Abstract
Objectives
Cerebral ischemia is a serious possible manifestation of diabetic vascular disease. Recurrent hypoglycemia (RH) enhances ischemic brain injury in insulin-treated diabetic (ITD) rats. In the present study, we determined the role of ischemic acidosis in enhanced ischemic brain damage in RH-exposed ITD rats.
Methods
Diabetic rats were treated with insulin and mild/moderate RH was induced for 5 days. Three sets of experiments were performed. The first set evaluated the effect of RH exposure on global cerebral ischemia-induced acidosis in ITD rats. The second set evaluated the effects of an alkalizing agent (Tris-(hydroxymethyl)-aminomethane: THAM) on ischemic acidosis-induced brain injury in RH-exposed ITD rats. The third experiment evaluated the effect of the glucose transporter (GLUT) inhibitor on ischemic acidosis- induced brain injury in RH-exposed ITD rats. Hippocampal pH and lactate were measured during ischemia and early reperfusion for all three experiments. Neuronal survival in Cornu Ammonis 1 (CA1) hippocampus served as a measure of ischemic brain injury.
Findings
Prior RH exposure increases lactate concentration and decreases pH during ischemia and early reperfusion when compared to controls. THAM and GLUT inhibitor treatments attenuated RH-induced increase in ischemic acidosis. GLUT inhibitor treatment reduced the RH-induced increase in lactate levels. Both THAM and GLUT inhibitor treatments significantly decreased ischemic damage in RH-exposed ITD rats.
Conclusions
Ischemia causes increased acidosis in RH-exposed ITD rats via a GLUT-sensitive mechanism. Exploring downstream pathways may help understand mechanisms by which prior exposure to RH increases cerebral ischemic damage.
1. Introduction
Diabetes mellitus is a serious metabolic disease associated with chronic hyperglycemia. Worldwide, 415 million people suffer from this disease (International Diabetes Federation, 2015). Stroke and heart disease are serious complications of diabetes (Morrish et al., 2001; Mozaffarian et al., 2016). The prevalence of ischemic stroke and associated mortality is higher in diabetic individuals (Almdal et al., 2004; Centers for Disease and Prevention, 2003; Jorgensen et al., 1994; Kissela et al., 2005; Ottenbacher et al., 2004). The role of hyperglycemia in exacerbation of ischemic stroke in diabetics is well-established (Gilmore and Stead, 2006; Huang et al., 2013; Pulsinelli et al., 1983). However, continued use of intensive glucose lowering therapies increases risk of hypoglycemia in diabetics (Cryer, 2007; Van den Berghe et al., 2006; van den Berghe et al., 2001; Yuan et al., 2015). Continuous blood glucose monitoring has shown that type 1 diabetics experience hypoglycemia daily for a period of 60 to 89 minutes (Tamborlane et al., 2008). Similarly, treated type 2 diabetics also experience hypoglycemia (Donnelly et al., 2005; Gehlaut et al., 2015).
The American Diabetes Association defines hypoglycemia as a clinical condition characterized by a plasma glucose level of ≤70 mg/dL (≤3.9 mmol/L) (ADA Workgroup on Hypoglycemia, 2005). Hypoglycemia activates many counterregulatory mechanisms like decrease in insulin, increase in glucagon and epinephrine secretion, and modulation of the autonomic nervous system (Fanelli et al., 1994; Jensen et al., 2014; Mitrakou et al., 1991; Schwartz et al., 1987; Tesfaye and Seaquist, 2010). Repeated episodes of hypoglycemia referred to as recurrent hypoglycemia (RH) blunts certain glucose counterregulatory responses (Dagogo-Jack et al., 1993; Davis et al., 1997; Heller and Cryer, 1991). “Hypoglycemia unawareness” is defined as “syndromes of defective glucose counterregulation and hypoglycemia without warning symptoms” (Cryer, 2004). This increases the risk of both symptomatic and asymptomatic hypoglycemia manifested in diabetics under intensive anti-diabetic therapy (Amiel et al., 1988; Bolli et al., 1984; Clarke et al., 1995; Conget et al., 2016; Cryer, 2006; White et al., 1983). Previously, we observed that a prior exposure to moderate RH increases cerebral ischemic brain damage in insulin-treated diabetic (ITD) rats (Dave et al., 2011b).
Hypoglycemia activates homeostatic mechanisms that help maintain the normal physiology of brain during stress (Frier, 2009; Rosenthal et al., 2001). Hypoglycemia enhances hexokinase utilization index and activity of hexokinase Type II isoenzyme (Crane et al., 1981; Kaur et al., 1983). Hypoglycemia upregulates mRNA and protein levels of glucose transporter 1 (GLUT1) in the blood-brain barrier and brain (Boado and Pardridge, 1993; Koranyi et al., 1991; Kumagai et al., 1995). Repeated episodes of hypoglycemia increases mRNA and protein levels of glucose transporter 3 (GLUT3) in brain (Antony et al., 2010; Lee et al., 2000). A cell culture study has shown that hypoglycemia with serum depletion in culture media increases glucose uptake (Russo et al., 2004). Chronic hypoglycemia causes increased transportation, utilization and preservation of glucose in brain (McCall et al., 1986; Pelligrino et al., 1990). Hyperglycemia-induced aggravation of ischemic brain injury is associated with increase in the glucose concentration in brain (Hoxworth et al., 1999; Wagner and Lanier, 1994). RH increases lactate and glucose oxidation in brain during hypoglycemia (Herzog et al., 2013). During cerebral ischemia, increased glycolytic synthesis of lactate in brain is observed (Biros et al., 1986; Combs et al., 1990; Rehncrona et al., 1981). A lactate shuttle between glia and neurons mediates post-ischemic survival of neurons (Schurr and Rigor, 1998). However, increase in lactate levels above a critical threshold causes a severe drop in pH in ischemic brain, leading to damage (Combs et al., 1990; Schurr et al., 1997; Schurr and Rigor, 1998).
The potential interplay between the effect of RH on the role of GLUT, acidosis and ischemic brain injury in ITD rats is unknown. Therefore, the first experiment tested the hypothesis that prior exposure to RH increases ischemia-induced acidosis in brain of RH-exposed ITD rats. The second experiment tested whether this increased acidosis is responsible for RH-induced increase in ischemic brain injury. The third experiment tested whether RH-induced increase in pH drop, and increased ischemic injury are mediated via GLUT.
2. Materials and methods
2.1 Animals
Experimental procedures on animals were carried out as per the Guide for the Care and Use of Laboratory Animals laid down by the National Institutes of Health and in accordance with the protocols approved by the Animal Care and Use Committee of the University of Miami.
2.2 Induction of diabetes
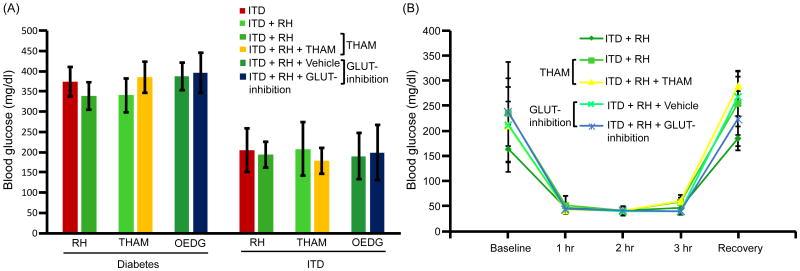
Streptozotocin (Sigma-Aldrich, St Louis, MO), the β-cell toxin, was intraperitoneally administered at a dose of 58 mg × kg-1 to induce diabetes in male Wistar rats (Charles River Laboratories International, Inc, Wilmington, MA). Streptozotocin was dissolved in citrate buffer and prepared immediately before use. After induction of diabetes, rats were monitored twice a week for their blood glucose levels by tail pricking using a portable glucose meter between 9 AM and noon (FreeStyle Freedom, Abbott Diabetes Care Inc, CA) (Dave et al., 2011b). The results presented in Figure 2A for diabetic groups are blood glucose levels at the time of insulin pellet implantation (i.e. at last reading of untreated diabetes). The animals were considered diabetic if their blood glucose levels was >300 mg × dl-1 after streptozotocin administration.
Figure 2.
Blood glucose levels (A) after diabetes induction and insulin treatment and (B) during hypoglycemia. Experiment 1: ITD (n = 13), ITD + RH (n = 8); Experiment 2: ITD + RH (n = 10), ITD + RH + THAM (n = 8); Experiment 3: ITD + RH + Vehicle (n = 6) and ITD + RH + GLUT-inhibition (n = 8).
2.3 Insulin treatment
After 2-3 weeks of diabetes induction, subcutaneous implantation of insulin pellet(s) (each pellet releasing 2 U of insulin in 24 hours) (Linplant; LinShin, Toronto, Canada) was carried out to control the blood glucose levels to euglycemic levels. Twice a week monitoring of blood glucose levels was continued after insulin pellet implantation. During this monitoring period, if blood glucose levels were not within the target range, amount of insulin pellets was adjusted. The results presented in Figure 2A for ITD groups are blood glucose levels at the time of cerebral ischemia surgery (i.e. last reading of treated diabetic condition). The, thus treated rats are referred to as “insulin-treated diabetic” (ITD) animals (Dave et al., 2011b). This group represents diabetics on insulin therapy.
2.4 Induction of recurrent hypoglycemia
The ITD rats were then used for further experimentation about 2-3 weeks post-insulin pellet implantation. In the RH-exposed ITD group, an additional subcutaneous administration of an appropriate dose of insulin (Novolog Insulin aspart, Novo Nordisk, AIS, Denmark) was used to elicit moderate hypoglycemia for a 3 hour period once a day for 5 consecutive days (Dave et al., 2011b). Blood glucose levels were monitored (as described in section above) at baseline and at every 60 minutes post-insulin injection until completion of a 3-hour period of hypoglycemia. To avoid glycemic excursions, food was withdrawn during the period of hypoglycemia. Hypoglycemia was then normalized by a subcutaneous injection of dextrose and euglycemia was confirmed by assessing glucose level in blood samples obtained 30 minutes post-dextrose injection. The drop of blood glucose levels to below 70 mg/dL was considered as hypoglycemia (ADA Workgroup on Hypoglycemia, 2005). This group represents insulin-treated diabetics experiencing RH.
2.5 Induction of global cerebral ischemia
Rats were subjected to an episode of global cerebral ischemia overnight after the last episode of hypoglycemia or equivalent time in the naïve and ITD groups. Briefly, rats were anesthetized using isoflurane, paralyzed with rocuronium and artificially ventilated. Physiological parameters; viz., body temperature, head temperature and mean arterial blood pressure were maintained in their normal ranges. A cut was made in the skin of the anterior aspect of the neck and the carotid arteries were isolated from the adjoining tissue and vagus nerve. Ligatures (Polyethylene-10 tubing) were passed around the carotid arteries and loosely secured using a bilumen tube. An eight minute of global cerebral ischemia was then elicited by occluding the two common carotid arteries along with simultaneous transient hypotension (∼50 mmHg) produced by controlled hemorrhage using a syringe connected to a cannulated femoral artery. Carotid ligatures were then removed and the withdrawn blood was re-infused into the systemic circulation. Both carotid arteries were physically inspected to ensure recirculation (Dave et al., 2011b). The wound was then sutured back and animals were given appropriate post-operative care.
2.6 Assessment of pH and lactate concentration
Burr holes of 2 mm2 area were made over the left and right aspect of the skull approximately 3.5 mm posterior and 2.5 mm lateral to the bregma. Using a stereotaxic apparatus, a pH probe was inserted 3 mm deep through the burr hole into the CA1 region of the hippocampus in the right hemisphere of brain. Hippocampal pH was measured using a pH-1 micro fiber optic pH meter (Precision Sensing GmbH, Regensburg, Germany). Probes were calibrated as per manufacturer's instructions. pH assessment was done continuously at a frequency of one Hertz from 15 minutes before onset of cerebral ischemia to 65-80 minutes of reperfusion using pH1-View software (V1.0.0). pH data is presented in terms of Δ pH in comparison to mean basal pH, and area under the curve obtained from a plot of Δ pH versus time. A 2 mm microdialysis probe (IBR combination infusion and microdialysis brain probe - Bioanalytical Systems, Inc., West Lafayette, Indiana, USA) was inserted through the burr hole 3 mm deep into the CA1 region of left hippocampus. This probe was perfused with Ringer's solution (147 mM NaCl, 4 mM KCl, and 1.3 mM CaCl2) using a micro-infusion pump at a flow rate of 0.3 μl × min-1 (Carnegie Medicine, Stockholm, Sweden). A 30-min stabilization period was allowed before collecting baseline samples. Samples of microdialysis perfusate, representing extracellular fluid were immediately analyzed using a lactate plus meter (Nova Biomedical Corporation, Waltham, MA, USA). These samples were obtained immediately before, 4 and 8 minutes after the onset of ischemia, and 10, 20, 30, 45, 60 and 75 minutes after the completion of ischemia. Lactate concentration results are presented as percentage change of baseline values and area under the curve calculated using a plot of percentage change in concentration versus time.
2.7 Administration of alkalizing agent
Tris-(hydroxymethyl)-aminomethane (THAM) is a biologically inert weak base amino alcohol of low toxicity, which buffers carbon dioxide and acids in vivo as reviewed previously (Nahas, 1962; Nahas et al., 1998). THAM, when administered, is well distributed in the extracellular fluid, accepts protons in a stoichiometric manner and is excreted by kidneys in protonated form. THAM rapidly restores pH and acid-base regulation during acidosis produced by metabolic acid accumulation. THAM has been used earlier to inhibit ischemic acidosis in brain (Kuyama et al., 1994; Nagao et al., 1996). Therefore, we used THAM as an alkalizing agent in the present study to test the effect of chemically induced decrease in ischemic acidosis on RH-induced increase in ischemic brain injury. The previously reported dose of THAM (Nagao et al., 1996) was adjusted for inhibition of RH-induced increase in ischemic pH drop in ITD rats based on preliminary studies. THAM was administered in form of a 0.3 M solution in distilled water (3 ml × kg-1 × hr-1, i.v.) from ten to fifteen minutes prior to the induction of cerebral ischemia to eighty minutes after onset of reperfusion (Nagao et al., 1996).
2.8 Administration of GLUT inhibitor
To test the potential role of GLUTs in RH-induced acidotic aggravation of ischemic brain injury, we determined the effect of chemical inhibition of GLUT on ischemic acidosis and extent of the brain injury in RH-exposed ITD rats. 4,6-O-Ethylidene-α-D-glucose (OEDG) is an asymmetric non-transported competitive inhibitor of glucose transport receptor systems in membranes (Baker and Widdas, 1973; Barnett et al., 1975; Dick et al., 1984; Holman and Rees, 1982). The extent of OEDG-induced inhibition of GLUTs is dependent on the dose (Baker and Widdas, 1973). The minimum dose of OEDG effective in inhibiting the intra-ischemic decrease in pH in the CA1 hippocampus of RH-exposed ITD rats was identified by pilot experiments. OEDG was infused intravenously in the form of a solution in citrate buffer (2 mmol × kg-1 bolus followed by 0.2 mmol × kg-1 × min-1 maintenance infusion) from ten to fifteen minutes before onset of cerebral ischemia to eighty minutes of reperfusion after ischemia (Kawada et al., 1987; Kawada et al., 1989).
2.9 Histological Assessment
After 7 days of reperfusion following ischemia, animals underwent perfusion (at a pressure of 120 mm Hg) with saline until the blood was washed out of the body, followed by perfusion with a mixture of formaldehyde, glacial acetic acid and methanol (in a ratio of 1:1:8), and brain samples were then harvested. Coronal sections (10 μm thick, 200 μm apart) of processed brains were collected from 2.8 to 4.0 mm posterior from bregma. Hematoxylin and eosin staining was performed on the sections. Assessment was made using a Nikon microscope (Nikon Microphot-SA; Nikon Corporation, Tokyo, Japan), and a computer system (MCID Elite 6.0 software; InterFocus Imaging Ltd., Cambridge, UK). Ischemic brain injury was measured in terms of the number of normal neurons in CA1 hippocampus. Sections of CA1 hippocampus were visualized at a magnification of 40×. Counting of normal neurons was done manually at various fields sequentially along the medial to lateral aspect of the CA1 hippocampus on both sides on three consecutive sections. Total neuronal counts from both hemispheres of brain were added and the resulting values obtained from three sequential slides were averaged to compute the number of normal neurons in CA1 hippocampus. Samples were coded and mixed prior to analysis. However, our exploratory study design did not include blinding.
2.10 Experimental Protocol
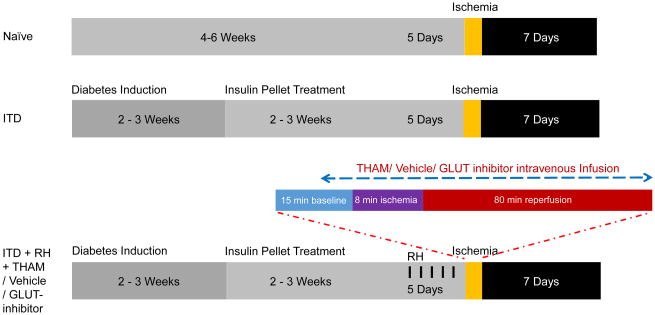
Rats were randomly assigned to various study groups in the three experiments (Figure 1).
Figure 1.
Synopsis of time course and experimental design of the study. Experiment 1: Study of the effect of RH on ischemic acidosis in ITD rats. Study groups were Naïve, ITD, and ITD + RH. Experiment 2: Study of the effect of inhibition of acidosis on ischemic injury in RH-exposed ITD rats. Study groups were ITD + RH, and ITD + RH + THAM. Experiment 3: Study of the effect of GLUT-inhibition on ischemic injury in RH-exposed ITD rats. Study groups were ITD + RH + Vehicle, and ITD + RH + GLUT inhibitor. ITD: insulin-treated diabetic, RH: recurrent hypoglycemia, THAM: Tris-(hydroxymethyl)-aminomethane.
Experiment 1
The effect of RH exposure on intra-ischemic acidosis in ITD rats. Groups included naïve (non-diabetic controls), ITD (insulin-treated streptozotocin-diabetic rats), and ITD + RH (ITD rats exposed to RH).
Experiment 2
The effect of an alkalizing agent (THAM) on RH-induced increase in intra-ischemic acidosis and ischemic brain injury in ITD rats. Groups included ITD + RH (control), and ITD + RH + THAM (treatment).
Experiment 3
The effect of GLUT-inhibition (OEDG) on RH-induced increase in intra-ischemic acidosis and ischemic brain injury in ITD rats. Groups included ITD + RH + vehicle (ITD + RH rats that received citrate buffer treatment), and ITD + RH + GLUT-inhibitor (ITD + RH rats that received GLUT-inhibitor treatment).
2.11 Statistical Analysis
Statistical analysis was carried out using Graph Pad prism software version 5. Significant outlier data points, if any, as identified by Grubbs' test were excluded from further analysis. Moreover, animals with blood glucose levels (during weekly measurements), those with recurrent hypoglycemia, and those with values of physiological parameters (during surgical procedures) to be outside the normal range were excluded. In addition, issues with tissue processing for histology led to the exclusion of animals from assessment of neuronal count. Formal randomization was not a part of this exploratory study design. The physiological parameter results include the data obtained from all of the animals in each group. More than two groups were compared using one-way ANOVA followed by post-hoc Tukey's test for multiple comparisons. Two group comparisons were carried out using Student's t-test. The percentage change of lactate concentration in experiment 1 and experiment 3 were analyzed using the Kruskal-Wallis test and rank sum test, respectively. Only groups belonging to same experiment were compared. Comparisons were made between naïve, ITD and ITD+RH groups for the first set of experiments, between ITD + RH + THAM group and its respective ITD + RH control group for the second set of experiments, and between the ITD + RH + GLUT-inhibition and respective ITD + RH + vehicle groups for the thirdatment and at the time set of experiments. The results are presented as mean ± SEM. A p value less than 0.05 was considered statistically significant.
3. Results
The experimental protocol is described in Figure 1. No statistically significant difference in blood glucose levels was observed either prior to insulin treatment or at the time of cerebral ischemia induction in all study groups (Figure 2A). In the ITD groups blood glucose level was maintained slightly above euglycemia to avoid any unwanted hypoglycemia. We did not observe any significant difference between blood glucose levels in various RH-exposed ITD groups at different time points of assessment during hypoglycemia (Figure 2B). Physiological parameters assessed; viz. body weight, body temperature, head temperature, pH of blood, partial pressure of carbon dioxide (pCO2) in blood, partial pressure of oxygen (pO2) in blood and mean arterial blood pressure (MABP) were assessed. There was a minor yet significant difference between the THAM and GLUT-inhibitor treatment groups versus their respective ITD + RH control groups in terms of blood pO2 and MABP levels. However, there was no other statistical difference between the assessed physiological parameters' data in all experimental groups. In addition, we did not observe any intergroup difference in body weight data among various study groups when compared with the respective control groups. Pre-ischemia values are presented in Table 1, and during ischemia and post-ischemia values are presented in Supplementary Table 1.
Table 1. Physiological parameters.
| Experiment | Group | Body Weight (Grams) | Body Temperature (°C) | Head Temperature (°C) | pH | pCO2 (mmHg) | pO2 (mmHg) | MABP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| 1 | Naïve (n=7) | 431 ± 30 | 37.0 ± 0.0 | 36.6 ± 0.1 | 7.43 ± 0.03 | 36 ± 3 | 142 ± 31 | 104 ± 5 |
| ITD (n=5) | 363 ± 28 | 37.0 ± 0.0 | 36.7 ± 0.2 | 7.42 ± 0.07 | 34 ± 3 | 148 ± 44 | 102 ± 6 | |
| ITD + RH (n=8) | 384 ± 50 | 37.0 ± 0.0 | 36.7 ± 0.1 | 7.46 ± 0.07 | 35 ± 5 | 145 ± 20 | 106 ± 5 | |
| 2 | ITD + RH (n=10) | 359 ± 54 | 36.7 ± 0.5 | 35.9 ± 0.6 | 7.45 ± 0.06 | 34 ± 3 | 133 ± 13 | 106 ± 15 |
| ITD + RH + THAM (n=8) | 332 ± 33 | 37.0 ± 0.0 | 36.0 ± 0.5 | 7.44 ± 0.07 | 35 ± 4 | 135 ± 37 * | 104 ± 17 | |
| 3 | ITD + RH + Vehicle (n=6) | 338 ± 25 | 37.0 ± 0.0 | 37.0 ± 0.2 | 7.45 ± 0.03 | 37 ± 2 | 124 ± 19 | 99 ± 16 |
| ITD + RH + OEDG (n=8) | 322 ± 23 | 37.0 ± 0.3 | 37.0 ± 0.3 | 7.42 ± 0.02 | 39 ± 4 | 116 ± 10 | 107 ± 7 * |
p<0.05 vs respective ITD + RH control
3.1 RH exposure increases levels of lactate in CA1 hippocampus during ischemia
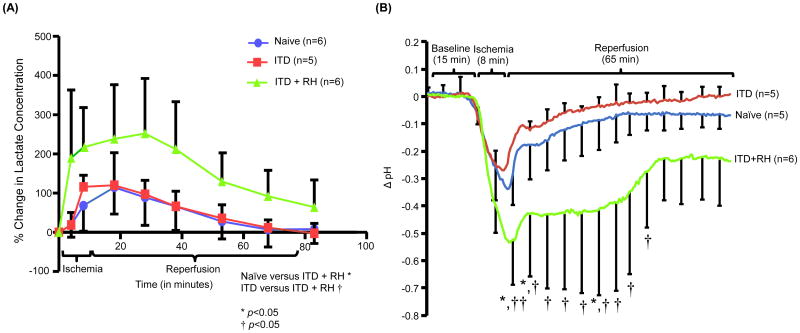
Because exposure to hypoglycemia results in enhanced mRNA (Antony et al., 2010) and protein levels of glucose transporters, and increases the extent of glucose (Kumagai et al., 1995; McCall et al., 1986) as well as lactate uptake (Herzog et al., 2013; Lee et al., 2000), we investigated if prior exposure of ITD rats to RH had any effect on cerebral ischemia-induced increase in hippocampal lactate levels. Ischemia increased hippocampal lactate levels in all three experimental groups. Compared to pre-ischemia baseline, percentage increase in hippocampal lactate concentration in naïve animals (19 ± 13% and 65 ± 27% at 4 and 8 minutes after induction of ischemia, respectively) was significantly lower than the increase seen in RH-exposed ITD animals when quantified during ischemia and early reperfusion (p<0.05). The lactate levels in ITD group was significantly lower than the levels observed in RH-exposed ITD animals when quantified during ischemia and early reperfusion (p<0.05). (Figure 3A).
Figure 3.
The effect of RH on ischemia-induced (A) increase in lactate concentration and (B) decrease in pH in CA1 hippocampus of ITD rats. Lactate levels in terms of percentage change in lactate concentration. Hippocampal (CA1) pH (Δ pH) versus time curve of rats belonging to naïve (n = 5), ITD (n = 5), and ITD + RH (n = 6) groups. †<0.05 vs ITD; *p<0.05 vs naïve; **p<0.01 vs naïve; †p<0.05 vs ITD; ††<0.01 vs ITD.
Furthermore, the area under the curve (AUC) obtained from the percentage change in hippocampal lactate concentration in animals belonging to the naïve (72 ± 58 % change in mM × hr) and ITD groups (78 ± 25 % change in mM × hr) was lower than that of animals belonging to the ITD + RH group (227 ± 113 % change in mM × hr). These results indicate that a transient increase in lactate concentration is observed during ischemia and early reperfusion in RH-exposed ITD rats.
3.2 Prior RH exposure enhances ischemic acidosis in CA1 hippocampus
Because we observed a significant increase in lactate concentrations during ischemia in ITD + RH rats, we hypothesized that RH-related increase in lactate levels in ischemic brain might also be associated with an enhanced ischemia-induced pH drop in CA1 hippocampus. To test this hypothesis, we measured pH in CA1 hippocampus. The reduction in hippocampal pH values in animals belonging to the naïve group was significantly different from that of animals belonging to the ITD + RH group for all time points from 7 minutes after the onset of ischemia to 7 minutes after the onset of reperfusion (decrease in pH in ITD + RH group versus naïve group was 0.175 to 0.254) and also from 18 minutes to 26.5 minutes after the onset of reperfusion (decrease in pH in ITD + RH group versus naïve group was 0.299 to 0.329). The observed drop in hippocampal pH values in animals belonging to the ITD group was noted to be significantly different from that of animals belonging to the ITD + RH group for all time points from 4.5 minutes after the onset of ischemia to 62 minutes after onset of reperfusion (decrease in pH in ITD + RH group versus naïve group was 0.147 to 0.381). Similarly, the reduction seen in the naïve group was not significantly different from that observed in the ITD + RH group during the first 6.5 minutes of ischemia, between 7.5 and 17.5 minutes of reperfusion and after 27 minutes of reperfusion (Figure 3B). However, the drop in hippocampal pH in the ITD group was not significantly different from that seen in the ITD + RH group during the first 4 minutes of ischemia. The ischemia-related fall in hippocampal pH in the ITD group was not significantly different from that of the naïve group throughout the observation period. Our results indicate that prior exposure to RH results in larger ischemia-induced pH drop when compared to the two control groups.
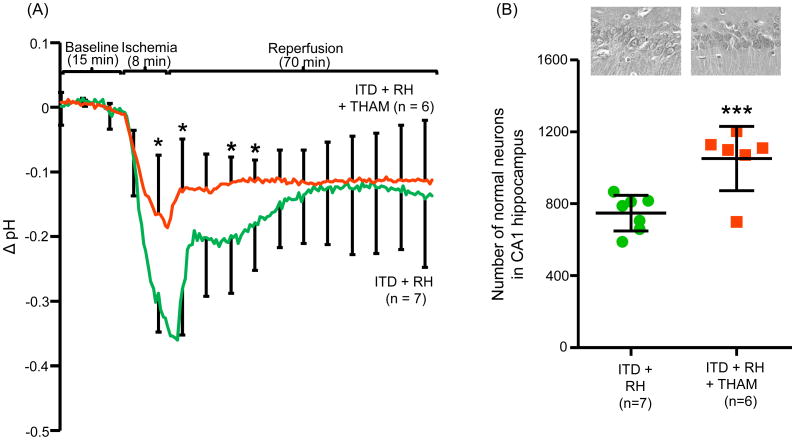
3.3 THAM treatment attenuates increased intra-ischemic acidosis in ITD rats exposed to RH
As we observed an enhanced decrease in hippocampal pH in ITD rats exposed to RH, we tested if an alkalizing agent (tris-hydroxymethyl-aminomethane: THAM) can prevent RH-induced increase in ischemic drop in pH in CA1 hippocampus. We determined pH before, during and after (during reperfusion) global cerebral ischemia in ITD rats exposed to RH with or without THAM treatment. The ischemia-associated drop in hippocampal pH in ITD + RH animals treated with THAM was significantly lower than that observed in animals belonging to the ITD + RH control group for all time points from 4 minutes after the onset of ischemia to 4 minutes after onset of reperfusion and 11 minutes to 18 minutes after the onset of reperfusion (drop in pH in ITD + RH + THAM group versus ITD + RH control group was 0.11 to 0.22). However, the drop in hippocampal pH in the ITD + RH + THAM treatment group was not significantly different from that seen in the ITD + RH control group during the first 4 minutes of ischemia, between 4 and 11 minutes of reperfusion and after 18 minutes of reperfusion (Figure 4A). Therefore, it is concluded that systemic THAM treatment prevents pronounced ischemic acidosis observed in ITD rats exposed to RH.
Figure 4.
The effect of THAM on ischemia-induced (A) Decrease in hippocampal (CA1) pH and (B) damage in CA1 hippocampus in ITD rats subjected to RH. Hippocampal (CA1) pH (Δ pH) versus time curve and number of normal neurons in CA1 hippocampus of rats belonging to ITD + RH (n = 7) and ITD + RH + THAM (n = 6) groups. *p<0.05 vs ITD + RH, ***p<0.005 vs ITD + RH.
3.4 THAM treatment attenuates RH-induced ischemic brain injury in ITD rats
As systemic THAM treatment prevents RH-induced increase in ischemic acidosis, we assessed whether this also prevents increased ischemic damage in RH-exposed ITD rats. Thus, we assessed the extent of ischemic brain damage in RH-exposed ITD rats with or without THAM treatment. Rats were euthanized after 7 days of reperfusion. The CA1 hippocampal neuronal count served as a measure of ischemic brain injury. As compared to the RH-exposed ITD control group, THAM treatment to RH-exposed ITD rats reduced ischemic brain injury by 20% (p<0.05) (Figure 4B). This result demonstrates that prevention of RH-induced increase in ischemic acidosis reduces the extent of ischemic brain injury in ITD rats.
3.5 GLUT-inhibition decreases ischemic increase in CA1 hippocampal lactate levels in ITD rats exposed to RH
Considering the effects of RH on brain GLUTs and glucose transport (Antony et al., 2010; Herzog et al., 2013; Lee et al., 2000), we tested the hypothesis that partial blockade of GLUTs (using OEDG) (Barnett et al., 1973; Wheeler and Hauck, 1985) will prevent ischemia-induced increase in lactate levels in CA1 hippocampus of ITD rats previously exposed to RH. Lactate levels were quantified in microdialysate samples obtained at various time points during the ischemia-reperfusion period in RH-exposed ITD rats receiving either vehicle or GLUT inhibitor treatment. We observed that the ischemia-induced percentage change in hippocampal lactate concentration during ischemia and early reperfusion in animals belonging to the GLUT inhibitor-treated group was significantly lower than that of animals belonging to the ITD + RH + vehicle control group (p<0.05) after the induction of cerebral ischemia (Figure 5A).
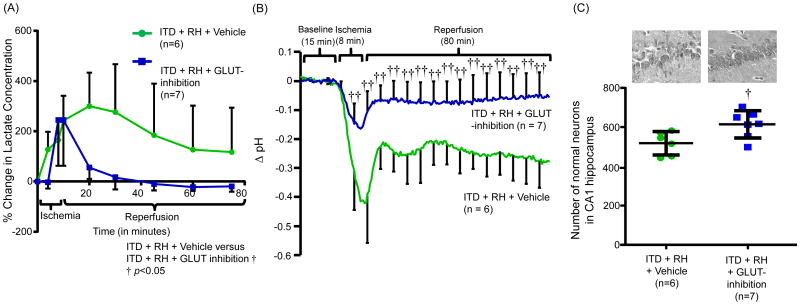
Figure 5.
The effect of GLUT-inhibition on ischemia-induced (A) increase in hippocampal (CA1) lactate concentration, (B) decrease in hippocampal (CA1) pH and (C) damage in CA1 hippocampus in ITD rats exposed to RH. Lactate levels in terms of percentage change in lactate concentration. Hippocampal (CA1) pH (Δ pH) versus time curve and number of normal neurons in CA1 hippocampus of rats belonging to ITD + RH + vehicle (n = 6) and ITD + RH + GLUT-inhibition (n = 7) groups. †p<0.05 vs ITD + RH + Vehicle, ††p<0.01 vs ITD + RH + Vehicle.
The AUC calculated from the percentage change in lactate concentration in the hippocampus versus time curve of animals belonging to GLUT inhibitor-treated ITD + RH group (87 ± 54 % change in mM × hr) was lower than that of animals belonging to the vehicle-treated ITD + RH group (268 ± 181 % change in mM × min). Therefore, it may be deduced that GLUTs mediate enhanced intra-ischemic lactic acidosis in RH-exposed ITD rats.
3.6 GLUT inhibition decreases enhanced intra-ischemic acidosis in ITD rats exposed to RH
We also confirmed whether GLUT inhibition prevents increased ischemic acidosis in CA1 hippocampus of RH-exposed ITD rats. The hippocampal pH was recorded during the ischemia and early reperfusion period in RH-exposed ITD rats receiving either vehicle or GLUT inhibitor treatment. GLUT inhibitor treatment prevented increased hippocampal acidosis observed in RH-exposed ITD rats during ischemia and initial 80 min of reperfusion (Figure 5B). Thus, it is deduced that GLUT inhibition prevents RH-induced increased ischemic acidosis in ITD rats.
3.7 GLUT inhibition decreases RH-induced ischemic brain injury in ITD rats
In view of the above results, we also evaluated the effect of GLUT inhibition on the extent of ischemic brain injury in RH-exposed ITD rats. Compared to the vehicle-treated RH-exposed ITD group, GLUT inhibitor treatment of RH-exposed ITD rats resulted in a small but significant reduction (17%, de that pronounced ischp<0.05) in the extent of ischemic brain injury (Figure 5C). These results indicate that GLUTs participate in the observed increased ischemic brain damage in RH-exposed ITD rats.
Discussion
Diabetes mellitus is an important risk factor for stroke as well as stroke-related morbidity and mortality (Abbott et al., 1987; Barrett-Connor and Khaw, 1988; Chukwuma and Tuomilehto, 1993). Therapeutic interventions available to control diabetic hyperglycemia are unable to achieve tight euglycemia and results in transient hypoglycemia (Leese et al., 2003; Nathan et al., 1993). Hypoglycemic episodes inhibit intrinsic counter-regulatory mechanisms of blood glucose regulation leading to increased risk of RH (Cryer, 2013; Leese et al., 2003). Using in vivo and in vitro models, we have previously shown that prior exposure to RH aggravates ischemic brain damage (Dave et al., 2011a; Dave et al., 2011b). The present study investigated whether prior exposure to RH increases ischemia-induced acidosis via GLUT, which precipitates increased ischemic brain damage in RH-exposed ITD rats. We selected hippocampus for assessment of ischemic injury as it is a brain area that is highly susceptible to ischemic damage (Bartsch et al., 2015; Kirino and Sano, 1984; Paschen et al., 1988; Petito et al., 1987; Schmidt-Kastner, 2015; Schmidt-Kastner and Freund, 1991; Schmidt-Kastner et al., 1990). However, comparison of other brain areas like the cortex remains to be determined. In addition, corroborating the presently reported findings in models of focal cerebral ischemia also remains to be investigated.
Herzog et al. (Herzog et al., 2013) have shown that RH induces a modest increase in lactate uptake in brain and thus contributes to more efficient cerebral utilization of glucose during hypoglycemia. RH causes increase in the levels of monocarboxylate transporter in brain (Vavaiya et al., 2007). Cerebral ischemia also increases lactate levels in brain and thus causes a precipitous drop in extracellular pH, more acidosis leading to the activation of number of deleterious mechanisms contributing to ischemic neuronal damage (Combs et al., 1990; Poittevin et al., 2015; Rehncrona et al., 1981). While a short period of acidosis exerts a protective effect on ischemic brain (Lam et al., 2013; Simon et al., 1993), a sustained and relatively longer period of more severe acidosis is known to be detrimental instead (Kraig et al., 1987; Plum, 1983). Levels of lactic acid in ischemic brain are associated with pH drop and an attainment of a threshold level of lactic acid causes a substantial drop in pH (Combs et al., 1990). We observed that ITD rats exposed to RH displayed a higher increase in lactate concentration in CA1 hippocampus during ischemia and early reperfusion. Further, this increase in lactate levels was concomitant with a profound drop in hippocampal pH. Therefore, it is plausible that increased ischemia-induced lactic acidosis in brain might be causing RH-induced exacerbation of ischemic injury. This contention was further supported by our observation that chemical attenuation of ischemic acidosis in RH-exposed ITD rats prevented RH-related pronounced ischemic brain injury.
Several mechanisms have been proposed to mediate the detrimental consequences of ischemic acidosis. Lactic acidosis inhibits glutathione by modulating its metabolism and thus increases sensitivity of cells against oxidative glutamate toxicity (Lewerenz et al., 2010). Acidosis accelerates hydroxyl free radical production by facilitating the Fenton reaction and thus causes oxidative injury during cerebral ischemia (Ying et al., 1999). Intraischemic acidosis activates the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-2 which causes the generation of free radicals in neurons (Brennan-Minnella et al., 2015). The drop in extracellular pH observed during ischemic acidosis in brain causes development of severe oxidative stress in neurons (Pekun et al., 2013). Therefore, it is possible that ischemia-induced acidosis activates mechanisms of cell death in RH-exposed ITD rats. However, further studies are required to identify the downstream mechanisms responsible for the acidotic mediation of ischemic brain injury in RH-exposed ITD rats. Not confirming cerebral ischemic damage using specific immunohistochemical markers is a limitation of our study.
Glucose is an essential respiratory substrate in brain which enters from blood by facilitated diffusion across blood brain barrier via the glucose transporters (Simpson et al., 1999; Simpson et al., 2007). Sustained or chronic hypoglycemia increases the levels of GLUTs in rat brain and cerebral vasculature (Kumagai et al., 1995; Lee et al., 2000; Uehara et al., 1997). The rate of glucose uptake in brain stays at normal levels after RH (Boyle et al., 1995; Boyle et al., 1994). Sustained hypoglycemia causes enhanced glucose transport in brain via increase in the levels of GLUTs and glucose uptake, and therefore causes adaptive increase in brain functions (Boyle et al., 1994; Kumagai et al., 1995; McCall et al., 1986; Pelligrino et al., 1990). Antecedent RH causes an increase in brain glucose metabolism during eventual euglycemia and a decrease in glucose metabolism during hypoglycemia (Jiang et al., 2009). In line with the above study, a group has demonstrated that during euglycemia, animals previously exposed to RH show better spatial learning but their learning ability was worsened when they were subjected to hypoglycemia (McNay and Sherwin, 2004). The reported effect of RH on performance on memory tests is associated with a higher brain glucose uptake (Criego et al., 2005; Herzog et al., 2008; McNay and Sherwin, 2004). Acidosis is one of the important factors associated with the detrimental effect of hyperglycemia on ischemic brain (Li et al., 1994; Smith et al., 1986; Widmer et al., 1992). Therefore, it is plausible that, during ischemia, an RH-induced increased level of GLUTs in brain causes increased uptake of glucose, which anaerobically mediates the observed lactic acidosis-related brain injury in ITD rats. This hypothesis was supported by our observation that inhibition of GLUTs attenuated RH-induced increase in ischemic acidosis and brain injury in ITD rats. Therefore, the current data shows that GLUTs may be causing ischemic acidosis-induced increase in brain damage in RH-exposed ITD rats. Our study shows that the ischemic drop in pH is increased and relatively prolonged in RH-exposed ITD rats but was nevertheless transient. Therefore, a later inhibition of acidosis, either directly by an alkalizing agent or indirectly by inhibition of glucose transporters, is not expected to demonstrate efficacy in either the animal model or in a clinical situation. However, identifying increased acidosis-induced activation of downstream pathways during the late reperfusion phase may provide better therapeutic targets with clinical translational potential.
Overall, we conclude that ischemia-induced pronounced acidosis, possibly via GLUTs, participates in RH-induced aggravation of ischemic brain damage. Nevertheless, further studies are needed to identify how increased acidosis mediates enhanced ischemic brain damage in ITD animals exposed to RH.
Supplementary Material
Highlights.
Recurrent hypoglycemia (RH) enhances ischemic brain damage in insulin-treated diabetic rats.
RH increases ischemic acidosis in insulin-treated diabetic rats.
Prevention of increased ischemic acidosis reduces RH-related increase in ischemic damage.
Glucose transporter (GLUT) inhibition decreases RH-induced ischemic brain injury.
Ischemia increases acidotic brain injury in RH-exposed ITD rats via GLUT.
Acknowledgments
We would like to thank Dr. Brant Watson for critical reading of this manuscript. We would also like to thank the Biostatistics Collaboration and Consulting Core, Division of Biostatistics, Department of Public Health Sciences, Clinical and Translational Science Institute, University of Miami, Miami for help with selection of statistical methods of analysis for data in the manuscript.
Funding: This work was supported by National Institutes of Health grant number NS073779. The funding agency was not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- AUC
area under the curve
- CA
Cornus ammonis
- GLUT
Glucose transporter
- ITD
Insulin treated diabetic
- MABP
mean arterial blood pressure
- NADPH
reduced form of nicotinamide adenine dinucleotide phosphate
- OEDG
4,6-O-Ethylidene-α-D-glucose
- pCO2
partial pressure of carbon dioxide in blood
- pO2
partial pressure of oxygen in blood
- RH
Recurrent hypoglycemia
- THAM
Tris-(hydroxymethyl)-aminomethane
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RD, Donahue RP, MacMahon SW, Reed DM, Yano K. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA. 1987;257:949–952. [PubMed] [Google Scholar]
- ADA Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes. 1988;37:901–907. doi: 10.2337/diab.37.7.901. [DOI] [PubMed] [Google Scholar]
- Antony S, Peeyush Kumar T, Mathew J, Anju TR, Paulose CS. Hypoglycemia induced changes in cholinergic receptor expression in the cerebellum of diabetic rats. J Biomed Sci. 2010;17:7. doi: 10.1186/1423-0127-17-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker GF, Widdas WF. The permeation of human red cells by 4,6-O-ethylidene- -D-glucopyranose (ethylidene glucose) J Physiol. 1973;231:129–142. doi: 10.1113/jphysiol.1973.sp010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JE, Holman GD, Chalkley RA, Munday KA. Evidence for two asymmetric conformational states in the human erythrocyte sugartransport system. Biochem J. 1975;145:417–429. doi: 10.1042/bj1450417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JE, Holman GD, Munday KA. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973;131:211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT. Diabetes mellitus: an independent risk factor for stroke? Am J Epidemiol. 1988;128:116–123. doi: 10.1093/oxfordjournals.aje.a114934. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Dohring J, Reuter S, Finke C, Rohr A, Brauer H, Deuschl G, Jansen O. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35:1836–1845. doi: 10.1038/jcbfm.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biros MH, Dimlich RV, Barsan WG. Postinsult treatment of ischemia-induced cerebral lactic acidosis in the rat. Ann Emerg Med. 1986;15:397–404. doi: 10.1016/s0196-0644(86)80174-3. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. Glucose deprivation causes posttranscriptional enhancement of brain capillary endothelial glucose transporter gene expression via GLUT1 mRNA stabilization. J Neurochem. 1993;60:2290–2296. doi: 10.1111/j.1471-4159.1993.tb03516.x. [DOI] [PubMed] [Google Scholar]
- Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Benedetti MM, Santeusanio F, Gerich JE, Brunetti P. A reliable and reproducible test for adequate glucose counterregulation in type I diabetes mellitus. Diabetes. 1984;33:732–737. doi: 10.2337/diab.33.8.732. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Kempers SF, O'Connor AM, Nagy RJ. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:1726–1731. doi: 10.1056/NEJM199512283332602. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Nagy RJ, O'Connor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A. 1994;91:9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan-Minnella AM, Won SJ, Swanson RA. NADPH oxidase-2: linking glucose, acidosis, and excitotoxicity in stroke. Antioxid Redox Signal. 2015;22:161–174. doi: 10.1089/ars.2013.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C., Prevention. Self-reported heart disease and stroke among adults with and without diabetes--United States, 1999-2001. MMWR Morb Mortal Wkly Rep. 2003;52:1065–1070. [PubMed] [Google Scholar]
- Chukwuma C, Sr, Tuomilehto J. Diabetes and the risk of stroke. J Diabetes Complications. 1993;7:250–262. [PubMed] [Google Scholar]
- Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–522. doi: 10.2337/diacare.18.4.517. [DOI] [PubMed] [Google Scholar]
- Combs DJ, Dempsey RJ, Maley M, Donaldson D, Smith C. Relationship between plasma glucose, brain lactate, and intracellular pH during cerebral ischemia in gerbils. Stroke. 1990;21:936–942. doi: 10.1161/01.str.21.6.936. [DOI] [PubMed] [Google Scholar]
- Conget I, Avila D, Gimenez M, Quiros C, Salaverria V, Duenas B. Impaired awareness of hypoglycaemia in subjects with type 1 diabetes. Results of an online survey in a diabetes web site. Endocrinol Nutr. 2016;63:121–125. doi: 10.1016/j.endonu.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Crane PD, Pardridge WM, Braun LD, Nyerges AM, Oldendorf WH. The interaction of transport and metabolism on brain glucose utilization: a reevaluation of the lumped constant. J Neurochem. 1981;36:1601–1604. doi: 10.1111/j.1471-4159.1981.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res. 2005;79:42–47. doi: 10.1002/jnr.20296. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog Brain Res. 2006;153:361–365. doi: 10.1016/S0079-6123(06)53021-3. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117:868–870. doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Handb Clin Neurol. 2013;117:295–307. doi: 10.1016/B978-0-444-53491-0.00023-7. [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91:819–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Pileggi A, Raval AP. Recurrent hypoglycemia increases oxygen glucose deprivation-induced damage in hippocampal organotypic slices. Neurosci Lett. 2011a;496:25–29. doi: 10.1016/j.neulet.2011.03.079. [DOI] [PubMed] [Google Scholar]
- Dave KR, Tamariz J, Desai KM, Brand FJ, Liu A, Saul I, Bhattacharya SK, Pileggi A. Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke. 2011b;42:1404–1411. doi: 10.1161/STROKEAHA.110.594937. [DOI] [PubMed] [Google Scholar]
- Davis SN, Shavers C, Mosqueda-Garcia R, Costa F. Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes. 1997;46:1328–1335. doi: 10.2337/diab.46.8.1328. [DOI] [PubMed] [Google Scholar]
- Dick AP, Harik SI, Klip A, Walker DM. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A. 1984;81:7233–7237. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, Band MM, Reekie G, Leese GP, Collaboration DM. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22:749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, Di Vincenzo A, Annibale B, Lepore M, Lalli C, et al. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia. 1994;37:797–807. doi: 10.1007/BF00404337. [DOI] [PubMed] [Google Scholar]
- Frier BM. In: Contemporary Diabetes: Diabetes and the brain. Biessels GJL, JA, editors. Humana Press; 2009. pp. 133–135. [Google Scholar]
- Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia in Type 2 Diabetes--More Common Than You Think: A Continuous Glucose Monitoring Study. J Diabetes Sci Technol. 2015;9:999–1005. doi: 10.1177/1932296815581052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore RM, Stead LG. The role of hyperglycemia in acute ischemic stroke. Neurocrit Care. 2006;5:153–158. doi: 10.1385/ncc:5:2:153. [DOI] [PubMed] [Google Scholar]
- Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Chan O, Yu S, Dziura J, McNay EC, Sherwin RS. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology. 2008;149:1499–1504. doi: 10.1210/en.2007-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RI, Jiang L, Herman P, Zhao C, Sanganahalli BG, Mason GF, Hyder F, Rothman DL, Sherwin RS, Behar KL. Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J Clin Invest. 2013;123:1988–1998. doi: 10.1172/JCI65105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman GD, Rees WD. Side-specific analogues for the rat adipocyte sugar transport system. Biochim Biophys Acta. 1982;685:78–86. doi: 10.1016/0005-2736(82)90037-2. [DOI] [PubMed] [Google Scholar]
- Hoxworth JM, Xu K, Zhou Y, Lust WD, LaManna JC. Cerebral metabolic profile, selective neuron loss, and survival of acute and chronic hyperglycemic rats following cardiac arrest and resuscitation. Brain Res. 1999;821:467–479. doi: 10.1016/s0006-8993(98)01332-8. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu B, Yang C, Chen H, Eunice D, Yuan Z. Acute hyperglycemia worsens ischemic stroke-induced brain damage via high mobility group box-1 in rats. Brain Res. 2013;1535:148–155. doi: 10.1016/j.brainres.2013.08.057. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. [accessed 03.30.17];IDF diabetes atlas-7th edition. 2015 http://www.diabetesatlas.org/ [PubMed]
- Jensen VF, Bogh IB, Lykkesfeldt J. Effect of insulin-induced hypoglycaemia on the central nervous system: evidence from experimental studies. J Neuroendocrinol. 2014;26:123–150. doi: 10.1111/jne.12133. [DOI] [PubMed] [Google Scholar]
- Jiang L, Herzog RI, Mason GF, de Graaf RA, Rothman DL, Sherwin RS, Behar KL. Recurrent antecedent hypoglycemia alters neuronal oxidative metabolism in vivo. Diabetes. 2009;58:1266–1274. doi: 10.2337/db08-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen H, Nakayama H, Raaschou HO, Olsen TS. Stroke in patients with diabetes. The Copenhagen Stroke Study. Stroke. 1994;25:1977–1984. doi: 10.1161/01.str.25.10.1977. [DOI] [PubMed] [Google Scholar]
- Kaur G, Singh R, Baquer NZ. Changes in hexokinase isoenzymes in regions of rat brain during insulin-induced hypoglycemia. J Neurochem. 1983;41:594–596. doi: 10.1111/j.1471-4159.1983.tb04780.x. [DOI] [PubMed] [Google Scholar]
- Kawada J, Okita M, Nishida M, Yoshimura Y, Toyooka K, Kubota S. Protective effect of 4,6-O-ethylidene glucose against the cytotoxicity of streptozotocin in pancreatic beta cells in vivo: indirect evidence for the presence of a glucose transporter in beta cells. J Endocrinol. 1987;112:375–378. doi: 10.1677/joe.0.1120375. [DOI] [PubMed] [Google Scholar]
- Kawada J, Okita M, Nukatsuka M, Toyooka K, Naito S, Nabeshima A, Tsujihara K, Yoshimura Y, Nishida M. Ethylidene glucose-substituted new analogue of streptozotocin cannot induce diabetes: study on the basis of structure and activity relationship. Mol Cell Endocrinol. 1989;62:153–159. doi: 10.1016/0303-7207(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28:355–359. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- Koranyi L, Bourey RE, James D, Mueckler M, Fiedorek FT, Jr, Permutt MA. Glucose transporter gene expression in rat brain: Pretranslational changes associated with chronic insulin-induced hypoglycemia, fasting, and diabetes. Mol Cell Neurosci. 1991;2:244–252. doi: 10.1016/1044-7431(91)90051-o. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Petito CK, Plum F, Pulsinelli WA. Hydrogen ions kill brain at concentrations reached in ischemia. J Cereb Blood Flow Metab. 1987;7:379–386. doi: 10.1038/jcbfm.1987.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–1404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- Kuyama H, Kitaoka T, Fujita K, Nagao S. The effect of alkalizing agents on experimental focal cerebral ischemia. Acta Neurochir Suppl (Wien) 1994;60:325–328. doi: 10.1007/978-3-7091-9334-1_87. [DOI] [PubMed] [Google Scholar]
- Lam TI, Brennan-Minnella AM, Won SJ, Shen Y, Hefner C, Shi Y, Sun D, Swanson RA. Intracellular pH reduction prevents excitotoxic and ischemic neuronal death by inhibiting NADPH oxidase. Proc Natl Acad Sci U S A. 2013;110:E4362–4368. doi: 10.1073/pnas.1313029110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Chung MY, Lee JU, Kang DG, Paek YW. Changes of glucose transporters in the cerebral adaptation to hypoglycemia. Diabetes Res Clin Pract. 2000;47:15–23. doi: 10.1016/s0168-8227(99)00107-2. [DOI] [PubMed] [Google Scholar]
- Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD, Collaboration DM. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Dargusch R, Maher P. Lactacidosis modulates glutathione metabolism and oxidative glutamate toxicity. J Neurochem. 2010;113:502–514. doi: 10.1111/j.1471-4159.2010.06621.x. [DOI] [PubMed] [Google Scholar]
- Li PA, Shamloo M, Smith ML, Katsura K, Siesjo BK. The influence of plasma glucose concentrations on ischemic brain damage is a threshold function. Neurosci Lett. 1994;177:63–65. doi: 10.1016/0304-3940(94)90045-0. [DOI] [PubMed] [Google Scholar]
- McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB. Chronic hypoglycemia increases brain glucose transport. Am J Physiol. 1986;251:E442–447. doi: 10.1152/ajpendo.1986.251.4.E442. [DOI] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53:418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260:E67–74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics, C., Stroke Statistics, S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitaoka T, Fujita K, Kuyama H, Ohkawa M. Effect of tris-(hydroxymethyl)-aminomethane on experimental focal cerebral ischemia. Exp Brain Res. 1996;111:51–56. doi: 10.1007/BF00229555. [DOI] [PubMed] [Google Scholar]
- Nahas GG. The pharmacology of tris(hydroxymethyl) aminomethane (THAM) Pharmacol Rev. 1962;14:447–472. [PubMed] [Google Scholar]
- Nahas GG, Sutin KM, Fermon C, Streat S, Wiklund L, Wahlander S, Yellin P, Brasch H, Kanchuger M, Capan L, Manne J, Helwig H, Gaab M, Pfenninger E, Wetterberg T, Holmdahl M, Turndorf H. Guidelines for the treatment of acidaemia with THAM. Drugs. 1998;55:191–224. doi: 10.2165/00003495-199855020-00003. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C, Diabetes C Complications Trial Research, G. The effect of intensive treatment of diabetes on the development and progression of longterm complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Ostir GV, Peek MK, Markides KS. Diabetes mellitus as a risk factor for stroke incidence and mortality in Mexican American older adults. J Gerontol A Biol Sci Med Sci. 2004;59:M640–645. doi: 10.1093/gerona/59.6.m640. [DOI] [PubMed] [Google Scholar]
- Paschen W, Rohn G, Meese CO, Djuricic B, Schmidt-Kastner R. Polyamine metabolism in reversible cerebral ischemia: effect of alpha-difluoromethylornithine. Brain Res. 1988;453:9–16. doi: 10.1016/0006-8993(88)90138-2. [DOI] [PubMed] [Google Scholar]
- Pekun TG, Lemeshchenko VV, Lyskova TI, Waseem TV, Fedorovich SV. Influence of intra- and extracellular acidification on free radical formation and mitochondria membrane potential in rat brain synaptosomes. J Mol Neurosci. 2013;49:211–222. doi: 10.1007/s12031-012-9913-3. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Segil LJ, Albrecht RF. Brain glucose utilization and transport and cortical function in chronic vs. acute hypoglycemia. Am J Physiol. 1990;259:E729–735. doi: 10.1152/ajpendo.1990.259.5.E729. [DOI] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Plum F. What causes infarction in ischemic brain?: The Robert Wartenberg Lecture. Neurology. 1983;33:222–233. doi: 10.1212/wnl.33.2.222. [DOI] [PubMed] [Google Scholar]
- Poittevin M, Bonnin P, Pimpie C, Riviere L, Sebrie C, Dohan A, Pocard M, Charriaut-Marlangue C, Kubis N. Diabetic microangiopathy: impact of impaired cerebral vasoreactivity and delayed angiogenesis after permanent middle cerebral artery occlusion on stroke damage and cerebral repair in mice. Diabetes. 2015;64:999–1010. doi: 10.2337/db14-0759. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Levy DE, Sigsbee B, Scherer P, Plum F. Increased damage after ischemic stroke in patients with hyperglycemia with or without established diabetes mellitus. Am J Med. 1983;74:540–544. doi: 10.1016/0002-9343(83)91007-0. [DOI] [PubMed] [Google Scholar]
- Rehncrona S, Rosen I, Siesjo BK. Brain lactic acidosis and ischemic cell damage: 1. Biochemistry and neurophysiology. J Cereb Blood Flow Metab. 1981;1:297–311. doi: 10.1038/jcbfm.1981.34. [DOI] [PubMed] [Google Scholar]
- Rosenthal JM, Amiel SA, Yaguez L, Bullmore E, Hopkins D, Evans M, Pernet A, Reid H, Giampietro V, Andrew CM, Suckling J, Simmons A, Williams SC. The effect of acute hypoglycemia on brain function and activation: a functional magnetic resonance imaging study. Diabetes. 2001;50:1618–1626. doi: 10.2337/diabetes.50.7.1618. [DOI] [PubMed] [Google Scholar]
- Russo VC, Kobayashi K, Najdovska S, Baker NL, Werther GA. Neuronal protection from glucose deprivation via modulation of glucose transport and inhibition of apoptosis: a role for the insulin-like growth factor system. Brain Res. 2004;1009:40–53. doi: 10.1016/j.brainres.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R. Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience. 2015;309:259–279. doi: 10.1016/j.neuroscience.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Ophoff BG, Hossmann KA. Pattern of neuronal vulnerability in the cat hippocampus after one hour of global cerebral ischemia. Acta Neuropathol. 1990;79:444–455. doi: 10.1007/BF00308722. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res. 1997;744:105–111. doi: 10.1016/s0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- Schurr A, Rigor BM. Brain anaerobic lactate production: a suicide note or a survival kit? Dev Neurosci. 1998;20:348–357. doi: 10.1159/000017330. [DOI] [PubMed] [Google Scholar]
- Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. 1987;79:777–781. doi: 10.1172/JCI112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RP, Niro M, Gwinn R. Brain acidosis induced by hypercarbic ventilation attenuates focal ischemic injury. J Pharmacol Exp Ther. 1993;267:1428–1431. [PubMed] [Google Scholar]
- Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, von Hanwehr R, Siesjo BK. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J Cereb Blood Flow Metab. 1986;6:574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- Tesfaye N, Seaquist ER. Neuroendocrine responses to hypoglycemia. Ann N Y Acad Sci. 2010;1212:12–28. doi: 10.1111/j.1749-6632.2010.05820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Nipper V, McCall AL. Chronic insulin hypoglycemia induces GLUT-3 protein in rat brain neurons. Am J Physiol. 1997;272:E716–719. doi: 10.1152/ajpendo.1997.272.4.E716. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Vavaiya KV, Paranjape SA, Briski KP. Testicular regulation of neuronal glucose and monocarboxylate transporter gene expression profiles in CNS metabolic sensing sites during acute and recurrent insulin-induced hypoglycemia. J Mol Neurosci. 2007;31:37–46. doi: 10.1007/BF02686116. [DOI] [PubMed] [Google Scholar]
- Wagner SRt, Lanier WL. Metabolism of glucose, glycogen, and high-energy phosphates during complete cerebral ischemia. A comparison of normoglycemic, chronically hyperglycemic diabetic, and acutely hyperglycemic nondiabetic rats. Anesthesiology. 1994;81:1516–1526. doi: 10.1097/00000542-199412000-00028. [DOI] [PubMed] [Google Scholar]
- Wheeler TJ, Hauck MA. Reconstitution of the glucose transporter from bovine heart. Biochim Biophys Acta. 1985;818:171–182. doi: 10.1016/0005-2736(85)90559-0. [DOI] [PubMed] [Google Scholar]
- White NH, Skor DA, Cryer PE, Levandoski LA, Bier DM, Santiago JV. Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med. 1983;308:485–491. doi: 10.1056/NEJM198303033080903. [DOI] [PubMed] [Google Scholar]
- Widmer H, Abiko H, Faden AI, James TL, Weinstein PR. Effects of hyperglycemia on the time course of changes in energy metabolism and pH during global cerebral ischemia and reperfusion in rats: correlation of 1H and 31P NMR spectroscopy with fatty acid and excitatory amino acid levels. J Cereb Blood Flow Metab. 1992;12:456–468. doi: 10.1038/jcbfm.1992.63. [DOI] [PubMed] [Google Scholar]
- Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. 1999;73:1549–1556. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Liu T, Zhang X, Si Y, Ye Y, Zhao C, Wang Q, Shen X. Intensive Versus Conventional Glycemic Control in Patients with Diabetes During Enteral Nutrition After Gastrectomy. J Gastrointest Surg. 2015;19:1553–1558. doi: 10.1007/s11605-015-2871-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.