Abstract
Interleukin (IL)-17A is a pro-inflammatory cytokine in mice and humans. It is recognized as a key factor for the protection of mice against various pathogens, but it also underlies pathogenic inflammatory responses in numerous mouse models. The inborn errors of IL-17A- and IL-17F-mediated immunity identified in humans in the last decade have revealed that IL-17A and IL-17F are key players in mucocutaneous immunity to Candida albicans, and, to a lesser extent, Staphylococcus aureus. By contrast, there is currently no genetic evidence for a causal link between excess of IL-17 and autoimmunity, autoinflammation, or allergy in humans. We discuss here the physiological and pathological roles of mouse and human IL-17A and IL-17F in host defense and excessive inflammation. We highlight recent advances in our understanding of the consequences deficient or excessive IL-17 immunity at various mucocutaneous sites, including the oral cavity, skin, intestine, lungs, and vagina.
Keywords: cytokines, inborn errors of immunity, infectious diseases, autoimmune diseases, inflammatory diseases
IL-17 cytokines and receptors
The IL-17 cytokine family has six members in mice and humans: IL-17A (also known as IL-17), IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F (Figure 1). The founding member, IL-17A, was originally called cytotoxic T-lymphocyte-associated antigen 8 (CTLA-8) when it was first cloned in 19931. It was subsequently renamed IL-172, and, more recently, IL-17A3. The IL-17F protein is the protein of this family most closely related to IL-17A, and the genes encoding these two cytokines are located on the same chromosome, in both mice and humans4. TH17 cells, a distinct lineage of CD4+ T helper cells discovered in mice in 2005, have been identified as the principal source of IL-17A and IL-17F5–7 (Figure 2). IL-22 and IL-26 (absent in mice) are the other effector cytokines preferentially produced by TH17 cells8, 9. CD8+ T cells have also been reported to produce IL-17A and IL-17F, and are known as Tc17 cells10, 11 (Figure 2). The other cells capable of producing IL-17A and IL-17F include γδ T cells12, 13, invariant natural killer T (iNKT) cells14, 15, natural killer (NK) cells16, 17, and type 3 innate lymphoid cells (ILC3s)18–21 (Figure 2). It remains unclear whether neutrophils produce IL-17A22–25. IL-17A and IL-17F have a similar pattern of expression in cells, driven by signal transducer and activator of transcription 3 (STAT3), RAR-related orphan receptor γT (RORγT), and RORα26–28. They also display a similar cysteine knot configuration4, acting both as IL-17A/IL-17A and IL-17F/IL-17F homodimers and as IL-17A/IL-17F heterodimers29–31. IL-17A homodimers have been shown to be more potent than IL-17F homodimers in fibroblasts, macrophages, and epithelial cells, in terms of their ability to induce pro-inflammatory cytokines and chemokines, such as IL-6 and growth-related oncogene-α (GRO-α); IL-17A/IL-17F heterodimers have intermediate activity29–31.
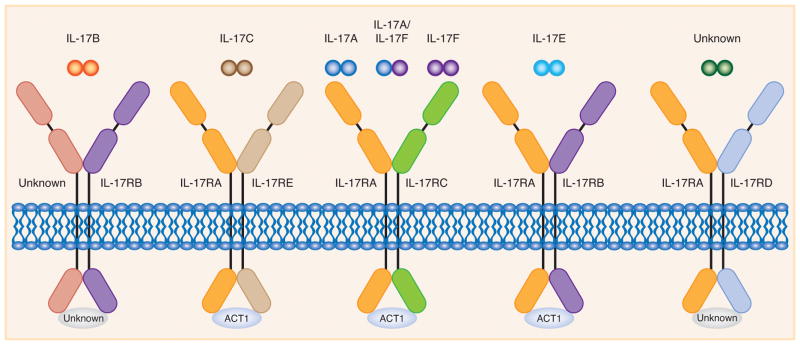
Figure 1. The IL-17 cytokine and receptor family.
The IL-17 cytokine family has six members (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F), whereas the IL-17 receptor family has five members (IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE). IL-17RA forms heterodimeric complexes with other subunits to induce signaling by IL-17 cytokines. The IL-17RA/IL-17RC complex binds to IL-17A/IL-17A and IL-17F/IL-17F homodimers and IL-17A/IL-17F heterodimers. The IL-17RA/IL-17RB and IL-17RA/IL-17RE receptors recognize IL-17E and IL-17C, respectively. The ligand for the IL-17RA/IL-17RD receptor complex and the receptors for IL-17B have not yet been identified. ACT1 serves as a key adaptor molecule, which is recruited to the known IL-17 receptors.
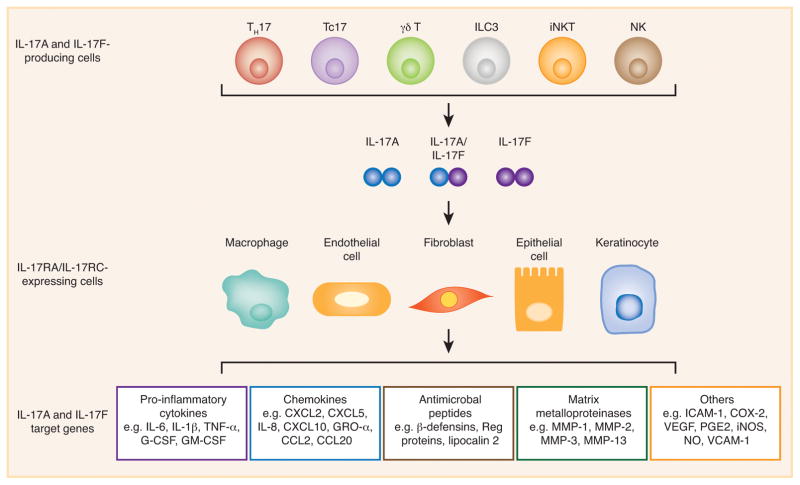
Figure 2. IL-17A- and IL-17F-mediated immunity.
TH17, Tc17, γδ T, ILC3s, iNKT, and NK cells are the major IL-17A- and IL-17F-producing cells, whereas macrophages, fibroblasts, keratinocytes, endothelial and epithelial cells are the main IL-17RA/IL-17RC-expressing cells. The target genes of IL-17A and IL-17F include pro-inflammatory cytokines, chemokines, antimicrobial peptides, and matrix metalloproteinases (MMPs). ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1; COX-2, cyclooxygenase 2; VEGF, vascular endothelial growth factor; PGE2, prostaglandin E2; iNOS, inducible nitric oxide synthase; NO, nitric oxide.
Soon after the discovery of IL-17A, IL-17RA (also known as IL-17R) was identified as the founding member of the IL-17 receptor family in mice and humans2. This family has five members: IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE (Figure 1). The subunits of the IL-17 receptor, containing a conserved SEFIR (similar expression of fibroblast growth factor and IL-17R) domain, form various heterodimeric complexes with IL-17RA to induce signaling by IL-17A and IL-17F (IL-17RA/IL-17RC), IL-25 (IL-17RA/IL-17RB), and IL-17C (IL-17RA/IL-17RE)32 (Figure 1). In each case, functional mouse and human IL-17 receptors bind SEFIR domain-containing adaptor nuclear factor-κB (NF-κB) activator 1 (ACT1) to mediate the downstream NF-κB, mitogen-activated protein kinase (MAPK), and CCAAT/enhancer-binding protein (C/EBP) signaling pathways32. The receptors for IL-17B and the ligand for the IL-17RA/IL-17RD receptor complex have not yet been identified32 (Figure 1). IL-17RA and IL-17RC are the best-characterized members of the IL-17 receptor family, largely because of their interaction with IL-17A and IL-17F. IL-17RA is ubiquitously expressed, whereas IL-17RC expression is mostly restricted to non-hematopoietic cells33. In humans, the affinity of IL-17RA binding is much higher for IL-17A than for IL-17F34. By contrast, IL-17RC and IL-17RA/IL-17RC bind IL-17A and IL-17F with similar affinities34. In mice, IL-17RA binds both IL-17A and IL-17F, but IL-17RC binds strongly only to IL-17F34. The affinity of IL-17RA/IL-17RC binding for IL-17A and IL-17F has not been tested34. IL-17A and IL-17F therefore mostly activate non-hematopoietic cells (Figure 2). Genes encoding pro-inflammatory cytokines and chemokines are the major targets of IL-17A and IL-17F33 (Figure 2). For example, IL-6, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) regulate myeloid cell functions and inflammatory responses, whereas GRO-α, CXC chemokine 2 (CXCL2), and CXCL5 act as chemoattractants for monocytes and neutrophils33. Antimicrobial peptides, including β-defensins in particular, are also induced by IL-17A and IL-17F at the epithelial barrier, to protect the host against a wide range of microorganisms33 (Figure 2). CC chemokine 20 (CCL20) recruits IL-17A- and IL-17F-producing cells expressing CC chemokine receptor 6 (CCR6)33 (Figure 2).
IL-17 in the oral cavity and skin
In mice, IL-17A, IL-17F, and their receptors, IL-17RA/IL-17RC, have been shown to play a major role in host defense against experimental infections of the oral cavity and skin caused by C. albicans and S. aureus. In a mouse model of oropharyngeal candidiasis (OPC), IL-17RA- and IL-17RC-deficient mice, like anti-IL-17A/IL-17F antibody (Ab)-treated mice, were found to have higher fungal burdens on the tongue than wild-type controls35–37. Neutrophil recruitment and function, which have been reported to be important for host defense against OPC in mice38, were essentially normal in these mice37. A recent study, using mice with a conditional deficiency of IL-17RA in superficial oral and esophageal epithelial cells, suggested that the IL-17RA-dependent antifungal response was mediated by the production of β-defensin 339. Moreover, ILCs, γδ T and thymus-derived TH17 cells (natural TH17 cells) have been shown to be major sources of IL-17A and IL-17F in response to C. albicans in mice, and indispensable for IL-17-mediated antifungal immunity in the oral mucosa40–42. IL-17A-deficient mice are more susceptible to cutaneous infection with C. albicans than wild-type mice43. Mice lacking IL-12p40 or IL-23p19, the two subunits of IL-23, which is essential for TH17 cell development and maintenance in mice44, also have high fungal burdens in the skin when challenged with C. albicans43. These results suggest that the immunity mediated by IL-17A and IL-17F is the key to mucocutaneous protection against C. albicans in mice, at least in experimental conditions. However, C. albicans is a commensal in humans, but not in mice. In addition, IL-17RA- and γδ T cell-deficient mice have larger skin lesions with higher bacterial counts upon cutaneous S. aureus infection, and these phenotypes can be rescued by the administration of recombinant IL-17A in γδ T cell-deficient mice45. Furthermore, mice lacking both IL-17A and IL-17F are particularly susceptible to S. aureus infection, developing mucocutaneous abscesses around the nose and mouth46. These findings highlight the crucial importance of IL-17A and IL-17F in protective immunity to experimental infection with S. aureus, which is commensal in mice47.
Humans with inborn errors of IL-17 immunity present chronic mucocutaneous candidiasis (CMC)48, 49. CMC is characterized by recurrent or persistent lesions of the skin, nails, oral, and genital mucosae caused by Candida spp., mostly C. albicans50–52. Autosomal recessive (AR) IL-17RA and autosomal dominant (AD) IL-17F deficiencies were the first genetic etiologies identified in patients with ‘isolated’ inherited CMC, who develop CMC with no other prominent clinical signs except staphylococcal skin lesions and pulmonary bacterial diseases in some cases53. AR IL-17RA deficiency has been reported in 23 patients from 13 unrelated kindreds, and shown to be caused by 12 different homozygous IL17RA mutations and one large homozygous deletion encompassing IL-17RA and adenosine deaminase 2 (ADA2)53–55. These patients lack fibroblast responses to IL-17A and IL-17F homodimers and heterodimers and leukocyte responses to IL-2553, 55. These cellular responses are also impaired in patients with AR ACT1 deficiency (2 patients from 1 kindred)56. By contrast, patients with AD IL-17F (5 patients from 1 kindred) or AR IL-17RC (3 patients from unrelated kindreds) deficiencies display impaired or abolished responses to IL-17A and IL-17F homodimers and heterodimers in fibroblasts, but their leukocytes respond normally to IL-2553, 57. Interestingly, staphylococcal diseases are frequently seen in patients with AR IL-17RA or ACT1 deficiencies, but not in those with AD IL-17F or AR IL-17RC deficiencies48, 49. It has thus been suggested that compromised responses to IL-25 or another IL-17RA-dependent cytokine may account for staphylococcal diseases in patients with inborn errors of IL-17 immunity. Taken together, these data reveal that human IL-17 immunity is indispensable for mucocutaneous immunity to C. albicans and S. aureus in natural conditions, especially in the oral cavity and skin.
Psoriasis is an autoimmune and inflammatory skin disorder characterized by red scaly patches of hyperproliferating keratinocytes and hyperkeratinosis58–61. Following the intradermal injection of IL-23, wild-type mice develop erythema, epidermal hyperplasia, and massive neutrophil infiltration62. By contrast, IL-17RA- or IL-17A- (or even IL-22-)deficient mice are resistant to IL-23-induced psoriasis-like epidermal hyperplasia and inflammation63, 64. Dermal γδ T cells, which constitutively express the IL-23 receptor (IL-23R), have been shown to be the principal producers of IL-17A in mouse skin following stimulation with IL-2363. Ablation of the IL-17RA or ACT1 gene also protects mice from imiquimod-induced psoriasis-like skin inflammation65–67. Furthermore, K5.Stat3C transgenic mice, which express STAT3 constitutively in keratinocytes, develop psoriasiform lesions following treatment with 12-O-tetradecanoylphorbol-13-acetate (TPA), whereas the Ab-mediated neutralization of IL-12p40, IL-23p19, or IL-17A, or deletion of the IL-17A gene attenuates the development of psoriasis-like lesions68. These data suggest that the IL-23-TH17-IL-17A axis contributes to the pathogenesis of skin lesions in mouse models of psoriasis. Consistent with this hypothesis, several TH17-associated molecules, including IL-17A, IL-17F, IL-22, IL-26, and RORγT, are strongly expressed in the psoriatic skin lesions of patients, probably due to the production of IL-23 by dendritic cells (DCs) in the skin9, 69. Genome-wide association studies (GWAS) in humans have also identified polymorphisms weakly associated with psoriasis close to the IL23R and ACT1 genes70, 71. Genetic proof of the involvement of IL-17 in the pathogenesis of human psoriasis is, however, lacking, in the absence of known gain-of-function (GOF) mutations. The known monogenic forms of psoriasis include AR deficiency of IL-36-receptor antagonist (DITRA) and AD caspase recruitment domain-containing protein 14 (CARD14) GOF72–76. Nevertheless, IL-23 and IL-17A have been identified as potential treatment targets in psoriasis. Human monoclonal Abs targeting IL-23p19 (risankizumab, guselkumab, and tildrakizumab) are currently in phase III trials, and have yielded encouraging early efficacy and safety results77–79. Anti-IL-17A (secukinumab and ixekizumab) and anti-IL-17RA (brodalumab) Abs have recently been approved by the US Food and Drug Administration (FDA) for psoriasis treatment, and have been shown to clear up skin lesions totally after 12 weeks of treatment in more than a quarter of the participants in the phase III trial80–82.
IL-17 in the intestine
Not only is the immunity mediated by IL-17A and IL-17F crucial for protection against C. albicans and S. aureus, it also protects mice against experimental infection of the intestine with Citrobacter rodentium. IL-12p40-, IL-23p19-, IL-17A-, IL-17F-, IL-17A/IL-17F double-, and IL-22-deficient mice are more susceptible to C. rodentium infection than wild-type mice46, 83, 84. Mortality is higher in IL-12p40-, IL-23p19-, or IL-22-deficient mice challenged with C. rodentium than in wild-type mice challenged with the same bacterium83, 84. IL-22 is induced by IL-23 in the colon and maintains colonic epithelial integrity during the early phase of C. rodentium infection in mice, by inducing the regenerating islet-derived (Reg) family of antimicrobial proteins84. Likewise, in response to C. rodentium infection, IL-17A-, IL-17F-, and IL-17A/IL-17F double-deficient mice have higher bacterial burdens and display more severe colonic inflammation, probably due to the impairment of β-defensin production46. The specific disruption of IL-17RA in mouse intestinal epithelial cells is associated with segmented filamentous bacteria (SFB) overgrowth and lower mRNA levels of α-defensins, NADPH oxidase 1 (Nox1), and polymeric immunoglobulin receptor (pIgR), as well as poor fecal secretory IgA production85. However, it remains unclear whether IL-17A and IL-17F protect against experimental infection of the intestine with Salmonella enterica serovar Typhimurium. In a mouse model of streptomycin-pretreated S. typhimurium infection, IL-17RA-deficient mice challenged with strain ATCC 14028 display higher levels of bacterial translocation from the intestine to the mesenteric lymph nodes and spleen86. By contrast, IL-17A-, IL-17F-, or IL-17RA-deficient mice, and mice treated with anti-IL-17A plus IL-17F Abs, display levels of bacterial dissemination and cecal inflammation similar to those of wild-type mice following challenge with strain SL134487. Local neutralization of IL-17A in the intestinal lumen decreases bacterial clearance and exacerbates epithelial damage in mice infected with S. typhimurium strain LT288. Further studies are, therefore, required, to clarify the contribution of IL-17 immunity to intestinal protection against experimental S. typhimurium infection in mice. In humans, deficiencies of IL-17 immunity do not seem to cause a predisposition to pathogenic infections in the intestine, at least among the patients identified to date48, 49.
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a group of autoinflammatory disorders characterized by inflammation of the colon and small intestine58. The precise role of IL-17A- and IL-17F-mediated immunity in experimental inflammatory colitis in mice remains a matter of debate. In a mouse model of acute trinitrobenzenesulfonic acid (TNBS)-induced colitis, IL-17RA-deficient mice are protected from weight loss and colonic inflammation, and the administration of an IL-17RA IgG1 fusion protein improves established colonic inflammation in wild-type mice89. Mice lacking IL-17A or IL-17F are also resistant to dextran sodium sulfate (DSS)-induced colitis, displaying milder acute intestinal inflammation90, 91. However, some studies have suggested that the neutralization or genetic ablation of IL-17A, and the epithelial-specific deletion of ACT1 result in severe DSS-induced colitis in mice91–94. These conflicting findings may result from differences in experimental setting, including the genetic background and intestinal microbiota of the mice. Moreover, in a mouse model of T cell-mediated colitis, the adoptive transfer of IL-17A- or IL-17RA-deficient T cells in recombination activating gene 1 (Rag1)-deficient mice, which lack mature T and B cells, led to exacerbated colitis95. Multidrug resistance 1a (Abcb1a)-deficient mice infected with Helicobacter bilis develop spontaneous colitis, and the neutralization of IL-17A or IL-17RA accelerates death, by greatly weakening the intestinal epithelial barrier and increasing colonic inflammation96. It has been suggested that the principal function of IL-17A and IL-17RA in experimental colitis is the maintenance of intestinal barrier integrity rather than driving pathogenic inflammation. Clinical trials of anti-IL-17A (secukinumab) and anti-IL-17RA (brodalumab) Abs in patients with moderate-to-severe CD have reported no improvement, or even a worsening of disease, on treatment97, 98. The high levels of mRNA for TH17 signature cytokines, including IL-17A, IL-17F, IL-22, and IL-26, in the intestinal mucosa of patients with IBD may therefore be beneficial99–102.
IL-17 in the lungs
The protective role of IL-17 cytokines and receptors in host defense against microorganisms was first described in a mouse model of Klebsiella pneumoniae infection. IL-17RA-deficient mice display high mortality rates, delayed neutrophil recruitment, and poor G-CSF and CXCL2 expression in the lungs103. This observation was supported by the findings of a recent study of mice with a conditional deficiency of IL-17RA or IL-17RC in the lung epithelium. These mice display compromised K. pneumoniae clearance, with impaired neutrophil recruitment in response to IL-17A and low levels of CXCL5 production104. Similarly, mice lacking IL-17A due to a genetic deficiency or Ab-mediated neutralization are more susceptible to K. pneumoniae infection than wild-type mice105–107. It has been shown that IL-17A- and IL-17F-producing γδ T cells are essential to protect mice against K. pneumoniae106, whereas ILC3s also play a crucial role in activating inflammatory monocytes during infection107. In a mouse model of S. aureus pneumonia, IL-17A-, IL-17F-, and IL-17RA-deficient mice have been shown to have large bacterial burdens in the lungs108. In addition, the genetic deletion of IL-17A or IL-17RA leads to higher bacterial counts in the lungs, associated with lower levels of G-CSF production and an impairment of IL-12-dependent interferon-γ (IFN-γ) responses in mice infected with a live vaccine strain (LVS) of Francisella tularensis109. IL-17A induces the production of IL-12 and IFN-γ in antigen-presenting cells (APCs) and enhances bacterial clearance following infection with F. tularensis LVS109. Furthermore, upon challenge with Chlamydia muridarum, mice in which IL-17A has been neutralized display compromised Chlamydia-specific TH1 responses, with greater bacterial growth in the lungs, and lower survival110, 111. In a DC/T-cell coculture system, DCs isolated from mice in which IL-17A was neutralized induced lower levels of IFN-γ production by Chlamydia-specific T cells than those derived from wild-type mice110. Collectively, these findings suggested that the immunity mediated by IL-17A and IL-17F makes a major contribution to the protection of mice against experimental bacterial infections of the lungs. Consistent with this hypothesis, various bacterial infections of the respiratory tract have been seen in patients with AR IL-17RA deficiency55.
Asthma is classically considered to be a TH2-mediated allergic disorder characterized by eosinophilic inflammation and airway hyperresponsiveness (AHR) that ultimately gives rise to obstructive airway and breathing problems58. In a mouse model of ovalbumin (OVA)-induced asthma, IL-17RA-deficient mice display impaired eosinophil recruitment and compromised TH2 responses112. Likewise, IL-17A-deficient mice have lower levels of OVA-induced AHR and pulmonary inflammation113. The adoptive transfer of OVA-specific TH17 cells in recipient mice leads to an influx of neutrophils in the airways and AHR, whereas the transfer of OVA-specific TH2 cells results in the migration of lymphocytes and eosinophils into the lungs and AHR, following OVA challenge114. Treatment with dexamethasone attenuates the airway inflammation and AHR induced by TH2 cells, but not those induced by TH17 cells114. These results suggest a potential role for TH17 cells in steroid-resistant asthma in mice. In a mouse model of allergen-induced asthma, the neutralization of both IL-13 and IL-17A protects mice from eosinophilic and neutrophilic inflammation and AHR115, suggesting that the combined targeting of TH2 and TH17 cells may maximize therapeutic efficacy. Moreover, a subset of IL-17A-producing TH2 (TH2/TH17) cells has been shown to induce higher levels of heterogeneous inflammatory leukocyte infiltration and to exacerbate asthma relative to classical TH2 and TH17 cells116. Consistently, several studies have shown that IL-17A is upregulated in the sputum and bronchial biopsies of patients with asthma and that the production of this cytokine is correlated with disease severity117. It has also been suggested that a higher frequency of TH2/TH17 cells in bronchoalveolar lavage fluid is associated with a more severe form of asthma in humans118. However, a clinical trial showed that anti-IL-17RA Ab (brodalumab) alone had no effect in patients with moderate-to-severe asthma119.
Chronic obstructive pulmonary disease (COPD), encompassing chronic bronchitis and emphysema, is a progressive and largely irreversible disorder resulting in airflow limitation58. It is caused by long-term exposure to toxic inhalants, and is most often associated with cigarette smoke58. In mice, cigarette smoke promotes TH17 cell differentiation in vitro and in vivo via the aryl hydrocarbon receptor120, 121. IL-17A- or IL-17RA-deficient mice are resistant to emphysema120, 121, whereas transgenic mice displaying lung-specific IL-17A expression (Cc10-Il17a) develop severe emphysema in response to cigarette smoke121. The adoptive transfer of lung APCs isolated from mice with emphysema induces emphysema and an increase in inflammation after 12 weeks in the absence of smoke in wild-type, but not in IL-17A-deficient recipient mice121. In addition, the neutralization of IL-17A attenuates smoke-induced neutrophilic inflammation of the airways in mice122. Large numbers of TH17 cells and the overproduction of IL-17A have also been observed in the lungs of mice treated with porcine pancreatic elastase123. IL-17A-deficient mice display lower levels of elastase-induced pulmonary inflammation and emphysema than wild-type mice123. Furthermore, in two mouse models of airway fibrosis triggered by COPD-relevant stimuli, exposure to an adenoviral IL-1β vector (Ad-IL-1β) or cigarette smoke in combination with viral mimetic poly(I:C), the knockout of IL-17RA or the neutralization of IL-17A or IL-17RA results in lower levels of airway fibrosis and inflammation124. Finally, there is growing evidence to suggest that patients with COPD have higher proportions of TH17 cells among their peripheral blood mononuclear cells (PBMC) and high levels of IL-17A and IL-17F on bronchial biopsies125, 126. High levels of RORγT mRNA have also been reported in the lungs of patients with COPD127. However, a phase II trial of anti-IL-17A Ab (CNTO 6785) did not show any significant efficacy in the treatment of patients with symptomatic moderate-to-severe COPD128.
IL-17 in the vagina
There is increasing evidence to suggest that IL-17A- and IL-17-mediated immunity may be involved in host defense in the mouse vagina. For instance, mice in which IL-17A or IL-17RA has been neutralized are more susceptible to Neisseria gonorrhoeae infection than wild-type mice129. They die more rapidly and have higher bacterial burdens and delayed neutrophil recruitment to the vagina during N. gonorrhoeae infection129. By contrast, ablation of the IL-17A gene results in a lower bacterial load in the vagina upon vaginal infection with C. muridarum130, whereas the knockout of IL-17RA does not131. Both types of mutant mouse display lower levels of neutrophil influx into the vagina130, 131, but IL-17A-deficient mice have high levels of Chlamydia-neutralizing Ab in the serum130, whereas IL-17RA-deficient mice display higher levels of macrophage influx and tumor necrosis factor-α (TNF-α) production, possibly compensating for the impaired Chlamydia-specific TH1 response and IFN-γ production131. However, the role of IL-17 immunity in vaginal C. albicans infection in mice remains unclear. In a mouse model of estrogen-induced vulvovaginal candidiasis (VVC), wild-type mice produce larger amounts of IL-17A and display massive neutrophil influx into the vagina upon C. albicans chanllenge132, and treatment with halofuginone, a specific inhibitor of mouse and human TH17 cell differentiation133, results in a higher fungal load and greater β-defensin 2 production132. By contrast, wild-type and IL-23p19-, IL-17RA-, and IL-22-deficient mice have similar vaginal fungal burdens and similar levels of neutrophil infiltration following inoculation with C. albicans134. Humans with inborn errors of IL-17 immunity do not seem to be susceptible to pathogenic infections of the vagina48, 49.
Concluding remarks
The discovery of TH17 cells has greatly expanded and advanced our understanding of the roles of IL-17A, IL-17F, and their receptors in autoimmunity, autoinflammation, allergy, and host defense (Figure 3). However, the role of these molecules in malignancy, the fifth broad category of phenotypes associated with inborn errors of immunity, has not been studied. The development of mucocutaneous carcinomas in patients with CMC and inborn errors of IL-17 immunity suggests that IL-17 may play a role in immunosurveillance, at least indirectly, via host defense against C. albicans. Treatments with IL-17A and IL-17RA inhibitors have been shown to be beneficial in diseases characterized by excessive inflammation, such as psoriasis, but human genetic studies have shown that IL-17A and IL-17F play an essential role in mucocutaneous immunity to C. albicans and, to a lesser extent, S. aureus. A higher percentage of fungal infections, properly managed with antifungal treatments, has been reported in some trials of the blockade of IL-17-mediated immunity (e.g. anti-IL-17A or anti-IL-17RA Abs in patients with psoriasis or psoriatic arthritis)135, 136. Thus, fine control of balance between pathogenic and protective IL-17 immunity will be of crucial importance in the development of future therapeutic strategies for treating inflammatory and infectious diseases.
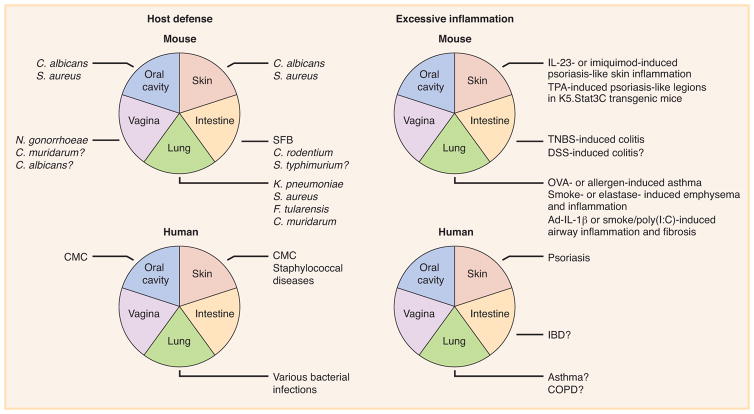
Figure 3. IL-17A and IL-17F in host defense and excessive inflammation.
In mice, deficient IL-17A- and IL-17F-mediated immunity predisposes to various pathogenic infections of the oral cavity, skin, intestine, lungs, and vagina, whereas excessive IL-17A- and IL-17F-mediated immunity induces pathological inflammation in the skin, intestine, and lungs. Human inborn errors of IL-17 immunity present chronic mucocutaenous candidiasis (CMC) in the oral cavity, CMC and staphylococcal diseases in the skin, and various bacterial infections in the lungs. Treatments with anti-IL-17A and IL-17RA inhibitors are beneficial for patients with psoriasis characterized by excessive inflammation in the skin.
Acknowledgments
We thank the members of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions. We also thank Yelena Nemirovskaya and Cécile Patissier for their assistance. This work was supported in part by the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID); the French National Research Agency (ANR) under the “Investments for the future” program (grant number ANR-10-IAHU-01); ANR HGDIFD (ANR-14-CE15-0006-01); eRARE EURO-CMC (ANR-14-RARE-0005-02); the National Institute of Allergy and Infectious Diseases (R01AI127564); the Jeffrey Modell Foundation Translational Research Program; the St. Giles Foundation, the Rockefeller University; Institut National de la Santé et de la Recherche Médicale (INSERM); University Paris Descartes.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–5456. [PubMed] [Google Scholar]
- 2.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 3.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20(19):5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 6.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 10.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182(9):5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 11.Guillot-Delost M, Le Gouvello S, Mesel-Lemoine M, Cherai M, Baillou C, Simon A, et al. Human CD90 identifies Th17/Tc17 T cell subsets that are depleted in HIV-infected patients. J Immunol. 2012;188(3):981–991. doi: 10.4049/jimmunol.1101592. [DOI] [PubMed] [Google Scholar]
- 12.Umemura M, Kawabe T, Shudo K, Kidoya H, Fukui M, Asano M, et al. Involvement of IL-17 in Fas ligand-induced inflammation. Int Immunol. 2004;16(8):1099–1108. doi: 10.1093/intimm/dxh111. [DOI] [PubMed] [Google Scholar]
- 13.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1. 1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel ML, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, Dy M, et al. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105(50):19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184(4):1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandya AD, Al-Jaderi Z, Hoglund RA, Holmoy T, Harbo HF, Norgauer J, et al. Identification of human NK17/NK1 cells. PLoS One. 2011;6(10):e26780. doi: 10.1371/journal.pone.0026780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10(1):66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 19.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 22.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170(4):2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 23.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15(2):143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huppler AR, Verma AH, Conti HR, Gaffen SL. Neutrophils Do Not Express IL-17A in the Context of Acute Oropharyngeal Candidiasis. Pathogens. 2015;4(3):559–572. doi: 10.3390/pathogens4030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 28.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17(5):435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 30.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 31.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282(18):13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 32.Amatya N, Garg AV, Gaffen SL. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017 doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179(8):5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206(2):299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho AW, Shen F, Conti HR, Patel N, Childs EE, Peterson AC, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol. 2010;185(2):1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trautwein-Weidner K, Gladiator A, Nur S, Diethelm P, LeibundGut-Landmann S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015;8(2):221–231. doi: 10.1038/mi.2014.57. [DOI] [PubMed] [Google Scholar]
- 38.Huppler AR, Conti HR, Hernandez-Santos N, Darville T, Biswas PS, Gaffen SL. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol. 2014;192(4):1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conti HR, Bruno VM, Childs EE, Daugherty S, Hunter JP, Mengesha BG, et al. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe. 2016;20(5):606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10(10):1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190(2):521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 42.Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211(10):2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185(9):5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Tavakkol Z, Samuelson D, deLancey Pulcini E, Underwood RA, Usui ML, Costerton JW, et al. Resident bacterial flora in the skin of C57BL/6 mice housed under SPF conditions. J Am Assoc Lab Anim Sci. 2010;49(5):588–591. [PMC free article] [PubMed] [Google Scholar]
- 48.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35(8):696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada S, Puel A, Casanova JL, Kobayashi M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin Transl Immunology. 2016;5(12):e114. doi: 10.1038/cti.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22(4):467–474. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616–622. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25(6):736–747. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fellmann F, Angelini F, Wassenberg J, Perreau M, Arenas Ramirez N, Simon G, et al. IL-17 receptor A and adenosine deaminase 2 deficiency in siblings with recurrent infections and chronic inflammation. J Allergy Clin Immunol. 2016;137(4):1189–1196. e1181–1182. doi: 10.1016/j.jaci.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 55.Levy R, Okada S, Beziat V, Moriya K, Liu C, Chai LY, et al. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci U S A. 2016;113(51):E8277–E8285. doi: 10.1073/pnas.1618300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212(5):619–631. doi: 10.1084/jem.20141065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621. doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 60.Arakawa A, Siewert K, Stohr J, Besgen P, Kim SM, Ruhl G, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212(13):2203–2212. doi: 10.1084/jem.20151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen YL, et al. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213(11):2399–2412. doi: 10.1084/jem.20160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186(3):1495–1502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- 65.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 66.El Malki K, Karbach SH, Huppert J, Zayoud M, Reissig S, Schuler R, et al. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J Invest Dermatol. 2013;133(2):441–451. doi: 10.1038/jid.2012.318. [DOI] [PubMed] [Google Scholar]
- 67.Ha HL, Wang H, Pisitkun P, Kim JC, Tassi I, Tang W, et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc Natl Acad Sci U S A. 2014;111(33):E3422–3431. doi: 10.1073/pnas.1400513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakajima K, Kanda T, Takaishi M, Shiga T, Miyoshi K, Nakajima H, et al. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol. 2011;186(7):4481–4489. doi: 10.4049/jimmunol.1000148. [DOI] [PubMed] [Google Scholar]
- 69.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160(2):319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 70.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80(2):273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet. 2010;42(11):996–999. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365(7):620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 73.Onoufriadis A, Simpson MA, Pink AE, Di Meglio P, Smith CH, Pullabhatla V, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89(3):432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fuchs-Telem D, Sarig O, van Steensel MA, Isakov O, Israeli S, Nousbeck J, et al. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. 2012;91(1):163–170. doi: 10.1016/j.ajhg.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jordan CT, Cao L, Roberson ED, Duan S, Helms CA, Nair RP, et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am J Hum Genet. 2012;90(5):796–808. doi: 10.1016/j.ajhg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90(5):784–795. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2017;376(16):1551–1560. doi: 10.1056/NEJMoa1607017. [DOI] [PubMed] [Google Scholar]
- 78.Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A Phase 2 Trial of Guselkumab versus Adalimumab for Plaque Psoriasis. N Engl J Med. 2015;373(2):136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- 79.Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222–226. doi: 10.1038/nature14175. [DOI] [PubMed] [Google Scholar]
- 80.Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75(3):329–338. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- 81.Markham A. Ixekizumab: First Global Approval. Drugs. 2016;76(8):901–905. doi: 10.1007/s40265-016-0579-y. [DOI] [PubMed] [Google Scholar]
- 82.Greig SL. Brodalumab: First Global Approval. Drugs. 2016;76(14):1403–1412. doi: 10.1007/s40265-016-0634-8. [DOI] [PubMed] [Google Scholar]
- 83.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 84.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 85.Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 2016;44(3):659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14(4):421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Songhet P, Barthel M, Rohn TA, Van Maele L, Cayet D, Sirard JC, et al. IL-17A/F-signaling does not contribute to the initial phase of mucosal inflammation triggered by S. Typhimurium. PLoS One. 2010;5(11):e13804. doi: 10.1371/journal.pone.0013804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayuzumi H, Inagaki-Ohara K, Uyttenhove C, Okamoto Y, Matsuzaki G. Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology. 2010;131(3):377–385. doi: 10.1111/j.1365-2567.2010.03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12(5):382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 90.Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377(1):12–16. doi: 10.1016/j.bbrc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 91.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205(5):1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110(1):55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 93.Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43(4):727–738. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song X, Dai D, He X, Zhu S, Yao Y, Gao H, et al. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 2015;43(3):488–501. doi: 10.1016/j.immuni.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 95.O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M, et al. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 2015;43(4):739–750. doi: 10.1016/j.immuni.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 97.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Targan SR, Feagan B, Vermeire S, Panaccione R, Melmed GY, Landers C, et al. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn’s Disease. Am J Gastroenterol. 2016;111(11):1599–1607. doi: 10.1038/ajg.2016.298. [DOI] [PubMed] [Google Scholar]
- 99.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rovedatti L, Kudo T, Biancheri P, Sarra M, Knowles CH, Rampton DS, et al. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58(12):1629–1636. doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- 101.Sugihara T, Kobori A, Imaeda H, Tsujikawa T, Amagase K, Takeuchi K, et al. The increased mucosal mRNA expressions of complement C3 and interleukin-17 in inflammatory bowel disease. Clin Exp Immunol. 2010;160(3):386–393. doi: 10.1111/j.1365-2249.2010.04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, Ricks DM, et al. IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense against K. pneumoniae. Cell Host Microbe. 2016;20(5):596–605. doi: 10.1016/j.chom.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murakami T, Hatano S, Yamada H, Iwakura Y, Yoshikai Y. Two Types of Interleukin 17A-Producing gammadelta T Cells in Protection Against Pulmonary Infection With Klebsiella pneumoniae. J Infect Dis. 2016;214(11):1752–1761. doi: 10.1093/infdis/jiw443. [DOI] [PubMed] [Google Scholar]
- 107.Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG. Innate Lymphocyte/Ly6C(hi) Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance. Cell. 2016;165(3):679–689. doi: 10.1016/j.cell.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186(3):1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183(9):5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 111.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol. 2009;183(2):1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203(12):2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17(3):375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 114.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7(301):301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 116.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190(10):1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 118.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134(5):1175–1186. e1177. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 120.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6(5):e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, et al. Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Sci Transl Med. 2012;4(117):117ra119. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen N, Wang J, Zhao M, Pei F, He B. Anti-interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke-exposed mice. Inhal Toxicol. 2011;23(4):212–218. doi: 10.3109/08958378.2011.559603. [DOI] [PubMed] [Google Scholar]
- 123.Kurimoto E, Miyahara N, Kanehiro A, Waseda K, Taniguchi A, Ikeda G, et al. IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir Res. 2013;14:5. doi: 10.1186/1465-9921-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yanagisawa H, Hashimoto M, Minagawa S, Takasaka N, Ma R, Moermans C, et al. Role of IL-17A in murine models of COPD airway disease. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L122–L130. doi: 10.1152/ajplung.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vargas-Rojas MI, Ramirez-Venegas A, Limon-Camacho L, Ochoa L, Hernandez-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(11):1648–1654. doi: 10.1016/j.rmed.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 126.Chang Y, Nadigel J, Boulais N, Bourbeau J, Maltais F, Eidelman DH, et al. CD8 positive T cells express IL-17 in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:43. doi: 10.1186/1465-9921-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chu S, Zhong X, Zhang J, Lao Q, He Z, Bai J. The expression of Foxp3 and ROR gamma t in lung tissues from normal smokers and chronic obstructive pulmonary disease patients. Int Immunopharmacol. 2011;11(11):1780–1788. doi: 10.1016/j.intimp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 128.Eich A, Urban V, Jutel M, Vlcek J, Shim JJ, Trofimov VI, et al. A Randomized, Placebo-Controlled Phase 2 Trial of CNTO 6785 in Chronic Obstructive Pulmonary Disease. COPD. 2017;14(5):476–483. doi: 10.1080/15412555.2017.1335697. [DOI] [PubMed] [Google Scholar]
- 129.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3(3):312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andrew DW, Cochrane M, Schripsema JH, Ramsey KH, Dando SJ, O’Meara CP, et al. The duration of Chlamydia muridarum genital tract infection and associated chronic pathological changes are reduced in IL-17 knockout mice but protection is not increased further by immunization. PLoS One. 2013;8(9):e76664. doi: 10.1371/journal.pone.0076664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scurlock AM, Frazer LC, Andrews CW, Jr, O’Connell CM, Foote IP, Bailey SL, et al. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun. 2011;79(3):1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, et al. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One. 2011;6(7):e22770. doi: 10.1371/journal.pone.0022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324(5932):1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yano J, Kolls JK, Happel KI, Wormley F, Wozniak KL, Fidel PL., Jr The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS One. 2012;7(9):e46311. doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2016 doi: 10.1111/bjd.15015. [DOI] [PubMed] [Google Scholar]
- 136.Papp KA, Bachelez H, Blauvelt A, Winthrop KL, Romiti R, Ohtsuki M, et al. Infections from 7 Clinical Trials of Ixekizumab, an Anti-Interleukin-17A Monoclonal Antibody, in Patients with Moderate-to-Severe Psoriasis. Br J Dermatol. 2017 doi: 10.1111/bjd.15723. [DOI] [PubMed] [Google Scholar]