Abstract
Background
Nociceptin is a key regulator linking environmental stress and alcohol drinking. In a genome-wide methylation analysis we recently identified an association of a methylated region in the OPRL1 gene with alcohol-use disorders.
Methods
Here we investigate the biological basis of this observation by analysing psychosocial stressors, methylation of the OPRL1 gene, brain response during reward anticipation and alcohol drinking, in 660 14-year old adolescents of the IMAGEN study. We validate our findings in marchigian sardinian (msP) alcohol-preferring rats that are genetically selected for increased alcohol drinking and stress sensitivity.
Results
We found that low methylation levels in intron 1 of OPRL1 are associated with higher psychosocial stress and higher frequency of binge drinking, an effect mediated by OPRL1 methylation. In individuals with low methylation of OPRL1 frequency of binge drinking is associated with stronger BOLD-response in the ventral striatum during reward anticipation. In msP rats we found that stress results in increased alcohol intake and decreased methylation of OPRL1 in the nucleus accumbens.
Conclusions
Our findings describe an epigenetic mechanism that helps to explain how psychosocial stress influences risky alcohol consumption and reward processing, thus contributing to the elucidation of biological mechanisms underlying risk for substance abuse.
Keywords: OPRL1 methylation, stressful life events, adolescence, binge drinking, nucleus accumbens
Introduction
Adolescence is a critical period for brain development and maturation. It is characterised by changes in synaptic density and connectivity, fine-tuning of neurotransmitter systems, and integration between brain structures that together lead to increased efficiency of neural function (Keshavan et al., 2014). However, as a consequence of these dynamic changes the adolescent brain is particularly vulnerable to the exposure to traumatic life events (e.g. physical and psychological abuse) but also to more common life events (e.g. parental divorce, difficulties with authority and body changes) that are perceived as stressful by adolescents (Loth et al., 2014). Early exposure to such psychosocial stressors has been consistently associated with hazardous drinking behaviours in adolescence (Arpawong et al., 2015) that in turn predict increased risk for alcohol-use disorders in adulthood (Palmer et al., 2009, Nees et al., 2012).
Nociceptin/Orphanin FQ (N/OFQ) is a 17 amino acid peptide, that binds to its cognate G protein-coupled receptor (GPCR) Opioid Receptor Like-1 (OPRL1) also known as NOP (Meunier et al., 1995, Reinscheid et al., 1995). Several animal studies, mostly conducted on marchigian sardinian (msP) rats, an animal line genetically selected for excessive alcohol drinking and preference, have shown that activation of NOP by N/OFQ or by selective synthetic agonists reduces alcohol drinking and blunt withdrawal symptoms and according to the alcohol intoxication history and withdrawal time course, it results in anxiolytic or anxiogenic effects (Witkin et al., 2014), indicating a complex effect of N/OFQ on the regulation of anxiety and alcohol drinking.
In a recent genome-wide methylation analysis of monozygotic discordant twins we have identified a genome-wide significant association of alcohol-use disorders with a differentially methylated region in the gene body of OPRL1 (Ruggeri et al., 2015). Others reported an association of the promoter OPRL1 methylation with child adversity both in alcohol-use disorder cases and healthy controls (Zhang et al., 2013). These data suggest that the N/OFQ system could be mediating alcohol problems induced by early exposure to psychosocial stress.
We hypothesise that changes in the methylation profile of OPRL1 mediates the effect of early stress exposure on hazardous drinking behaviour in adolescents by modulating the processing of rewarding stimuli. To test our hypothesis we assessed the association of the previously identified differentially methylated region (DMR) in intron 1 (Ruggeri et al., 2015) with early stressful life events and binge drinking. We also measured its interaction with ventral striatal response during reward anticipation in a functional neuroimaging epigenetics dataset of 660 adolescents from the IMAGEN study (Schumann et al., 2010). To obtain mechanistic information and to evaluate the translational value of our results, we then assessed brain Oprl1 methylation changes induced by early stress in alcohol preferring msP rats.
Method
IMAGEN study participants
We selected a randomly picked subset of 660 14-year old adolescents (50.8 % female; Table 1) of the IMAGEN study, a European multi-centre imaging genetic/epigenetic study. Participants were tested in eight IMAGEN assessment centres. The study was approved by local ethics research committees at each site. Written informed consent was obtained from all participants as well as from their legal guardians. A detailed description of recruitment and assessment procedures as well as inclusion/exclusion criteria has previously been published (Schumann et al., 2010). Behavioural information, blood and an fMRI scan were collected during a single visit to the recruitment centre when participants were 14 years old.
Table 1.
Demographics of the IMAGEN participants
| Sample Size | % | |
|---|---|---|
| Males | 325 | 49.2 |
| Mean | SE | |
|
| ||
| ESPAD-Lifetime frequency of binge drinking (n=606) | 0.58 | 0.05 |
| LEQ-Lifetime stressful events (n=626) | 6.63 | 0.11 |
Abbreviations: SE, standard error; ESPAD, European School Survey Project on Alcohol and Other Drugs; LEQ, life event questionnaire. ESPAD scores are on a five-level ordinal scale. Stressful life events are the sum of 20 events.
Behavioural characterization
Behavioural interviews were presented by Psytools software. Lifetime stressful life events were measured with an adapted version of the Life Events Questionnaire (Newcomb et al., 1981) and were defined based on the quality ratings of adolescents who had experienced the event (Supplementary Table S1.) as previously described (Loth et al., 2014). Data about lifetime frequency of binge drinking at the age of 14 were obtained from a 5 Likert scale question of the European School Survey Project on Alcohol and Other Drugs (ESPAD) (Hibell et al., 2004). Data quality was controlled by context checks administered at the start of each task.
OPRL1 methylation analysis
DNA was extracted from whole blood and was bisulphite treated using the EZ 96 DNA methylation kit and DNA methylation of OPRL1 was assessed using bisulfite-PCR and the Sequenom EpiTYPER system (Supplementary Table S2). The signal of two CpG sites, located in the first intron of the OPRL1 gene and in the shelf of a CpG island (CGI) (chr20:62718473-62719016, hg19) previously found associated with alcohol-use disorders (Ruggeri et al., 2015), was studied. The Sequenom technology provided a mean signal for the two CpG sites.
OPRL1 genotype analysis
Genotype information was collected for 582,982 markers using DNA extracted from whole blood and the Illumina HumanHap610 Genotyping BeadChip. Genotype data of 11 SNPs contained in OPRL1 gene (rs1335579, rs6062342, rs6090041, rs7271530, rs2229205, rs13036542, rs4809401, rs35368587, rs910883, rs3003155, rs13039817) was extracted, and tested for association with OPRL1 methylation profile. Data for rs6010718, previously associated with alcohol-use disorders (Huang et al., 2008), was imputed as previously described and tested for association with OPRL1 methylation.
OPRL1 gene expression analysis
Total RNA was extracted from whole blood cells collected at the age of 14. Gene expression profiling was performed using Illumina HumanHT-12 v4 Expression BeadChips as previously described (Ruggeri et al., 2015).
Monetary incentive delay task
The participants performed a modified version of the Monetary Incentive Delay task to study neural responses to reward anticipation and reward outcome where candies were used as reward instead of money as previously described (Nees et al., 2012).
Functional magnetic resonance imaging data acquisition and analysis
Structural and functional magnetic resonance imaging (fMRI) data were acquired at eight IMAGEN assessment sites with 3T-MRI scanners of different manufacturers. Full details of MRI acquisition protocols and quality checks have been described previously, including standardization across MRI scanners (Schumann et al., 2010). We extracted regions of interest (ROIs) using the Marsbar toolbox (http://marsbar.sourceforge.net). We used the left and right ventral striatum as ROIs and extracted mean blood-oxygen-level dependent (BOLD) responses in 9-mm spheres centred at x, y, z = ±15, −9, 9 in Montreal Neurological Institute space. These coordinates were based on previous findings (Yacubian et al., 2006).
Animals
Genetically selected msP male rats weighing 250–300 g (7–8 weeks old) at the beginning of the experiments were used (Ciccocioppo et al., 2006) and were kept in controlled conditions. All the procedures were conducted in adherence with the European Community Council Directive for Care and Use of Laboratory Animals.
Early stress exposure
A total of 32 msP rats were subdivided in 2 groups, a control group that remained in the home cage throughout the procedure and a treated group receiving one of the following stressful procedure, every 7 days for 3 weeks, acute restrain stress (Ciccocioppo et al., 2014), forced swimming stress (Guan et al., 2014) and sleep deprivation stress (Youngblood et al., 1997). One week after the last exposure to stress, the two groups were divided in 2 subgroups (n=8), one stressed and one control group were sacrificed, brains were dissected and the nucleus accumbens was frozen in dry ice and stored at −80°C. The other two subgroups were tested for voluntary EtOH intake for a total of 7 days and one week after the last exposure to alcohol.
Voluntary EtOH intake
To test voluntary EtOH intake, we used the 2-bottle choice test in msP rats as previously described (Ciccocioppo et al., 2006). Briefly, animals were exposed to an intermittent 10% (v/v) ethanol solution for seven consecutive days. Alcohol was offered 24 hours a day. Alcohol, water, and food consumption were monitored 24 hours from the moment that EtOH was made available. Food intake was measured by weighing the food containers and taking into account spillage.
Oprl1 methylation and gene expression analysis
DNA and RNA were simultaneously extracted from the left and right nucleus accumbens. DNA was bisulphite-treated using the EZ DNA methylation kit and DNA methylation of Oprl1 was assessed using bisulphite-PCR and the Sequenom EpiTYPER system (Supplementary Table S2). Gene expression of Oprl1 and the housekeeping gadph were measured via quantitative polymerase-chain-reaction (Supplementary Table S2). The ABI Prism® SDS 2.1 software was used to analyse the specificity (dissociation curve) and relative quantification of the Oprl1 amplicon (ΔCt).
Statistical analyses
The general linear model was used to determine associations among human OPRL1 methylation and OPRL1 genotype and gene expression, negative life events, drinking behaviour and BOLD responses correcting for gender and recruitment site (handiness was corrected for BOLD responses).
A mediation analysis was carried out to test if the OPRL1 methylation mediated the association of lifetime stressful events and frequency of binge drinking using PROCESS (Hayes, 2013), a SPSS macro that uses a path analytical framework for estimating direct and indirect effects based on OLS regression models. This approach involves bootstrapping the sampling distribution of the indirect effect and obtaining its confidence interval. The analysis is based on 5,000 bootstraps with stressful events as a predictor variable, OPRL1 methylation as a mediator, and binge drinking as the dependent variable.
Methyl-eQTL analysis was performed using the R package (R version 3.1.2) Matrix expression QTL (Shabalin, 2012). The analysis was performed on 34,833 gene expression probes and OPRL1 methylation data generated on the Sequenom platform. We run a linear regression model adjusted for gender and recruitment centre.
The database for annotation, visualisation and integrated discovery (DAVID) (Huang da et al., 2009) was used to determine enrichment of biological processes and disease classes in the gene list generated from the methyl-eQTL analysis with p<0.05. Enrichment for specific biological functions and disease classes was determined using a modified Fisher's test at a significance level of p<0.05.
The effect of the interaction of OPRL1 methylation and ventral striatum BOLD response on frequency of binge drinking was tested with a general linear model.
A principal component analysis was applied on the methylation data collected across the rat Oprl1 gene, where the missing data were replaced by means and a varimax rotation was also applied to increase interpretability of outcomes. Components with eigenvalue > 1 were selected for further analyses.
All analysis were carried out using the software IBM SPSS statistics (version 22) unless otherwise is stated. P-values corrected for multiple testing are reported as pcorrected.
Results
OPRL1 methylation mediates the association of early exposure to psychosocial stress and frequency of binge drinking at the age of 14
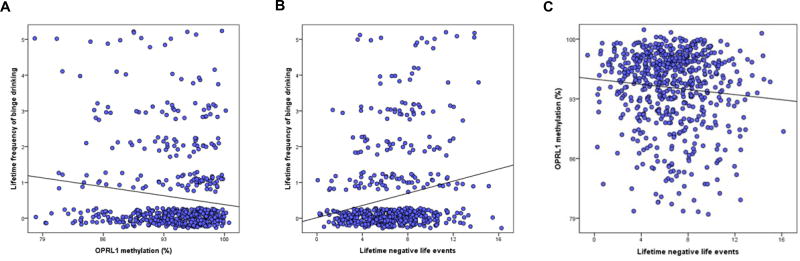
We found a negative association of OPRL1 methylation and lifetime frequency of binge drinking in participants aged 14 years (t=−3.56, p=2.2×10−04, η2p=0.021) (Figure 1A, Table 1). We then investigated whether lifetime stressful events were associated with lifetime frequency of binge drinking, and with OPRL1 methylation. We found a positive association of stressful life events and lifetime binge drinking at the age of 14 (t=4.56, p=8.7×10−06, η2p=0.036) (Figure 1B), and a negative association of stressful life events with OPRL1 methylation (t=−2.50, p=0.013, η2p=0.010) (Figure 1C). A subsequent mediation analysis revealed that the indirect effect of psychosocial stressors on binge drinking through OPRL1 methylation was significant (z=2.17, p=0.030) and that the 95% confidence interval did not include 0 (95% CI [0.002, 0.019]).
Figure1. Association of OPRL1 methylation with lifetime frequency of binge drinking (N= 609) and stressful life events (N= 626) in adolescents.
Negative association of OPRL1 methylation and lifetime frequency of binge drinking (p=2.2×10−04) (panel A). Positive association of stressful life events and lifetime frequency of binge drinking (p=8.7×10−06) in 14-year-old adolescents (panel B). Negative association of lifetime frequency of binge drinking and OPRL1 methylation (p=0.013) (panel C).
To investigate if OPRL1 methylation is dependent on OPRL1 genotype, we used a general linear model to test twelve SNPs contained in the OPRL1 gene, including rs6010718, which was previously associated with alcohol-use disorders (Huang et al., 2008). We found no significant association between the OPRL1 methylation profile and any of the SNPs taken in consideration neither we found any significant association of SNPs and lifetime binge drinking (Supplementary Table S3).
OPRL1 methylation and gene expression
To explore the relationship between OPRL1 methylation and gene expression, we performed a methyl-eQTL analysis and we found a total of 1856 probes mapping 1245 genes associated with OPRL1 methylation (p<0.05; Supplementary Table S4); no OPRL1 cis-gene expression was observed. We investigated whether common pathways, biological functions or pathologies were over-represented in the list of 1245 genes associated to OPRL1 methylation with p<0.05. The analysis using DAVID, a database and tool for functional annotation (Huang da et al., 2009), revealed enrichment for phenotypically relevant gene networks, including the ‘disorder categories’ “neurological” and “psychological” disorders (p=0.003, n=48; p=0.004, n=46, respectively) and genes involved in dopamine, acetylcholine and glutamate signalling (Supplemtary Tables S5 and S6).
Ventral striatal activation during reward anticipation is associated with frequency of binge drinking and interacts with OPRL1 methylation
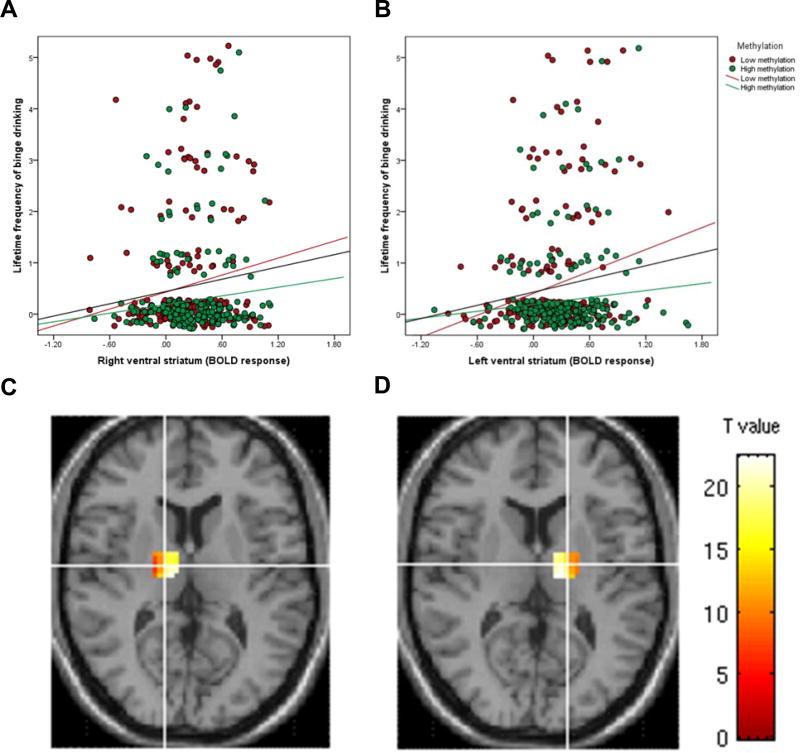
The reinforcing properties of alcohol are dependent on activation of the ventral striatum, a brain area, which is central to reward processing, including reward anticipation. We measured reward anticipation in the Monetary-Incentive-Delay task in 393 14-year old IMAGEN participants where neuroimaging, methylation and drinking data were available, and found a positive association of frequency of binge drinking with BOLD response in the left ventral striatum (t=2.87, p=0.004, pcorrected=0.008, η2p=0.022) and the right ventral striatum (t=2.33, p=0.020, pcorrected=0.040, η2p=0.015). We found no significant association of OPRL1 methylation and BOLD response in the left ventral striatum (t=2.02, p=0.044, pcorrected=0.088, η2p=0.010) and the right ventral striatum (t=1.86, p=0.064, pcorrected=0.128, η2p=0.009) after correction for multiple comparison. Since both OPRL1 methylation and ventral striatal response during reward anticipation are associated with binge drinking we investigated if there was an interaction of these variables on binge drinking. We found a significant interaction between OPRL1 methylation and the left ventral striatum activation (t=−2.69, p= 0.007, pcorrected=0.014, η2p=0.020; Figure 2B and 2D) and a trend in the right ventral striatum (t=−2.18, p=0.030, pcorrected=0.060, η2p=0.013; Figure 2A and 2C). Our data suggest that the association of ventral striatum activation and frequency of binge drinking is observed only in individuals with low OPRL1 methylation levels.
Figure2. Association of activation of the ventral striatal activation during Monetary Incentive Delay Task and lifetime binge drinking in adolescents (N=393) and interaction with OPRL1 methylation.
Significant interaction between OPRL1 methylation and the left VS activation (p= 0.007, pcorrected=0.014) while the interaction was only a trend in the right VS (p=0.030, pcorrected=0.060). Methylation values in panel A and B are separated into low and high methylation using a median slip. Coronal section shows methylation differences in right and left ventral activation during reward anticipation (xyz: ±9, 11, −2) (panel C and D).
Early stress and differential methylation are associated with oprl1 gene expression in the nucleus accumbens of msP rats
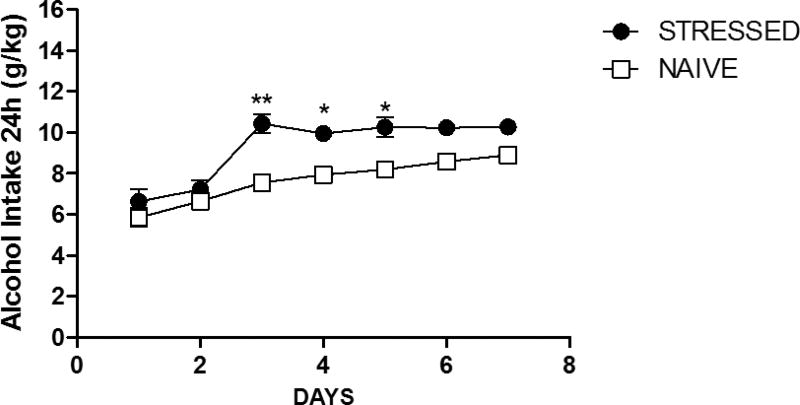
We next investigated if the relation between psychosocial stress, alcohol abuse and OPRL1 methylation in peripheral blood can be validated in the ventral striatum, a brain structures that is central to reward processing and anticipation. It is not possible to obtain post mortem brain materials of the ventral striatum from a sufficient number of human adolescents to carry out association analyses; we therefore used an established animal model, namely msP rats, for our experiments. We exposed msP rats to a stress protocol and, similar to humans, found a significant overall effect of stress exposure on voluntary alcohol intake (F (1, 13) = 45.05, p<0.0001) (Figure 3). Water and food consumption were not significantly affected by stress exposure (Supplementary Tables S7 and S8). In stressed vs. non-stressed animals we then compared Oprl1 methylation and gene expression levels in the nucleus accumbens, the core region of the ventral striatum (Galtress and Kirkpatrick, 2010). We assessed methylation levels of the entire Oprl1 gene locus and detected 39 methylation sites. We then carried out a principal component analysis of these sites identifying 11 principal components (eigenvalue>1) that explained 82% of the variance (Supplementary Table S9). An exploratory analysis in stressed animals, revealed a significant association of Oprl1 gene expression and one methylation component explaining 8% of the variance (r=0.799, p=0.001, n=13); no association was observed in non-stressed animals (r=0.054, p=0.861, n=13). A subsequent moderation analysis showed an interaction effect of stress and methylation on gene expression of Oprl1 in the nucleus accumbens (z=2.33, p=0.020; two-tailed test; Supplementary Figure S1).
Figure3. Association of early stress and intermittent alcohol intake in the msP rats.
One-way ANOVA revealed a significant overall effect of the stress exposure on voluntary alcohol intake in msP rats (p<0.0001). The effect appeared from the third day of alcohol exposure and remained significant on the fourth and fifth day of alcohol exposure as shown by Newman–Keuls tests.* p<0.05.
Discussion
In a population-based sample of 14 year old adolescents of the IMAGEN cohort (Schumann et al., 2010), we showed that decreased methylation in the first intron of OPRL1 gene is associated with increased early psychosocial stress exposure and higher frequency of binge drinking, a risk factor for future alcohol-use disorders (Nees et al., 2012). Among individuals with lower methylation of OPRL1 we found association of high frequency of binge drinking with increased BOLD response in the ventral striatum during reward anticipation, reflecting enhanced reward sensitivity. The OPRL1 gene encodes for the N/OFQ receptor (NOP), a G protein-coupled receptor that together with its ligand N/OFQ has been widely studied for its role in alcohol-related behaviour both in humans and in animal models (Witkin et al., 2014). Providing corroborative evidence in msP rats, an animal model of innate vulnerability to excessive drinking and high sensitivity to stress (Witkin et al., 2014), we show that stress is associated with increased alcohol intake of these animals and interacts with a component of differentially methylated regions resulting in association with altered gene expression of the Oprl1 in the nucleus accumbens.
The ventral striatum and nucleus accumbens are subcortical areas involved in reward processing that are heavily innervated by mesolimbic dopamine neurons (Tritsch and Sabatini, 2012). Increased BOLD response in this region may reflect an increase dopaminergic activity leading to enhanced sensitivity to reward. However, striatal activity is also influenced by other neurocircuitries, including glutamatergic efferents from the basolateral amygdala and the frontal cortex (Fudge et al., 2002, Haber et al., 2006). The NOP receptor is functionally coupled to inhibition of adenylate cyclase, activation of MAP kinase, activation of K+ conductance, and inhibition of Ca+2 conductance and induces inhibition of neurotransmitter release, including of dopamine, glutamate, gamma-amino butyric acid (GABA), acetylcholine, and others (Ciccocioppo et al., 1999). NOP receptor activity thus results in a comprehensive direct and indirect regulatory system of reward processing (Neal et al., 1999). Our observation of a trans-regulation of genes involved in dopamine and glutamate signalling by OPRL1 methylation in human blood is in keeping with these findings.
Previous studies, including our own findings in adult alcohol abusing discordant twins reported a positive association between OPRL1 hypermethylation and alcohol-use disorders (Ruggeri et al., 2015), as well as a positive association with child adversity in both alcohol-use disorders cases and healthy controls (Zhang et al., 2013). In addition to the severity of the environmental stressor, we looked at stressful events while in a previous study they investigated severe physical and emotional abuse (Ruggeri et al., 2015, Zhang et al., 2013), the most striking difference of our study is that we investigated adolescents at age 14 years where binge drinking is less intense and frequent as in adult alcohol abusers. In fact, it is a reflection of personality traits involving anxiety sensitivity, sensation seeking and/or impulsiveness (Nees et al., 2012, Woicik et al., 2009). While these traits are associated with psychosocial stress and alcohol drinking (Whelan et al., 2014), our young participants have not yet developed alcohol-use disorders neither have they been exposed to protracted consumption of alcohol that is known to alter the methylation profile (Weng et al., 2015). Possibly OPRL1 hypomethylation may occur as a result of adverse life events and may contribute to enhanced reward sensitivity and propensity to binge drinking. This epigenetic change may subsequently be reversed following protracted exposure to alcohol, and possibly contribute to decreased striatal activation often observed in patients suffering from alcohol-use disorders (Chen et al., 2011).
Epigenetic regulation of gene expression can be species-specific (Zeng et al., 2012) and is contingent on several factors, including epigenetic imprinting (Adalsteinsson and Ferguson-Smith, 2014), biological age (Talens et al., 2012) as well as tissue-specificity (Grunau et al., 2000). It is a limitation of our study that in humans we were only able to measure methylation and gene expression in peripheral blood, as there are not sufficient adolescent post mortem brain data from human ventral striatum available to analyse this brain region. A validation in the brain of the stress-induced OPRL1 hypomethylation found in the human blood was provided by our findings in the nucleus accumbens of stressed msP rats, which showed not only a similar association of stress and Oprl1 methylation but also an association between Oprl1 methylation and its gene expression.
Methylation in the promoter area is often negatively correlated with gene expression, however, correlation of methylation in the gene body and cis-gene expression does not show a specific directionality (Lee et al., 2015). Our findings show that stress-induced hypomethylation of the Oprl1 gene body in msP rats is associated with a decrease in Oprl1 gene expression. It is possible that a similar correlation between OPRL1 methylation and gene expression exists in human brain, consistent with the decreased level of OPRL1 mRNA found in the brain of alcohol-use disorders patients (Kuzmin et al., 2009).
A previous study reported an association of the OPRL1 genetic variant rs6010718 in intron 1 with alcohol-use disorders (Huang et al., 2008). There is evidence that the methylation of certain CpG sites is correlated with single nucleotide polymorphisms (SNPs) (Shoemaker et al., 2010) suggesting that an interplay of genetic and epigenetics mechanisms could underlie complex disorder. The absence of association in our data suggests independence of genetic and epigenetic mechanisms related to alcohol drinking in this gene locus.
Together our findings describe an epigenetic mechanism that helps to explain how psychosocial stress influences risky alcohol consumption and reward processing, thus contributing to the elucidation of biological mechanisms underlying risk for substance abuse.
Supplementary Material
Table S1. Frequency of stressful life events.
Table S2. Human and rat OPRL1 methylation and rat oprl1 gene expression assessment.
Table S3. Association of OPRL1 genetic variants and OPRL1 methylation or lifetime binge drinking.
Table S4. List of OPRL1 methyl-eQTL in the blood of 14-year-old adolescent.
Table S5. Enriched disease categories (p<0.05) for OPRL1 methyl-eQTLs in blood of 14-year-old adolescents.
Table S6. Enriched GO biological processes categories (p<0.05) for OPRL1 methyl-eQTLs in blood of 14-year-old adolescents.
Table S7. PCA loadings of 39 CpG sites of the rat oprl1 gene (N=16).
Table S8. Water consumption (in g/kg) of stressed versus naïve rats during intermittent 10% alcohol exposure.
Table S9. Food consumption (in g/kg) of stressed versus naïve rats during intermittent 10% alcohol exposure.
Figure S1. The Scatter plot of rat Oprl1 methylation factor and Oprl1 gene expression conditioned on the status of stress in the nucleus accumbens.
Acknowledgments
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (LSHM-CT- 2007-037286), the FP7 projects IMAGEMEND (602450), AGGRESSOTYPE (602805) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant “Developmental pathways into adolescent substance abuse” (93558) and Consortium on Vulnerability to Externalizing Disorders and Addictions [c-VEDA] (MR/N000390/1), the Swedish funding agencies VR, FORTE and FORMAS, the Medical Research Council and the Wellcome Trust (Behavioural and Clinical Neuroscience Institute, University of Cambridge), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1), the National Institutes of Health, U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence. TB has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. JG has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen-Cilag, and Bristol-Myers Squibb; he has received speaking fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb. Dr Barker has received honoraria from General Electric for teaching on scanner programming courses.
Footnotes
Conflict of interest statement: See acknowledgements for disclosures.
The remaining authors have declared that they have no competing or potential conflicts of interest.
References
- Adalsteinsson BT, Ferguson-smith AC. Epigenetic control of the genome-lessons from genomic imprinting. Genes (Basel) 2014;5:635–655. doi: 10.3390/genes5030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpawong TE, Sussman S, Milam JE, Unger JB, Land H, Sun P, Rohrbach LA. Post-traumatic growth, stressful life events, and relationships with substance use behaviors among alternative high school students: a prospective study. Psychol Health. 2015;30:475–494. doi: 10.1080/08870446.2014.979171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cuzon carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011;35:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, Roberto M, Weiss F, Teshima K. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology. 2014;39:2601–2610. doi: 10.1038/npp.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Galtress T, Kirkpatrick K. The role of the nucleus accumbens core in impulsive choice, timing, and reward processing. Behav Neurosci. 2010;124:26–43. doi: 10.1037/a0018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau C, Hindermann W, Rosenthal A. Large-scale methylation analysis of human genomic DNA reveals tissue-specific differences between the methylation profiles of genes and pseudogenes. Hum Mol Genet. 2000;9:2651–2663. doi: 10.1093/hmg/9.18.2651. [DOI] [PubMed] [Google Scholar]
- Guan XT, Shao F, Xie X, Chen L, Wang W. Effects of aspirin on immobile behavior and endocrine and immune changes in the forced swimming test: comparison to fluoxetine and imipramine. Pharmacol Biochem Behav. 2014;124:361–366. doi: 10.1016/j.pbb.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis A Regression-Based Approach. Guilford Press; 2013. [Google Scholar]
- Hibell B CENTRALFÖRBUNDET FÖR ALKOHOL- OCH NARKOTIKAUPPLYSNING & EUROPEAN SCHOOL SURVEY PROJECT ON ALCOHOL AND OTHER DRUGS. The ESPAD report 2003: alcohol and other drug use among students in 35 European countries. Stockholm: Swedish Council for Information on Alcohol and Other Drugs (Centralförb. för alkohol- och narkotikaupplysning)(CAN); 2004. [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang J, Young B, Pletcher MT, Heilig M, Wahlestedt C. Association between the nociceptin receptor gene (OPRL1) single nucleotide polymorphisms and alcohol dependence. Addict Biol. 2008;13:88–94. doi: 10.1111/j.1369-1600.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Bazov I, Sheedy D, Garrick T, Harper C, Bakalkin G. Expression of pronociceptin and its receptor is downregulated in the brain of human alcoholics. Brain Res. 2009;1305(Suppl):S80–85. doi: 10.1016/j.brainres.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Choi WY, Lee J, Kim YJ. The regulatory mechanisms of intragenic DNA methylation. Epigenomics. 2015;7:527–531. doi: 10.2217/epi.15.38. [DOI] [PubMed] [Google Scholar]
- Loth E, Poline JB, Thyreau B, Jia T, Tao C, Lourdusamy A, Stacey D, Cattrell A, Desrivieres S, Ruggeri B, Fritsch V, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, Conrod PJ, Fauth-Buehler M, Flor H, Gallinat J, Garavan H, Heinz A, Bruehl R, Lawrence C, Mann K, Martinot JL, Nees F, Paus T, Pausova Z, Poustka L, Rietschel M, Smolka M, Struve M, Feng J, Schumann G CONSORTIUM, I. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol Psychiatry. 2014;76:367–376. doi: 10.1016/j.biopsych.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Neal CR, JR, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., JR Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999;412:563–605. [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-klein S, Steiner S, Poustka L, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, Garavan H, Heinz A, Gallinat J, Lathrop M, Mann K, Artiges E, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka MN, Spanagel R, Struve M, Loth E, Schumann G, Flor H CONSORTIUM, I. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb MD, Huba GJ, Bentler PM. A Multidimensional Assessment of Stressful Life Events among Adolescents - Derivation and Correlates. Journal of Health and Social Behavior. 1981;22:400–415. [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, JR, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Ruggeri B, Nymberg C, Vuoksimaa E, Lourdusamy A, Wong CP, Carvalho FM, Jia T, Cattrell A, Macare C, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Buchel C, Conrod PJ, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot JL, Nees F, Pausova Z, Paus T, Rietschel M, Robbins T, Smolka MN, Spanagel R, Bakalkin G, Mill J, Sommer WH, Rose RJ, Yan J, Aliev F, Dick D, Kaprio J, Desrivieres S, Schumann G CONSORTIUM, I. Association of Protein Phosphatase PPM1G With Alcohol Use Disorder and Brain Activity During Behavioral Control in a Genome-Wide Methylation Analysis. Am J Psychiatry. 2015;172:543–552. doi: 10.1176/appi.ajp.2014.14030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Mallik C, Mann K, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Reed L, Smolka M, Spanagel R, Speiser C, Stephens DN, Strohle A, Struve M CONSORTIUM, I. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–1358. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R, Deng J, Wang W, Zhang K. Allele-specific methylation is prevalent and is contributed by CpG-SNPs in the human genome. Genome Res. 2010;20:883–889. doi: 10.1101/gr.104695.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talens RP, Christensen K, Putter H, Willemsen G, Christiansen L, Kremer D, Suchiman HE, Slagboom PE, Boomsma DI, Heijmans BT. Epigenetic variation during the adult lifespan: cross-sectional and longitudinal data on monozygotic twin pairs. Aging Cell. 2012;11:694–703. doi: 10.1111/j.1474-9726.2012.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JT, Wu LS, Lee CS, Hsu PW, Cheng AT. Integrative epigenetic profiling analysis identifies DNA methylation changes associated with chronic alcohol consumption. Comput Biol Med. 2015;64:299–306. doi: 10.1016/j.compbiomed.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, Conrod PJ, Flor H, Fauth-Buhler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot JL, Nees F, Ortiz N, Paillere-martinot ML, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Strohle A, Schumann G, Garavan H CONSORTIUM, I. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther. 2014;141:283–299. doi: 10.1016/j.pharmthera.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Stewart SH, Pihl RO, Conrod PJ. The Substance Use Risk Profile Scale: a scale measuring traits linked to reinforcement-specific substance use profiles. Addict Behav. 2009;34:1042–1055. doi: 10.1016/j.addbeh.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RB. Sleep deprivation by the "flower pot" technique and spatial reference memory. Physiol Behav. 1997;61:249–256. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]
- Zeng J, Konopka G, Hunt BG, Preuss TM, Geschwind D, Yi SV. Divergent whole-genome methylation maps of human and chimpanzee brains reveal epigenetic basis of human regulatory evolution. Am J Hum Genet. 2012;91:455–465. doi: 10.1016/j.ajhg.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang F, Kranzler HR, Zhao H, Gelernter J. Profiling of childhood adversity-associated DNA methylation changes in alcoholic patients and healthy controls. PLoS One. 2013;8:e65648. doi: 10.1371/journal.pone.0065648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Frequency of stressful life events.
Table S2. Human and rat OPRL1 methylation and rat oprl1 gene expression assessment.
Table S3. Association of OPRL1 genetic variants and OPRL1 methylation or lifetime binge drinking.
Table S4. List of OPRL1 methyl-eQTL in the blood of 14-year-old adolescent.
Table S5. Enriched disease categories (p<0.05) for OPRL1 methyl-eQTLs in blood of 14-year-old adolescents.
Table S6. Enriched GO biological processes categories (p<0.05) for OPRL1 methyl-eQTLs in blood of 14-year-old adolescents.
Table S7. PCA loadings of 39 CpG sites of the rat oprl1 gene (N=16).
Table S8. Water consumption (in g/kg) of stressed versus naïve rats during intermittent 10% alcohol exposure.
Table S9. Food consumption (in g/kg) of stressed versus naïve rats during intermittent 10% alcohol exposure.
Figure S1. The Scatter plot of rat Oprl1 methylation factor and Oprl1 gene expression conditioned on the status of stress in the nucleus accumbens.