Abstract
Introduction
“Partners in Dementia Care” (PDC) tested a care-coordination program based on partnerships between Veterans Affairs (VA) medical centers and Alzheimer's Association chapters. The hypothesis posited PDC would reduce the likelihood and number of veterans' hospital admissions and emergency department (ED) visits, particularly for those with more cognitive impairment or behavioral symptoms.
Methods
The sample included 328 veterans with dementia and their primary family or friend caregivers from five matched sites (two randomly selected treatment sites). Data came from VA records; supplemented by caregiver research interviews. Regression analyses using the likelihood and number of hospital and ED visits as outcomes tested for overall treatment-comparison group differences and statistical interactions with cognitive impairment and behavioral symptoms.
Results
Consistent with the hypothesis, three significant interactions showed treatment-group veterans, with more cognitive impairment and behavioral symptoms, had fewer hospital admissions and ED visits than comparison-group veterans. There were no differences in the likelihood of hospital or ED use.
Discussion
PDC, a low-cost program for veterans and caregivers, was effective in reducing the number, but not the likelihood, of hospital admissions and ED visits. Reductions in service use were greater when caregivers reported more difficulties with veterans' symptoms, which in the absence of PDC would place veterans at risk of being high-volume, high-cost service users.
Clinical Trial Registration: ClinicalTrials.gov: NCT00291161.
Keywords: Care-coordination, Support for veterans and caregivers, Hospital and ED use, Partnering VA medical centers and community agencies, Veteran outcomes, Telephone and computer support
1. Introduction
More than 300,000 veterans with diagnosed dementia receive care from the Department of Veterans Affairs (VA), the largest healthcare system in the US [1]. “Partners in Dementia Care” (PDC) was one program being tested as a possible component of the VA's system of support services for veterans with dementia and their informal caregivers [2], [3]. PDC was designed to coordinate healthcare and community services, which is a goal of the National Plan to Address Alzheimer's Disease [4] and other state and federal initiatives [5]. Care coordination was facilitated by a formal partnership between a healthcare organization (e.g., VA) and community service organization (e.g., Alzheimer's Association Chapters). This partnership addresses a number of limitations of dementia care by promoting: holistic, less fragmented care for medical, and nonmedical needs of individuals with dementia and their caregivers [6]; increased attention to informal caregivers as the lynchpin of long-term care [7]; greater access to information and educational resources [8]; and improved management of coexisting medical conditions [9]. PDC was a version of the evidence-based program, “BRI (Benjamin Rose Institute) Care Consultation,” which was developed through a series of studies led by the Benjamin Rose Institute on Aging [10].
Previously published results showed PDC improved psychosocial outcomes for both veterans and their caregivers, including depression, strain, and unmet needs [11], [12]. The hypothesis tested in this analysis posited PDC will reduce the likelihood of veterans having any hospital admission or emergency department (ED) visit during the 1-year study period, and will decrease the number of hospital admissions and ED visits. Differences in the likelihood and number of admissions and visits were tested by comparing veterans in PDC with a matched comparison group that received usual care (UC).
Examining the number of admissions and visits is related to the growing interest in readmissions and return ED visits, which can be avoided with quality postdischarge transitional care [13]. Reducing readmissions and return visits is at the core of attempts to lower healthcare costs, including financial penalties in reimbursement for hospitals with high risk-standardized readmission rates [13], [14].
Hospital and ED use by individuals with dementia account for a sizeable portion of the higher costs for dementia care than for other chronic conditions [15]. Individuals with dementia have hospitalization rates 1.5 to 3 times higher than persons with other chronic conditions [15], [16], [17], [18]. Excess utilization often results from complications in coexisting conditions caused by dementia; care management problems; lack of care alternatives during crises; unmet need for home and community services; insufficient family support; and lack of care coordination [15], [18], [19], [20]. Individuals with dementia also have more preventable hospitalizations and ED visits [15], [21], many of which are due to poor postdischarge and transitional care [17].
Hospital and ED use have unintended negative consequences for individuals with dementia, such as increased delirium, aggression, falls, incontinence, confusion, functional decline, and the use of feeding tubes and urinary catheters [22], [23], [24], [25], [26]. Moreover, family members often identify a hospital admission as a turning point, after which preadmission levels of functioning are never regained [27], [28], and the likelihood of nursing home placement increases [29].
The Stress Process Model [30], [31] guided this research, with hospital admissions and ED visits conceptualized as “well-being outcomes” that are determined by: (1) primary stressors, (2) support resources, and (3) background and context characteristics. “Primary stressors” are perceived difficulties with symptoms; symptoms perceived as causing more difficulties have more negative effects on outcomes. Cognitive and behavioral symptoms of dementia represented primary stressors. “Support resources” are coping mechanisms that can have direct benefits, regardless of the severity of primary stressors; or conditional benefits that are only realized when primary stressors are appraised causing more difficulties [32], [33]. In this research, PDC was conceived as a support resource hypothesized to provide direct and/or conditional reductions in hospital and ED service use. “Background and context” are demographic and social characteristics. For this analysis, these were restricted to characteristics that significantly differed at baseline between PDC-UC groups, despite the matching sites on key organizational characteristics. Background and context also included baseline functional status as an indicator of veterans' general health. Functional status reflected the cumulative effects of all veterans' chronic health conditions, which in this sample averaged over five.
2. Method
2.1. Design
Five study sites included: Boston, MA; Houston, TX; Providence, RI; Oklahoma City, OK; and Beaumont, TX. Northeast and southwest sites were in the same Veterans Integrated Service Networks that provided a common overarching administrative structure. Additionally, matched VA medical centers were similar in: size; inpatient, and outpatient services; academic affiliations; research missions; and medical residency programs. Matched Alzheimer's Association Chapters were similar in size and programs. The study was approved by the Institutional Review Boards of the Providence VA Medical Center, VA Boston Healthcare System, University of Oklahoma Health Sciences Center, and Baylor College of Medicine, and is registered at ClinicalTrials.gov (NCT00291161).
One of the matched sites from each region was randomly selected to deliver PDC; the other provided UC. Boston was the randomly selected PDC site in the Northeast; Providence was its matched UC site. Houston was the randomly selected PDC site in the Southwest; Oklahoma City and Beaumont were matched UC sites. Matching, rather than within-site randomization, was used to allow PDC implementation throughout partnering organizations, without concerns about diffusion to UC veterans.
The study included veterans and the unpaid primary family or friend caregiver, who provided the most assistance with daily activities and health-related decisions. Veterans and caregivers participated for 12 months. Intervention-site participants received PDC and dementia educational materials; UC-site participants received the same dementia educational materials. Other than access to PDC, there were no restrictions on services study participants could use from the VA, Alzheimer's Association, or other community organizations.
Study data came from three structured telephone interviews conducted at 6-month intervals with caregivers and veterans' VA medical records. The first caregiver interview was at baseline, before distributing dementia educational materials and implementing PDC at intervention sites.
2.2. Sample
Eligible veterans received primary healthcare from the VA; resided outside a residential care facility at enrollment; lived within a partnering Alzheimer's Association Chapter's service area; were 60 and above; and had at least one International Classification of Diseases, 9th Revision dementia diagnostic code (290.41–43,291.2, 292.82, 294.1, 294.8, and 331.0) in the medical record. There were no restrictions based on severity of dementia, other health conditions, or availability of a caregiver. VA primary care physicians confirmed veterans’ eligibility before recruitment, which occurred from January 2007 to July 2009.
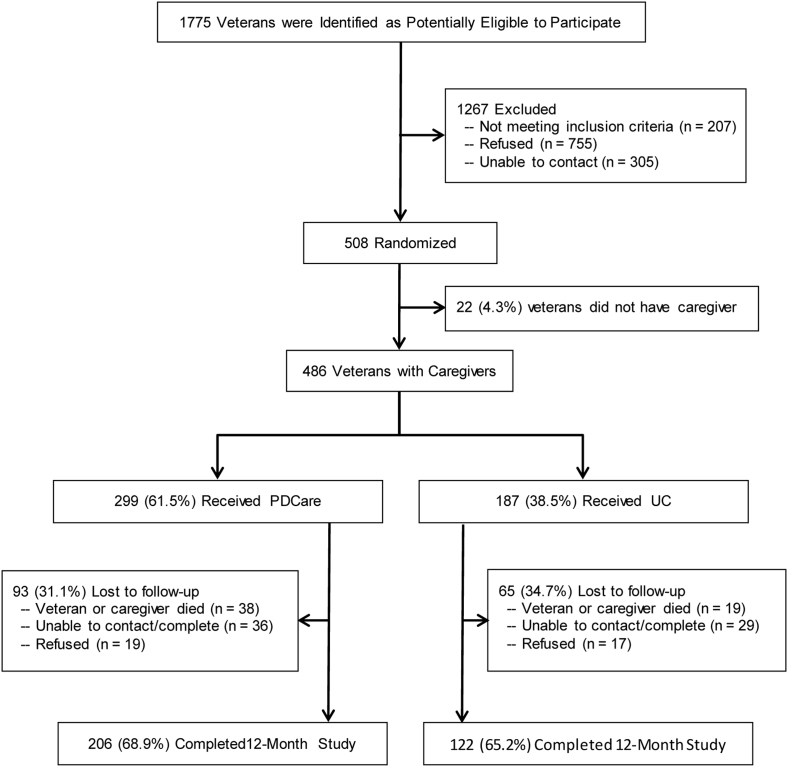
The recruitment and sampling processes for the PDC are depicted in Fig. 1. A total of 1775 veterans were referred and mailed Institutional Review Board-approved invitations; 508 veterans (28.6%) consented, of which 486 had a consenting caregiver. Nearly all veterans were men (97.5%) and caregivers were women (94.9%; 75% were veterans' wives). Most veterans (76.4%) completed at least high school, with 24.8% having a college degree; 19.0% were members of a minority group; and received their dementia diagnosis 2.03 years (SD = 1.97) before the study.
Fig. 1.
Consort diagram.
This analysis was based on 328 veterans with caregivers who completed the full 12-month study and follow-up interview. As shown in Fig. 1, a small number of veterans without caregivers were excluded (n = 22; 4.3%), because a number of key measures (e.g., non-VA service use, cognitive and behavioral symptoms) came from caregiver interviews. Other excluded veterans did not complete the full 12-month study and follow-up interviews due to: death (n = 57, 11.2%), inability to contact after repeated telephone and mail attempts (n = 65, 12.8%), and refusal (n = 36, 7.1%).
Sample-size was based on: a one-tailed significance test; a medium effect size of 0.12, 0.80 power, 0.05 alpha, 10 independent variables in a regression equation; and a squared multiple correlation of 0.55. These assumptions meant a minimum sample of 247 was required [34].
2.3. PDC care coordination program
A detailed description of PDC, its service delivery protocol, and conceptual foundation is available in previously published work [35]. This section provides a brief overview. PDC was a coaching model driven by the preferences of veterans and caregivers. Coordinators offered guidance in finding solutions to the concerns that were priorities of veterans and caregivers. PDC had a standardized protocol, with a minimum of one contact between coordinators and veteran/caregiver dyads per month, with more frequent contacts as needed. Two half-time care coordinators delivered PDC; one from the VA medical center and one from the partnering Alzheimer's Association Chapter. The two coordinators worked as a team, sharing the electronic Care Consultation Information System. Coordinators had bachelor's or master's degrees in social work, nursing, or other helping professions.
VA coordinators had primary responsibility for veterans' medical-related concerns (e.g., medications, disease management, VA services and benefits); Alzheimer's Association coordinators had primary responsibility for caregivers' nonmedical concerns (e.g., care-related strain, community service use).
PDC was low-cost because it was delivered by telephone, mail, and e-mail. Two partnering half-time coordinators (1 full time equivalent) maintained caseloads of 100 to 125 families. Although economies of scale came from larger caseloads and exact program costs depended on salaries and benefits of care consultants, all expenses to deliver PDC typically (i.e., salaries, benefits, equipment, supplies, training, software, licensing, supervision, overhead) were $60 to $80 per month per family. PDC's main components were: (1) initial assessment, (2) action plan, and (3) ongoing monitoring and reassessment.
2.3.1. Initial assessment
Initial assessment was completed gradually during the first 4 weeks. It was brief but broad, covering 23 domains for veterans (e.g., coordinating and accessing VA services, medication management, understanding the diagnosis) and 16 domains for caregivers (e.g., finding community services, care-related strains, depression).
2.3.2. Action plan
The action plan was the core of PDC. It included simple behavioral tasks called action steps. If accomplished, action steps moved veterans and caregivers toward solutions to their concerns. Action steps should be easy to complete and include, for example, calling an organization to enquire about the availability of a service, making a list of questions to ask the doctor, reading an educational resource provided by care coordinators, or contacting another family member or friend to ask whether he or she is willing to do a caregiving task.
With coaching and support from care coordinators, veterans and caregivers determined the content of action steps, identified the person who will be responsible for completion, and specified the projected completion dates. New action steps were continuously added and built on prior action steps. Multiple action steps, spread over a period of weeks or months, often were needed to find solutions to specific problems. Copies of action plans were mailed to veterans and caregivers and summarized in the larger medical record.
On average, each veteran and his/her caregiver had over seven action steps. The most common pertained to accessing and coordinating services. However, action steps address any type of care-related issue. The percentage of veterans and caregivers with the most common action-step content areas included: 78% accessing and/or coordinating VA services or benefits; 76% accessing services from other community organizations; 59% accessing Alzheimer's Association services; 57% getting family and friends to work together more effectively; 40% managing symptoms of dementia or other coexisting medical conditions at home; 33% communicating effectively with healthcare providers; and 29% making the home environment safe.
2.3.3. Ongoing monitoring and reassessment
Coordinators became a trusted and knowledgeable resource person with whom an ongoing relationship was maintained. Facilitators of this relationship were: a minimum of at least one monthly call, multiple reassessments of veteran and caregiver domains, ongoing addition of new action steps, and checking the disposition of pending action steps.
2.4. Measures
2.4.1. Service use outcomes
Data on hospital and ED use (including urgent care) from, or paid for by, the VA were obtained from the VA National Patient Care Database maintained at the Austin Automation Center. Utilization records were extracted electronically for 1-year post each participant's baseline interview. Data on non-VA hospital and ED use (including urgent care) came from the structured caregiver research interviews. Caregivers were given dates of their baseline and 6-month interviews to use as reference points when reporting service use between months 1 to 6 and 7 to 12. Combining VA and non-VA service use was necessary to get a full picture of utilization because most veterans do not exclusively use the VA [36]. Prior studies show a high correspondence between extracted record data and interview data for hospital and ED use, when interview data are based on a recall period that is no more than 6 months [37], [38].
Two dichotomous and two continuous outcome measures were created to represent utilization during 12 months after the baseline. Dichotomous measures represented whether veterans had any hospital admissions and any ED visits; continuous measures represented the number of hospital admissions and ED visits by veterans with at least one admission/visit.
2.4.2. Symptoms of dementia as primary stressors
Multi-item scales represented difficulties with veterans' cognitive and behavioral symptoms at baseline and 6-month post baseline. Data to construct these two measures came from caregiver responses to a previously published 22-item instrument [39], [40]. Factor analyses confirmed the independence and structural validity of two scales, with individual-item loadings between 0.43 and 0.81 on their respective factors and low cross-loadings [8], [9].
Cognitive symptoms were the sum of seven items, scored from (0) to (2) (“no,” “some,” or “a great deal” of difficulty), that asked caregivers about the amount of difficulty veterans had with: tracking current events; knowing the day of the week; repeating things; paying attention; and remembering addresses, people, and appointments. It had good reliability as represented by a Cronbach's alpha of 0.82. Behavioral symptoms represented one part of the broad category of neuropsychiatric symptoms that can be particularly stressful for caregivers. It was the sum of four items, scored from (0) to (3) (“none of the time” to “most or all the time”), that asked about the frequency of veterans: complaining or criticizing, interfering with family members, yelling or swearing, and being agitated. It had a Cronbach's alpha of 0.79.
2.4.3. Background and context
Measures of background and context controlled for significant PDC-UC baseline differences and included: whether caregivers were veterans' spouses, caregiver age, and whether veterans were at Northeast or Southwest study sites. Number of personal care dependencies was a six-item scale, based on caregiver reports, that measured veterans' functional status [41]. Each item was scored from (0) to (2) (“no difficulty” to “a great deal of difficulty”) and asked about the amount of difficulty veterans had with: bathing, dressing, grooming, toileting, eating, and mobility inside the home. It had a Cronbach's alpha of 0.87.
2.5. Analytic strategy
Logistic regression was used with dichotomous-dependent variables representing the likelihood of hospital and ED use; ordinary least-squares regression was used with continuous dependent variables representing the number of hospital admissions and ED visits. Logistic regression equations included the full sample (n = 328) and tested for differences between veterans who did and did not use each service type. Ordinary least-squares regression equations included only veterans with at least one service use episode and focused on differences in the total number of admissions and visits, as distinct from the user versus nonuser comparison.
All regression equations included variables: distinguishing PDC-UC groups (1 = PDC/0 = UC); baseline and 6-month cognitive and behavioral symptoms; the four background and context characteristics; and four interaction or product terms to test for conditional effects. Interaction terms were constructed by multiplying the PDC-UC group variable by baseline and 6-month cognitive and behavioral symptoms [42]. Only statistically significant interaction terms were maintained in final equations. Analyses were conducted using IBM SPSS Statistics, Version 21.
Results consistent with the hypothesis were indicated by significant negative regression coefficients for the PDC-UC group variable and four interaction terms. When there were no significant interaction terms (i.e., no conditional effects), the coefficient for PDC-UC variable reflected the average difference in outcomes between groups, controlling for cognitive and behavioral symptoms (i.e., direct effect of PDC). When there was a significant interaction term, it meant an outcome differed for the PDC and UC groups, depending on the level of difficulties with cognitive and/or behavioral symptoms (i.e., conditional effect of PDC) (see [42] for detail on interpreting interaction terms). All significant interaction terms in regressions will be illustrated by dividing the sample into veterans with high and low levels of the modifying symptom and showing simple percent-differences in hospital and ED use between PDC and UC groups.
3. Results
Table 1 describes the four service outcomes. Just over one-third (35.0%) of veterans had at least one hospital admission, with a mean of 1.79 admissions among those with at least one. Over half had at least one ED visit (55.2%), with a mean of 2.46 among veterans with at least one. Although Table 1 presents figures separately for PDC and UC groups, differences between groups were formally tested and discussed later based on regression analyses.
Table 1.
Descriptive statistics for veteran hospital admissions and ED visits for PDC and UC groups
| Service use during 12-month study period | Total |
PDC group |
UC group |
|||
|---|---|---|---|---|---|---|
| % or mean | SD | % or mean | SD | % or mean | SD | |
| Any hospital admissions (n = 328) | 35.0% | 0.48 | 38.0% | 0.49 | 29.9% | 0.46 |
| Number hospital admissions (veterans with one or more admissions; n = 118) | 1.79 | 1.29 | 1.80 | 1.22 | 1.77 | 1.46 |
| Any ED visits (n = 328) | 55.2% | 0.50 | 54.0% | 0.50 | 57.3% | 0.50 |
| Number ED visits (veterans with one or more visits; n = 175) | 2.46 | 1.78 | 2.38 | 1.69 | 2.58 | 1.52 |
Abbreviations: ED, emergency department; PDC, Partners in Dementia Care; UC, usual care; SD, standard deviation.
Table 2 describes symptoms and covariates. Means showed cognitive symptoms were more common than behavioral symptoms due, in part, to a sizeable portion of veterans with no behavioral symptoms (22.2% at baseline; 22.0% at 6 months).
Table 2.
Descriptive statistics for independent variables used in the analysis of veteran hospital admissions and ED visits
| Total (n = 328) |
PDC group (n = 206) |
UC group (n = 122) |
Difference PDC-UC groups |
||||
|---|---|---|---|---|---|---|---|
| % or mean | SD | % or mean | SD | % or mean | SD | P | |
| Dementia symptoms | |||||||
| Baseline cognitive symptoms (0–14, low to high) | 6.77 | 3.73 | 6.77 | 3.78 | 6.77 | 3.65 | 1.00 |
| 6-month cognitive symptoms (0–14, low to high) | 6.83 | 3.65 | 6.93 | 3.61 | 6.64 | 3.74 | .50 |
| Baseline behavioral symptoms (0–12, low to high) | 2.66 | 2.61 | 2.60 | 2.56 | 2.49 | 2.32 | .58 |
| 6-month behavioral symptoms (0–12, low to high) | 2.59 | 2.42 | 2.66 | 2.49 | 2.77 | 2.70 | .55 |
| Covariates | |||||||
| Baseline personal care dependencies (0–12, low to high) | 2.77 | 3.23 | 2.76 | 0.69 | 2.80 | 3.39 | .93 |
| Spouse caregiver | 75.0% | 0.43 | 68.9% | 0.46 | 85.2% | 0.36 | .02 |
| Northeast region | 47.8% | 0.50 | 42.2% | 0.50 | 57.4% | 0.50 | .001 |
| Caregiver age | 69.76 | 11.94 | 68.57 | 12.64 | 71.77 | 10.39 | .01 |
Abbreviations: ED, emergency department; PDC, Partners in Dementia Care; UC, usual care; SD, standard deviation.
Table 3 summarizes regression equations for hospital admissions. The first equation is for the likelihood of any admission. The only significant (P ≤ .05) predictor was the number of personal care dependencies (b = 0.14; P = .001); veterans with more personal care dependencies were more likely to have a hospital admission. There were no direct or conditional effects of PDC, and the overall equation was not significant (P = .11). The second equation was for the number of hospital admissions, which included 118 veterans who had at least one admission. There were three significant interaction terms in this equation. “PDC-UC group × baseline cognitive impairment” had a significant positive coefficient (b = 0.32; P = .004). The positive direction meant it was opposite the hypothesis; PDC veterans who had more difficulties with cognitive symptoms at baseline had more hospital admissions. The two other significant interaction terms were negative and consistent with the hypothesis; “PDC-UC group × 6-month cognitive impairment” and “PDC-UC group × baseline behavioral symptoms” (−0.29; P = .01 and −0.26; P = .01, respectively). PDC veterans who had more difficulties with cognitive symptoms at 6 months, and those who had more difficulties with behavioral symptoms at baseline, had fewer admissions. The overall equation was significant accounting for 12% of the variance (P = .01).
Table 3.
Multiple regression equations for hospital admissions for PDC and UC groups
| Any hospital admissions (n = 328)∗ |
Number hospital admissions (n = 118) |
|||||
|---|---|---|---|---|---|---|
| Logistic regression coefficients | Odds ratio | P | Unstandardized regression coefficients | Standardized regression coefficients | P | |
| Predictive factors | ||||||
| PDC-UC group | 0.45 | 1.57 | .09 | 0.50 | 0.18 | .38 |
| Intervention × baseline cognitive symptoms | – | – | – | 0.32 | 1.10 | .004 |
| Intervention × 6-month cognitive symptoms | – | – | – | −0.29 | −0.98 | .01 |
| Intervention × baseline behavioral symptoms | – | – | – | −0.26 | −0.49 | .01 |
| Dementia symptoms | ||||||
| Baseline cognitive symptoms | 0.003 | 1.00 | .96 | −0.20 | −0.60 | .02 |
| 6-month cognitive symptoms | −0.08 | 0.92 | .14 | 0.22 | 0.64 | .01 |
| Baseline behavioral symptoms | −0.02 | 0.98 | .78 | 0.22 | 0.43 | .02 |
| 6 month behavioral symptoms | 0.10 | 1.11 | .12 | −0.05 | −0.09 | .48 |
| Covariates | ||||||
| Baseline personal care dependencies | 0.14 | 1.15 | .001 | 0.07 | 0.19 | .05 |
| Spouse caregiver | −0.38 | 0.68 | .33 | −0.21 | −0.07 | .60 |
| Northeast region | 0.23 | 1.26 | .35 | 0.05 | 0.02 | .83 |
| Caregiver age | 0.02 | 1.02 | .11 | 0.01 | 0.08 | .54 |
| Overall explained variance | 0.01 | 0.11 | .11 | – | 0.12 | .01 |
Abbreviations: ED, emergency department; PDC, Partners in Dementia Care; UC, usual care.
Eleven cases were excluded from this analysis due to missing information on one or more variables.
Table 4 summarizes regression equations for ED visits. The first equation was for any ED visits, where there were no significant direct or conditional effects of PDC. The second equation was for the number of ED visits, which included 175 veterans with at least one visit. This equation had one significant interaction term; “PDC-UC group × 6-month behavioral symptoms” (b = −0.27; P = .02). Consistent with the hypotheses, PDC veterans who had more difficulties with behavioral symptoms at 6 months had fewer ED visits. The overall equation also was significant (P = .01), explaining approximately 12% of the variance.
Table 4.
Multiple regression equations for ED visits for PDC and UC groups
| Any ED visit (n = 328)∗ |
Number ED visits (n = 175) |
|||||
|---|---|---|---|---|---|---|
| Logistic regression coefficients | Odds ratio | P | Unstandardized regression coefficients | Standardized regression coefficients | P | |
| Intervention and interactions | ||||||
| Intervention Group | 0.04 | 1.04 | .88 | 0.60 | 0.16 | .15 |
| Intervention × 6-month behavioral symptoms | – | – | – | −0.27 | −0.35 | .02 |
| Dementia symptoms | ||||||
| Baseline cognitive symptoms | 0.01 | 0.95 | .90 | 0.05 | 0.10 | .46 |
| 6-month cognitive symptoms | −0.004 | 1.00 | .94 | −0.08 | −0.16 | .27 |
| Baseline behavioral symptoms | 0.01 | 1.01 | .90 | −0.03 | −0.04 | .68 |
| 6-month behavioral symptoms | −0.01 | 1.00 | .94 | 0.36 | 0.47 | .001 |
| Covariates | ||||||
| Baseline personal care dependencies | 0.13 | 1.14 | .003 | 0.08 | 0.14 | .08 |
| Spouse caregiver | 0.52 | 1.69 | .17 | −0.21 | −0.05 | .63 |
| Northeast region | 0.88 | 2.41 | .00 | 0.46 | 0.13 | .10 |
| Caregiver age | −0.02 | 0.98 | .23 | 0.02 | 0.14 | .16 |
| Overall explained variance | 0.09 | – | .01 | – | 0.12 | .01 |
Abbreviations: ED, emergency department; PDC, Partners in Dementia Care; UC, usual care.
Twelve cases were excluded from this analysis due to missing information on one or more variables.
Table 5 offers an easy-to-interpret illustration of the four significant conditional PDC-UC group differences indicated by interaction terms in regression equations. Column 1 gives the average number of hospital admissions and ED visits, among veterans who had more difficulties with cognitive and/or behavioral symptoms; columns 2 and 3 give the mean difference and percent difference between groups, respectively.
Table 5.
Illustration of statistically significant PDC-UC group differences for number of hospital admissions and ED visits
| Hospital readmissions | Mean number admissions | Mean difference admissions | Percent difference admissions |
|---|---|---|---|
| High baseline cognitive symptoms | |||
| UC group | 1.5 | ||
| PDC group | 1.9 | +0.4 | +26.7% |
| High baseline behavioral symptoms | |||
| UC group | 2.5 | ||
| PDC group | 1.7 | −0.8 | −32.0% |
| High 6-month cognitive symptoms | |||
| UC group | 2.6 | ||
| PDC group | 1.9 | −0.7 | −26.9% |
| Return emergency dept. visits | Mean number ED visits | Mean difference ED visits | Percent difference ED visits |
| High 6-month behavioral symptoms | |||
| UC group | 3.5 | ||
| PDC group | 2.5 | −1.0 | −28.6% |
Abbreviations: PDC, Partners in Dementia Care; UC, usual care; ED, emergency department.
As noted in regression results, the first significant conditional effect was opposite the hypothesis. This is illustrated in Table 5 by the PDC group having 26.7% more hospital admissions than the UC group. This contrasted to the other three significant conditional effects, where the PDC group had fewer admissions or ED visits. Specifically, for those with high baseline behavioral symptoms, there were 32.0% fewer admissions in the PDC group; for those with more difficulties with cognitive symptoms at 6 months, there were 26.9% fewer admissions in the PDC group; and for those with more behavioral symptoms at 6 months, there were 28.6% fewer ED visits in the PDC group.
4. Discussion
Individuals with dementia have higher rates of hospital and ED use and experience more unintended negative consequences from utilization episodes, including nursing home placement, falls, and medical complications [15], [18], [22], [23], [24], [25], [26]. Excess use of hospital and ED services account for a substantial portion of the higher total cost of dementia care [15] and is stressful for caregivers, especially when individuals with dementia need continuous supervision and/or have behavioral symptoms [43].
This investigation showed PDC was effective in decreasing the number of hospital admissions and ED visits, but had no effect on the likelihood of an initial admission and visit. Decreases pertained to situations where caregivers reported more difficulties with cognitive symptoms after 6 months in PDC, and more behavioral symptoms at both enrollment and after 6 months.
The effectiveness of PDC for decreasing number of hospital admissions and ED visits was conditional to situations where caregivers reported more difficulties with veterans' symptoms of dementia. This finding was noteworthy because veterans with more symptom difficulties are predisposed to being high-volume, high-cost service users, who economically strain the healthcare system [44]. This finding also was consistent with published results for PDC's impact on psychosocial outcomes (e.g., care-related strains), suggesting that across all types of outcomes, PDC was most beneficial for vulnerable veterans and caregivers with more problematic symptoms [11], [12].
Having more difficulties with symptoms may motivate veterans and caregivers to accept assistance from care coordinators and elevate their readiness to complete tasks in the Action Plan [45]. Difficulties with symptoms also may provide care coordinators with a clear problem to target for improvement, which if successful, could further veterans' and caregivers' commitment to the program.
Although three of the four significant results suggested PDC decreased service use, one indicated the opposite. When caregivers reported more difficulties with baseline cognitive symptoms, veterans in PDC had more, rather than less, hospital admissions. One possible explanation for this finding is that families who had more difficulties with cognitive symptoms at enrollment may have been receiving less than optimal care before PDC. After enrollment, care coordinators may have attempted to improve the quality of care by aggressively mobilizing VA and community resources, including the increased use of hospital services.
There were two notable study limitations. First, matched sites, rather than within-site randomization, made it less certain that PDC and UC groups were equivalent at baseline; although statistically significant baseline differences were controlled. However, the use of matched sites had the advantage of assuring UC subjects were not exposed to the intervention, when using sustainable procedures at PDC sites for the recruitment and integration of PDC within existing services. The second limitation pertained to service-use data and included a focus on a relatively short 1-year window, and the use of caregiver reports for non-VA hospital and ED use that may have been less accurate than medical record data.
The impact on number of, rather than initial, admissions and visits suggested PDC was particularly helpful in preventing subsequent readmissions and return ED visits after veterans were discharged from a prior admission or visit. This finding is of particular interest, as the period after discharge often is characterized by more acute problems, the need to adjust to changes in symptoms or care, and high rates of preventable readmissions and return visits [46], [47]. Additionally, the finding has noteworthy clinical and practice implications that align with a key goal of the Patient Protection and Affordability Act, to reduce avoidable hospital readmissions and return ED visits by promoting the development of “care transition programs.” The benefits of PDC indicated by these results may reflect similarities in its functions to care transition programs [48], [49], such as encouraging follow-up appointments with primary and specialty healthcare providers; facilitating the use of community services and resources; and providing information about medications, treatments, and care tasks at home.
However, PDC has several advantages over most care transition programs by its broader, more holistic focus that includes: giving equal attention to psychosocial and utilization outcomes; establishing a longer-term relationship with patients and families; providing more comprehensive caregiver support; and linking healthcare and community services to address both medical and nonmedical needs [35]. Postdischarge action steps also commonly focused on preventing future hospital admissions and ED visits by adjusting the involvement of informal helpers and preparing caregivers to deal with emergencies and unexpected problems. Additionally, PDC was designed explicitly for dementia; a condition originally excluded from most care transition programs [47], [50].
This investigation suggests PDC can reduce the excess use of hospitals and EDs, and corresponding healthcare costs, for an important segment of individuals with dementia. A next step will be to test whether PDC is feasible to implement and can achieve similar outcomes, when delivered on a larger scale outside a research study. This will require more information on practical issues related to implementation costs and financing options, marketing strategies, willingness of families to use the program, and the ability to integrate PDC with existing services. Many of these translational issues are being examined in a replication study of PDC being conducted in Ohio, with the support by Administration for Community Living (grant 90DS0001), Ohio Department of Aging, Benjamin Rose Institute on Aging, Louis Stokes Cleveland VA Medical Center, the Western Reserve Area Agency on Aging, and the Greater East Ohio Area Alzheimer's Association Chapter.
Research in context.
-
1.
Systematic review: “Partners in Dementia” (PDC), a version of the evidence-based BRI (Benjamin Research Institute) Care Consultation, is based on partnerships between healthcare and community-service organizations. It was intended to: reduce fragmentation, coordinate medical and nonmedical services, decrease the excess use of costly hospital and emergency department (ED) services, and support caregivers.
-
2.
Interpretation: PDC improved psychosocial outcomes for veterans and caregivers. This study showed PDC also reduced excess hospital and ED use in terms of the number of admissions and ED visits for high-risk veterans, who had more cognitive impairment and behavioral symptoms. PDC may be an effective, longer-term care-transition program for decreasing readmissions and return ED visits.
-
3.
Future directions: Feasibility studies are needed to determine if PDC can be cost-effective and sustained on a larger scale and outside the VA. Improved methods need to be developed and tested for: sharing confidential patient information across partnering organizations, hiring and training staff, and interfacing PDC and other services.
Acknowledgments
The authors wish to thank Sonora Hudson, MA, for assistance with medical editing and input on the manuscript.
Financial disclosure: This work was supported by a grant from the Department of Veterans Affairs, Health Services Research and Development (HR 04-238-3) and grants from the Alzheimer's Association (IIRG-08-89058) and the Robert Wood Johnson Foundation (#57816). This work was partly supported by the Houston VA HSR&D Center for Innovations in Quality, Effectiveness & Safety (CIN 13-413). The attitudes expressed are those of the authors and do not necessarily reflect those of the Department of Veterans Affairs/US government or Baylor College of Medicine. The funders had no role in the study design, data collection analysis, decision to publish, or preparation of the manuscript.
Competing interests: The Benjamin Rose Institute on Aging holds the copyright to BRI Care Consultation and licenses organizations to deliver the program.
References
- 1.Office of Assistant Deputy Under-Secretary for Health . Department of Veterans Affairs; Washington, DC: 2004. Projections of the prevalence and incidence of dementias including Alzheimer's disease for the total, enrolled, and patient veteran populations age 65 and over.http://www4.va.gov/HEALTHPOLICYPLANNING/dementia/Dem022004.pdf Available from: [Google Scholar]
- 2.Sheets C.J., Mahoney-Gleason H. Caregiver support in the Veterans Health Administration: caring for those who care. Generations. 2010;34:92–98. [Google Scholar]
- 3.Cooley S.G., Asthana S. Dementia care for veterans: enhancing comprehensive, coordinated services. Generations. 2010;34:57–63. [Google Scholar]
- 4.US Department of Health and Human Services. The national plan to address Alzheimer’s disease. Available from: http://alzheimers.gov/pdf/NationalPlantoAddressAlzheimersDisease.pdf.
- 5.Craig C., Eby D., Whittington J. Institute for Healthcare Improvement; Cambridge, Massachusetts: 2011. Care coordination model: better care at lower cost for people with multiple health and social needs.http://www.ihi.org/resources/Pages/IHIWhitePapers/IHICareCoordinationModelWhitePaper.aspx IHI Innovation Series white paper. Available from: [Google Scholar]
- 6.Maslow K., Selstad J. Chronic care networks for Alzheimer’s disease: approaches for involving and supporting family caregivers in an innovative model of dementia care. Alzheimers Care Q. 2001;2:33–46. [Google Scholar]
- 7.Shay K., Burris J.F. Setting the stage for a new strategic plan for geriatrics and extended care in the Veterans Health Administration: summary of the 2008 VA State of the Art Conference, ”The Changing Faces of Geriatrics and Extended Care: Meeting the Needs of Veterans in the Next decade.“. J Am Geriatr Soc. 2008;56:2330–2339. doi: 10.1111/j.1532-5415.2008.02079.x. [DOI] [PubMed] [Google Scholar]
- 8.Hepburn K.W., Tornatore J., Center B. Dementia family caregiver training: affecting beliefs about caregiving and caregiver outcomes. J Am Geriatr Soc. 2001;49:450–457. doi: 10.1046/j.1532-5415.2001.49090.x. [DOI] [PubMed] [Google Scholar]
- 9.Coon D.W., Williams M.P., Moore R.J., Edgerly E.S., Steinbach C.M., Roth S.P. The Northern California Chronic Care Network for Dementia. J Am Geriatr Soc. 2004;52:150–156. doi: 10.1111/j.1532-5415.2004.52026.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosalynn Carter Institute for Caregiving . Caregiver Intervention Database; 2009. Care consultation telephone-based empowerment intervention.http://www.rosalynncarter.org/caregiver_intervention_database/dimentia/care_consultation_telephone-based_empowerment_intervention/ Available at: [Google Scholar]
- 11.Bass D.M., Judge K.S., Snow A.L., Wilson N.L., Morgan R., Looman W.J. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61:1377–1386. doi: 10.1111/jgs.12362. [DOI] [PubMed] [Google Scholar]
- 12.Bass D.M., Judge K.S., Snow A.L., Wilson N.L., Morgan R.O., Maslow K. A controlled trial of Partners in Dementia Care: veteran outcomes after six and twelve months. Alzheimers Res Ther. 2014;6:9. doi: 10.1186/alzrt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen L.O., Young R.S., Hinami K., Leung A., Williams M.V. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 14.US Congress. House Committee on Ways and Means. Committee on Energy and Commerce. Committee on Education and Labor . US Government Printing Office; Washington, DC: 2010. Compilation of Patient Protection and Affordable Care Act: As amended through 1 November 2010, including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. xxiii. [Google Scholar]
- 15.Bynum J.P., Rabins P.V., Weller W., Niefeld M., Anderson G.F., Wu A.W. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 16.Bynum JPW. Characteristics, costs, and health service use for Medicare beneficiaries with a dementia diagnosis. Contract report prepared for the Alzheimer’s Association, January 2009.
- 17.Callahan C.M., Arling G., Tu W., Rosenman M.B., Counsell S.R., Stump T.E. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60:813–820. doi: 10.1111/j.1532-5415.2012.03905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan L.L., Peterson N.J., Snow A.L., Cully J.A., Schulz P.E., Graham D.P. Prevalence of dementia among veterans affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20:245–253. doi: 10.1159/000087345. [DOI] [PubMed] [Google Scholar]
- 19.Kunik M.E., Snow A.L., Molinari V.A., Menke T.J., Slouchek J., Sullivan G. Health care utilization in dementia patients with psychiatric comorbidity. Gerontologist. 2003;43:86–91. doi: 10.1093/geront/43.1.86. [DOI] [PubMed] [Google Scholar]
- 20.Lin P.J., Fillit H.M., Cohen J.T., Neumann P.J. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimers Dement. 2013;9:30–38. doi: 10.1016/j.jalz.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Wolinsky F.D., Liu L., Miller T.R., An H., Geweke J.F., Kaskie B. Emergency department utilization patterns among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:204–209. doi: 10.1093/gerona/63.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maslow K., Mezey M. Adverse health events in hospitalized patients with dementia. Am J Nurs. 2008;108:40–49. doi: 10.1097/01.NAJ.0000304475.80530.a6. [DOI] [PubMed] [Google Scholar]
- 23.Clevenger C.K., Chu T.A., Yang Z., Hepburn K.W. Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60:1742–1748. doi: 10.1111/j.1532-5415.2012.04108.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuo S., Rhodes R.L., Mitchell S.L., Mor V., Teno J.M. Natural history of feeding-tube use in nursing home residents with advanced dementia. J Am Med Dir Assoc. 2009;10:264–270. doi: 10.1016/j.jamda.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sands L.P., Yaffe K., Covinsky K., Chren M.M., Counsell S., Palmer R. Cognitive screening predicts magnitude of functional recovery from admission to 3 months after discharge in hospitalized elders. J Gerontol A Biol Sci Med Sci. 2003;58:37–45. doi: 10.1093/gerona/58.1.m37. [DOI] [PubMed] [Google Scholar]
- 26.Bejjani C., Rumph G., Kunik M. Addressing psychiatric problems of dementia in the emergency room. The Internet Journal of Emergency Medicine. 2012;7 [Google Scholar]
- 27.Gill T.M., Williams C.S., Tinetti M.E. The combined effects of baseline vulnerability and acute hospital events on the development of functional dependence among community-living older persons. J Gerontol A Biol Sci Med Sci. 1999;54:M377–M383. doi: 10.1093/gerona/54.7.m377. [DOI] [PubMed] [Google Scholar]
- 28.Gill T.M., Gahbauer E.A., Han L., Allore H.G. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci. 2009;64:1296–1303. doi: 10.1093/gerona/glp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong T.G., Jones R.N., Marcantonio E.R., Tommet D., Gross A.L., Habtemariam D. Adverse outcomes after hospitalization and delirium in persons with Alzheimer’s disease. Ann Intern Med. 2012;156:848–856. doi: 10.7326/0003-4819-156-12-201206190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearlin L.I., Mullan J.T., Semple S.J., Skaff M.M. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 31.Judge K.S., Menne H.L., Whitlatch C.J. Stress process model for individuals with dementia. Gerontologist. 2010;50:294–302. doi: 10.1093/geront/gnp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin N. Modeling the effects of social support. In: Lin N., Dean A., Ensel W., editors. Social support, life events, and depression. Academic; Orlando, FL: 1986. pp. 173–209. [Google Scholar]
- 33.Lazarus R.S., Folkman S. Springer; New York: 1984. Stress, appraisal, and coping. [Google Scholar]
- 34.Cohen J., Cohen P. Lawrence Erlbaum; Mahwah, NJ: 1975. Applied multiple regression/correlation analysis for the behavioral sciences. [Google Scholar]
- 35.Judge K.S., Bass D.M., Snow A.L., Wilson N.L., Morgan R., Looman W.J. Partners in dementia care: a care coordination intervention for individuals with dementia and their family caregivers. Gerontologist. 2011;51:261–272. doi: 10.1093/geront/gnq097. [DOI] [PubMed] [Google Scholar]
- 36.Nelson K.M., Starkebaum G.A., Reiber G.E. Veterans using and uninsured veterans not using Veteran Affairs (VA) health care. Public Health Rep. 2007;122:93–100. doi: 10.1177/003335490712200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritter P.L., Stewart A.L., Kaymaz H., Sobel D.S., Block D.A., Lorig K.R. Self-reports of health care utilization compared to provider records. J Clin Epidemiol. 2001;54:136–141. doi: 10.1016/s0895-4356(00)00261-4. [DOI] [PubMed] [Google Scholar]
- 38.Roberts R.O., Bergstralh E.J., Schmidt L., Jacobsen S.J. Comparison of self-reported and medical record health care utilization measures. J Clin Epidemiol. 1996;49:989–995. doi: 10.1016/0895-4356(96)00143-6. [DOI] [PubMed] [Google Scholar]
- 39.Bass D.B., McClendon M.J., Deimling G.T., Mukherjee S. The influence of diagnosed mental impairment on family caregiver strain. J Gerontol B Psychol Sci Soc Sci. 1994;49B:S146–S155. doi: 10.1093/geronj/49.3.s146. [DOI] [PubMed] [Google Scholar]
- 40.Bass D.M., Judge K.S., Snow A.L., Wilson N.L., Looman W.J., McCarthy C. Negative caregiving effects among caregivers of veterans with dementia. Am J Geriatr Psychiatry. 2012;20:239–247. doi: 10.1097/JGP.0b013e31824108ca. [DOI] [PubMed] [Google Scholar]
- 41.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 42.McClendon M. Waveland; Prospect Heights, IL: 1994. Multiple regression and causal analysis. [Google Scholar]
- 43.Silverstein N.M. In search of dementia-friendly hospitals: a survey of patient care directors in Massachusetts. In: Silverstein N.M., Maslow K., editors. Improving hospital care for persons with dementia. Springer; New York: 2005. pp. 23–33. [Google Scholar]
- 44.Dying in American: improving quality and honoring individual preferences near the end of life. Institute of Medicine; Washington, DC: 2014. Committee on approaching death: addressing key end-of-life issues. Policies and payment systems to support high-quality end-of-life care. [Google Scholar]
- 45.Prochaska J.O., Velicer W.F. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 46.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 47.Epstein-Lubow G., Fulton A.T., Gardner R., Gravenstein S., Miller I.W. Post-hospital transitions: special considerations for individuals with dementia. Med Health R I. 2010;93:125–127. [PubMed] [Google Scholar]
- 48.Jack B.W., Chetty V.K., Anthony D., Greenwald J.L., Sanchez G.M., Johnson A.E. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman E.A., Parry C., Chalmers S., Min S.J. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 50.Peikes D., Chen A., Schore J., Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]