Introduction
The Four-and-a-half LIM (FHL)-only protein subfamily belongs to the LIM-only protein family. The proteins within the group might originated by gene duplicate from a common ancestor and are sharing a high degree of homology all over their amino acid sequence (Fimia et al., 2000). As indicated by its nomenclature, these proteins are signified by the presence of the four and a half cysteine-rich LIM homeodomain with the half-domain always located in the N-terminus (Kurakula et al., 2015). The domain is generally having the consensus sequence CX2CX16–23HX2CX2CX2CX16–21CX2 (C/H/D) with X denotes any amino acid and is comprised of a double zinc finger motif (Schmeichel and Beckerle, 1994). The name LIM was derived from the first letter of the transcription factors LIN-11, ISL-1 and MEC-3, from which the domain were originally characterized (Way and Chalfie, 1988). However, the in vivo evidence that point towards the direct interactions of LIM domain and DNA is still elusive. Reports from different research groups suggested that LIM domain functions as a specific scaffold supporting the LIM-containing proteins to complex with diverse proteinaceous binding partners (Schmeichel and Beckerle, 1994; Breen et al., 1998; Kadrmas and Beckerle, 2004; Frank et al., 2006). Thus far, five members have been categorized into the FHL subfamily, which are FHL1, FHL2, FHL3, FHL4 and activator of CREM in testis (ACT) in human (Johannessen et al., 2006). FHL1, FHL2 and FHL3 are predominantly expressed in muscle, whereas FHL1 and FHL2 can also be found in tissues of different origins (Samson et al., 2004). FHL4 and ACT are expressed exclusively in testis (Morgan and Madgwick, 1999).
The transcription of FHL2
Amongst the different FHL proteins, FHL2 is best studied within the subfamily. The protein is encoded by the fhl2 gene in human which is mapped in the region of chromosome 2q12-q14 (Chan et al., 1998). The fhl2 gene consists of seven exons, in which the first three exons are non-coding while the rest are translated into a protein of 279 amino acids. Five transcript variants of fhl2 have been reported and all of them are translated into the identical FHL2 protein. Two alternative promoters, 1a and 1b, of fhl2 have been reported which are responsible for regulating the transcription of transcript variant 4 and variants 1,2,3,5 respectively (Ng et al., 2011b). Promoter 1a and b are located at exon 1a (−2139 to +375) and exon 1b (−2268 to +397) of fhl2 which is 40 kb downstream of exon 1a respectively (Fig.1). By using luciferase activity analysis, Xu et al found that 1b promoter shows a higher activity than 1a promoter in the HEK 293 cell line (Xu et al., 2014). Both of these promoters involve in fhl2 expression in various cells such as liver, kidney, lung, ovary, pancreas, prostate, stomach, colon and cortex, in particular, the heart. However, its expression in some immune-related tissues like the spleen, thymus and blood leukocytes has not been documented (Zheng and Zhao, 2007). FHL2 exhibits diverse expression patterns in a cell/tissue-specific manner (Johannessen et al., 2006). Its expression patterns are significantly difference between different types of cancer. For example, fhl2 is predicted to be a potential tumor suppressor gene, since it is downregulated in prostate cancer, rhabdomyosarcoma and hepatocellular carcinoma (Genini et al., 1997; Kinoshita et al., 2005; Xu et al., 2014). On the contrary, higher expression of FHL2 is observed in breast cancer, ovarian cancer, lung cancer, colon cancer, human melanoma which imply the oncogenic property of FHL2 in these cancers(Chan et al., 2000; Tanahashi and Tabira, 2000; Chen et al., 2003; Gabriel et al., 2004). Many transcription factors are governing the FHL2 expression which may account for the bivalent functions acting as both the inducer and suppressor during the course of carcinogenesis.
Fig.1. Schematic diagram of FHL1 1a and 1b promoter.
Promoter 1a and 1b are located in transcript variant 4 and variants 1,2,3,5 respectively. Exon 1a is about 40kb upstream of Exon 1b.
Transcriptional regulation of FHL2 expression by tumorigenesis-related genes
P53 is an important tumor suppressor protein playing a significant role in cell cycle regulation and the maintenance of genome integrity by mediating biological processes which include cell-cycle arrest, apoptosis, and senescence under environmental stress (Meng et al., 2014). Although there is no direct interaction between p53 and FHL2 as demonstrated by a yeast two-hybrid screening assay, the expression of FHL2 is significantly associated with the cellular level of p53 (Tanahashi and Tabira, 2000). In an in vitro model of rhabdomyosarcoma RD which expresses mutated p53, the FHL2 expression of the cell line is highly downregulated upon γ-irradiation exposure when compared to the A33GM primary myoblast control (Scholl et al., 2000). When the RD cells are transfected with temperature-sensitive P53 (tsp53) with p53 synthesis inactivated at temperature higher than 37°C, FHL2 mRNA expression was upregulated when the temperature was shifted to 32°C (Scholl et al., 2000). Such p53-dependent FHL2 expression pattern has also been observed in the osteosarcoma cell line SaOS-2, in which the FHL2 expression of the p53-ablated cell line is hampered (Amaar et al., 2002). The 1a instead of 1b promoter appeared to be the putative interacting site of p53, even though the 1b promoter exists in more transcript variants and displays a higher activity (Xu et al., 2014).
Serum response factor (SRF) is a transcription factor that regulates the immediate-early genes (IEGs), cytoskeletal, and muscle-specific gene expression (Treisman, 1995; Miano, 2003). Since FHL2 is preferentially expressed in muscle, and functionally resembling SRF, which also contributes to the upkeep of cytoskeleton organization and control of transcription, SRF and FHL2 may interact closely with each other for achieving these purposes (Kadrmas and Beckerle, 2004). FHL2 is also an androgen receptor (AR)-coactivator, therefore is an active participant of the molecular machinery for AR activities (Culig et al., 2004). In fact, the expression of FHL2 is related to the coordination of AR signaling with SRF. In an investigation based on the use of androgen-sensitive prostate adenocarcinoma cell lines LNCaP and C4-2 cell lines, the expression of FHL2 is found to be induced by androgen with the effect mediated by action of SRF on the FHL2 proximal promoter (Heemers et al., 2007). The participation of SRF in FHL2 expression is also found in embryonic stem cells (ESCs). FHL2 expression is increased during ESCs differentiation by either the removal of leukaemia inhibitory factor (LIF) or addition of retinoic acid to the cell culture system. This FHL2 upregulation of differentiating ESCs is mediated by the direct binding of SRF and its interacting partner Nkx2.5 to the FHL2 promoter in response to RhoA activation (Philippar et al., 2004). The lack of FHL2 responses in SRF−/− ESCs under the same experimental setting further confirms the functional role of SRF in FHL2 expression (Philippar et al., 2004).
Other transcription factors for FHL2 which are not well recorded as p53 and SRF have also been mentioned, for instant, specificity protein 1 (Sp1) that highly expresses in many cancer cells. Guo et al showed that FHL2 expression is regulated by interaction of Sp1 with the regulatory element located fifty nucleotides (nts) upstream of the FHL2 translation starting codon at 1058–1049nts (Guo et al., 2010). The pleiotropic factor IL-1β also significantly downregulates FHL2 mRNA and protein level as confirmed by the IL-1β-treated human chondrocyte model. P38 mitogen-activated protein kinase (MAPK) counteracts the IL-1β-induced effect causing an upregulation of FHL2 mRNA level (Joos et al., 2008). Transcriptional factors such as MEF-2 (Johannessen et al., 2006), and activator protein-1 (AP-1) (Morlon and Sassone-Corsi, 2003) likewise take part in FHL2 transcriptional regulation with their putative binding sites found at FHL2 promoter. In addition, there are a few transcription factors that can regulate FHL2 expression with as yet unclear reasons. For example, Lbx1−/− embryos shows a strong attenuated FHL2 mRNA level when compared with the wild-type embryo, however, no putative binding sites could be detected in FHL2 promoter (Schafer et al., 2003; Johannessen et al., 2006).
Molecular function of FHL2
Apart from being regulated by different transcription factors, FHL2 is involved extensively in regulating the expression of other genes per se. Since FHL2 does not bind to DNA or nucleic acid but having a higher affinity to other proteins, its transcriptional regulatory effects is most likely mediated by protein-protein interaction with FHL2 function as an adaptor protein. Growing evidence are suggesting that LIM domains is the platform for multimeric protein complexes formation (Bach, 2000). An in vitro study with the use of yeast two-hybrid library screen indicated that FHL2 protein is able to shuttle between cytoplasm and nucleus and conjugates with the human DNA-binding nuclear protein (hNP220) within the nucleus. Domain studies further designated the binding site to be the second, third and fourth LIM domains of FHL2 (Ng et al., 2002). In another yeast two-hybrid screening experiment, the interacting partner of FHL2 in myoblasts was investigated through co-immunoprecipitation and direct in vitro pull-down assays. β-catenin was identified as the binding target which together with FHL2 modulate myogenic differentiation through the modulation of LEF/TCF-induced transcriptional activity. The four LIM domains of the FHL2 protein are required for its interaction with β-catenin as verified by using deletion mutant (Martin et al., 2002). The FHL2 proteins are participating in various biological functions such as cell adhesion, invasion, proliferation, apoptosis, and differentiation through the regulation of gene transcription, which involve the maintenance of homeostasis of various fundamental and developmental processes. Therefore, FHL2 contributes to human carcinogenesis by interacting with cancer-related proteins which modulates the signaling pathways underlying the expression of genes related to malignant transformation of normal cells. In this review, we will discuss several types of these FHL2-related cancers.
Cancers related to FHL2
Breast cancer is the most common cancer in adult female (Jemal et al., 2011). FHL2 was first elucidated in 2003 as a molecular culprit critical for the pathogenesis of breast cancer by Yan et al. FHL2 interacts with the breast cancer type 1 susceptibility gene (BRCA1) and enhances the transactivation of BRCA1 (Yan et al., 2003). Clinicopathological studies detected a higher FHL2 expression in breast cancer and lower levels in four out of the five types of premalignant ductal carcinoma in situ (DCIS). In contrast, FHL2 was undetectable in normal breast tissue. Higher intratumoral FHL2 expression in breast cancer patients was significantly associated with their worse survival (Gabriel et al., 2006).
At the molecular level, FHL2 regulates the differentiation of a breast cancer cell line Michigan Cancer Foundation-7 (MCF-7) by cooperating with c-FOS and FRA-1 via the ERK signaling pathway (Saeki et al., 2009). FHL2 also interacts upstream with the receptor that initiates the molecular pathway associated with breast cancer. The estrogen receptor (ER), which consists of the subtypes ERα and ERβ, has been a hot field in FHL2 and breast cancer research. The half LIM domain located at the N-terminal or a single LIM domain of FHL2 can physically and functionally interacts with ERα and ERβ. FHL2 suppresses ER transcriptional activity by enhancing the interaction of ERα and mothers against decapentaplegic homolog 4 (smad4) which is a corepressor of ERα (Xiong et al., 2010). FHL2 also participates in other important signaling pathways in breast cancer acting as a transcriptional cofactor. The protein mediates transcriptional activation of MAPK target gene such as p21 which regulates the cell cycle and contributes to breast cancer development (Martin et al., 2007). Chen and colleagues also showed that FHL2 significantly suppressed Id3-promoted cell proliferation and invasive growth of human MCF-7 breast cancer cells (Chen et al., 2012). Of note, recent findings of Putnik et al showed that DNA methylation can directly repress FHL2 expression in MCF-7 cells, implicating the significance of FHL2 epigenetic modifications in breast cancer progression (Putnik et al., 2012). Most of the above mechanistic data suggest an antiproliferative role of FHL2 which appear to be contradictory to the clinical observation that a poor survival in breast cancer is associated with FHL2 expression. These seemingly discordant findings may be resulted from the yet-to-be discovered tumorigenic properties of FHL2 counteract and outweigh the tumor suppressive effects of FHL2.
Gastrointestinal (GI) cancers represent malignant tumors of the GI tract and accessory digestive organs including oral cavity, esophagus, stomach, liver, gall bladder, pancreas, small intestine, large intestine, rectum, and anus (Rokavec et al., 2014). FHL2 is highly expressed in gastrointestinal cancers such as colon cancer in which FHL2 is the cell cycle and growth modulator of cancer cells. Wu et al found that transduction of rAAV-FHL2-shRNA into human LoVo colon cancer cell line can inhibit cyclin D1 and CDK6 expression which led to significant G0/G1 phase accumulation (Wu et al., 2013). Consistent with Wu’s study, the reduced level of FHL2 inhibits serum-dependent, anchorage-dependent and -independent cell growth resulting in the suppression of de novo tumor formation in nude mice xenograft (Wang et al., 2007). Interestingly, overexpression of FHL2 in HT-29 colon cancer cells will also led to cell cycle arrest but at G2/M phase and leading to both anchorage-dependent and -independent growth inhibition (Amann et al., 2010). However, the overexpression of FHL2 in HT-29 significantly increases E-cadherin expression, which may stimulate differentiation and cell-cell adhesion in HT-29 cells (Chartier et al., 2006; Amann et al., 2010). Accordingly, FHL2 functions as an oncogene in colon cancer which induces cell differentiation in colon cancer cell lines.
FHL2 is crucial to cancer cell invasion, migration and adhesion to extracellular matrix. Before acquiring the migratory and invasive properties, cancer cells must first adopt a mesenchymal/fibroblast-like phenotype through epithelial-mesenchymal transition (EMT) (Turley et al., 2008). Transforming growth factor beta 1 (TGF-β1), a signaling molecule promoting invasion of colorectal cancer, induces EMT and deprives cancer cells of cellular polarity and cell-cell adhesion to become mesenchymal stem cells(van Zijl et al., 2009; Calon et al., 2012). FHL2 expression is positively regulated by TGF-β1 and therefore, responsible for endowing cancer cells metastatic properties (Zhang et al., 2010). In sporadic colon cancer, TGF-β1 could change FHL2 subcellular localization of intratumoral fibroblast to focal adhesions which mediate the TGF-β1-activated polymerization of alpha smooth muscle actin (α-SMA) to give rise to the stress fibers structure (Gullotti et al., 2011). Such process induces the fibroblast to take up a myofibroblast phenotype which is critical to cancer invasion. The tumor-secreted TGF-β1 then induces FHL2 expression in myofibroblasts facilitating the dissemination of cancer cells by promoting a highly mobile tumor stroma formation (Gullotti et al., 2011). On the other hand, FHL2 could also inhibit the formation of membrane-associated E-cadherin-β-catenin complex by modulating the phosphorylation status of the protein complex. The structural integrity of membrane-associated E-cadherin-β-catenin complex is an important determinant for the induction of EMT and therefore indicates the role of FHL2 in cancer migration and invasion (Zhang et al., 2010).
Liver cancer is classified mainly into three classes, including (1) the most common hepatocellular carcinoma (HCC), (2) the rare hepatoblastoma which developed from immature liver cells and (3) those derived from cellular components other than hepatocyte, e.g., immune cells and bile duct. FHL2 is always downregulated in the clinical samples of HCC, suggesting a tumor-suppressive property of fhl2. Similar to FHL2, p53 is also downregulated in the majority of HCC samples is significantly interacting with fhl2 (Xu et al., 2014). P53 is activated by DNA damage, which arrests the cell cycle by inhibiting cyclin D/CDK4/6 complex with p21/Waf1, thereby providing time necessary for cellular repairs (Kleiber et al., 2007). In the HCC cell line Hep3B, overexpression of FHL2 could exhibit anti-proliferative activity by decreasing cyclin D1 expression and increasing P21 and P27 expression (Ng et al., 2011a).
Recently, our laboratory has established a database of FHL2-regulated genes in murine liver by using microarray and bioinformatics analysis. Most of the pathways and new genes related to FHL2 have been identified. Information from our database will be useful to future experimental design related to FHL2 research works which help to broaden our understating about the field (Ng et al., 2014).
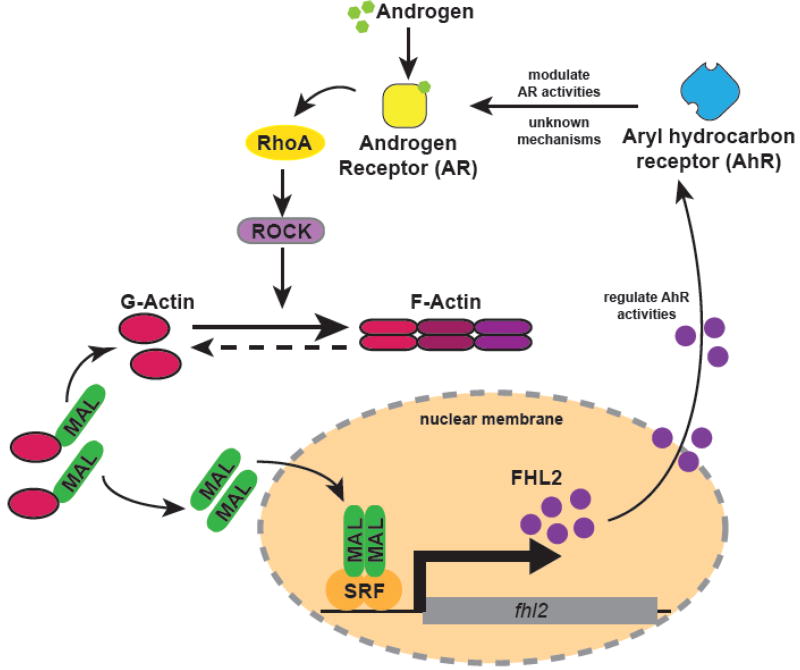
Prostate cancer progression is critically mediated by the androgen receptor (AR) and the AR-coactivator FHL2 is related to aggressiveness and biomedical recurrence of prostate specific antigen (PSA) (Heemers et al., 2009; Uchio et al., 2010). The Heemers’s group initially found that androgens can strongly induce the expression of FHL2 with SRF as the transcription factor acting on the FHL2 proximal promoter (Heemers et al., 2007). They then discovered that this androgen responsiveness can be regulated by the RhoA/actin/megakaryocytic acute leukemia (MAL) signaling axis which is the activating pathway upstream of SRF. Mediated by the activity of RhoA effector Rho-associated coiled-coil containing protein kinase 1 (ROCK), the nuclear translocation of MAL and its recruitment to the FHL2 promoter is increased during androgen-induced RhoA activation (Fig.2) (Schmidt et al., 2012). Reciprocally, FHL2 is able to modulate AR signaling by altering the effect of Aryl hydrocarbon receptor (AhR) imposing AR activity. However, the precise mechanisms between the crosstalk of AR and AhR are still enigmatic (Kollara and Brown, 2010). FHL2 is found to correlate with filamin which promotes actin fiber formation in cytoplasm. Truncated filamin directly corepresses AR in the nucleus. Calpain cleavage of cytoskeletal protein filamin which is increased in prostate cancer could induce the nuclear translocation of FHL2, and this subsequently increase AR coactivation (McGrath et al., 2013).
Fig.2. The molecular communication between Androgen Receptor (AR) and FHL2.
After engagement with AR, Androgen can activate the RhoA/Rho-associated coiled-coil containing protein kinase 1 (ROCK) signaling which promotes polymerization of monomeric globular actin (G-actin) and initiate filamentous actin (F-actin) formation. Megakaryocytic acute leukemia (MAL) is the coactivator of SRF and its nucleus translocation is halted when the protein is binding with G-actin in the cytoplasm. Once released from G-actin, MAL binds to SRF which mediates the interaction between SRF and fhl2 at the proximal promoter eventually activating the FHL2 expression. Reciprocally, FHL2 modulates AR responsiveness by alternating aryl hydrocarbon receptor (AhR) activity.
Conclusion
FHL2 expression levels vary greatly depending on the cancer type that the protein is expressed. For example, FHL2 transcript levels are strongly enhanced in patient samples derived from squamous carcinoma, glioblastoma, melanoma, chronic myelogenous leukaemia and cancers of the cervix, colon, lungs and kidneys. Low or undetectable FHL2 transcript levels can be observed in lymphoblastic leukaemia, promyelocytic leukaemia and Burkitt’s lymphoma cells. In this review, we focused on explaining such phenomena by examining the differential mechanistic regulations of FHL2 transcription in the various types of cancer. However, it is worth notice that the mutations of fhl2 and FHL2 posttranslational modifications may also contribute to cancinogenesis. In fact, an FHL2 mutation (Gly48Ser) with functional alterations has been identified in a patient with familial dilated cardiomyopathy (DCM) in 2007. FHL2 can bind to the N2B and is2 regions of the metabolic enzyme titin/connectin, and the Gly48Ser mutation abrogates the interactions of FHL2 with the two proteins impairing the recruitment of titin/connectin to cardiac sarcomere which may lead to cardiac dysfunction and heart failure. (Arimura et al., 2007). The case of cardiac dysfunction tempted us to question if fhl2 mutation may also profoundly affect the carcinogenesis of the different types of cancer, although not much records is available about the effects of FHL2 mutations on cancer progression. Posttranslational modification may alter FHL2 interactions with partners or its subcellular translocation ability. However, literatures demonstrate that FHL2 is interacting with mitogen-activated protein kinase ERK2 both in vitro and in vivo without phosphorylation (Purcell et al., 2004). Muller et al also failed to detect FHL2 phosphorylation although the protein contains several potential phosphorylation sites and one O-glycosylation site (El Mourabit et al., 2003). To date, the existence of posttranslational modifications other than phosphorylation that taken place on FHL2 such as ubiquitination, sumoylation, methylation and acetylation is still a mystery. Since, FHL2 exerts its functions almost exclusively through protein-protein interactions (Fig. 3), research works in this direction would be of great importance.
Fig.3. Diagram depicted the protein-protein interactions network of FHL2 with its binding partners generated from STRING 9.1.
The different colors of line indicate the mode of molecular activities: binding (blue), post-translation modification (purple), expression (yellow), reaction (black), inhibition (red) and activation (green).
Subcellular localization of FHL2 protein is also a field that deserves future investigation. FHL2 is present either predominantly in the nuclear or distributed uniformly in different cellular compartments. The expression patterns of FHL2 are dependent on the cell types that they are expressed, the differentiation stages, cancer progressive stages or responses to stimulation. The molecular mass of FHL2 is 32kDa which is well below the 50-kDa cut-off for transportation through nuclear pores (Chan et al., 1998). FHL2 also interacts with many signal transduction proteins which belong to different functional classes including structural proteins, transcription factors, signal transducers, DNA replication and repair enzymes. Therefore, FHL2 translocation could play an important role in controlling signaling pathways and is inducible by using different biochemical or physical stimulating factors. Philip et al found that FHL2 could translocate into the nucleus by stimulating the Rho signaling pathway which leads eventually to aggressiveness and recurrence of prostate cancer (Kahl et al., 2006). The translocation of FHL2 into the nucleus of NIH 3T3 cells after serum or ultraviolet light stimulation has also been reported. Inside the NIH 3T3 nucleus, FHL2 upregulate the expression and accumulation of the oncoproteins Fos and Jun in the nucleus which further elicit the expression of cyclin D1 (Brown et al., 1998; Zheng and Zhao, 2007). Similar evidence has been identified in A7FIL+ cells, in which FHL2 shuttles from cytoskeleton into the nucleus upon lysophosphatidic acid stimulation (McGrath et al., 2013). In sporadic colon cancer and human melanoma, FHL2 are highly expressed by peritumoral fibroblasts and melanoma cells respectively at the invasion front and are infrequently localized in the nuclear. TGF-β1 co-express with FHL2 in both cases, therefore, is believed to be one of the mediators of FHL2 nuclear translocation (Gullotti et al., 2011; Westphal et al., 2015). Apparently, the pleiotropic functions of FHL2 as an adaptor protein may rely on its subcellular localization when traffic between nucleus and cytoplasm. However, the detailed molecular mechanisms and networks underpinning the FHL2 nuclear translocation process still need to be explored.
Taken together, the expression of FHL2 is regulated by a numbers of transcription factors. The protein itself also takes part in governing the expression of other genes by acting as an adaptor protein interacting with other transcription factors. The posttranslational modifications and mutations analysis of FHL2 appeared to be a valuable research niche, as findings may probably explain many questions concerning the molecular functioning of FHL2, e.g., the intricate machinery of the intracellular trafficking of FHL2. Since, FHL2 is highly related to cancer progression, in-depth investigation will provide insight for the search of novel pharmaceutical targets, as well as methods for the development of cancer interventions.
Research Highlights.
This review concludes transcriptional regulation of FHL2 by tumorigenesis-related genes.
It also gives useful synopsis on various function of FHL2 in a tissue specific manner.
This review eventually discusses valuable research niche on FHL2 research.
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the CardiacGene Wiki Review series--a series resulting from a collaboration between the journal GENE, the Gene Wiki Initiative, and the BD2K initiative. The Cardiac Gene Wiki Initiative is supported by National Institutes of Health (GM089820 and GM114833). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The corresponding Gene Wiki entry for this review can be found here: http://en.wikipedia.org/wiki/FHL2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaar YG, Thompson GR, Linkhart TA, Chen ST, Baylink DJ, Mohan S. Insulin-like growth factor-binding protein 5 (IGFBP-5) interacts with a four and a half LIM protein 2 (FHL2) J Biol Chem. 2002;277:12053–60. doi: 10.1074/jbc.M110872200. [DOI] [PubMed] [Google Scholar]
- Amann T, Egle Y, Bosserhoff AK, Hellerbrand C. FHL2 suppresses growth and differentiation of the colon cancer cell line HT-29. Oncol Rep. 2010;23:1669–74. doi: 10.3892/or_00000810. [DOI] [PubMed] [Google Scholar]
- Arimura T, Hayashi T, Matsumoto Y, Shibata H, Hiroi S, Nakamura T, Inagaki N, Hinohara K, Takahashi M, Manatsu SI, Sasaoka T, Izumi T, Bonne G, Schwartz K, Kimura A. Structural analysis of four and half LIM protein-2 in dilated cardiomyopathy. Biochem Biophys Res Commun. 2007;357:162–7. doi: 10.1016/j.bbrc.2007.03.128. [DOI] [PubMed] [Google Scholar]
- Bach I. The LIM domain: regulation by association. Mech Dev. 2000;91:5–17. doi: 10.1016/s0925-4773(99)00314-7. [DOI] [PubMed] [Google Scholar]
- Breen JJ, Agulnick AD, Westphal H, Dawid IB. Interactions between LIM domains and the LIM domain-binding protein Ldb1. J Biol Chem. 1998;273:4712–7. doi: 10.1074/jbc.273.8.4712. [DOI] [PubMed] [Google Scholar]
- Brown JR, Nigh E, Lee RJ, Ye H, Thompson MA, Saudou F, Pestell RG, Greenberg ME. Fos family members induce cell cycle entry by activating cyclin D1. Mol Cell Biol. 1998;18:5609–19. doi: 10.1128/mcb.18.9.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massague J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–84. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KK, Tsui SK, Lee SM, Luk SC, Liew CC, Fung KP, Waye MM, Lee CY. Molecular cloning and characterization of FHL2, a novel LIM domain protein preferentially expressed in human heart. Gene. 1998;210:345–50. doi: 10.1016/s0378-1119(97)00644-6. [DOI] [PubMed] [Google Scholar]
- Chan KK, Tsui SK, Ngai SM, Lee SM, Kotaka M, Waye MM, Lee CY, Fung KP. Protein-protein interaction of FHL2, a LIM domain protein preferentially expressed in human heart, with hCDC47. J Cell Biochem. 2000;76:499–508. [PubMed] [Google Scholar]
- Chartier NT, Laine M, Gout S, Pawlak G, Marie CA, Matos P, Block MR, Jacquier-Sarlin MR. Laminin-5-integrin interaction signals through PI 3-kinase and Rac1b to promote assembly of adherens junctions in HT-29 cells. J Cell Sci. 2006;119:31–46. doi: 10.1242/jcs.02698. [DOI] [PubMed] [Google Scholar]
- Chen D, Xu W, Bales E, Colmenares C, Conacci-Sorrell M, Ishii S, Stavnezer E, Campisi J, Fisher DE, Ben-Ze'ev A, Medrano EE. SKI activates Wnt/beta-catenin signaling in human melanoma. Cancer Res. 2003;63:6626–34. [PubMed] [Google Scholar]
- Chen YH, Wu ZQ, Zhao YL, Si YL, Guo MZ, Han WD. FHL2 inhibits the Id3-promoted proliferation and invasive growth of human MCF-7 breast cancer cells. Chin Med J (Engl) 2012;125:2329–33. [PubMed] [Google Scholar]
- Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:265–71. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- El Mourabit H, Muller S, Tunggal L, Paulsson M, Aumailley M. Characterization of recombinant and natural forms of the human LIM domain-containing protein FHL2. Protein Expr Purif. 2003;32:95–103. doi: 10.1016/S1046-5928(03)00211-0. [DOI] [PubMed] [Google Scholar]
- Fimia GM, De Cesare D, Sassone-Corsi P. A family of LIM-only transcriptional coactivators: tissue-specific expression and selective activation of CREB and CREM. Mol Cell Biol. 2000;20:8613–22. doi: 10.1128/mcb.20.22.8613-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med (Berl) 2006;84:446–68. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Gabriel B, Fischer DC, Orlowska-Volk M, zur Hausen A, Schule R, Muller JM, Hasenburg A. Expression of the transcriptional coregulator FHL2 in human breast cancer: a clinicopathologic study. J Soc Gynecol Investig. 2006;13:69–75. doi: 10.1016/j.jsgi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Gabriel B, Mildenberger S, Weisser CW, Metzger E, Gitsch G, Schule R, Muller JM. Focal adhesion kinase interacts with the transcriptional coactivator FHL2 and both are overexpressed in epithelial ovarian cancer. Anticancer Res. 2004;24:921–7. [PubMed] [Google Scholar]
- Genini M, Schwalbe P, Scholl FA, Remppis A, Mattei MG, Schafer BW. Subtractive cloning and characterization of DRAL, a novel LIM-domain protein down-regulated in rhabdomyosarcoma. DNA Cell Biol. 1997;16:433–42. doi: 10.1089/dna.1997.16.433. [DOI] [PubMed] [Google Scholar]
- Gullotti L, Czerwitzki J, Kirfel J, Propping P, Rahner N, Steinke V, Kahl P, Engel C, Schule R, Buettner R, Friedrichs N. FHL2 expression in peritumoural fibroblasts correlates with lymphatic metastasis in sporadic but not in HNPCC-associated colon cancer. Lab Invest. 2011;91:1695–705. doi: 10.1038/labinvest.2011.109. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang W, Xia G, Niu L, Zhang Y, Wang X, Zhang Y, Jiang B, Wang J. Sp1 upregulates the four and half lim 2 (FHL2) expression in gastrointestinal cancers through transcription regulation. Mol Carcinog. 2010;49:826–36. doi: 10.1002/mc.20659. [DOI] [PubMed] [Google Scholar]
- Heemers HV, Regan KM, Dehm SM, Tindall DJ. Androgen induction of the androgen receptor coactivator four and a half LIM domain protein-2: evidence for a role for serum response factor in prostate cancer. Cancer Res. 2007;67:10592–9. doi: 10.1158/0008-5472.CAN-07-1917. [DOI] [PubMed] [Google Scholar]
- Heemers HV, Regan KM, Schmidt LJ, Anderson SK, Ballman KV, Tindall DJ. Androgen modulation of coregulator expression in prostate cancer cells. Mol Endocrinol. 2009;23:572–83. doi: 10.1210/me.2008-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Moller S, Hansen T, Moens U, Van Ghelue M. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell Mol Life Sci. 2006;63:268–84. doi: 10.1007/s00018-005-5438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos H, Albrecht W, Laufer S, Reichel H, Brenner RE. IL-1beta regulates FHL2 and other cytoskeleton-related genes in human chondrocytes. Mol Med. 2008;14:150–9. doi: 10.2119/2007-00118.Joos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–31. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schule R, Buettner R. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66:11341–7. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Nakagawa T, Shimizu A, Katsuoka Y. Differently regulated androgen receptor transcriptional complex in prostate cancer compared with normal prostate. Int J Urol. 2005;12:390–7. doi: 10.1111/j.1442-2042.2005.01093.x. [DOI] [PubMed] [Google Scholar]
- Kleiber K, Strebhardt K, Martin BT. The biological relevance of FHL2 in tumour cells and its role as a putative cancer target. Anticancer Res. 2007;27:55–61. [PubMed] [Google Scholar]
- Kollara A, Brown TJ. Four and a half LIM domain 2 alters the impact of aryl hydrocarbon receptor on androgen receptor transcriptional activity. J Steroid Biochem Mol Biol. 2010;118:51–8. doi: 10.1016/j.jsbmb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Kurakula K, Sommer D, Sokolovic M, Moerland PD, Scheij S, van Loenen PB, Koenis DS, Zelcer N, van Tiel CM, de Vries CJ. LIM-only protein FHL2 is a positive regulator of liver X receptors in smooth muscle cells involved in lipid homeostasis. Mol Cell Biol. 2015;35:52–62. doi: 10.1128/MCB.00525-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Schneider R, Janetzky S, Waibler Z, Pandur P, Kuhl M, Behrens J, von der Mark K, Starzinski-Powitz A, Wixler V. The LIM-only protein FHL2 interacts with beta-catenin and promotes differentiation of mouse myoblasts. J Cell Biol. 2002;159:113–22. doi: 10.1083/jcb.200202075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BT, Kleiber K, Wixler V, Raab M, Zimmer B, Kaufmann M, Strebhardt K. FHL2 regulates cell cycle-dependent and doxorubicin-induced p21Cip1/Waf1 expression in breast cancer cells. Cell Cycle. 2007;6:1779–88. doi: 10.4161/cc.6.14.4448. [DOI] [PubMed] [Google Scholar]
- McGrath MJ, Binge LC, Sriratana A, Wang H, Robinson PA, Pook D, Fedele CG, Brown S, Dyson JM, Cottle DL, Cowling BS, Niranjan B, Risbridger GP, Mitchell CA. Regulation of the transcriptional coactivator FHL2 licenses activation of the androgen receptor in castrate-resistant prostate cancer. Cancer Res. 2013;73:5066–79. doi: 10.1158/0008-5472.CAN-12-4520. [DOI] [PubMed] [Google Scholar]
- Meng X, Franklin DA, Dong J, Zhang Y. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 2014;74:7161–7. doi: 10.1158/0008-5472.CAN-14-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–93. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Madgwick AJ. The fourth member of the FHL family of LIM proteins is expressed exclusively in the testis. Biochem Biophys Res Commun. 1999;255:251–5. doi: 10.1006/bbrc.1999.0180. [DOI] [PubMed] [Google Scholar]
- Morlon A, Sassone-Corsi P. The LIM-only protein FHL2 is a serum-inducible transcriptional coactivator of AP-1. Proc Natl Acad Sci U S A. 2003;100:3977–82. doi: 10.1073/pnas.0735923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CF, Ng PK, Lui VW, Li J, Chan JY, Fung KP, Ng YK, Lai PB, Tsui SK. FHL2 exhibits anti-proliferative and anti-apoptotic activities in liver cancer cells. Cancer Lett. 2011a;304:97–106. doi: 10.1016/j.canlet.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Ng CF, Xu JY, Li MS, Tsui SK. Identification of FHL2-regulated genes in liver by microarray and bioinformatics analysis. J Cell Biochem. 2014;115:744–53. doi: 10.1002/jcb.24714. [DOI] [PubMed] [Google Scholar]
- Ng CF, Zhou WJ, Ng PK, Li MS, Ng YK, Lai PB, Tsui SK. Characterization of human FHL2 transcript variants and gene expression regulation in hepatocellular carcinoma. Gene. 2011b;481:41–7. doi: 10.1016/j.gene.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Ng EK, Chan KK, Wong CH, Tsui SK, Ngai SM, Lee SM, Kotaka M, Lee CY, Waye MM, Fung KP. Interaction of the heart-specific LIM domain protein, FHL2, with DNA-binding nuclear protein, hNP220. J Cell Biochem. 2002;84:556–66. [PubMed] [Google Scholar]
- Philippar U, Schratt G, Dieterich C, Muller JM, Galgoczy P, Engel FB, Keating MT, Gertler F, Schule R, Vingron M, Nordheim A. The SRF target gene Fhl2 antagonizes RhoA/MAL-dependent activation of SRF. Mol Cell. 2004;16:867–80. doi: 10.1016/j.molcel.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Purcell NH, Darwis D, Bueno OF, Muller JM, Schule R, Molkentin JD. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol Cell Biol. 2004;24:1081–95. doi: 10.1128/MCB.24.3.1081-1095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnik M, Zhao C, Gustafsson JA, Dahlman-Wright K. Global identification of genes regulated by estrogen signaling and demethylation in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2012;426:26–32. doi: 10.1016/j.bbrc.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Rokavec M, Li H, Jiang L, Hermeking H. The p53/microRNA connection in gastrointestinal cancer. Clin Exp Gastroenterol. 2014;7:395–413. doi: 10.2147/CEG.S43738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y, Endo T, Ide K, Nagashima T, Yumoto N, Toyoda T, Suzuki H, Hayashizaki Y, Sakaki Y, Okada-Hatakeyama M. Ligand-specific sequential regulation of transcription factors for differentiation of MCF-7 cells. BMC Genomics. 2009;10:545. doi: 10.1186/1471-2164-10-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson T, Smyth N, Janetzky S, Wendler O, Muller JM, Schule R, von der Mark H, von der Mark K, Wixler V. The LIM-only proteins FHL2 and FHL3 interact with alpha- and beta-subunits of the muscle alpha7beta1 integrin receptor. J Biol Chem. 2004;279:28641–52. doi: 10.1074/jbc.M312894200. [DOI] [PubMed] [Google Scholar]
- Schafer K, Neuhaus P, Kruse J, Braun T. The homeobox gene Lbx1 specifies a subpopulation of cardiac neural crest necessary for normal heart development. Circ Res. 2003;92:73–80. doi: 10.1161/01.res.0000050587.76563.a5. [DOI] [PubMed] [Google Scholar]
- Schmeichel KL, Beckerle MC. The LIM domain is a modular protein-binding interface. Cell. 1994;79:211–9. doi: 10.1016/0092-8674(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Duncan K, Yadav N, Regan KM, Verone AR, Lohse CM, Pop EA, Attwood K, Wilding G, Mohler JL, Sebo TJ, Tindall DJ, Heemers HV. RhoA as a mediator of clinically relevant androgen action in prostate cancer cells. Mol Endocrinol. 2012;26:716–35. doi: 10.1210/me.2011-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, McLoughlin P, Ehler E, de Giovanni C, Schafer BW. DRAL is a p53-responsive gene whose four and a half LIM domain protein product induces apoptosis. J Cell Biol. 2000;151:495–506. doi: 10.1083/jcb.151.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi H, Tabira T. Alzheimer's disease-associated presenilin 2 interacts with DRAL, an LIM-domain protein. Hum Mol Genet. 2000;9:2281–9. doi: 10.1093/oxfordjournals.hmg.a018919. [DOI] [PubMed] [Google Scholar]
- Treisman R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 1995;14:4905–13. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280–90. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170:1390–5. doi: 10.1001/archinternmed.2010.262. [DOI] [PubMed] [Google Scholar]
- van Zijl F, Mair M, Csiszar A, Schneller D, Zulehner G, Huber H, Eferl R, Beug H, Dolznig H, Mikulits W. Hepatic tumor-stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene. 2009;28:4022–33. doi: 10.1038/onc.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yang Y, Xia HH, Gu Q, Lin MC, Jiang B, Peng Y, Li G, An X, Zhang Y, Zhuang Z, Zhang Z, Kung HF, Wong BC. Suppression of FHL2 expression induces cell differentiation and inhibits gastric and colon carcinogenesis. Gastroenterology. 2007;132:1066–76. doi: 10.1053/j.gastro.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Way JC, Chalfie M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell. 1988;54:5–16. doi: 10.1016/0092-8674(88)90174-2. [DOI] [PubMed] [Google Scholar]
- Westphal P, Mauch C, Florin A, Czerwitzki J, Olligschlager N, Wodtke C, Schule R, Buttner R, Friedrichs N. Enhanced FHL2 and TGF-beta1 Expression Is Associated With Invasive Growth and Poor Survival in Malignant Melanomas. Am J Clin Pathol. 2015;143:248–56. doi: 10.1309/AJCPXEC6CIT2TXAF. quiz 307. [DOI] [PubMed] [Google Scholar]
- Wu Y, Guo Z, Zhang D, Zhang W, Yan Q, Shi X, Zhang M, Zhao Y, Zhang Y, Jiang B, Cheng T, Bai Y, Wang J. A novel colon cancer gene therapy using rAAVmediated expression of human shRNA-FHL2. Int J Oncol. 2013;43:1618–26. doi: 10.3892/ijo.2013.2090. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Ding L, Sun J, Cao J, Lin J, Lu Z, Liu Y, Huang C, Ye Q. Synergistic repression of estrogen receptor transcriptional activity by FHL2 and Smad4 in breast cancer cells. IUBMB Life. 2010;62:669–76. doi: 10.1002/iub.367. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhou J, Li MS, Ng CF, Ng YK, Lai PB, Tsui SK. Transcriptional regulation of the tumor suppressor FHL2 by p53 in human kidney and liver cells. PLoS One. 2014;9:e99359. doi: 10.1371/journal.pone.0099359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhu J, Zhong H, Lu Q, Huang C, Ye Q. BRCA1 interacts with FHL2 and enhances FHL2 transactivation function. FEBS Lett. 2003;553:183–9. doi: 10.1016/s0014-5793(03)00978-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, Jiang B, Guo Z, Sardet C, Zou B, Lam CS, Li J, He M, Lan HY, Pang R, Hung IF, Tan VP, Wang J, Wong BC. Four-and-a-half LIM protein 2 promotes invasive potential and epithelial-mesenchymal transition in colon cancer. Carcinogenesis. 2010;31:1220–9. doi: 10.1093/carcin/bgq094. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Zhao Y. The diverse biofunctions of LIM domain proteins: determined by subcellular localization and protein-protein interaction. Biol Cell. 2007;99:489–502. doi: 10.1042/BC20060126. STRING : http://string-db.org/newstring_cgi/show_network_section.pl. [DOI] [PubMed] [Google Scholar]