Abstract
Background
The aim of this study was to evaluate the efficacy, safety, and cost of treatment of the traditional Chinese herbal medicine, catalpol, in patients following surgical resection for locally advanced colon cancer.
Material/Methods
The 345 patients who had undergone surgical resection for locally advanced colon adenocarcinoma, were divided into three groups: a placebo-treated group (n=115); patients treated with an intraperitoneal injection of 10 mg/kg catalpol twice a day for 12 weeks (treatment group) (n=115); patients treated with 5 mg/kg intravenous bevacizumab twice a week for 12 weeks (control group) (n=115). Serum levels of carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA), matrix metalloproteinases-2 (MMP-2), and matrix metalloproteinases-9 (MMP-9) were measured. Patient overall survival (OS), cancer-free survival (CFS), adverse effects, and cost of therapy were evaluated. Statistical analysis included the Wilcoxon rank sum test and Tukey’s test for clinicopathological response at 95% confidence interval (CI).
Results
Patients in the catalpol-treated group had significantly reduced serum levels of CA 19-9 (p=0.0002, q=3.202), CEA (p=0.0002, q=3.007), MMP-2 (p≤0.0001, q=6.883), and MMP-9 (p<0.0001, q=3.347). Only non-fatal adverse effects occurred in the catalpol treatment group (p<0.0001, q=5.375). OS and CFS were significantly increased in the catalpol treatment group compared with the placebo group (p<0.0001 q=7.586). The cost of catalpol treatment compared favorably with other treatments (p<0.0001, q=207.17).
Conclusions
In this preliminary study, treatment with the Chinese herbal medicine, catalpol, showed benefits in clinical outcome, at low cost, and with no serious complications.
MeSH Keywords: Angiogenesis Inducing Agents; Antigen Presentation; Antigens, Tumor-Associated, Carbohydrate; Inflammation Mediators
Background
Worldwide, colon and rectal cancer are the third major types of cancer [1]. All stages of colon cancer originally arise from gene mutation in the large bowel epithelial cells, with local growth, invasion, and metastasis, as represented by the tumor stage (Figure 1) [2]. Current treatment for colon cancer includes surgical resection [3], chemotherapy [4], and/or radiation therapy [5].
Figure 1.
Diagrammatic illustration of the staging of colon cancer. A: Stage I. B: Stage II. C: Stage III. D: Stage IV, with lymph node involvement. Source: http://symptoms-colon-cancer.blogspot.in.
The etiology of colon cancer is complex [6]. There are several factors that affect the pathophysiology of colon cancer [2]. However, tumor angiogenesis [7] and inflammation [8] are known to be important. Therefore, treatment using anti-inflammatory agents and angiogenesis suppressor agents are potential treatment approaches for colon cancer [2].
There are several traditional Chinese herbal medicines that have anti-inflammatory and tumor angiogenesis suppressor effects, some of which are officially included in the Chinese Pharmacopoeia for the treatment of colon cancer [9]. Catalpol is an iridoid glucoside extracted from Rehmannia glutinosa (Chinese name: Di Huang), which has anti-inflammatory and anti-proliferative activity [10]. Catalpol is used at a dose of 400–500 μg/kg/day dose for anti-inflammatory and anti-proliferative activity [11]. Recently published studies have provided evidence that freeze-dried catalpol administered intravenously at 10 mg/kg twice a day is safe for human use without significant toxicity and without associated anaphylactic reactions [12,13]. At present, published studies have evaluated the anti-cancer effect of catalpol in vitro, using the MCF-7 breast cancer cell line [11], the OVCAR-3 ovarian cancer cell line [10], and the T24 bladder cancer cell line [14].
Therefore, the aim of this study was to evaluate the efficacy, safety, and cost of treatment of the traditional Chinese herbal medicine, catalpol, in patients following surgical resection for locally advanced colon cancer.
Material and Methods
Ethical statement
The Oncology Research Ethics Committee of Pingshan District Peoples’ Hospital of Shenzhen approved the experimental protocol. This study followed the ethical guidelines of the Jingshan Maternal and Child Health Family Planning Service Center, Jingshan, China for oncological research on human subjects, in accordance with Chinese legal requirements for research studies [15]. All patients who participated in the study provided signed written informed consents.
Materials
The freeze-dried samples of catalpol were donated from Xi’an Helin Biological Engineering Co., Xi-an, Shanxi, Peoples’ Republic of China. Bevacizumab (Avastin®) (25 mg×1 mL) was purchased from Roche Holding AG, Basel, Switzerland. Normal saline was purchased from Baxter International Inc. USA.
Study design
The primary aim or endpoint of the study was to evaluate the efficacy, safety, and cost of treatment of the traditional Chinese herbal medicine, catalpol, in patients following surgical resection for locally advanced colon cancer, including patient overall survival (OS), and cancer-free survival (CFS). The secondary endpoints were the evaluation of adverse events, and cost of treatment with catalpol.
Patient groups
Statistical evaluation of the required study sample size was 115 for each group in the study. There were 345 patients included in the study who had undergone surgical resection for locally advanced colon adenocarcinoma, who were divided into three groups: a placebo-treated group (n=115); patients treated with an intraperitoneal injection of 10 mg/kg catalpol twice a day for 12 weeks (treatment group) (n=115); and patients treated with 5 mg/kg intravenous bevacizumab twice a week for 12 weeks (control group) (n=115).
Study inclusion criteria
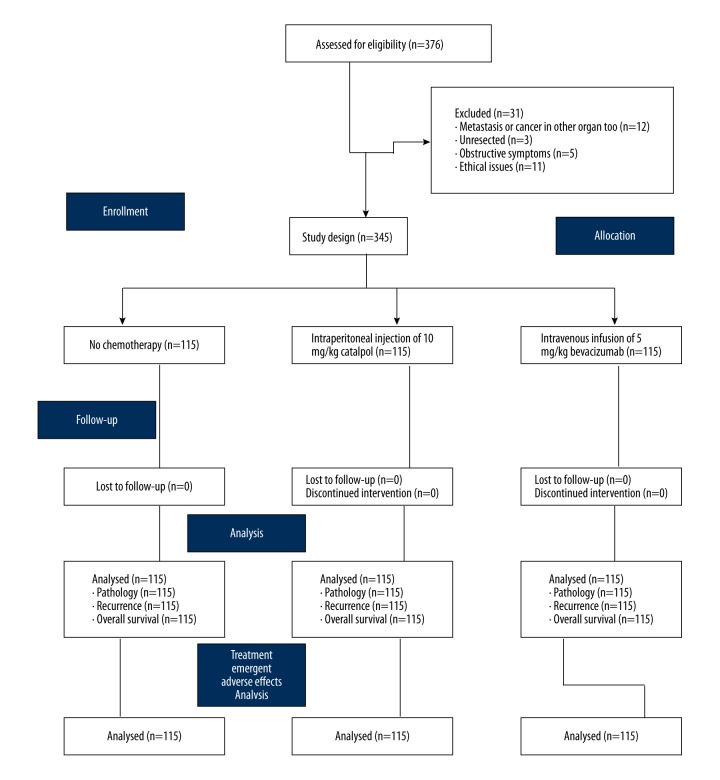
Patients included in the study were aged between 18–70 years and all were admitted to the Jingshan Maternal and Child Health Family Planning Service Center and Pingshan District People’s Hospital of Shenzhen, China between January 2012–July 2017. All patients underwent surgical resection for locally advanced colon cancer. Patients who had no previous exposure to chemotherapy or immunotherapy in the previous two years were included in the study. Patients who had adequate renal, liver, bone marrow, and hepatic function and histopathologically proven colon cancer were included in the study. A randomized, parallel design was applied for the experimental clinical study. The flow diagram of the study design is shown in Figure 2. The clinicopathological and demographic characteristics of patients with colon cancer enrolled for treatment at baseline are shown in Table 1.
Figure 2.
Flowchart showing the study design, including treatment with catalpol. Study population: 345. Confidence limit: 95±5%. Sample size (n)=115.
Table 1.
Clinicopathological and demographic characteristics of colon cancer patients enrolled for treatment.
| Group | I (Placebo) | II (Treatment) | III (Control) | Statistical comparison p value* | |
|---|---|---|---|---|---|
| Interventions | No chemotherapy | Intraperitoneal injection of 10 mg/kg catalpol | Intravenous infusion of 5 mg/kg bevacizumab | ||
| Sample size (n) | 115 | 115 | 115 | ||
| Gender | Male | 71 (62) | 68 (59) | 75 (65) | 0.6364 |
| Female | 44 (38) | 47 (41) | 40 (35) | ||
| Age (years, Mean ±SD) | 52.2±4.46 | 52.32±4.45 | 52.6±4.3 | 0.7725 | |
| Ethnicity | Chinese | 113 (98) | 112 (97) | 111 (97) | 0.2239 |
| Non-Chinese | 2 (2) | 3 (3) | 4 (3) | ||
| Cancer stage | I | 25 (22) | 29 (25) | 35 (30) | 0.2264 |
| II | 41 (36) | 47 (41) | 41 (36) | ||
| III | 49 (42) | 39 (34) | 39 (34) | ||
| Tumor location | Left | 27 (23) | 23 (20) | 31 (30) | 0.03 |
| Right | 45 (39) | 41 (36) | 42 (37) | ||
| Transverse | 43 (38) | 51 (44) | 42 (33) | ||
| Differentiation | Well differentiated | 24 (21) | 27 (23) | 35 (30) | 0.02 |
| Moderately differentiated | 38 (33) | 31 (27) | 59 (51) | ||
| Poorly differentiated | 53 (46) | 57 (50) | 21 (19) | ||
| Lymph node involvement | Positive | 54 (47) | 51 (44) | 62 (54) | 0.3264 |
| Negative | 61 (53) | 64 (56) | 53 (46) | ||
| Metastasis | Positive | 13 (11) | 11 (10) | 9 (8) | 0.6709 |
| Negative | 102 (89) | 104 (90) | 106 (92) | ||
Constant data are represented as a number (percentage) and continuous data are represented as mean ±SD. No statistical discriminations between groups at baseline (p≥0.01).
p<0.01 was considered as significant.
Study exclusion criteria
Patients with confirmed metastasis, or primary malignancy of other organs, were excluded from the study. Patients who refused to provide written informed consent, and who did not attend regular post-treatment follow-up, patient age <18 years and >70 years were excluded from the study.
Surgical resection and treatment
Computed tomography (CT) imaging was used to determine whether patients who were diagnosed with colon cancer required laparoscopic-assisted or open complete mesocolic excision (CME) for the treatment of colon cancer [16]. The resected tumor was examined by the pathologist who confirmed the diagnosis, grade, and stage of the tumor, in combination with imaging findings.
Following surgery, patients who did not receive chemotherapy of any kind during the next two years of survival were included in the placebo group (n=115). Patients in the treatment group (n=115) had an intraperitoneal injection of 10 mg/kg catalpol twice a day for 12 weeks [2]. Patients in the control group (n=115) were treated with a two-hour intravenous infusion of 5 mg/kg bevacizumab, twice in a week for 12 weeks [17,18].
Laboratory investigations
Elevated serum levels of carbohydrate antigen 19-9 (CHA19-9) and serum carcinoembryonic antigen (CEA) are biomarkers for colorectal cancer [19]. Also, elevated serum levels of matrix metalloproteinases-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) are biomarkers for recurrence of colon cancer [20]. Blood samples from all patients who participated in the study were collected using Vacuette® blood collection tubes (Greiner Bio-One International GmbH, Germany) and the laboratory investigations were interpreted by a pathologist who was blinded to the patient study group assignment [21].
Cancer-free survival (CFS) and overall survival (OS)
All patients underwent surgical follow-up with planned visits up to 48 months. Cancer-free survival (CFS) was recorded by clinicopathological measurements. However, overall survival (OS) was considered to be a live patient, irrespective of the presence of disease [17]. The response to the outcomes of each treatment followed the Response Evaluation Criteria In Solid Tumors (RECIST) guidelines [22].
Treatment-related adverse effects
Patients were monitored for non-fatal treatment-related adverse events, including, diarrhea, nausea, vomiting, gastrointestinal ulcers, allergy, constipation, alopecia, and peripheral neurotoxicity. Patients were also monitored for fatal treatment-related adverse events, including neutropenia, thrombocytopenia, anemia, hypertension, proteinuria, and gastrointestinal bleeding [17].
Treatment costs
Treatment costs were analyzed and compared, including surgery, imaging costs, diagnostic pathology costs, costs associated with duration of hospital stay, and follow-up treatment [23].
Statistical analysis
One-way ANOVA was used for analysis of clinicopathological and demographic characteristics of the enrolled patients at baseline at 99% of confidence interval (CI) [2]. Two-tailed t-tests (considering β=0.1 and α=0.05) [24], following Tukey’s post hoc test, with the critical value of q>0.314 considered as significant [25], were used for fatal and non-fatal treatment-associated adverse events, CFS, OS, and cost of the therapy. Wilcoxon rank sum test following Tukey post hoc test, with the critical value of q>3.344 considered as significant [25], were performed to compare clinicopathological responses between baseline and at the end of the 12-week treatment period [26]. All tests were performed using GraphPad Instat software (GraphPad, Inc., CA, USA). The results of parameters analyzed were considered significant at 95% confidence interval (CI).
Results
This study included 345 patients who had undergone surgical resection for locally advanced colon cancer, who were divided into three groups: a placebo-treated group (n=115); patients treated with catalpol (treatment group) (n=115); and patients treated with bevacizumab (control group). There were no significant differences in the clinicopathological and demographic characteristics between the three patient groups (all, p>0.01).
The serum values of carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA), matrix metalloproteinases-2 (MMP-2), and matrix metalloproteinases-9 (MMP-9), measured at baseline were elevated in all patients, consistent with the presence of colon cancer. Following surgery, catalpol treatment reduced the serum levels of CHA19-9, CE, MMP-2, and MMP-9 (Table 2).
Table 2.
Effect of interventions on pathological parameters.
| Tumor markers | Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | For placebo* | Treatment | For treatment* | Control | For control* | ||||||||
| Level | BL | EP | BL | EP | BL | EP | |||||||
| n | 115 | 115 | p | q | 115 | 115 | p | q | 115 | 115 | p | q | |
| CHA 19-9 | ≤27 ng/L | 12 (10) | 17 (15) | 0.0625 | N/A | 14 (12) | 27 (23) | 0.0002 | 3.202 | 11 (10) | 29 (25) | <0.0001 | 4.53 |
| >27 ng/L | 103 (90) | 98 (85) | 101 (88) | 88 (77) | 104 (90) | 86 (75) | |||||||
| CE | ≤5 ng/L | 18 (16) | 23 (20) | 0.0313 | 0.529 | 24 (21) | 36 (31) | 0.0002 | 3.007 | 16 (14) | 41 (36) | <0.0001 | 4.83 |
| >5 ng/L | 97 (84) | 92 (80) | 91 (79) | 79 (69) | 99 (86) | 74 (64) | |||||||
| MMP-2 (kDa) | 93.17 ±10.05 | 92.09 ±9.87 | 0.0413 | 0.342 | 92.27 ±10.21 | 87.55 ±5.39 | <0.0001 | 6.883 | 91.61 ±9.84 | 91.39 ±9.82 | 0.0625 | N/A | |
| MMP-6 (kDa) | 220.7 ±11.86 | 220.02 ±11.76 | 0.001 | 1.217 | 225.25 ±6.23 | 223.15 ±5.31 | <0.0001 | 3.347 | 220.44 ±6.99 | 219.99 ±6.73 | <0.0001 | 2.12 | |
Constant data were represented as a number (percentage) and continuous data were represented as mean ±SD. n – sample size; BL – baseline; EP – after the end of the treatment of 12-weeks; CHA19-9 – carbohydrate antigen; CE – carcinoembryonic antigen; MMP-2 – matrix metalloproteinases-2; MMP-9 – matrix metalloproteinases-9.
Statistical Analysis between BL and EP.
N/A – Not applicable. A p-value for Wilcoxon rank sum test, q value for Turkey post hoc test. A p<0.05 and q>3.314 were considered as significant.
At the end of the post-surgical and post-treatment follow-up period of 48 months for all patients in the three groups, showed significant non-fatal treatment-related adverse events in the catalpol treatment group (p<0.0001, q=5.375) and the bevacizumab-treated control group (p<0.0001, q=24.242) (Figure 3). However, critical fatal treatment-related adverse events were found in the bevacizumab-treated control group only (p<0.0001, q=42.071) (Figure 4).
Figure 3.

Non-fatal adverse events in the study. For all groups, n=115. For statistical analysis, non-fatal adverse effects were considered to be 1; no adverse effect considered to be 0. Non-fatal adverse events were significantly associated with the catalpol treatment group (p<0.0001, q=5.375), compared with the control group (p<0.0001, q=24.242). p<0.05 and q>3.314 were considered to be significant.
Figure 4.

Fatal adverse events in the study. For all groups, n=115. For statistical analysis, fatal adverse effects were considered to be 1; no adverse effect considered to be 0. Only treatment with bevacizumab resulted in fatal adverse events (p<0.0001, q=42.071). p<0.05 and q>3.314 were considered to be significant.
With increasing follow-up duration, the percentage of cases of recurrence of colon cancer significantly increased in the placebo group (p<0.0001, q=8.655) and catalpol treatment group (p=0.0052, q=3.541), when compared with the control group (Figure 5). Overall survival (OS), irrespective of disease-free status, was significantly increased in the catalpol treatment group compared with the placebo group (p<0.0001, q=7.586), and was the same as the control group (p=0.3434, q=1.362) (Figure 6). The cost of treatment of colon cancer was significantly greater in the bevacizumab-treated control group when compared with the placebo group (p<0.0001, q=125.71) and with the catalpol treatment group (p<0.0001, q=207.17) (Figure 7).
Figure 5.

Recurrence of colon cancer following treatment. The response to the outcomes of each treatment followed the Response Evaluation Criteria In Solid Tumors (RECIST) guidelines [22]. For all groups, n=115. For statistical analysis, the percentage of recurrence of colon cancer in patients was considered to be 1; no recurrence of colon cancer in patients was considered to be 0. The percentage of cases of recurrence of colon cancer in patients was increased in the placebo group (p<0.0001, q=8.655) and the catalpol treatment group (p=0.0052, q=3.541) compared with the bevacizumab treatment group. p<0.05 and q>3.314 were considered to be significant.
Figure 6.

Overall survival (OS) associated with treatment. For all groups, n=115. For statistical analysis, survival was considered to be 1; patient death was considered to be 0. The catalpol treatment group significantly improved OS compared with the placebo group (p 0.0001, q=0.586), but did not have significantly improved OS when compared with the control group (p=0.3434, q1.362). p<0.05 and q>3.314 were considered to be significant.
Figure 7.

Comparative treatment costs. All data are shown as the mean ± standard deviation (SD), n=115. The bootstrap method of analysis was used. p<0.05 and q>3.314 were considered to be significant.
Discussion
The aim of this study was to evaluate the efficacy, safety, and cost of treatment of patients following surgical resection for locally advanced colon cancer, with the traditional Chinese herbal medicine, catalpol. This study included three groups of patients, a placebo-treated group, a catalpol treatment and a control group treated with bevacizumab (control group). There were no significant differences in the clinicopathological and demographic characteristics between the three patient groups, but overall survival (OS) and cancer-free survival (CFS) were significantly increased in the catalpol treatment group when compared with the placebo group and the control group.
In the present study, patients were treated with an intraperitoneal injection of catalpol. Until this in vivo study, the effects of catalpol have previously been reported in an in vitro study using CT26 colon cancer cell lines [2]. The present study was designed and conducted to investigate the effects of catalpol in patients who were diagnosed and surgically treated for locally advanced colon cancer, and was the first study to show an effect of catalpol in patients with colon cancer.
At the end of the follow-up period of 48 months, in patients who were treated with catalpol, only mild non-fatal treatment-associated adverse events were found, supporting the safe use of catalpol injection in human subjects [12,13]. The findings of this preliminary study are encouraging for the safe and effective use of catalpol as an adjuvant chemotherapeutic agent following surgical resection in colon cancer patients. However, further large-scale, controlled clinical studies are required to support these findings.
This study also showed that catalpol significantly reduced the levels of the serum biomarkers of colon cancer, including carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA), matrix metalloproteinases-2 (MMP-2), and matrix metalloproteinases-9 (MMP-9) [19]. These findings supported the effects of catalpol in patients with colon cancer.
Patients with locally advanced colon cancer, treated with the traditional Chinese herbal medicine, catalpol, were shown in this study to have significantly fewer tumor recurrences and increased OS, irrespective of other concurrent diseases, when compared with placebo and bevacizumab. The reduction of serum levels of MMP-2 and MMP-9 following catalpol treatment might explain the prevention of metastases in patients with colon cancer [27], which has implications for the anti-cancer chemotherapeutic effects of catalpol, including for colon cancer [10]. This study also showed that the cost of treatment with catalpol for patients with locally advanced colon cancer with was competitive when compared with the other chemotherapeutic agent, bevacizumab.
This study was preliminary and had several limitations. The study evaluated the effects of the traditional Chinese herbal medicine, catalpol with only one other chemotherapeutic agent, bevacizumab, in post-operative patients with locally advanced colon cancer. This study did not evaluate the effects of catalpol in patients treated with radiotherapy. Also, this study did not include detailed histopathologic tumor grading and classification and did not undertake sub-analysis of clinical aspects of the patients during diagnosis. The study did not include an analysis of the dose-dependent effects of catalpol. In future studies, analysis of individualized chemotherapeutic treatment with catalpol in a large study population is recommended, with a longer follow-up period, to improve the evaluation of adverse events, and of clinical outcome.
Conclusions
The findings of this preliminary study on the efficacy, safety, and cost of treatment of patients following surgical resection for locally advanced colorectal cancer were that treatment with the Chinese herbal medicine, catalpol, showed benefits in clinical outcome, at low cost, and with no serious complications.
Acknowledgements
The authors thank the medical and non-medical staff of Pingshan District Peoples’ Hospital of Shenzhen and Jingshan Maternal and Child Health Family Planning Service Center, the Peoples’ Republic of China. The authors also thank the pharmacists of Jingshan Maternal and Child Health Family Planning Service Center, Peoples’ Republic of China, for preparation of freeze-dried catalpol for injection.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Wang S, Wang H, Lu Y. Tianfoshen oral liquid: a CFDA approved clinical traditional Chinese medicine, normalizes major cellular pathways disordered during colorectal carcinogenesis. Oncotarget. 2017;8:14549–69. doi: 10.18632/oncotarget.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu P, Wu Y, Yang A, et al. Catalpol suppressed proliferation, growth, and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed Pharmacother. 2017;95:68–76. doi: 10.1016/j.biopha.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 3.Kim NK, Kim YW, Han YD, et al. Complete mesocolic excision and central vascular ligation for colon cancer: Principle, anatomy, surgical technique, and outcomes. Surg Oncol. 2016;25:252–62. doi: 10.1016/j.suronc.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Manjelievskaia J, Brown D, McGlynn KA, et al. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg. 2017;152:452–59. doi: 10.1001/jamasurg.2016.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren Y, Fleischmann D, Foygel K, et al. Antiangiogenic and radiation therapy early effects on in vivo computed tomography perfusion parameters in human colon cancer xenografts in mice. Invest Radiol. 2012;47:25–32. doi: 10.1097/RLI.0b013e31823a82f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favoriti P, Carbone G, Greco M, et al. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Wai Man GC, Liu Y, et al. Physiological and pathological angiogenesis in endometrium at the time of embryo implantation. Am J Reprod Immunol. 2017:78. doi: 10.1111/aji.12693. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Zhao Y, Wang X, et al. Wogonoside prevents colitis-associated colorectal carcinogenesis and colon cancer progression in inflammation-related microenvironment via inhibiting NF-κB activation through PI3K/Akt pathway. Oncotarget. 2016;7:34300–15. doi: 10.18632/oncotarget.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy – from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–31. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 10.Gao N, Tian JX, Shang YH, et al. Catalpol Suppresses proliferation and facilitates apoptosis of OVCAR-3 ovarian cancer cells through upregulating MicroRNA-200 and downregulating MMP-2 expression. Int J Mol Sci. 2014;15:19394–405. doi: 10.3390/ijms151119394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C, Wu F, Liu Y, Meng C. Catalpol suppresses proliferation and facilitates apoptosis of MCF-7 breast cancer cells through upregulating microRNA-146a and downregulating matrix metalloproteinase-16 expression. Mol Med Rep. 2015;12:7609–15. doi: 10.3892/mmr.2015.4361. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Wang Q, Yuan H, et al. [Effect of catalpol and puerarin freeze-dried powder on coagulability, hemorheology and no in rats with Qi-deficiency and blood-stasis syndrome]. Zhongguo Zhong Yao Za Zhi. 2012;37(10):1472–76. [in Chinese] [PubMed] [Google Scholar]
- 13.Zhang A, Hao S, Bi J, et al. Effects of catalpol on mitochondrial function and working memory in mice after lipopolysaccharide-induced acute systemic inflammation. Exp Toxicol Pathol. 2009;61:461–69. doi: 10.1016/j.etp.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Jin D, Cao M, Mu X, et al. Catalpol inhibited the proliferation of T24 human bladder cancer cells by inducing apoptosis through the blockade of akt-mediated anti-apoptotic signaling. Cell Biochem Biophys. 2015;7:1349–56. doi: 10.1007/s12013-014-0355-0. [DOI] [PubMed] [Google Scholar]
- 15.Benson AB, 3rd, Bekaii-Saab T, Chan E, et al. National Comprehensive Cancer Network. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:5519–28. doi: 10.6004/jnccn.2013.0069. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang C, Fengm Y-F, et al. Comparison of short-term outcomes between laparoscopic-assisted and open complete mesocolic excision (CME) for the treatment of transverse colon cancer. Chin Clin Oncol. 2016;6:6. doi: 10.21037/cco.2017.01.01. [DOI] [PubMed] [Google Scholar]
- 17.Matsui T, Nagata N, Hirata K, et al. Bi-weekly capecitabine-oxaliplatin (XELOX) plus bevacizumab as first-line treatment of metastatic colorectal cancer – The PHOENiX trial. Anticancer Res. 2016;36:3437–43. [PubMed] [Google Scholar]
- 18.Yeh YS, Tsai H, Huang CW, et al. Prospective analysis of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patients treated with bevacizumab plus FOLFIRI as the first-line setting: study protocol for a randomized controlled trial. Trials. 2016;17:46. doi: 10.1186/s13063-016-1153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokunaga R, Sakamoto Y, Nakagawa S, et al. The utility of tumor marker combination, including serum P53 antibody, in colorectal cancer treatment. Surg Today. 2016;47:636–42. doi: 10.1007/s00595-016-1464-8. [DOI] [PubMed] [Google Scholar]
- 20.Waas ET, Lomme RM, DeGroot J, et al. Tissue levels of active matrix metalloproteinase-2 and -9 in colorectal cancer. Br J Cancer. 2002;86:1876–83. doi: 10.1038/sj.bjc.6600366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L-N, Xiao WW, Xi S-Y, et al. Pathological assessment of the AJCC tumor regression grading system after preoperative chemoradiotherapy for Chinese locally advanced rectal cancer. Medicine (Baltimore) 2016;95(3):e2272. doi: 10.1097/MD.0000000000002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stattaus J. Oncological imaging for therapy response assessment. Radiologe. 2014;54:69–78. doi: 10.1007/s00117-013-2586-2. [DOI] [PubMed] [Google Scholar]
- 23.Tse VC, Ng WT, Lee V, et al. Cost-analysis of XELOX and FOLFOX4 for treatment of colorectal cancer to assist decision-making on reimbursement. BMC Cancer. 2011;11:288. doi: 10.1186/1471-2407-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endharti AT, Wulandari A, Listyana A, et al. Dendrophthoe pentandra (L.) Miq extract effectively inhibits inflammation, proliferation and induces p53 expression on colitis-associated colon cancer. BMC Complem Altern Med. 2016;16:374. doi: 10.1186/s12906-016-1345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouanet P, Rullier E, Lelong B, et al. the GRECCAR Study Group. Tailored treatment strategy for locally advanced rectal carcinoma based on the tumor response to induction chemotherapy: Preliminary results of the French phase II multicenter GRECCAR4 trial. Dis Colon Rectum. 2017;60:653–63. doi: 10.1097/DCR.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 27.Cepeda MA, Evered CL, Pelling JJH, Damjanovski S. Inhibition of MT1-MMP proteolytic function and ERK1/2 signalling influences cell migration and invasion through changes in MMP-2 and MMP-9 levels. J Cell Commun Signal. 2017;11:167–79. doi: 10.1007/s12079-016-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]