Abstract
Introduction
Several studies have tested the N-methyl-D-aspartate–receptor antagonist memantine as an add-on to pre-existing treatment with acetylcholinesterase inhibitors. The objective of this study was to evaluate the efficacy and safety of a combined memantine and galantamine-CR de novo regimen compared with galantamine-CR only treatment in never treated patients with mild-to-moderate Alzheimer's disease (AD).
Methods
Antidementia drug–naïve participants (n = 232) with probable, mild-to-moderate AD, and mini-mental state examination scores between 15 and 26 (inclusive) were randomized to receive either 20 mg/day memantine plus 24 mg/day galantamine-CR or 24 mg/day galantamine-CR plus placebo in a 52-week, prospective, double-blind, controlled trial. The primary outcome measurement was the change on the Alzheimer's disease assessment scale-cognition score. Secondary measures comprised the Alzheimer's Disease Cooperative Study-activities of daily living inventory and the clinical dementia rating.
Results
At the end of the trial, there were no statistically significant differences between the galantamine-CR/memantine combination and galantamine-CR only group in primary and secondary outcome measurements. The incidence and the severity of adverse events were similar between the groups.
Discussion
In this trial, memantine in combination with galantamine-CR did not show an advantage with respect to cognition, function, and behavior in previously never treated patients with mild-to-moderate AD. There were no significant differences in tolerability and safety between the groups. Thus, a de novo combination treatment results in no significant improvement in disease progression (current controlled trials number: NCT01921972).
Keywords: Memantine, Galantamine-CR, Combination treatment, Acetylcholinesterase inhibitor, Alzheimer's disease, Dementia
1. Introduction
Although there is a great hope that disease-modifying therapies can be developed in the near future, until today, acetylcholinesterase inhibitors (AChEIs), such as galantamine, rivastigmine, donepezil, and the N-methyl-D-aspartate (NMDA) receptor antagonist memantine, remain the only approved drugs for the treatment of Alzheimer's disease (AD). The rationale to combine the different modes of action, namely an AChEI and an NMDA receptor antagonist, to increase the therapeutic benefit appears promising. However, until now, there is an ongoing debate about the benefit of memantine as an add-on strategy to AChEI treatment in AD [1], [2].
Studies supporting efficacy and safety of combination treatment in moderate (mini-mental state examination [MMSE] = 10–20), and specially, severe AD (MMSE <10), have been reported [3], [4]. In contrast, findings in mild-to-moderate AD revealed that the combination strategy does not show consistent benefits. A double-blind trial with different AChEIs [5], an open-label trial with rivastigmine [6] and a cohort-observational study with donepezil [7], did not report significant cognitive benefits of combination treatment with memantine. Only an open-label trial with rivastigmine found greater symptomatic improvements with add-on memantine [8]. So far, one might speculate that the efficacy of this treatment throughout AD stages is inversely proportional to the scores on the MMSE: the lower the score, the more effective the add-on strategy appears to be. In all these studies, memantine was subsequently added to an existing AChEI monotreatment.
In summary, studies to date have primarily focused on the possible usefulness of an add-on treatment. However, the benefit of a de novo combination treatment in previously never treated mild-to-moderate AD (MMSE: 15–26) patients had not yet been tested in a randomized, double-blind, controlled trial. Based on these considerations, the first objective of the present study was to assess the long-term efficacy, safety, and tolerability of galantamine-continuous-release (CR) and concomitant memantine in treating antidementia drug–naïve patients with mild-to-moderate AD for a longer period of time (52 weeks). Furthermore, we explored whether memantine add-on treatment, as compared with galantamine-CR only, had an effect on disease progression.
2. Methods
2.1. Participants
A total of 232 community-dwelling participants were recruited at 12 centers in Germany. The patients met the following inclusion criteria: age ≥50 years; diagnosis of probable AD according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) and the German Dementia Competence Network [9]; results of an MRI or CT within the past 12 months consistent with a diagnosis of probable AD; MMSE score = 15–26 at screening and baseline; absence of previous treatments with AChEIs or memantine; vision and hearing sufficient to permit compliance with assessments; and an informed and reliable caregiver to accompany the participant to all study visits and to supervise the administration of the study drug. Exclusion criteria included presence of clinically significant medical, psychiatric, neurodegenerative, or intracerebral diseases. Vitamin B12 and folate deficiency as well as active pulmonary, gastrointestinal, renal, hepatic, endocrine, or cardiovascular disease were explicitly excluded. This study was approved by the ethics committee of the Charité Medical School. Written informed consent was provided by the patient's caregiver and either the patient (if possible) or a legally acceptable representative (if not the caregiver) before initiation of study-specific procedures.
2.2. Trial design
A 52-week, prospective, randomized, double-blind, controlled trial was conducted. At the baseline visit, participants were randomly assigned in a 1:1 ratio to one of two treatment groups: (1) galantamine only: galantamine-CR 24 mg/day with dose-titration over 12 weeks (maintenance phase starting at week 9) and placebo capsules; (2) galantamine/memantine combination: a combination of galantamine-CR 24 mg/day plus memantine 10 mg b.i.d. with a dose-titration phase over 16 weeks (12 weeks for galantamine-CR [maintenance phase starting at week 9], additional 4 weeks for memantine). Participants received one capsule (galantamine-CR) and two pills (memantine or placebo) each day. The galantamine-CR group received memantine placebo pills. Memantine and placebo pills were equal with regard to shape, color, and size. The randomization was performed in blocks with a block length of six. The participants assigned to the galantamine-CR only group received over 4 weeks 8 mg/day galantamine-CR, followed by 4 weeks of 16 mg/day and 24 mg/day starting in week 9 until the end of the trial. The patients assigned to the galantamine-CR/memantine combination group received a memantine titration over 4 weeks in steps of 5 mg/day up to 20 mg/day (10 mg b.i.d.). Half of this group received galantamine-CR first, the other half received memantine first to allow for differential qualitative evaluation of tolerability of a combination treatment. Any change in dosage or discontinuation of galantamine-CR was recorded, and the patients were discontinued from the study if the inclusion criterion of concomitant memantine dosing was no longer met. All patients on memantine were required to receive the target dose of 20 mg/day at the beginning of weeks 4 or 16 (depending on the group type). Patients, who did not tolerate the target dose, were excluded. Compliance and adherence with the study medication were monitored by an inventory of individually returned blister packs and routine assessment of concomitant medication use. The overall education, experience, and training of study personnel were adequate to conduct clinical trials according to good clinical practice, and the researchers were qualified and trained in the treatment of AD. The primary outcome measurement was the change from baseline on the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) score. Secondary measures comprised the Alzheimer Disease Cooperative Study-activities of daily living scale (ADCS-ADL) and clinical dementia rating (CDR). The outcome measurements were reassessed in weeks 16, 26, and 52. No individual participant randomization code had to be revealed during the trial.
2.3. Sample size
The number of patients to be enrolled into this trial was calculated under the following assumptions: (1) The number of patients allocated to the individual arms of the trial should be balanced; (2) the maximum tolerable risk for an error of the second kind is 15%, corresponding to a power of at least 85%; (3) for both groups, the underlying distributions are normal (4); the difference between the means of the two normal distributions under comparison equals δ = 40% of the square root of the common population variance. Based on these assumptions, the usual procedure for the exact computation of the sample size required in the one-sided two-sample t test with α = 0.05 showed the minimum sample size required under the above conditions to be n = 70 per group. To be on the safe side, we allowed for a drop-out rate of up to 40%. Accordingly, the total number of AD patients to be recruited to this trial was fixed at 2 × 120 = 240.
2.4. Statistical analysis
The analyses were based on the intention-to-treat (ITT) population (patients who were randomized to receive either galantamine-only or memantine add-on treatment, and who completed at least one baseline and one post-baseline ADAS-cog assessment) and per-protocol (PP) set (patients who completed the 52 weeks as planned and had measurements for all efficacy variables with no major protocol violations). The statistical analyses were done using SPSS software (version 21) for Windows and were conducted at the two-sided, 5% significance level. Results were expressed as mean ± standard error. The outcome differences in ADAS-cog, ADCS-ADL, and CDR scores were compared by means of an unpaired Student t test analysis. The baseline and different time-point measurements were compared using the paired Student t test. Differences in frequencies in adverse events (AEs) were tested using Pearson's χ2 test.
2.5. Sponsoring
This study was sponsored by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung). Galantamine-CR and memantine were provided by Janssen-Cilag and Merz.
3. Results
3.1. Study population
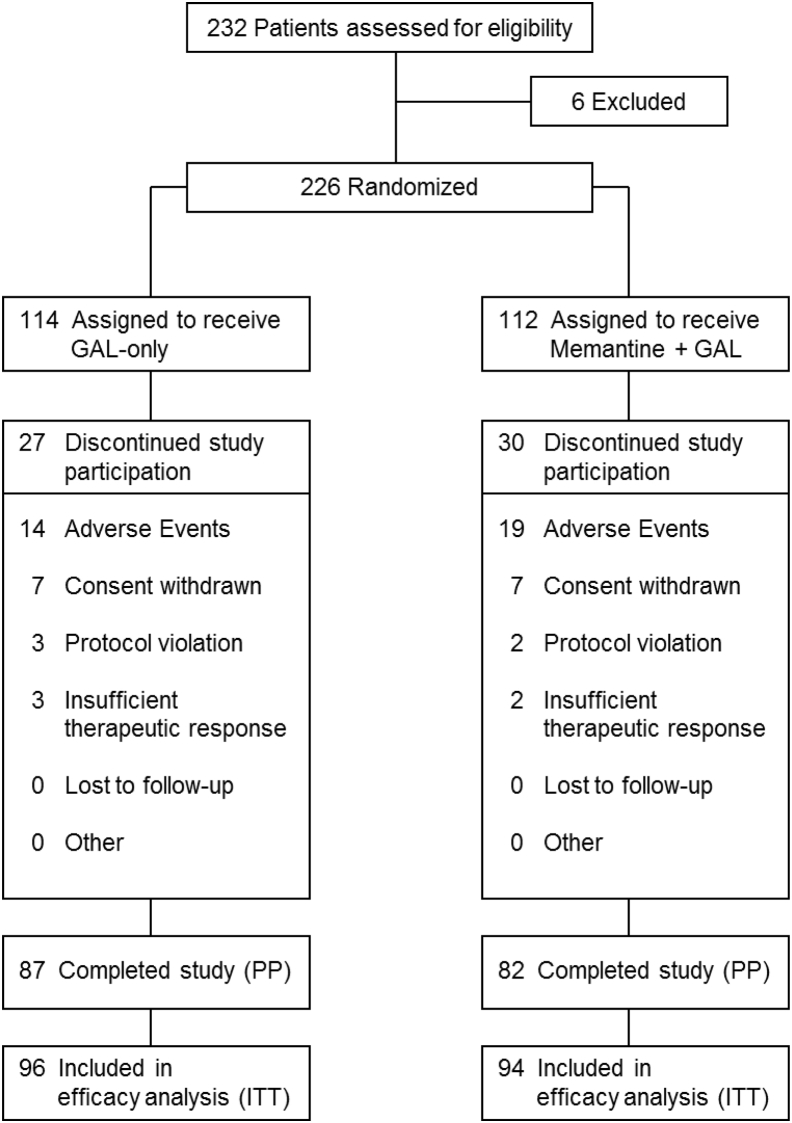
The study assessed 232 patients from the German Dementia Competence Network (DCN) cohort [9] for eligibility, 6 of whom were found not suitable based on the inclusion and exclusion criteria (Fig. 1). When the last patients were recruited, it could be foreseen that the overall drop-out rate in the study will end up far below calculation; therefore, recruitment was already halted closely before 240 patients were recruited. A total of 226 participants were randomly assigned (1:1) to receive either galantamine-CR only treatment (n = 114) or galantamine-CR/memantine combination treatment (n = 112), with 87 (76.31%) and 82 (73.21%) participants completing the trial, respectively. Thereby, the overall drop-out rate of 27.15% was substantially lower than calculated. The ITT population comprised 190 patients (96 galantamine-CR only; 94 galantamine-CR/memantine combination). The PP population comprised 169 patients (87 galantamine-CR only; 82 galantamine-CR/memantine combination). AEs were the most frequent reason for discontinuation in both groups. The treatment groups were well matched for demographic and clinical characteristics at baseline (Table 1). A small but only statistically significant difference was noticed in the MMSE scores: galantamine-CR only, 22.6 ± 3.1; galantamine-CR/memantine combination, 21.7 ± 3.2; P = .029. All participants were in the range of mild-to-moderate AD.
Fig. 1.
Study flow. Abbreviations: GAL-only, galantamine-CR; PP, per protocol; ITT, intention to treat.
Table 1.
Demographic and clinical characteristics of both study groups at baseline
| Characteristics | Galantamine-CR | Galantamine-CR/memantine combination |
|---|---|---|
| n | 114 | 112 |
| Age | 72.6 (7.8) | 72.1 (8.5) |
| Female % | 68.4 | 58.9 |
| MMSE | 22.6 (3.1) | 21.7 (3.2)* |
| ADAS-cog | 18.9 (6.6) | 20.2 (7.0) |
| ADCS-ADL | 62.1 (12.9) | 62.1 (10.5) |
| NPI | 7.9 (9.7) | 5.7 (6.7) |
| CDR (SOB) | 4.8 (1.4) | 5 (1.5) |
| MADRS | 6.8 (4.7) | 7.2 (5.4) |
Abbreviations: MMSE, mini-mental state examination; ADAS-cog, Alzheimer's disease assessment scale; ADCS-ADL, Alzheimer's Disease Cooperative Study ADL; NPI, neuropsychiatric inventory; CDR-SOB, clinical dementia rating scale–sum of boxes; MADRS, Montgomery-Åsberg depression rating scale.
NOTE. *P < .05.
3.1.1. Primary outcome measure
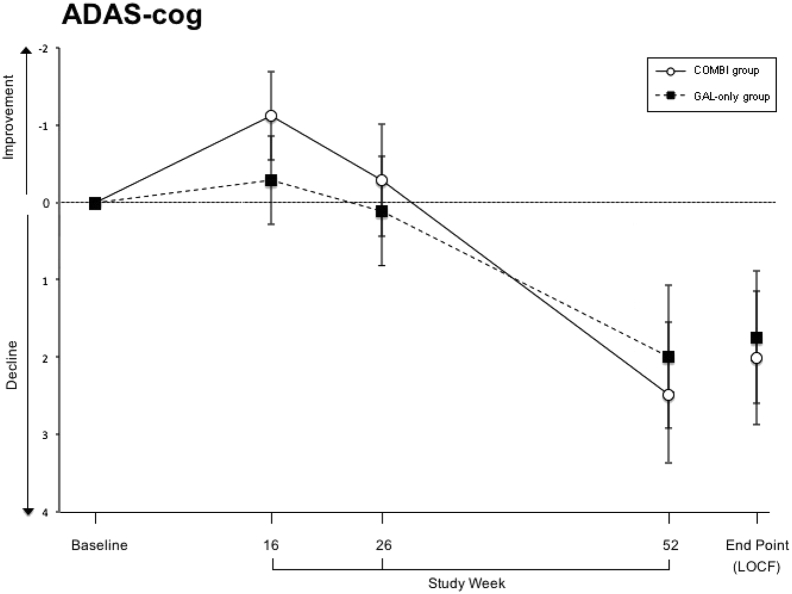
Both ITT and PP analyses were performed to assess efficacy. Analyses using the last observation carried forward (LOCF) approach showed no statistically significant differences between the treatment groups in the ADAS-cog during any visit throughout the trial (at the end point: P = .831). At baseline, the galantamine-CR only and the galantamine-CR/memantine combination groups showed mean values of 18.9 ± 6.6 and 20.2 ± 7, respectively (P = .165). In week 16, both groups—especially the galantamine-CR/memantine combination group—showed a statistically insignificant improvement in the primary outcome (Fig. 2). From week 16 to 52, the mean values of both groups approximated each other over time and increased significantly from baseline to the end point (P = .025 and P = .038, respectively). Similarly, analyses using the PP approach showed no statistically significant differences between the treatment groups at the end of the treatment (P = .781) and significant differences between the baseline and week 52 (P = .015 and P = .023, respectively).
Fig. 2.
Primary outcome measure Alzheimer's Disease Assessment Scale (ADAS-cog). Sample size at week 16 (Combi/GAL-only): 184 (91/93); at week 26: 175 (90/85) and at week 52: 170 (86/84). Data are presented as mean and standard deviation. Abbreviations: COMBI group, galantamine-CR/memantine combination; GAL-only group, galantamine-CR; LOCF, last observation carried forward.
3.1.2. Secondary outcome measurements
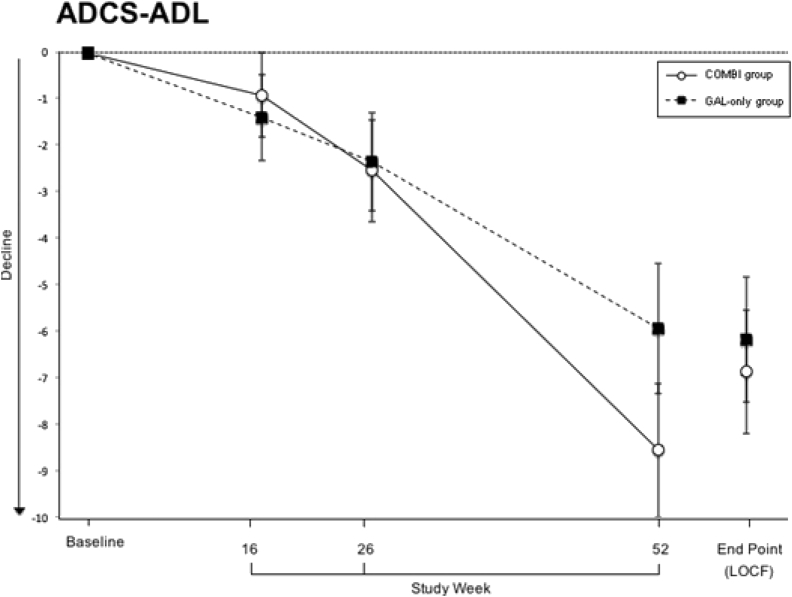
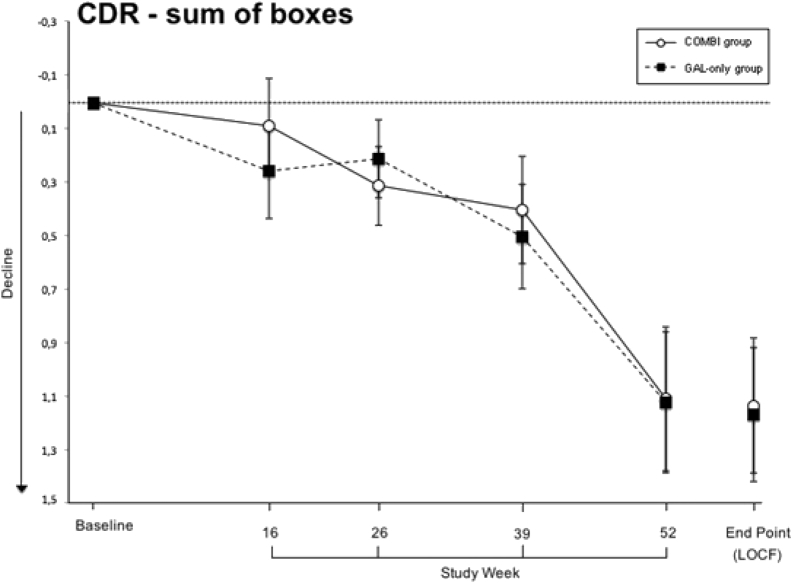
The secondary outcomes included the ADCS-ADL and the CDR and the neuropsychological inventory (NPI). At baseline, there were no statistically significant differences between the groups in mean values (ADCS-ADL: 62.1 ± 12.9 and 62.1 ± 10.5, P = .980; CDR: 4.8 ± 1.4 and 5 ± 1.5, P = .297; NPI: 7.9 ± 9.7 and 5.7 ± 6.7, P = .067). The mean ADCS-ADL and CDR values of both treatment groups remained below baseline throughout the trial (Fig. 3, Fig. 4). LOCF analyses showed that at the end point the scores of both tests worsened significantly compared with baseline in the galantamine-CR only group (P < .001) and in the galantamine-CR/memantine combination group (P < .001) and no significant differences were detected between the treatment groups (ADCS-ADL: P = .719; CDR: P = .921). The mean NPI score had slightly improved, but not statistically significantly, in the galantamine-CR only group at the end of treatment (P = .064). At the end point, there were no significant differences in both groups compared with baseline (galantamine-CR only: P < .344; galantamine-CR/memantine combination: P < .078).
Fig. 3.
Secondary outcome measure Alzheimer's Disease Cooperative Study-activities of daily living scale (ADCS-ADL). Sample size at week 16 (Combi/GAL-only): 151 (77/74); at week 26: 149 (72/77) and at week 52: 136 (66/70). Data are presented as mean and standard deviation. Abbreviations: COMBI group, galantamine-CR/memantine combination; GAL-only group, galantamine-CR; LOCF, last observation carried forward.
Fig. 4.
Secondary outcome measure clinical dementia rating scale–sum of boxes. Sample size at week 16 (Combi/GAL-only): 188 (94/94); at week 26: 177 (87/90); at week 39: 177 (86/91) and at week 52: 172 (84/88). Data are presented as mean and standard deviation. Abbreviations: COMBI group, galantamine-CR/memantine combination; GAL-only group, galantamine-CR; LOCF, last observation carried forward.
Once again, the analysis of results of the PP approach followed the same trend as the ITT approach. In week 52, the scores of the ADCS-ADL and CDR worsened significantly compared with the baseline in the galantamine-CR only group (P < .001 and P < .001, respectively) and in the galantamine-CR/memantine group (P < .001 and P < .001, respectively) and no significant differences were detected between the treatment groups (ADCS-ADL: P = .149; CDR: P = .802). The mean NPI score did not differ between groups in week 52 (P = .106). At the end of the treatment, there were no significant differences in both groups compared with the baseline (galantamine-CR only: P < .390; galantamine-CR/memantine: P < .138).
3.2. Safety and tolerability
A summary of AEs is listed in Table 2. The most common AEs involved the nervous system (galantamine-CR only: 42 [23.6%], galantamine-CR/memantine combination: 42 [22.2%]). A total of 80 galantamine-CR only treated patients (70.17%) and 89 galantamine-CR/memantine combination treated patients (79.4%) reported AEs, with both groups showing, in general, a similar AE profile (P = .108). Serious AEs were experienced by 15 galantamine-CR only treated (13.1%) and 15 galantamine-CR/memantine combination treated patients (13.3%). One patient of 226 (0.44%) died during the trial in the galantamine-CR/memantine combination group. This death was judged to be not related or even remotely related to treatment in the memantine-treated group.
Table 2.
Summary of adverse events
| Patients at risk for AE/unique patients with AE | Galantamine-CR |
Galantamine-CR/memantine combination |
||
|---|---|---|---|---|
| n = 114 | n = 80 | n = 112 | n = 89 | |
| Total (serious) adverse events | 178 (19) | % | 189 (20) | % |
| Nervous system events | 42 (3) | 23.6 | 42 (4) | 22.2 |
| Gastrointestinal system events | 37 (3) | 20.7 | 36 (3) | 19.0 |
| Cardiac and vascular events | 22 (4) | 12.3 | 27 (4) | 14.3 |
| Fall | 14 (3) | 7.9 | 18 (4) | 9.5 |
| Otolaryngologic events | 7 | 4.0 | 10 (1) | 5.3 |
| Skin events | 5 | 2.8 | 13 | 6.9 |
| Endocrine events | 1 | 0.5 | 3 | 1.6 |
| Urinary system events | 8 (2) | 4.5 | 7 | 3.7 |
| Metabolic-nutritional events | 3 | 1.7 | 2 | 1.0 |
| Respiratory, thoracic, and mediastinal events | 9 (1) | 5.0 | 8 (3) | 4.2 |
| Other | 30 (3) | 16.8 | 23 (1) | 12.1 |
Abbreviation: AE, adverse events.
NOTE. The number of serious adverse events is indicated in parentheses.
4. Discussion
This is the first prospective, randomized, double-blind, controlled trial examining the efficacy of a combination of an AChEI (galantamine-CR) and the NMDA receptor antagonist (memantine) in antidementia-naïve patients with mild-to-moderate AD. A treatment of 52 weeks with 24 mg/day galantamine-CR in combination with 20 mg/day memantine had no significant benefit over galantamine-CR alone on cognitive function, activities of daily living, and disease progression.
Benefits of a memantine monotreatment in patients with mild AD using standard cognitive and functional measurements have not been consistently reported [10], [11], [12], [13]. Similarly, most of the recent studies found that a memantine add-on treatment to prior, stable AChEI administration resulted in no cognitive enhancement in mild-to-moderate AD patients when compared with AChEIs alone [5], [6], [7]. Likewise, a double-blind, placebo-controlled trial revealed that memantine did not show an advantage over placebo, based on protocol-specified primary (ADAS-cog and Clinician's Interview-Based Impression of Change with caregiver input [CIBIC-plus]) or secondary outcomes (ADCS-ADL, NPI, MMSE, the Resource Utilization in Dementia (RUD)) in patients with mild-to-moderate AD on stable AChEI medication [5]. Our study supports these findings and extends them to antidementia-naïve, mild-to-moderately severe AD patients and a much longer trial period (24 vs. 52 weeks, respectively).
To date, there is only one published study suggesting a cognitive benefit of a combination strategy in mild-to-moderate AD: Riepe et al. [8] reported in a small open-label study that a combination of memantine with rivastigmine resulted in a statistically significant improvement in both the ADAS-cog total score and the MMSE score in mild-to-moderate AD. However, it is noteworthy that the change in ADAS-cog score (1.7 points) was less than the 4-point change considered clinically relevant by the US Food and Drug Administration [14].
On the other hand, two randomized, double-blind, placebo-controlled trials in moderate-to-severe AD (MMSE scores from 3–5 to 13–17) supported the notion that memantine, when added to prior, stable AChEIs may have significant benefits in reducing decline in cognition, functioning, and global status [3], [4]. However, other studies disagree. In a double-blind, placebo-controlled trial (n = 295; MMSE: 5–13), Howard et al. [15] found that AChEI treatment was discontinued before the efficacy of an AChEI and memantine did not differ significantly in the presence or absence of the other; furthermore, there were no significant benefits of the combination of AChEI and memantine over AChEI alone.
In conclusion, a combination strategy does not seem to be cognitively or functional advantageous in mild-to-moderately affected AD patients who had never before received antidementia drugs. More specifically, the slopes of cognitive decline were similar between the two groups. This strategy may be considered safe and well tolerated in this kind of patients.
Research in context.
-
1.
Systematic review: Studies supporting efficacy and safety of memantine as an add-on strategy to AChEI treatment in moderate-to-severe AD, have been reported. However, findings in mild-to-moderate AD revealed no consistent benefits.
To our knowledge, this is the first randomized controlled trial (RCT) of a large number of antidementia-naïve, mild-to-moderately severe Alzheimer's patients receiving either a combination treatment of memantine and galantamine (AD COMBI) or galantamine (AD GAL) alone for 52 weeks.
-
2.
Interpretation: In this RCT, we did not find any clinically relevant or statistically significant benefits of the combination treatment. Furthermore, the slopes of cognitive, functional, and global decline were similar between the AD-COMBI and the AD-GAL groups. Interestingly, a significant global decline was noted in both groups starting at week 26. Thus, we conclude that in previously antidementia naïve patients with Alzheimer's disease, combining the N-methyl-D-aspartate modulator memantine with the acetylcholine-inhibitor galantamine results in no improvement over galantamine alone.
-
3.
Future directions: We feel that our study would be of great interest to the reader of your journal, given the need for an effective “palliative” treatment for Alzheimer's disease, until, hopefully in the not so distant future, a disease-modifying strategy is available. Also, this is one of the very few trials where patients were treated with an antidementia drug combination for a duration of as long as 52 weeks.
Acknowledgments
The Bundesministerium für Bildung und Forschung is gratefully acknowledged for financial support (01GI0420). The authors thank Dr Brigitte Haas for her expert technical assistance. This article is dedicated to Prof Dr H Hippius for his tremendous work in psychopharmacology.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2015.10.001.
Supplementary data
References
- 1.Patel L., Grossberg G.T. Combination therapy for Alzheimer's disease. Drugs Aging. 2011;28:539–546. doi: 10.2165/11591860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt R., Hofer E., Bouwman F.H., Buerger K., Cordonnier C., Fladby T. EFNS-ENS/EAN Guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer's disease. Eur J Neurol. 2015;22:889–898. doi: 10.1111/ene.12707. [DOI] [PubMed] [Google Scholar]
- 3.Tariot P.N., Farlow M.R., Grossberg G.T., Graham S.M., McDonald S., Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: A randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 4.Grossberg G.T., Manes F., Allegri R.F., Gutierrez-Robledo L.M., Gloger S., Xie L. The safety, tolerability, and efficacy of once-daily memantine (28 mg): A multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer's disease taking cholinesterase inhibitors. CNS Drugs. 2013;27:469–478. doi: 10.1007/s40263-013-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porsteinsson A.P., Grossberg G.T., Mintzer J., Olin J.T., Memantine MEM-MD-12 Study Group Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: A randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5:83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 6.Choi S.H., Park K.W., Na D.L., Han H.J., Kim E., Shim Y.S. Tolerability and efficacy of memantine add-on therapy to rivastigmine transdermal patches in mild to moderate Alzheimer's disease: A multicenter, randomized, open-label, parallel-group study. Curr Med Res Opin. 2011;27:1375–1383. doi: 10.1185/03007995.2011.582484. [DOI] [PubMed] [Google Scholar]
- 7.Schneider L.S., Insel P.S., Weiner M.W. Alzheimer's Disease Neuroimaging Initiative: Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer's Disease Neuroimaging Initiative. Arch Neurol. 2011;68:58–66. doi: 10.1001/archneurol.2010.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riepe M.W., Adler G., Ibach B., Weinkauf B., Gunay I., Tracik F. Adding memantine to rivastigmine therapy in patients with mild-to-moderate Alzheimer's disease: Results of a 12-week, open-label pilot study. Prim Care Companion J Clin Psychiatry. 2006;8:258–263. doi: 10.4088/pcc.v08n0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornhuber J., Schmidtke K., Frolich L., Perneczky R., Wolf S., Hampel H. Early and differential diagnosis of dementia and mild cognitive impairment: Design and cohort baseline characteristics of the German Dementia Competence Network. Dement Geriatr Cogn Disord. 2009;27:404–417. doi: 10.1159/000210388. [DOI] [PubMed] [Google Scholar]
- 10.Peskind E.R., Potkin S.G., Pomara N., Ott B.R., Graham S.M., Olin J.T. Memantine treatment in mild to moderate Alzheimer disease: A 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14:704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 11.Knopman D.S. Commentary on “Meta-analysis of six-month memantine trials in Alzheimer's disease”. Memantine has negligible benefits in mild to moderate Alzheimer's disease. Alzheimers Dement. 2007;3:21–22. doi: 10.1016/j.jalz.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Bakchine S., Loft H. Memantine treatment in patients with mild to moderate Alzheimer's disease: Results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2008;13:97–107. doi: 10.3233/jad-2008-13110. [DOI] [PubMed] [Google Scholar]
- 13.Schneider L.S., Dagerman K.S., Higgins J.P., McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68:991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration . Department of Health and Human Services, Public health Service; Rockville, MD: 1989. Peripheral and Central Nervous System Drugs Advisory Committee Meeting; p. 227. [Google Scholar]
- 15.Howard R., McShane R., Lindesay J., Ritchie C., Baldwin A., Barber R. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med. 2012;366:893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.