Abstract
Hydrogen sulfide (H2S) has emerged as an important physiological and pathophysiological signaling molecule in the cardiovascular system influencing vascular tone, cytoprotective responses, redox reactions, vascular adaptation, and mitochondrial respiration. However, bioavailable levels of H2S in its various biochemical metabolite forms during clinical cardiovascular disease remain poorly understood. We performed a case-controlled study to quantify and compare the bioavailability of various biochemical forms of H2S in patients with and without cardiovascular disease (CVD). In our study, we used the reverse-phase high performance liquid chromatography monobromobimane assay to analytically measure bioavailable pools of H2S. Single nucleotide polymorphisms (SNPs) were also identified using DNA Pyrosequencing. We found that plasma acid labile sulfide levels were significantly reduced in Caucasian females with CVD compared with those without the disease. Conversely, plasma bound sulfane sulfur levels were significantly reduced in Caucasian males with CVD compared with those without the disease. Surprisingly, gender differences of H2S bioavailability were not observed in African Americans, although H2S bioavailability was significantly lower overall in this ethnic group compared to Caucasians. We also performed SNP analysis of H2S synthesizing enzymes and found a significant increase in cystathionine gamma-lyase (CTH) 1364 G-T allele frequency in patients with CVD compared to controls. Lastly, plasma H2S bioavailability was found to be predictive for cardiovascular disease in Caucasian subjects as determined by receiver operator characteristic analysis. These findings reveal that plasma H2S bioavailability could be considered a biomarker for CVD in an ethnic and gender manner. Cystathionine gamma-lyase 1346 G-T SNP might also contribute to the risk of cardiovascular disease development.
Keywords: Hydrogen sulfide, HPLC, Monobromobimane, Coronary artery disease, Peripheral artery disease
Highlights
-
•
Baseline plasma sulfide metabolite levels are significantly different in an ethnic dependent manner.
-
•
Reductions in sulfide metabolites are predictive of cardiovascular disease in an ethnic dependent manner.
-
•
Differences in acid labile versus bound sulfane sulfur metabolites during cardiovascular disease are gender dependent.
-
•
Single nucleotide polymorphism of CTH 1364 G>T is significantly associated with increased cardiovascular disease.
1. Introduction
With recognition and definition of physiologic effects of nitric oxide, there has been increasing interest in the biological activity of the “other” gaseous signaling molecules, namely hydrogen sulfide (H2S) and carbon monoxide (CO). H2S is produced endogenously via enzymes of the transsulfuration pathway including cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL, CTH or CSE), as well as the mitochondrial enzyme 3-mercaptopyruvate sulfurtransferase (MST). H2S may also be generated through a non-enzymatic process from glucose (via glycolysis, NADPH oxidase), glutathione (direct reduction), inorganic and organic polysulfides (present in foods) or through elemental sulfur (direct reduction) [1], [2]. Alteration of H2S bioavailability and metabolism through many of these pathways are known to influence cardiovascular function and health in experimental models [3]. Unfortunately, the relationship of H2S bioavailability with clinical cardiovascular disease conditions remains poorly defined [4], [5].
Atherosclerotic cardiovascular disease is still the most common and costly cause of death in the United States and much of the world [6], [7]. Chronic vascular inflammation and sub-endothelial accumulation of foam cells stimulate occlusion and stenosis of blood vessels, which is a common culprit underlying peripheral and coronary arterial disease [8]. Studies have reported that metabolic dysfunction involving reduced production of cellular H2S may be a critical factor in the progression of experimental cardiovascular disease [2]. Endogenous H2S production is significantly reduced in the CTH (CSE) knockout mouse model, which is associated with impaired endothelial vasodilation and hypertension, increased production of reactive oxygen species, increased vascular inflammatory responses, and enhanced vascular atherosclerosis [9]. Further, H2S therapy using sulfide donors has been reported to diminish vascular inflammatory responses, decrease reactive oxygen species, and promote ischemic vascular remodeling/angiogenesis involving increased NO production [1].
Congruent with the current literature, H2S exists in different biochemical forms, including free or unbound sulfide (S2-, HS- or H2S), acid labile sulfide (ALS), and bound sulfane sulfur (BSS) [10], [11], [12]. These sulfur pools are crucial in regulating the total amount of bioavailable sulfide. ALS exists primarily in the form of iron–sulfur (Fe–S) complexes that modulate cellular functions including mitochondrial respiration and cytoplasmic redox reactions. BSS includes various compounds such as persulfides, polysulfides, thiosulfate, polythionates, thiosulfonates, bisorganylpolysulfanes or monoarylthiosulfonates, elemental sulfur, and many others. BSS compounds such as per/polysulfides can release H2S under reducing conditions suggesting that the cellular redox state is important for regulating its bioavailability. The precise chemistry through which these different biological pools of H2S interact to affect their pathophysiological functions is an area of active research. However, differences in bioavailability of these biochemical pools of sulfide remain largely unknown in part due to difficulties in measuring them. Overall, the sulfide field has been limited by controversies related to measurements of H2S in various biological systems. Our lab has established and validated analytical chemistry methods to accurately detect and quantify discrete H2S pools using a monobromobimane (MBB) assay coupled with reverse-phase high performance liquid chromatography (RP-HPLC), which was verified by electrospray ionization mass spectrometry [11], [13], [14].
In this study, we report findings of a clinical case-control study to accurately measure the amounts of different sulfide biochemical pools, namely ALS (with free sulfide combined), BSS, and total sulfide in subjects with coronary artery disease (CAD) or peripheral artery disease (PAD) compared to those without disease (controls). By combining clinically validated diagnoses with thoroughly established analytical chemistry techniques, these data provide important new insight regarding variations in bioavailability of sulfide biochemical pools and their association with cardiovascular disease states.
2. Materials & methods
2.1. Study design
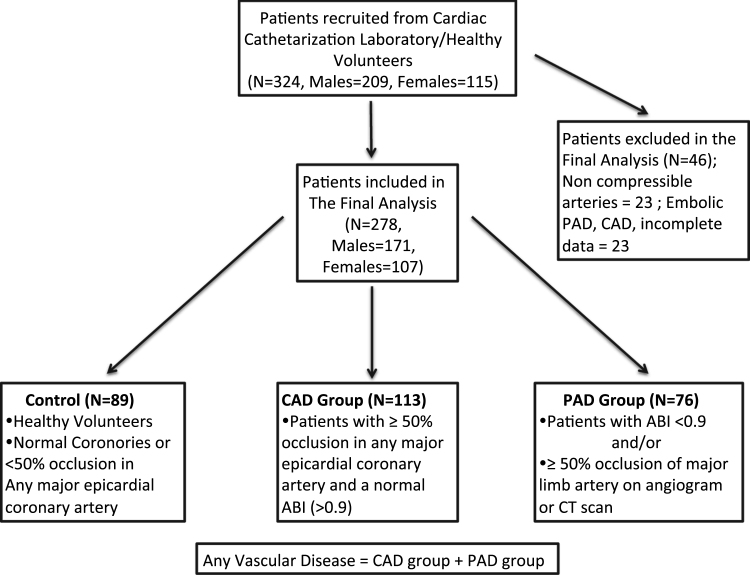
This was a case-control study approved by the Institutional Review Board (IRB) of Louisiana State University Health Sciences Center at Shreveport (LSUHSC-S). Patients over 40 years of age who presented to the cardiac catheterization laboratory at LSUHSC-S for coronary or peripheral angiography were recruited for this study. Healthy, age-matched volunteers were also enrolled as controls. Each patient's ankle brachial index (ABI) was measured as we previously described [6] and each patient was also administered the San Diego Claudication Questionnaire prior to angiography. Following exclusion criteria, the total study population consisted of 278 Caucasian and African American (AA) subjects categorized into three basic subgroups (Fig. 1):
Healthy controls: healthy volunteers and patients with less than 50% occlusion of all major coronary or peripheral arteries and a normal ABI (1.4 > ABI > 0.9).
Coronary arterial disease (CAD): patients with greater than or equal to 50% occlusion of any major coronary artery and a normal ABI (1.4 > ABI > 0.9).
Peripheral arterial disease (PAD): patients with greater than 50% occlusion of a major limb artery and/or an abnormal ABI (ABI < 0.9).
Fig. 1.
Study organization flow chart. A total of 324 patients were used for analysis, including 209 males and 115 females. This subject population was subsequently diagnosed with or without CAD or PAD after cardiac catheterization. CAD indicates coronary artery disease; PAD, peripheral artery disease.
2.2. Exclusion criteria
Volunteers who were excluded from this study were those who could not provide informed consent, were participating in another clinical trial involving experimental therapeutics, or were pregnant or nursing. Patients with ST elevated myocardial infarction or cardiogenic shock were not included to avoid interference with time-sensitive revascularization and confusion of pathophysiological events. Additional exclusion criteria included patients with an ABI > 1.4 (due to non-compressible arteries) or patients with Buerger's disease (non-atherosclerotic PAD).
2.3. History and blood collection
Patients were interviewed and medical record data were collected for analysis of typical cardiovascular risk factors such as hypertension, diabetes, obesity, tobacco use, and dyslipidemia. Blood samples were collected from already-established catheterization into 6 mL BD vacutainer tubes with lithium heparin. Samples were transported to the lab within 15 min on ice and were centrifuged at 1500 RCF for 4 min at 4 °C.
2.4. Measurement of biological pools of H2S
Plasma samples were analyzed for free sulfide, ALS, BSS, and total sulfide levels as we have previously reported [11], [13]. Free sulfide was measured using the MBB method as previously reported [11]. For detection of ALS and BSS, 50 μl of plasma was added separately into two sets of 4 mL BD vacutainer tubes. Four hundred fifty microliters of 100 mM phosphate buffer (pH 2.6, 0.1 mM DTPA) was added to one tube [acid labile reaction] and 450 μl of 100 mM phosphate buffer (pH 2.6, 0.1 mM DTPA) plus 1 mM TCEP was added to the second tube [total sulfide reaction]. Following a 30-min incubation on a nutator, the reaction liquid was removed and sulfide gas subsequently trapped by adding 500 μl of 100 mM Tris-HCl buffer (pH 9.5, 0.1 mM DTPA) into the BD vacutainer tube and incubated again for 30 min on a nutator mixer. The trapping solutions were removed and sulfide levels measured using the MBB method as we have previously reported [13]. Determination of ALS was made by reacting plasma samples with acidic phosphate buffer alone and subsequent trapping of evolved sulfide. Measurement of BSS was determined by subtracting the acid labile value from the total sulfide protocol containing TCEP reductant treatment under acidic conditions. Total sulfide levels were directly obtained from the total sulfide reaction.
2.5. MBB assay and RP-HPLC detection
Thirty microliters of reaction buffer with trapped sulfide was transferred to a PCR tube and mixed with 70 μl of H2S stabilization buffer (100 mM Tris-HCl, 0.1 mM DTPA, pH 9.5) and 50 μl MBB solution (10 mM). Samples were then incubated in a hypoxic chamber (1% O2) for 30 min at room temperature. The reaction was stopped by adding 50 μl of 200 mM sulfosalicylic acid, followed by centrifugation at 12,000 rpm for 10 min at 4 °C. One hundred microliters of supernatant was collected for RP-HPLC. Ten microliters of the supernatant was transferred into the RP-HPLC system with an Agilent Eclipse XDB-C18 column (5 µm, 80 Å, 4.6 mm × 250 mm) equilibrated with 15% CH3CN in water containing 0.1% (v/v) TFA for fluorescence detection (excitation: 390 nm; emission: 475 nm).
MBB and sulfide-dibimane were separated using the gradient of two mobile phases: (A) water containing 0.1% (v/v) TFA and (B) 99.9% CH3CN, 0.1% (v/v) TFA at a flow rate of 0.6 mL/min. Retention time for sulfide-dibimane is 16.5 min and MBB is 17.6 min. The amount of H2S was measured from linear plots of the HPLC peak areas of sulfide-dibimane versus standard concentration of sulfide solution.
2.6. Statistical analysis
Levels of ALS, BSS and total H2S in the three subgroups were first assessed by group means and standard deviations with subsequent pairwise comparison using analysis of variance (ANOVA). Multiple linear regression analysis was conducted to delineate the relationship between sulfide pools and the dependent variables including race, gender, diagnosis of CAD and PAD, and cardiovascular risk factors. Receiver-operating characteristic analysis (ROC) was conducted to assess the predictive accuracy in correlating sulfide levels with CAD or PAD diagnosis. Cutoff values for positive classification were included in the curve, with a nonparametric distribution assumption and a confidence level of 95%. All statistical analyses were performed using GraphPad Prism 5.0.
3. Results
3.1. Sulfide bioavailability associated with cardiovascular disease
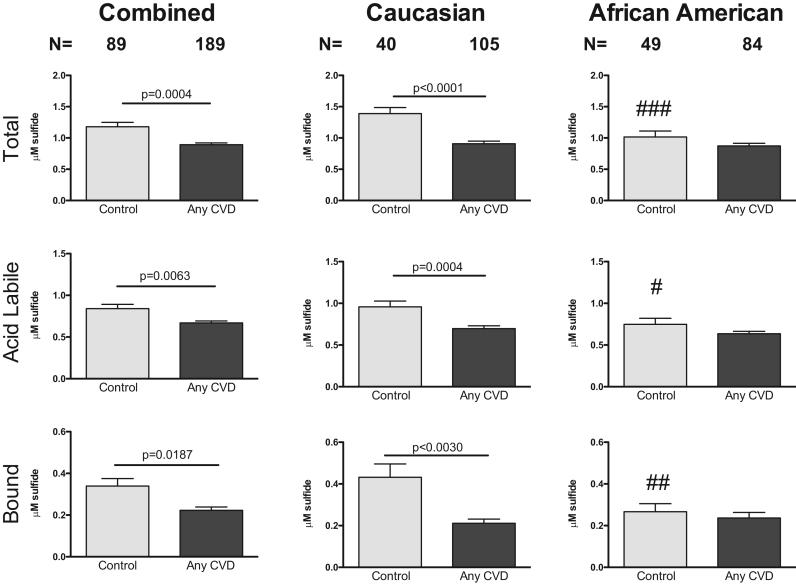
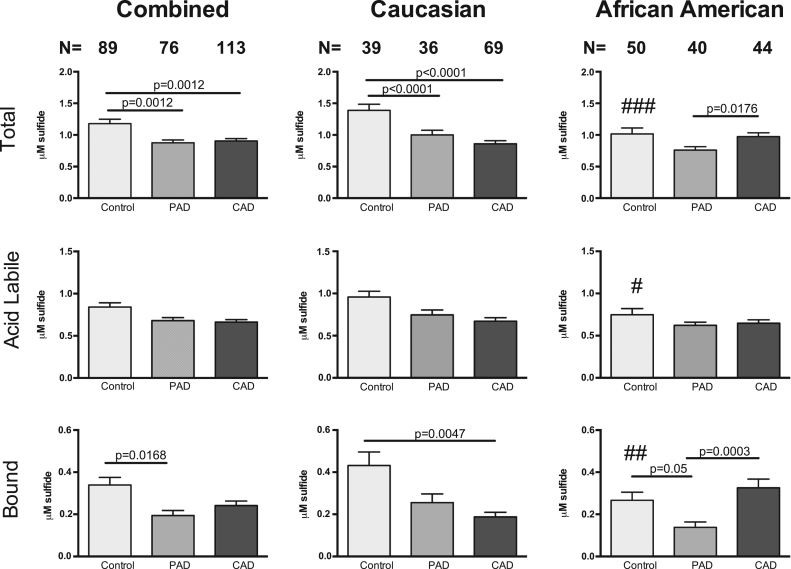
Plasma sulfide bioavailability was initially compared between control subjects and patients with any form of cardiovascular disease (any CVD). Fig. 2 illustrates that total, acid labile, and bound sulfide were all significantly reduced in patients with any CVD. Stratifying subjects based on ethnicity revealed a significant reduction in plasma total, ALS, and BSS in CVD patients compared to controls among Caucasian subjects, which was not observed in African Americans. However, comparison of control cohorts of African Americans to Caucasians revealed a significant reduction in plasma total sulfide (1.015 vs 1.389) and BSS levels (0.431 vs 0.266) in the African Americans.
Fig. 2.
Plasma sulfide bioavailability by ethnicity. Total sulfide, acid labile pools, and bound sulfide in total subject populations (combined), Caucasian and African American subjects respectively with any form of CVD. CVD indicates cardiovascular disease. Control Caucasian vs African Americans ### p=0.0001; #p=0.0028; ##p=0.0326.
3.2. Sulfide bioavailability as a function of gender
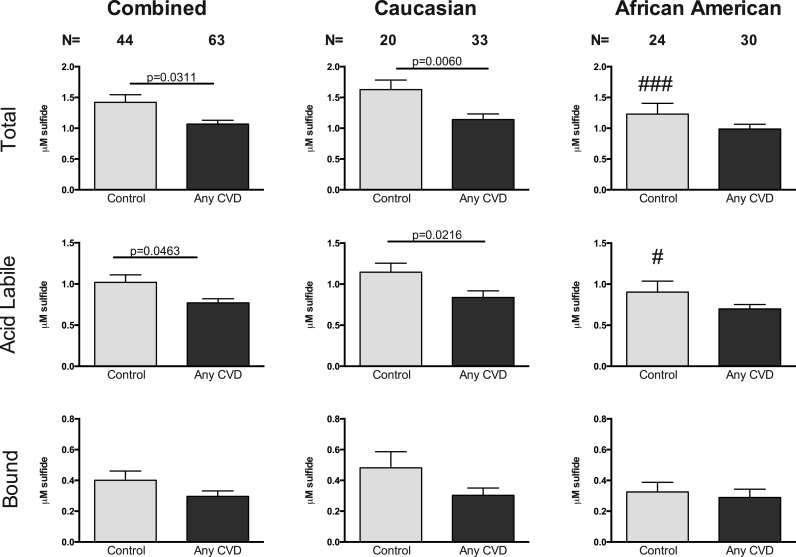
The association of plasma sulfide levels was compared between the subject population with and without CVD based on gender. Fig. 3 illustrates that women with any CVD display a significant reduction in total sulfide and ALS levels. There were no significant differences in BSS levels. Stratifying by race and gender further revealed that Caucasian female CVD patients had significantly reduced total sulfide and ALS levels compared to African American female CVD patients. Fig. 4 demonstrates that all males with any CVD had significantly reduced plasma total and BSS levels. Segregation by race and gender again revealed that plasma total and BSS levels were significantly reduced only in Caucasian males with CVD. Together, these data reveal discrete novel differences in plasma sulfide metabolites during cardiovascular disease between males and females.
Fig. 3.
Plasma sulfide pools in Women by ethnicity. Total sulfide, acid labile pools, and bound sulfide levels of Plasma have been displayed in female subjects with and without any CVD. CVD indicates cardiovascular disease. Control Caucasian vs African Americans ###p=0.0031; #p=0.06555.
Fig. 4.
Plasma sulfide pools in Men by ethnicity. Total sulfide, acid labile pools, and bound sulfide levels of Plasma have been displayed in male subjects with and without any CVD. CVD indicates cardiovascular disease. Control Caucasian vs African Americans ###p=0.0338; #p=0.03085.p = .0338; #p = .03085.
3.3. Sulfide bioavailability as a function of coronary or peripheral artery disease
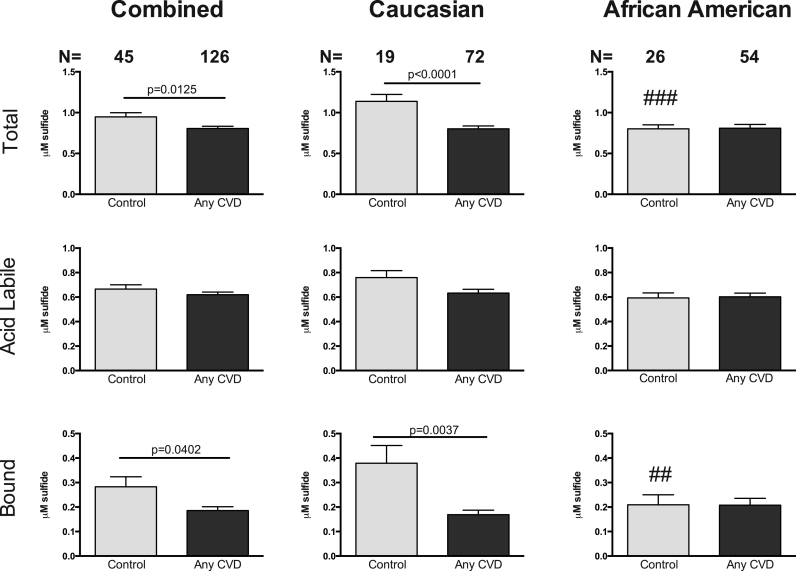
Plasma sulfide metabolite levels were next analyzed based on the diagnosis of either CAD or PAD. Importantly, a majority of patients (>85%) diagnosed with PAD were also diagnosed with CAD indicating broad CVD, which has been previously reported [4], [15]. Stratifying CVD subjects diagnosed as PAD or CAD revealed a significant reduction in plasma total and BSS levels (Fig. 5). Plasma total and BSS levels in Caucasian subjects with either CAD or PAD were significantly lower compared to controls, while levels of ALS were not significantly reduced. African American subjects with PAD had reduced BSS compared to controls. Conversely, BSS levels were significantly elevated in African Americans with CAD compared to PAD subjects but not that of controls. Together, these data reveal that plasma sulfide bioavailability is predominantly reduced in Caucasian subjects with either CAD or PAD. A comparison of different ethnicities between control cohorts revealed significantly reduced base levels of plasma sulfide in African Americans to Caucasians.
Fig. 5.
Sulfide bioavailability in CAD and PAD subjects. Plasma total, acid labile and bound sulfane sulfide pools in a combined or total subject population, Caucasian and African American subjects with either CAD or PAD. CAD indicates Coronary arterial disease; PAD, Peripheral arterial disease. Control Caucasian vs African Americans ###p=0.0001; #p=0.0028; ##p=0.0326.
3.4. Sulfide as an indicator of cardiovascular disease
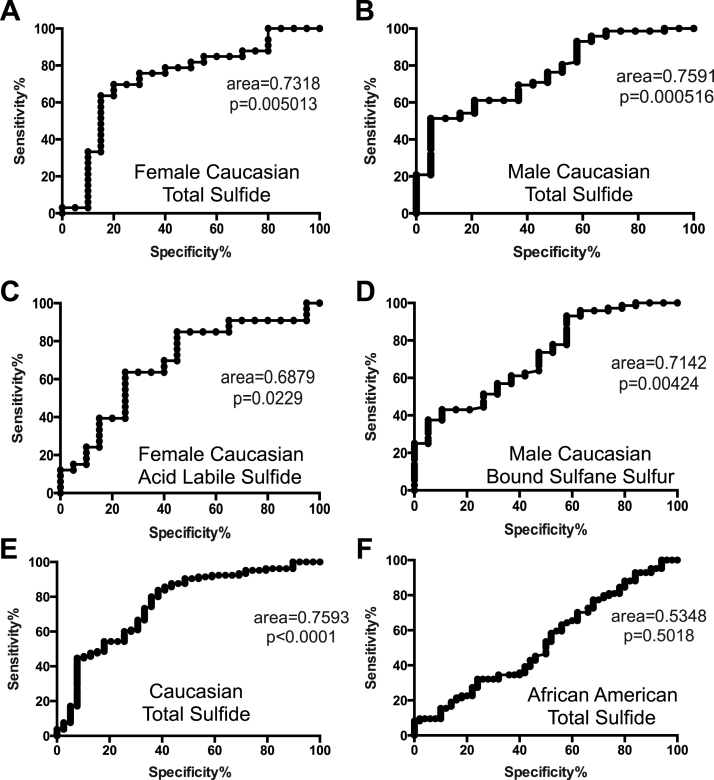
Based on the observed differences in plasma sulfide measurements between CVD patients and controls in a gender and ethnic manner, we next performed receiver operator analysis to determine the accuracy of reduced sulfide levels as an indicator for CVD. Caucasian male and female plasma total sulfide levels were analyzed separately revealing an area under the curve (AUC) of 0.7591 (p = 0.0005) for males and 0.7318 (p = 0.005) for females (Figs. 6A and 6B). Having observed significant gender differences of Caucasian plasma sulfide metabolite pools, these data were further analyzed using plasma ALS in females and plasma BSS in males. Fig. 6C shows the female Caucasian ALS AUC was 0.6879 (p=0.022), whereas the male Caucasian BSS was 0.7142 (p=0.0042) (Fig. 6D). Lastly, plasma total sulfide levels were analyzed based on ethnicity and found to be a statistically significant indicator of CVD in Caucasian patients regardless of gender, with an AUC of 0.76 (p < 0.0001) (Fig. 6E). However, total sulfide levels were not identified as an indicator for CVD in African American patients (Fig. 6F).
Fig. 6.
Receiver-operating characteristic analysis (ROC) in subjects with CVD. ROC curves with area under the curve of Caucasian population A. total sulfide levels in females B. total sulfide levels in males C. Acid labile pools in females D. Bound sulfane sulfur in males and E. total sulfide. F. Total sulfide levels in African American CVD patients. CVD indicates cardiovascular disease.
Linear regression analysis was further utilized to determine relationships between sulfide biochemical metabolite pools with various dependent variables related to cardiovascular disease including age, sex, diagnosis, BMI, tobacco use, and dyslipidemia based on subject demographics (Table 1). Regression analysis of CVD risk factors for all subjects revealed significant associations with ethnicity, diagnosis, and gender. Importantly, in both Caucasian and African American subjects, females were associated with higher levels of total sulfide, ALS, and BSS levels than males (Table 2, Table 3). The most consistent linear regression trends were seen in Caucasian subjects with cardiovascular disease (Table 2). Among this subgroup, total sulfide, ALS, and BSS levels were significant and most strongly associated with a diagnosis of CVD, along with other significant yet weaker associations of gender, hypertension, and smoking status. While a weak association with diagnosis was also observed in African Americans, these results were not as significant compared to Caucasians (Table 3, Table 4).
Table 1.
Subject Demographics.
|
Controls |
PAD |
CAD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 54.44 ± 8.27 |
58.73 ± 7.94 |
57.83 ± 8.86 |
||||||
| Total | AA | Cauc | Total | AA | Cauc | Total | AA | Cauc | |
| 89 | 50 | 39 | 76 | 40 | 36 | 113 | 44 | 69 | |
| Males | 45(51%) | 26 | 19 | 60(78%) | 35 | 25 | 66(58%) | 19 | 47 |
| Females | 44(40%) | 24 | 20 | 16(22%) | 5 | 11 | 47(42%) | 25 | 22 |
| DM | 27(30%) | 19 | 8 | 29(38%) | 14 | 15 | 48(42%) | 26 | 22 |
| HTN | 70(78%) | 47 | 23 | 62(81%) | 39 | 33 | 104(92%) | 61 | 43 |
| Smokers | 29(32%) | 16 | 13 | 37(49%) | 20 | 17 | 38(34%) | 10 | 28 |
| BMI>30 | 43(48%) | 31 | 12 | 25(32%) | 12 | 13 | 64(56%) | 23 | 31 |
AA = African American; Cauc = Caucasian; PAD = Peripheral Arterial Disease; CAD = Coronary Artery Disease.
DM = Diabetes Mellitus; HTN = Hypertension; BMI = Body Mass Index.
Table 2.
Regression analysis of CVD risk factors for all subjects.
| Dependent Variable | Total Sulfide |
Acid Labile Sulfide |
Bound Sulfide |
|||
|---|---|---|---|---|---|---|
| β1 | p value | β1 | p value | β1 | p value | |
| Age | −0.911 | 0.363 | −0.743 | 0.572 | −2.684 | 0.162 |
| Sex | 0.329 | 0.001 | 0.270 | 0.001 | 0.414 | 0.001 |
| Race | −0.104 | 0.075 | −0.195 | 0.006 | −0.074 | 0.507 |
| Diagnosis | −0.661 | 0.001 | −0.835 | 0.001 | −0.930 | 0.004 |
| BMI>30 | 1.487 | 0.136 | 1.460 | 0.272 | 2.322 | 0.215 |
| Smoking Status | −0.222 | 0.015 | −0.234 | 0.053 | −0.354 | 0.044 |
| Dyslipidemia | −0.013 | 0.828 | −0.017 | 0.823 | −0.032 | 0.765 |
| Hypertension | −0.056 | 0.088 | −0.070 | 0.106 | −0.087 | 0.166 |
Table 3.
Regression analysis of CVD risk factors for Caucasian subjects.
| Dependent Variable | Total Sulfide |
Acid Labile Sulfide |
Bound Sulfide |
|||
|---|---|---|---|---|---|---|
| β1 | p value | β1 | p value | β1 | p value | |
| Age | −0.057 | 0.965 | 0.413 | 0.804 | −2.671 | 0.259 |
| Sex | 0.391 | 0.001 | 0.251 | 0.005 | 0.442 | 0.001 |
| Diagnosis | −1.218 | 0.001 | −1.327 | 0.001 | −1.780 | 0.001 |
| BMI>30 | 3.946 | 0.0141 | 5.342 | 0.010 | 2.064 | 0.459 |
| Smoking Status | −0.310 | 0.0120 | −0.308 | 0.054 | −0.482 | 0.032 |
| Dyslipidemia | −0.111 | 0.158 | −0.109 | 0.357 | −0.225 | 0.113 |
| Hypertension | −0.127 | 0.0246 | −0.150 | 0.043 | −0.175 | 0.091 |
Table 4.
Regression analysis of CVD risk factors for African American subjects.
| Dependent Variable | Total Sulfide |
Acid Labile Sulfide |
Bound Sulfide |
|||
|---|---|---|---|---|---|---|
| β1 | p value | β1 | p value | β1 | p value | |
| Age | −1.922 | 0.217 | −2.323 | 0.274 | −2.748 | 0.382 |
| Sex | 0.276 | 0.001 | 0.338 | 0.003 | 0.383 | 0.023 |
| Diagnosis | −0.164 | 0.503 | −0.386 | 0.247 | 0.178 | 0.720 |
| BMI>30 | −0.222 | 0.861 | −1.596 | 0.354 | 2.591 | 0.309 |
| Smoking Status | −0.159 | 0.247 | −0.200 | 0.284 | −0.206 | 0.458 |
| Dyslipidemia | 0.0850 | 0.303 | 0.060 | 0.592 | 0.2143 | 0.198 |
| Hypertension | 0.0408 | 0.165 | 0.057 | 0.157 | 0.0421 | 0.478 |
3.5. Single Nucleotide Polymorphisms (SNPs)
A study by Wang et al. suggests that genetic variation in cystathionine gamma-lyase (CTH) is associated with elevated plasma homocysteine levels [16]. To further identify any such associations with CVD, we screened a subset of our patient population for SNPs in three enzymes related to H2S and NO metabolism to determine if variations in allele frequencies existed between CVD patients and controls. After screening for 7 SNPs in the CTH, CBS, and eNOS (endothelial nitric oxide synthase) genes, we identified 2 polymorphisms with considerable variation in allele frequencies among our patient population: 1 SNP in the NOS3 gene and 1 in the CTH gene.
The CTH 1364 G>T SNP (Rs1021737) involves a point mutation in exon 12 of the CTH gene that was previously identified in patients with hyperhomocysteinemia [16], [17]. In our patient population, the mutant 1364 T allele frequency was higher in patients with CVD (0.265) and either CAD or PAD (0.256 or 0.273) compared to controls (0.107) (Table 5). We also identified an SNP in exon 8 of the eNOS gene (894 G>T) with considerable allelic variation among our patient population. The 894 G>T mutation (Rs1799983) has been previously identified in several studies among patients with hypertension, cerebrovascular disease, and CAD [18], [19], [20]. The allele frequency of this mutation was highest in patients with CAD compared to controls (0.273 vs. 0.143, respectively; Table 5). These data suggest that the CTH 1364 G>T SNP is a potential risk factor with increasing mutation allele frequency with CVD including PAD and CAD, whereas eNOS 894 G>T was associated with increased risk of CAD.
Table 5.
Mutant allele frequencies of CTH, CBS, and NOS3 single-nucleotide polymorphisms. CTH: Cystathionine γ-lyase; CBS: cystathionine β-synthase; NOS3: endothelial NO synthase; PAD: Peripheral artery disease; CAD: Coronary Artery Disease; CVD: Cardiovascular Disease (PAD and/or CAD); N/A: not applicable – mutant allele not detected within cohort.
| Gene symbol | Chromosome | Polymorphism | dbSNP | Mutant Allele Frequency |
|||
|---|---|---|---|---|---|---|---|
| Controls | PAD | CAD | CVD | ||||
| N = 28 | N = 41 | N = 44 | N = 85 | ||||
| CTH | 1p31 | 1364 G > T | Rs1021737 | 0.107 | 0.256 | 0.273 | 0.265 |
| CTH | 1p31 | 356 C > T | Rs28941785 | N/A | N/A | 0.023 | 0.011 |
| CTH | 1p31 | 874 C > G | Rs28941786 | N/A | N/A | N/A | N/A |
| CBS | 21q22 | 833 T > CT | Rs5742905 | 0.035 | 0.02 | N/A | 0.021 |
| CBS | 21q22 | 1058 C > | Rs121964972 | N/A | N/A | 0.012 | 0.006 |
| NOS3 | 7q36 | 894 G > T | Rs1799983 | 0.143 | 0.159 | 0.273 | 0.224 |
| NOS3 | 7q36 | − 786 T > C | Rs2070744 | 0.232 | 0.159 | 0.318 | 0.241 |
4. Discussion
Hydrogen sulfide metabolism and its bioavailability have been associated with vascular dysfunction and disease, which has been demonstrated in several experimental models [5], [9], [21]. However, the relationship between sulfide bioavailability and clinically validated cardiovascular disease remains poorly understood due to lack of a reliable and sensitive analytical measurement of sulfide in its various biochemical forms coupled with well defined, clinically validated subjects. For the first time, our study indicates an association of polysulfide to any form of vascular disease. We have reported the levels of total, acid labile and bound sulfane sulfide in plasma samples from clinical subjects of vascular disease using validated analytical chemistry methodology that others and we have extensively characterized [10], [11], [12], [13], [14]. Importantly, we found that subjects with CVD have lower bound and total sulfide metabolites compared to control subjects, which were observed in an ethnic and gender specific manner. As we have previously reported, the relevance of different sulfide biochemical forms including ALS and BSS represent biochemical sulfide reservoirs. These sulfide equivalents may be important under various pathophysiological conditions, which might be inter-convertible depending on pH and redox balance [1], [2]. Thus, the ALS and BSS pools are postulated to be a ‘reversible sulfide sink’ as free H2S is ephemeral, making it difficult to account for its prolonged physiological actions.
The biological effects of H2S are increasingly attributed to per/polysulfides that are produced endogenously in many cells and tissues of mammalian origin. These per/polysulfides can reversibly generate H2S by their degradation, which could play critical physiological roles [22], [23], [24]. Additionally, conversion of a thiol to the corresponding hydropersulfide can result in a change of catalytic activity within the protein. The physiological significance of BSS is not completely understood but has recently been appreciated beyond being a storage form of sulfide. Persulfides such as alkyl hydropersulfides may be generated via H2S-independent mechanisms and can have a greater biological activity [22], [25]. As mentioned earlier, the literature suggests that BSS includes compounds such as per/polysulfides. However, the role of BSS in clinical complications such as cardiovascular disease has not been identified. Here, we observed lower levels of BSS in subjects with vascular disease and those with major cardiovascular risk factors. This could be either a manifestation of the disease state itself or a “compensatory mechanism” that enables movement of sulfide to more bioavailable pools (H2S/S2-/HS-) that exert beneficial effects including anti-oxidation, vasodilation or angiogenesis. In support of the latter theory, BSS has been shown to release H2S in reducing conditions [2]. This BSS-derived sulfide could then have biological implications that are known to be associated with H2S.
BSS also appears to be a major product of the cysteine aminotransferase and MST pathway and has been proposed to be the major pathway for sulfide signaling in the brain [26], [27]. H2S can be liberated from MST – BSS by the ubiquitous reductant, thioredoxin and by dihydrolipoic acid, both present in cells. Additionally, CTH and CBS can also generate BSS in tissues using thiol substrates including homocysteine, cysteine, cystathionine, and cystine, with cysteine hydropersulfide (Cys-SSH) as an intermediate [22]. A very recent study has demonstrated that cysteine tRNA synthase (CARS), which is involved in cysteine metabolism and aminoacyl-tRNA synthesis is another major source of cysteinepersulfide in vivo [28]. Interestingly, the kinetics of these enzymes vary with conditions such as oxidative stress, that influence changes in the generation of reactive persulfide species [29].
A previous study from our lab reported elevated levels of plasma free H2S in subjects with vascular disease [4]. Supplementary table 1 shows that free sulfide levels were not significantly different within this current cohort of subjects. An explanation for this observation may be due to the inclusion of subjects with acute coronary syndromes (ACS) or critical limb ischemia (CLI) in our previous study [4], which are not included here. Importantly, alteration of free H2S is known to occur where hypoxia is an active component of tissue dysfunction and may contribute to our previous findings [2], [9]. Additional studies are planned to examine the relationship between ACS or CLI and plasma sulfide metabolites.
It is well known through numerous studies that African American (AA) subjects are more predisposed to vascular disease [30], [31], [32], [33]. Interestingly, we observed low levels BSS in male AA subjects. Specifically, AA subjects with PAD had reduced BSS compared to controls. Low BSS could potentially be a marker for increased risk for vascular disease in AA subjects. Conversely, lower BSS levels in Caucasian subjects with vascular disease could indicate that BSS has a protective effect in Caucasians that is lost in vascular disease. These observations suggest that BSS may serve as a dynamic sulfide metabolite influencing vascular disease, which requires further study.
An alternative hypothesis is that reduced sulfide metabolites represent deficient endogenous production of sulfide due to the development of CVD itself. Low sulfide levels have been shown to accelerate experimental atherosclerosis, supporting the notion that low BSS is a manifestation of the diseased vasculature. In a study by Mani et al., it was shown that decreased endogenous production of H2S led to accelerated atherosclerosis [34]. In another study, Zavaczki et al. has shown that hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells and suggested that low H2S could promote vascular calcification seen in atherosclerosis [35]. Several studies demonstrate an increase in production of reactive oxygen species (ROS) in atherosclerosis [36]. BSS is known to be an antioxidant and its low levels could be a manifestation of its utilization and consumption in redox reactions. This may explain the low BSS levels as well as the protective effect of sulfide. That low H2S is a manifestation of the disease state is also supported by our observation of low BSS under CVD. However, future studies are required to better understand specific pathophysiological relationships between sulfide bioavailability and vascular disease.
In conjunction with these observations, ethnicity and gender were found to be significant variables associated with total sulfide, ALS, and BSS with a diagnosis of CVD. Regression analyses show a significant reduction in plasma sulfide levels with onset of CVD in Caucasian patients. Notably, a significant association is observed with total sulfide, ALS, and BSS levels in females in comparison to the males in both Caucasians and African Americans. Additionally, SNP analysis of H2S synthesis enzymes revealed that polymorphisms in CTH (but not CBS) are a potential risk factor for vascular disease development. It is known that CTH is a key enzyme for production of H2S in the cardiovascular system. Others and we have previously reported that endogenous H2S production is significantly reduced in CTH (CSE) deficient mice, which could translate to clinical implications for the development of vascular disease [9], [34]. Deficiency in bioavailable sulfide may be associated with polymorphic variants of CTH as a previous report revealed an association of CTH SNP with increased serum homocysteine levels [16]. Our findings regarding CTH 1364 G-T allele frequency in patients with CVD advances the hypothesis that a CTH SNP 403Ser to 403Ile is associated with decreased sulfide bioavailability and has clinical associations in patients with chronic vascular disease conditions. However, future studies are needed to identify how this missense mutation alters enzyme function in vivo compared to a previous enzymatic study that did not find alterations in CTH pyridoxal-5’-phosphate cofactor content or steady state kinetic properties [36].
4.1. Study limitations
Our study is not without limitations. All subjects were included who presented for cardiac or peripheral arterial catheterization, and for whom an accurate diagnosis could be made. In general, subjects who were scheduled for cardiac catheterization, even if they do not have the clinically defined disease, are usually those with greater cardiovascular risk factors. In an attempt to mitigate this limitation, normal volunteers with less than 2 cardiovascular risk factors and no history of cardiovascular disease were also included in the control group but not subjected to catheterization, as doing this procedure in healthy subjects is not justifiable (for obvious reasons). A second limitation is the fact that ABI was used to detect PAD. Even though ABI is a good screening tool its utility in diagnosing PAD accurately may be limiting, in that a few patients who have PAD could have been missed. Some of our patients had indeterminate ABIs, which would require further clinical workup with an ultrasound modality to rule out PAD if there is high clinical suspicion, which was not done in this study. A third limitation is that we could not assess the role of hypertension as an important risk factor for CVD. This is due to the fact that more than 90% of patients scheduled for cardiac catheterization had hypertension and we were unable to establish a meaningful “normotensive” group. A final limitation was the smaller sample size associated with the genetic polymorphism study. However, this study produced convincing preliminary evidence that mutations in CTH and eNOS genes are higher among patients with CVD, with these initial findings substantiating the need for further investigation. These limitations aside, our findings reveal that reduced sulfide metabolite bioavailability is significantly associated with cardiovascular disease along with increased single nucleotide polymorphisms of CTH. Lastly, additional studies distinguishing specific inorganic and organic per/polysulfides such as cysteine or glutathione-related sulfur species in CVD are needed to better understand the relationship of these metabolites with CVD.
Acknowledgements
This work was funded in part by a Translational Research Grant from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center-Shreveport to Dr. Saurabh Rajpal and NIH grant HL113303 to Dr. Christopher G Kevil.
Acknowledgments
Disclosures
Xinggui Shen and Christopher G Kevil have intellectual property on the use of gasotransmitter measurements for clinical cardiovascular disease. Christopher G Kevil has intellectual property regarding H2S measurement and equity in Innolyzer, LLC. Xinggui Shen has intellectual property regarding H2S measurement and is a consultant for Innolyzer, LLC.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.01.007.
Appendix A. Supplementary material
Supplementary material
References
- 1.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 2.Kolluru G.K., Shen X., Bir S.C., Kevil C.G. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide: Biol. Chem. / Off. J. Nitric Oxide Soc. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvert J.W., Coetzee W.A., Lefer D.J. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid. Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peter E.A., Shen X., Shah S.H., Pardue S., Glawe J.D., Zhang W.W., Reddy P., Akkus N.I., Varma J., Kevil C.G. Plasma free H2S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2013;2:e000387. doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bir S.C., Kolluru G.K., McCarthy P., Shen X., Pardue S., Pattillo C.B., Kevil C.G. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J. Am. Heart Assoc. 2012;1:e004093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faxon D.P., Creager M.A., Smith S.C., Jr., Pasternak R.C., Olin J.W., Bettmann M.A., Criqui M.H., Milani R.V., Loscalzo J., Kaufman J.A., Jones D.W., Pearce W.H., American Heart A. atherosclerotic vascular disease conference: executive summary: atherosclerotic vascular disease conference proceeding for healthcare professionals from a special writing group of the American heart association. Circulation. 2004;109:2595–2604. doi: 10.1161/01.CIR.0000128517.52533.DB. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group M., Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., de Ferranti S., Despres J.P., Fullerton H.J., Howard V.J., Huffman M.D., Isasi C.R., Jimenez M.C., Judd S.E., Kissela B.M., Lichtman J.H., Lisabeth L.D., Liu S., Mackey R.H., Magid D.J., McGuire D.K., Mohler E.R., 3rd, Moy C.S., Muntner P., Mussolino M.E., Nasir K., Neumar R.W., Nichol G., Palaniappan L., Pandey D.K., Reeves M.J., Rodriguez C.J., Rosamond W., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Woo D., Yeh R.W., Turner M.B. American heart association statistics C and stroke statistics S. heart disease and stroke statistics-2016 update: a report From the American heart association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 8.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 9.Kolluru G.K., Bir S.C., Yuan S., Shen X., Pardue S., Wang R., Kevil C.G. Cystathionine gamma-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc Res. 2015;107:590–600. doi: 10.1093/cvr/cvv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ubuka T., Abe T., Kajikawa R., Morino K. Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2001;757:31–37. doi: 10.1016/s0378-4347(01)00046-9. [DOI] [PubMed] [Google Scholar]
- 11.Shen X., Peter E.A., Bir S., Wang R., Kevil C.G. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic. Biol. Med. 2012;52:2276–2283. doi: 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogasawara Y., Ishii K., Togawa T., Tanabe S. Determination of trace amounts of sulphide in human red blood cells by high-performance liquid chromatography with fluorimetric detection after derivatization with p-phenylenediamine and iron(III) Analyst. 1991;116:1359–1363. doi: 10.1039/an9911601359. [DOI] [PubMed] [Google Scholar]
- 13.Shen X., Kolluru G.K., Yuan S., Kevil C.G. Measurement of H2S in vivo and in vitro by the monobromobimane method. Methods Enzymol. 2015;554:31–45. doi: 10.1016/bs.mie.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolluru G.K., Shen X., Kevil C.G. Detection of hydrogen sulfide in biological samples: current and future. Expert Rev. Clin. Pharmacol. 2011;4:9–12. doi: 10.1586/ecp.10.132. [DOI] [PubMed] [Google Scholar]
- 15.Golomb B.A., Dang T.T., Criqui M.H. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Hegele R.A. Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH) Hum. Genet. 2003;112:404–408. doi: 10.1007/s00439-003-0906-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Huff A.M., Spence J.D., Hegele R.A. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin. Genet. 2004;65:483–486. doi: 10.1111/j.1399-0004.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 18.Hingorani A.D., Liang C.F., Fatibene J., Lyon A., Monteith S., Parsons A., Haydock S., Hopper R.V., Stephens N.G., O'Shaughnessy K.M., Brown M.J. A common variant of the endothelial nitric oxide synthase (Glu298-->Asp) is a major risk factor for coronary artery disease in the UK. Circulation. 1999;100:1515–1520. doi: 10.1161/01.cir.100.14.1515. [DOI] [PubMed] [Google Scholar]
- 19.Casas J.P., Bautista L.E., Humphries S.E., Hingorani A.D. Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23028 subjects. Circulation. 2004;109:1359–1365. doi: 10.1161/01.CIR.0000121357.76910.A3. [DOI] [PubMed] [Google Scholar]
- 20.Berger K., Stogbauer F., Stoll M., Wellmann J., Huge A., Cheng S., Kessler C., John U., Assmann G., Ringelstein E.B., Funke H. The glu298asp polymorphism in the nitric oxide synthase 3 gene is associated with the risk of ischemic stroke in two large independent case-control studies. Hum. Genet. 2007;121:169–178. doi: 10.1007/s00439-006-0302-2. [DOI] [PubMed] [Google Scholar]
- 21.Jha S., Calvert J.W., Duranski M.R., Ramachandran A., Lefer D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Fujii S., Matsunaga T., Yamamoto M., Ono K., Devarie-Baez N.O., Xian M., Fukuto J.M., Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono K., Akaike T., Sawa T., Kumagai Y., Wink D.A., Tantillo D.J., Hobbs A.J., Nagy P., Xian M., Lin J., Fukuto J.M. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014;77:82–94. doi: 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greiner R., Palinkas Z., Basell K., Becher D., Antelmann H., Nagy P., Dick T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toohey J.I. Sulfur signaling: is the agent sulfide or sulfane? Anal. Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 26.Pan L.L., Liu X.H., Gong Q.H., Yang H.B., Zhu Y.Z. Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxid. Redox Signal. 2012;17:106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensley K., Denton T.T. Alternative functions of the brain transsulfuration pathway represent an underappreciated aspect of brain redox biochemistry with significant potential for therapeutic engagement. Free Radic. Biol. Med. 2015;78:123–134. doi: 10.1016/j.freeradbiomed.2014.10.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akaike T., Ida T., Wei F.Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., Nishimura A., Morita M., Tomizawa K., Nishimura A., Watanabe S., Inaba K., Shima H., Tanuma N., Jung M., Fujii S., Watanabe Y., Ohmuraya M., Nagy P., Feelisch M., Fukuto J.M., Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez L., Bianco C.L., Toscano J.P., Lin J., Akaike T., Fukuto J.M. Chemical Biology of Hydropersulfides and Related Species: possible Roles in Cellular Protection and Redox Signaling. Antioxid. Redox Signal. 2017 doi: 10.1089/ars.2017.7081. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons G.H. Physiology, genetics, and cardiovascular disease: focus on African Americans. J. Clin. Hypertens. 2004;6:11–18. doi: 10.1111/j.1524-6175.2004.03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajjar I., Kotchen T.A. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 32.Mata-Greenwood E., Chen D.B. Racial differences in nitric oxide-dependent vasorelaxation. Reprod. Sci. 2008;15:9–25. doi: 10.1177/1933719107312160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris A.A., Patel R.S., Binongo J.N., Poole J., Al Mheid I., Ahmed Y., Stoyanova N., Vaccarino V., Din-Dzietham R., Gibbons G.H., Quyyumi A. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J. Am. Heart Assoc. 2013;2:e002154. doi: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mani S., Li H., Untereiner A., Wu L., Yang G., Austin R.C., Dickhout J.G., Lhotak S., Meng Q.H., Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 35.Zavaczki E., Jeney V., Agarwal A., Zarjou A., Oros M., Katko M., Varga Z., Balla G., Balla J. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int. 2011;80:731–739. doi: 10.1038/ki.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santilli F., D'Ardes D., Davi G. Oxidative stress in chronic vascular disease: from prediction to prevention. Vasc. Pharmacol. 2015;74:23–37. doi: 10.1016/j.vph.2015.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material