Abstract
Introduction
Pyroglutamate-amyloid-β (pE-Aβ) peptides are major components of Aβ-oligomers and Aβ-plaques, which are regarded as key culprits of Alzheimer's disease (AD) pathology. PQ912 is a competitive inhibitor of the enzyme glutaminyl cyclase (QC), essential for the formation of pE-Aβ peptides.
Methods
A randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study investigated the safety, pharmacokinetics, and pharmacodynamics of PQ912 in healthy nonelderly and elderly subjects.
Results
PQ912 was considered safe and well tolerated with dose-proportional pharmacokinetics up to doses of 200 mg. At higher doses up to 1800 mg, exposure was supraproportional and exposure in elderly subjects was approximately 1.5- to 2.1-fold higher. Exposure in cerebrospinal fluid (CSF) was approximately 20% of the unbound drug in plasma, and both serum and CSF QC activity was inhibited in a dose-related manner.
Discussion
This first-in-man study of a compound-targeting QC inhibition justifies further development of PQ912 for the treatment of AD.
Keywords: Alzheimer's disease, Glutaminyl cyclase, QC inhibitor, Amyloid-beta, Pyroglutamate, pE-Aβ, Cerebrospinal fluid
1. Introduction
Current approved drugs for Alzheimer's disease (AD) only treat symptoms of the disease and neither halt the progression nor provide sustainable improvement of the disease. The positive effects of these treatments on cognitive functions and activities of daily living are at best modest and transient and may have side effects [3], [4]. Thus, there is an important need for new medicines to either improve cognition or reduce the progression of symptoms long term in AD patients.
Pathologic hallmarks of AD are intracellular tau tangles and deposition of extracellular amyloid plaques within the brain [5], [6]. The amyloid plaques are composed of variants of amyloid-β (Aβ) peptides, resulting in a mixture of Aβ1–40/1–42 peptides and N- and C-terminally truncated Aβ peptides [7], [8]. The truncated species Aβ3–40/3–42 and Aβ11–40/11–42 bearing an N-terminal glutamate moiety undergo posttranslational modification to their respective oxo-prolyl (pyroglutamyl [pE])–containing amyloid-β (pE-Aβ) peptides [8], [9], [10]. This process is catalyzed by the enzymes glutaminyl cyclase (QC) and iso-glutaminyl cyclase (isoQC) [9], [10], [11]. Both pE-Aβ peptides and QC expression have been found abundantly in both sporadic and familial AD [7], [8], [10], [12], [13], [14], [15], and have been shown to increase with AD severity [14], [16]. The presence of pE in pE-Aβ peptides changes their physicochemical properties, profoundly increasing their ability to initiate and sustain self- and co-aggregation with unmodified Aβ peptides, leading to highly toxic Aβ-oligomers [17], [18], [19], [20], [21], [22]. The seeding effect of pE-Aβ relates to the modified Aβ-hypothesis, where Aβ-oligomers are considered the toxic culprit in AD pathology [23]. New research findings have demonstrated that pE-Aβ/Aβ-oligomers appear to be centrally involved in human AD-like pathology in animal models and that the pathology and behavioral deficits could be rescued by means of QC inhibitors [10], [24], [25]. Thus, the inhibition of QC in the brain could represent an important new therapeutic target. PQ912 is a heterocyclic compound (Fig. 1A) bearing the ability to coordinate QC's active zinc-ion [26], while being selective against other metal-containing enzymes. Its Ki values for human QC and isoQC enzymes are 29 and 5 nM, respectively. In preclinical studies, PQ912 showed an attractive drug-like profile (unpublished data) and to investigate this further, the pharmacokinetic (PK) and safety profile of single and multiple PQ912 doses were defined in this phase 1 clinical study.
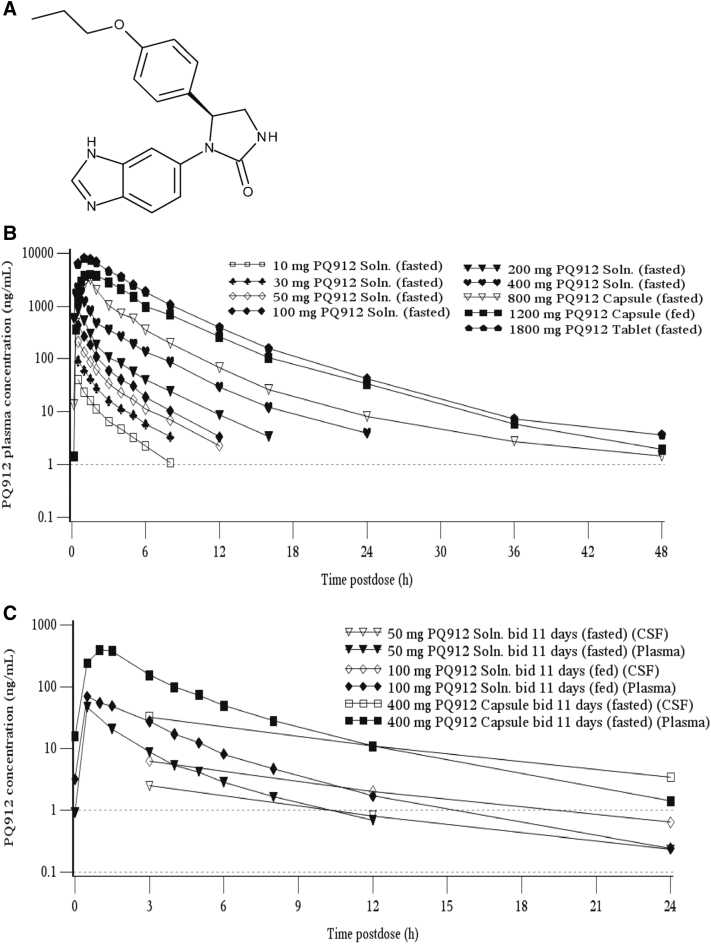
Fig. 1.
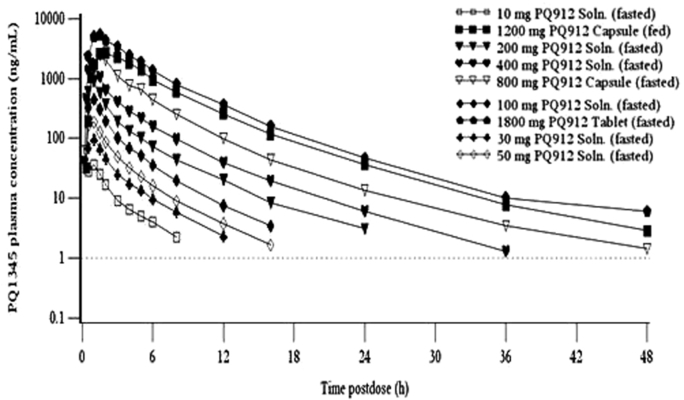
The structural formula of PQ912 and the plasma and CSF concentrations of PQ912 following oral administration. (A) A schematic of the structural form of PQ912 as a free base. (B) Arithmetic mean concentrations of PQ912 for plasma on day 1 after single oral administration of PQ912 in healthy nonelderly subjects. (C) Free/protein unbound concentrations of PQ912 in plasma (fub-plasma = 0.22) and CSF at steady-state (day 11) after multiple bid oral doses in nonelderly subjects. The lower limit of quantification (dashed line) is 1.00 ng/mL for plasma and 0.1 ng/mL for CSF. Abbreviations: CSF, cerebrospinal fluid; bid, twice daily (dose interval of 12 hours); fub, free/protein unbound; Soln., solution.
2. Methods
2.1. Study design
This was a randomized, single- and multiple-ascending oral dose study conducted in two parts. Part 1 comprised a double-blind, placebo-controlled, ascending single-dose, sequential-cohort study, incorporating three cohorts with crossover arms to evaluate a food-effect and formulation-effect. Part 2 comprised a double-blind, placebo-controlled, ascending multiple-dose, sequential-cohort study in nonelderly and elderly subjects. The study was conducted at Covance Clinical Research Unit (CRU) in Basel, Switzerland and Covance CRU in Leeds, UK.
In part 1, subjects were randomized to receive single oral doses of 10–3600 mg of PQ912 or placebo in each period (3600 mg was administered as two doses of 1800 mg taken 12hours apart). In part 2, subjects were randomized to receive twice daily (bid; dose interval of 12 hours) doses, of 20–800 mg of PQ912 or placebo for 11 days or in case of 200- and 300-mg dose levels; doses were administered to elderly subjects for 7 days (see Supplementary Methods 2.1 for further details).
2.2. Study subjects
Healthy male and female subjects were enrolled; subjects were aged between 18 and 55 years in the nonelderly cohorts and between 65 and 80 years in the elderly cohorts.
The study protocol was approved by institutional review boards for both sites and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. In addition, the cohorts studied in Basel were conducted in accordance with Swiss Federal Laws on Medicinal Products and Medical Devices (December 2000) and Swiss Regulation on Clinical Trials with Medicinal Products (October 2001). All subjects provided written informed consent before participating.
2.3. PK and pharmacodynamic assessments
Blood samples were collected for PK and pharmacodynamic (PD) analysis predose and up to 72 hours postdose for single-dose cohorts; and predose on day 1, day 7/11, and specified days between days 2 and 7/11, and up to 96 hours after the last dose for multiple-dose cohorts.
Cerebrospinal fluid (CSF) samples were collected for PK and PD analysis from specific dose cohorts (50, 100, and 400 mg single-dose cohorts; 50, 100, and 400 mg nonelderly multiple-dose cohorts; and 300 and 500 mg elderly multiple-dose cohorts) at various time points up to 3 hours (50 and 100 mg single-dose cohorts), up to 12 hours (400 mg single-dose cohort) postdose, and at day -1 and up to 24 hours after the final dose for multiple-dose cohorts. The rationale for the CSF sampling scheme after repeated dosing was to determine (1) maximum CSF concentration (Cmax; the time to maximum concentration [tmax] was expected between 2 and 3 hours based on single-dose data), (2) Cmin, i.e., trough concentration (12 hours after the last dose), and (3) the concentration 24 hours after dosing for estimating CSF apparent terminal half-life (t1/2). Based on the PK data acquired during the single-dose study, CSF sampling time points for the multiple-dose cohorts were adjusted accordingly to assess PQ912. Single-tap lumbar punctures were performed on each occasion by an anesthetist, and subjects were not sampled more than four times. Samples were inspected visually before analysis.
Urine samples were collected for PK analysis from all dose cohorts over various time intervals. For single doses of 10 to 200 mg of PQ912, urine was collected over a 6-hour period postdose (day 1), for single doses of 1800 and 3600 mg, urine was collected over a 12-hour period, and for single doses of 400, 800, and 1200 mg PQ912, urine was collected over various time intervals up to 48 hours postdose. For multiple-dose cohorts of 20–800 mg of PQ912, urine was collected over various time intervals over a 12-hour period postdose on day 7/11, as appropriate.
Plasma, urine, and CSF samples were analyzed for PQ912 and the major metabolite, PQ1345, using a validated liquid chromatography with tandem mass spectrometric detection method by Swiss BioQuant, Switzerland (see Supplementary Methods 2.21 for sample preparation). The lower and upper limits of quantification for the analysis of PQ912 and PQ1345 were 1.00–2000 ng/mL for plasma samples, 10–10,000 ng/mL for urine samples, and 0.1–100 ng/mL for CSF samples. PK parameter estimates for PQ912 and PQ1345 were calculated using standard noncompartmental methods of analysis using Phoenix WinNonlin version 6.2.1 (Pharsight, Mountain View, CA, USA). PQ1345 results are presented in the Supplementary Data. The unbound (free) concentrations of PQ912 were estimated in vitro in human plasma using equilibrium dialysis (Supplementary Methods 2.2.1.1).
The QC activity was measured with a coupled assay using glutaminyl-7-amino-4-methylcoumarin as the substrate and pyroglutamyl aminopeptidase as the auxiliary enzyme (Probiodrug AG, Germany or Evotec AG, Germany [27]; Supplementary Methods 2.2.2).
Assessment of the PK/PD relationship was performed on multiple-dose cohort data. The sigmoidal concentration response curve was fitted by nonlinear regression according to the following Emax model: QC activity (% of baseline) = 100 × EC50/(EC50 + C), where C represents PQ912 concentration.
2.4. Safety assessments
Safety was assessed by physical examinations, clinical laboratory evaluations, vital signs, electrocardiograms (ECGs; 12-lead and telemetry), and by monitoring of treatment-emergent adverse events (TEAEs).
2.5. Statistical analysis
No formal statistical assessment of sample size was conducted. However, the number of subjects is common in early clinical pharmacology studies and was considered sufficient to achieve the objectives of the study.
See Supplementary Methods 2.5 for further details on the statistical analyses performed.
2.6. Investigational Medicinal Product formulations
Three immediate release formulations of PQ912 were applied during the study: solution, capsule, and tablets. In the earlier cohorts when only the noncrystalline free base of PQ912 was available, initially a reconstituted solution and later a formulation as a liquid-containing gelatin capsule (Licaps Technology) were used. With the progression of the manufacturing process of the active pharmaceutical ingredient and the identification of a crystalline hydrochloride salt, the development of a solid formulation was accomplished and PQ912 hydrochloride was administered as a film-coated tablet.
3. Results
3.1. Demographics and disposition
A total of 206 subjects participated in the study, with 205 subjects completing the study. One hundred and four subjects aged between 22 and 55 years received single doses of PQ912 or placebo, and 102 subjects aged between 23 and 55 years for the nonelderly dose cohorts and aged between 65 and 77 years for the elderly dose cohorts received multiple doses of PQ912 or placebo (Supplementary Table 1). One subject (500-mg bid, 11 days; capsule, fed) was withdrawn from the study because of a TEAE of urticaria papular, which started approximately 3.5 hours after the 18th dose and was suspected to be related to the study drug.
Overall, 83 subjects were exposed to single doses of up to 3600 mg of PQ912, and 80 subjects were exposed to multiple bid doses of up to 800 mg for up to 11 days as specified.
3.2. PK analyses
3.2.1. Plasma concentrations of PQ912 after single oral administration
After single oral solution administration in the fasted state, PQ912 was rapidly absorbed with mean maximum plasma concentration (Cmax) attained 0.5 hours postdose (tmax) for the 10–400 mg doses. At the higher doses of up to 1800 mg, where formulation was tablet or capsule, mean tmax was reached approximately 1.0–1.5 hours postdose (Fig. 1B and Table 1). After Cmax, plasma concentrations of PQ912 showed a multiexponential decline. The terminal t1/2 varied between 2–8 hours. However, the half-life for the concentration decline from tmax to 12 hours postdose (t1/2,eff), representing more than 90% of the area under the concentration versus time curve (AUC), was between 2 and 3 hours and showed less interindividual and across-the-dose range variation.
Table 1.
Summary of plasma pharmacokinetic parameters of PQ912 on day 1 after single doses and on day 7 or 11 after multiple doses
| Parameters | Single dose of PQ912 (mg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 (n = 6) | 30 (n = 6) | 50 (n = 6) | 100 (n = 8) | 200 (n = 6) | 400 (n = 12) | 800 capsule (n = 6) | 1200 (fed) capsule (n = 6) | 1800 tablet (n = 6) | |
| AUC0–tlast (ng.h/mL) | 61.0 (38.9) | 168 (19.3) | 348 (47.0) | 685 (31.1) | 1343 (21.8) | 3542 (49.8) | 8546 (20.7) | 17,891 (39.8) | 32,166 (50.8) |
| AUC0–12 h (ng.h/mL) | 65.1 (35.3) | 171 (17.5) | 346 (44.6) | 680 (30.5) | 1309 (21.0) | 3421 (48.6) | 8186 (19.8) | 16,793 (35.2) | 30,525 (48.5) |
| AUC (ng.h/mL) | 66.1 (35.3) | 174 (17.8) | 355 (46.1) | 692 (30.8) | 1350 (21.7) | 3556 (49.7) | 8576 (20.8) | 17,914 (39.9) | 32,211 (50.8) |
| Cmax (ng/mL) | 36.2 (33.1) | 91.4 (17.8) | 199 (43.5) | 421 (27.0) | 818 (19.4) | 2152 (31.6) | 3444 (36.7) | 4714 (16.8) | 9676 (31.9) |
| tmax∗ (h) | 0.500 (0.417–0.517) | 0.500 (0.500–0.500) | 0.500 (0.500–0.500) | 0.500 (0.500–0.533) | 0.500 (0.500–0.533) | 0.500 (0.333–0.500) | 1.50 (0.750–1.50) | 1.25 (1.00–3.00) | 1.26 (0.500–2.05) |
| t1/2 (h) | 2.11 (22.6) | 2.28 (6.4) | 2.62 (29.1) | 2.47 (14.4) | 3.16 (12.4) | 3.98 (35.2) | 7.77 (85.3) | 7.47 (87.5) | 5.56 (37.2) |
| t1/2,eff (h) | 2.05 (16.6) | 2.30 (7.6) | 2.25 (18.0) | 2.16 (3.6) | 2.43 (10.5) | 2.43 (8.8) | 2.28 (14.9) | 2.45 (25.0) | 2.28 (21.3) |
| CL/f (mL/min) | 2520 (35.3) | 2872 (17.8) | 2349 (46.1) | 2407 (30.8) | 2469 (21.7) | 1875 (49.7) | 1555 (20.8) | 1116 (39.9) | 931 (50.8) |
| Multiple twice-daily doses (dose interval of 12 h) of PQ912 (mg) for 11 d |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nonelderly healthy subjects |
Elderly healthy subjects |
|||||||||
| 20 (n = 8) | 50 (n = 8) | 100 (fed) (n = 8) | 200 (n = 8) | 400 capsule (n = 8) | 500 (fed) capsule (n = 7) | 200† capsule (n = 6) | 300† capsule (n = 8) | 500 tablet (n = 12) | 800 tablet (n = 6) | |
| AUC0–12 h (ng.h/mL) | 120 (32.5) | 413 (37.3) | 969 (26.5) | 1833‡ (35.1) | 5946 (16.1) | 9302 (63.0) | 3891‡ (35.1) | 5658 (48.1) | 11,851 (39.4) | 24,549 (46.0) |
| Cmax (ng/mL) | 68.4 (36.5) | 229 (20.8) | 358 (34.6) | 1049‡ (28.1) | 2397 (15.8) | 1870 (55.3) | 1581‡ (17.1) | 2058 (37.2) | 4460 (30.0) | 6454 (32.8) |
| tmax∗ (h) | 0.500 (0.500–0.500) | 0.500 (0.500–0.500) | 0.750 (0.500–1.50) | 0.500 (0.500–0.500) | 1.00 (0.500–1.50) | 3.00 (1.00–4.05) | 1.00 (1.00–1.50) | 1.00 (0.500–1.00) | 0.500 (0.500–1.00) | 1.00 (0.500–1.50) |
| t1/2 (h) | 2.36 (13.9) | 2.73 (18.3) | 3.46 (16.7) | 6.36 (119.7) | 5.74 (63.2) | 6.82 (61.9) | 7.55 (93.3) | 6.28 (37.4) | 7.58 (24.7) | 7.67 (30.0) |
| t1/2,eff (h) | 2.46 (23.2) | 2.62 (10.2) | 2.44 (7.8) | 2.72 (11.6) | 2.49 (15.4) | 3.01 (28.6) | 3.08 (18.6) | 2.85 (21.2) | 2.78 (15.9) | 3.22 (17.9) |
| CL/f (mL/min) | 2778 (32.5) | 2018 (37.3) | 1721 (26.5) | 1818 (35.1) | 1121 (16.1) | 896 (63.0) | 857 (35.1) | 884 (48.1) | 703 (39.4) | 543 (46.0) |
| Day 7/11: day 1 ratio | 1.15 (9.8) | 1.10 (12.8) | 1.13 (9.0) | 1.19 (16.6) | 1.30 (19.4) | 1.71 (36.0) | 1.21 (11.1) | 1.34 (16.6) | 1.30 (17.4) | 1.57 (19.9) |
Abbreviations: n, number of subjects; AUC0–tlast, area under the concentration versus time curve from time 0 h to the last data point tlast after drug administration above the limit of quantitation on day 1; AUC0–12h = area under the concentration versus time curve from time 0 h to the available and quantifiable sampling point closest to 12 h after dosing; AUC, area under the concentration versus time curve extrapolated to infinity; Cmax, maximum observed concentration; tmax, time to reach maximum concentration; t1/2, apparent terminal half-life; t1/2,eff, apparent half-life in the range of 2–12 h postdose; CL/f, total body clearance; CV, coefficient of variation.
NOTE. All data presented are for doses administered as solutions under fasted conditions unless stated otherwise. Geometric mean (CV%) data are presented.
Median (min–max).
Doses administered for 7 d for these cohorts.
The ratios and 90% confidence intervals for the geometric least squares means of elderly: nonelderly subjects are 2.02 (1.43–2.85) and 1.72 (1.31–2.26) for day 1, and 2.12 (1.51–2.99) and 1.51 (1.15–1.98) for day 7/11, for AUC0–12 h and Cmax, respectively.
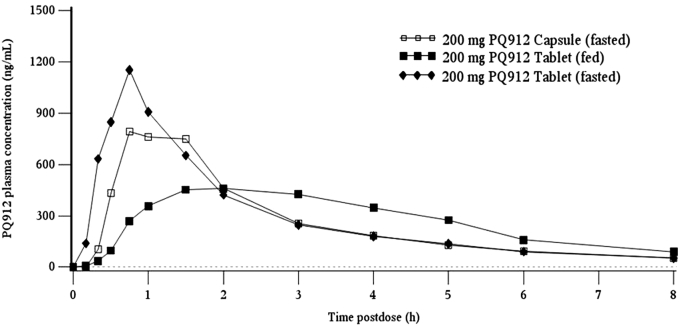
The effect of food (tablets: fasted vs. fed) and different formulations (tablet, fasted vs. capsule, fasted) were investigated at the 200-mg dose level using a crossover design. Compared to the fasted state, Cmax in the fed state was 52.7% lower and was reached later (least squares mean ratio of 0.473; 90% confidence intervals [CIs], 0.399–0.559). However, total systemic exposure to PQ912 (AUC) was similar between dietary states indicating that the extent of absorption was not affected by food (least squares mean ratio of 0.995, 90% CIs, 0.938–1.05; Supplementary Table 2 and Supplementary Fig. 1).
Supplementary Fig.1.
Arithmetic mean plasma concentrations of PQ912 following single oral doses of tablet and capsule formulations of PQ912 in healthy subjects under fed and fasted conditions. Lower limit of quantification (- - -) was 1.00 ng/mL.
In addition, tablet (hydrochloride) and capsule (free base) formulations showed absorption profiles with similar Cmax, tmax, and AUC (least squares mean ratios [90% CIs] of 0.827 [0.699–0.979] and 0.909 [0.857–0.964] for Cmax and AUC, respectively; Supplementary Table 2 and Supplementary Fig. 1). Compared to the solution formulation (Table 1), exposure appeared to be similar, but Cmax was reached later. However, in view of the number of variables (different formulations, doses, and age), it cannot be stated whether the latter was solely due to differences in formulation.
3.2.2. Dose proportionality and multiple-dose kinetics
Over the 10–200 mg of PQ912 dose range, systemic exposure of PQ912 increased in a dose-proportional manner, with the 20-fold dose increment resulting in increases of approximately 20- and 23-fold for AUC and Cmax, respectively. At higher doses, systemic exposure to PQ912 increased in a supraproportional manner, with a ninefold increase in dose from 200–1800 mg resulting in a 24-fold increase in AUC (Table 1).
After bid doses of PQ912, the disposition of PQ912 in plasma was similar to that after single dosing, with a multiexponential decline after Cmax, and t1/2,eff values of approximately 2–3 hours (Table 1). Visual examination of the morning trough PQ912 concentrations for individual subjects showed steady-state was achieved within 2 days after start of the multiple bid dosing. After repeated dosing up to 400 mg, accumulation (AUC from time 0–12 hours after dosing [AUC0–12 h] day 11/day 1 ratio) of PQ912 was minimal. In addition, systemic exposure to PQ912 on day 11 appeared to increase in a supraproportional manner from 20 to 200 mg, with the 10-fold dose increment resulting in increases of approximately 15-fold for AUC0–12 h and Cmax (Table 1).
Healthy elderly subjects were also administered multiple bid doses of PQ912, demonstrating a similar t1/2,eff to nonelderly subjects. Analysis showed a statistically significant 1.5- to 2.1-fold increase in AUC0–12 h and Cmax values of elderly subjects (capsule) compared with nonelderly subjects (solution) on day 1 and day 7/11 at the 200-mg dose (Table 1). However, because of the different PQ912 formulations administered at this dose, it cannot be excluded that differences in formulation contributed to the observed increase in exposure.
3.2.3. Comparison of PQ912 concentrations in CSF and plasma
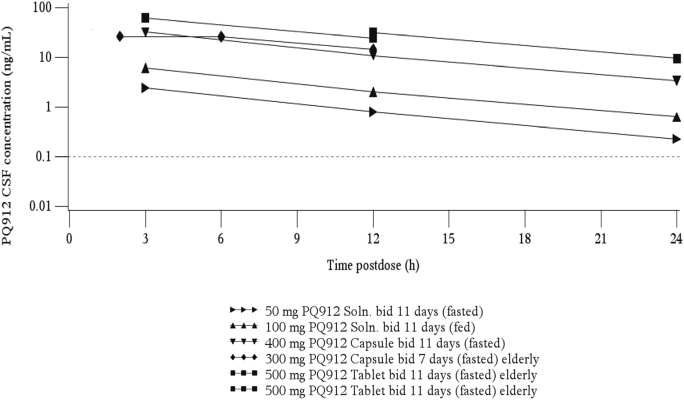
After single oral solution administration, the highest concentrations of PQ912 in the CSF were observed after 3 hours (CSF sampling times: 1, 1.5, and 3 hours; data not shown). In addition, concentrations after repeated dosing (CSF sampling times: 2, 3, 6, 12, and 24 hours after final dose on day 7/11) indicated that peak CSF concentrations were reached at between 2 and 3 hours (Supplementary Fig. 2 and Supplementary Table 3). Thus, Cmax in the CSF was reached considerably later than in plasma (tmax: 0.5–1 hours; Table 1). In contrast to plasma, concentrations in CSF showed a monoexponential decline (Fig. 1C), with a t1/2 between 6 and 7 hours (Table 2). This was similar to the terminal plasma t1/2 (doses ≥200-mg bid), but much longer than the t1/2,eff of 2–3 hours in plasma (Fig. 1B and Table 1). Thus, after repeated bid dosing (≥50 mg), trough values (12 hours postdose) in plasma were up to 61-fold lower than peak levels, but only approximately threefold lower in CSF (plasma trough values for the 20-mg bid group were generally below the limit of quantification).
Supplementary Fig.2.
Arithmetic mean CSF concentrations of PQ912 following multiple oral doses of PQ912 in healthy subjects. Lower limit of quantification (- - -) was 0.1 ng/mL. Abbreviations: bid = twice daily (dose interval of 12 h); Soln. = solution.
Table 2.
Estimation of QC target inhibition in the CSF using plasma AUC
| Multiple dose (mg) | AUC(0–12 h) day 7/11 (ng.h/mL) |
CSF t1/2 (h) | CSF concentration (ng/mL) |
CSF: QC inhibition (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (AUC/12 h) | Max (C 3 h) | Min (C 12 h) | Mean | Max | Min | ||||||
| Plasma | CSF | CSF/AUC (%) | |||||||||
| Nonelderly healthy subjects | 20 (n = 8) | 120 (32.5) | 6 | 5 | 0.5 | 0.8 | 0.3 | 5 | 8 | 3 | |
| 50 (n = 8) | 413 (37.3) | 17.3 (34.8) | 4.19 (13.0) | 6.21 (7.9) | 1.7 | 2.32 (34.5) | 0.75 (40.4) | 15 | 19 | 7 | |
| 100 (n = 8; fed) | 969 (26.5) | 44.7 (27.2) | 4.65 (12.1) | 6.38 (6.9) | 4.0 | 6.02 (27.4) | 1.92 (29.6) | 29 | 38 | 16 | |
| 200 (n = 8) | 1833 (35.1) | 91.7 | 5 | 7.6 | 12.2 | 4.0 | 44 | 56 | 29 | ||
| 400 (n = 8) capsule | 5946 (16.1) | 236 (25.0) | 3.97 (12.6) | 6.45 (9.7) | 24.8 | 31.4 (23.3) | 10.5 (33.0) | 72 | 76 | 52 | |
| 500 (n = 8; fed) capsule | 9302 (63.0) | 465 | 5 | 38.8 | 62.0 | 20.5 | 80 | 86 | 68 | ||
| Elderly healthy subjects | 200 (n = 6) capsule | 3891 (35.1) | 195 | 5 | 16.2 | 25.9 | 8.6 | 62 | 73 | 47 | |
| 300 (n = 8) capsule | 5658 (48.1) | 275 (40.7) | 4.86 (11.8) | 6.22 (42.6) | 23.6 | 37.7 | 12.5 | 71 | 79 | 56 | |
| 500 (n = 12) tablet | 11,851 (39.4) | 530 (36.6) | 5.27 (9.7) | 6.91 (5.2) | 49.4 | 79.0 | 26.2 | 84 | 89 | 73 | |
| 800 (n = 6) tablet | 24,549 (46.0) | 1230 | 5 | 102 | 163.7 | 54.2 | 91 | 94 | 85 | ||
Abbreviations: QC, glutaminyl cyclase; CSF, cerebrospinal fluid; AUC, area under the concentration versus time curve; AUC0–12 h, area under the concentration versus time curve from time 0 h to the available and quantifiable sampling point closest to 12 h after dosing; t1/2, apparent terminal half-life; n, number of subjects; CV, coefficient of variation.
NOTE. Doses administered as solutions under fasted conditions unless stated otherwise. Multiple doses administered twice daily (dose interval of 12 h). Geometric mean (CV%) data are presented and calculated/estimated values are presented in bold. Assumptions of model: AUC in CSF is 5% of that in plasma (CSF AUC = plasma AUC × 0.05); CSF mean concentrations defined as CSF AUC0–12h/12 h. CSF 3 h (max) and 12 h (min) concentrations were estimated by multiplying mean concentrations by 1.6 and 0.53, respectively. CSF target inhibition: Inhibition was calculated as %inhibition = 100×C/(Ki + C), where Ki = 29 nM and C is the concentration of PQ912 in the CSF.
Concentrations of PQ912 in CSF were lower than those in plasma, with the AUC approximately 4%–5% of the plasma AUC for total PQ912 (Table 2) and about 20% when compared with the AUC of the unbound fraction of PQ912 (0.22).
The CSF/plasma AUC ratios were found to be independent of dose, and when compared with the AUCs, these ratios showed only small interindividual differences (Table 2). Thus, if unknown, the AUC in CSF can be estimated from plasma AUC with reasonable accuracy.
It should be noted that the number of CSF samples per subject was limited; thus, calculation of AUC was based on three time points only (3, 12, and 24 hours postdose; Fig. 1C and Table 2). For simplicity, it was assumed that the 3-hour value represented the maximum concentration; however, concentrations of samples taken at earlier time points in single-dose groups suggested that maximum levels were reached earlier, between 2 and 3 hours. Because of this, the value for the AUC in CSF was considered as a low estimate, with its “true” value likely to be 10%–15% higher (Table 2).
3.3. QC activity in serum and CSF at predose and after PQ912 administration
To study the effect of PQ912 on the target enzyme, QC, its activity was determined in serum and CSF.
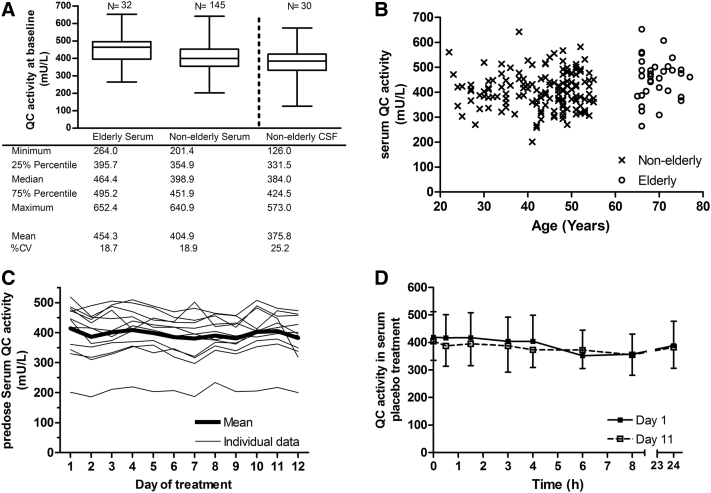
The mean baseline serum QC activity in elderly subjects was approximately 12% higher than in nonelderly subjects (Fig. 2A; unpaired t test, P = .0012). Although this difference suggests a slight increase of QC activity with age, in view of the much larger interindividual differences (Fig. 2B), no definite statement can be made in this respect.
Fig. 2.
Serum and CSF QC activity profiles. (A) Serum and CSF QC activity at baseline in elderly and nonelderly subjects. (B) Scatter plot of serum QC activity at baseline and subject age. (C) Daily mean serum QC activity premorning dose of subjects who received placebo bid for 11 days. (D) Serum QC activity on day 1 and day 11 for subjects who received placebo bid for 7 of 11 days. Abbreviations: CSF, cerebrospinal fluid; QC, glutaminyl cyclase; CV, coefficient of variation; bid, twice daily (dose interval of 12 hours).
Day-to-day variation in QC activity was minor i.e., premorning dose activities in subjects receiving placebo differed on average less than 10% over the study period of 11 days (Fig. 2C). Minor within-day differences were observed, with the activity at 8 hours after first dose on day 1 and day 11 on average 13% lower (P < .01) than the respective predose value (Fig. 2D).
The mean baseline activity of QC in CSF was 376 mU/L and, thus, similar to that observed in serum with a CSF/serum ratio of 0.94 (n = 30; 95% CI, 0.84–1.03; Fig. 2A).
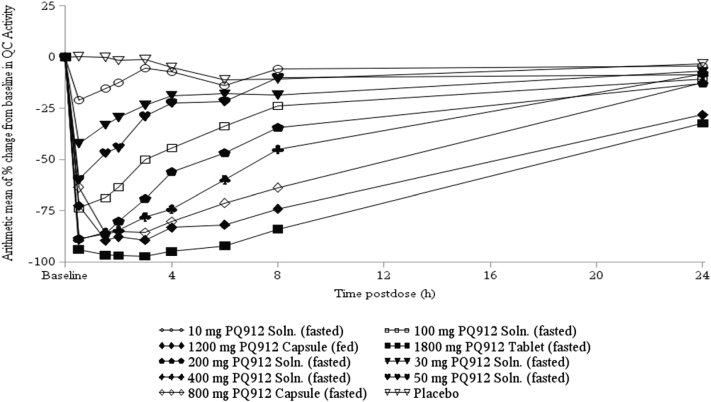
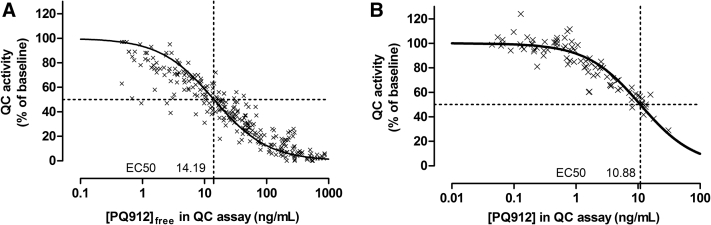
After single and multiple doses of PQ912, QC activity in serum and CSF decreased in a dose-related manner (Fig. 3). For an estimate of half maximal effective concentration (EC50) in serum and CSF, changes in QC activity were plotted versus PQ912 concentrations and fitted to an Emax model (Fig. 4A and B, respectively). This model provided a good description of the data, with the exception of a cluster of serum data points at low PQ912 concentrations that showed higher than predicted inhibition. We believe that this may be due to diurnal fluctuations of QC activity, which were also observed in placebo-treated subjects where QC activity was approximately 13% lower at 6 and 8 hours compared with predose values (Fig. 2D). For low inhibitor concentrations (low doses and late time points), this would, therefore, result in the observed stronger decrease in QC activity. This model provided EC50 values of 14 and 11 ng/mL (42 and 32 nM, respectively) for serum and CSF, respectively, and thus was in good agreement with the Ki value of 29 nM determined in vitro, in matrix-free solutions.
Fig. 3.
Arithmetic mean percentage changes from baseline in serum QC activity after single oral doses of PQ912 in healthy nonelderly subjects. Abbreviations: QC, glutaminyl cyclase; Soln., solution.
Fig. 4.
The pharmacokinetic/pharmacodynamic relationship between PQ912 concentration and QC inhibition in serum and CSF. (A) Correlation of PQ912 free plasma concentrations versus serum QC inhibition. (B) Correlation of PQ912 CSF concentration versus CSF QC inhibition. Abbreviations: CSF, cerebrospinal fluid; QC, glutaminyl cyclase.
The data collected within this study were used to establish a simple relationship between plasma AUC and CSF concentration, with the plasma AUC used to estimate the likely target inhibition in CSF (Table 2). The assumptions for this model were as follows: (1) The AUC in CSF is 5% of that in plasma. This value was somewhat higher than the overall mean value of 4.6% derived from experimental data, but for reasons outlined in Section 3.2.3, this is believed to be justified; (2) CSF concentrations declined in a monoexponential fashion with a t1/2 of approximately 6 hours. From this it can be derived that CSF concentrations after 3 and 12 hours are approximately 1.6- and 0.53-fold the mean concentration, respectively; (3) QC inhibition by PQ912 was assumed to follow a simple binding model using a Ki of 29 nM determined in vitro. This Ki value was in good agreement with the experimental EC50 value of 32 nM (Fig. 4B).
Thus, we believe that concentrations of PQ912 and the target inhibition in CSF can be estimated with reasonable accuracy from the plasma AUC without the need of CSF sampling. According to the estimates presented in Table 2, the mean target inhibition varied from 5% to 91%, across the dose range. As predicted by the binding model, the within-dose interval target inhibition varied at low doses by almost a factor of 3 (similar to CSF concentrations), but at 800 mg, only between 85% and 94%. In contrast, the variation in plasma was much larger with steady-state PQ912 peak levels being up to 61-fold higher than that of trough concentrations.
3.4. Safety and tolerability
There were no apparent trends in the number of subjects reporting TEAEs across the doses of PQ912 administered (Table 3). After single doses of PQ912, the incidence of drug-related TEAEs was low with 25 events reported by 16 of 104 subjects, and after multiple doses, 113 drug-related TEAEs were reported by 47 of 102 subjects.
Table 3.
Treatment-emergent adverse events after single and multiple oral administration of PQ912
| Single dose of PQ912 or placebo (mg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled placebo (n = 21) | 10 solution (n = 6) | 30 solution (n = 6) | 50 solution (n = 6) | 100 solution (n = 8) | 200 solution (n = 6) | 400 solution (n = 12) | 800 capsule (n = 6) | 1200 (fed) capsule (n = 6) | 1800 tablet (n = 6) | 3600∗ tablet (n = 6) | |
| Subjects with AEs | 8 (38.1) | — | 2 (33.3) | 5 (83.3) | 3 (37.5) | — | 4 (33.3) | 1 (16.7) | 1 (16.7) | 2 (33.3) | — |
| Number of AEs | 12 | — | 2 | 11 | 7 | — | 7 | 2 | 2 | 2 | — |
| Most frequent TEAEs (≥2 subjects) | |||||||||||
| Nausea | — | — | — | 1 [1] | — | — | — | 1 [1] | — | 2 [2] | — |
| Vomiting | — | — | — | 1 [2] | — | — | — | 1 [1] | — | — | — |
| Headache | 4 [5] | — | — | 2 [3] | — | — | 1 [1] | — | 1 [1] | — | — |
| Back pain | — | — | 1 [1] | 1 [1] | 3 [3] | — | 2 [2] | — | — | — | — |
| Fatigue | — | — | — | 1 [1] | 1 [1] | — | — | — | — | — | — |
| Presyncope | 1 [1] | — | — | — | — | — | 1 [1] | — | — | — | — |
| Injection site pain | — | — | — | 1 [1] | — | — | 1 [1] | — | — | — | — |
| Nasopharyngitis | 2 [2] | — | — | — | — | — | — | — | — | — | — |
| Multiple twice-daily doses (dose interval of 12 h) of PQ912 or placebo (mg) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonelderly healthy subjects |
Elderly healthy subjects |
|||||||||||
| Pooled placebo (n = 12) | 20 solution (n = 8) | 50 solution (n = 8) | 100 (fed) solution (n = 8) | 200 solution (n = 8) | 400 capsule (n = 8) | 500 (fed) capsule (n = 8) | Pooled placebo (n = 10) | 200† capsule (n = 6) | 300† capsule (n = 8) | 500 tablet (n = 12) | 800 tablet (n = 6) | |
| Subjects with AEs | 6 (50.0) | 7 (87.5) | 7 (87.5) | 8 (100.0) | 6 (75.0) | 5 (62.5) | 8 (100.0) | 6 (60.0) | 3 (50.0) | 6 (75.0) | 9 (75.0) | 6 (100) |
| Number of AEs | 19 | 16 | 22 | 16 | 11 | 9 | 30‡ | 11 | 3 | 13 | 32 | 34 |
| Most frequent TEAEs (≥3 subjects) | ||||||||||||
| Nausea | 1 [1] | — | 1 [1] | — | 1 [1] | — | 1 [1] | — | — | — | 3 [4] | 2 [2] |
| Flatulence | 1 [1] | — | — | — | 1 [1] | — | — | 1 [1] | — | — | — | 3 [3] |
| Constipation | — | — | — | — | — | — | — | 1 [1] | — | — | 2 [2] | 2 [2] |
| Abdominal pain | — | 1 [1] | 1 [1] | — | 1 [1] | 1 [1] | 1 [1] | — | — | — | 1 [1] | — |
| Diarrhea | — | — | — | — | 1 [1] | — | 1 [1] | — | — | — | 1 [1] | 1 [1] |
| Headache | 3 [4] | 3 [7] | 4 [8] | 3 [4] | — | 3 [4] | 3 [3] | 1 [1] | — | 4 [4] | 2 [4] | 2 [2] |
| Back pain | 1 [1] | — | — | 1 [1] | 1 [1] | 1 [1] | 4 [5] | 2 [2] | — | 1 [1] | 1 [1] | — |
| Pain in extremity | 1 [1] | — | — | — | — | — | 2 [2] | — | — | 1 [2] | — | — |
| Neck pain | 1 [1] | 1 [1] | — | — | 1 [1] | — | — | — | — | — | — | 1 [1] |
| Abnormal sensation in eye | 1 [1] | — | — | — | — | — | 2 [2] | — | — | — | — | — |
| Pollakiuria | — | — | — | — | — | — | — | 1 [1] | — | — | — | 2 [2] |
| Presyncope | — | — | — | 6 [6] | — | 1 [1] | — | — | — | — | — | — |
| Dizziness | 1 [1] | — | 1 [1] | — | — | — | 2 [2] | — | — | — | 1 [2] | — |
| Injection site pain | — | — | 5 [6] | 3 [3] | — | — | — | 2 [2] | — | 4 [4] | 1 [1] | — |
| Chest pain | — | — | 1 [1] | 1 [1] | 1 [1] | — | — | — | — | — | — | 1 [2] |
| Contusion | — | — | — | — | — | — | — | — | — | — | 2 [3] | 1 [1] |
Abbreviations: n, number of subjects; AEs, adverse events; TEAEs, treatment-emergent adverse events.
NOTE. Pooled placebo includes all placebo subjects for the dose cohorts presented. All active doses are fasted unless stated otherwise. Values in [ ] refer to the number of TEAEs, and values in ( ) refer to the percentage of total subjects.
Administered as two doses of 1800 mg 12 h apart.
Dosed for 7 d.
One subject was discontinued from the study because of a TEAE.
Most TEAEs reported after PQ912 administration were mild or moderate in severity and resolved without treatment and there were no serious adverse events reported during the study. Three severe adverse events were reported; two events of presyncope (placebo solution, fasted and 100-mg bid solution, fed; neither were considered to be related to study drug) and an event of pyrexia (500-mg bid solution, fed). The event of pyrexia was reported by the subject who was withdrawn from the study due to a TEAE of urticaria papular (Section 3.1).
The most frequently reported type of TEAE across all dose cohorts were gastrointestinal disorders. The incidence remained similar across all nonelderly dose cohorts, but at the two highest dose levels in elderly subjects incidence was slightly higher. In addition, the most frequently reported individual TEAE was headache, although the incidence was not dose dependent (Table 3).
There were no safety concerns for any of the clinical laboratory parameters assessed; although, four subjects experienced changes in clinical laboratory parameters, which were considered to be clinically significant, with increases observed in serum potassium (one subject; 50-mg solution, fed), erythrocytes (one subject; 500-mg bid capsule fed), and leukocytes (two subjects; 200-mg bid capsule, fasted, elderly and 500-mg bid capsule, fed).
There were no clinically significant findings in the safety assessments of vital signs, 12-lead ECGs, telemetry, or physical examinations during the study.
4. Discussion
PQ912 is a first-in-class compound addressing the novel target QC, which appears to be very relevant in the Aβ pathology of AD [10]. This present study was a first-in-man single- and multiple-ascending oral dose study, which assessed the safety, PK, and PD of PQ912.
Eighty-three subjects were exposed to single doses of PQ912 up to 3600 mg (within a period), and 80 subjects were exposed to multiple bid doses of PQ912 up to 800 mg. Overall, PQ912 was considered to be safe and well tolerated across all doses, with most adverse events mild or moderate in severity. The most frequently reported group of TEAEs were classified as gastrointestinal disorders, and the most frequently reported TEAE was headache. No correlations were noted between the frequency and severity of TEAEs and the dose level, age, sex, or dietary state, except at the higher doses (500- and 800-mg bid) where a slightly higher incidence of gastrointestinal TEAEs was reported in elderly subjects. The reason for this is not known at present; however, the higher number of tablets taken, the higher systemic or local concentration of the drug, and/or the total mass of the drug released in the gastrointestinal tract may have contributed to this observation.
In addition to being safe and well tolerated, this study has demonstrated that oral administration of various formulations provides dose-proportional or supraproportional exposure of PQ912 in both plasma and CSF. At the 200-mg dose, an increase in exposure was also apparent in the elderly compared with the nonelderly population. Although differences in formulation may have contributed to this increase, it could indicate a decrease in clearance with age, possibly due to a reduced liver blood flow in elderly or due to an age-related decrease in the capacity to metabolize this drug. Overall, absorption was rapid, with tmax observed generally 0.5–1.5 hours postdose. The t1/2,eff of PQ912 in plasma was 2–3 hours, but approximately 6 hours for CSF (t1/2). Thus, after repeated dosing, trough concentrations were up to 61-fold lower than peak levels in plasma and approximately threefold lower in CSF. The finding that AUC in CSF was only approximately 20% of the AUC in plasma for unbound PQ912 suggested some active efflux, which according to in vitro studies could have been P-glycoprotein mediated (unpublished data).
Due to the longer t1/2 in the clinically relevant CSF compartment, a relatively constant target inhibition of QC can be achieved at the higher bid doses. The CSF QC target inhibition was estimated to be approximately 70% after bid dosing of 400 mg in nonelderly subjects, and 90% at 800-mg bid in elderly subjects (Table 2), with only small within-dose interval fluctuations.
Information on in vivo QC activity in plasma and CSF is limited, with Gontsarova et al. [28] being the first to publish evidence of QC activity in human CSF. To our knowledge, the present study was the first to quantify QC activity in human plasma and CSF. The QC activity profile showed minimal change with age and no or little within-day or day-to-day fluctuations. This is an important preparatory data set for further evaluating QC activity in AD patients, as currently only QC messenger RNA (mRNA) expression and protein levels have been shown to be increased in the blood and brain of early AD patients [14], [16], [29]. In these studies, levels of QC mRNA and protein levels correlate with an accumulation of pE-Aβ in human brains and with cognitive decline, and thus, in future patient studies we will further evaluate QC activity in plasma and CSF and pE-Aβ levels in CSF to learn about their potential as stratification and/or therapeutic biomarkers.
The potential of QC inhibition for the treatment of AD and thus, the potential of PQ912, has been demonstrated by a number of preclinical studies. Using AD mouse models, the potent cytotoxicity of pE-Aβ peptides and the potential role that these peptides play in the initial stages of AD pathology have been demonstrated [30]. In addition, other recent studies have demonstrated the effect of QC inhibition in reducing pE-Aβ peptides and total Aβ levels in similar AD mouse models, with long-term administration resulting in decreased soluble pE-Aβ and plaque density in the brain and significantly improved memory and cognition [10], [14], [25].
To develop an active central nervous system drug for treatment of chronic diseases such as AD, characterization of its brain penetration and target inhibition combined with its safety and tolerability is important for further clinical development. Therefore, emphasis was placed on estimating target engagement by means of a PK/PD correlation in CSF, as a measure of brain exposure [31].
Overall, the study established a PK/PD correlation between plasma and CSF, which allows estimation of the target inhibition in CSF from plasma AUC without the need for CSF sampling. The results of this study suggest that clinical doses that provide 70%–90% QC inhibition are well tolerated and, therefore, justify the further evaluation of PQ912 in patients.
Research in context.
-
1.
Systematic review: The authors reviewed relevant literature using traditional sources and meeting abstracts/presentations, which are cited appropriately.
-
2.
Interpretation: This was the first time that a compound targeting glutaminyl cyclase (QC) enzyme inhibition had been assessed in a clinical setting for the inhibition of pyroglutamate-amyloid-β (pE-Aβ) formation and, thus, potentially the treatment of Alzheimer's disease (AD). Our results show that PQ912 was safe and well tolerated in healthy nonelderly and elderly subjects, and that it had dose-dependent inhibition of QC in blood and cerebrospinal fluid (CSF).
-
3.
Future directions: A major objective of a phase 2 study will be to evaluate the safety of PQ912 in the patient population and to explore the hypotheses that inhibition of QC interferes with Aβ pathology and leads to cognitive improvement. In addition, we will further evaluate QC activity and pE-Aβ levels in CSF to learn about their potential as AD biomarkers and how PQ912 can be of benefit in modifying these molecules.
Acknowledgments
The authors acknowledge the investigators and subjects who participated in this study as well as the following individuals:
The authors thank all people of Probiodrug Halle, Covance Basel, and Covance Leeds who were involved in the organization and execution of this study and contributed to its successful completion. Furthermore, the authors recognize Mark Enzler and his team at Swiss BioQuant in Basel who were responsible for the compound analytics. The authors especially thank Livia Böhme and her team at Probiodrug and Florian Krieger and his coworkers at Evotec Hamburg for the measurement of QC activity.
This study was funded by Probiodrug.
Footnotes
The authors declared a potential conflict of interest (e.g., a financial relationship with the commercial organizations or products discussed in this article) as follows: I.L., A.M., F.W., K.K.-W., U.H., T.H., and K.G. are employees of and serve as authors for Probiodrug. U.B. serves as a pharmacokinetic advisor to Probiodrug. I.L. and K.G. also hold stock/shares in Probiodrug. S.M. is no longer an employee of Probiodrug. J.C. is an employee of Covance Ltd. R.P. was a former employee of Covance Ltd.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2015.08.002.
Appendix
Supplementary Fig.3.
Arithmetic mean plasma concentrations of PQ1345 on Day 1 following single oral administration of PQ912. Lower limit of quantification (- - -) was 1.00 ng/mL. Abbreviations: Soln. = solution.
Supplementary data
References
- 1.Black R., Lues I., Weber F., Meyer A., Hoffman T., Pokorny R. Safety, pharmacokinetics and pharmacodynamics of PQ912, the first glutaminyl cyclase (QC) inhibitor to treat Alzheimer's disease, in healthy elderly subjects. Alzheimers Dement. 2013;9(supplement):280. [Google Scholar]
- 2.Weber F, Lues I, Meyer A, Hoffmann T, Pokorny R, Lopez L, et al. A phase 1 study assessing safety, pharmacokinetics and pharmacodynamics of PQ912, the first glutaminyl cyclase (QC) inhibitor to treat AD. International conference on Alzheimer's and Parkinson's disease 2013; abstract 1453.
- 3.Cummings J., Reynders R., Zhong K. Globalization of Alzheimer's disease clinical trials. Alzheimers Res Ther. 2011;3:24. doi: 10.1186/alzrt86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghezzi L., Scarpini E., Galimberti D. Disease-modifying drugs in Alzheimer's disease. Drug Des Devel Ther. 2013;7:1471–1478. doi: 10.2147/DDDT.S41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy J.A., Higgins G.A. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 7.Liu K., Solano I., Mann D., Lemere C., Mercken M., Trojanowski J. Characterization of Aß11-40/42 peptide deposition in Alzheimer's disease and young Down's syndrome brains: Implication of the N-terminally truncated Aβ species in the pathogenesis of Alzheimer's disease. Acta Neuropathol. 2006;112:163–174. doi: 10.1007/s00401-006-0077-5. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsubo T., M Mann D., Odaka A., Suzuki N., Ihara Y. Amyloid beta protein (A beta) deposition: A beta 42(43) precedes A beta 40 in Down syndrome. Ann Neurol. 1995;37:294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- 9.Schilling S., Hoffmann T., Manhart S., Hoffmann M., Demuth H.-U. Glutaminyl cyclases unfold glutamyl cyclase activity under mild acid conditions. FEBS Lett. 2004;563:191–196. doi: 10.1016/S0014-5793(04)00300-X. [DOI] [PubMed] [Google Scholar]
- 10.Schilling S., Zeitschel U., Hoffmann T., Heiser U., Francke M., Kehlen A. Glutaminyl cyclase inhibition attenuates pyroglutamate Aß and Alzheimer's disease–like pathology. Nat Med. 2008;14:1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- 11.Stephan A., Wermann M., von Bohlen A., Koch B., Cynis H., Demuth H.-U. Mammalian glutaminyl cyclases and their isoenzymes have identical enzymatic characteristics. FEBS J. 2009;276:6522–6536. doi: 10.1111/j.1742-4658.2009.07337.x. [DOI] [PubMed] [Google Scholar]
- 12.Wirths O., Bethge T., Marcello A., Harmeier A., Jawhar S., Lucassen P.J. Pyroglutamate Abeta pathology in APP/PS1KI mice, sporadic and familial Alzheimer's disease cases. J Neural Transm. 2010;117:85–96. doi: 10.1007/s00702-009-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morawski M., Hartlage-Rübsamen M., Jäger C., Waniek A., Schilling S., Schwab C. Distinct glutaminyl cyclase expression in Edinger–Westphal nucleus, locus coeruleus and nucleus basalis Meynert contributes to pGlu-Aß pathology in Alzheimer's disease. Acta Neuropathol. 2010;120:195–207. doi: 10.1007/s00401-010-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morawski M., Schilling S., Kreuzberger M., Waniek A., Jager C., Koch B. Glutaminyl cyclase in human cortex: Correlation with (pGlu)-amyloid-β load and cognitive decline in Alzheimer's disease. J Alzheimers Dis. 2014;39:385–400. doi: 10.3233/JAD-131535. [DOI] [PubMed] [Google Scholar]
- 15.Gunn A.P., Masters C.L., Cherny R.A. Pyroglutamate-Aß: Role in the natural history of Alzheimer's disease. Int J Biochem Cell Biol. 2010;42:1915–1918. doi: 10.1016/j.biocel.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 16.De Kimpe L., Bennis A., Zwart R., van Haastert E.S., Hoozemans J.J., Scheper W. Disturbed Ca2+ homeostasis increases glutaminyl cyclase expression; connecting two early pathogenic events in Alzheimer's disease in vitro. PLoS One. 2012;7:9. doi: 10.1371/journal.pone.0044674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo C., Violani E., Salis S., Venezia V., Dolcini V., Damonte G. Pyroglutamate-modified amyloid beta-peptides-AbetaN3(pE)-strongly affect cultured neuron and astrocyte survival. J Neurochem. 2002;82:1480–1489. doi: 10.1046/j.1471-4159.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 18.Schlenzig D., Rönicke R., Cynis H., Ludwig H.-H., Scheel E., Reymann K. N-Terminal pyroglutamate formation of Aß38 and Aß40 enforces oligomer formation and potency to disrupt hippocampal long-term potentiation. J Neurochem. 2012;121:774–784. doi: 10.1111/j.1471-4159.2012.07707.x. [DOI] [PubMed] [Google Scholar]
- 19.Saido T.C. Alzheimer's disease as proteolytic disorders: Anabolism and catabolism of beta-amyloid. Neurobiol Aging. 1998;19:S69–75. doi: 10.1016/s0197-4580(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 20.D'Arrigo C., Tabaton M., Perico A. N-terminal truncated pyroglutamyl amyloid peptide Aβpy3-42 shows a faster aggregation kinetics than the full-length Aβ1-42. Biopolymers. 2009;91:861–873. doi: 10.1002/bip.21271. [DOI] [PubMed] [Google Scholar]
- 21.Matos J.O., Goldblatt G., Jeon J., Chen B., Tatulian S.A. Pyroglutamylated amyloid-β peptide reverses cross β–sheets by a prion-like mechanism. J Phys Chem B. 2014;118:5637–5643. doi: 10.1021/jp412743s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun N., Hartmann R., Lecher J., Stoldt M., Funke S.A., Gremer L. Structural analysis of the pyroglutamate-modified isoform of the Alzheimer's disease-related amyloid-β using NMR spectroscopy. J Pept Sci. 2012;18:691–695. doi: 10.1002/psc.2456. [DOI] [PubMed] [Google Scholar]
- 23.Viola K.L., Klein W.L. Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jawhar S., Wirths O., Schilling S., Graubner S., Demuth H.-U., Bayer T.A. Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate Aβ formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J Biol Chem. 2011;286:4454–4460. doi: 10.1074/jbc.M110.185819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H., J Chang Y., Moon M., Park S.K., Tran P.-T., Hoang V.-H. Inhibition of glutaminyl cyclase ameliorates amyloid pathology in an animal model of Alzheimer's disease via the modulation of γ-secretase activity. J Alzheimers Dis. 2015;43:797–807. doi: 10.3233/JAD-141356. [DOI] [PubMed] [Google Scholar]
- 26.Huang K.F., Liu Y.L., Cheng W.J., Ko T.P., Wang A.H. Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. Proc Natl Acad Sci U S A. 2005;102:13117–13122. doi: 10.1073/pnas.0504184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schilling S., Hoffmann T., Wermann M., Heiser U., Wasternack C., Demuth H.-U. Continuous spectrometric assays for glutaminyl cyclase activity. Anal Biochem. 2002;303:49–56. doi: 10.1006/abio.2001.5560. [DOI] [PubMed] [Google Scholar]
- 28.Gontsarova A., Kaufmann E., Tumani H., Dressel A., Mandel F., Wiesmüller K.H. Glutaminyl cyclase activity is a characteristic feature of human cerebrospinal fluid. Clin Chim Acta. 2008;389:152–159. doi: 10.1016/j.cca.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 29.T Valenti M., Bolognin S., Zanatta C., Donatelli L., Innamorati G., Pampanin M. Increased glutaminyl cyclase expression in peripheral blood of Alzheimer disease patients. J Alzheimers Dis. 2013;34:263–271. doi: 10.3233/JAD-120517. [DOI] [PubMed] [Google Scholar]
- 30.M Nussbaum J., Schilling S., Cynis H., Silva A., Swanson E., Wangsanut T. Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-ß. Nature. 2012;485:651–655. doi: 10.1038/nature11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J.H. CSF as a surrogate for assessing CNS exposure: An industrial perspective. Curr Drug Metab. 2008;9:46–59. doi: 10.2174/138920008783331077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.