Abstract
Background
The oxidative stress environment of pathological tissue has an adverse effect on the survival of bone marrow mesenchymal stem cells (BMSCs) transplantation. Ginkgo biloba L. extract (EGB) has a potent antioxidant effect. In this research, we assessed the protective effects of EGB and EGB-Containing Serum (EGB CS) on BMSCs against injury induced by hydrogen peroxide (H2O2).
Material/Methods
BMSCs were pretreated with EGB or EGB CS and treated with H2O2. The cell counting kit-8 (CCK-8) method was utilized to detect cell viability. The DCFH-DA Fluorescent Kit method was used to detect intracellular ROS level. Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and (CAT) were determined. The Hoechst staining assay and qRT-PCR assay were utilized to evaluate the effect of EGB on cell apoptosis. Mitogen-activated protein kinases (MAPKs) signaling pathway were detected by western blot analysis.
Results
Compared to the H2O2 group, the number of apoptotic cells in the EGB and EGB CS pretreated groups significantly decreased. The mRNA expression ratio of Bax/Bcl-2 was also decreased. EGB and EGB CS can reduce the production of ROS in BMSCs exposed to H2O2. SOD, GSH-Px and CAT activities were significantly higher compared with those with H2O2 group. Furthermore, EGB or EGB CS pretreatment decreased the protein levels of p-p38MAPK and p-JNK in BMSCs compared to the H2O2 group.
Conclusions
Our findings suggested that EGB and EGB CS have protective effect on BMSCs against oxidative stress injury and increase the survival rate of BMSCs transplantation by regulating p38MAPK and JNK signaling.
MeSH Keywords: Apoptosis, Ginkgo biloba, MAP Kinase Signaling System, Mesenchymal Stromal Cells, Oxidative Stress
Background
Bone marrow mesenchymal stem cells (BMSCs) are pluripotent stem cells that present therapeutic potential in regenerative medicine owing to their ability to self-duplicate, self-renew, proliferate, and differentiate into multilineage differentiation into bone, cartilage, muscle, endothelial cells, and islet-like cells, homing to diseased tissue and repairing it [1]. Bone marrow mesenchymal stem cells (BMSCs) was investigated as a method of regenerating these tissues for OA therapy [2]. Transplantation of BMSCs was a reported to be a safe and effective treatment for T2DM in a macaque and rat model. The transplanted BMSCs also improved numerous bodily and cellular functions and repaired damaged tissue [3]. However, oxidative stress plays a vital role in osteoarthritis, diabetes, and other diseases. The study found a trend towards a positive correlation between radiographic severity of osteoarthritis and insulin resistance, and the results indicate that osteoarthritis might be correlated with impaired glucose metabolism. The study shows the importance of oxidative stress and metabolic disturbances in the pathogenesis of osteoarthritis [4]. The features of regulation of free radical oxidation in the blood and the synovial fluid have been studied in patients with knee osteoarthritis [5]. Over 90% of diabetic patients are classified as type 2 diabetes cases (T2DM), and one of the primary factors underlying the etiology of T2DM is insulin resistance caused by oxidative stress [6]. The persistent oxidative stress and inflammation environment in the lesion area is adverse to the survival of BMSCs, and over 80% of the transplanted cells die within the first 24 h after transplantation [7,8]. Therefore, promoting the survival of the transplanted BMSCs is a critical step to improve the treatment of osteoarthritis and diabetes.
Ginkgo biloba L. extract (EGB) is a traditional herbal medicine that has been shown to remove free radicals and has been used in neurology to improve circulation and blood supply [9,10]. EGB has antioxidant properties, and can reduce the hydrogen peroxide level in cerebellar neurons and protect cultured cortical neurons from iron-induced injury [11]. Previous studies have emphasized that Ginkgo biloba extract reduced generation of high-glucose-induced endothelial reactive oxygen species by regulating Akt/eNOS and p38 MAP kinase pathways [12].
It has been reported that the MAPK signaling pathway plays a crucial role in cell survival and oxidative stress [13–15]. Our previous study found that EGB can protect BMSCs against oxidative stress and decrease cell death rates of BMSCs in vitro [16]. However, whether the MAPK signaling pathway is involved in this process remain unclear. In the present study, we used cultured BMSCs and hydrogen peroxide (H2O2)-induced BMSCs apoptosis, demonstrating the protect effect of EGB and determined that EGB mediated the protective effect by altering the MAPK signaling pathway.
Material and Methods
BMSC culture
This research was approved by the Ethics Committee of Jilin University 2nd hospital. The BMSCs were collected from the long bones of 5-day-old Wistar rats (Laboratory Animal Center of Jilin University, China). The rats were euthanized by exposure to CO2 and submerged in 75% ethanol for 5 min at room temperature to disinfect them. The femurs and tibias were removed from the muscles and connective tissue, and subsequently flushed with DMEM/F12 medium using a syringe. Cell suspension was centrifuged for 10 min at 1000 rpm. Then, the supernatant was removed and the cells were resuspended in DMEM/F12 medium supplemented with 10% FBS (GIBCO, USA) and adhered on a 100-ml flask at 1×106 cells/flask. The culture medium was half-refreshed every 3 days and the cells were passaged into 2 flasks when the confluence reached 70–80%.
BMSC surface marker detection
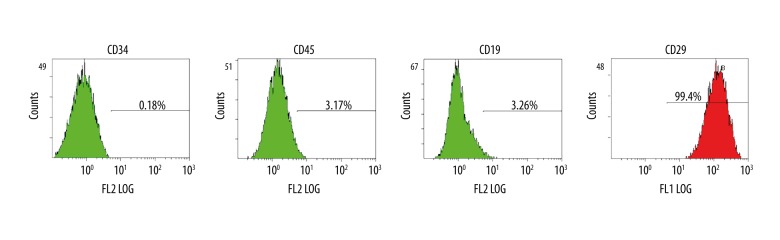
BMSCs at passage 3 were collected to analyze the expression of CD34, CD45, CD19, and CD29 by flow cytometry. About 5×104 cells were collected and incubated with mAbs against CD34, CD45, CD19, and CD29 for 30 min at room temperature, and then analyzed by flow cytometry. Flow cytometry was used to detect the expression of CD34, CD45, CD19, and CD29 of BMSCs.
Preparation of EGB-containing serum
EGB was obtained from Taiwan Chi Sheng Chemical Corp. The rat medicated serum was prepared according to the published protocols [17–19]. All procedures were approved by Jilin University Experimentation Committee and were carried out in accordance with the National Institute of Health guidelines of China for the care and use of laboratory animals. Briefly, 100 Wistar rats, aged 6–8 weeks old and weighing 200±20 g, were divided into experimental and control groups. The animals were supplied by the Laboratory Animal Center of Jilin University, China. Rats had free access to food and water and were kept in a 12/12 h light/dark cycle. Rats received an intragastric dose of EGB (12 mg/kg, which corresponds to 10 times the adult clinical dose). The control group received intragastric injections of physiological saline. At 60 min after administration, abdominal aortic blood was collected after the final drug administration, followed by centrifugation at 3500 rpm for 15 min. The serum samples were heat-inactivated at 56°C for 30 min, filtered with a 0.22-μm Millipore membrane filter, and stored at −80°C until use.
Cell treatment
The BMSCs were treated with either H2O2 at a final concentration of 400 μmmol/l, EGB (Chi Sheng Chemical Corporation, Taiwan) at final concentration of 100 μg/ml or H2O2 (final concentration 400 μmmol/l), EGB (final concentration of 100 μg/ml), EGB CS (final concentration of 10%) and EGB CS at final concentration of 10% or H2O2 (final concentration 400 μmmol/l). BMSCs without any treatment were used as control. After 12-h treatment, the cells were collected for further analyses.
Assessment of cell viability
BMSC viability was determined with the Cell Counting Kit-8 (CCK-8) assay. After treatment with EGB or EGB CS for 12 h followed by H2O2 for 12 h, we added 10 μl of CCK-8 solution to each well and incubated them at 37°C, 5% CO2 for 4 h. The absorbance at 450 nm was measured using a microplate reader (Thermo, MA, USA).
Preparation of BMSCs homogenates
The collected BMSCs were washed in PBS and lysed in lysis buffer (20 mM Tris base, 10 mM EDTA, 1% NP40, 10% glycerol, and 140 mM NaCl, pH 7.5) containing protease inhibitor (1 mM PMSF) for 30 min. Then, the homogenized cells were centrifuged at 4°C and 14 000 rpm for 15 min and the supernatants were subjected to MDA, SOD, CAT, and GSH-Px analysis.
Measurement of MDA, SOD, CAT, and GSH-Px
The MDA, SOD, CAT, and GSH-Px levels were measured using assay kits purchased from Nanjing Jiancheng Biotechnology Company (China). The MDA concentration and the SOD, CAT, and GSH-Px activity were calculated.
Measurement of oxidative stress
The levels of intracellular ROS were determined using an ROS assay kit (Beyotime Biotech, China) following the manufacturer’s protocol. The peroxide-sensitive fluorescent probe DCFH-DA was used to measure intracellular levels of ROS in H2O2-induced BMSCs. Cells were harvested and then washed twice with PBS and incubated with DCFH-DA (10 mmol/L) at 37°C for 40 min in the dark for final analysis by fluorescence microscopy.
Hoechst 33258 staining assay
A Hoechst 33258 cell apoptosis staining kit (Beyotime, Nanjing, China) was used to confirm morphological changes in the nuclei. The transfected cells were seeded onto sterile glass coverslips placed in 6-well plates and incubated for 24 h. The cells were fixed, washed twice with PBS, and stained with Hoechst 33258 staining solution for 5 min at room temperature in the dark. The cells were then washed twice with PBS, examined, and immediately photographed under a fluorescence microscope with an excitation wavelength of 350 nm. Apoptotic cells were defined by the condensation of nuclear chromatin or fragmentation to the nuclear membrane.
Quantitative real-time PCR assay
Total RNA was extracted from each group using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. DNase I was used to eliminate genomic DNA, the contaminant. The concentrations of isolated RNA samples were determined using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized using a M-MuLV First-Strand cDNA Synthesis Kit (Sangon Company, Shanghai, China) according to the manufacturer’s instructions. The total reaction volume was 20 μL, which included 1 μg of total RNA, 10 ng/μL of random primer, and RNase-free distilled water.
qRT-PCR was performed using 2X SG Fast qPCR Master Mix (Sangon Company, Shanghai, China). The qRT-PCR instrument was equipped with a Roche LightCycler 480 detection system (Roche Diagnostics, Germany) according to the manufacturer’s instructions. Gene primer sequences are as follows: Bax, sense 5′-AGACACCTGAGCTGACCTTGGAG-3′ and anti-sense 5′-GTTGAAGTTGCCATCAGCAAACA-3′. Bcl-2, sense 5′-TGAACCGGCATCTGCACAC-3′ and anti-sense 5′-CGTCTTCAGAGACAGCCAGGAG-3′. GAPDH, sense 5′-GGCACAGTCAAGGCTGAGAATG-3′ and anti-sense 5′-ATGGTGGTGAAGACGCCAGTA-3′. The PCR reaction of each sample was repeated with 3 holes. Then, the mean threshold cycle was calculated. The results of fluorescence quantitative analysis were calculated by 2-ΔΔCt, using the relative quantification (RQ) value statistics.
Western blot assay
The collected BMSCs were washed with cold PBS 3 times and then were lysed with lysis buffer containing protease and phosphatase inhibitors at 4°C, then centrifuged at 13 000 rpm for 15 min at 4°C. The protein concentration was measured using a BCA protein assay kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instructions. Equal amounts of protein (20 μg/well) were loaded and separated by 10% polyacrylamide gel containing SDS electrophoresis and transferred to polyvinylidene difluoride membranes. After being blocked with 5% BSA in Tris-buffered saline containing Tween-20 for 2 h, the following primary antibodies were used: rabbit anti-P38 MAPK (1: 1000, CST, USA), rabbit anti-Phospho-P38 MAPK(Thr180/Tyr182) (1: 1000, CST, USA), rabbit anti-JNK (1: 1000, CST, USA), rabbit anti-Phospho-JNK (1: 1000, CST, USA), rabbit anti-ERK1/2 (1: 1000, CST, USA), rabbit anti-Phospho-ERK1/2 (1: 1000, CST, USA), rabbit anti-GAPDH (1: 1000, CST, USA), and HRP-conjugated secondary antibody (1: 2000, CST, USA). Immunoreactivity signals were developed using the chemiluminescence of ECL reagent (PIERCE Chemical Co, IL, USA). The protein bands were quantitatively analyzed using a QuantityOne image densitometer.
Statistical analysis
Results are expressed as the mean ±SD from an appropriate number of experiments. One-way ANOVA followed by Bonferroni correction was used to compare the data among 3 or more groups, and the t test was also used. All statistical analyses were performed using the SPSS 17.0 software package for Windows (SPSS Inc., Chicago, IL) and a value of p<0.05 was considered significant.
Results
Cell surface marker detection
Adherent cells expressed CD29, but not the hematopoietic cell phenotype CD34, CD34, or CD19 (Figure 1). This confirmed that the adherent cells that were isolated and cultured from bone marrow were MSCs.
Figure 1.
Characterization of Rat BMSCs by flow cytometry. The majority of the cells are CD34−, CD45−, CD19−, and CD29+, which are typical characteristic phenotypes of BMSCs.
EGB or EGB CS increased BMSC viability upon H2O2 injury
To determine whether EGB plays a role in protecting BMSCs from oxidative stress-induced cell death, dissociated BMSCs were pretreated with EGB or EGB CS for 12 h followed by H2O2 treatment for 12 h, and cell viability was determined using the CCK-8 assay (Figure 2). BMSCs exposed to H2O2 had significantly lower cell viability compared with BMSCs protected by EGB or EGB CS (Figure 2).
Figure 2.
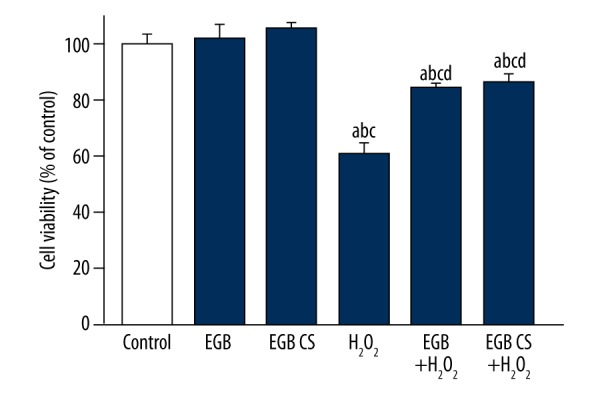
Cell viability changes exposed to H2O2. BMSCs were exposed to either H2O2 or H2O2 and EGB or H2O2 and EGB CS to investigate the protective effects of EGB or EGB CS against oxidative stress. a) p<0.05, significantly different from control; b) p<0.05, significantly different from EGB; b) p<0.05, significantly different from EGB CS; d) p<0.05, significantly different from H2O2. One-way ANOVA.
EGB or EGB CS ameliorated H2O2-induced apoptosis
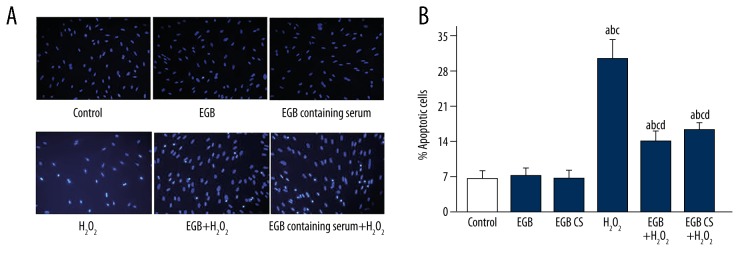
To further assess the protective effect of the EGB or EGB CS against apoptosis in H2O2-induced BMSCs, we performed Hoechst 33258 staining assay (Figure 3). Results showed that BMSCs treated with H2O2 displayed the typical changes of apoptosis. Pretreatment with the EGB or EGB CS for 12 h led to a significant decrease in the number of cells with condensed and fragmented nuclei. These results demonstrate EGB or EGB CS can decrease the development of apoptosis in BMSCs exposed to H2O2.
Figure 3.
(A, B) Cell apoptosis in BMSCs exposed to H2O2, EGB and EGB CS (×200). BMSCs were exposed to either H2O2, EGB, EGB CS, both H2O2 and EGB or both H2O2 and EGB CS. Hoechst 33258 staining assay was used to detect the cell apoptosis in BMSCs. a) p<0.05, significantly different from control; b) p<0.05, significantly different from EGB; c) p<0.05, significantly different from EGB CS; d) p<0.05, significantly different from H2O2. one-way ANOVA.
Effects of EGB or EGB CS on the mRNA levels of Bcl-2 and Bax in BMSCs
The Bcl-2 and Bax gene products were amplified by qRT-PCR and analyzed. Figure 4 shows that treatment with H2O2 elevated the ratio of Bax to Bcl-2. However, pretreatment with the EGB or EGB CS reduced the ratio of Bax to Bcl-2. These results show that EGB or EGB CS decreased the apoptosis of BMSCs caused by H2O2.
Figure 4.
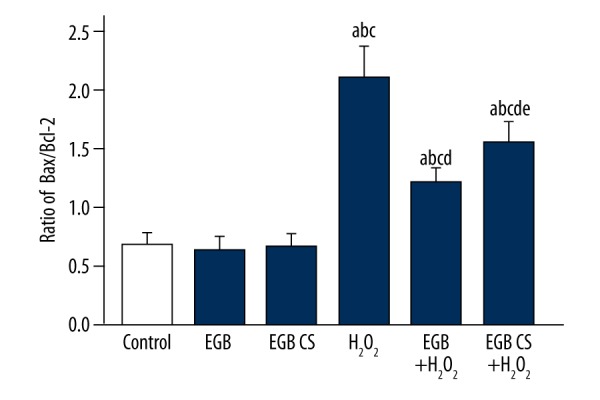
EGB or EGB CS inhibited the expression of apoptosis-related mRNA in BMSCs. The mRNA expression was detected via real-time RT-PCR. The data are reported as the mean ±SD, n=3. a) p<0.05, significantly different from control; b) p<0.05, significantly different from EGB; c) p<0.05, significantly different from EGB CS; d) p<0.05, significantly different from H2O2; e) p<0.05, significantly different from EGB + H2O2. one-way ANOVA.
Increased intracellular reactive oxygen species (ROS) levels in BMSCs exposed to H2O2
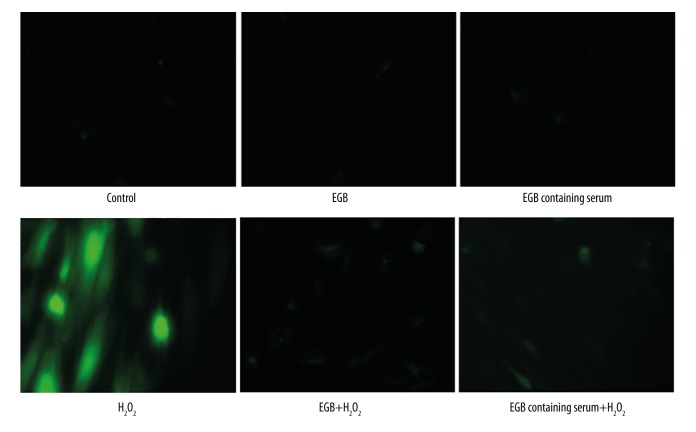
Intracellular ROS production plays important roles in the proliferation and apoptosis of various cell types. It has been reported that ROS production regulates aging, senescence, and osteogenic differentiation in BMSCs [20–22]. To further evaluate the effects of the EGB or EGB CS against ROS production in H2O2-induced BMSCs, we performed DCFH-DA staining assay. As shown in Figure 5, we found that H2O2 treatment induced ROS production in BMSCs. Pretreatment with the EGB or EGB CS for 12 h led to a significant decrease in the number of cells with ROS production. These results demonstrate EGB or EGB CS decreased the intracellular ROS generation in BMSCs exposed to H2O2.
Figure 5.
Effects of EGB or EGB CS on the generation of ROS in BMSCs exposed to H2O2.
EGB or EGB CS lowered oxidative stress in BMSCs exposed to H2O2
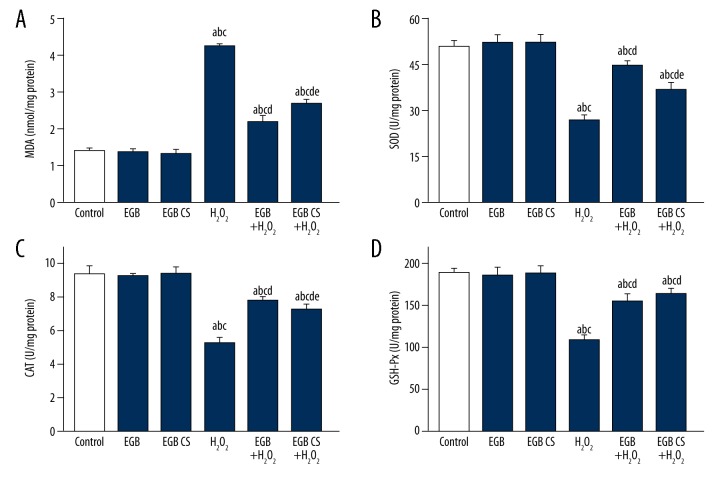
BMSCs exposed to H2O2 had significantly higher levels of MDA and lower activities of SOD, CAT, and GSH-Px compared with those pretreated with the EGB or EGB CS for 12 h (Figure 6).
Figure 6.
Oxidative stress in BMSCs exposed to H2O2 was alleviated by EGB or EGB CS. (A) MDA level changes in BMSCs treated with EGB or EGB CS; (B) SOD activities changes in BMSCs treated with either EGB or EGB CS; (C) CAT activities changes in BMSCs treated with either EGB or EGB CS. (D) GSH-Px activities changes in BMSCs treated with EGB or EGB CS. All data are presented as mean value ±SD and n=3 in each group. a) p<0.05, significantly different from control; b) p<0.05, significantly different from EGB; c) p<0.05, significantly different from EGB CS; d) p<0.05, significantly different from H2O2; e) p<0.05, significantly different from EGB + H2O2. one-way ANOVA.
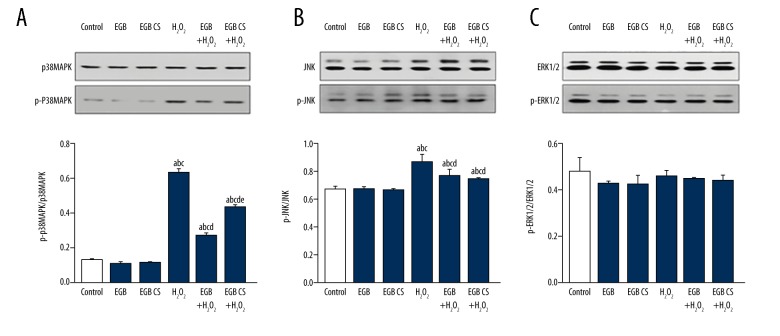
Effect of EGB or EGB CS on MAPK signaling pathways in BMSC cells
To further study the protective mechanism of EGB or EGB CS against BMSC oxidative damage, we analyzed the expression of p38MAPK, p-p38MAPK, JNK, p-JNK, ERK1/2, and p-ERK1/2 proteins by Western blot (Figure 7). The results indicated that the expressions of p-p38MAPK and p-JNK protein were significantly increased after the addition of H2O2. EGB or EGB CS pretreatment decreased the protein levels of p-p38MAPK and p-JNK in BMSCs compared to the H2O2 group. There was no significant difference in the protein expression of p38MAPK, JNK, ERK1/2, and p-ERK1/2. EGB or EGB CS-mediated protection of BMSCs from apoptosis induced by H2O2 was abolished. These results indicated that EGB or EGB CS prevented BMSC apoptosis mediated by oxidative stress through p38MAPK and JNK signaling pathways.
Figure 7.
Effects of EGB or EGB CS on MAPK signaling pathways in BMSCs. Western blot analysis of p38MAPK, p-p38MAPK, JNK, p-JNK, ERK1/2 and p-ERK1/2. Quantitative analysis of p-p38MAPK/ p38MAPK, p-JNK/JNK, and p-ERK1/2 /ERK1/2 relative level. All data are presented as mean value ±SD and n=3 in each group. a) p<0.05, significantly different from control; b) p<0.05, significantly different from EGB; c) p<0.05, significantly different from EGB CS; d) p<0.05, significantly different from H2O2; e) p<0.05, significantly different from EGB +H2O2. One-way ANOVA.
Discussion
EGB is an ideal antioxidant that has the ability to remove free radicals. In recent years, BMSC transplantation has been considered a promising treatment for diabetes [23]. After transplantation, BMSCs can settle down in the lesion area, and differentiate into islet-like cells to repair the damaged tissue and promote the recovery of islet function. It has been reported that reduction of oxidative stress can reduce cell apoptosis, increase the survival rate of transplanted cells, and delay disease progression [24]. In our previous study, we found that EGB can enhance the effectiveness of BMSCs in lowering blood glucose levels and reversing oxidative stress in diabetic rats [16]. However, EGB is a traditional herbal medicine, and it is difficult to study their pharmacological functions and mechanisms. Serum pharmacology is a scientifically feasible method that could be used to study traditional herbal medicines at the cellular and molecular levels. This method avoids the interference of nonspecific chemicals in the complex components of TCMs and reflects internal processes such as absorption, distribution, metabolism, and excretion of these components [25,26]. In the present study, we evaluated the protective effect and mechanisms of EGB or EGB CS against H2O2-induced bone marrow mesenchymal stem cell cytotoxicity.
As one of the most important ROS, hydrogen peroxide (H2O2) had been extensively used to induce oxidative stress in vitro models [27,28]. Apoptosis is triggered by changes in oxidative stress. Oxidative stress and subsequent ROS generation are 2 critical factors involved in protecting BMSCs from H2O2-induced apoptosis, and the imbalance between ROS generation and the innate ability of cells to scavenge the reactive species leads to increased oxidative stress [29,30]. ROS damage to polyunsaturated fatty acids and lead to higher levels of MDA that a major end product from membrane lipid peroxidation. H2O2 causes cell oxidative damage, mainly related to it attacking the antioxidant system [31]. The present results show that ROS production, MDA, and the total apoptosis were significantly increased, while the activities of antioxidant enzymes were significantly inhibited in BMSCs treated with H2O2. We administered EGB or EGB CS before H2O2 treatment, thereby protecting BMSCs from H2O2-induced apoptosis. We found that the nuclei of H2O2-treated BMSCs were degraded or condensed. However, a marked reduction in the number of apoptotic cells was found when BMSCs were pretreated with EGB or EGB CS for 12 h. These results confirmed that EGB or EGB CS could promote BMSCs proliferation, mainly by reducing apoptosis. Our results are similar to those mentioned in previous studies, showing that H2O2 treatment significantly decreased the activities of SOD, CAT, and GSH-Px in BMSCs, while the activity of these antioxidant enzymes was significantly increased in H2O2-induced BMSCs after EGB or EGB CS treatment. We found that multi-targeted antioxidant activity of EGB might protect tissues from oxygen radical damage.
Apoptosis is a highly regulated and intrinsic cell-suicide program in which the Bcl-2 family plays a crucial regulatory role. The Bcl-2 family is a category of cytoplasmic proteins; Bax and Bcl-2 proteins are 2 main members of this family that are functionally opposed, which promote apoptosis in various systems, depending on the Bax/Bcl-2 ratio [32]. In the present study, we demonstrated that EGB or EGB CS reduced apoptosis of BMSC treated with H2O2. Furthermore, EGB or EGB CS increased Bcl-2 and decreased Bax mRNA expressions in BMSCs, and EGB contributed to the attenuation of H2O2-induced apoptosis through regulation of the Bax/Bcl-2 ratio.
Many signaling molecules and pathways, such as NF-κB, PI3K/Akt, and MAPK, participate in oxidative stress-induced cell apoptosis [12,33]. Members of the MAPK family, one of the most important downstream signaling pathways of ROS, are generally considered to function as modulators of cell survival, proliferation, and apoptosis. The MAPK signaling pathway consists of 3 parts – ERK1/2, JNK, and p38MAPK – and is recognized to be closely related to cell apoptosis. Activation of the MAPK signaling pathway leads to enhanced oxidative damage [34]. The ERK1/2 cascade is involved in cell differentiation and proliferation, the JNK cascade modulates cell differentiation, apoptosis, and inflammation, and the p38 cascade may be involved in apoptosis and modulating the reactions of cytokines [35,36]. EGB or EGB CS-mediated protection of BMSCs from apoptosis induced by H2O2 was abolished. However, whether the protective effect of EGB or EGB CS on BMSCs is related to the MAPK signaling pathway is still unknown. Therefore, we examined the effect of EGB or EGB CS on MAPK signaling in BMSC oxidative injury. We first pretreated BMSCs with EGB or EGB CS for 12 h and then treated them with H2O2 for 12 h. The results indicated that EGB or EGB CS can reverse the decreased level of p-p38MAPK and p-JNK caused by H2O2 damage, but the protein expression of p-ERK1/2 was not significantly different.
These outcomes demonstrate that EGB or EGB CS can reduce the apoptosis of BMSCs by activating the p38MAPK/JNK signaling pathway. These findings also suggest that EGB or EGB CS may be combined with BMSCs transplantation in treatment of osteoarthritis, diabetes, and other diseases to promote the survival of transplanted BMSCs owing to its anti-apoptotic and antioxidant properties. However, the exact mechanism of this protective effect is not entirely clear, and we will assess the role of other regulatory factors involved in the p38MAPK/JNK signaling pathway in a future study.
Conclusions
Our findings suggest that EGB and EGB CS have protective effects on BMSCs against oxidative stress injury and can be used to improve the oxidative stress environment of osteoarthritis or diabetes and promote the survival rate of transplanted BMSCs by regulating p38MAPK and JNK signaling.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81503531), the Health Scientific Research Foundation of Jilin Province, P.R.C. (No. 2015Q024), the Science and Technology Department of Jilin Province, P.R.C. (No. 20180520111JH), the Education Department of Jilin Province, P.R.C. (No. JJKH20170853KJ), and the Norman Bethune Program of Jilin University, P.R.C. (No. 2015404)
References
- 1.da Costa Goncalves F, Grings M, Nunes NS, et al. Antioxidant properties of mesenchymal stem cells against oxidative stress in a murine model of colitis. Biotechnol Lett. 2017;39:613–22. doi: 10.1007/s10529-016-2272-3. [DOI] [PubMed] [Google Scholar]
- 2.Snyder TN, Madhavan K, Intrator M, et al. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng. 2014;8:10. doi: 10.1186/1754-1611-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan XH, Song QQ, Dai JJ, et al. Transplantation of bone marrow mesenchymal stem cells for the treatment of type 2 diabetes in a macaque model. Cells Tissues Organs. 2013;198:414–27. doi: 10.1159/000358383. [DOI] [PubMed] [Google Scholar]
- 4.Tootsi K, Martson A, Kals J, et al. Metabolic factors and oxidative stress in osteoarthritis: A case-control study. Scand J Clin Lab Invest. 2017;77:520–26. doi: 10.1080/00365513.2017.1354255. [DOI] [PubMed] [Google Scholar]
- 5.Vnukov VV, Panina SB, Krolevets IV, et al. [Features of oxidative stress in the blood and the synovial fluid associated with knee osteoarthritis]. Adv Gerontol. 2015;28:284–89. [in Russian] [PubMed] [Google Scholar]
- 6.Styskal J, Van Remmen H, Richardson A, Salmon AB. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52:46–58. doi: 10.1016/j.freeradbiomed.2011.10.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Porcel M, Gheysens O, Paulmurugan R, et al. Antioxidants improve early survival of cardiomyoblasts after transplantation to the myocardium. Mol Imaging Biol. 2010;12:325–34. doi: 10.1007/s11307-009-0274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong L, Hu Y, Yao Y, et al. The coumarin derivative osthole stimulates adult neural stem cells, promotes neurogenesis in the hippocampus, and ameliorates cognitive impairment in APP/PS1 transgenic mice. Biol Pharm Bull. 2015;38:1290–301. doi: 10.1248/bpb.b15-00142. [DOI] [PubMed] [Google Scholar]
- 9.Ran K, Yang DL, Chang YT, et al. Ginkgo biloba extract postconditioning reduces myocardial ischemia reperfusion injury. Genet Mol Res. 2014;13:2703–8. doi: 10.4238/2014.April.8.14. [DOI] [PubMed] [Google Scholar]
- 10.Cao S, Gao M, Wang N, et al. Prevention of selenite-induced cataratogenesis by Ginkgo biloba extract (Egb761) in wistar rats. Curr Eye Res. 2015;40:1028–33. doi: 10.3109/02713683.2014.980005. [DOI] [PubMed] [Google Scholar]
- 11.Belviranli M, Okudan N. The effects of Ginkgo biloba extract on cognitive functions in aged female rats: the role of oxidative stress and brain-derived neurotrophic factor. Behav Brain Res. 2015;278:453–61. doi: 10.1016/j.bbr.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Tsai HY, Huang PH, Lin FY, et al. Ginkgo biloba extract reduces high-glucose-induced endothelial reactive oxygen species generation and cell adhesion molecule expression by enhancing HO-1 expression via Akt/eNOS and p38 MAP kinase pathways. Eur J Pharm Sci. 2013;48:803–11. doi: 10.1016/j.ejps.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Chun J, Jung HA, et al. Capillarisin attenuates exercise-induced muscle damage through MAPK and NF-kappaB signaling. Phytomedicine. 2017;32:30–36. doi: 10.1016/j.phymed.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Kello M, Kulikova L, Vaskova J, et al. Fruit peel polyphenolic extract-induced apoptosis in human breast cancer cells is associated with ROS production and modulation of p38MAPK/Erk1/2 and the Akt signaling pathway. Nutr Cancer. 2017;69:920–31. doi: 10.1080/01635581.2017.1339819. [DOI] [PubMed] [Google Scholar]
- 15.NaveenKumar SK, Hemshekhar M, Sundaram MS, et al. Cell-free methemoglobin drives platelets to apoptosis via mitochondrial ROS-mediated activation of JNK and p38 MAP kinase. Biochem Biophys Res Commun. 2017;491:183–91. doi: 10.1016/j.bbrc.2017.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Ren M, Yang S, Li J, et al. Ginkgo biloba L. extract enhances the effectiveness of syngeneic bone marrow mesenchymal stem cells in lowering blood glucose levels and reversing oxidative stress. Endocrine. 2013;43:360–69. doi: 10.1007/s12020-012-9745-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Qu F. Treating gynaecological disorders with traditional Chinese medicine: A review. Afr J Tradit Complement Altern Med. 2009;6:494–517. doi: 10.4314/ajtcam.v6i4.57181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sze SC, Tong Y, Zhang YB, et al. A novel mechanism: Erxian Decoction, a Chinese medicine formula, for relieving menopausal syndrome. J Ethnopharmacol. 2009;123:27–33. doi: 10.1016/j.jep.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Yin D, Liu Z, Peng D, et al. Serum containing Tao-Hong-Si-Wu decoction induces human endothelial cell VEGF production via PI3K/Akt-eNOS signaling. Evid Based Complement Alternat Med. 2013;2013:195158. doi: 10.1155/2013/195158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–70. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 21.Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015;24:1150–63. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, Yan G, Gong R, et al. Effects of blue light emitting diode irradiation on the proliferation, apoptosis and differentiation of bone marrow-derived mesenchymal stem cells. Cell Physiol Biochem. 2017;43:237–46. doi: 10.1159/000480344. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro ML, Moreira LM, Arcari DP, et al. Protective effects of chronic treatment with a standardized extract of Ginkgo biloba L. in the prefrontal cortex and dorsal hippocampus of middle-aged rats. Behav Brain Res. 2016;313:144–50. doi: 10.1016/j.bbr.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J Clin Invest. 2003;111:163–69. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han XH, Wang CL, Xie Y, et al. Anti-metastatic effect and mechanisms of Wenshen Zhuanggu Formula in human breast cancer cells. J Ethnopharmacol. 2015;162:39–46. doi: 10.1016/j.jep.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Ding K, Wang Y, Jiang W, et al. Qian Yang Yu Yin Granule-containing serum inhibits angiotensin II-induced proliferation, reactive oxygen species production, and inflammation in human mesangial cells via an NADPH oxidase 4-dependent pathway. BMC Complement Altern Med. 2015;15:81. doi: 10.1186/s12906-015-0619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Anders F, Thanos S, et al. Hydrogen sulfide protects retinal ganglion cells against glaucomatous injury in vitro and in vivo. Invest Ophthalmol Vis Sci. 2017;58:5129–41. doi: 10.1167/iovs.17-22200. [DOI] [PubMed] [Google Scholar]
- 28.Reissig K, Silver A, Hartig R, et al. Chk1 promotes DNA damage response bypass following oxidative stress in a model of hydrogen peroxide-associated ulcerative colitis through JNK inactivation and chromatin binding. Oxid Med Cell Longev. 2017;2017:9303158. doi: 10.1155/2017/9303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alam MB, Ju MK, Lee Sh. DNA protecting activities of Nymphaea nouchali (Burm. f) flower extract attenuate t-BHP-induced oxidative stress cell death through Nrf2-mediated induction of heme Oxygenase-1 expression by activating MAP-kinases. Int J Mol Sci. 2017;18(10) doi: 10.3390/ijms18102069. pii: E2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farias JG, Molina VM, Carrasco RA, et al. Antioxidant therapeutic strategies for cardiovascular conditions associated with oxidative stress. Nutrients. 2017;9(9) doi: 10.3390/nu9090966. pii: E966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergara R, Parada F, Rubio S, Perez FJ. Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J Exp Bot. 2012;63:4123–31. doi: 10.1093/jxb/ers094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saberi Firouzi S, Namazi Sarvestani N, Bakhtiarian A, et al. Sildenafil protective effects on high glucose-induced neurotoxicity in PC12 cells: The role of oxidative stress, apoptosis, and inflammation pathways in an in vitro cellular model for diabetic neuropathy. Neurol Res. 2018 doi: 10.1080/01616412.2018.1458813. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Ma ZN, Liu Z, Wang Z, et al. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-kappaB signaling pathways. Food Chem Toxicol. 2017;110:62–73. doi: 10.1016/j.fct.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Tomar A, Vasisth A, Khan SI, et al. Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling cascade. Phytomedicine. 2017;34:154–61. doi: 10.1016/j.phymed.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 36.Keshet Y, Seger R. The MAP kinase signaling cascades: A system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]