Abstract
Background
The role of multidrug resistance 1 gene (MDR1 or ABCB1) polymorphism G2677T was studied in relation to paroxetine therapeutic efficacy and its side effects, as well as its association with selected demographic and clinical characteristics of patients with depressive disorder.
Material/Methods
To evaluate therapeutic efficacy, all patients (n=61) were rated at week 0, 2, 4, and 6 using the Hamilton Rating Scale for Depression (HAMD-21). They were labelled as “responders” (a decrease in HAMD ≥50%) and “nonresponders”. The frequency of the side effects of nausea and sexual dysfunction were assessed using the Utvalg for Kliniske Undersogelser rating scale. The PCR-restriction fragment length polymorphism method was used for genotyping.
Results
A significantly enhanced therapeutic efficacy of paroxetine was observed in patients carrying at least one T allele at week 4 (GG versus GT: 0.049; GG versus GT+TT: 0.035) and week 6 (GG versus TT: 0.001; GG versus GT+TT: 0.016; GG+GT versus TT: 0.003; G versus T: 0.001). On the other hand, carriers of the T allele showed only a nonsignificant increase in HAMD-21 score reduction. In the present study, no significant association between G2677T polymorphism and side effects was detected. However, we found a marginally significant difference between GG and GT genotypes regarding family history of depressive disorder (p=0.049).
Conclusions
Our study provided evidence for the potential effect of MDR1 G2677T polymorphism on paroxetine therapeutic efficacy, and eventually on depressive disorder family history. Larger multicenter studies and studies across other ethnic groups are needed to elucidate the contradictory implications of G2677T polymorphism with depressive disorder and its treatment.
MeSH Keywords: Depression; Genotype; P-Glycoprotein; Paroxetine; Polymorphism, Genetic; Polymorphism, Restriction Fragment Length
Background
Treatment-resistant depression is a common problem in the treatment of major depressive disorder (DD), with 60–70% of all patients meeting the criteria for treatment-resistant depression [1,2]. It has been hypothesized that genetic factors contribute to the variability of antidepressant drug efficacy [3,4]. Additionally, it has been shown that genetic markers involved in the brain bioavailability of antidepressants and/or toxic substances seem to be better predictors of clinical response than those related to antidepressant plasma concentrations [5].
P-glycoprotein (P-gp) is the transmembrane efflux pump coded by the gene of multidrug resistance 1 (MDR1 or ABCB1), which was initially discovered as the precursor to a protein associated with failure of cancer chemotherapy. It has been confirmed that over-expression of MDR1 causes resistance in cultured tumor cells [6]. Generally, P-gp plays an important role in regulating absorption, distribution, and elimination of drugs [7]. It is strategically positioned to “barrier localizations” (the apical membrane of the gastrointestinal tract, the biliary canalicular membrane of hepatocytes, blood cells, and the luminal membranes of proximal tubular epithelial cells in the kidney), including blood-brain barrier and blood-cerebrospinal fluid (CSF) barrier, and thus modulates the accumulation of different xenobiotics in the brain [8–10].
Many drugs have been shown to be P-gp substrates, and P-gp activity may influence their pharmacokinetic parameters, interactions, and finally therapeutic efficacy, as well as occurrence of drug side effects [11–17]. Similarly, many antidepressants interact with P-gp [4,18]. Some in vitro studies 19] and in vivo studies [20–22] have also demonstrated the involvement of paroxetine, as a selective serotonin reuptake inhibitor (SSRI), in P-gp inhibitory activity as well as a substrate of P-gp.
Currently, more than 100 variants in the MDR1 gene have been reported. One of these, the nonsynonymous single nucleotide polymorphism (SNP) (Ala893Ser/Thr) localized in exon 21 and codon 893, G2677T/A (rs2032582) has been found to be associated with altered expression, activity, and the substrate specificity of P-gp [23–26].
Recent reports showed that individuals who had the TT genotype had a lower P-gp messenger RNA expression than those carrying GG genotype [25]. Moreover, variant alleles of the G2677T/A polymorphism were nonsignificantly associated with lower P-gp expression in the placenta [23]. On the contrary, some pharmacokinetic studies reported an opposite effect of the 2677T variant allele, i.e., an increase in efflux activity or impact on plasma and CSF concentration of antidepressants compared with the G2677 allele [24,27].
Only a few clinical studies have evaluated the associations between G2677T/A polymorphism and the therapeutic response to paroxetine, with mixed and inconsistent results [28–33]. Additionally, evidence of a better response in TT carriers of G2677T/A polymorphism (OR=0.75, 95% CI: 0.58–0.97; p=0.03) observed in a meta-analysis evaluating MDR1 variants in relation to antidepressant treatment outcomes [34], was not confirmed in a later meta-analysis [35]. However, only five studies included in the meta-analysis were studies on paroxetine.
Based on earlier published data, our study focused on determining the relevance of the MDR1 (G2677T) polymorphism to treatment response of paroxetine (efficacy and side effects) as well as its association with selected demographic and clinical characteristics of patients with DD in the Slovakian population.
Material and Methods
The study sample was comprised of patients with a diagnosis of DD (first depressive episodes and recurrent depressive disorder) according to ICD-10: (n=61; 40 females and 21 males, mean age=40.85; SD=12.828; females/males ratio: 1.90). All patients were of Slovakian origin (Caucasians) from different regions of Eastern Slovakia. Research protocol was approved by ethical committee of P. J. Safarik University and written informed consent was obtained from each participant prior to inclusion. Diagnostic assessments and ratings were made by two experienced psychiatrists who were kept blind to the diagnosis made by one to another and to the genotypes.
Inclusion criteria were: age 18–65 years, diagnosis of DD, six weeks of continuous paroxetine treatment, at least 18 points at baseline on the Hamilton Rating Scale for Depression, 21-item version (HAMD-21) (Hamilton, 1960). Exclusion criteria included: drug or alcohol abuse, organic brain syndrome and personality disorders, psychotic symptoms, pregnancy, electroconvulsive therapy within the previous six months, and therapy with another known P-gp substrate. Likewise, concomitant psychotropic drugs were not allowed, except a low dose of symptomatic benzodiazepine treatment for a minimal duration. The patients were assessed according to HAMD-21 scale at week 0, 2, 4, and 6. They were either drug free or post washout phase of an ineffective antidepressants (three weeks for fluoxetine and one week for other antidepressants). Paroxetine was administered at an initial dose of 10–20 mg/day and increased to reach a dose of 40 mg/day from day 12–15 until the end of the trial. Participants were labelled as “responders” (REs; a decrease in HAMD-21 ≥50%) and “nonresponders” (NREs). Remission was defined as a score ≤7 points on HAMD-21 at week 6. Paroxetine tolerance was assessed through the Utvalg for Kliniske Undersogelser (UKU) rating scale of side effects at baseline and after 2, 4, and 6 weeks of treatment, with a focus on the frequency of the side effects of nausea and sexual dysfunction.
DNA extraction and genotyping
Anti-coagulated (Na2EDTA) blood samples were obtained from the antecubital vein of patients with DD, and DNA was extracted and purified using the Wizard® Genomic DNA Purification Kit (Promega Corporation, USA). The G2677T polymorphism (rs 2032582) in the MDR1 gene was analyzed by PCR-RFLP assay using the primer sequences F: 5′-TTACCCAGAATATAGCAAATCTTGG-3′ and R: 5′-CATATTTAGTTTGACTCACCTTCTCAG-3′. The PCR reaction mixture contained: approximately 200 ng of genomic DNA, 1×PCR Buffer with 1.5 mM MgCl2 (Solis BioDyne, Estonia), 200 μM deoxynucleotide triphosphate (dNTP) mix (Jena Bioscience, Germany), 0.4 μM of each primer (Sigma-Aldrich, Germany) and 1U HOT FIREPol® DNA Polymerase (Solis BioDyne, Estonia). PCR-grade water was added to bring the final volume to 25 μL. The amplification consisted of an initial polymerase activation step for 15 minutes at 95°C and initial denaturation step for 30 seconds at 95°C followed by 40 cycles of denaturation at 95 °C for 30 seconds, annealing at 56°C for 30 seconds, and extension at 72°C for 30 seconds. Terminal elongation was performed at 72°C for three minutes. The PCR products were digested at 37°C overnight using 10 units Hpy188I restriction endonuclease (New England BioLabs, UK). The restriction fragments obtained were separated by electrophoresis on a 3% agarose gel for 90 minutes at 140 V and analyzed after staining with GelRed (Biotium, USA) under ultraviolet light. Electrophoretic pattern showed one band (198 bp) for homozygous GG genotype, two bands (198 bp, 173 bp) for heterozygous GT genotype and one band (173 bp) for homozygous TT genotype. Due to the low population frequency of the more recently described 2677A allele (<2% in Caucasians) [33,37,38], this variant was not genotyped in the present study.
Statistical analyses
SPSS software for Windows (version 15.0, USA) and GraphPad Prism 5 (GraphPad Software, Inc., USA) were used for statistical analyses. A p value of <0.05 was taken as statistically significant. Chi-square or Fisher’s exact tests were performed to compare contingency tables. The Hardy-Weinberg equilibrium (HWE) assumption was assessed for tested group by comparing the observed numbers of each genotype with those expected under the HWE for the estimated allele frequency. Codominant, dominant, recessive, and over dominant genetic models were used to analyze the association between a polymorphism and phenotype. The most commonly used model, the codominant model, was used for all SNPs. Odds ratios (OR) and the corresponding 95% confidence intervals (95% CI) were used for calculating the relative associations. All quantitative changes were evaluated using Mann-Whitney U test, or Kruskal-Wallis H test, for comparison of more than two independent groups.
Results
Genotype and allelic distribution of MDR1 (G2677T) polymorphism
All obtained blood samples were successfully genotyped for the MDR1 (G2677T) polymorphism. The G allele was found to be more frequent in our study. Overall frequencies of the G2677T genotypes GG, GT, and TT in the patient samples were 23 (37.71%), 29 (47.54%), and nine (14.75%), respectively. The genotype distribution among patients was in accordance with HWE law (p=1.000), likewise, in the sample of REs (p=1.000) and among NREs (p=0.332).
The demographic and clinical characteristics of the patients (sex, mean age, family history of DD, smoking history, suicide history, suicide ideation, first episode, admission, severity of episodes and remission, and HAMD-21 baseline) were differentiated according to MDR1 genotypes and alleles are shown in Table 1. We observed significant associations between G2677T polymorphism and family history of depression (Table 1). Overall positive family history of DD was found to be more frequent among patients carrying GT genotype than patients carrying GG genotype (OR=3.49; 95% CI=1.07–11.40; p=0.049; codominant model), or GG+TT genotypes (OR=3.69; 95% CI=1.25–10.92; p=0.020; over dominant model).
Table 1.
Demographic and clinical characteristics of depressive patients in relation to genotypes of MDR1 (G2677T) polymorphism.
| Variable (%) | Genotypes | p-Value (OR; 95% CI) | ||
|---|---|---|---|---|
| GG (n=23) | GT (n=29) | TT (n=9) | ||
| Sex | ||||
| Male | 8 (38.1) | 11 (52.4) | 2 (9.5) | Ns |
| Female | 15 (37.5) | 18 (45.0) | 7 (17.5) | |
| Age in study | 38.91±11.21 | 41.03±14.15 | 45.22±12.38 | Ns |
| DD in family history | ||||
| No | 17 (45.95) | 13 (35.13) | 7 (18.92) | 0.049* (3.49; 1.07–11.40)a |
| Yes | 6 (25.0) | 16 (66.7) | 2 (8.3) | 0.020* (3.69; 1.25–10.92)b |
| Smoking history | ||||
| No | 16 (34.8) | 23 (50.0) | 7 (15.2) | Ns |
| Yes | 7 (46.7) | 6 (40.0) | 2 (13.3) | |
| Suicide history | ||||
| No | 23 (39.65) | 27 (46.55) | 8 (13.8) | Ns |
| Yes | 0 (0.00) | 2 (66.7) | 1 (14.3) | |
| Suicide ideation | ||||
| No | 21 (38.88) | 25 (46.29) | 8 (14.8) | Ns |
| Yes | 2 (28.6) | 4 (57.1) | 1 (14.3) | |
| First episode | ||||
| No | 9 (29.0) | 15 (48.4) | 7 (22.6) | Ns |
| Yes | 14 (46.7) | 14 (46.7) | 2 (6.6) | |
| Admission | ||||
| No | 12 (34.3) | 18 (51.4) | 5 (14.3) | Ns |
| Yes | 11 (42.3) | 11 (42.3) | 4 (15.4) | |
| Severity of episodes | ||||
| Mild | 6 (35.3) | 7 (41.2) | 4 (23.5) | Ns |
| Moderate | 10 (34.5) | 16 (55.2) | 3 (10.3) | |
| Severe | 7 (46.7) | 6 (40.0) | 2 (13.3) | |
| Remission | ||||
| No | 16 (45.7) | 14 (40.0) | 5 (14.3) | Ns |
| Yes | 7 (26.9) | 15 (57.7) | 4 (15.4) | |
| HAMD-21 baseline | 27.13±10.48 | 26.72±8.44 | 25.22±9.56 | Ns |
DD in family history – family history of depression; suicide history – lifetime suicide attempts; suicide ideation – suicide ideation present by current episode; first episode – first episode of depression; admission “Yes” – inpatients, “No” – outpatients; HAMD-21 – Hamilton Rating Scale for Depression; Ns – non-significant;
codominant model (GG vs. GT);
recessive model;
a significant association.
Allele and genotype associations of MDR1 (G2677T) with therapeutic efficacy (TE) of paroxetine
The association between MDR1 G2677T polymorphism in exon 21 and therapeutic response to paroxetine (according to HAMD-21 scale) at weeks 2, 4, and 6 of treatment were assessed in all patients (n=61). Generally, there was a gradual increase in the RE/NRE ratio during paroxetine therapy. The RE/NRE ratio was 0.196 at week 2 (RE: 10; 16.39%) and it increased to 0.794 at week 4 (REs: 27; 44.26%; p<0.001; OR=4.05; 95% CI=1.74–9.43), and to 1.259 at week 6 (REs: 34; 55.74%; p<0.0001; OR=6.42; 95% CI=2.76–14.96).
A statistically significant association was found between REs and NREs according to the polymorphism of MDR1 G2677T and successful treatment with paroxetine. Statistical analysis showed a significantly increased chance of treatment response in patients carrying at least one T allele at week 4 (OR=3.49; 95% CI=1.07–11.40; p=0.049; codominant model: GG versus GT) and week 6 (OR=34.65; 95% CI=1.79–672.0; p=0.001; codominant model: GG versus TT; OR=20.49; 95% CI=1.13–370.6; p=0.003; recessive model). The difference in allele frequencies between NREs and REs was also statistically significant at week 6 (OR=3.71; 95% CI=1.67–8.25; p=0.001). On the other hand, patients carrying GG genotype had lower chance to be responders at week 4 (OR=0.29; 95% CI=0.09–0.88; p=0.035; dominant model) and week 6 (OR=0.25; 95% CI=0.08–0.74; p=0.016; dominant model). Data are shown in the Table 2.
Table 2.
Distributions of genotypes and alleles of MDR1 (G2677T) gene polymorphism in relation to therapeutic efficacy (according HAMD-21) to paroxetine.
| Genotype | RE | NRE | OR (95% CI) | p Value |
|---|---|---|---|---|
| Week 2 | ||||
| GG | 20 (39.2) | 3 (30.0) | 1.00 (Ref.)a | |
| GT | 23 (45.1) | 6 (60.0) | 1.74 (0.38–7.88) | 0.714 |
| TT | 8 (15.7) | 1 (10.0) | 0.83 (0.08–9.26) | 1.000 |
| G allele | 63 (61.8) | 12 (60.0) | 1.00 (Ref.) | |
| T allele | 39 (38.2) | 8 (40.0) | 1.08 (0.40–2.87) | 0.882 |
| Week 4 | ||||
| GG | 17 (50.0) | 6 (22.2) | 1.00 (Ref.)a | |
| GT | 13 (38.2) | 16 (59.3) | 3.49 (1.07–11.40) | 0.049* |
| TT | 4 (11.8) | 5 (18.5) | 3.54 (0.70–17.74) | 0.213 |
| GT+TT | 17 (50.0) | 21 (77.8) | 1.00 (Ref.)b | |
| GG | 17 (50.0) | 6 (22.2) | 0.29 (0.09–0.88) | 0.035* |
| G allele | 47 (69.1) | 28 (51.9) | 1.00 (Ref.) | |
| T allele | 21 (30.9) | 26 (48.1) | 2.08 (0.99–4.36) | 0.052 |
| Week 6 | ||||
| GG | 15 (55.6) | 8 (23.5) | 1.00 (Ref.)a | |
| GT | 12 (44.4) | 17 (50.0) | 2.66 (0.86–8.25) | 0.103 |
| TT | 0 (0.00) | 9 (26.5) | 34.65 (1.79–672.0) | 0.001* |
| GT+TT | 12 (44.4) | 26 (76.5) | 1.00 (Ref.)b | |
| GG | 15 (55.6) | 8 (23.5) | 0.25 (0.08–0.74) | 0.016* |
| GG+GT | 27 (100.0) | 25 (73.5) | 1.00 (Ref.)c | |
| TT | 0 (0.00) | 9 (26.5) | 20.49 (1.13–370.6) | 0.003* |
| G allele | 42 (77.8) | 33 (48.5) | 1.00 (Ref.) | |
| T allele | 12 (22.2) | 35 (51.5) | 3.71 (1.67–8.25) | 0.001* |
RE – responders; NRE – non-responders; OR – odds ratio; CI – confidence interval;
codominant model;
dominant model;
recessive model;
a significant association.
On the other hand, we found no statistically significant differences in both HAMD-21 score at week 0, week 2, week 4, and week 6 and the percentage of HAMD-21 score reduction from baseline to week 2, week 4, and week 6 among alleles and genotypes (Table 3; Figure 1). However, the percentage of HAMD-21 score reduction was the highest in the patients carrying the TT genotype or T allele at week 2, week 4 and week 6.
Table 3.
MDR1 (G2677T) polymorphism in relation to HAMD-21 scores and percentage of HAMD-21 score reduction from baseline to weeks 2, 4, 6 during paroxetine treatment (Kruskal-Wallis test and Mann-Whitney test).
| HAMD-21 | p Value | HAMD-21 (%) | p Value | |
|---|---|---|---|---|
| Week 2 | ||||
| GG | 21.87±9.93 | 16.49±27.04 | ||
| GT | 21.41±11.71 | χ2=0.742 | 19.20±41.80 | χ2=1.896 |
| TT | 18.33±6.38 | df=2; p=0.690 | 24.54±21.87 | df=2; p=0.388 |
| G | 21.69±10.52 | MW U=1599.50 | 17.54±33.13 | MW U=1599.50 |
| T | 20.23±9.99 | Z=−0.86; p=0.390 | 21.25±35.17 | Z=−0.86; p=0.390 |
| Week 4 | ||||
| GG | 19.17±11.46 | 25.32±51.78 | ||
| GT | 17.17±14.08 | χ2=2.363 | 36.03±50.82 | χ2=2.005 |
| TT | 13.33±6.93 | df=2; p=0.307 | 47.56±19.36 | df=2; p=0.367 |
| G | 18.40±12.42 | MW U=1481.50 | 29.46±50.98 | MW U=1599.50 |
| T | 15.70±11.87 | Z=−1.48; p=0.139 | 40.44±41.65 | Z=−0.86; p=0.390 |
| Week 6 | ||||
| GG | 15.48±11.71 | 38.08±58.36 | ||
| GT | 14.31±13.23 | χ2=1.584 | 45.26±52.84 | χ2=1.339 |
| TT | 9.89±4.62 | df=2; p=0.453 | 61.04±9.32 | df=2; p=0.512 |
| G | 15.11±12.14 | MW U=1527.50 | 40.86±55.62 | MW U=1599.50 |
| T | 12.74±10.87 | Z=−1.24; p=0.215 | 51.31±42.30 | Z=−0.86; p=0.390 |
HAMD-21 – Hamilton Rating Scale for Depression; MW – Mann-Whitney.
Figure 1.
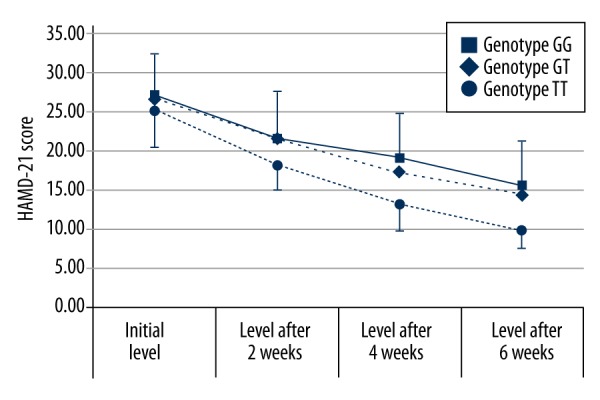
Average HAMD-21 scores of patients with DD in three groups according their genotypes of MDR1 G2677T polymorphism at weeks 0, 2, 4, 6.
Furthermore, there were no differences in sex, age, family history, smoking history, suicide history, suicide ideation, first episode, admission, severity of episodes and remission, and HAMD-21 baseline score between REs and the NREs (data not shown).
MDR1 gene variants and side effects of paroxetine
The relation between G2677T polymorphism of MDR1 gene and the occurrence of the two most frequent adverse side effects of paroxetine therapy (sexual dysfunction and nausea) in our patient sample were also evaluated. The frequency of nausea at week 0, week 2, week 4, and week 6 was: 22 patients (36.07%; 18 females and four males), 17 patients (27.87%; 12 females and five males), 13 patients (21.31%; 10 females and three males), 11 patients (18.03%; 10 females and one male), respectively, while frequency of sexual dysfunction was 17 patients (27.87%; 10 females and seven males); 22 patients (36.07%, 13 females and nine males), 24 patients (39.34%; 13 females and 11 males), and 17 patients (27.87%; nine females and eight males), respectively.
There was no significant difference in side effects of paroxetine between females and males. Similarly, no significant differences between the occurrence of these two side effects were found in samples differentiated by the genotype and allele frequencies of MDR1 G2677T polymorphism (statistical data not shown).
Discussion
Genomic medicine, which is the use of information from genomes and their derivatives to guide medical decision-making, is a key component of personalized medicine, and is a rapidly advancing field of healthcare [39].
The main concern of our study was to investigate whether MDR1 (G2677T, rs2032582) polymorphism influence short-term response to paroxetine, its selected side effects, as well as selected demographics and clinical characteristics of the patients with DD. To our knowledge, this is the first study that evaluated MDR1 G2677T polymorphism in relation to paroxetine response and its side effects (nausea and sexual dysfunction) in the same patient sample, as well as the first study assessing this association in a Slovakian population.
Recently, MDR1 genotyping has attracted research attention regarding the possibility of personalized treatment through identification of REs and NREs to a certain class of pharmacotherapy. The selection of the tested SNP in DD patients in this analysis was based on knowledge that paroxetine belongs to P-gp substrates and/or inhibitors [19–22]. From the two most common assessed SNPs (MDR1 C3435T and G2677T/A) in relation to antidepressants, we chose the latter. In contrast to C3435T polymorphism, G2677T/A leads to amino acid exchange [23]. Additionally, several studies have found an association with altered expression, activity, the substrate specificity of P-gp or pharmacokinetics of paroxetine [23–26]. Due to the low population frequency of the more recently described 2677A allele (< 2% in Caucasians) [33,37,38], this variant was not genotyped in the present study. We found that patients carrying at least one T allele had a significantly increased chance of therapeutic efficacy at week 4 and week 6. The difference in allele frequencies between the REs and the NREs was statistically significant at week 6. On the other hand, participants carrying the GG genotype had a lower chance of being a responder at week 4 and week 6. However, these results should be interpreted with caution. Taking a 50% cutoff point, the obtained results may depend on the initial value. Therefore, we also evaluated the percentage of HAMD-21 score reduction from baseline to week 2, week 4, and week 6 among alleles and genotypes. Despite nonsignificant differences, we observed the highest percentage of HAMD-21 score reduction in the patients carrying the TT genotype or T allele at week 2, week 4, and week 6. In accordance with our results, Kato et al. showed a significant decrease in HAM-D score reduction (%) in patients carrying the variant alleles (T/A) of the G2677T/A polymorphism at week 6 of paroxetine treatment in a Japanese study population [29]. Moreover, the same authors found that the wild variants haplotype (3435C-2677G-1236T) was significantly associated with poor response. Other authors have reported that MDR1 variants of G2677T polymorphism were not associated with therapeutic response to paroxetine using the HAMD-17 scale in Croatian patients with DD [30]. Similarly, studies performed in Germany, Switzerland, and the United States failed to show the importance of G2677T polymorphism for paroxetine treatment [28,31,32].
No study aimed at MDR1 G2677T/A in relation to paroxetine outcomes reported an importance of G allele for better therapeutic response. On the other hand, Nikish et al. reported that GG/GT genotypes were associated with a better treatment response and higher plasma and CSF concentrations in depressive patients [27]. However, the study evaluated citalopram, another drug belonging to the SSRI group. Recently, two meta-analyses evaluating MDR1 variants in relation to antidepressant treatment outcomes have been published. The authors of the one meta-analysis [35] could not confirm better therapeutic response to paroxetine in TT carriers of G2677T/A polymorphism which had been observed in a previous meta-analysis [34]. Besides paroxetine, other SSRIs have been assessed in relation to G2677T polymorphism. Gassó et al. demonstrated that the T allele was significantly associated with higher clinical improvement with fluoxetine therapy in children and adolescent patients from Spain using scales including the Clinical Global Impression-Improvement scale [5]. The study patients were diagnosed with DD, obsessive-compulsive disorder, or generalized anxiety disorder. Surprisingly, the authors failed to show a significant association between the polymorphism G2677T and plasma levels of fluoxetine or its active metabolite (S)-norfluoxetine. Moreover, Taiwanese DD patients with GG genotype had a worse antidepressant response to fluoxetine, as well to venlafaxine [40].
From the literature, it is well known that the effect of SSRIs is noticeable after a few weeks. In our study, we observed therapeutic response already at week 2 in 10 participants (16.39%), whereas the RE/NRE ratio significantly increased from week 2 to week 4 (p<0.001), and to week 6 (p<0.0001). Other authors reported a larger number of REs at week 2 (58.62%) after paroxetine therapy for depression [41]. Overall, an increased therapeutic efficacy over time was found in other short-term treatment analyses [29,30]. Since paroxetine can act as a P-gp inhibitor [4], long-term studies are needed in the future.
The occurrence of adverse side effects after antidepressant therapy is another important problem. As the prevalence and severity of side effects follow interindividual variations, it is reasonable to hypothesize a genetic basis for drug tolerability [42]. Therefore, we performed a statistical analysis of the association of MDR1 G2677T polymorphism with two side effects of paroxetine (nausea and sexual dysfunction) referred to in the summary of product characteristics as very common (≥1/10). The occurrence of other drug side effects was too low to evaluate. Despite better therapeutic response to paroxetine in T allele carriers in our analysis, we did not find any evidence that G2677T polymorphism could influence the occurrence and severity of sexual dysfunction and nausea during paroxetine therapy. It is difficult to ascertain whether negative findings are due to a lack of influence of the tested SNP on the adverse effects or due to distinct characteristics of our sample (overall low frequency of side effects). However, the frequency of side effects of paroxetine in our study was slightly higher than the reported adverse effects by other authors [43,44]. Another explanation of nonsignificant results could be due to the possibility of different impacts of the polymorphism on various tissues and organs. Moreover, these side effects could also be symptoms of depression and not explicitly side effects of paroxetine. A recently published small Czech study analyzed G2677T polymorphism in relation to selected symptoms of sexual dysfunction after paroxetine treatment in women with bulimia nervosa and anxiety disorders [33]. It was demonstrated that G allele carriers were significantly at higher risk of orgasm disorder and lubrication development problems. No significant differences in the distribution of the MDR1 G2677T/A genotypes between patients with and without drug side effects were confirmed in the study evaluating therapeutic response to several SSRIs including paroxetine [45]. Regarding other antidepressants, Ozbey et al. showed significantly higher frequencies in venlafaxine-induced akathisia in TT/TA carriers of G2677T/A polymorphism [46]. This relationship was not observed for the therapeutic efficacy of venlafaxine.
Additionally, selected demographic and clinical patient characteristics were assessed in relation to G2677T polymorphism. We found only marginally significant differences between GG and GT genotypes regarding family history of depression. Positivity of family history of DD was more often observed in patients with GT genotype. Based on findings that P-gp also protects the brain not only from many drugs but also from neurotoxic substances, the association between MDR1 polymorphisms and susceptibility to DD was evaluated. First, a Japanese study showed that the frequencies of GA and AA genotypes, as well as A allele of MDR1 G2677T/A polymorphism were significantly higher in patients with mood disorders than in controls [47]. Another Japanese analysis reported no significant difference in genotype or allele distribution regarding G2677T polymorphism in DD patients [48]. On the other hand, a significant protective role of T allele of G2677T/A polymorphism and 1236T-2677T-3435T haplotype was found in male Portuguese individuals with DD [4]. Furthermore, Chinese individuals carrying TG haplotype of rs1045642-rs2032582 had significantly (53%) lower risk of developing DD [50]. These findings support the need of other case-control studies of the impact of G2677T polymorphism in relation to susceptibility to DD.
There are a few other issues that should be discussed regarding the inconsistent results of studies evaluating MDR1 G2677T/A polymorphism in relation to antidepressants or DD. First, studies were done in patients with different ethnicities. It should be noted that P-gp expression and influence of MDR1 polymorphisms may vary depending on ethnicity and environmental factors [51]. Enrollment of mixed patient sample regarding ethnicity in the relevant study was also reported [28]. Second, the findings from several studies, including meta-analyses, looked at other antidepressant agent or evaluated several different antidepressants in the same sample. However, it was hypothesized that the substitution of Thr or Ser for Ala due to MDR1 G2677T polymorphism would affect the geometric precision of the interaction site and the secondary structure of P-gp [23]. Thus, the contradictory results might be due to different effects of variant alleles on specific drugs [52]. Therefore, antidepressants should be evaluated individually in the future. Moreover, previous analyses differed in their end-points; and equally important, several studies included patients not only with DD but also with other psychiatric disorders. Thus, other factors might have influenced previous study results, such as non-antidepressant drug and food interactions.
Overall, the main limitation of our study was the relatively small sample size. Therefore, we cannot exclude the possibility of bias and our preliminary results must be interpreted with caution. Additionally, paroxetine plasma concentrations were not measured in this study. However, it has been reported that the serum concentration of the antidepressant does not have to correlate with the therapeutic response in human studies, which may be because the brain concentrations depend mainly on blood-brain efflux [5,53]. Also, the lack of analysis of other pharmacokinetic genes, such as CYP2D6, that might have a possible effect to therapeutic response could be a limitation; however, several studies reported that polymorphisms such as CYP2D6 are not always linked with paroxetine response [28,54,55]. It is also possible that the functional effects of MDR1 variants may in fact be in linkage disequilibrium with the true causative variants, as it was shown in recent studies [29,31,50].
Conclusions
Our findings confirmed the potential significant influence of T allele of MDR1 G2677T polymorphism on paroxetine short-term treatment response but did not find the same significance for percentage HAMD-21 score reduction and side effect occurrence. Larger multicenter prospective studies and well-designed pharmacokinetic studies of paroxetine are needed to elucidate contradictory implications of MDR1 G2677T/A polymorphism in patients with DD.
Acknowledgments
We thank Silvia Kitkova and Eva Palova for their skillful technical assistance.
Footnotes
Conflict of interest
None.
Source of support: This research project was supported by the Slovak Grant Agency for Science (VEGA grant No. 1/0302/10)
References
- 1.Fava GA, Fabbri S, Sonino N. Residual symptoms in depression: An emerging therapeutic target. Prog Neuropsychopharmacol Biol Psych. 2003;26:1019–27. doi: 10.1016/s0278-5846(02)00226-9. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with Citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Crisafulli C, Fabbri C, Porcelli S, et al. Pharmacogenetics of antidepressants. Front Pharmacol. 2011;2:6. doi: 10.3389/fphar.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien FE, Dinan TG, Griffin TB, Cryan JF. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: Clinical significance of in vitro and in vivo findings. Br J Pharmacol. 2012;165(2):289–312. doi: 10.1111/j.1476-5381.2011.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gassó P, Rodríguez N, Mas S, et al. Effect of CYP2D6, CYP2C9 and ABCB1 genotypes on fluoxetine plasma concentrations and clinical improvement in children and adolescent patients. Pharmacogenomics J. 2014;14(5):457–62. doi: 10.1038/tpj.2014.12. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann U, Roots I, Eichelbaum M. Pharmacogenetics of the human drug transporter gene MDR1: Impact of polymorphisms on pharmacotherapy. Drug Discov Today. 2001;6(16):835–39. doi: 10.1016/s1359-6446(01)01892-x. [DOI] [PubMed] [Google Scholar]
- 7.Lin JH, Yamazaki M. Role of p-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet. 2003;42:59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hitzl M, Drescher S, Van der Kuip H, et al. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics. 2001;11:293–98. doi: 10.1097/00008571-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Silverman JA. P-glycoprotein. In: Levy RH, editor. Metabolic drug interactions. Philadelphia: Lippincott Williams & Willkins; 2000. pp. 135–44. [Google Scholar]
- 10.Thuerauf N, Fromm MF. The role of the transporter P-glycoprotein for disposition and effects of centrally acting drugs and for the pathogenesis of CNS diseases. Eur Arch Psychiatry Clin Neurosci. 2006;256:281–86. doi: 10.1007/s00406-006-0662-6. [DOI] [PubMed] [Google Scholar]
- 11.Eichelbaum M, Fromm MF, Schwab M. Clinical aspects of the MDR1 (ABCB1) gene polymorphism. Ther Drug Monit. 2004;26:180–85. doi: 10.1097/00007691-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Giessmann T, May K, Modess C, et al. Carbamazepine regulates intestinal P-glycoprotein and multidrug resistance protein MRP2 and influences disposition of talinolol in humans. Clin Pharmacol Ther. 2004;76:192–200. doi: 10.1016/j.clpt.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB2) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43(9):553–76. doi: 10.2165/00003088-200443090-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kakumoto M, Takara K, Sakaeda T, et al. MDR1-mediatet interaction of digoxin with antiarrhythmic or antianginal drugs. Biol Pharm Bull. 2002;25:1604–7. doi: 10.1248/bpb.25.1604. [DOI] [PubMed] [Google Scholar]
- 15.Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther. 2002;301:7–14. doi: 10.1124/jpet.301.1.7. [DOI] [PubMed] [Google Scholar]
- 16.Okamura N, Hirai M, Tanigawara Y, et al. Digoxincyclosporin A interaction: Modulation of the multidrug transporter P-glycoprotein in the kidney. J Pharmacol Exp Ther. 1993;266:1614–19. [PubMed] [Google Scholar]
- 17.Westphal K, Weinbrenner A, Giessmann T, et al. Oral bioavailability of digoxin is enhanced by talinolol: Evide.nce for involvement of intestinal P-glycoprotein. Clin Pharmacol Ther. 2000;68(1):6–12. doi: 10.1067/mcp.2000.107579. [DOI] [PubMed] [Google Scholar]
- 18.Lee G, Bendayan R. Functional expression and localization of P-glycoprotein in the central nervous system: Relevance to the pathogenesis and treatment of neurological disorders. Pharm Res. 2004;21:1313–30. doi: 10.1023/b:pham.0000036905.82914.8e. [DOI] [PubMed] [Google Scholar]
- 19.Feng B, Mills JB, Davidson RE, et al. In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos. 2008;36:268–75. doi: 10.1124/dmd.107.017434. [DOI] [PubMed] [Google Scholar]
- 20.Uhr M, Grauer MT, Holsboer F. Differential enhancement of antidepressant penetration into the brain in mice with ABCB1ab (MDR1ab) P-glycoprotein gene disruption. Biol Psychiatry. 2003;54:840–46. doi: 10.1016/s0006-3223(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 21.Doran A, Obach RS, Smith BJ, et al. The impact of P-glycoprotein on the disposition of drugs targeted for indications for the central nervous system: Evaluation using the MDR1A/1B knockout mouse model. Drug Metab Dispos. 2005;33:165–74. doi: 10.1124/dmd.104.001230. [DOI] [PubMed] [Google Scholar]
- 22.Yasui-Furukori N, Saito M, Niioka T, et al. Effect of itraconazole on pharmacokinetics of paroxetine: the role of gut transporters. Ther Drug Monit. 2007;29(1):45–48. doi: 10.1097/FTD.0b013e31802bb20d. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe M, Ieiri I, Nagata N, et al. Expression of P-glycoprotein in human placenta: Relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297(3):1137–43. [PubMed] [Google Scholar]
- 24.Kurata Y, Ieiri I, Kimura M, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002;72(2):209–19. doi: 10.1067/mcp.2002.126177. [DOI] [PubMed] [Google Scholar]
- 25.Lamba J, Strom S, Venkataramanan R, et al. MDR1 genotype is associated with hepatic cytochrome P450 3A4 basal and induction phenotype. Clin Pharmacol Ther. 2006;79(4):325–38. doi: 10.1016/j.clpt.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai A, Onishi I, Hirano H, et al. Quantitative structure-activity relationship analysis and molecular Dynamics simulation to functionally validate nonsynonymous polymorphisms of human ABC transporter ABCB1 (P-glycoprotein/MDR1) Biochemistry. 2007;43(26):7678–93. doi: 10.1021/bi700330b. [DOI] [PubMed] [Google Scholar]
- 27.Nikisch G, Eap CB, Baumann P. Citalopram enantiomers in plasma and cerebrospinal fluid of ABCB1 genotyped depressive patients and clinical response: A pilot study. Pharmacol Res. 2008;58(5–6):344–47. doi: 10.1016/j.phrs.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Gex-Fabry M, Eap CB, Oneda B, et al. CYP2D6 and ABCB1 genetic variability: Influence on paroxetine plasma level and therapeutic response. Ther Drug Monit. 2008;30:474–82. doi: 10.1097/FTD.0b013e31817d6f5d. [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Fukuda T, Serretti A, et al. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psych. 2008;32:398–404. doi: 10.1016/j.pnpbp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Mihaljevic Peles A, Bozina N, Sagud M, et al. MDR1 gene polymorphism: therapeutic response to paroxetine among patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1439–44. doi: 10.1016/j.pnpbp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Uhr M, Tontsch A, Namendorf C, et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;24:203–9. doi: 10.1016/j.neuron.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Sarginson JE, Lazzeroni LC, Ryan HS, et al. ABCB1 (MDR1) polymorphisms and antidepressant response in geriatric depression. Pharmacogenet Genomics. 2010;20(8):467–75. doi: 10.1097/FPC.0b013e32833b593a. [DOI] [PubMed] [Google Scholar]
- 33.Zourková A, Slanař O, Jarkovský J, et al. MDR1 in paroxetine-induced sexual dysfunction. J Sex Marital Ther. 2013;39(1):71–78. doi: 10.1080/0092623X.2012.668514. [DOI] [PubMed] [Google Scholar]
- 34.Niitsu T, Fabbri C, Bentini F, Serretti A. Pharmacogenetics in major depression: A comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183–94. doi: 10.1016/j.pnpbp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Breitenstein B, Brückl TM, Ising M, et al. ABCB1 gene variants and antidepressant treatment outcome? A meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(4):274–83. doi: 10.1002/ajmg.b.32309. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cascorbi I, Gerloff T, Johne A, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69(3):169–74. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- 38.Pechandová K, Buzková H, Slanar O, Perlík F. Polymorphisms of the MDR1 gene in the Czech population. Folia Biol (Praha) 2006;52:184–89. [PubMed] [Google Scholar]
- 39.Ginsburg GS, Willard HF. Genomic and personalized medicine: Foundations and applications. Transl Res. 2009;154:277–87. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Chang HH, Chen-Hsi Ch, Yen KY, et al. Association between ABCB1 polymorphisms and antidepressant treatment response in taiwanese major depressive patients. Clin Psychopharmacol Neurosci. 2015;13(3):250–55. doi: 10.9758/cpn.2015.13.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inagaki T, Furuya M, Kawamukai T, et al. Prediction of response within the first 3 days to treatment with paroxetine for depression. Prim Care Companion J Clin Psychiatry. 2008;10(2):129–32. doi: 10.4088/pcc.v10n0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy GM, Jr, Hollander SB, Rodrigues HE, et al. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry. 2004;61(11):1163–69. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- 43.Dueņas H, Lee A, Brnabic AJM, et al. Frequency of treatment-emergent sexual dysfunction and treatment effectiveness during SSRI or duloxetine therapy: 8-week data from a 6-month observational study. Int J Psychiatry Clin Pract. 2011;15:80–90. doi: 10.3109/13651501.2011.572169. [DOI] [PubMed] [Google Scholar]
- 44.Dechant KL, Clissold SP. Paroxetine: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depressive illness. Drugs. 1991;41:225–53. doi: 10.2165/00003495-199141020-00007. [DOI] [PubMed] [Google Scholar]
- 45.Bly MJ, Bishop JR, Thomas KL, Ellingrod VL. P-glycoprotein (PGP) polymorphisms and sexual dysfunction in female patients with depression and SSRI-associated sexual side effects. J Sex Marital Ther. 2013;39(3):280–88. doi: 10.1080/0092623X.2011.615896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozbey G, Celikel FC, Cumurcu BE, et al. Influence of ABCB1 polymorphisms and serum concentrations on venlafaxine response in patients with major depressive disorder. Nord J Psychiatry. 2017;71(3):230–37. doi: 10.1080/08039488.2016.1268203. [DOI] [PubMed] [Google Scholar]
- 47.Qian W, Homma M, Itagaki F, et al. MDR1 gene polymorphism in japanese patients with schizophrenia and mood disorders including depression. Biol Pharm Bull. 2006;29(12):2446–50. doi: 10.1248/bpb.29.2446. [DOI] [PubMed] [Google Scholar]
- 48.Fujii T, Ota M, Hori H, et al. Association between the functional polymorphism (C3435T) of the gene encoding P-glycoprotein (ABCB1) and major depressive disorder in the Japanese population. J Psychiatr Res. 2012;46(4):555–59. doi: 10.1016/j.jpsychires.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Santos M, Carvalho S, Lima L, et al. Common genetic polymorphisms in the ABCB1 gene are associated with risk of major depressive disorder in male Portugese individuals. Genet Test Mol Biomarkers. 2014;18(1):12–19. doi: 10.1089/gtmb.2013.0197. [DOI] [PubMed] [Google Scholar]
- 50.Xie WW, Zhang L, Wu RR, et al. Case-control association study of ABCB1 gene and major depressive disorder in a local Chinese Han population. Neuropsychiatr Dis Treat. 2015;11:1967–71. doi: 10.2147/NDT.S87175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakaeda T. MDR1 genotype-related pharmacokinetics: Fact or fiction? Drug Metab Pharmacokinet. 2005;20(6):391–414. doi: 10.2133/dmpk.20.391. [DOI] [PubMed] [Google Scholar]
- 52.Kim RB, Leake BF, Choo EF, et al. Identification of functionally variant MDR1 alleles among European Americans and African americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 53.Ferner RE. ABCB1 (P-glycoprotein) and the clinical pharmacology of adverse drug reactions. Adverse Drug React Bull. 2010;2010:1011–14. [Google Scholar]
- 54.Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255:387–400. doi: 10.1007/s00406-005-0579-5. [DOI] [PubMed] [Google Scholar]
- 55.Van Nieuwerburgh FC, Denys DA, Westenberg HG, Deforce DL. Response to serotonin reuptake inhibitors in OCD is not influenced by common CYP2D6 polymorphisms. Int J Psychiatry Clin Pract. 2009;13(1):345–48. doi: 10.3109/13651500902903016. [DOI] [PMC free article] [PubMed] [Google Scholar]


