Abstract
Diastolic dysfunction (DD), an abnormality in cardiac left ventricular (LV) chamber compliance, is associated with an increased risk of morbidity and mortality. While DD has been extensively studied in older populations, co-morbidity patterns are less well characterized among middle-aged individuals. We screened 156,434 subjects with transthoracic echocardiogram reports available through Vanderbilt’s de-identified electronic heath record data resource and identified 6,612 individuals 40–55 years old with LV ejection fraction ≥50% and diastolic function staging. We tested 452 incident and prevalent clinical diagnoses for associations with early stage (grade 1: impaired LV relaxation) DD versus normal function. Among 1,676 subjects with grade 1 DD we identified associations (FDR q<0.01) between DD and 44 co-morbid diagnoses including hypertension (OR=2.02 [95% CI:1.78–2.28], p<5.3×10−29), type 2 diabetes (OR=1.96, [1.68–2.29], p=2.1×10−17), tachycardia (OR=1.38 [0.53–2.19], p=2.9×10−6) and obesity (OR=1.76 [1.51–2.06], p=1.7×10−12) and clinical end-points including end-stage renal disease (OR=3.29 [2.19 – 4.96], p=1.2×10−8) and stroke (OR=1.5 [1.12 – 2.02], p=6.9×10−3). Among the 60 incident clinical diagnoses associated with DD at FDR q<0.01, the most significant associations were with heart failure with preserved EF (HFpEF) (OR=4.63 [3.39–6.32] p=6.3×10−22). Among individuals with normal diastolic function and blood pressure at baseline, a blood pressure measurement in the hypertensive range at the time of a d second echocardiogram was associated with progression to stage 1 DD (p=0.04). In conclusion, DD was common in a EHR-based clinical population of individuals 40–55 years old and was associated with a heavy burden of co-morbidities and cardiovascular diseases.
Keywords: diastolic dysfunction, heart failure, risk factors, electronic health record
INTRODUCTION
Diastolic dysfunction (DD) is an abnormality in left ventricular (LV) chamber compliance contributing to elevated filling pressures.1 Individuals with DD are at increased risk for heart failure (HF), particularly HF with preserved ejection fraction (HFpEF),2–8 and death.9 As no available therapies improve mortality in established HFpEF, strategies directed toward preventing DD progression are one approach to reduce morbidity and mortality.10 While the epidemiology of DD is most extensively described in older subjects participating in prospective cohorts,2,11,12 findings from the CARDIA cohort demonstrate that DD present in early life also associates with increased future morbidity and mortality.13 DD is not well-characterized in younger individuals outside of prospective cohorts, and the relevance of the risk factors in real world medical populations is unclear. We used Vanderbilt University Medical Center’s (VUMC) electronic health record (EHR) to identify and characterize subjects between 40 and 55 years old with early stage DD and a preserved ejection fraction (LVEF≥50%).
METHODS
All individuals were identified from VUMC’s Synthetic Derivative database, a research tool developed to enable observational studies using de-identified clinical data.14 This IRB-approved resource comprises clinical data including demographics (age, gender, race), billing codes (ICD-9 codes15), text from inpatient and outpatient clinical notes, laboratory values, procedural reports (e.g., echocardiography), inpatient and outpatient medications and laboratory values extracted from ~2.7 million individual clinical records. Selection of the final study population is outlined Supplementary Figure S1. We used natural language processing to identify inpatient and outpatient transthoracic echocardiography (TTE) reports, generated between April 1997 and February 2014, with a cardiologist-defined staging of LV diastolic function (Supplementary Table S1). Diastolic function stage was assigned by an expert clinical echocardiographer who provided an integrated interpretation of echocardiogram parameters to assign a diastolic function grade as follows: normal (stage 0), impaired LV relaxation, (stage 1), a “pseudonormal” filling (stage 2) and restrictive filling (stage 3).16 Among 156,434 TTE reports analyzed, 77,235 reports from 53,638 unique subjects had been assigned a diastolic function stage. Subjects with acute cardiac diagnoses (e.g. endocarditis), congenital/structural heart disease (e.g. secundum atrial-septal defect) and who received chemotherapy prior to or at the time of their echocardiogram were excluded (see Supplementary Table S2 for a list of ICD-9 codes used for exclusions). After exclusions, there were 6,612 unique individuals whose TTE was performed between the ages of 40 and 55 years old, who had a LV ejection fraction (LVEF)≥50% and whose race was coded as white, black or unknown. TTEs were measured in outpatient (n=3,930), inpatient (n=1,519) and undetermined settings (n=1,163). There were 572 individuals with normal diastolic function or stage 1 DD who also had a follow-up TTE with LVEF≥50%. This study was approved by Vanderbilt University Medical Center’s IRB. Informed consent was not required. The first author (JDM) had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Gender and race (white, black or other) were extracted from a structured demographics table. Outpatient clinic visits and dates were extracted from scheduling records. Systolic and diastolic blood pressure (BP), heart rate and body mass index (BMI) values were extracted from vital signs tables. Lab values for creatinine and glucose were extracted from structured labs tables. For BP and heart rate, the median for all recorded measurements taken within the 14 days prior to the TTE was used for analysis. For BMI, glucose and creatinine values, the median for all measurements taken within the 30 days prior was used for analysis. Systolic BP measurements <50 or >275 mmHg and diastolic BP measurements <30 or >200 mmHg were excluded as these were thought likely to be entry errors or represent acute clinical decompensation. Continuous measurements were not available for all subjects (see Supplementary Table S3 for percentages of individuals with available data for each variable).
Clinical diagnoses were based on ICD-9 CM (International Classification of Disease, Ninth revision) billing codes. Related ICD-9 codes were aggregated using PheWAS diagnosis code definitions, which have been previously described and validated (https://phewas.mc.vanderbilt.edu/).17,18 We identified all diagnosis codes that were present in an individual’s EHR at any time before or after the TTE. Many individuals had multiple diagnoses, and diagnoses were not explicitly linked to the clinical indication for the TTE. For each of these diagnoses, each individual was assigned to be a case if they had 1 or more instances of the diagnosis appearing in their record or a control if that code, or a closely related code, did not appear in their record (e.g. all individuals with a diagnoses of hypertension were designated hypertension cases and individuals without a hypertension diagnosis were designated controls for the hypertension diagnosis). There were 452 PheWAS diagnoses codes with >100 cases (our minimum threshold for inclusion).
Logistic regression was used to serially test associations between diastolic dysfunction (independent variable) and each clinical phenotype and comorbidity (the dependent variable) to identify diagnoses associated with normal versus stage 1 DD. All analyses were adjusted for age at the time of the TTE, gender, race (coded as white versus other) and the setting where the TTE was performed (inpatient, outpatient or unknown). To adjust for multiple testing, a Benjamini-Hochberg false discovery rate (FDR) correction was applied to each analysis and the FDR-adjusted q-values are presented.19
For the analyses identifying clinical diagnoses associated with DD, association testing was performed separately for diagnoses that were assigned (1) prior to the TTE and (2) on or after the date of the TTE. Since clinical diagnoses are highly correlated, a reduced set of diagnoses present before the TTE was identified using a two-step approach. First, all diagnoses associated with stage 1 DD with FDR q<0.01 were selected. Second, a multivariable logistic model incorporating each selected diagnosis in conjunction with a stepwise selection feature (using Proc Logistic in SAS) was used to identify an independent set of diagnoses associated with stage 1 DD. For analyses looking at incident diagnoses after the initial TTE, subjects with a diagnosis code assigned prior to the TTE (i.e. subjects with prevalent disease at the time of the TTE) were excluded from the analysis.
To test the association between continuous variables and normal versus stage 1 DD, a linear regression model adjusting for age, gender, self-reported race and the echocardiogram setting was used. Setting was included as a covariate to adjust for the potential impact of acute illness on the continuous measures. Log-transformed creatinine and glucose level variables were used since these variables were highly skewed. A p<0.05 was considered significant.
Kaplan-Meier curves showing progression to a diagnosis of HFpEF (defined by PheWAS code 428.4) by DD stage were generated using the Proc Lifetest function (SAS). The p-value testing for equality across strata is based on a log-rank test statistic.
For subjects with multiple TTEs, multivariable linear regression, adjusting for age, gender, self-reported race and time between the first and second TTE was used to calculate the average difference in the continuous variables (e.g. systolic BP) at the time of the second and first TTEs. The independent variables were either progression (from normal to stage 1 DD) or regression (from stage 1 DD to normal) between the first and second TTE. A p<0.05 was considered statistically significant. A Fisher’s exact test was used to compare the proportions of individuals whose diastolic function either remained unchanged or changed between their first and second TTEs and their BP control status (normotensive versus hypertensive) at the time of the first and second TTE. Hypertension was defined as a median systolic BP>140 or diastolic BP>90, and used all measurements within 14 days prior to the TTE.
Analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
We screened 156,343 TTE reports and identified 6,612 individuals between the ages of 40 and 55 with an echocardiographer-assigned diastolic function staging and LVEF≥50% (Supplementary Figure S1). The majority of subjects (69.4%) had normal diastolic function and 1,676 (25.3%) had stage 1 DD. The remaining ~5% had stage 2 (n=277) or stage 3 (n=72) DD. Stage 1 DD, as compared to normal diastolic function, was associated with older age, male gender and black race (Table 1). The proportion of individuals with prevalent Stage 1 DD was constant across age groups among individuals whose echo was performed after 2005, but was lower in the 5% of individuals with TTEs in earlier years (Supplementary Table S4).
Table 1.
Demographics of the echocardiogram population.
| Characteristic | Normal (n=4587) | Stage 1 DD (n=1676) | p-value |
|---|---|---|---|
| Ages (years) | |||
| 40–45 | 1449 (32%) | 257 (15%) | <.00011 |
| 45–50 | 1605 (35.0%) | 475 (28%) | |
| 50–55 | 1533 (33%) | 944 (57%) | |
| Men | 1920 (42%) | 769 (46%) | 0.0091 |
| Women | 2663 (58%) | 907 (54%) | |
| White | 3617 (79%) | 1261 (75%) | <.00011 |
| Black | 635 (14%) | 306 (18%) | |
| Unknown | 335 (7%) | 109 (7%) | |
| Duration prior to TTE (years)2 | 2.9 (0.6–5.5) | 2.6 (0.4–5.3) | |
| Duration post TTE (years)2 | 2.7 (0.1–7.7) | 2.4 (0.1–7.6) | |
| Systolic blood pressure (mmHg)3 | 123 ± 17 | 130 ± 20 | <0.00014 |
| Diastolic blood pressure (mmHg)3 | 75 ± 11 | 79 ± 13 | <0.00014 |
| Heart rate (beats per minute)3 | 79 ± 16 | 85 ± 17 | <0.00014 |
| Body mass index (kg/m2)3 | 30.4 ± 7.9 | 33.5 ± 8.4 | <0.00014 |
| Creatinine level (mg/dl)3 | 0.85 (0.70–1.03) | 0.92 (0.77–1.16) | <0.00014 |
| Serum glucose (mg/dl)3 | 97 (87–114) | 106 (92–136) | <0.00014 |
Notes:
P-values are from a chi-square test evaluating the distributions across age, gender and racial groups among individuals with normal versus stage 1 DD.
Median (interquartile range) number of years of patient data available prior to or after the TTE.
Values are mean (standard deviation) except for creatinine and glucose which are median (interquartile range).
P-value from a linear regression model adjusting for age, gender, self-reported race and setting. Log-transformed values were analyzed for creatinine and glucose.
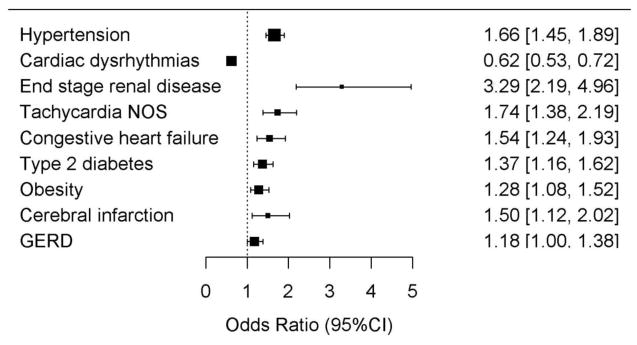
We evaluated 452 prevalent clinical diagnoses (assigned any time prior to the date the TTE was performed) and found that 44 were associated with stage 1 DD (FDR q<0.01) (Supplementary Figure 2 and Supplementary Table S5). DD was associated with an increased prevalence for the majority of these diagnoses. The most significant associations were with diagnoses of hypertension (OR=2.02 [1.78–2.28], p<5.3×10−29), T2D (OR=1.96, [1.68–2.29], p=2.1×10−17), chronic kidney disease (OR=2.55 [1.97–3.29], p=8.2×10−13 and obesity (OR=1.76 [1.51–2.06], p=1.7×10−12). We used a multivariable selection model to identify a reduced collection of independently associated prevalent diagnoses among the 44 diagnoses. Nine diagnoses were independently associated with stage 1 DD (Figure 1 and Supplementary Table S6). Hypertension remained the most significantly associated factor. DD was also associated with end-stage renal disease (OR=3.29 [2.19 - 4.96], p=1.2×10−8) and stroke (OR=1.5 [1.12 – 2.02], p=6.9×10−3). DD was associated with a decreased odds of a “Cardiac dysrhythmias” diagnosis (OR=0.62 [0.53–0.72], p=1.1×10−9), an umbrella diagnosis that incorporates diagnoses including palpitations and atrial fibrillation. However, DD was associated with an increased odds for the more specific arrhythmia diagnosis of “Tachycardia” (OR=1.38 [0.53–2.19], p=2.9×10−6). Consistent with the clinical associations, stage 1 DD was positively associated with systolic and diastolic BP, heart rate, BMI, serum creatinine, and serum glucose level (Table 1 and Supplementary Table S7). Individuals with stage 1 DD were also more likely to attend specialty clinics including nephrology (OR= 2.91 [95% CI: 1.86–4.54], p=2.7×10−6), diabetes/endocrine (OR=1.54 [1.25–1.88], p=3.4×10−5) and hypertension (OR=2.25 [1.36–3.70], p=1.5×10−3) clinics (Supplementary Figure S3) (FDR q<0.05 in each case).
Figure 1. Clinical diagnoses present before the echocardiogram independently associated with stage 1 DD.
An independent set of diagnoses was identified by taking each of the 44 diagnoses associated with stage 1 DD at FDR q<0.01 and applying a multivariable logistic selection model incorporating all 44 diagnoses and covariates. Shown are the 9 diagnoses retained in the final selection model.
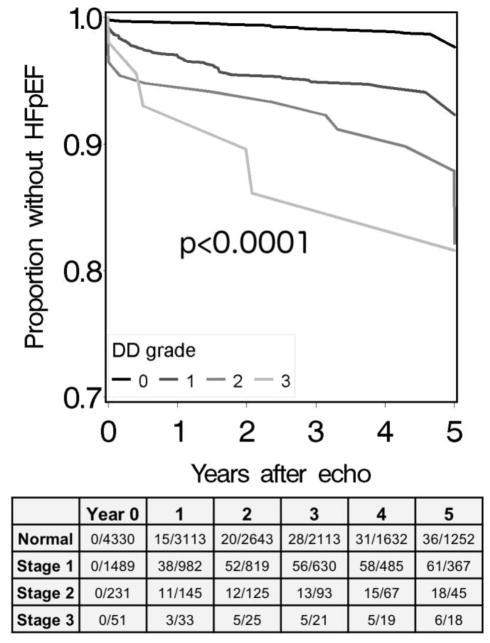
There were 60 incident diagnoses (assigned after the date of the TTE), among 452 evaluated, that were associated with stage 1 DD (FDR q<0.01). DD was associated with an increased prevalence of all but three of these diagnoses (Figure 2 and Supplementary Table S8). The most significant associations were with heart failure diagnoses, with the HFpEF subtype demonstrating the strongest association (OR=4.63 [3.39–6.32] p=6.3×10−22). The prevalence of HFpEF increased across increasing DD stages (Figure 3). Additional associations were seen with hypertension and related sequela (e.g. hypertensive heart and kidney disease, OR=2.24, [1.79–2.80], p=2.1×10−12), ischemic heart disease (OR=1.64 [1.38–1.96], p=3.6×10−8), obesity (OR=1.89 [1.56–2.29], p=4.5×10−11), sleep apnea (OR=1.58 (1.31–1.91), p=2.3×10−6) and sequelae of kidney disease.
Figure 2. Clinical diagnoses assigned after the echocardiogram associated with DD stage 1 versus normal diastolic function.
Subjects assigned a diagnosis prior to the echocardiogram were excluded from the analysis of that diagnosis. Logistic regression models were used to test for associations with each diagnosis and stage 1 DD and adjusted for age, gender, self-reported race and setting. Shown are all diagnoses associated with DD at FDR q<0.01. EF=ejection fraction.
Figure 3. Kaplan-Meier curves, stratified by DD stage, showing time to an incident clinical diagnosis of HFpEF.
Subjects with any clinical heart failure diagnosis prior to the echocardiogram were excluded. The table shows the cumulative counts of HFpEF diagnoses and of subjects remaining at each year. Data were censored at 5 years. The p-value is based on a log-rank test statistic.
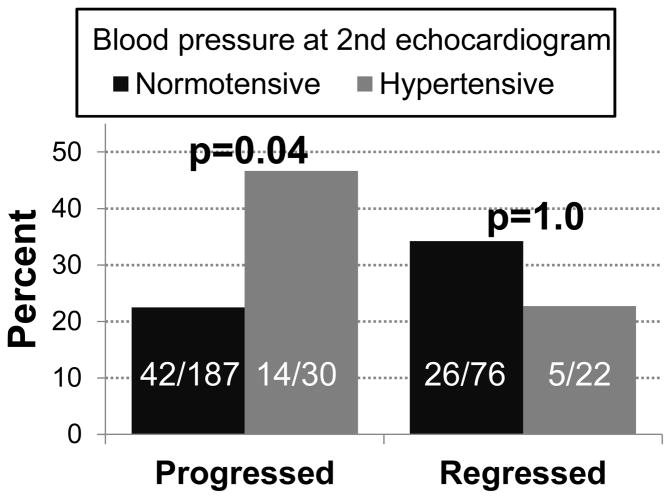
There were 572 subjects who had a second TTE (Supplementary Table S9). For the majority of subjects (n=410), the diastolic function stage did not change between echoes. However, there were 101 subjects who progressed from normal diastolic function to stage 1 DD and 61 subjects who regressed from stage 1 to normal. Subjects who progressed to stage 1 DD, as compared to those who maintained normal diastolic function, had significantly larger increases in systolic BP (mean difference=4.2 mmHg [0.4 – 8.0], p=0.03) and heart rate (mean difference=8.3 bpm [4.2 – 12.4], p=0.0001) between their baseline and second a TTE (Supplementary Table S10). Subjects whose diastolic function normalized, as compared to those who stayed at stage 1, had significantly larger decreases in systolic BP (mean difference=−8.4 mmHg [−14.7 – −2.0], p=0.01) and diastolic BP (mean difference=−6.5 mmHg [−10.5 – −2.5], p=0.002) between their baseline and second TTE (Supplementary Table S10). When we restricted the analyses to individuals with >1.5 years between TTEs, the associations between progression and systolic BP and heart rate were attenuated, while the associations between BP and regression were stronger (Supplementary Table S11). Finally, we examined the impact of transitioning from BPs in the normotensive range to the hypertensive range (systolic BP>140 mmHg or diastolic BP>90 mmHg) on change in diastolic function. Among subjects with normal diastolic function and who were normotensive at their first TTE, a significantly higher portion of those who were hypertensive at the follow-up echocardiogram progressed to stage 1 DD versus those who remained normotensive (46.7% vs. 22.5%, Fisher’s p=0.04) (Figure 4).
Figure 4. Association between DD progression or regression and hypertension status.
All subjects in these analyses were normotensive at their first echocardiogram. The first pair of columns (Progressed) show the percentages of subjects, who progressed from normal diastolic function to grade 1 DD at the second echocardiogram, stratified by hypertension status at the second echocardiogram. The second pair of columns (Regressed) lists the percentages of subjects who went from grade 1 DD to normal diastolic function at their second echo. The numbers represent the counts underlying the percentages. P-values are for differences in proportions by Fisher’s exact test.
DISCUSSION
We employed a discovery-oriented approach to characterize the clinical and epidemiological profiles and health care utilization patterns associated with early stage DD among individuals ages 40–55. The study population was identified through VUMC’s de-identified EHR, which enabled us to rapidly acquire a large number of individuals with diastolic function status characterized by TTE. We demonstrate that this EHR population has an epidemiological profile consistent with that described using traditional study designs with standardized data collection. For instance, prevalent stage 1 DD was most strongly associated with hypertension, diabetes, obesity, hyperlipidemia, kidney disease, tachycardia and sleep apnea, consistent with findings among older individuals.2–7 Also in agreement with prior reports, we found that the prevalence of DD increased with age11,20 and, overall, 30% of individuals that met our selection criteria had stage 1 or higher DD. Individuals with, stage 1 DD were also more likely to receive care at specialty clinics managing important risk factors including hypertension, diabetes and kidney function. Collectively, our data indicate that DD in younger individuals share a similar risk profile as older individuals, and DD at an early age is driven by earlier and more severe manifestations of known risk factors, rather than an intrinsic predisposition to diastolic disease.
Early stage DD is a plastic measure and can progress or regress over time.2,12 This has important implications for younger individuals with DD, as it opens the possibility that their disease course can not only be delayed, but can potentially be reversed. When we examined subjects whose diastolic function progressed or regressed, we found that the direction of association of clinical factors at the time of the second echocardiogram was consistent with an attenuated risk profile. For instance, individuals who were hypertensive at their second echocardiogram, but not their first, were more likely to progress, while those with lower BPs were more likely to regress. A similar association between progression and hypertension has been observed in other cohorts,4 and clinical studies have demonstrated that aggressive treatment of hypertension can improve both left ventricular hypertrophy and diastolic function.21
The associations we report were based on diagnoses tied to clinical billing, indicating that individuals with DD were being actively treated for the reported conditions. Clinical care was also more frequently through specialty clinics which treat resistant risk factors or advanced disease, suggesting that aggressive risk modification in a younger population with DD may require specialty care. For almost all of the associations, DD was associated with an increased prevalence of the diagnosis, highlighting that DD is associated with an increased burden of a broad range of co-morbidities.
There are several limitations to this study. VUMC is a tertiary care center and may represent a sicker cohort than the general population. The study design was observational and analyses used data generated during the course of delivering clinical care. Thus, the availability of data was influenced by clinical reasoning, which can lead to biased ascertainment and differential rates of missing data for clinical factors. This bias can impact prevalence estimates and risk factor comparisons derived from this population. There was no active follow-up with participants, and the durations of follow-up were variable across patients, which can lead to differential censoring of subjects related to the outcomes and risk factors of interest. The diagnosis and staging of diastolic function was based on echocardiographic interpretation conducted by trained cardiologists who had access to a patient’s clinical history. Echocardiographic criteria for grading diastolic function evolved over the course of the study period (1997–2014).22 Thus, our approach of accepting the grade assigned by the interpreting cardiologist reflects their integration of the available data and reflects standards of clinical practice at the time the echocardiograms were interpreted. However, interobserver variability is a potential source of bias in analyses examining individuals with repeated TTE assessments.
In conclusion, we show that DD in middle-aged population is associated with an increased burden of comorbidities and adverse sequelae including heart failure. Importantly, this work supports the validity of EHR cohorts to rapidly identify epidemiologically relevant populations. We show that DD, diagnosed in a clinical setting, is a biomarker which integrates a range of cardiovascular risk factors. Targeting screening, risk modification and treatment strategies toward younger individuals with early stage DD and DD risk factors may be important for reducing the heavy burden of co-morbidity associated with this biomarker in older adults.
Supplementary Material
Acknowledgments
This work was supported by a career development award from the Vanderbilt Faculty Research Scholars Fund (JDM), American Heart Association (AHA) 15MCPRP25620006 (JDM), AHA 13FTF16810038 (QSW), AHA 17IBDG33780015 (QSW), NIH/NHLBI 1R01HL140074 (QSW), NIH/NHLBI K23HL128928-02 (DKG) and AHA 13FTF16070002 (ELB). VUMC’s Synthetic Derivative is supported by numerous sources: institutional funding, private agencies, and federal grants including the NIH funded Shared Instrumentation Grant S10RR025141; and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975.
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeong E-M, Dudley SC. Diastolic dysfunction. Circ J. 2015;79:470–477. doi: 10.1253/circj.CJ-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam CSP, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa de Sa DD, Hodge DO, Slusser JP, Redfield MM, Simari RD, Burnett JC, Chen HH. Progression of preclinical diastolic dysfunction to the development of symptoms. Heart. 2010;96:528–532. doi: 10.1136/hrt.2009.177980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel MW, Slusser JP, Hodge DO, Chen HH. The natural history of preclinical diastolic dysfunction: a population-based study. Circ Heart Fail. 2012;5:144–151. doi: 10.1161/CIRCHEARTFAILURE.110.959668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–305. doi: 10.1016/j.jacc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren X, Ristow B, Na B, Ali S, Schiller NB, Whooley MA. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am J Cardiol. 2007;99:1643–1647. doi: 10.1016/j.amjcard.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbate A, Arena R, Abouzaki N, Van Tassell BW, Canada J, Shah K, Biondi-Zoccai G, Voelkel NF. Heart failure with preserved ejection fraction: Refocusing on diastole. Int J Cardiol. 2015;179C:430–440. doi: 10.1016/j.ijcard.2014.11.106. [DOI] [PubMed] [Google Scholar]
- 9.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 10.Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJV. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;60:2349–2356. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 11.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, Thomas JD, Jaber WA. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788. doi: 10.1161/CIRCULATIONAHA.111.066423. [DOI] [PubMed] [Google Scholar]
- 13.Desai CS, Colangelo LA, Liu K, Jacobs DR, Cook NL, Lloyd-Jones DM, Ogunyankin KO. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: the coronary artery risk development in young adults study. Am J Epidemiol. 2013;177:20–32. doi: 10.1093/aje/kws224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, Masys DR. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israel RA. The International Classification of Disease. Two hundred years of development. Public Health Rep. 1978;93:150–152. [PMC free article] [PubMed] [Google Scholar]
- 16.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, Wang D, Masys DR, Roden DM, Crawford DC. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26:1205–1210. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, Field JR, Pulley JM, Ramirez AH, Bowton E, Basford MA, Carrell DS, Peissig PL, Kho AN, Pacheco JA, Rasmussen LV, Crosslin DR, Crane PK, Pathak J, Bielinski SJ, Pendergrass SA, Xu H, Hindorff LA, Li R, Manolio TA, Chute CG, Chisholm RL, Larson EB, Jarvik GP, Brilliant MH, McCarty CA, Kullo IJ, Haines JL, Crawford DC, Masys DR, Roden DM. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31:1102–1110. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- 20.Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods M-C, Fagard RH, Díez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 21.Mattioli AV, Zennaro M, Bonatti S, Bonetti L, Mattioli G. Regression of left ventricular hypertrophy and improvement of diastolic function in hypertensive patients treated with telmisartan. Int J Cardiol. 2004;97:383–388. doi: 10.1016/j.ijcard.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen-Torvik LJ, Colangelo LA, Lima JAC, Jacobs DR, Rodriguez CJ, Gidding SS, Lloyd-Jones DM, Shah SJ. Prevalence and Predictors of Diastolic Dysfunction According to Different Classification Criteria: The Coronary Artery Risk Development in Young in Adults Study. Am J Epidemiol. 2017:1–7. doi: 10.1093/aje/kww214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.