Abstract
Epilepsy, characterized by recurrent seizures and abnormal electrical activity in the brain, is one of the most prevalent brain disorders. Over 2 million people in the United States have been diagnosed with epilepsy and 3 percent of the general population will be diagnosed with epilepsy at some point in their lives. While most developmental epilepsies occur due to genetic predisposition, a class of “acquired” epilepsies result from a variety of brain insults. A leading etiological factor for epilepsy, currently on the rise, is traumatic brain injury which accounts for up to 20% of all symptomatic epilepsies. Remarkably, the presence of an identified early insult that constitutes a risk for development of epilepsy provides a therapeutic window in which the pathological processes associated with brain injury could be manipulated to limit the subsequent development of recurrent seizure activity and epilepsy. Recent studies have revealed diverse pathologies including enhanced excitability, activated immune signaling, cell death and enhanced neurogenesis within a week after injury suggesting a period of heightened adaptive and maladaptive plasticity. An integrated understanding of these processes and their cellular and molecular underpinnings could lead to novel targets to arrest epileptogenesis after trauma. This review attempts to highlight and relate the diverse early changes after trauma and their role in development of epilepsy and suggests potential strategies to limit neurological complications in the injured brain.
Keywords: traumatic brain injury, dentate gyrus, neurogenesis, excitotoxicity, neuroinflammation
Traumatic brain injury (TBI) is a growing silent epidemic and a major contributor to increases in the long-term risk for neurological dysfunction including drug-resistant acquired temporal lobe epilepsies (Annegers and Coan 2000; Gupta et al. 2014; Kharatishvili and Pitkanen 2010b; Lowenstein 2009). A recent report by the Centers for Disease Control and Prevention (CDC 2015) estimates that in the US, TBI accounts for over 2.5 million civilian emergency room visits and more than 200,000 hospitalizations annually, while those in service have a 4% risk of sustaining TBI. Brain injuries ranging from mild trauma due to falls in elderly, to severe penetrating injuries from motor vehicle accidents and explosive devices heighten the risk for epilepsy (Chen et al. 2009; Kovacs et al. 2014). There appears to be a strong correlation between injury severity and the risk for developing spontaneous seizures with a two-fold increase following mild TBI and over seven to fourteen fold increase with severe TBI (Annegers and Coan 2000; Christensen et al. 2009; Ferguson et al. 2010; Yeh et al. 2013). A variety of experimental paradigms have been used to model physically diverse human brain injuries, but the most widely used models are the Fluid Percussion model (FPI) and Cortical Contusion injury (CCI) (Reviewed in Johnson et al. 2015). Fluid percussion injury (FPI) models closed head, concussive brain trauma by delivering a brief fluid pulse, generated by the impact of a pendulum on one end of a fluid filled piston, to the exposed but intact dura (Dixon et al. 1987; Toth et al. 1997). The Cortical contusion injury (CCI) simulates impact or penetrating injuries by means of controlled impact of a piston driven at a certain speed to a defined depth (Dixon et al. 1991; Hunt et al. 2009). Other methods employed to mimic traumatic brain injury include weight drop models of impact injury, primary blast wave TBI, rotational TBI and denervation injury from cortical undercut (Adelson et al. 1997; Cullen et al. 2016; Prince et al. 2009; Sundaramurthy et al. 2012). Both FPI and CCI lead to spontaneous epileptic seizures and reduce threshold for chemically evoked seizures (Bolkvadze and Pitkanen 2012; D’Ambrosio et al. 2005). While differences in injury modality, severity and rodent strains used in various studies make it difficult to directly compare epileptogenesis across different injury models, in comparative studies in adult C57BL/6S mice, CCI results in greater incidence of spontaneous seizures FPI (Bolkvadze and Pitkanen 2012). Long term EEG monitoring of rats after FPI has revealed 50% incidence of limbic epilepsy after a prolonged latency of 7 weeks to one year while cortical epilepsies are observed as early as 2 weeks after injury (Curia et al. 2011; Kharatishvili et al. 2006). Whereas, about 20–36 % of rats and mice develop behavioral epileptic seizures over 9–10 weeks after penetrating CCI (Hunt et al. 2009; Kelly et al. 2015). Both clinical and experimental data suggest that, like adults, the developing brain is also susceptible to post-traumatic epileptogenesis (Keret et al. 2017; Semple et al. 2017). The link between injury severity, histopathology and epilepsy is particularly well characterized after FPI (Kharatishvili and Pitkanen 2010a). Curiously, the risk for epilepsy after blast wave exposure is still unknown (Kovacs et al. 2014). Rising incidences of civilian and military TBI highlight a pressing need to identify the mechanisms of posttraumatic epileptogenesis. The progressive increase in risk for epilepsy with injury severity and long latency to seizures suggest that convergence of early pathological responses to trauma culminate in epilepsy (Kharatishvili and Pitkanen 2010a; Lowenstein 2009). Here we focus on the period within a week after injury to identify potentially epileptogenic alterations and shared mechanisms that may be targeted to prevent posttraumatic epilepsy.
Cellular and circuit mechanisms of posttraumatic epilepsy
TBI has been shown to contribute to development of neocortical and limbic epileptic foci (Curia et al. 2011). Among neocortical circuits, principal neurons in layer 2/3 of the prefrontal cortex exhibit increased intrinsic excitability and glutamatergic synaptic drive, and a reduction in inhibitory synaptic inputs within a week after FPI (Smith et al. 2015). Moreover, studies conducted 8–10 weeks after FPI have identified that the cortical tissue around the site of injury exhibits enhanced excitability indicating lasting perturbations that could underlie the development of cortical epileptic foci after TBI (D’Ambrosio et al. 2004). Interestingly, while reports that CCI augments epileptogenesis following amygdala kindling are consistent with the reduction in inhibition and increase in cholinergic excitation after CCI (Eslami et al. 2016), amygdala excitability appears to be reduced after FPI suggesting that injury modality may dictate specific circuit changes after TBI (Almeida-Suhett et al. 2014; Palmer et al. 2016). In contrast, both FPI and CCI have been consistently shown to augment excitability of the hippocampal dentate gyrus.
Brain injury leads to a cascade of early cellular and circuit changes in the hippocampus (Bolkvadze and Pitkanen 2012; Hunt et al. 2013; Lowenstein 2009). The dentate gyrus, a functional gate to the hippocampal trisynaptic circuit (Heinemann et al. 1992; Lothman et al. 1991), and the focus of circuit reorganizations in temporal lobe epilepsy, is particularly sensitive to TBI (Bruton 1988; Lowenstein et al. 1992). Dentate hilar and hippocampal neuronal degeneration characterize both experimental TBI and epilepsy (Kelly et al. 2015; Lowenstein et al. 1992; Saatman et al. 2006; Toth et al. 1997). However, the tightly packed dentate granule cells (GCs) are generally preserved after brain injury and, paradoxically, show transient increases in numbers with enhanced generation of newborn granule cells from the subgranular neurogenic niche 3–7 days after FPI, as is reported after seizures (Jessberger et al. 2007; Neuberger et al. 2017). Although GCs survive the trauma, they show atrophy of dendrites and spines as early as 72 hours after trauma (Gao et al. 2011). GC axons known as mossy fibers, show evidence of abnormal sprouting of molecular layer collaterals targeting GC dendrites within a week after injury, replicating a pathological hallmark of temporal lobe epilepsy (Hunt et al. 2009; Jeub et al. 1999; Kharatishvili et al. 2006; Santhakumar et al. 2001). This is significant because, although sprouting may not be necessary for epileptogenesis (Buckmaster and Lew 2011), modeling data indicate that sprouting is sufficient to promote seizure-like activity (Santhakumar et al. 2005). Neuronal injury after FPI occurs almost instantaneously due to impact and a majority of hilar neurons undergo subsequent degeneration during the course of days to a week due to secondary injury processes (Hunt et al. 2013; Toth et al. 1997). The focal impact of CCI generates more extensive loss of hippocampal pyramidal neurons than concussive injuries (Peterson et al. 2015). In contrast, the limited data on blast TBI suggests little acute cell loss within days after trauma (Kovacs et al. 2014). Using a programmable FPI device, we found that rapidly rising injury waveforms result in less neuronal injury at 24 hours followed by more progressive cell loss in the ensuing week than slower injuries (Neuberger et al. 2014). These findings suggest that the evolution of cell loss following rapid blast TBI may differ from that of slower impact injuries.
At a global level, brain injury impairs hippocampal rhythms reducing the power of theta (4–8 Hz) oscillations (Paterno et al. 2016) and leading to the emergence of pathological high frequency oscillations (pHFO) in the cortex within weeks after FPI (Bragin et al. 2016; Reid et al. 2016). Emergence of pHFO correlates with injury severity and results in progression to spontaneous seizures indicating that pHFO could serve as an EEG biomarker for development of epilepsy after brain injury (Bragin et al. 2016). At the level of hippocampal circuits, the dentate consistently shows enhanced afferent evoked excitability within two hours to one week following brain injury while CA1 appears less excitable (Gupta et al. 2012; Hunt et al. 2009; Reeves et al. 1995; Schwarzbach et al. 2006; Titus et al. 2013; Witgen et al. 2005; Wolf et al. 2017). Increases in dentate excitability result from convergence of a more excitable glutamatergic circuit and the loss of GABAergic hilar neurons that mediate feedback inhibition (Hunt et al. 2009; Lowenstein et al. 1992; Pavlov et al. 2011; Santhakumar et al. 2000; Toth et al. 1997; Witgen et al. 2005). Although GC intrinsic firing properties are largely preserved after trauma, their synaptic inputs undergo significant reorganization (Hunt et al. 2009; Santhakumar et al. 2000). In studies conducted 5–9 days after FPI, afferent activation evokes enhanced polysynaptic glutamatergic currents in GCs with increases occurring exclusively from changes in AMPA, and not NMDA currents (Li et al. 2015; Santhakumar et al. 2000). Consistent with these data, in vitro stretch injury models have identified a potential role for enhanced calcium permeable AMPA currents in excitotoxic neuronal loss after trauma (Spaethling et al. 2008). GCs also show enhanced spontaneous and evoked bursting activity as well as functional GC synaptic interconnections mediated by sprouted mossy fiber collaterals after CCI (Hunt et al. 2009). Unlike excitability, changes in inhibition vary with injury modality and severity. Action potential independent miniature inhibitory postsynaptic currents (mIPSCs) in GCs are consistently decreased across animal models reflecting the loss of hilar GABAergic neurons (Gupta et al. 2012; Hunt et al. 2011; Pavlov et al. 2011; Toth et al. 1997; Witgen et al. 2005). However, spontaneous IPSC frequency increases within a week after mild to moderate FPI reflecting a move towards homeostatic plasticity (Gupta et al. 2012; Santhakumar et al. 2001) while it remains depressed following severe and penetrating injuries (Hunt et al. 2011; Pavlov et al. 2011). Similarly, extrasynaptic, tonic GABA currents in GCs are enhanced a week after FPI but not at later time points, and are persistently reduced after CCI (Boychuk et al. 2016; Gupta et al. 2012; Pavlov et al. 2011). In addition, there is a depolarizing shift in GC GABA reversal potential one week after brain injury (Bonislawski et al. 2007) which could further compromise the efficacy of both synaptic and tonic inhibition and impact brain rhythms (Proddutur and Santhakumar 2015).
There is limited data on the effect of trauma on functional properties of neurons other than GCs. Hilar glutamatergic mossy cells, which have expansive septo-temporal projections to GCs, are lost in significant numbers after trauma and in epilepsy. Although their intrinsic excitability is unchanged, surviving mossy cells show altered intrinsic currents, depolarized membrane potential and enhanced spontaneous and evoked glutamatergic inputs one week after FPI (Howard et al. 2007; Li et al. 2015; Santhakumar et al. 2000). Semilunar granule cells, a morphologically and functionally distinct excitatory cell type with CA3 projections, show reduced synaptic and tonic GABAergic inhibition and are the only excitatory neuron with increased excitability after FPI (Gupta et al. 2012). Like mossy cells, the intrinsic physiology of CA1 Pyramidal cells is largely unchanged after brain injury (Cao et al. 2006). However, CA1 Pyramidal cells show reduced glutamate currents and long term potentiation and enhanced inhibition after TBI (Johnson et al. 2014; Schwarzbach et al. 2006; Titus et al. 2013; Witgen et al. 2005). A subset of inhibitory neurons in the granule cell layer survive the initial injury, are depolarized and fire at higher rates for hours to days after moderate FPI (Ross and Soltesz 2000). Similarly, hilar GABAergic neurons receive more glutamatergic synaptic inputs and have increased spontaneous firing a week after CCI (Hunt et al. 2011). Together, the early cellular and synaptic changes after TBI likely drive increases in dentate excitability and promote epileptogenesis (Fig. 1A).
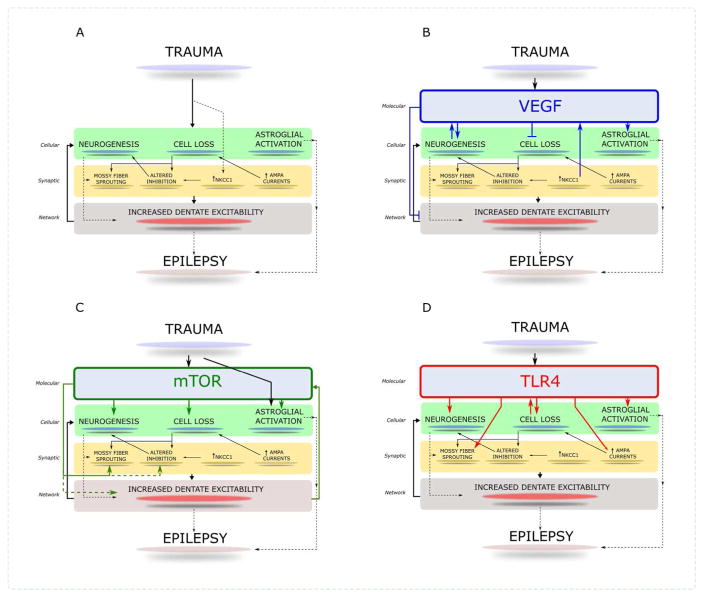
Figure 1. Schematic of converging molecular and cellular processes initiated early after TBI.
A. The schematic illustrates the major cellular and synaptic alterations in the dentate gyrus initiated within days to weeks after brain injury. The convergence of the processes on early dentate excitability and long term epileptogenesis is indicated. B. Activation and influence of VEGF receptor signaling on early posttraumatic pathology. Illustration of proposed roles for VEGF signaling in modifying cellular pathology and excitability after brain injury. C. The schematic indicates how the cellular and signaling processes illustrated in panel A could be influenced by activation of mTOR signaling after brain injury. D. Illustration of known roles for TLR4 signaling in modifying cellular pathology and excitability after brain injury. Established pathways are indicated as solid arrows and potential interactions are denoted with dashed lines.
Neurogenesis after brain injury
The dentate gyrus is a niche region for neural stem cells which support constitutive birth of granule cells throughout postnatal life. Similar to epilepsy, dentate neurogenesis is enhanced in the first week following TBI (Dash et al. 2001; Kernie and Parent 2010; Yu et al. 2008). GCs born after injury survive and mature into adult granule cells and have been proposed to contribute to long-term recovery (Sun et al. 2007; Villasana et al. 2015). Although the basic intrinsic properties of GCs born early after CCI are not different from controls, they exhibit enhanced dendritic growth and branching, and outward migration of somata 2–4 weeks later that could compromise their role in the network (Villasana et al. 2015). In recordings from granule cells born after injury and examined four weeks after trauma miniature excitatory and inhibitory synaptic events in GCs were found to be comparable to controls (Villasana et al. 2015). This is surprising because these GCs born after injury exist alongside GCs with trauma-induced alterations in mIPSC and mEPSC frequency and in a fundamentally altered dentate network with mossy fiber sprouting, hilar interneuron and mossy cell loss (Hunt et al. 2013). Indeed, while physiological increases in neurogenesis can improve cognitive performance and addition of new neurons may be beneficial, abnormal functional integration of GCs born after experimental seizures has been proposed to promote epileptogenesis (Danzer 2012; Kernie and Parent 2010). Consistent with this proposal, we recently demonstrated that limiting early posttraumatic neurogenesis to control levels reverses long term increases in seizure susceptibility and exhaustion of neuroproliferative capacity after FPI (Neuberger et al. 2017). Similarly, rapamycin treatment has been shown to reduce both neurogenesis and epileptogenesis after CCI (Butler et al. 2015).
How do transient increases in neurogenesis after brain injury contribute to epileptogenicity? It is possible that, while neurogenesis in the normal physiological range is reparative, additional increases after TBI enhance excitability. Indeed, restoring post-injury neurogenesis to control levels reduced dentate hyperexcitability observed early after FPI (Neuberger et al. 2017). Concurrently, studies have also reported that ablating post-injury neurogenesis impairs recovery of cognitive function after TBI (Blaiss et al. 2011) demonstrating that basal neurogenesis is essential for recovery. Immature GCs are more excitable than mature GCs and show accelerated network integration after seizures (Dieni et al. 2016; Pugh et al. 2011). Thus, an increase in highly excitable and plastic newborn GCs, with accelerated network integration could contribute to the early dentate hyperexcitability after TBI. It should be noted that hilar inhibitory neurons provide early and robust inhibitory innervation of newborn GCs (Markwardt et al. 2009) and may be subject to posttraumatic loss exacerbating the excitability of the GCs born after injury. Additionally, whether GCs born after injury show abnormal basal dendrites and axonal sprouting as observed in some epilepsy models remains to be tested (Kron et al. 2010). While there are several parallels between post-injury and post-seizure neurogenesis, seizures lead to ectopic hilar localization of some newborn GCs (McCloskey et al. 2006) while hilar ectopics are rarely reported after brain injury (Neuberger et al. 2017; Yu et al. 2008). It is notable that posttraumatic increase in neurogenesis is transient and leads to an accelerated decline in neurogenesis within a month (Neuberger et al. 2017). Thus, transient excesses in neurogenesis after TBI likely converge on and reinforce posttraumatic alterations to the mature circuit and augment excitability (Fig. 1A).
Molecular regulators of post-traumatic Neurogenesis
Understanding the mechanisms underlying regulation of neurogenesis after TBI could provide ways to support recovery while preventing epilepsy. Neurovascular interactions mediated by vascular endothelial growth factor (VEGF) derived from both the vascular endothelium and neural precursors appear to be key regulators of posttraumatic neurogenesis (Jin et al. 2002; Kirby et al. 2015; Licht et al. 2016; Lu et al. 2011). In the adult hippocampus, neurogenesis and angiogenesis are closely linked processes (Fournier et al. 2012; Licht et al. 2016). Neuronal precursors proliferate in clusters around dividing vascular endothelial cells that release factors which can induce neural precursor differentiation, defining a neurovascular niche (Palmer et al. 2000). VEGF-A, a hypoxia-induced angiogenic protein, has been shown to promote the survival and integration of newborn neurons by stimulating production of endothelial cells which release neurotropic factors (Louissaint et al. 2002). Although angiogenesis may be required to sustain enhancements in neurogenesis (Licht et al. 2016). VEGF augments neurogenesis in vitro (Jin et al. 2002) suggesting that VEGF may acutely enhance neurogenesis by direct effects on neural precursor cells even in the absence of angiogenesis. Among the multiple VEGF receptor subtypes, VEGF receptor type 2 (VEGFR2) is exclusively expressed in neural stem and progenitor cells and could mediate the direct effects of VEGF on neurogenesis (Kirby et al. 2015). Additionally, expression of the chloride transporter NKCC1 is upregulated within a week after brain injury and contributes to brain edema. This has been shown to trigger VEGF expression through the Raf-MEK-ERK signaling pathway and increase neurogenesis (Fournier et al. 2012). Consistent with the NKCC1-VEGFR2 regulation of neurogenesis, Bumetanide, an NKCC1 antagonist, SU1498, a VEGFR2 antagonist, and MAPK inhibitors all reduced increases in neurogenesis when administered immediately after TBI (Fournier et al. 2012; Lu et al. 2011; Neuberger et al. 2017). Posttraumatic increases in NKCC1 coupled with decreases in KCC2, a chloride extruder, could contribute to the depolarized chloride/GABA current reversal potential reported one week after TBI (Bonislawski et al. 2007; Fournier et al. 2012). In this regard, GABA currents have also been shown to modulate neural stem cell proliferation (Song et al. 2012). Thus, post-injury changes in GABA currents and chloride transporters could simultaneously compromise inhibition and enhance generation of excitable newborn GCs. Interestingly, VEGF acutely reduces neuronal excitability (Ma et al. 2009) and prolonged antagonism of VEGFR2 promotes excitotoxic hilar neuronal loss after TBI (Lee and Agoston 2009). In contrast, brief SU1498 treatment after FPI reduces both neurogenesis and dentate hyperexcitability without altering excitability in uninjured controls (Neuberger et al. 2017). Additionally, VEGF supports neuroinflammation by promoting blood brain barrier permeability (Abdul-Muneer et al. 2015). Taken together, VEGF signaling has complex effects on the cellular and physiological processes (Fig. 1B) which, acting together, influence dentate excitability. Temporally regulated early antagonism of VEGFR2 signaling after TBI offers the unique ability to suppress neurogenesis, network excitability and inflammation and thereby preempt epileptogenesis.
Another pathway at the crossroads of trauma, excitability, neurogenesis and epileptogenesis is the mammalian target of rapamycin (mTOR) pathway (Chen et al. 2007; Park et al. 2012; van Vliet et al. 2012). mTOR is an evolutionarily conserved serine threonine protein kinase which forms the catalytic core of two functionally distinct complexes mTORC1 and mTORC2. At the cellular level, mTOR complexes control several aspects of metabolism, survival, proliferation and differentiation and play a crucial role in a number of diseases (Bhaskar and Hay 2007). mTOR is activated primarily through PI3K/Akt pathways but can also be modulated through the highly interconnected Raf/MEK/ERK pathway (Saini et al. 2013). Activating mTOR signaling enhances neurogenesis and reverses age-related decline in neurogenesis, although overstimulation reduces proliferative capacity (Tee et al. 2016). mTOR phosphorylation is transiently enhanced within minutes after TBI and recovers to control levels by three days (Chen et al. 2007; Nikolaeva et al. 2016). Suppressing mTOR signaling after TBI reduces cell death, astrogliosis, mossy fiber sprouting and neurogenesis (Butler et al. 2015; Nikolaeva et al. 2016). mTOR inhibitors also limit post-TBI changes in synaptic and tonic GABA currents (Butler et al. 2016). Although whether mTOR drives or is regulated by network excitability remains to be resolved (Santos et al. 2017; Shima et al. 2015), mTOR signaling does converge on structural and functional plasticity in experimental epilepsy. Interaction of the mTOR pathway with multiple aspects of early posttraumatic alterations (Fig. 1C) makes it a promising target to prevent epileptogenesis.
Neuroimmune Interactions in Brain Injury
Brain injury, regardless of injury type, leads to an increase in permeability of the blood brain barrier and early inflammatory responses (Abdul-Muneer et al. 2013; Schmidt and Grady 1993). The resident microglia are typically the first to respond within minutes following a focal brain injury and are recruited to the injury site where they undergo morphological and functional transformation and contribute to release of cytokines (reviewed in Jassam et al. 2017). There is also an early increase in reactive astrocytes within 24 hours after injury and recruitment of peripheral immune cells (Chiu et al. 2016; Li et al. 2015). The cellular inflammatory response is accompanied by activation of innate immune receptors in response to damage-associated molecular patterns (DAMPs) released by injured and dying cells. HMGB1, a prototypic DAMP, is increased after seizures and contributes to epileptogenesis through activation of the innate immune receptor Toll-like receptor 4 (TLR4) (Maroso et al. 2010). HMGB1-TLR4 signaling is increased after TBI and augments production of pro-inflammatory cytokines (Ahmad et al. 2013; Laird et al. 2014). We recently demonstrated that the transient increase in TLR4 in the dentate gyrus occurring four hours to three days after FPI is neuronal and contributes to enhanced GC AMPA currents (Li et al. 2015). Notably, TLR4 antagonists reversed the early posttraumatic dentate hyperexcitability in ex vivo slices suggesting a role for TLR4 signaling in excitability after brain injury (Li et al. 2015). TLR4 is also expressed in neural progenitor cells and regulates neurogenesis (Rolls et al. 2007). Recent studies have identified a strong temporal correlation between TLR4 expression and enhanced neurogenesis after CCI (Ye et al. 2014). Additionally, TLR4 suppression decreases mossy fiber sprouting in experimental models of epilepsy (Sedaghat et al. 2017). Thus, TLR4 signaling converges on neuroinflammation, neuronal excitability, neuroproliferation and mossy fiber sprouting after TBI (Fig. 1D) and is a potential target to prevent epileptogenesis.
TLR4, VEGF and mTOR are far from independent signaling pathways. mTOR signaling has been shown to enhance TLR4 expression (Yu et al. 2011; Yu et al. 2016) while TLR4 was found to activate mTOR through Akt phosphorylation (Fang et al. 2017). VEGF acting through VEGFR2 converges on the PI3K/Akt pathway to increase mTOR signaling while mTOR activation enhances VEGF transcription (Liu et al. 2017). Moreover, studies in non-neuronal tissues have reported a causal association between TLR4 activation and increases in VEGF expression (Botero et al. 2006; Cho et al. 2013). These studies highlight the highly interconnected molecular signaling between VEGF, mTOR and TLR4 pathways and their convergence on various aspects of post-traumatic cellular and synaptic responses.
Interacting mechanisms reinforce pathological plasticity and offer therapeutic targets
We have focused on a subset of cellular, synaptic and signaling mechanisms activated within hours to a week after brain injury, which we propose, set the stage for development of spontaneous epileptic activity. Increase in inhibition of glutamatergic neurons (Gupta et al. 2012; Howard et al. 2007) and excitation of GABAergic neurons (Hunt et al. 2011) may represent attempts to restore homeostasis, However, mutually reinforcing maladaptive alterations after TBI could augment dentate excitability (Fig. 1). The molecular pathways activated after injury regulate critical cellular and synaptic responses to injury and many of these processes enhance excitotoxicity, cell death, neurogenesis and sprouting which, in turn, augment dentate excitability. The intertwined molecular pathways offer novel targets to limit early cellular changes and dentate hyperexcitability and could prevent development of epilepsy. In this regard, it is promising that suppressing either mTOR or VEGF signaling is sufficient to reverse the lowered seizure threshold after brain injury (Butler et al. 2015; Neuberger et al. 2017). Whether TLR4 antagonism or combinational therapy transiently suppressing mTOR, VEGF and TLR4 signaling within days after brain injury could reduce the reinforcing maladaptive molecular and cellular interactions and prevent epileptogenesis remains to be determined.
Conclusion
The studies reviewed here suggest a strong reinforcing association between the molecular pathways which regulate cell loss, synaptic plasticity and neurogenesis. The converging influence of these pathways on cellular and synaptic processes contribute to early increases in dentate excitability following TBI and lead to the development of epilepsy. While mechanistic studies generally focus on a specific cellular or molecular pathway, the genetic and pharmacological manipulations adopted in experimental analyses inevitably modulate multiple interacting pathways which act in concert to promote or limit the pathologies associated with TBI. The new-found convergence of multiple molecular pathways contributing to early TBI-induced pathologies may present an opportunity to target a combination of mechanisms to prevent post-injury epileptogenesis
Significance Statement.
Brain injury is a leading risk factor for acquired epilepsies which are refractory to treatments emphasizing the need for better mechanistic understanding and preventive strategies. The review discusses the reinforcing associations between molecular pathways which regulate cell loss, synaptic plasticity and neurogenesis after brain injury and explores the potential for modulating specific pathways early after brain injury to prevent progression to epilepsy.
Acknowledgments
Grant Support: The project was supported by NIH R01NS097750 and R01NS069861 and NJCBIR CBIR16IRG017, NJCBIR CBIR14RG024 and CURE Foundation CF 259051 to V.S.
Footnotes
Author Contributions: E.J.N., A.G., A.K. authored sections of the manuscript and reviewed relevant literature; D.S. prepared figures; E.J.N., A.G., D.S., A.K and VS edited and approved the manuscript. V.S: conception and scope of review.
References
- Abdul-Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2015;51(3):966–979. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson PD, Dixon CE, Robichaud P, Kochanek PM. Motor and cognitive functional deficits following diffuse traumatic brain injury in the immature rat. J Neurotrauma. 1997;14(2):99–108. doi: 10.1089/neu.1997.14.99. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Crupi R, Campolo M, Genovese T, Esposito E, Cuzzocrea S. Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS One. 2013;8(3):e57208. doi: 10.1371/journal.pone.0057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Suhett CP, Prager EM, Pidoplichko V, Figueiredo TH, Marini AM, Li Z, Eiden LE, Braga MF. Reduced GABAergic inhibition in the basolateral amygdala and the development of anxiety-like behaviors after mild traumatic brain injury. PLoS One. 2014;9(7):e102627. doi: 10.1371/journal.pone.0102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure. 2000;9(7):453–457. doi: 10.1053/seiz.2000.0458. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12(4):487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31(13):4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkvadze T, Pitkanen A. Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma. 2012;29(5):789–812. doi: 10.1089/neu.2011.1954. [DOI] [PubMed] [Google Scholar]
- Bonislawski DP, Schwarzbach EP, Cohen AS. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol Dis. 2007;25(1):163–169. doi: 10.1016/j.nbd.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero TM, Shelburne CE, Holland GR, Hanks CT, Nor JE. TLR4 mediates LPS-induced VEGF expression in odontoblasts. Journal of endodontics. 2006;32(10):951–955. doi: 10.1016/j.joen.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Boychuk JA, Butler CR, Halmos KC, Smith BN. Enduring changes in tonic GABAA receptor signaling in dentate granule cells after controlled cortical impact brain injury in mice. Exp Neurol. 2016;277:178–189. doi: 10.1016/j.expneurol.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Bragin A, Li L, Almajano J, Alvarado-Rojas C, Reid AY, Staba RJ, Engel J., Jr Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia. 2016;57(5):735–745. doi: 10.1111/epi.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. The neuropathology of temporal lobe epilepsy. New York: Oxford UP; 1988. [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(6):2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Boychuk JA, Smith BN. Effects of Rapamycin Treatment on Neurogenesis and Synaptic Reorganization in the Dentate Gyrus after Controlled Cortical Impact Injury in Mice. Frontiers in systems neuroscience. 2015;9:163. doi: 10.3389/fnsys.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Boychuk JA, Smith BN. Differential effects of rapamycin treatment on tonic and phasic GABAergic inhibition in dentate granule cells after focal brain injury in mice. Exp Neurol. 2016;280:30–40. doi: 10.1016/j.expneurol.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Hasuo H, Ooba S, Akasu T, Zhang X. Facilitation of glutamatergic synaptic transmission in hippocampal CA1 area of rats with traumatic brain injury. Neurosci Lett. 2006;401(1–2):136–141. doi: 10.1016/j.neulet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- CDC. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. Atlanta, GA: National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention; 2015. [Google Scholar]
- Chen JW, Ruff RL, Eavey R, Wasterlain CG. Posttraumatic epilepsy and treatment. Journal of rehabilitation research and development. 2009;46(6):685–696. doi: 10.1682/jrrd.2008.09.0130. [DOI] [PubMed] [Google Scholar]
- Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27(5):939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Liao YE, Yang LY, Wang JY, Tweedie D, Karnati HK, Greig NH, Wang JY. Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods. 2016;272:38–49. doi: 10.1016/j.jneumeth.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Kang JH, Han IH, Um JY, Lee HM. Activation of TLR4 induces VEGF expression via Akt pathway in nasal polyps. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2013;43(9):1038–1047. doi: 10.1111/cea.12165. [DOI] [PubMed] [Google Scholar]
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373(9669):1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Harris JP, Browne KD, Wolf JA, Duda JE, Meaney DF, Margulies SS, Smith DH. A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration. Methods in molecular biology. 2016;1462:289–324. doi: 10.1007/978-1-4939-3816-2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Levitt M, Fender JS, Miller JW, Ojemann J, D’Ambrosio R. Impact of injury location and severity on posttraumatic epilepsy in the rat: role of frontal neocortex. Cereb Cortex. 2011;21(7):1574–1592. doi: 10.1093/cercor/bhq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127(Pt 2):304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128(Pt 1):174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC. Depression, stress, epilepsy and adult neurogenesis. Exp Neurol. 2012;233(1):22–32. doi: 10.1016/j.expneurol.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63(4):313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dieni CV, Panichi R, Aimone JB, Kuo CT, Wadiche JI, Overstreet-Wadiche L. Low excitatory innervation balances high intrinsic excitability of immature dentate neurons. Nat Commun. 2016;7:11313. doi: 10.1038/ncomms11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39(3):253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. JNeurosurg. 1987;67(1):110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Eslami M, Ghanbari E, Sayyah M, Etemadi F, Choopani S, Soleimani M, Amiri Z, Hadjighassem M. Traumatic brain injury accelerates kindling epileptogenesis in rats. Neurol Res. 2016;38(3):269–274. doi: 10.1179/1743132815Y.0000000086. [DOI] [PubMed] [Google Scholar]
- Fang W, Bi D, Zheng R, Cai N, Xu H, Zhou R, Lu J, Wan M, Xu X. Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264.7 macrophages. Sci Rep. 2017;7(1):1663. doi: 10.1038/s41598-017-01868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson PL, Smith GM, Wannamaker BB, Thurman DJ, Pickelsimer EE, Selassie AW. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia. 2010;51(5):891–898. doi: 10.1111/j.1528-1167.2009.02384.x. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology. 2012;63(4):642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Deng P, Xu ZC, Chen J. Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS One. 2011;6(9):e24566. doi: 10.1371/journal.pone.0024566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Elgammal FS, Proddutur A, Shah S, Santhakumar V. Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain injury. J Neurosci. 2012;32(7):2523–2537. doi: 10.1523/JNEUROSCI.4141-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK, Sayed N, Ding K, Agostini MA, Van Ness PC, Yablon S, Madden C, Mickey B, D’Ambrosio R, Diaz-Arrastia R. Subtypes of post-traumatic epilepsy: clinical, electrophysiological, and imaging features. J Neurotrauma. 2014;31(16):1439–1443. doi: 10.1089/neu.2013.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy ResSuppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Howard AL, Neu A, Morgan RJ, Echegoyen JC, Soltesz I. Opposing modifications in intrinsic currents and synaptic inputs in post-traumatic mossy cells: evidence for single-cell homeostasis in a hyperexcitable network. JNeurophysiol. 2007;97(3):2394–2409. doi: 10.1152/jn.00509.2006. [DOI] [PubMed] [Google Scholar]
- Hunt RF, Boychuk JA, Smith BN. Neural circuit mechanisms of post-traumatic epilepsy. Front Cell Neurosci. 2013;7:89. doi: 10.3389/fncel.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Posttraumatic epilepsy after controlled cortical impact injury in mice. Experimental neurology. 2009;215(2):243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(18):6880–6890. doi: 10.1523/JNEUROSCI.0032-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron. 2017;95(6):1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27(35):9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeub M, Lie A, Blumcke I, Elger CE, Beck H. Loss of dynorphin-mediated inhibition of voltage-dependent Ca2+ currents in hippocampal granule cells isolated from epilepsy patients is associated with mossy fiber sprouting. Neuroscience. 1999;94(2):465–471. doi: 10.1016/s0306-4522(99)00249-3. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BN, Palmer CP, Bourgeois EB, Elkind JA, Putnam BJ, Cohen AS. Augmented Inhibition from Cannabinoid-Sensitive Interneurons Diminishes CA1 Output after Traumatic Brain Injury. Front Cell Neurosci. 2014;8:435. doi: 10.3389/fncel.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Meaney DF, Cullen DK, Smith DH. Animal models of traumatic brain injury. Handbook of clinical neurology. 2015;127:115–128. doi: 10.1016/B978-0-444-52892-6.00008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Miller ER, Lepsveridze E, Kharlamov EA, McHedlishvili Z. Posttraumatic seizures and epilepsy in adult rats after controlled cortical impact. Epilepsy Res. 2015;117:104–116. doi: 10.1016/j.eplepsyres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Keret A, Bennett-Back O, Rosenthal G, Gilboa T, Shweiki M, Shoshan Y, Benifla M. Posttraumatic epilepsy: long-term follow-up of children with mild traumatic brain injury. Journal of neurosurgery Pediatrics. 2017;20(1):64–70. doi: 10.3171/2017.2.PEDS16585. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140(2):685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkanen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy research. 2010a;90(1–2):47–59. doi: 10.1016/j.eplepsyres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkanen A. Posttraumatic epilepsy. Current opinion in neurology. 2010b;23(2):183–188. doi: 10.1097/WCO.0b013e32833749e4. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci U S A. 2015;112(13):4128–4133. doi: 10.1073/pnas.1422448112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs SK, Leonessa F, Ling GS. Blast TBI Models, Neuropathology, and Implications for Seizure Risk. Frontiers in neurology. 2014;5:47. doi: 10.3389/fneur.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci. 2010;30(6):2051–2059. doi: 10.1523/JNEUROSCI.5655-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, Youssef P, Yanasak N, Vender JR, Dhandapani KM. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62(1):26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Agoston DV. Inhibition of VEGF receptor 2 increased cell death of dentate hilar neurons after traumatic brain injury. Experimental neurology. 2009;220(2):400–403. doi: 10.1016/j.expneurol.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Li Y, Korgaonkar AA, Swietek B, Wang J, Elgammal FS, Elkabes S, Santhakumar V. Toll-like receptor 4 enhancement of non-NMDA synaptic currents increases dentate excitability after brain injury. Neurobiol Dis. 2015;74:240–253. doi: 10.1016/j.nbd.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Rothe G, Kreisel T, Wolf B, Benny O, Rooney AG, Ffrench-Constant C, Enikolopov G, Keshet E. VEGF preconditioning leads to stem cell remodeling and attenuates age-related decay of adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2016;113(48):E7828–E7836. doi: 10.1073/pnas.1609592113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen Z, Cheng H, Chen J, Qian J. Gremlin promotes retinal pigmentation epithelial (RPE) cell proliferation, migration and VEGF production via activating VEGFR2-Akt-mTORC2 signaling. Oncotarget. 2017;8(1):979–987. doi: 10.18632/oncotarget.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, 3rd, Stringer JL. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37(1):1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50(Suppl 2):4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12(12):4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Sun CL, Wo PY, Yen HH, Tang TH, Ng MC, Huang ML, Yang YL. Hippocampal neurogenesis after traumatic brain injury is mediated by vascular endothelial growth factor receptor-2 and the Raf/MEK/ERK cascade. J Neurotrauma. 2011;28(3):441–450. doi: 10.1089/neu.2010.1473. [DOI] [PubMed] [Google Scholar]
- Ma YY, Li KY, Wang JJ, Huang YL, Huang Y, Sun FY. Vascular endothelial growth factor acutely reduces calcium influx via inhibition of the Ca2+ channels in rat hippocampal neurons. J Neurosci Res. 2009;87(2):393–402. doi: 10.1002/jnr.21859. [DOI] [PubMed] [Google Scholar]
- Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci. 2009;29(48):15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. NatMed. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24(8):2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger EJ, Abdul-Wahab R, Jayakumar A, Pfister BJ, Santhakumar V. Distinct effect of impact rise times on immediate and early neuropathology after brain injury in juvenile rats. Journal of Neuroscience Research. 2014 doi: 10.1002/jnr.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger EJ, Swietek B, Corrubia L, Prasanna A, Santhakumar V. Enhanced Dentate Neurogenesis after Brain Injury Undermines Long-Term Neurogenic Potential and Promotes Seizure Susceptibility. Stem Cell Reports. 2017 doi: 10.1016/j.stemcr.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaeva I, Crowell B, Valenziano J, Meaney D, D’Arcangelo G. Beneficial Effects of Early mTORC1 Inhibition after Traumatic Brain Injury. J Neurotrauma. 2016;33(2):183–193. doi: 10.1089/neu.2015.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CP, Metheny HE, Elkind JA, Cohen AS. Diminished amygdala activation and behavioral threat response following traumatic brain injury. Exp Neurol. 2016;277:215–226. doi: 10.1016/j.expneurol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Park J, Zhang J, Qiu J, Zhu X, Degterev A, Lo EH, Whalen MJ. Combination therapy targeting Akt and mammalian target of rapamycin improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2012;32(2):330–340. doi: 10.1038/jcbfm.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterno R, Metheny H, Xiong G, Elkind J, Cohen AS. Mild Traumatic Brain Injury Decreases Broadband Power in Area CA1. J Neurotrauma. 2016;33(17):1645–1649. doi: 10.1089/neu.2015.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I, Huusko N, Drexel M, Kirchmair E, Sperk G, Pitkanen A, Walker MC. Progressive loss of phasic, but not tonic, GABAA receptor-mediated inhibition in dentate granule cells in a model of post-traumatic epilepsy in rats. Neuroscience. 2011;194:208–219. doi: 10.1016/j.neuroscience.2011.07.074. [DOI] [PubMed] [Google Scholar]
- Peterson TC, Maass WR, Anderson JR, Anderson GD, Hoane MR. A behavioral and histological comparison of fluid percussion injury and controlled cortical impact injury to the rat sensorimotor cortex. Behav Brain Res. 2015;294:254–263. doi: 10.1016/j.bbr.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Parada I, Scalise K, Graber K, Jin X, Shen F. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50(Suppl 2):30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proddutur A, Santhakumar V. Marching Towards a Seizure: Spatio-Temporal Evolution of Preictal Activity. Epilepsy Curr. 2015;15(5):267–268. doi: 10.5698/1535-7511-15.5.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh P, Adlaf E, Zhao CS, Markwardt S, Gavin C, Wadiche J, Overstreet-Wadiche L. Enhanced integration of newborn neurons after neonatal insults. Front Neurosci. 2011;5:45. doi: 10.3389/fnins.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves TM, Lyeth BG, Povlishock JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. ExpBrain Res. 1995;106(2):248–256. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- Reid AY, Bragin A, Giza CC, Staba RJ, Engel J., Jr The progression of electrophysiologic abnormalities during epileptogenesis after experimental traumatic brain injury. Epilepsia. 2016;57(10):1558–1567. doi: 10.1111/epi.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. NatCell Biol. 2007;9(9):1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Ross ST, Soltesz I. Selective depolarization of interneurons in the early posttraumatic dentate gyrus: involvement of the Na(+)/K(+)-ATPase. J Neurophysiol. 2000;83(5):2916–2930. doi: 10.1152/jn.2000.83.5.2916. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23(8):1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39(8):935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. JNeurophysiol. 2005;93(1):437–453. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, Soltesz I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ’irritable mossy cell’ hypothesis. J Physiol. 2000;524(Pt 1):117–134. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth K, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. AnnNeurol. 2001;50(6):708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Santos VR, Pun RYK, Arafa SR, LaSarge CL, Rowley S, Khademi S, Bouley T, Holland KD, Garcia-Cairasco N, Danzer SC. PTEN deletion increases hippocampal granule cell excitability in male and female mice. Neurobiol Dis. 2017 doi: 10.1016/j.nbd.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RH, Grady MS. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotrauma. 1993;10(4):415–430. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16(6):541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat R, Taab Y, Kiasalari Z, Afshin-Majd S, Baluchnejadmojarad T, Roghani M. Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: Underlying mechanisms. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;87:200–208. doi: 10.1016/j.biopha.2016.12.109. [DOI] [PubMed] [Google Scholar]
- Semple BD, O’Brien TJ, Gimlin K, Wright DK, Kim SE, Casillas-Espinosa PM, Webster KM, Petrou S, Noble-Haeusslein LJ. Interleukin-1 Receptor in Seizure Susceptibility after Traumatic Injury to the Pediatric Brain. J Neurosci. 2017;37(33):7864–7877. doi: 10.1523/JNEUROSCI.0982-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima A, Nitta N, Suzuki F, Laharie AM, Nozaki K, Depaulis A. Activation of mTOR signaling pathway is secondary to neuronal excitability in a mouse model of mesio-temporal lobe epilepsy. Eur J Neurosci. 2015;41(7):976–988. doi: 10.1111/ejn.12835. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Xiong G, Elkind JA, Putnam B, Cohen AS. Brain Injury Impairs Working Memory and Prefrontal Circuit Function. Frontiers in neurology. 2015;6:240. doi: 10.3389/fneur.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, Deisseroth K, Luscher B, Christian KM, Ming GL, Song H. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489(7414):150–154. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethling JM, Klein DM, Singh P, Meaney DF. Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J Neurotrauma. 2008;25(10):1207–1216. doi: 10.1089/neu.2008.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204(1):264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Sundaramurthy A, Alai A, Ganpule S, Holmberg A, Plougonven E, Chandra N. Blast-induced biomechanical loading of the rat: an experimental and anatomically accurate computational blast injury model. J Neurotrauma. 2012;29(13):2352–2364. doi: 10.1089/neu.2012.2413. [DOI] [PubMed] [Google Scholar]
- Tee AR, Sampson JR, Pal DK, Bateman JM. The role of mTOR signalling in neurogenesis, insights from tuberous sclerosis complex. Semin Cell Dev Biol. 2016;52:12–20. doi: 10.1016/j.semcdb.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus DJ, Furones C, Kang Y, Atkins CM. Age-dependent alterations in cAMP signaling contribute to synaptic plasticity deficits following traumatic brain injury. Neuroscience. 2013;231:182–194. doi: 10.1016/j.neuroscience.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci. 1997;17(21):8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Burger JC, Sinjewel A, de Vries HE, Aronica E, Gorter JA. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia. 2012;53(7):1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- Villasana LE, Kim KN, Westbrook GL, Schnell E. Functional Integration of Adult-Born Hippocampal Neurons after Traumatic Brain Injury(1,2,3) eNeuro. 2015;2(5) doi: 10.1523/ENEURO.0056-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience. 2005;133(1):1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Johnson BN, Johnson VE, Putt ME, Browne KD, Mietus CJ, Brown DP, Wofford KL, Smith DH, Grady MS, Cohen AS, Cullen DK. Concussion Induces Hippocampal Circuitry Disruption in Swine. J Neurotrauma. 2017;34(14):2303–2314. doi: 10.1089/neu.2016.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Xu H, Zhang X, Li Z, Jia Y, He X, Huang JH. Association between toll-like receptor 4 expression and neural stem cell proliferation in the hippocampus following traumatic brain injury in mice. International journal of molecular sciences. 2014;15(7):12651–12664. doi: 10.3390/ijms150712651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CC, Chen TL, Hu CJ, Chiu WT, Liao CC. Risk of epilepsy after traumatic brain injury: a retrospective population-based cohort study. Journal of neurology, neurosurgery, and psychiatry. 2013;84(4):441–445. doi: 10.1136/jnnp-2012-302547. [DOI] [PubMed] [Google Scholar]
- Yu M, Kang X, Xue H, Yin H. Toll-like receptor 4 is up-regulated by mTOR activation during THP-1 macrophage foam cells formation. Acta Biochim Biophys Sin (Shanghai) 2011;43(12):940–947. doi: 10.1093/abbs/gmr093. [DOI] [PubMed] [Google Scholar]
- Yu R, Bo H, Villani V, Spencer PJ, Fu P. The Inhibitory Effect of Rapamycin on Toll Like Receptor 4 and Interleukin 17 in the Early Stage of Rat Diabetic Nephropathy. Kidney Blood Press Res. 2016;41(1):55–69. doi: 10.1159/000368547. [DOI] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. JNeurosci. 2008;28(48):12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]