Abstract
(1) Objectives
To investigate whether urinary levels of macrophage migration inhibitory factor (MIF) are elevated in Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) patients with Hunner lesions and also whether urine MIF is elevated in other forms of inflammatory cystitis.
(2) Methods
Urine samples were assayed for MIF by ELISA. Urine samples from three female groups were examined: IC/BPS patients without (N=55) and with Hunner lesions (N=43); Non-IC/BPS patients (N=100; control group; no history of IC/BPS; cancer or recent bacterial cystitis). Urine samples from three male groups were examined: patients with bacterial cystitis (N=50), radiation cystitis (N=18) and non-cystitis patients (N = 119; control group; negative for bacterial cystitis).
(3) Results
Urine MIF (Mean MIF pg/ml ± SEM) was increased in female IC/BPS patients with Hunner lesions (2159 ± 435.3) compared to IC/BPS patients without Hunner lesions (460 ± 114.5) or non-IC/BPS patients (414 ± 47.6). Receiver-operating curve analyses showed that urine MIF levels discriminated between the two IC groups (AUC = 72%; CI: 61–82%). Male patients with bacterial and radiation cystitis had elevated urine MIF levels (2839 ± 757.1 and 4404 ± 1548.1; respectively) compared to non-cystitis patients (681 ± 75.2).
(4) Conclusions
Urine MIF is elevated in IC/BPS patients with Hunner lesions and also in patients with other bladder inflammatory and painful conditions. MIF also may serve as a noninvasive biomarker to select IC/BPS patients more accurately for endoscopic evaluation and possible anti-inflammatory treatment.
Introduction
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a debilitating condition with between 3 and 8 million women and approximately 2 million men in the US having symptoms 1,2. The condition has poorly defined etiology, variable histological findings and no consistently effective treatments. Different clinical definitions have been proposed, with a common theme of pain or discomfort perceived to arise from the bladder 3. These patients are commonly subdivided into those with gross inflammatory disease (Hunner lesions) and those without, the former often requiring more aggressive management.
Cytoscopy is necessary to identify Hunner lesions; however, this examination is not mandated in the early evaluation of afflicted patients. A urine biomarker that identifies IC/BPS patients in general and those with Hunner lesions in particular, would potentially streamline diagnosis and direct the most appropriate therapeutic options without the need for invasive procedures. Furthermore, such a marker might provide prognostic information and pose a target for future interventions.
Macrophage migration inhibitory factor (MIF) is a soluble factor produced by T-lymphocytes with the ability to stop migration of macrophages and was one of the first cytokines to be identified 4. Subsequently, MIF has been shown to be expressed in a variety of immune and non-immune cells. Ample experimental evidence shows that MIF is a regulator of immunity that appears to be active in generalized inflammatory events such as sepsis and autoimmune diseases such as rheumatoid arthritis 5.
MIF is constitutively expressed in human urothelial cells 6 and stored MIF pools in the urothelium are released into the bladder lumen after bladder insults, as demonstrated by various experimental rodent models of cystitis 7,8. Released MIF then binds to MIF receptors on the bladder surface to mediate bladder inflammation and bladder pain 9–11. Based on this experimental evidence, we hypothesized that urinary MIF levels would be increased in patients with inflammatory and painful conditions of the bladder. Our primary aim was to measure urine MIF levels in IC/BPS patients with and without Hunner lesions and compare them to controls (non-IC/BPS individuals). In addition, we investigated whether urine MIF may be a useful biomarker for IC/BPS patients with Hunner lesions. Our secondary aim in this study was to extend previous findings 12 by examining a larger cohort of adult patients with confirmed bacterial cystitis and comparing them to a non-Cystitis group. In addition, we added a cohort of radiation cystitis patients, as a positive control cohort for bladder inflammation.
Methods
Subject Populations
Urine samples were collected from the following groups of patients:
-
Female Non-IC/BPS (Control group): No history of IC/BPS, cancer or recent (3 month) bacterial cystitis.
Banked samples (N=92): Samples were obtained from participants in the Kentucky Women Health Registry and were collected as part of routine visits to healthcare providers and distributed by the Biospecimens Core of the Kentucky Center for Clinical and Translational Science, University of Kentucky. Procedures were approved by the Institutional Review Board at the University of Kentucky
Collected samples (N=8): Obtained (after IRB approval and informed consent) during routine visit to urologists’ clinics (Zucker School of Medicine at Hofstra-Northwell).
-
Women with IC/BPS without Hunner lesions (IC/BPS-NH): IC/BPS was diagnosed using the same criteria that are described in the American Urological Association Guideline for Diagnosis and Treatment of IC/BPS. All subjects had symptom duration > 1 year and were symptomatic at the time of urine collection and underwent cystoscopy.
Banked samples (N=14): Samples were obtained from existing samples (University of Gothenburg; collected between 1994–1997).
-
Collected Samples: Samples were collected (with IRB approval from respective institutions) during office visits at:
Zucker School of Medicine at Hofstra-Northwell (N=38)
University of Kentucky (N=3)
-
Women with IC/BPS with Hunner lesions (IC/BPS-H): IC/BPS was diagnosed using the same criteria that are described in the American Urological Association Guideline for Diagnosis and Treatment of IC/BPS. All subject had symptom duration > 1 year and were symptomatic at the time of urine collection and underwent cystoscopy.
Banked samples (N=26): Samples were obtained from existing samples (University of Gothenburg; collected between 1994–1997).
-
Collected Samples: Samples were collected (with IRB approval from respective institutions) during office visits at:
Zucker School of Medicine at Hofstra-Northwell (N=14)
University of Kentucky (N=3)
Male Non-Cystitis (Control group; N=119): negative for bacterial cystitis; no obvious urologic pathology on cystoscopic exam or any studies performed (subjects gave informed consent of diagnostic procedures and urine specimens were collected as part of the procedure; Lexington VAMC).
Men with Bacterial Cystitis (N=50): confirmed by culture; no clinical signs of pyelonephritis (patients gave informed consent of diagnostic procedures and urine specimens were collected as part of the procedure; Lexington VAMC).
Men with Radiation Cystitis (N=18): confirmed by clinical observation, history and cystoscopy (patients gave informed consent and samples collected during office visits; The Bay Pines VA Healthcare System).
Urine MIF assay and analyses
Upon receipt, all samples were centrifuged, pellet discarded, treated with protease inhibitors (Thermo Scientific, 1%), aliquoted and stored at −80 °C until analysis. Samples were assayed in duplicate for urine MIF levels using a commercially available ELISA kit (R&D; DY289). Urine creatinine levels were measured using a commercially available kit (Enzo, ADI-907-030).
The following parameters were obtained for each group: applicable demographics, age (at time of urine collection); urine MIF levels (pg MIF/ml); urine MIF normalized to creatinine (MIF pg/mg Creat). Values reported are mean ± standard error of the mean, SEM.
The study was designed so that an N = 50 in each group would result in 78% power for analysis of variance to detect a significant difference (p = 0.05; medium effect size = .25) or for individual group tests to have 80% power in detecting significant differences (p =0.05, medium effect size = 0.5; 13). Statistical differences were determined using analysis of variance followed by Tukey tests using R 14. Area under the curve (AUC) and Receiver Operating Characteristic Curve (ROC) analyses were performed using the pROC package 15 for R.
Results
Patient Demographics
Table 1 shows the demographic characteristics for all groups studied. Most of the female subjects (91%) identified themselves as White, while 4% identified themselves African-American or Hispanic and 1% identified themselves as Asian. Most of the male subjects (86%) identified themselves as White, while 11% and 1.6% identified themselves as African-Americans or Hispanic, respectively. Patients without Hunner lesions were younger (43 ± 1.9) than those in the non-IC/BPS (control) group (54 ± 1.2) while patients with Hunner lesions were older (64 ± 2.0) than the other two subject groups and these differences were statistically significant (Table 1). Male non-cystitis samples came from subjects with a mean age of years, while patients with bacterial cystitis had a mean age of 67 ± 1.1 years and patients with radiation cystitis had a mean age of 64 ± 0.7 years. Non-cystitis patients (63 ± 0.9) were younger than bacterial cystitis patients. Age of radiation cystitis patients was not different from non-cystitis patients. (Table 1).
Table 1.
Demographics for all groups
| White | African-American | Asian | Hispanic | Age (years) | p | |
|---|---|---|---|---|---|---|
| FEMALES | ||||||
| Non-IC/BPS (N=100) | 88 (88%) | 8 (8%) | 1 (1%) | 3 (3%) | 54 ± 1.2 | |
| IC/BPS-Non Hunner’s (N=55) | 53 (96%) | 0 | 0 | 2 (4%) | 43 ± 1.9 | (p < 0.0001) |
| IC/BPS-Hunner’s (N=43) | 39 (91%) | 0 | 1 (2%) | 3 (7%) | 64 ± 2.0 | (p < 0.0001) |
| Totals N=198 | 180 (91%) | 8 (4%) | 2 (1%) | 8 (4%) | ||
| MALES | ||||||
| Non-Cystitis (N=119) | 107 (90%) | 9 (8%) | 0 | 3 (2%) | 63 ± 0.9 | |
| Bacterial Cystitis (N=50) | 37 (74%) | 13 (26%) | 0 | 0 | 67 ± 1.2 | (p = 0.04) |
| Radiation Cystitis (N=18) | 16 (89%) | 2 (11%) | 0 | 0 | 64 ± 0.7 | |
| Totals N=187 | 160 (86%) | 24 (11%) | 0 | 3 (1.6%) | ||
MIF levels in female patients: Non-IC/BPS (Control group), IC/BPS without Hunner lesions, IC/BPS with Hunner lesions
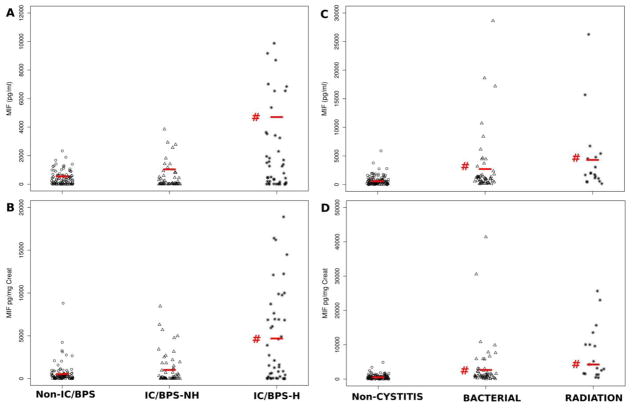
Table 2A and Figure 1A; B summarize the findings on urinary MIF levels in Non-IC/BPS female samples, and those from female IC/BPS patients with or without Hunner lesions.
Table 2.
Urine MIF values in all patient groups
| A: Female patients without and with IC/BPS | |||||
|---|---|---|---|---|---|
| Groups | N | Urine MIF (pg/ml) | p | Urine MIF pg/mg Creat | p |
| Non-IC/BPS | 100 | 414 ± 47.6 | 585 ± 112.0 | ||
| IC/BPS-NH | 55 | 460 ± 114.5 | 1074 ± 250.0 | ||
| IC/BPS-H | 43 | 2159 ± 435.3 | (p < .0001) | 4742 ± 815.2 | (p < 0.0001) |
| B: Male patients without cystitis, with bacterial and radiation cystitis | |||||
|---|---|---|---|---|---|
| Groups | N | Urine MIF (pg/ml) | p | Urine MIF pg/mg Creat | p |
| Non-Cystitis | 119 | 681 ± 75.2 | 649 ± 64.5 | ||
| Bacterial | 50 | 2839 ± 757.1 | (p = 0.0009) | 3505 ± 1028.0 | (p = 0.0006) |
| Radiation | 18 | 4404 ± 1548.1 | (p = 0.0001) | 7360 ± 1823.0 | (p < 0.0001) |
Figure 1.
Urine MIF levels in all patient groups. A: Urine MIF concentration (pg/ml) in non-IC/BPS patients (control group) and IC/BPS patients with (IC/BPS-H) and without (IC/BPS-NH) Hunner lesions. Urine MIF was significantly elevated (#; p < 0.001) in IC/BPS-H patients. B: Urine MIF levels normalized to creatinine (MIF pg/mg Creat). IC/BPS patients with Hunner lesions also had significantly elevated (#; p <0.001) levels compared to non-IC/BPS patients. C; D: Urine MIF levels, either as concentration (pg/ml; C) or MIF normalized to creatinine (MIF pg/mg Creat) were significantly elevated (#; p < 0.001) in both patients with bacterial and radiation cystitis when compared to non-cystitis patients.
Urine MIF concentrations (pg/ml) were not significantly different between IC/BPS patients without Hunner lesions (IC/BPS-NH; mean= 460 ± 114.5; N = 55) and non-IC/BPS samples (mean = 414 ± 47.6; N = 100). On the other hand, urine MIF concentrations in IC/BPS patients with Hunner lesions (IC/BPS-H) were significantly higher (approximately 5-fold; mean MIF pg/ml = 2159 ± 435.3; N = 43) compared to non-IC/BPS samples (Fig. 1A). When normalized to creatinine concentrations, urine MIF levels in IC/BPS-NH patients were not significantly different from non-IC/BPS values while urine MIF levels in IC/BPS-H patients were significantly increased (approximately 8-fold) compared to non-IC/BPS (Table 2A; Fig 1B).
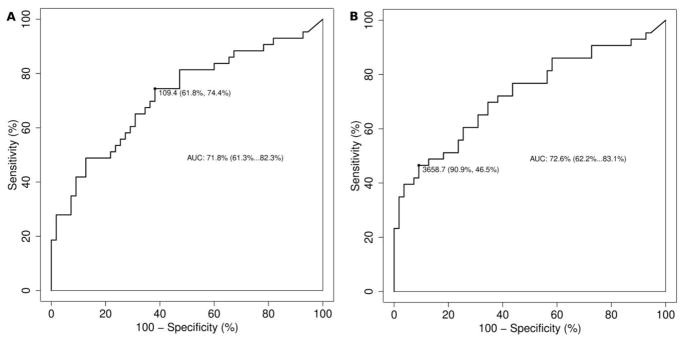
ROC analysis showed that an optimal cut-off urine MIF level of 109.4 pg MIF/ml had a 61.8% specificity and 74.4% sensitivity in detecting IC/BPS-H vs IC/BPS-NH patients (Figure 2A). The area under the curve (AUC) was found to be 71.8% (95% CI 61.3–82.3%). In the case of urine MIF normalized to creatinine, ROC analysis showed that a cut-off value of 3659 MIF pg/mg Creat had a 91% specificity and 47% sensitivity in detecting IC/BPS-H patients (Figure 2B). The AUC was 73% (95% CI 62.2–83.1%).
Figure 2.
Receiver operating curve for urine MIF concentration (pg MIF/ml; A) and urine MIF normalized to creatinine (MIF pg/mg Creat; B). Area under the curve (AUC) presented with confidence intervals. Most appropriate cut-off point displayed along with sensitivity and specificity.
MIF levels in male patients: Non-Cystitis (control group), Bacterial Cystitis and Radiation Cystitis
Table 2B shows urine MIF levels observed in male non-cystitis samples. Patients with bacterial cystitis had significantly elevated MIF levels in the urine, either as MIF concentration (mean MIF pg/ml = 2839 ± 757.1; p=0.0009; Fig 1C) or normalized to creatinine (mean MIF pg/mg Creat= 3505 ± 1028.0; p=0.0006) when compared to non-cystitis samples (mean MIF pg/ml= 681 ± 75.2; MIF pg/mg Creat = 649 ± 64.5; Fig 1D). Patients with radiation cystitis also had higher urine MIF concentrations (mean MIF pg/ml = 4404 ± 1548.1; p= 0.0001) compared to non-cystitis (Table 2B; Fig. 1C). In addition, radiation cystitis patients had significantly increased levels of urine MIF normalized to creatinine (mean MIF pg/mg Creat = 7360 ± 1823.0) when compared to non-cystitis patients (Table 2B; Fig 1D).
Discussion
Based on MIF’s pro-inflammatory and pro-nociceptive roles we hypothesized that urinary MIF would be elevated in bladder disease states marked by inflammation (and pain) such as IC/BPS with Hunner lesions, bacterial cystitis and radiation cystitis.
Our primary aim was to measure urine MIF levels in IC/BPS patients with and without Hunner lesions and compare them to non-IC/BPS individuals. Our findings show that urine MIF levels in female patients with IC/BPS and Hunner lesions are approximately five-fold higher than urine levels in female non-IC/BPS patients or IC/BPS patients without Hunner lesions, thus supporting our original hypothesis. Moreover, ROC analyses showed that urine MIF levels were able to discriminate between IC/BPS patients with Hunner lesions and those without Hunner lesions, with an urine MIF threshold value of 109.4 pg/ml achieving 62% specificity and 74% sensitivity in separating the two groups. Normalizing urine MIF concentration to creatinine was less useful, since it had a greater sensitivity but lower specificity than urine MIF concentration (in pg/ml). This is an interesting and potentially clinically useful finding that needs validation in a prospective study to determine whether urine MIF may aid IC/BPS diagnosis.
Presently it is not known whether patients with and without Hunner lesions have different pathophysiology or mechanisms or represent two points in a continuum. Mounting evidence indicates that they represent separate clinical conditions 16,17. From the clinician’s perspective, identification or exclusion of these regions of focal inflammatory disease has important treatment implications. For example, their presence might prompt an early recommendation for an ablative procedure, while this would not be considered in other IC/BPS patients 18,19. Furthermore, immunotherapy such as cyclosporine appears to have a more profound clinical benefit in those patients with visible inflammation 20. Having a urine marker to distinguish these groups has the potential to direct care more effectively and may obviate the need for more invasive procedures.
Previously it was assumed that Hunner lesions were rare, but modern evidence suggests a much higher prevalence 21. Detection of Hunner lesions depends on attention and training. Therefore, non-invasive methods other than cystoscopy, are needed to increase the detection rate. Determination of intravesical evaporation of nitric oxide (NO) is one non-cystoscopic method to differentiate the classic Hunner type from other IC/BPS presentations 22. This method is easy and simple to use but a disadvantage is that a special device is required; so far it is not generally used. Another potential biomarker (etiocholan-3α-ol-17-one sulfate) has been recently reported as being able to identify IC/BPS patients 23. Unfortunately, the use of sophisticated assay procedures currently preclude their clinical usefulness. Detection of urine MIF would be an attractive alternative in the sense that it only requires urine samples and instrumentation common to most facilities for analysis.
Developing non-invasive markers that may separate these two groups of IC/BPS patients is clinically important 24. A recent study described several chemokines that were increased in the urine of IC/BPS patients with Hunner lesions including nerve growth factor (NGF) 25. Interestingly, experimental evidence suggests that MIF release is upstream of NGF release after bladder injury 7. Furthermore, bladder NGF levels correlated well with pain scores in experimental animals while MIF antagonism prevented both bladder pain and increases in bladder NGF levels 8.
Although MIF itself was not reported as being upregulated in IC/BPS patients with Hunner lesions, CD74 (cognate receptor for MIF 26) was indeed upregulated 16. Previous findings showed that CD74 is upregulated and expressed in urothelial cells after experimental bladder injury and MIF binds to these receptors to mediate bladder inflammation 11. Based on the experimental evidence, it is tempting to speculate that increased MIF urine levels in patients with Hunner lesions would be available to bind to CD74 receptors and thus mediate bladder inflammation and pain in these patients. Our current findings of increased urine MIF levels in patients with Hunner lesions and published evidence of upregulated chemokines and/or receptors that are mediated by MIF strongly indicate that MIF is a crucial component of bladder inflammation (and pain) in this cohort of patients.
In terms of our secondary aim, our current findings in bacterial cystitis patients show that urinary MIF is increased in this population approximately 4-fold compared to non-cystitis values. These findings confirm and extend previous results from a much smaller data set than the current study 12. Similarly, urine MIF levels in radiation cystitis patients were increased approximately 6-fold over non-cystitis patients. Acute, uncomplicated bacterial cystitis is a common occurrence in urologic practice and treatment regimens are well-delineated 27. Radiation cystitis is common side-effect of pelvic radiation for malignancies and management of this condition is also well described 28. These two conditions result in frank bladder inflammation and thus serve as a good test of our original hypothesis that urine MIF levels are elevated in patients with bladder inflammation from these causes.
The current study has a number of limitations. First, we used patients without the selected diagnoses as controls (non-IC/BPS; non-cystitis) rather than normal healthy volunteers. Urine MIF levels in normal healthy volunteers (N=12) are reported to be in range with our current findings 29 or lower than our findings (N=20) 30. A larger study of normal healthy volunteers of both sexes is needed to determine a more accurate range of urine MIF concentration in normal individuals. Second, we did not determine or control for possibly co-morbidities that may affect urine MIF levels. Our group of IC/BPS (and bacterial cystitis) patients was heterogeneous. Most are afflicted by a wide variety of comorbid conditions, some of which may have an inflammatory component. Third, there is a significant age difference in the female groups. IC/BPS patients without Hunner lesions were younger (43 ± 1.9) than those with Hunner lesions (64 ± 2.0) or non-IC/BPS (54 ± 1.2). This age difference has been observed in previous reports 17. It is unlikely that increased age in the IC/BPS patients with Hunner lesions accounts for the increased urine MIF levels we found, since non-IC/BPS patients were also older than IC/BPS patients without Hunner lesions and yet their urine MIF levels were very similar. Fourth, there was no assessment of the relationship between IC/BPS symptoms (for example, pain) and urine MIF levels. Although all patients were symptomatic (including pain) at the time of collection, questionnaires were not obtained in this preliminary study. Our goal was to identify specific urine changes in MIF between the IC/BPS with Hunner lesions patients and the non-Hunner patients. Fifth, it should be noted that two of the groups did not reach full enrollment and therefore comparisons are likely underpowered and require a larger study. Lastly, samples were obtained from several institutions. Possible differences between institutions were not tested due to unequal sample sizes. For these reasons, our findings should be considered as preliminary and awaiting validation from future studies addressing these issues.
Conclusions
Our findings demonstrate that urine MIF is elevated in patients with bladder inflammatory conditions, supporting our original hypothesis. Our findings of a difference in urine MIF levels between the two IC/BPS groups add to the growing body of evidence that these patients may represent different disease entities and/or etiologies 16,17. In addition, this difference raises the possibility that urine MIF may have diagnostic value in separating the clinical presentation of these two groups.
Acknowledgments
This study is funded by NIH (DK0093496; PLV). We would like to acknowledge grant support from NIH CTSA UL1TR000117. This material is the result of work supported with resources and the use of facilities at the Lexington (Kentucky) Veterans Affairs Medical Center. Judy Glass and Xiu Xu provided excellent technical assistance. Barbara Kahn, MD provided excellent assistance in the collection of bacterial cystitis and non-cystitis samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suskind AM, Berry SH, Ewing BA, et al. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141–145. doi: 10.1016/j.juro.2012.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 4.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grieb G, Merk M, Bernhagen J, et al. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 2010;23:257–264. doi: 10.1358/dnp.2010.23.4.1453629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer-Siegler KL, Vera PL. Substance P induced release of macrophage migration inhibitory factor from rat bladder epithelium. J Urol. 2004;171:1698–1703. doi: 10.1097/01.ju.0000115883.49365.1a. [DOI] [PubMed] [Google Scholar]
- 8.Vera PL, Iczkowski KA, Howard DJ, et al. Antagonism of macrophage migration inhibitory factor decreases cyclophosphamide cystitis in mice. Neurourol Urodyn. 2010;29:1451–1457. doi: 10.1002/nau.20878. [DOI] [PubMed] [Google Scholar]
- 9.Kouzoukas DE, Ma F, Meyer-Siegler KL, et al. Protease-Activated Receptor 4 Induces Bladder Pain through High Mobility Group Box-1. PLoS One. 2016;11:e0152055. doi: 10.1371/journal.pone.0152055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouzoukas DE, Meyer-Siegler KL, Ma F, et al. Macrophage Migration Inhibitory Factor Mediates PAR-Induced Bladder Pain. PLoS One. 2015;10:e0127628. doi: 10.1371/journal.pone.0127628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera PL, Wang X, Bucala RJ, et al. Intraluminal blockade of cell-surface CD74 and glucose regulated protein 78 prevents substance P-induced bladder inflammatory changes in the rat. PLoS One. 2009;4:e5835. doi: 10.1371/journal.pone.0005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer-Siegler KL, Iczkowski KA, Vera PL. Macrophage migration inhibitory factor is increased in the urine of patients with urinary tract infection: macrophage migration inhibitory factor-protein complexes in human urine. J Urol. 2006;175:1523–1528. doi: 10.1016/S0022-5347(05)00650-6. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. Statistical power analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: [Google Scholar]
- 14.R Core Team. R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [Google Scholar]
- 15.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blalock EM, Korrect GS, Stromberg AJ, et al. Gene expression analysis of urine sediment: evaluation for potential noninvasive markers of interstitial cystitis/bladder pain syndrome. J Urol. 2012;187:725–732. doi: 10.1016/j.juro.2011.09.142. [DOI] [PubMed] [Google Scholar]
- 17.Peeker R, Fall M. Toward a precise definition of interstitial cystitis: further evidence of differences in classic and nonulcer disease. J Urol. 2002;167:2470–2472. [PubMed] [Google Scholar]
- 18.Hillelsohn JH, Rais-Bahrami S, Friedlander JI, et al. Fulguration for Hunner ulcers: long-term clinical outcomes. J Urol. 2012;188:2238–2241. doi: 10.1016/j.juro.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Peeker R, Aldenborg F, Fall M. Complete transurethral resection of ulcers in classic interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:290–295. doi: 10.1007/s001920070019. [DOI] [PubMed] [Google Scholar]
- 20.Cox A, Golda N, Nadeau G, et al. CUA guideline: Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2016;10:E136–E155. doi: 10.5489/cuaj.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logadottir Y, Fall M, Kabjorn-Gustafsson C, et al. Clinical characteristics differ considerably between phenotypes of bladder pain syndrome/interstitial cystitis. Scand J Urol Nephrol. 2012;46:365–370. doi: 10.3109/00365599.2012.689008. [DOI] [PubMed] [Google Scholar]
- 22.Logadottir YR, Ehren I, Fall M, et al. Intravesical nitric oxide production discriminates between classic and nonulcer interstitial cystitis. J Urol. 2004;171:1148–1150. doi: 10.1097/01.ju.0000110501.96416.40. discussion 1150-1141. [DOI] [PubMed] [Google Scholar]
- 23.Parker KS, Crowley JR, Stephens-Shields AJ, et al. Urinary Metabolomics Identifies a Molecular Correlate of Interstitial Cystitis/Bladder Pain Syndrome in a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network Cohort. EBioMedicine. 2016;7:167–174. doi: 10.1016/j.ebiom.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doiron RC, Tolls V, Irvine-Bird K, et al. Clinical Phenotyping Does Not Differentiate Hunner Lesion Subtype of Interstitial Cystitis/Bladder Pain Syndrome: A Relook at the Role of Cystoscopy. J Urol. 2016;196:1136–1140. doi: 10.1016/j.juro.2016.04.067. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi P, Killinger K, Tyagi V, et al. Urinary chemokines as noninvasive predictors of ulcerative interstitial cystitis. J Urol. 2012;187:2243–2248. doi: 10.1016/j.juro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grigoryan L, Trautner BW, Gupta K. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA. 2014;312:1677–1684. doi: 10.1001/jama.2014.12842. [DOI] [PubMed] [Google Scholar]
- 28.Browne C, Davis NF, Mac Craith E, et al. A Narrative Review on the Pathophysiology and Management for Radiation Cystitis. Adv Urol. 2015;2015:346812. doi: 10.1155/2015/346812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong MY, Tseng CC, Chuang CC, et al. Urinary macrophage migration inhibitory factor serves as a potential biomarker for acute kidney injury in patients with acute pyelonephritis. Mediators Inflamm. 2012;2012:381358. doi: 10.1155/2012/381358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto K, Kanmatsuse K. Urinary levels of macrophage migration inhibitory factor in patients with IgA nephropathy. Nephron. 2002;92:309–315. doi: 10.1159/000063297. [DOI] [PubMed] [Google Scholar]