Abstract
Introduction
Solanezumab is an anti-amyloid monoclonal antibody in clinical testing for treatment of Alzheimer's disease (AD). Its mechanism suggests the possibility of slowing the progression of AD.
Methods
A possible disease-modifying effect of solanezumab was assessed using a new statistical method including noninferiority testing. Performance differences were compared during the placebo-controlled period with performance differences after the placebo patients crossed over to solanezumab in the delayed-start period.
Results
Noninferiority of the 14-item Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog14) and Alzheimer's Disease Cooperative Study Activities of Daily Living inventory instrumental items (ADCS-iADL) differences was met through 132 weeks, indicating that treatment differences observed in the placebo-controlled period remained, within a predefined margin, after the placebo group initiated solanezumab. Solanezumab was well tolerated, and no new safety concerns were identified.
Discussion
The results of this secondary analysis show that the mild subgroup of solanezumab-treated patients who initiated treatment early, at the start of the placebo-controlled period, retained an advantage at most time points in the delayed-start period.
Keywords: Solanezumab, Delayed-start, Clinical trials, Alzheimer's disease, Anti-amyloid-β antibody
1. Introduction
Alzheimer's disease (AD) is an age-related neurodegenerative disorder characterized by a progressive decline in cognitive function. Currently approved treatments attenuate the symptoms of AD but have not been shown to affect the underlying pathology [1]; thus, they are sometimes termed “symptomatic” treatments. Leber (1997) [2] proposed the delayed-start design as a method for demonstrating a disease-modification drug effect; that is, an effect that slows the progression of disease by modifying the underlying biological pathology, rather than only attenuating symptoms.
The delayed-start, also known as randomized-start, study design is one in which patients are randomized to the same active treatment but starting at different times, resulting in two treatment periods: a placebo-controlled period followed by a delayed-start period. During the placebo-controlled period, patients receive either an active treatment or placebo. During the delayed-start period, placebo patients are switched to active treatment and thus become delayed-start patients. Patients on active treatment during the placebo-controlled period continue to receive active treatment during the delayed-start period and are labeled as early-start patients. Thus, in a delayed-start study, patients are randomized at the beginning of the placebo-controlled period to be either early- or delayed-start patients. During the entire length of the study (that is, both the placebo-controlled and delayed-start periods), this randomization to treatment group is blinded to all patients and study personnel. If the treatment difference observed at the end of the placebo-controlled period was preserved at the end of the delayed-start period (that is, delayed-start patients do not “catch up” with the early start patients), the treatment effect is considered consistent with a disease-modifying effect.
There have been very limited published data on delayed-start studies; one example is the Attenuation of Disease Progression with Azilect Given Once-daily (ADAGIO) study, a study of rasagiline conducted to assess possible disease-modification effects in Parkinson's disease. However, the study included some methodological issues and a lack of dose response made the results difficult to interpret [3]. Other studies have reported the long-term use of symptomatic drugs in a framework similar to the delayed-start design, either with or without placebo wash-out periods [4], [5]. Although these studies suggested that continued treatment with symptomatic drugs may offer longer term benefit, no quantitative methods were applied to assess whether the differences between early-start and delayed-start patients were due to chance and whether delayed-start patients had caught up with early-start patients within a statistical margin.
We recently proposed a new method for delayed-start analyses that includes comparisons of treatment differences at the beginning and end of the delayed-start period using a noninferiority test. This method uses a single mixed-model repeated measure (MMRM) analysis model including all available data from all randomized patients from the beginning of the placebo-controlled period through the end of the delayed-start period. This new method was developed to mitigate some issues observed with previously applied methods [6], and its application to data from the solanezumab EXPEDITION program represents the first effort of a prespecified statistical delayed-start analysis in an AD study.
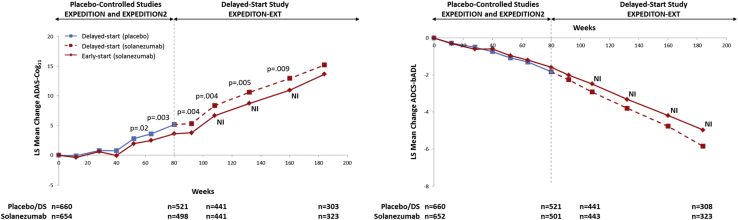
1.1. Solanezumab
Solanezumab is an IgG1 anti-amyloid monoclonal antibody that binds to the mid-domain of the amyloid-beta (Aβ) peptide and is thought to increase clearance of soluble Aβ. EXPEDITION and EXPEDITION2 were identical phase 3, 18-month, placebo-controlled studies investigating solanezumab treatment in patients with mild-to-moderate AD. EXPEDITION-EXT is an ongoing open-label extension study offered to patients who completed EXPEDITION or EXPEDITION2, in which all patients receive solanezumab; patients, investigators, and site personnel remain blinded to the original treatment assignment during the placebo-controlled period. Analyses from the two individual placebo-controlled studies did not show a significant benefit of solanezumab for the original coprimary outcomes: the 11-item Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog11) and the Alzheimer's Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL) in the mild-to-moderate AD population. In EXPEDITION, the treatment benefit for solanezumab at 80 weeks was 0.8 points (P = .24) for the ADAS-Cog11 and 0.4 points (P = .64) for the ADCS-ADL. In EXPEDITION2, the treatment benefit for solanezumab at 80 weeks was 1.3 points (P = .06) for the ADAS-Cog11 and 1.6 points (P = .08) for the ADCS-ADL [7]. However, a key prespecified secondary analysis of the mild AD population in EXPEDITION demonstrated a significant effect of solanezumab on cognition; based on this result, the statistical analysis plan for EXPEDITION2 was changed such that the primary outcome was cognition alone (the 14-item ADAS-Cog [ADAS-Cog14]) in the mild population. This single primary outcome did not reach statistical significance in the smaller mild-only subgroup in EXPEDITION2 (P = .06) [7]. When examining the mild subgroup in the larger pooled population from EXPEDITION and EXPEDITION2, the treatment benefit for solanezumab was 2.13 points (P = .001) for the ADAS-Cog14 and 1.21 points (P = .045) for the instrumental items of the ADCS-ADL (ADCS-iADL) [8]. Delayed-start analyses of the first interim data cut for EXPEDITION-EXT (through 20 June 2012, including 240 placebo and 232 solanezumab mild AD patients who had completed 28 weeks of treatment in the delayed-start period), showed a persistent benefit on cognition [6]. That is, the treatment difference in cognition between solanezumab and placebo observed at the end of the placebo-controlled studies was preserved at 28 weeks in the delayed-start period. Safety analyses from the placebo-controlled period (the first 18 months of the program) suggested that solanezumab is well tolerated [7].
Data have subsequently become available for all patients who have completed 2 years or discontinued in EXPEDITION-EXT. Delayed-start and safety analyses have been performed for the entire 3.5-year period (18 months in the placebo-controlled period, plus 2 years in the delayed-start period) in the EXPEDITION studies for the patients who were defined as mild at the start of the placebo-controlled period. This population was selected because a delayed-start analysis presupposes an effect at the end of the placebo-controlled period, which was not observed in the moderate population in the EXPEDITION program. As such, the current solanezumab development program focuses on mild and preclinical AD populations.
2. Methods
2.1. Study design
The designs of EXPEDITION and EXPEDITION2 (ClinicalTrials.gov numbers NCT00905372 and NCT00904683) have been described previously [7]. Briefly, both were multinational, randomized, double-blind, placebo-controlled, phase 3 studies of solanezumab given intravenously 400 mg every 4 weeks in outpatients with mild-to-moderate AD. Patients were at least 55 years old and met criteria for probable AD based on National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria [9]. Patients with Mini-Mental Status Examination (MMSE) [10] scores of 16–26 were allowed to participate. Mild AD was defined as screening visit MMSE scores of 20–26; moderate AD was defined as screening visit scores of 16–19. Randomization to treatment was stratified by AD severity to ensure a balance of treatment assignment within both mild and moderate AD patients. Patients were allowed to continue treatment with stable doses of standard-of-care AD treatments (for example, acetylcholinesterase inhibitors and memantine) throughout the studies.
EXPEDITION-EXT (NCT01127633) is an ongoing extension study in patients who completed EXPEDITION or EXPEDITION2. During EXPEDITION-EXT, all patients receive solanezumab 400 mg every 4 weeks starting at week 1 (which is the same as week 80 in the placebo-controlled studies); however, both patients and site personnel remain blinded to patients' original treatment assignment to solanezumab or placebo in the placebo-controlled studies. In EXPEDITION-EXT, patients who received solanezumab in the placebo-controlled studies remain on active treatment (early-start), whereas patients who received placebo in the placebo-controlled studies are switched to solanezumab (delayed-start).
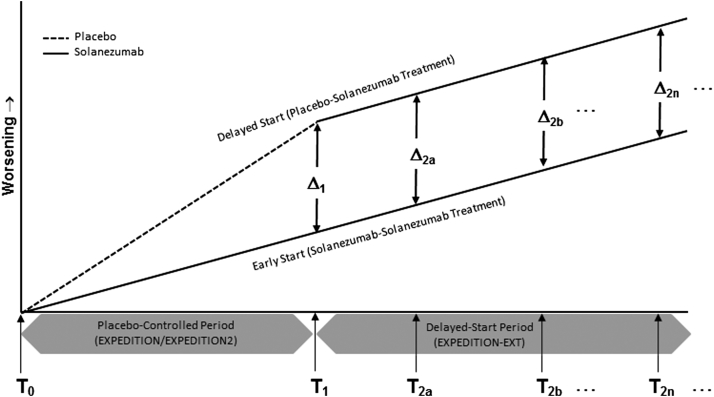
Together, these studies represent the placebo-controlled period (EXPEDITION and EXPEDITION2) and the delayed-start period (EXPEDITION-EXT) in the delayed-start analysis of the EXPEDITION program (Fig. 1).
Fig. 1.
Model of delayed-start design and analysis in the solanezumab EXPEDITION program. T0 = beginning time point in the placebo-controlled period; T1 = end time point in the placebo-controlled period/beginning time point in the delayed-start period; T2 = postbaseline time point in the delayed-start analysis (multiple time points are analyzed in the MMRM analysis; the primary time point for the delayed-start analysis in the EXPEDITION program was 108 weeks, that is, 28 weeks after the start of the delayed-start period); Δ1 = the true treatment difference at T1; Δ2 = the true treatment difference at T2. Abbreviation: MMRM, mixed-model repeated measure.
The trial protocols were approved by the ethical review board at each of the 221 study centers in EXPEDITION and EXPEDITION2 and the 206 study centers in EXPEDITION-EXT in 16 countries. These studies were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with good clinical practices and the applicable laws and regulations. A properly executed, signed informed consent form was obtained from each subject.
2.2. Statistical methods
All statistical analyses were conducted using SAS version 9.2.
2.2.1. Delayed-start analysis
The primary time point for the delayed-start analyses was the analysis at 108 weeks (that is, 28 weeks after the start of EXPEDITION-EXT), among the subgroup of patients with mild AD at baseline. We evaluated the persistence of the separation between active- and placebo-treated subjects during the delayed-start period with a noninferiority test and by determining the statistical significance of the treatment difference, using pooled data from the EXPEDITION, EXPEDITION2, and EXPEDITION-EXT studies. In addition, we conducted the delayed-start analysis for additional time points after 108 weeks, through 184 weeks in the mild AD population for a total of up to 3.5 years in the placebo-controlled and delayed-start periods. The statistical methodologies for the delayed-start analysis described in this article are described in detail elsewhere [6]; they were communicated to Food and Drug Administration (FDA) and were prespecified in the statistical analysis plan before database locks.
To test for differences in disease progression between the delayed-start and early-start groups, the change for each efficacy measure from the beginning of the placebo-controlled period through the delayed-start period was analyzed with an MMRM analysis model as described in the following; the schematics of the design and analysis are shown in Fig. 1. Efficacy measures included the ADAS-Cog14, ADCS-iADL, clinical dementia rating scale–sum of boxes (CDR-SB), MMSE, the 11-item ADAS-Cog (ADAS-Cog11), and the basic items of the ADCS-ADL (ADCS-bADL).
The MMRM analysis model included all randomized patients with mild AD and used data from the beginning of the placebo-controlled period (T0) through the delayed-start period (T2). The model included terms for seven fixed effects: baseline score, pooled investigator, treatment, visit, treatment-by-visit interaction, concomitant acetylcholinesterase inhibitor (AChEI) and/or memantine use at baseline (yes or no), and age at baseline (for all variables, baseline was the beginning of the placebo-controlled period); subject was considered a random effect. Visit was considered a categorical variable with values equal to the visit numbers at which the scales were assessed.
To determine whether the benefits of early treatment can be matched by later treatment (that is, whether delayed-start patients can “catch up” with early-start patients), a noninferiority test was conducted. The null and alternative hypotheses of the noninferiority test are described in the following, where Δ1 is the true treatment difference at the end of the placebo-controlled period, Δ2 is the true treatment difference at the postbaseline time point in the delayed-start period, and the noninferiority margin was specified as 50% of the treatment difference observed at the end of the placebo-controlled period (Δ1):
which is equivalent to
The noninferiority test was carried out by constructing a 90% one-sided confidence interval for Δ2–0.5Δ1. If the lower limit of the confidence interval is greater than 0, the null hypothesis is rejected and the noninferiority criterion is met, indicating that at least 50% of the treatment difference observed at the end of the placebo-controlled period has been preserved at the end of the delayed-start period.
In addition, a superiority analysis was conducted on Δ2 with two-sided P value of ≤.05 using the same MMRM model used for the noninferiority analysis mentioned previously.
2.2.2. Safety assessment
Safety was assessed by summarizing adverse events (AEs) and electrocardiograms for the delayed-start and early-start treatment groups across the entire EXPEDITION program (that is, the placebo-controlled and delayed-start periods). AEs were coded according to established MedDRA version 17.0 terms and summarized by MedDRA system organ class and preferred term. AEs of special interest included infusion-related reactions, suicidal ideation or behavior, hemorrhagic stroke, cardiac ischemic– and arrhythmia-related events, and amyloid-related imaging abnormality-hemorrhage/hemosiderin deposition (ARIA-H) and ARIA-effusion/edema (ARIA-E); these events were identified as being of special interest because of potential class effects (infusion reaction and ARIA), observations from prior studies (cardiac), and regulatory considerations (suicidal ideation or behavior). All safety variables were summarized by descriptive statistics and tests of significance between early-start and delayed-start treatment groups were calculated using Fisher exact tests. The baseline for safety analyses was defined as the last nonmissing visit or measurement before the first infusion during EXPEDITION or EXPEDITION2.
Changes in ARIA-H during the study were calculated based on the number of patients with both a baseline and at least one postbaseline magnetic resonance imaging scan and an increase in number or increase in size of preexisting ARIA-H. The magnitude of changes in ARIA-H was quantified by number of categorical increases (categories 0, 1, 2–5, 6–10, and >10). A categorical increase was defined as a shift to a higher category; any increase in the number of ARIA-H in the patient with >10 ARIA-H at baseline was also considered a categorical increase. ARIA-E cases were summarized by patient.
3. Results
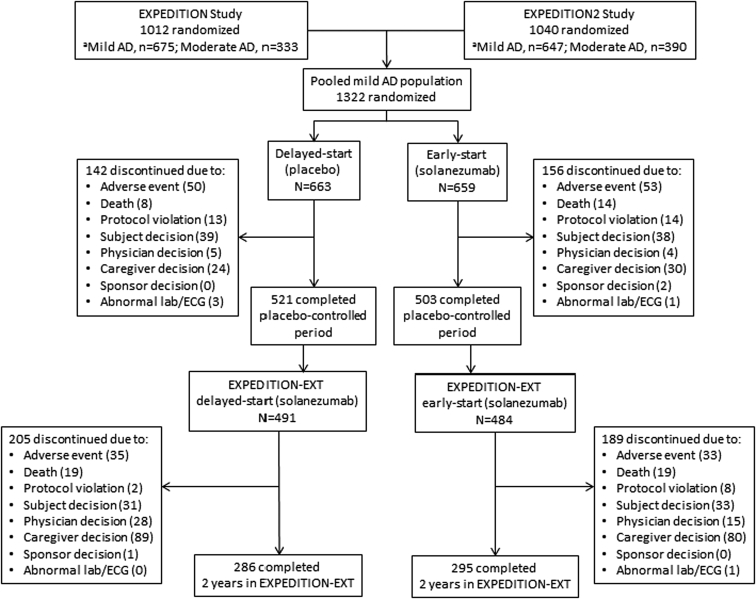
The flow of patients included in the delayed-start efficacy analyses are shown in Fig. 2. A total of 1322 patients with mild AD were randomized to be either delayed-start (n = 663) or early-start (n = 659) patients. Discontinuation rates during the placebo-controlled period were similar for placebo- and solanezumab-treated patients (21.4% and 23.7%, respectively). Of the 1024 patients who completed the placebo-controlled period (EXPEDITION and EXPEDITION2), 975 (95.2%) entered the delayed-start period (EXPEDITION-EXT) and 58.2% of delayed-start (n = 286) and 61.0% of early-start (n = 295) patients completed 2 years in the delayed-start period.
Fig. 2.
Flow of study participants. Abbreviations: AD, Alzheimer's disease; lab, laboratory measure; ECG, electrocardiogram; MMSE, Mini-Mental Status Examination. aDisease severity was based on MMSE score at baseline (mild, MMSE 20–26; moderate, MMSE 16–19). Baseline MMSE was outside of the study eligibility range (>26) for some randomized subjects, and these individuals were not categorized based on disease severity or included in pooled mild AD data set.
Demographic and clinical characteristics of the mild AD population at baseline of the EXPEDITION program are described in Table 1.
Table 1.
Baseline characteristics, mild AD patients in the Expedition program
| Characteristics | Delayed-start |
Early-start |
Total |
P value | ||
|---|---|---|---|---|---|---|
| n | n = 663 | n | n = 659 | n = 1322 | ||
| Age, mean (SD), y | 663 | 73 (7.9) | 659 | 73 (8.1) | 73 (8.0) | .169 |
| Female, n (%) | 663 | 362 (54.6) | 659 | 346 (52.5) | 708 (53.6) | .473 |
| APOE ε4 carriers, n (%) | 614 | 367 (59.8) | 595 | 329 (55.3) | 696 (57.6) | .117 |
| MMSE, mean (SD) | 660 | 22.5 (2.76) | 656 | 22.5 (2.79) | 22.5 (2.78) | .699 |
| AChEI or memantine use, n (%) | 663 | 587 (88.5) | 659 | 574 (87.1) | 1161 (87.8) | .450 |
Abbreviations: AD, Alzheimer's disease; SD, standard deviation; AChEI, acetylcholinesterase inhibitor; MMSE, Mini-mental status examination.
NOTE. The number of subjects included in each analysis varies based on the number of subjects with a baseline value for that measure.
3.1. Delayed-start
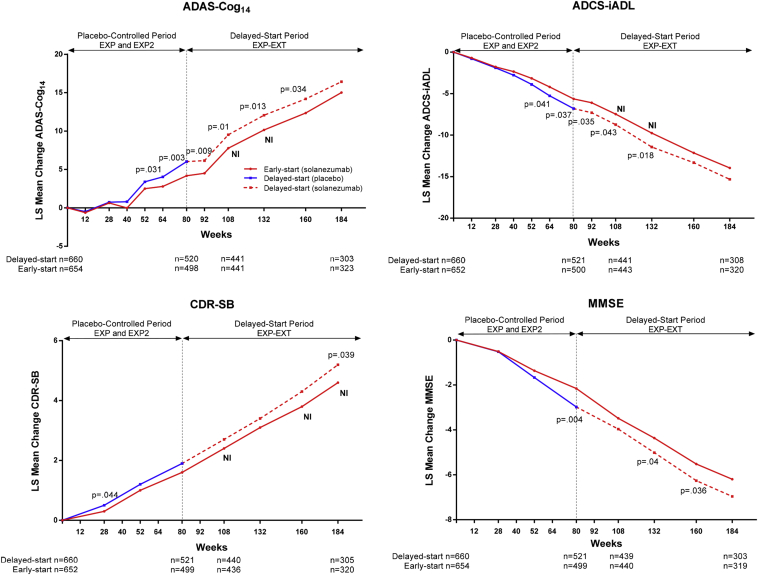
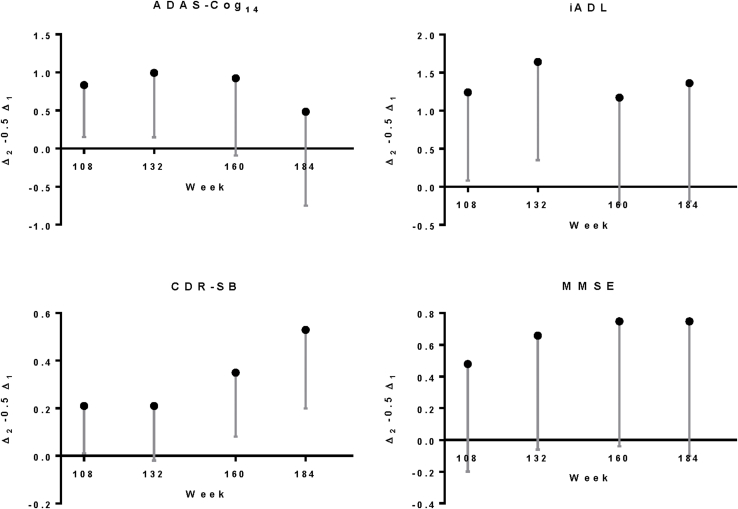
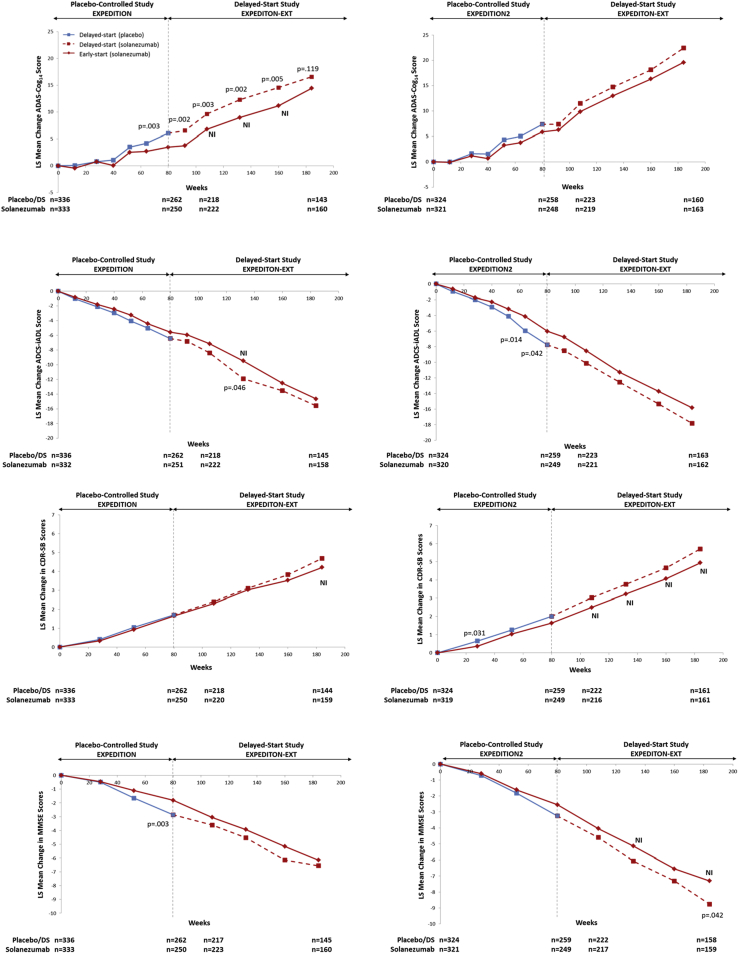
Treatment differences between early-start and delayed-start groups at 108 weeks since randomization (Δ2, that is, 28 weeks in the delayed-start period) for ADAS-Cog14 and ADCS-iADL were similar to differences at the end of the placebo-controlled period (Δ1, that is, 80 weeks since randomization; Fig. 3 and Table 2). The noninferiority and superiority criteria were both met, indicating the treatment differences in cognition and function at the end of the placebo-controlled period were preserved at 108 weeks within a predefined margin. Throughout the remainder of the delayed-start period, treatment differences for ADAS-Cog14 were significant through 160 weeks and noninferiority was met through 132 weeks. For ADCS-iADL, treatment differences were significant and noninferiority was met through 132 weeks. For ADAS-Cog14, ADCS-iADL, and MMSE, treatment differences increased steadily during the placebo-controlled period and remained stable in the delayed-start period. For the CDR-SB, treatment differences were small during the placebo-controlled period but increased over the delayed-start period (Fig. 4). Visual representations of the noninferiority statistics are presented in Supplementary Fig. 1.
Fig. 3.
Delayed-start analysis of ADAS-Cog14, ADCS-iADL, MMSE, and CDR-SB among patients with mild AD in the solanezumab EXPEDITION program through 3.5 years. P values for treatment difference shown at all time points at which P < .05. Abbreviations: ADAS-Cog14, 14-item Alzheimer's Disease Assessment Scale-Cognitive subscale; ADCS-iADL, Alzheimer's Disease Cooperative Study Activities of Daily Living inventory, instrumental items; MMSE, Mini-Mental Status Examination; CDR-SB, Clinical Dementia Rating Scale-Sum of Boxes; AD, Alzheimer's disease; NI, noninferiority criterion met (90% one-sided confidence interval for Δ2 – 0.5Δ1 >0); LS, least squares.
Table 2.
Treatment difference and noninferiority from delayed-start analyses of the solanezumab EXPEDITION program through 3.5 y among patients with mild AD
| Scale, time point | Delayed-start |
Early-start |
LS mean difference∗ | P value | Lower limit of 90% CI for Δ2–0.5Δ1 (SE) | ||
|---|---|---|---|---|---|---|---|
| n | LS mean change (SE) | n | LS mean change (SE) | ||||
| ADAS-Cog14, wk | |||||||
| Baseline | 660 | 654 | |||||
| 12 | 632 | −0.49 (0.441) | 613 | −0.62 (0.441) | 0.14 | .756 | |
| 28 | 588 | 0.73 (0.490) | 570 | 0.63 (0.491) | 0.10 | .852 | |
| 40 | 571 | 0.80 (0.492) | 552 | −0.01 (0.493) | 0.81 | .128 | |
| 52 | 551 | 3.39 (0.498) | 539 | 2.50 (0.498) | 0.90 | .100 | |
| 64 | 535 | 4.03 (0.514) | 519 | 2.79 (0.515) | 1.24 | .031 | |
| 80 | 520 | 6.02 (0.540) | 498 | 4.18 (0.542) | 1.83 | .003 | |
| 108 | 441 | 9.52 (0.577) | 441 | 7.77 (0.577) | 1.75 | .010 | 0.15 (0.531) |
| 132 | 389 | 12.06 (0.630) | 406 | 10.14 (0.627) | 1.91 | .013 | 0.15 (0.657) |
| 160 | 334 | 14.19 (0.698) | 357 | 12.35 (0.689) | 1.84 | .034 | −0.09 (0.793) |
| 184 | 303 | 16.42 (0.796) | 323 | 15.02 (0.782) | 1.40 | .169 | −0.75 (0.963) |
| ADCS-iADL, wk | |||||||
| Baseline | 660 | 652 | |||||
| 12 | 631 | −0.79 (0.434) | 609 | −0.70 (0.435) | 0.09 | .823 | |
| 28 | 589 | −1.90 (0.463) | 569 | −1.77 (0.465) | 0.12 | .795 | |
| 40 | 572 | −2.77 (0.471) | 547 | −2.34 (0.473) | 0.43 | .385 | |
| 52 | 554 | −3.98 (0.480) | 538 | −3.16 (0.481) | 0.72 | .158 | |
| 64 | 537 | −5.26 (0.486) | 517 | −4.19 (0.488) | 1.07 | .041 | |
| 80 | 521 | −6.80 (0.504) | 500 | −5.64 (0.507) | 1.16 | .037 | |
| 108 | 441 | −8.73 (0.542) | 443 | −7.47 (0.542) | 1.26 | .043 | 0.08 (0.463) |
| 132 | 390 | −11.42 (0.589) | 405 | −9.75 (0.587) | 1.66 | .018 | 0.35 (0.566) |
| 160 | 336 | −13.32 (0.628) | 358 | −12.13 (0.623) | 1.19 | .118 | −0.23 (0.650) |
| 184 | 308 | −15.33 (0.694) | 320 | −13.95 (0.689) | 1.38 | .111 | −0.19 (0.776) |
| MMSE, wk | |||||||
| Baseline | 660 | 654 | |||||
| 28 | 591 | −0.52 (0.232) | 572 | −0.51 (0.230) | 0.01 | .955 | |
| 52 | 554 | −1.67 (0.251) | 537 | −1.36 (0.251) | 0.31 | .217 | |
| 80 | 521 | −2.98 (0.265) | 499 | −2.16 (0.266) | 0.82 | .004 | |
| 108 | 439 | −3.96 (0.278) | 440 | −3.48 (0.278) | 0.48 | .115 | −0.20 (0.212) |
| 132 | 387 | −5.01 (0.287) | 404 | −4.36 (0.285) | 0.66 | .040 | −0.06 (0.242) |
| 160 | 335 | −6.26 (0.308) | 354 | −5.52 (0.305) | 0.75 | .036 | −0.04 (0.291) |
| 184 | 303 | −6.96 (0.329) | 319 | −6.20 (0.327) | 0.75 | .055 | −0.10 (0.342) |
| CDR-SB, wk | |||||||
| Baseline | 660 | 652 | |||||
| 28 | 587 | 0.52 (0.104) | 569 | 0.33 (0.104) | 0.19 | .044 | |
| 52 | 553 | 1.15 (0.120) | 536 | 0.96 (0.119) | 0.18 | .149 | |
| 80 | 521 | 1.85 (0.142) | 499 | 1.63 (0.143) | 0.22 | .189 | |
| 108 | 440 | 2.71 (0.166) | 436 | 2.39 (0.167) | 0.32 | .128 | 0.01 (0.149) |
| 132 | 387 | 3.43 (0.185) | 403 | 3.11 (0.185) | 0.32 | .170 | −0.02 (0.181) |
| 160 | 334 | 4.25 (0.204) | 352 | 3.79 (0.203) | 0.46 | .085 | 0.08 (0.211) |
| 184 | 305 | 5.21 (0.232) | 320 | 4.57 (0.230) | 0.64 | .039 | 0.20 (0.257) |
Abbreviations: AD, Alzheimer's disease; LS, least squares; SE, standard error; Δ2, treatment difference at the postbaseline time point in the delayed-start period; Δ1, treatment difference at the end of the placebo-controlled period; ADAS-Cog14, 14-item Alzheimer's Disease Assessment Scale-Cognitive subscale; CI, confidence interval; ADCS-iADL, Alzheimer's Disease Cooperative Study Activities of Daily Living inventory, instrumental items; MMSE, Mini-Mental Status Examination; CDR-SB, Clinical Dementia Rating Scale-Sum of Boxes.
LS mean difference values are absolute values representing less worsening in the early-start group relative to the delayed-start group.
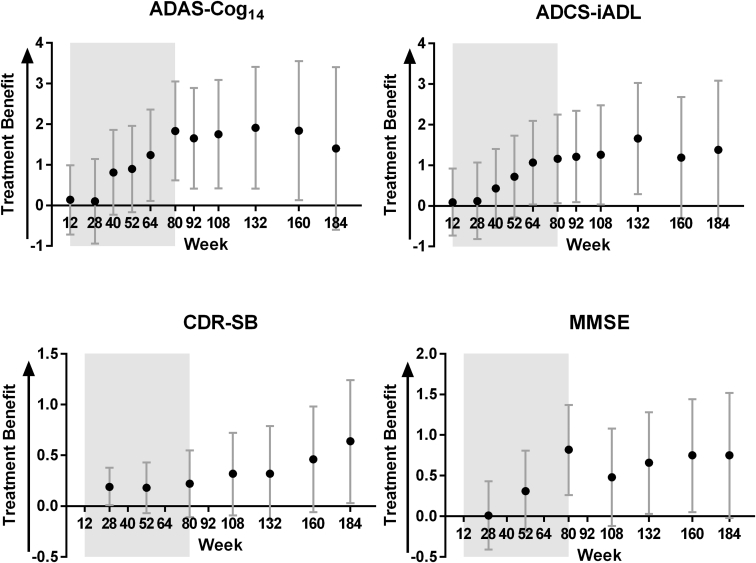
Fig. 4.
Differences in least squares means between delayed-start and early-start treatment groups and 95% confidence intervals for ADAS-Cog14, ADCS-iADL, CDR-SB, and MMSE among patients with mild AD in the solanezumab EXPEDITION program through 3.5 years. Shading represents the placebo-controlled period. Abbreviations: ADAS-Cog14, 14-item Alzheimer's Disease Assessment Scale-Cognitive subscale; ADCS-iADL, Alzheimer's Disease Cooperative Study Activities of Daily Living inventory, instrumental items; CDR-SB, Clinical Dementia Rating Scale-Sum of Boxes; MMSE, Mini-Mental Status Examination; AD, Alzheimer's disease.
Delayed-start analyses of the MMSE showed numerically similar treatment differences over the delayed-start period with statistical significance at 132 and 160 weeks. Noninferiority was not met at any time point (Fig. 3 and Table 2). Treatment differences on the CDR-SB increased over the delayed-start period, and statistical significance was reached at 184 weeks. Noninferiority was met at 108, 160, and 184 weeks; however, these results are limited in support of a delayed-start effect because a statistically significant treatment effect was not observed at the end of the placebo-controlled period for the CDR-SB.
The delayed-start results for ADAS-Cog11 and the ADCS-bADL are shown in Supplementary Fig. 2. Results for the ADAS-Cog11 were consistent with the ADAS-Cog14. Similar to the CDR-SB, the ADCS-bADL did not achieve statistical significance at the end of the placebo-controlled period but reached significance in the delayed-start period.
Results from the by-study analyses (that is, separate analyses for EXPEDITION + EXPEDITION-EXT and EXPEDITION2 + EXPEDITION-EXT) showed similar patterns in the outcomes between the two studies (Supplementary Fig. 3).
3.2. Safety
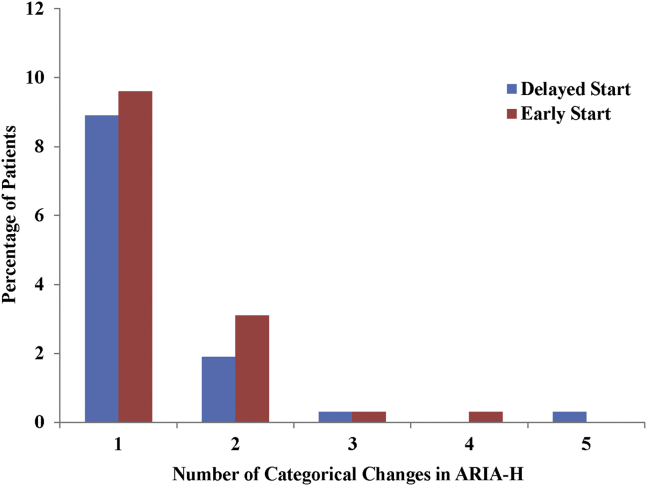
A total of 1322 mild AD patients enrolled in the EXPEDITION program, representing 3414.4 person-years of follow-up, with 79.9% of delayed-start and 77.5% of early-start patients having at least 18 months' of follow-up. Deaths, serious AEs, discontinuations due to an AE, and the incidence of increases in ARIA-H were evenly distributed across treatment regimen groups (Table 3). In addition, the severity of changes in ARIA-H (as represented by categorical increases in number) was evenly distributed across groups (Supplementary Fig. 4). Eight patients in the delayed-start group and 11 patients in the early-start group experienced ARIA-E; however, ARIA-E was not related to symptoms in any patient (see a summary table of the ARIA-E cases in Supplementary Table 1). Overall, cardiac disorders, as well as cardiac ischemic-related and cardiac arrhythmia–related events, were observed in similar proportions across groups (Table 3). Among the other safety topics of interest (suicidal ideation or behavior, infusion-related reactions, and hemorrhagic stroke), incidence did not differ significantly across treatment regimen groups (Supplementary Table 2).
Table 3.
Overview of adverse events in mild AD patients, EXPEDITION, EXPEDITION2, and EXPEDITION-EXT
| Events | Delayed-start, n = 663, n (%) | Early-start, n = 659, n (%) | P value |
|---|---|---|---|
| Death | 24 (3.6) | 32 (4.9) | .2776 |
| Serious adverse event | 239 (36.0) | 233 (35.4) | .8185 |
| Discontinuation due to adverse event | 106 (16.0) | 114 (17.3) | .5549 |
| ARIA-H (increase in number or size)∗ | 82 (12.7) | 88 (14.3) | .4108 |
| ARIA-E∗ | 8 (1.2) | 11 (1.8) | .4992 |
| Cardiac disorders SOC | 73 (11.0) | 92 (14.0) | .1140 |
| Cardiac ischemic–related events | 17 (2.6) | 25 (3.8) | .2134 |
| Myocardial infarction | 4 (0.6) | 6 (0.9) | .5462 |
| Angina pectoris | 6 (0.9) | 13 (2.0) | .1118 |
| Cardiac arrhythmia–related events | 78 (11.8) | 94 (14.3) | .1911 |
| Prolonged QTcF∗ | 2 (0.3) | 4 (0.6) | .4434 |
Abbreviations: AD, Alzheimer's disease; ARIA-H = amyloid-related imaging abnormality-hemosiderin deposition; ARIA-E = amyloid-related imaging abnormality-edema/effusion; n = number of patients enrolled in EXPEDITION and EXPEDITION2; SOC = system organ class; QTCF = QT interval corrected by the Fridericia method.
Percentages are calculated based on the number of patients with both a baseline and at least one postbaseline measurement (that is, magnetic resonance imaging [MRI] or electrocardiogram).
Additional AEs are presented in Supplementary Table 2.
4. Discussion
We have proposed a novel statistical methodology for delayed-start designs to provide evidence supporting a disease-modifying effect of an investigational treatment and have applied this methodology to the mild AD subgroup of patients from the EXPEDITION program. This is the first delayed-start analysis method that has been implemented and reported in the field of AD. Using this method, the results suggest that the possible drug effect demonstrated by solanezumab in the mild AD patients in the EXPEDITION program is consistent with a disease-modifying effect.
There has been very limited literature on delayed-start studies in neurodegenerative diseases. Previous studies have reported the long-term use of symptomatic drugs under a similar delayed-start or a withdrawal framework [4], [5]; however, these studies did not use the same rigorous statistical methodology that we have used to characterize the treatment effect. The use of a washout period following a placebo-controlled period can, in theory, also provide information regarding disease modification as in a withdrawal design [2], although the duration of the washout period required can be difficult to determine. The Donepezil study 302 used a 6-week placebo washout period, whereas the Donepezil study 301 used a 3-week washout period [4]; the longer washout period was necessary for patients on active treatment to decline to the same point as those patients originally randomized to placebo. Similarly, the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism study showed that a 1 month and perhaps a 2-month washout period were not sufficient given the prolonged pharmacodynamic effects of deprenyl [11]. Thus, complex pharmacokinetic/pharmacodynamic relationships can add challenges to both delayed-start and withdrawal study designs.
Until this present study, the ADAGIO study of rasagiline for the treatment of Parkinson's disease was the only published study that used the delayed-start design and applied prespecified statistical analyses to assess whether delayed-start patients caught up with early-start patients [3]. In 2011, a US FDA Advisory Committee meeting was held to review the disease-modification claim for rasagiline based on the ADAGIO study. The committee voted unanimously against a claim of disease modification for rasagiline, citing, among other reasons, methodological issues and lack of dose response [12]. One of the key issues was the use of a noninferiority comparison of slopes at the end of the delayed-start period, thus excluding data from patients who discontinued before the end of that period and resulting in potentially unbalanced treatment groups.
We recently proposed a new method for delayed-start analyses developed to ameliorate some of the methodological issues observed in previous applications of the delayed-start design [6]. Applied to the delayed-start analyses of the EXPEDITION program, this statistical method mitigates the key analysis issues identified in the ADAGIO trial. In particular, comparisons of treatment differences at the beginning and end of the delayed-start period using a noninferiority test eliminate the reliance on slopes, the proportional noninferiority margin avoids the issues associated with the absolute noninferiority margins, and a single MMRM analysis model including all available data from all randomized patients from the beginning of the placebo-controlled period through the end of the delayed-start period minimizes the potential for bias from unbalanced treatment groups in the delayed-start period. Extensive simulation studies have demonstrated that the methods used in our analyses of the EXPEDITION program control type I error (that is, controlling for false positives on the noninferiority test for any given time point; P values at different time points are not adjusted for multiplicity) and have good statistical properties [6].
No widely accepted noninferiority margin exists in the AD field. The rationale for a 50% noninferiority margin has been described elsewhere [6]. Briefly, it was selected because it represents a reasonable estimate of the largest clinically acceptable loss of the treatment benefit, indicating that no more than 50% of the treatment difference at the end of the placebo-controlled period would be lost at the end of the delayed-start period. In the event that a treatment had both symptomatic and disease-modifying effects, use of a 50% noninferiority margin would ensure that if noninferiority were met, at least half of the effect would be attributable to a disease-modifying effect. Furthermore, a 50% noninferiority margin has become standard in other therapeutic areas, such as cardiovascular [13].
The 28-week duration of the delayed-start period for the primary analysis time point was selected to balance the need to allow delayed-start patients sufficient time to experience potential symptomatic effects of solanezumab, but not so long that discontinuations would lead to small numbers and increased variability to the extent that results might be difficult to interpret. As previously reported, the half-life of solanezumab is approximately 28 days [14], thus 28 weeks, which is greater than five half-lives of solanezumab, was chosen as adequate for delayed-start patients to achieve pharmacokinetic equilibrium as well as likely to be long enough to observe any potential symptomatic effects (the effects of most approved symptomatic AD treatments peak in <6 months) [6]. Although pharmacodynamic equilibrium, as manifested by effects on clinical measures, could lag pharmacokinetic equilibrium, the additional analysis of time points after the 28 weeks allows assessment of that possibility. The current analyses including 2 years of the delayed-start period provide an additional benefit in that the delayed-start benefits of solanezumab may be examined beyond the primary analysis time point.
Results for the ADAS-Cog14 and the ADCS-iADL at the prespecified primary analysis time point of 108 weeks (that is, 28 weeks in the delayed-start period) demonstrated that starting solanezumab early had benefits that persisted after placebo-treated patients began solanezumab 18 months later. Although the MMSE showed a statistically significant difference between solanezumab and placebo at the end of the placebo-controlled studies, the separation was not statistically significant and the noninferiority criterion was not met at the 108-week time point. Generally, the results from the ADAS-Cog14, ADCS-iADL, and MMSE showed consistent benefits in the early-start group throughout the 3.5-year EXPEDITION program, although not always meeting statistical significance. Additionally, results from the by-study analyses (that is, EXPEDITION through EXPEDITION-EXT and EXPEDITION2 through EXPEDITION-EXT) showed similar results. Results for the CDR-SB were more difficult to interpret given the lack of statistical significance in the placebo-controlled period, followed by noninferiority and statistical significance at some points in the delayed-start period; the CDR-SB did not show a possible drug effect as clearly as the other scales measured. Safety analyses of the delayed-start period support an acceptable safety profile of solanezumab; no new safety signals were observed in the delayed-start patients.
Although patients, investigators, and site personnel were not blinded to treatments administered during the delayed-start period (because all patients received solanezumab), they were not informed of the individual treatment assigned in the placebo-controlled period. In addition, no observable effects of solanezumab, such as an AE or laboratory findings, are known that would inadvertently break the blind. Although the delayed-start period was an optional extension to the double-blind study, the fact that the vast majority (95%) of patients who finished the double-blind, both on drug and on placebo, enrolled in the extension strengthens the ability to compare treatment differences in the two phases. Taken together, maintaining the blind for treatment assignments during the placebo-controlled period and the large numbers of patients who continued in EXPEDITION-EXT create an opportunity to evaluate the data in total in a manner similar to the delayed-start design as originally proposed by Leber (1997) [2]. The EXPEDITION3 study incorporates a delayed-start design into a single protocol and will provide an opportunity to confirm or refute these findings.
Of the mild AD patients randomized in the EXPEDITION program, 43% of delayed-start patients (n = 286) and 45% of early-start patients (n = 295) completed the entire 3.5-year analysis period. Although this amount of attrition is not unexpected in a multiyear longitudinal study of this patient population, it does have an impact, particularly toward the later time points of the study, on power to detect noninferiority and treatment differences. As shown in Fig. 4 and Table 2, a pattern of increasing treatment differences in the placebo-controlled period, followed by more stable differences during the delayed-start period was observed for ADAS-Cog14, ADCS-iADL, and MMSE; however, increasing variability (as illustrated by increasing standard errors and widening confidence intervals) limited the ability to establish noninferiority at later time points despite largely similar differences between early-start and delayed-start treatment groups. Simulation studies have previously demonstrated the proposed statistical method is conservative, favoring control of type I error over power to detect noninferiority based on the given sample size. With similarly sized studies, approximately 50% power is estimated to detect noninferiority at week 108 [6]. However, it is noted that with approximately 600 patients in total completing the 3.5-year study duration, this delayed-start program represents one of the largest clinical trial cohorts in AD patients with long-term longitudinal data.
As noted previously, no new safety signals were identified and the safety profile was consistent with previous safety analyses of the EXPEDITION program [7]. Small differences in the frequency of individual AEs may be due to increased patient age relative to the placebo-controlled period and to longer exposure and observation time in this study.
Despite concerns about the analysis methods in ADAGIO, the delayed-start design remains as one possible option to assess a drug's effect on underlying disease progression. At the end of the ADAGIO FDA Advisory Committee, committee members acknowledged its utility [12], and FDA officials have reported elsewhere that results from an appropriately conducted delayed-start design could be interpreted and used to demonstrate a disease-modifying effect [15], [16]. In 2013, FDA issued draft guidance on developing drugs for early stages of AD. The guidance states that an effect on a biomarker in combination with clinical outcomes or an alternative trial design, such as a delayed-start design, could provide support for a disease-modifying effect of an investigational drug [17].
The results of the delayed-start analyses of the EXPEDITION studies suggest that for the ADAS-Cog14 and ADCS-iADL scales, the benefit observed at the end of the placebo-controlled portion of the study persisted during much of the delayed-start period, supporting initiation of treatment as early as possible. While the AD field has not arrived at a strong consensus on the definition of a clinically meaningful effect of a disease-modifying treatment, the 1.8-point treatment difference on the ADAS-Cog14 observed in pooled data from EXPEDITION and EXPEDITION2 at 80 weeks represents a 34% reduction in cognitive decline [8] and the effect was sustained through 132 weeks, as evidenced by the delayed-start analysis. Furthermore, the treatment effect in the placebo-controlled period is characterized by an increasing pattern over time compared with placebo-treated patients. The lack of statistically significant results on the MMSE and CDR-SB scales warrants some caution in drawing firm conclusions from these analyses. The safety and efficacy of solanezumab treatment will continue to be evaluated in the ongoing EXPEDITION3 study in patients with mild AD (NCT01900665), providing an opportunity to replicate these results.
These analyses further support the proposed method of delayed-start analysis as an appropriate methodology for ascertaining long-term effectiveness and possible disease-modifying effects of AD treatments.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations regarding implementation of delayed-start analysis methods and investigations of other treatments for Alzheimer's disease (AD).
-
2.
Interpretation: The results of the delayed-start analyses of the EXPEDITION studies suggest that for the 14-item Alzheimer's Disease Assessment Scale-Cognitive subscale and Alzheimer's Disease Cooperative Study Activities of Daily Living inventory, instrumental item scales, the benefit observed at the end of the placebo-controlled portion of the study persisted during much of the delayed-start period.
-
3.
Future directions: The safety and efficacy of solanezumab treatment will continue to be evaluated in the ongoing EXPEDITION3 study in patients with mild AD (NCT01900665); a delayed-start analysis has also been incorporated into this study.
Acknowledgments
The authors thank the patients, caregivers, study coordinators, and investigators who made the EXPEDITION Program possible. They also thank Karen Sundell (Eli Lilly and Company) for scientific review, Michael Case (Eli Lilly and Company) for statistical review, and Laura Ramsey (Eli Lilly and Company) for assistance with article preparation.
This study was funded by Eli Lilly and Company.
Footnotes
H.L.-S., E.S., K.C.H., S.W.A., C.C., G.S., and S.H. are full-time employees and minor shareholders of Eli Lilly and Company. E.S. is chair of the Alzheimer's Association Research Roundtable and has reviewed grants for the Alzheimer's Association. I.L. is a former full-time employee of Eli Lilly and Company and a current full-time employee of Quintiles. R.H. is a full-time employee of Quintiles. R.S.D. has consulted for AC Immune, AZ Therapies, Biogen, Biotie, Cerespir, Forum, Genentech, GlaxoSmithKline, Hoffman LaRoche, Merck, Nutricia, Shanghai Green Valley, Riovant Sciences, Suven, Transition, and Takeda about AD drug development or experimental agents and received one honorarium from Novartis for speaking in a CME program; she has served as PI for clinical trials funded by Avanir, Genentech, Lilly/NIA, Merck, NIH, Pfizer, and Takeda for which her institution receives payment and has stock options in AZ Therapies, QR Pharma, Sonexa, and Transition. P.A. has served as a consultant to the following companies: NeuroPhage, Elan, Eisai, Bristol-Myers Squibb, Eli Lilly, Merck, Roche, Amgen, Genentech, Abbott, Pfizer, Novartis, AstraZeneca, Janssen, Medivation, Ichor, Toyama, Lundbeck, Biogen Idec, iPerian, Probiodrug, Somaxon, Biotie, Cardeus, Anavex, Abbvie, and Cohbar. P.A. receives research support from Eli Lilly and Company, NIH (NIA U01-AG10483 [PI], NIA U01-AG024904 [Coordinating Center Director], NIA R01-AG030048 [PI], and R01-AG16381 [Co-I]).
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2015.06.006.
Supplementary data
Fig. S1.
Noninferiority statistics for early-start compared with delayed-start treatment groups for ADAS-Cog14, ADCS-iADL, CDR-SB, and MMSE among patients with mild AD in the solanezumab Expedition program through 3.5 years. Point estimates represent Δ2–0.5Δ1, and error bars represent the lower 90% confidence limit. Abbreviations: ADAS-Cog14, 14-item Alzheimer's disease assessment scale–cognitive subscale; ADCS-iADL, Alzheimer's Disease Cooperative Study activities of daily living inventory, instrumental items; CDR-SB, clinical dementia rating scale–sum of boxes; MMSE, mini-mental status examination; AD, Alzheimer's disease.
Fig. S2.
Delayed-start analysis of ADAS-Cog11 and ADCS-bADL among patients with mild AD in the solanezumab Expedition program through 3.5 years. Abbreviations: LS, least squares; ADAS-Cog11, 11-item Alzheimer's disease assessment scale–cognitive subscale; ADCS-bADL, Alzheimer's disease cooperative study activities of daily living inventory, basic items; NI, noninferiority criterion met (90% one-sided confidence interval for Δ2–0.5Δ1 >0); DS, delayed-start.
Fig. S3.
Delayed-start analysis of ADAS-Cog14, ADCS-iADL, MMSE, and CDR-SB among patients with mild AD in the solanezumab Expedition program by placebo-controlled study (Expedition and Expedition 2) through 3.5 years. Abbreviations: ADAS-Cog14, 14-item Alzheimer's disease assessment scale–cognitive subscale; ADCS-iADL, Alzheimer's disease cooperative study activities of daily living inventory, instrumental items; MMSE, mini-mental status examination; CDR-SB, clinical dementia rating scale–sum of boxes; AD, Alzheimer's disease; LS, least squares; DS, delayed-start; NI, noninferiority criterion met (90% one-sided confidence interval for Δ2–0.5Δ1 >0).
Fig. S4.
Categorical changes in ARIA-H. Categories: 0, 1, 2–5, 6–10, >10, and >10 with additional increase. Abbreviation: ARIA-H, amyloid-related imaging abnormality-hemorrhage/hemosiderin deposition.
References
- 1.Galimberti D., Scarpini E. Disease-modifying treatments for Alzheimer's disease. Ther Adv Neurol Disord. 2001;4:203–216. doi: 10.1177/1756285611404470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leber P. Slowing the progression of Alzheimer disease: Methodologicalissues. Alzheimer Dis Assoc Disord. 1997;11:S10–S21. [PubMed] [Google Scholar]
- 3.Olanow C.W., Rascol O., Hauser R., Feigin P.D., Jankovic J., Lang A. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 4.Doody R.S., Geldmacher D.S., Gordon B., Perdomo C.A., Pratt R.D., Donepezil Study Group Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427–433. doi: 10.1001/archneur.58.3.427. [DOI] [PubMed] [Google Scholar]
- 5.Winblad B., Wimo A., Engedal K., Soininen H., Verhey F., Waldemar G. 3-year study of donepezil therapy in Alzheimer's disease: Effects of early and continuous therapy. Dement Geriatr Cogn Disord. 2006;21:353–363. doi: 10.1159/000091790. [DOI] [PubMed] [Google Scholar]
- 6.Liu-Seifert H., Andersen S.W., Lipkovich I., Holdridge K.C., Siemers E. A novel approach to delayed-start analyses for demonstrating disease-modifying effects in Alzheimer's disease. PLoS ONE. 2015;10:e0119632. doi: 10.1371/journal.pone.0119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Alzheimer's Disease Cooperative Study Steering Committee; Solanezumab Study Group Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 8.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Pahse 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2015 doi: 10.1016/j.jalz.2015.06.1893. in press. [DOI] [PubMed] [Google Scholar]
- 9.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson Study Group Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Summary minutes of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting. October 17, 2011. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM284387.pdf. Accessed August 1, 2013.
- 13.Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Guidance for industry, non-inferiority clinical trials, draft guidance. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf. Accessed July 13, 2015.
- 14.Uenaka K., Nakano M., Willis B.A., Friedrich S., Ferguson-Sells L., Dean R.A. Comparison of pharmacokinetics, pharmacodynamics, safety and tolerability of the amyloidβ monoclonal antibody solanezumab in Japanese and white patients with mild to moderate Alzheimer disease. Clin Neuropharmacol. 2012;35:25–29. doi: 10.1097/WNF.0b013e31823a13d3. [DOI] [PubMed] [Google Scholar]
- 15.Katz R. FDA: evidentiary standards for drug development and approval. NeuroRx. 2004;1:307–316. doi: 10.1602/neurorx.1.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R.B. Mani. Establishing claims for long term therapeutic disease progression: A US regulatory perspective. In: ISCTM Autumn Meeting, Toronto, Canada, 6–7 October 2008, Session V. Nashville: ISCTM.
- 17.Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Guidance for industry, Alzheimer's disease: developing drugs for the treatment of early stage disease. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338287.pdf. Accessed June 12, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.