of the CIBIC-plus was analyzed by an ANCOVA model with the terms of treatment and study site with CIBIS score collected at baseline as a covariate. Longitudinal assessments of CIBIC-plus scores were analyzed by an MMRM analysis with the fixed effects of treatment, study site, visit, treatment-by-visit interaction, with the baseline CIBIS score as continuous covariate. The percentage of subjects with any improvement in the final CIBIC-plus assessment was compared between placebo and each ABT-126 dose group using Fisher's exact test.
Prespecified subgroup analyses were performed on the 11-item ADAS-Cog using an ANCOVA model with the terms of treatment, study site, and subgroup variable, and the treatment-by-subgroup variable interaction to determine any effect of baseline MMSE (≤19 or ≥20), gender, age (<65 or ≥65), country, and APOE ε4 allele status (ε4+ or ε4−). The baseline 11-item ADAS-Cog total score was included as a covariate. A treatment-by-subgroup interaction P value ≤.10 was considered significant.
Comparisons between each ABT-126 dose group and placebo for safety parameters were performed with two-sided tests at the significance level of .05. AEs were coded using the Medical Dictionary for Regulatory Activities [26].
2.6. Exposure-response analysis
Nonlinear mixed-effects analysis of the relationship between ABT-126 estimated plasma exposure (model estimated area under the plasma concentration time curve over a dosing interval at steady-state; AUC) and change from baseline in the ADAS-Cog scores was conducted using the software NONMEM (version 7.1; Icon, Ellicott City, MD, USA). The analysis accounted for the time course of placebo effect, ABT-126 effect relative to its exposure, inter-subject variability in placebo and ABT-126 effects, and residual random error. In this analysis, the Liklihood Ratio Test was used for comparing rival hierarchical models where a decrease in NONMEM objective function value (−2 log likelihood) of 3.84 points was necessary to consider the improvement in model performance statistically significant at α = 0.05 and 1 degree of freedom. The Akaike information criterion was used for comparing rival nonhierarchical models.
3. Results
3.1. Subjects
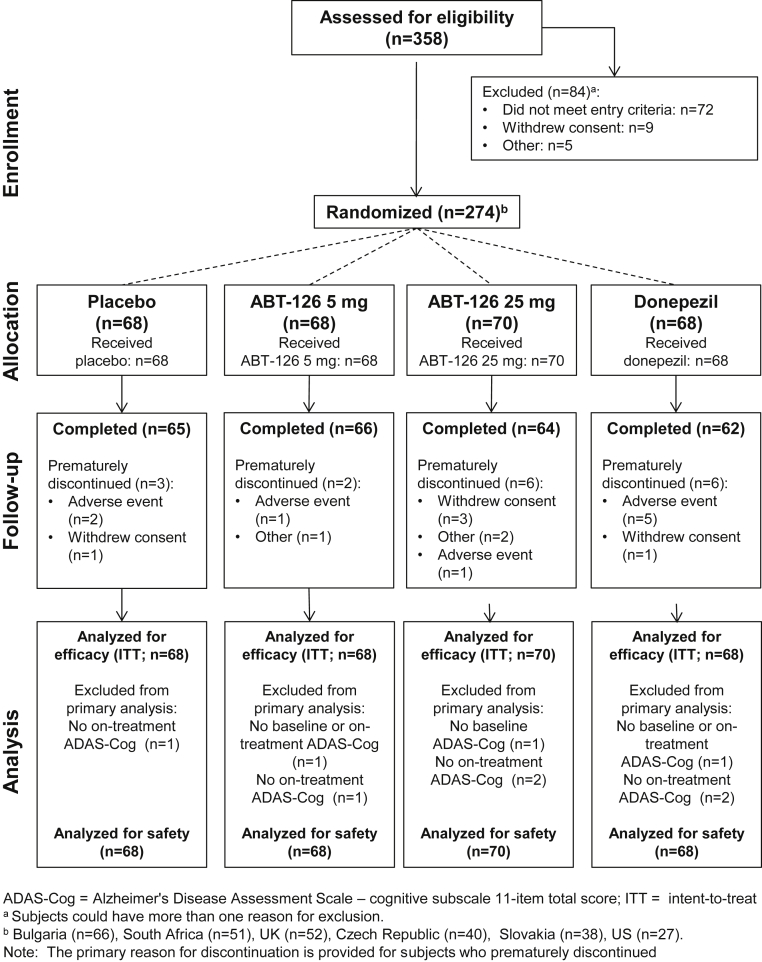
A total of 274 subjects were randomized from Bulgaria (n = 66), South Africa (n = 51), the United Kingdom (n = 52), the Czech Republic (n = 40), Slovakia (n = 38), and the United States (n = 27), with 93.8% (n = 257) completing the trial. Disposition is presented in Fig. 1. Demographic characteristics were generally well balanced among treatment groups with the exception of gender (Table 1). Approximately 40% of subjects had previously received pharmacotherapy for AD with the majority (28.8%) taking supplements, nootropic agents, or vitamins (e.g., Ginkgo biloba, piracetam, latrepirdine, tiapride, vitamin E). Fewer subjects had previously taken AChEIs (donepezil [8.8%], rivastigmine [4.7%], and galantamine [2.6%]; Table 1).
Fig. 1.
Subject disposition.
Table 1.
Baseline demographic characteristics
| Characteristics | Placebo | ABT-126, 5 mg | ABT-126, 25 mg | Donepezil, 10 mg | All subjects |
|---|---|---|---|---|---|
| Number of subjects | 68 | 68 | 70 | 68 | 274 |
| Age, y, mean ± SD | 73.6 ± 8.23 | 74.0 ± 7.47 | 75.7 ± 7.35 | 72.4 ± 8.42 | 73.9 ± 7.92 |
| Age <75 y, n (%) | 34 (50.0) | 33 (48.5) | 30 (42.9) | 41 (60.3) | 138 (50.4) |
| Age ≥75 y, n (%) | 34 (50.0) | 35 (51.5) | 40 (57.1) | 27 (39.7) | 136 (49.6) |
| Female, n (%)∗ | 42 (61.8) | 47 (69.1) | 48 (68.6) | 31 (45.6) | 168 (61.3) |
| Male, n (%) | 26 (38.2) | 21 (30.9) | 22 (31.4) | 37 (54.4) | 106 (38.7) |
| White, n (%) | 64 (94.1) | 66 (97.1) | 68 (97.1) | 65 (95.6) | 263 (96.0) |
| BMI, kg/m2, mean ± SD | 25.3 ± 4.63 | 26.3 ± 4.40 | 25.7 ± 4.05 | 25.7 ± 3.72 | 25.8 ± 4.20 |
| APOE ε4 positive | 38 (55.9) | 37 (56.1) | 26 (38.8) | 35 (53.0) | 136 (50.9) |
| Age at AD symptom onset, y, mean ± SD | 70.1 ± 8.62 | 70.6 ± 7.65 | 72.0 ± 7.69 | 69.3 ± 8.72 | 70.5 ± 8.20 |
| Years since AD symptom onset∗, mean ± SD | 3.5 ± 2.27 | 3.4 ± 2.24 | 3.7 ± 2.45 | 3.1 ± 2.01 | 3.4 ± 2.25 |
| Age at AD diagnosis, y, mean ± SD | 72.3 ± 8.47 | 72.5 ± 7.50 | 74.2 ± 7.41 | 71.2 ± 8.71 | 72.6 ± 8.07 |
| Years since AD diagnosis, mean ± SD | 1.3 ± 1.62 | 1.5 ± 1.71 | 1.5 ± 1.52 | 1.1 ± 1.28 | 1.3 ± 1.54 |
| Family history of AD, n (%) | 15 (22.1) | 12 (17.6) | 17 (24.3) | 11 (16.2) | 55 (20.1) |
| Medication used for treatment of AD†, n (%) | 25 (36.8) | 31 (45.6) | 28 (40.0) | 23 (33.8) | 107 (39.1) |
| Donepezil | 4 (5.9) | 7 (10.3) | 10 (14.3) | 3 (4.4) | 24 (8.8) |
| Galantamine | 3 (4.4) | 1 (1.5) | 3 (4.3) | 0 | 7 (2.6) |
| Memantine | 0 | 3 (4.4) | 5 (7.1) | 1 (1.5) | 9 (3.3) |
| Rivastigmine | 4 (5.9) | 3 (4.4) | 3 (4.3) | 3 (4.4) | 13 (4.7) |
| Other‡ | 17 (25.0) | 27 (39.7) | 17 (24.3) | 18 (26.5) | 79 (28.8) |
| ADAS-Cog (11-item), mean ± SD§ | 24.7 ± 11.65 | 25.6 ± 10.30 | 28.8 ± 10.84 | 25.1 ± 10.95 | 26.1 ± 11.01 |
| MMSE score, mean ± SD | 19.7 ± 3.95 | 18.7 ± 3.62 | 18.3 ± 3.72 | 19.6 ± 3.82 | 19.1 ± 3.81 |
Abbreviations: SD, standard deviation; BMI, body mass index; AD, Alzheimer's dementia; APOE, apolipoprotein E; ADAS-Cog, Alzheimer's Disease Assessment Scale-cognitive subscale; MMSE, Mini-Mental Status Examination.
NOTE. ∗P = .017 (Fisher's exact test).
Time from onset of AD symptoms or diagnosis to first dose of study drug.
Subjects were not allowed to take donepezil (unless randomized to that treatment) galantamine, memantine, or rivastigmine after enrolling in the study.
Other prestudy medications or supplements used by ≥5 subjects included Ginkgo biloba, piracetam, latrepirdine, tiapride, and vitamin E.
Baseline results based on a total of 271 subjects.
3.2. Efficacy
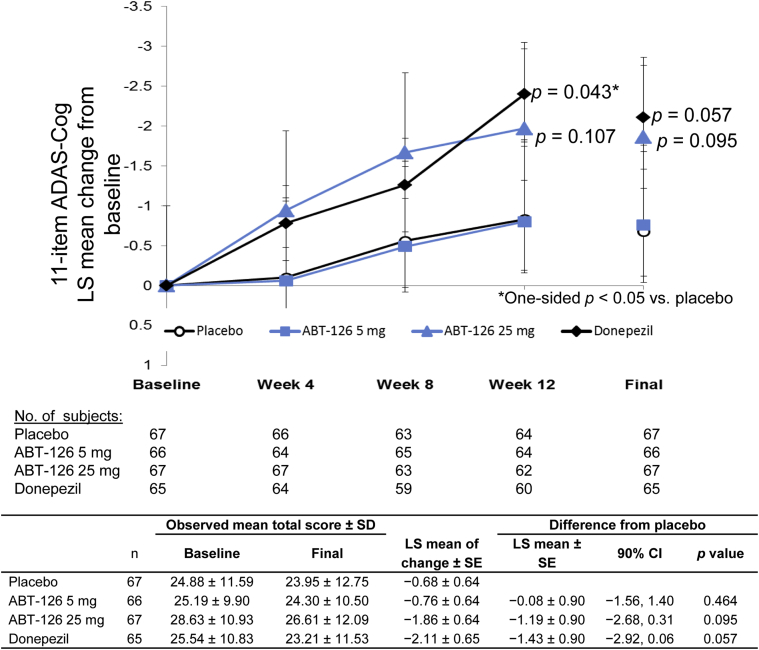
Nine subjects (3.3%) were not included in the primary efficacy analysis due to missing baseline and/or on-treatment 11-item ADAS-Cog total scores (Fig. 1). All interim efficacy evaluations were conducted but led to no changes to the study. The ANCOVA and MMRM analyses of the 11-item ADAS-Cog are presented in Fig. 2. In the primary efficacy analysis, a trend of greater LS mean changes from baseline (improvement) to the final evaluation in the 11-item ADAS-Cog total score was observed for ABT-126 25 mg (P = .095) and donepezil (P = .057) compared with placebo; ABT-126 5 mg was similar to placebo. In the MMRM analysis of the 11-item ADAS-Cog total score, the LS mean difference between ABT-126 25 mg and placebo increased over time: −0.84 at week 4 (90% confidence interval [CI] −1.92 to 0.23, P = .098), −1.11 at week 8 (90% CI −2.47 to 0.26, P = .091), and −1.14 at week 12 (90% CI −2.65 to 0.37, P = .107). Similarly, the LS mean difference between donepezil and placebo increased over time, reaching statistical significance at week 12: −0.68 at week 4 (90% CI −1.75 to 0.40, P = .149), −0.70 at week 8 (90% CI −2.06 to 0.66, P = .199), and −1.57 at week 12 (90% CI −3.08 to −0.07, P = .043). No differences were observed in the ABT-126 5 mg treatment group LS mean change compared with placebo over the 12 weeks. No treatment-by-subgroup interactions were observed for gender, age, country, or APOE ε4 allele status on the ADAS-Cog 11-item total score.
Fig. 2.
Least squares mean change from baseline over time in 11-item ADAS-Cog total score. The maximum likelihood, mixed-effect repeated-measures analysis of change from baseline to each study visit, and the primary analysis of covariance of change from baseline to the final evaluation for the ADAS Cog 11-item total score. The standard error of the least squares means are denoted by error bars. Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale-cognitive subscale; SD, standard deviation; LS, least squares; SE, standard error; CI, confidence interval.
ANCOVA results for secondary efficacy measures are presented in Table 2. Both ABT-126 25 mg- and donepezil-treated subjects had significant improvements in the 13-item ADAS-Cog total score in the ANCOVA analysis. In the MMRM analysis of the 13-item ADAS-Cog total score, the ABT-126 25 mg treatment group LS mean difference from placebo was −2.13 at week 4 (90% CI −3.36 to −0.89, P = .002), −2.06 at week 8 (90% CI −3.69 to −0.44, P = .018), and −1.61 at week 12 (90% CI −3.38 to 0.17, P = .068). The donepezil treatment group LS mean difference from placebo was −1.53 at week 4 (90% CI −2.75 to −0.31, P = .020), −1.85 at week 8 (90% CI −3.46 to −0.24, P = .030), and −2.48 at week 12 (90% CI −4.23 to −0.72, P = .010).
Table 2.
Analysis of covariance of change from baseline to the final evaluation in secondary efficacy variables
| Variables | n | Observed mean total score ± SD |
LS mean of change ± SE | Difference from placebo |
|||
|---|---|---|---|---|---|---|---|
| Baseline | Final | LS mean ± SE | 90% CI | P value (one-sided) | |||
| ADAS-Cog 13-item total score (↓ in score indicates improvement) | |||||||
| Placebo | 65 | 35.53 ± 12.19 | 34.28 ± 14.24 | −0.78 ± 0.74 | |||
| ABT-126 5 mg | 66 | 37.04 ± 11.32 | 35.92 ± 12.07 | −0.90 ± 0.73 | −0.13 ± 1.03 | −1.83 to 1.58 | .451 |
| ABT-126 25 mg | 64 | 40.85 ± 11.99 | 38.08 ± 13.81 | −2.60 ± 0.75 | −1.83 ± 1.05 | −3.57 to −0.09 | .042∗ |
| Donepezil | 64 | 37.44 ± 12.37 | 33.90 ± 13.37 | −3.27 ± 0.74 | −2.49 ± 1.04 | −4.21 to −0.78 | .009∗ |
| MMSE (↑ in score indicates improvement) | |||||||
| Placebo | 67 | 19.72 ± 3.98 | 20.43 ± 4.99 | 0.47 ± 0.33 | |||
| ABT-126 5 mg | 68 | 18.74 ± 3.62 | 19.65 ± 4.98 | 0.81 ± 0.32 | 0.34 ± 0.46 | −0.41 to 1.09 | .229 |
| ABT-126 25 mg | 69 | 18.30 ± 3.75 | 19.25 ± 4.92 | 0.88 ± 0.32 | 0.41 ± 0.46 | −0.34 to 1.17 | .183 |
| Donepezil | 66 | 19.50 ± 3.80 | 20.73 ± 4.86 | 1.00 ± 0.33 | 0.53 ± 0.46 | −0.22 to 1.29 | .122 |
| CIBIS and CIBIC-Plus† | |||||||
| Placebo | 66 | 3.91 ± 0.89 | 3.80 ± 0.85 | 3.83 ± 0.10 | |||
| ABT-126 5 mg | 68 | 3.85 ± 0.82 | 3.75 ± 0.89 | 3.76 ± 0.10 | −0.07 ± 0.14 | −0.31 to 0.16 | .306 |
| ABT-126 25 mg | 69 | 4.01 ± 0.81 | 3.83 ± 0.84 | 3.81 ± 010 | −0.02 ± 0.14 | −0.25 to 0.21 | .443 |
| Donepezil | 65 | 3.82 ± 0.83 | 3.62 ± 0.84 | 3.67 ± 0.10 | −0.16 ± 0.14 | −0.40 to 0.07 | .128 |
| NPI, 12-item total score (↓ in score indicates improvement) | |||||||
| Placebo | 67 | 8.67 ± 9.78 | 6.13 ± 7.94 | −3.00 ± 1.04 | |||
| ABT-126 5 mg | 68 | 8.38 ± 10.82 | 8.31 ± 15.62 | −0.57 ± 1.03 | 2.43 ± 1.45 | 0.04 to 4.82 | .953 |
| ABT-126 25 mg | 69 | 13.29 ± 13.40 | 10.52 ± 11.59 | −2.38 ± 1.03 | 0.62 ± 1.46 | −1.79 to 3.03 | .664 |
| Donepezil | 66 | 10.97 ± 13.19 | 9.29 ± 14.50 | −1.92 ± 1.04 | 1.07 ± 1.46 | −1.34 to 3.49 | .768 |
| ADCS-ADL total score (↑ in score indicates improvement) | |||||||
| Placebo | 67 | 53.01 ± 16.18 | 54.45 ± 17.31 | 1.58 ± 0.86 | |||
| ABT-126 5 mg | 68 | 52.71 ± 16.05 | 53.09 ± 16.89 | 0.42 ± 0.85 | −1.16 ± 1.19 | −3.12 to 0.81 | .834 |
| ABT-126 25 mg | 68 | 47.09 ± 16.89 | 47.68 ± 19.25 | 0.44 ± 0.85 | −1.14 ± 1.21 | −3.14 to 0.86 | .827 |
| Donepezil | 66 | 49.76 ± 16.93 | 52.71 ± 16.87 | 2.95 ± 0.86 | 1.37 ± 1.21 | −0.62 to 3.36 | .128 |
| QoL-AD, Subject total score (↑ in score indicates improvement) | |||||||
| Placebo | 63 | 34.65 ± 5.48 | 36.06 ± 6.14 | 1.19 ± 0.53 | |||
| ABT-126 5 mg | 67 | 35.58 ± 5.22 | 35.55 ± 5.19 | 0.12 ± 0.51 | −1.07, 0.72 | −2.26 to 0.13 | .929 |
| ABT-126 25 mg | 66 | 35.45 ± 4.88 | 35.09 ± 5.46 | −0.18 ± 0.51 | −1.37, 0.73 | −2.58 to −0.17 | .970 |
| Donepezil | 63 | 34.35 ± 5.19 | 34.92 ± 5.41 | 0.24 ± 0.52 | −0.95, 0.73 | −2.16 to 0.26 | .902 |
| QoL-AD, caregiver total score (↑ in score indicates improvement) | |||||||
| Placebo | 66 | 34.67 ± 5.66 | 34.73 ± 5.40 | 0.44 ± 0.47 | |||
| ABT-126 5 mg | 67 | 33.37 ± 5.84 | 33.87 ± 6.20 | 0.49 ± 0.46 | 0.06 ± 0.65 | −1.02 to 1.13 | .466 |
| ABT-126 25 mg | 66 | 31.21 ± 5.87 | 32.02 ± 5.65 | 0.35 ± 0.47 | −0.09 ± 0.67 | −1.20 to 1.02 | .552 |
| Donepezil | 63 | 32.35 ± 6.15 | 32.59 ± 6.06 | 0.02 ± 0.48 | −0.42 ± 0.67 | −1.53 to 0.69 | .735 |
Abbreviations: SD, standard deviation; LS, least squares; SE, standard error; CI, confidence interval; ADAS-Cog, Alzheimer's Disease Assessment Scale-cognitive subscale; MMSE, Mini-Mental Status Examination; CIBIS, Clinician Interview-Based Impression of Severity; CIBIC, Clinician Interview-Based Impression of Change; NPI, Neuropsychiatric Inventory; ADCS-ADL, Alzheimer's Disease Cooperative Study Activities of Daily Living; QoL-AD, Quality of Life-Alzheimer's Disease.
Statistically significant versus placebo (one-sided P value from analysis of covariance).
The CIBIS was conducted at baseline and the CIBIC-Plus at the final visit. CIBIC-Plus ratings range from 1, markedly improved to 7, markedly worse. LS means (SE) are presented instead of LS mean (SE) of change.
No statistically significant differences in CIBIC-plus scores were observed in the ANCOVA or MMRM analyses between either ABT-126 dose group and placebo. The donepezil treatment group had a trend in the percentage of subjects with any improvement (46.2%) compared with placebo (33.3%, P = .093) in the final CIBIC-plus rating; results for ABT-126 5 mg (36.8%) and ABT-126 25 mg (39.1%) were not statistically significant.
Subgroup analyses of the 11-item ADAS-Cog total score on the intent-to-treat data set indicated a statistically significant treatment-by-baseline MMSE category interaction based on a difference in the treatment response for donepezil between mild-and-moderate AD subjects (P = .097). Subjects treated with ABT-126 25 mg had similar LS mean differences from placebo of −1.9 (P = .066) and −1.3 (P = .178) in subgroups with baseline MMSE values ≤19 and ≥20, respectively, whereas donepezil subjects had LS mean differences from placebo of −3.8 (P = .003) and 0.5 (P = .661) in subgroups with baseline MMSE values ≤19 and ≥20, respectively.
3.3. Pharmacokinetics and the ADAS-Cog scores exposure-response analysis
The observed ABT-126 plasma concentrations were generally consistent with the expected exposures for the evaluated dose levels, indicating good adherence to study medication.
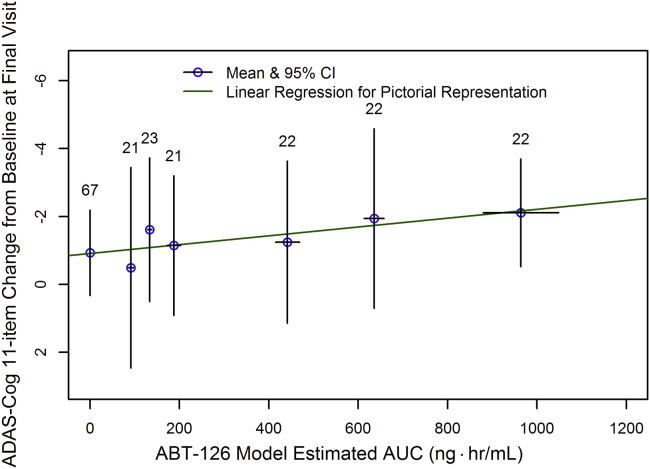
The exposure-response nonlinear mixed-effects analysis indicated a statistically significant relationship between ABT-126 exposure (plasma AUC over a dosing interval estimated based on the sparse pharmacokinetic samples) and the change from baseline in both the ADAS-Cog 11- and 13-item total scores (P < .05). The slope of the exposure-response relationship from the nonlinear mixed-effects analysis was in agreement with the slope from a simple linear regression of the data. A pictorial representation of the exposure-response relationship for the ADAS-Cog 11 scores is depicted in Fig. 3.
Fig. 3.
Relationship between ABT-126 exposure and the change from baseline in 11-item ADAS-Cog total score. Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale-cognitive subscale; CI, confidence interval; AUC, area under the plasma concentration time curve.
The analysis suggested that higher exposure of ABT-126 might be associated with higher efficacy because a plateau of the relationship between ABT-126 AUC and the change from baseline in the ADAS-cog scores could not be discerned based on available data.
3.4. Safety
The mean duration of treatment in this 12-week (84 day) trial was 81.5 days (SD = 13.09), with no statistically significant differences observed across treatment groups. Overall, study drug adherence was approximately 96% as determined by the investigators and was similar across the treatment groups.
Treatment-emergent AEs and serious adverse events (SAEs) are summarized in Table 3. When comparing subjects treated with ABT-126 versus placebo, no statistically significant differences were observed in the incidence of AEs or those considered possibly or probably related to study drug. The most frequently reported AEs in ABT-126-treated subjects overall were diarrhea, fall, and urinary tract infection (all 4.3%) compared with nausea (8.8%) and diarrhea and vomiting (both 7.4%) in the donepezil group (Table 3). Of the 112 subjects who experienced an AE, the maximum severity in 103 (92.0%) was mild or moderate. All SAEs were considered by the investigators as not related or probably not related to the study drug.
Table 3.
Summary of treatment-emergent adverse events
| Overall, n (%) | Placebo n = 68 | ABT-126 5 mg n = 68 | ABT-126 25 mg n = 70 | Donepezil 10 mg n = 68 | All subjects n = 274 |
|---|---|---|---|---|---|
| Any adverse event | 26 (38.2) | 29 (42.6) | 28 (40.0) | 29 (42.6) | 112 (40.9) |
| Discontinued due to AE | 2 (2.9) | 1 (1.5) | 1 (1.4) | 5 (7.4) | 9 (3.3) |
| Severe AE | 3 (4.4) | 0 | 2 (2.9) | 4 (5.9) | 9 (3.3) |
| Serious AE | 3 (4.4) | 2 (2.9) | 3 (4.3) | 5 (7.4) | 13 (4.7)∗ |
| Deaths† | 1 (1.5) | 0 | 1 (1.4) | 0 | 2 (0.7) |
| Adverse events reported by ≥5.0% of subjects in any treatment group, MedDRA preferred term | |||||
| Diarrhea | 2 (2.9) | 2 (2.9) | 4 (5.7) | 5 (7.4) | 13 (4.7) |
| Nausea | 1 (1.5) | 4 (5.9) | 1 (1.4) | 6 (8.8) | 12 (4.4) |
| Headache | 1 (1.5) | 1 (1.5) | 4 (5.7) | 4 (5.9) | 10 (3.6) |
| Vomiting | 1 (1.5) | 1 (1.5) | 3 (4.3) | 5 (7.4) | 10 (3.6) |
| Fall | 1 (1.5) | 2 (2.9) | 4 (5.7) | 2 (2.9) | 9 (3.3) |
| Urinary tract infection | 0 | 4 (5.9) | 2 (2.9) | 3 (4.4) | 9 (3.3) |
| Serious adverse events occurring during the double-blind period, MedDRA preferred term | |||||
| Atrial fibrillation | 1 (1.5) | 0 | 0 | 0 | 1 (0.4) |
| Bradycardia | 0 | 0 | 1 (1.4) | 0 | 1 (0.4) |
| Cholecystitis acute | 0 | 0 | 0 | 1 (1.5) | 1 (0.4) |
| Completed suicide | 1 (1.5) | 0 | 0 | 0 | 1 (0.4) |
| Hyponatremia | 0 | 1 (1.5) | 0 | 0 | 1 (0.4) |
| Lower respiratory tract infection | 0 | 0 | 0 | 1 (1.5) | 1 (0.4) |
| Nausea | 0 | 0 | 1 (1.4) | 0 | 1 (0.4) |
| Sinus arrhythmia | 1 (1.5) | 0 | 0 | 0 | 1 (0.4) |
| Vomiting | 0 | 0 | 1 (1.4) | 0 | 1 (0.4) |
| Withdrawal syndrome | 0 | 0 | 0 | 1 (1.5) | 1 (0.4) |
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities.
Nine subjects experienced serious AEs during the double-blind period and four subjects during the follow-up period.
One placebo-treated subject completed suicide and one subject who took ABT 126 25 mg died 18 d post-dose of cardiac failure. Neither death was considered related to study drug.
No consistent, clinically significant changes in laboratory or vital sign results were noted across treatment groups. Statistically significant differences between the ABT-126 25 mg group and placebo were observed in mean change from baseline to final for the Bazett QTc (QTcB) interval (10.4 milliseconds, standard error [SE] = 4.7, P = .026) and Fridericia QTc (QTcF) interval (9.5 milliseconds, SE = 4.4, P = .032). The significant differences from placebo emerged at week 12 and were related to increases in mean values in the ABT-126 25 mg group, as well as to decreases in the placebo group. Compared with placebo, no significant differences in QTcB or QTcF were observed in any treatment group at weeks 2, 4, and 8. A significant decrease in mean final heart rate (−5.3 bpm, SE = 1.8, P = .004) was noted for donepezil compared with placebo, consistent with data previously reported for donepezil [27]. In addition, the donepezil treatment group showed a significant increase in the QT interval at the final visit (11.5 milliseconds, SE = 5.5, P = .037) but this was not accompanied by a significant change in either QTcB or QTcF intervals. The incidence of potentially clinically significant changes in ECG parameters was low in the ABT-126 25 mg and donepezil treatment groups and similar to those observed across treatment groups.
Exploratory analyses indicated a statistically significantly higher LS mean PWC-20 score for the ABT-126 25 mg group (3.32, SE = 0.38) compared with placebo (1.64, SE = 0.38, P = .002). Although this suggested greater severity in withdrawal symptoms from ABT-126 25 mg treatment, reported symptoms were generally of mild intensity and there were no treatment-group differences in AEs spontaneously reported during the posttreatment period.
4. Discussion
The treatment effects in the donepezil group confirmed that the study design was sufficient to detect a trend toward improvement on the 11-item ADAS-Cog and significant improvement on the 13-item ADAS-Cog. The ABT-126 25 mg treatment group exhibited a 1.19-point improvement on the 11-item ADAS-Cog in this proof-of-concept study (P = .095), whereas the improvement in the donepezil group was −1.43 points (P = .057). There appeared to be some effect of dose on the ABT-126 treatment response, as the 5 mg dose group did not exhibit numerical improvement over placebo. Neither donepezil nor ABT-126 demonstrated improvement on secondary efficacy measures, although the sample size was intended to provide power only for the primary outcome.
Overall, ABT-126 was generally well tolerated in subjects with AD and exhibited an adequate safety profile to support continued development at higher doses. Few subjects discontinued due to AEs, and the incidence of AEs and SAEs was low and comparable across dose groups, and no SAEs were considered probably related or related to the study drug. Consistent clinically meaningful changes in laboratory or vital signs were not observed in ABT-126 treatment groups.
The difference in change from baseline in the QTcB and QTcF intervals between the ABT-126 25 mg group and placebo at week 12 is of undetermined clinical significance. It is likely that this finding would have been observed shortly after steady-state levels were reached (i.e., before the week 2 visit) if the changes were related to ABT-126 treatment. The emergence of QTc changes after several months of treatment is not typical of drug-induced QTc changes and therefore not considered related to ABT-126 administration. Other α7 nAChR agonists, including EVP-6124 [9] and MEM3454/RG3487 [10], also showed evidence of procognitive effects in subjects with mild-to-moderate AD. Phase 2 studies conducted with these agents also revealed no safety signals of concern.
Doses in this trial were selected to provide plasma exposures at or above those resulting in improved cognitive performance in preclinical models. The exposure-response data suggest higher doses may yield improved efficacy. Data from this trial helped to guide dose selection and target patient population for subsequent clinical studies of ABT-126 that explored a higher dose range. Two phase 2 studies exploring a dose range of 25–75 mg in patients with mild-to-moderate AD either as monotherapy or add-on therapy to AChEIs were recently completed, and preliminary data were disclosed [28], [29]. Initial results suggest that even at higher doses, the degree of efficacy exhibited by ABT-126 as monotherapy or add-on therapy is insufficient to support further development.
The present study had several limitations, including an uncharacteristically small donepezil response, short treatment duration, and disproportionate subject enrollment across the six participating countries. The donepezil improvement of −1.43 points was less than what has usually been observed in 24-week studies of donepezil where decreases were approximately 2.8 points (95% CI −3.74 to −2.10) [30], suggesting that the sensitivity of the trial to detect treatment effects was limited. The trial design included a relatively short 12-week treatment duration and no wash out period; it was adequately designed to detect the onset of treatment effect but did not provide useful information about the durability of this effect. In addition, the proportion of subjects enrolled from each country varied from approximately 0.1–0.25. Differences in application of the criteria for subject selection or the acquisition of the outcome measures may have created additional variability in the data that prevented detection of a treatment effect for donepezil that was more consistent with previous trial results.
Other α7 nAChR agonists, including encenicline (EVP-6124) [9] and MEM3454/RG3487 [10], also showed evidence of procognitive effects in subjects with mild-to-moderate AD, and phase 2 studies conducted with these agents also revealed no safety signals of concern. Ongoing randomized, placebo-controlled, phase 3 studies of encenicline (NCT01969123 and NCT01969136) will assess the safety and efficacy of high-dose and low-dose encenicline in subjects with mild-to-moderate AD who are currently or were previously taking stable doses of AChEIs. The results from these studies will provide additional evidence about the efficacy and safety of α7 nAChR agonists in the treatment of AD.
Research in context.
-
1.
Systematic review: Data regarding the preclinical and clinical effects of α7 nicotinic acetylcholine receptor agonists were acquired via searches of online databases such as PubMed and presentations from scientific meetings. The information obtained is summarized and cited.
-
2.
Interpretation: In this double-blind, randomized, placebo-controlled, proof-of-concept study, among the first with α7 nicotinic acetylcholine receptor agonists, no significant cognitive improvement was observed in mild-to-moderate Alzheimer's dementia patients.
-
3.
Future directions: Additional studies of ABT-126 and other α7 agonists as monotherapy or add-on therapy in the treatment of patients with mild-to-moderate Alzheimer's dementia should be performed.
Acknowledgments
This study was sponsored by Abbott, subsequently AbbVie. Abbott/AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. The authors were assisted in the preparation of this article by Muriel Cunningham, a professional medical writer compensated by AbbVie.
L.M.G., W.Z.R., and A.A.O. are employees of AbbVie and hold Abbott and/or AbbVie stock. Y.P. and R.A.L. are former employees of Abbott and hold Abbott and/or AbbVie stock. C.W.R. has received consultancies fees from AbbVie for attendance at Advisory Boards. He has also received consultancies fees from the following companies developing or marketing interventions for Alzheimer's disease: GSK, Roche, MSD, Sanofi-Aventis, Prana Biotechnology, Novartis, Eisai, Nutricia, Pfizer, Baxter, and Servier.
References
- 1.Yakel J.L. Cholinergic receptors: Functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch. 2013;465:441–450. doi: 10.1007/s00424-012-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lendvai B., Kassai F., Szajli A., Nemethy Z. α7 nicotinic acetylcholine receptors and their role in cognition. Brain Res Bull. 2013;93:86–96. doi: 10.1016/j.brainresbull.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie C.W., Zhinchin G. Low dose, high dose, or no dose: better prescribing of cholinesterase inhibitors for Alzheimer's disease. Int Psychogeriatr. 2013;25:511–515. doi: 10.1017/S1041610212002414. [DOI] [PubMed] [Google Scholar]
- 4.Beracochea D., Boucard A., Trocme-Thibierge C., Morain P. Improvement of contextual memory by S 24795 in aged mice: comparison with memantine. Psychopharmacology (Berl) 2008;196:555–564. doi: 10.1007/s00213-007-0987-5. [DOI] [PubMed] [Google Scholar]
- 5.Marighetto A., Valerio S., Desmedt A., Philippin J.N., Trocme-Thibierge C., Morain P. Comparative effects of the alpha7 nicotinic partial agonist, S 24795, and the cholinesterase inhibitor, donepezil, against aging-related deficits in declarative and working memory in mice. Psychopharmacology (Berl) 2008;197:499–508. doi: 10.1007/s00213-007-1063-x. [DOI] [PubMed] [Google Scholar]
- 6.Radek R.J., Robb H.M., Stevens K.E., Gopalakrishnan M., Bitner R.S. Effects of the novel α7 nicotinic acetylcholine receptor agonist ABT-107 on sensory gating in DBA/2 mice: Pharmacodynamic characterization. J Pharmacol Exp Ther. 2012;343:736–745. doi: 10.1124/jpet.112.197970. [DOI] [PubMed] [Google Scholar]
- 7.Rezvani A.H., Kholdebarin E., Brucato F.H., Callahan P.M., Lowe D.A., Levin E.D. Effect of R3487/MEM3454, a novel nicotinic alpha7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:269–275. doi: 10.1016/j.pnpbp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Prickaerts J., van Goethem N.P., Chesworth R., Shapiro G., Boess F.G., Methfessel C. EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors. Neuropharmacology. 2012;62:1099–1110. doi: 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Deardorff W.J., Shobassy A., Grossberg G.T. Safety and clinical effects of EVP-6124 in subjects with Alzheimer's disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev Neurother. 2015;15:7–17. doi: 10.1586/14737175.2015.995639. [DOI] [PubMed] [Google Scholar]
- 10.Memory Pharmaceuticals . 2007. Press release: Memory Pharmaceuticals announces positive Phase 2a results for MEM 3454 in Alzheimer's Disease.http://www.drugs.com/clinical_trials/memory-pharmaceuticals-announces-positive-phase-2a-results-mem-3454-alzheimer-s-2538.html Available at: Accessed May 1, 2014. [Google Scholar]
- 11.Masterman D., Awipi T., Ashford E., Nave S., Yoo K., Vellas B., editors. A nictotinic-alpha-7 partial agonist as adjunctive therapy to stable donepezil. Clinical Trials on Alzheimer's Disease; Monte Carlo: 2012. [Google Scholar]
- 12.Gopalakrishnan M., Bitner R., Anderson D., Drescher K., Kohlhaas K., Grønlien J., editors. Preclinical characterization of a selective α7 neuronal nicotinic acetylcholine receptor agonist ABT-126: A novel therapeutic agent for the treatment of cognitive impairment in Alzheimer's disease and schizophrenia. Alzheimer's Association International Conference; Boston MA: 2013. [Google Scholar]
- 13.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos G.S., Abrams R.C., Young R.C., Shamoian C.A. Cornell Scale for depression in dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 16.Hachinski V.C., Iliff L.D., Zilhka E., Du Boulay G.H., McAllister V.L., Marshall J. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 17.Mohs R.C., Knopman D., Petersen R.C., Ferris S.H., Ernesto C., Grundman M. Development of cognitive instruments for use in clinical trials of antidementia drugs: Additions to the Alzheimer's disease assessment scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S13–S21. [PubMed] [Google Scholar]
- 18.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 19.Boothby H., Mann A., Barker A. Factors determining interrater agreement with rating global change in dementia: The CIBIC-plus. Int J Geriatr Psychiatry. 1995;10:1037–1045. [Google Scholar]
- 20.Cummings J.L. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 21.Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 22.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 23.Logsdon R., Gibbons L., McCurry S., Teri L. Quality of life in Alzheimer's disease: Patient and caregiver reports. J Ment Health Aging. 1999;5:21–32. [Google Scholar]
- 24.Rickels K., Garcia-Espana F., Mandos L.A., Case G.W. Physician withdrawal checklist (PWC-20) J Clin Psychopharmacol. 2008;28:447–451. doi: 10.1097/JCP.0b013e31817efbac. [DOI] [PubMed] [Google Scholar]
- 25.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MedDRA Maintenance and Support Services Organization. Medical Dictionary for Regulatory Activities (MedDRA). 3975 Virginia Mallory Dr., Chantilly VA 20151Version 13.1.
- 27.Eisai Inc . 2015. Aricept highlights of prescribing information.http://www.aricept.com/docs/pdf/aricept_PI.pdf Available at: Accessed: May 11, 2015. [Google Scholar]
- 28.Gault L.M., Meier A., Florian H., Gauthier S., Lin Y., Tang Q. A Phase 2 trial of the efficacy and safety of the alpha7 agonist ABT-126 as an add-on treatment in mild-to-moderate Alzheimer's dementia. Alzheimers Dement. 2014;10:P917–P918. [Google Scholar]
- 29.Othman A., Meier A., Ritchie C.W., Florian H., Gault L.M., Tang Q. Efficacy and safety of the alpha7 agonist ABT-126 as a monotherapy treatment in mild-to-moderate Alzheimer's dementia: Results of a Phase 2b trial. Alzheimers Dement. 2014;10:P137. [Google Scholar]
- 30.Birks J., Harvey R.J. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2006;(1):CD001190. doi: 10.1002/14651858.CD001190.pub2. [DOI] [PubMed] [Google Scholar]