Abstract
Background
Soluble fibrin monomer complex (SFMC) is a biomarker of fibrin formation abnormally elevated in clinical situations of hypercoagulability.
Objective
We investigated the association and predictive performance of SFMC for stroke, adverse cardiovascular events, cardiovascular mortality and all-cause mortality in a cohort of patients with atrial fibrillation (AF) receiving vitamin K antagonist (VKA) anticoagulant therapy.
Design
During the second semester of 2007, we included 1226 AF outpatients stable on VKAs (INR 2.0–3.0) over a period of 6 months. SFMC levels were assessed at baseline. During 6.5 (IQR 4.4–8.0) years of follow-up, we recorded all ischemic strokes, adverse cardiovascular events (composite of stroke, acute heart failure, acute coronary syndrome and cardiovascular death), cardiovascular deaths and all-cause deaths.
Participants
All patients were recruited consecutively. We excluded patients with rheumatic mitral valves, prosthetic heart valves, acute coronary syndrome, stroke, hemodynamic instability, hospital admissions or surgical interventions within the preceding 6 months.
Main Measures
SFMC levels were measured in plasma by immunoturbidimetry in an automated coagulometer (STALiatestFM, Diagnostica Stago, Asnieres, France).
Key Results
We recorded 121 (1.52%/year) ischemic strokes, 257 (3.23%/year) cardiovascular events, 67 (0.84%/year) cardiovascular deaths and 486 (6.10%/year) all-cause deaths. SFMC >12 μg/mL was not associated with stroke but was associated with higher risk of cardiovascular events (HR 1.72, 95% CI 1.31–2.26), cardiovascular mortality (HR 2.16, 95% CI 1.30–3.57) and all-cause mortality (HR 1.26, 95% CI 1.03–1.55). When SFMC >12 μg/mL was added to the CHA2DS2-VASc, there were significant improvements in predictive performance, sensitivity and reclassification for adverse cardiovascular events (c-index: 0.645 vs. 0.660, p = 0.010; IDI = 0.013, p < 0.001; NRI = 0.121, p < 0.001) and cardiovascular mortality (c-index: 0.661 vs. 0.691, p = 0.006; IDI = 0.009, p = 0.049; NRI = 0.217, p < 0.001), but decision curves demonstrated a similar net benefit and clinical usefulness.
Conclusions
In AF patients taking VKAs, high SFMC levels were associated with the risk of adverse cardiovascular events, cardiovascular mortality and all-cause mortality. The addition of SFMC to the CHA2DS2-VASc score improved its predictive performance for these outcomes, but failed to show an improvement in clinical usefulness.
KEY WORDS: atrial fibrillation, anticoagulants, soluble fibrin monomer complex, biomarkers, thrombosis, mortality
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is characterized by a prothrombotic or hypercoagulable state.1,2 This state of hypercoagulability during AF causes alterations in hemostasis, with pro-fibrotic and pro-inflammatory responses in fibroblasts, and various alterations in inflammatory mediators and growth factors.3–5
Clinically, AF increases the risk of stroke and all-cause mortality,6,7 and the use of biomarkers as risk markers for stroke and bleeding in patients with AF treated with anticoagulants is an issue of continued debate. Several studies in recent years have demonstrated that AF also increases the risk of non-stroke cardiovascular events, including myocardial infarction (MI), heart failure and cardiovascular death.8–11
The soluble fibrin monomer complex (SFMC) is a biomarker of fibrin formation, and has been shown to be abnormally elevated in various clinical situations of hypercoagulability, such as deep vein thrombosis (DVT) or disseminated intravascular coagulation (DIC).12,13 However, the association between SFMC and cardiovascular events in patients with AF remains uncertain.
In the present study, we investigated the association between SFMC and ischemic stroke, adverse cardiovascular events, cardiovascular mortality and all-cause mortality in a “real-world” cohort of patients with AF treated with vitamin K antagonist (VKA) anticoagulants. Second, we investigated whether the predictive ability of the CHA2DS2-VASc score could be improved by the addition of SFMC as a biomarker.
METHODS
We included consecutive outpatients with paroxysmal, persistent or permanent AF from our anticoagulation clinic in a tertiary hospital in Murcia (southeast Spain). From May 1, 2007, to December 1, 2007, we enrolled all patients stable on VKAs (INR 2.0–3.0) for at least the previous 6 months, to ensure baseline homogeneity of the included cohort. This 6-months period of good anticoagulation control would ensure that the impact of the SFMC was not related to poor anticoagulation control, enabling us to investigate the “real” effect of this biomarker. Also, to avoid any possible fluctuation in SFMC due to an acute cardiovascular condition, patients who experienced acute coronary syndrome (ACS), stroke, hemodynamic instability, hospital admission or surgical intervention in the preceding 6 months were excluded. Patients with rheumatic mitral valves or prosthetic heart valves were also excluded.
The time in therapeutic range (TTR) was calculated at 6 months after entry, and stroke risk and bleeding risk were assessed using the CHA2DS2-VASc and HAS-BLED scores.14,15
Study Outcomes
The primary endpoints of this study were (i) ischemic stroke, (ii) the composite of adverse cardiovascular events including ischemic stroke, acute heart failure, ACS and cardiovascular death, (iii) cardiovascular mortality, and (iv) all-cause mortality. Ischemic stroke was defined as the sudden onset of a focal neurological deficit in a location consistent with the territory of a major cerebral artery resulting from an obstruction documented by imaging, surgery or autopsy. Acute heart failure was defined as a gradual or rapid change in the signs and symptoms of heart failure, resulting in a need for urgent therapy, whereas cardiovascular death was defined as sudden death, death caused by progressive congestive heart failure or fatal MI, or procedure-related death.
The investigators identified, confirmed and recorded all adverse events, and performed follow-up by personal interview at each visit to the anticoagulation clinic and through medical records. No patient was lost to follow-up.
The study protocol fulfilled the ethical standards laid down in the 1964 Declaration of Helsinki and was approved by the ethics committee of the University Hospital Morales Meseguer. All patients gave informed consent to participation in the study.
Blood Samples and Laboratory Analysis
Blood samples were drawn at baseline in an atraumatic manner and without stasis into syringes pre-loaded with trisodium citrate (0.011 M). Platelet-poor plasma fractions were obtained by centrifugation at 4 °C for 20 min at 2200×g. Aliquots were stored at −80 °C to allow batch analysis.
SFMC levels were determined by immunoturbidimetry in an automated coagulometer (STA-Liatest FM, Diagnostica Stago, Asnieres, France). The inter- and intra-assay variation coefficient was <3%, and the lower limit of detection was 0.03 μg/mL.
Statistical Analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test, and are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate. Categorical variables are expressed as absolute frequencies and percentages.
The Pearson chi-square test was used to compare proportions. Correlation between SFMC levels and TTR, CHA2DS2-VASc and HAS-BLED scores was assessed using Spearman’s rho, while differences in SFMC levels among patients who suffered an adverse event were assessed using the Mann–Whitney U test.
Receiver operating characteristic (ROC) curves were used to investigate predictive ability. Comparison of ROC curves was carried out using the method described by DeLong et al.16 The Youden index was used to determine the SFMC level with the best combination of sensitivity and specificity in order to establish a cut-off value.
Multivariate Cox proportional hazards regression models were used to determine the independent association between SFMC and primary endpoints. Only variables which showed a p value <0.15 in univariate analyses were included in the multivariate analyses. Differences in event-free survival were reflected by Kaplan–Meier curves.
The increment of the predictive performance was tested using the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI) measures, according to the methods described by Pencina et al.17 The clinical usefulness and net benefit of the original CHA2DS2-VASc and the modified CHA2DS2-VASc (after the addition of SFMC) was estimated using decision curve analyses (DCAs).18,19
A p value of <0.05 was accepted as statistically significant. Statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA), MedCalc version 16.4.3 (MedCalc Software bvba, Ostend, Belgium) STATA 12 (StataCorp LP, College Station, TX, USA) and the survIDINRI package for R 3.3.1 for Windows.
RESULTS
We included 1226 patients (49.7% male) with a median age of 76 (IQR 71–81) years. At entry, the median CHA2DS2-VASc was 4 (IQR 3–5), and the median HAS-BLED was 2 (IQR 2–3). The median baseline SFMC level was 4.9 (IQR 3.2–9.3) μg/mL. A summary of other clinical characteristic is shown in Table 1.
Table 1.
Baseline Clinical Characteristics of Patients with Atrial Fibrillation
| N = 1226 | |
|---|---|
| Demographics | |
| Male sex, no. (%) | 609 (49.7) |
| Age (years), median (IQR) | 76 (70–81) |
| Comorbidities, no. (%) | |
| Hypertension | 1002 (81.7) |
| Diabetes mellitus | 320 (26.1) |
| Heart failure | 391 (31.9) |
| History of stroke/TIA/TE | 232 (18.9) |
| Coronary artery disease | 229 (18.7) |
| Hyperlipidemia | 399 (32.5) |
| Renal impairment | 126 (10.3) |
| Baseline tobacco use | 187 (15.3) |
| Laboratory measurements, median (IQR) | |
| Hemoglobin (g/dL) | 13.8 (12.5–14.9) |
| Creatinine clearance (mL/min/1.73m2) | 97.1 (78.2–131.1) |
| Soluble fibrin monomer complex (μg/mL) | 4.9 (3.2–9.3) |
| Concomitant treatment, no. (%) | |
| Amiodarone | 71 (5.8) |
| Digoxin | 245 (20.0) |
| Calcium antagonist | 309 (25.2) |
| Beta-blockers | 423 (34.5) |
| Diuretics | 557 (45.4) |
| ACE inhibitors/ARBs | 629 (51.3) |
| Statins | 305 (24.9) |
| Antiplatelet therapy | 221 (18.0) |
| CHA2DS2-VASc score, median (IQR) | 4 (3–5) |
| HAS-BLED score, median (IQR) | 2 (2–3) |
ACE = angiotensin-converting-enzyme; ARB = angiotensin II receptor blocker; IQR = interquartile range; TIA = transient ischemic attack; TE = thromboembolism; CHA2DS2-VASc = cardiac failure or dysfunction, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled] – vascular disease, age 65–74 years and sex category [female]; HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly
At the 6-month follow-up, the cohort still demonstrated good anticoagulation control, with a median TTR of 80% (IQR 66–100).
During 6.5 (IQR 4.4–8.0) years of follow-up, we recorded 121 (9.9%, 1.52%/year) ischemic strokes, 257 (21%, 3.23%/year) composite cardiovascular events, 67 (5.5%, 0.84%/year) cardiovascular deaths and 486 (39.6%, 6.10%/year) all-cause deaths.
In terms of SFMC levels, no correlations were found with TTR (rho = 0.032, p = 0.438), CHA2DS2-VASc (rho = 0.002, p = 0.938) or HAS-BLED (rho = 0.015, p = 0.610). There were no differences in SFMC levels in patients who suffered ischemic stroke or all-cause death; however, SFMC levels were significantly higher in patients who suffered a composite cardiovascular event (5.3 μg/mL [IQR 3.2–15.3] vs. 4.7 μg/mL [IQR 3.2–8.8], p = 0.007) or a cardiovascular death (6.2 μg/mL [IQR 3.4–28.5] vs. 4.8 μg/mL [IQR 3.2–9.1], p = 0.032).
The ROC curves confirmed that SFMC modestly predicted composite cardiovascular events (c-index = 0.55, 95% CI 0.53–0.58, p < 0.001), with a level of 12.01 μg/mL demonstrating the best combination of sensitivity and specificity. Thus, we established the cut-off value for “a high level of SFMC” as >12 μg/mL.
Multivariate Cox Regression Analyses and Predictive Performance
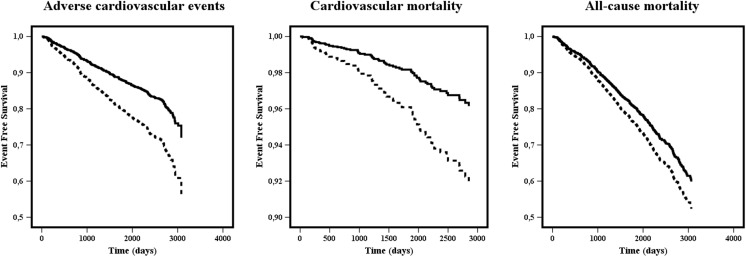
SFMC >12 μg/mL was not associated with ischemic stroke on univariate analysis (HR 1.28, 95% CI 0.83–1.96, p = 0.265), and therefore was not included in multivariate analysis. Multivariate analyses adjusted by comorbidity demonstrated that SFMC >12 μg/mL was independently associated with higher risk of composite adverse cardiovascular events (HR 1.75, 95% CI 1.34–2.30, p < 0.001), cardiovascular mortality (HR 2.16, 95% CI 1.30–3.57, p = 0.003) and all-cause mortality (HR 1.26, 95% CI 1.03–1.55, p = 0.028; Tables 2 and 3, Fig. 1).
Table 2.
Univariate Cox Regression Analyses
| Ischemic stroke | Adverse CV events | Cardiovascular mortality | All-cause mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis HR (95% CI) | p | Univariate analysis HR (95% CI) | p | Univariate analysis HR (95% CI) | p | Univariate analysis HR (95% CI) | p | |
| Age | 1.01 (1.05–1.11) | <0.001 | 1.09 (1.07–1.11) | <0.001 | 1.13 (1.09–1.17) | <0.001 | 1.11 (1.10–1.13) | <0.001 |
| Male sex | 0.77 (0.54–1.10) | 0.150 | 0.82 (0.64–1.05) | 0.113 | 0.91 (0.56–1.46) | 0.684 | 0.92 (0.77–1.10) | 0.370 |
| Hypertension | 2.18 (1.20–3.97) | 0.010 | 1.70 (1.17–2.46) | 0.005 | 1.05 (0.56–1.96) | 0.875 | 1.02 (0.81–1.28) | 0.862 |
| Diabetes mellitus | 0.83 (0.53–1.29) | 0.399 | 1.47 (1.13–1.92) | 0.004 | 2.30 (1.41–3.75) | <0.001 | 1.48 (1.22–1.79) | <0.001 |
| Previous stroke/TIA/TE | 3.42 (2.37–4.93) | <0.001 | 2.01 (1.53–2.63) | <0.001 | 1.53 (0.87–2.68) | 0.140 | 1.63 (1.33–2.00) | <0.001 |
| Heart failure | 1.00 (0.68–1.48) | 0.996 | 1.63 (1.27–2.08) | <0.001 | 3.03 (1.87–4.93) | <0.001 | 1.56 (1.30–1.87) | <0.001 |
| Coronary artery disease | 1.09 (0.69–1.72) | 0.708 | 1.61 (1.22–2.13) | 0.001 | 1.93 (1.14–3.25) | 0.014 | 1.20 (0.96–1.49) | 0.110 |
| Hyperlipidemia | 1.59 (1.05–2.42) | 0.029 | 1.00 (0.72–1.22) | 0.633 | 1.51 (0.87–2.62) | 0.143 | 1.43 (1.17–1.75) | <0.001 |
| Renal impairment | 1.17 (0.65–2.13) | 0.601 | 1.82 (1.27–2.60) | 0.001 | 2.28 (1.22–4.27) | 0.010 | 1.92 (1.50–2.46) | <0.001 |
| Baseline tobacco use | 0.81 (0.46–1.41) | 0.447 | 1.58 (1.16–2.16) | 0.004 | 2.02 (1.17–3.51) | 0.012 | 1.17 (0.92–1.49) | 0.197 |
| SMFC >12.00 μg/mL | 1.28 (0.83–1.96) | 0.265 | 1.79 (1.37–2.34) | <0.001 | 2.33 (1.41–3.84) | 0.001 | 1.35 (1.10–1.66) | 0.004 |
HR = hazard ratio; CI = confidence interval; TIA = transient ischemic attack; TE = thromboembolism
Table 3.
Multivariate Cox Regression Analyses
| Adverse cardiovascular events | Cardiovascular mortality | All-cause mortality | ||||
|---|---|---|---|---|---|---|
| Multivariate analysis HR (95% CI) | p | Multivariate analysis HR (95% CI) | p | Multivariate analysis HR (95% CI) | p | |
| Age | 1.07 (1.05–1.09) | <0.001 | 1.13 (1.08–1.17) | <0.001 | 1.10 (1.09–1.12) | <0.001 |
| Male sex | 0.80 (0.60–1.07) | 0.133 | 0.133 | |||
| Hypertension | 1.47 (1.01–2.14) | 0.046 | 0.046 | |||
| Diabetes mellitus | 1.39 (1.06–1.82) | 0.017 | 2.54 (1.54–4.20) | <0.001 | 1.63 (1.34–1.98) | <0.001 |
| Previous stroke/TIA/TE | 2.05 (1.55–2.70) | <0.001 | 1.66 (0.93–2.96) | 0.086 | 1.49 (1.21–1.84) | <0.001 |
| Heart failure | 1.39 (1.08–1.80) | 0.012 | 2.37 (1.43–3.94) | 0.001 | 1.30 (1.08–1.57) | 0.006 |
| Coronary artery disease | 1.42 (1.06–1.91) | 0.019 | 1.43 (0.81–2.52) | 0.221 | 1.13 (0.90–1.42) | 0.289 |
| Hyperlipidemia | – | 1.70 (0.95–3.04) | 0.076 | 1.36 (1.11–1.68) | 0.004 | |
| Renal impairment | 1.17 (0.81–1.69) | 0.399 | 1.24 (0.66–2.36) | 0.506 | 1.33 (1.03–1.71) | 0.030 |
| Baseline tobacco use | 1.82 (1.26–2.61) | 0.001 | 0.001 | 0.002 | – | |
| SMFC >12.00 μg/mL | 1.75 (1.34–2.30) | <0.001 | 2.16 (1.30–3.57) | 0.003 | 1.26 (1.03–1.55) | 0.028 |
Bold values are regarding to a p value of <0.05 which was accepted as statistically significant
HR = hazard ratio; CI = confidence interval; TIA = transient ischemic attack; TE = thromboembolism
Figure 1.
Kaplan–Meier curves for adverse cardiovascular events, cardiovascular mortality and all-cause mortality according to soluble fibrin monomer complex (SMFC) levels. Dashed lines = SMFC >12 μg/mL; solid lines = SMFC ≤12 μg/mL.
With regard to predictive ability, a high level of SFMC (i.e. >12 μg/mL) was a predictor of composite cardiovascular events, with a modest (but significant) c-index of 0.553 (95% CI 0.524–0.581, p < 0.001). Additionally, SFMC >12 μg/mL significantly predicted cardiovascular mortality (c-index: 0.581, 95% CI 0.553–0.609, p = 0.007) and all-cause mortality (c-index: 0.533, 95% CI 0.505–0.561, p = 0.006).
Improvement in Risk Stratification
Despite the lack of association between SFMC and ischemic stroke, we tested whether including SFMC >12 μg/mL in the CHA2DS2-VASc score would improve its predictive performance for ischemic stroke, cardiovascular events, cardiovascular mortality and all-cause mortality. Therefore, we added 1 point to the CHA2DS2-VASc score if the SFMC levels were higher than 12 μg/mL.
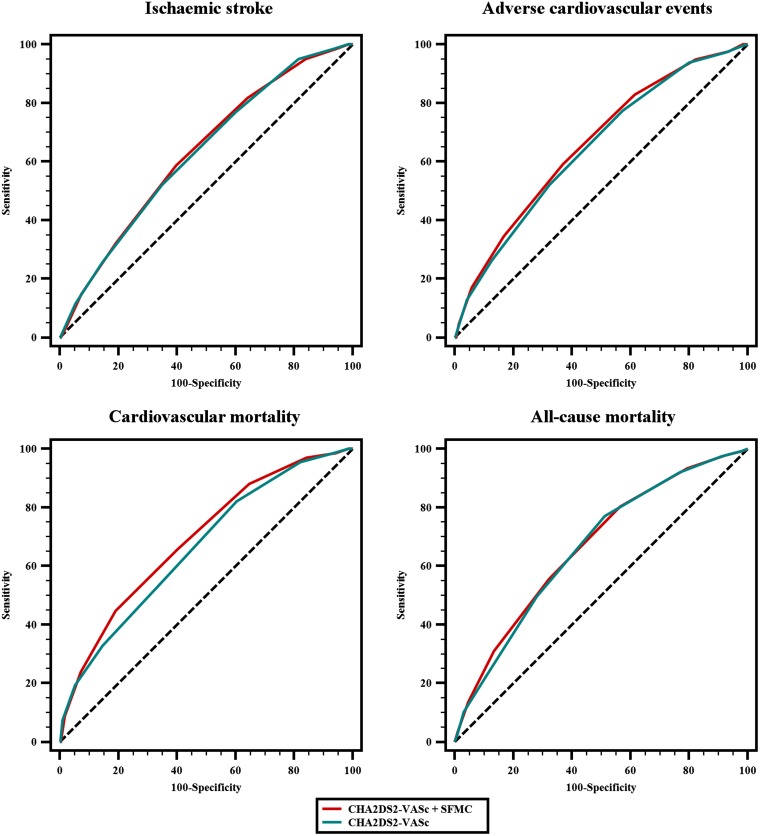
The SFMC-modified CHA2DS2-VASc showed a slight improvement in the c-index for all primary endpoints, which was significant for adverse cardiovascular events (0.645, 95% CI 0.618–0.672 vs. 0.660, 95% CI 0.632–0.686, p = 0.010) and cardiovascular mortality (0.661, 95% CI 0.634–0.688 vs. 0.691, 95% CI 0.664–0.717, p = 0.006; Table 4, Fig. 2). Additionally, the sensitivity and reclassification of CHA2DS2-VASc after the addition of SFMC for these events was increased, as assessed by the IDI (0.013, p < 0.001 for adverse cardiovascular events; 0.009, p = 0.049 for cardiovascular mortality) and NRI (0.121, p < 0.001 for adverse cardiovascular events; 0.217, p < 0.001 for cardiovascular mortality; Table 4).
Table 4.
ROC Curve Comparison, IDI and NRI Between CHA2DS2-VASc and a Modified CHA2DS2-VASc Including Soluble Fibrin Monomer Complex
| C-index | 95% CI | p * | IDI | 95% CI | p | NRI | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|---|
| (i) Ischemic stroke | |||||||||
| CHA2DS2-VASc | 0.627 | 0.599–0.654 | 0.685 | 0.002 | −0.004 / 0.010 | 0.557 | 0.060 | −0.167 / 0.157 | 0.289 |
| CHA2DS2-VASc + SFMC | 0.630 | 0.602–0.657 | |||||||
| (ii) Adverse cardiovascular events | |||||||||
| CHA2DS2-VASc | 0.645 | 0.618–0.672 | 0.010 | 0.013 | 0.004 / 0.022 | <0.001 | 0.121 | 0.050 / 0.247 | <0.001 |
| CHA2DS2-VASc + SFMC | 0.660 | 0.632–0.686 | |||||||
| (iii) Cardiovascular mortality | |||||||||
| CHA2DS2-VASc | 0.661 | 0.634–0.688 | 0.006 | 0.009 | <0.001 / 0.019 | 0.049 | 0.217 | 0.063 / 0.395 | <0.001 |
| CHA2DS2-VASc + SFMC | 0.691 | 0.664–0.717 | |||||||
| (iv) All-cause mortality | |||||||||
| CHA2DS2-VASc | 0.663 | 0.636–0.689 | 0.271 | 0.006 | 0.001 / 0.013 | 0.129 | 0.053 | 0.006 / 0.106 | 0.030 |
| CHA2DS2-VASc + SFMC | 0.667 | 0.640–0.694 | |||||||
*For c-index comparison
CI = confidence interval; IDI = integrated discrimination improvement; NRI = net reclassification improvement; SFMC = soluble fibrin monomer complex
Figure 2.
ROC curves for CHA2DS2-VASc and a modified CHA2DS2-VASc including soluble fibrin monomer complex.
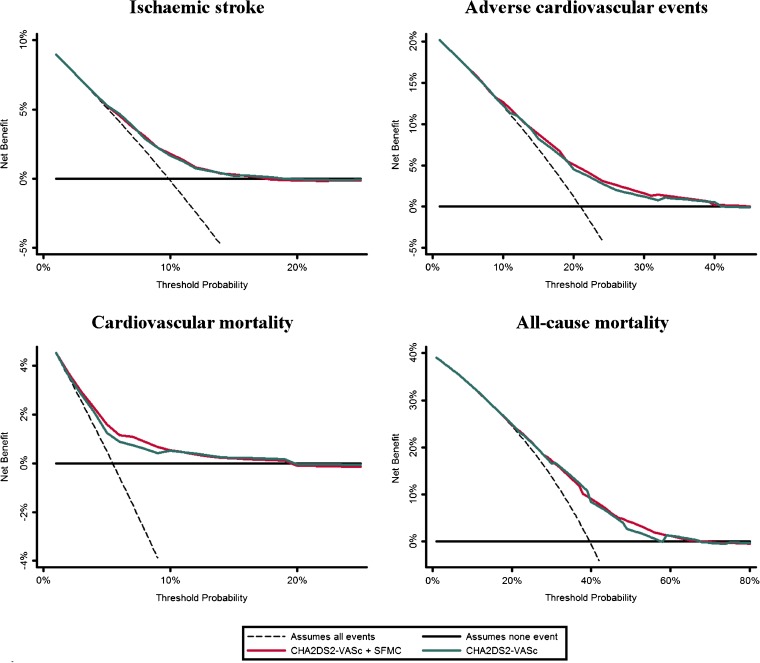
Finally, the DCAs demonstrated that the original CHA2DS2-VASc score and the SFMC-modified CHA2DS2-VASc score were broadly similar for all events, thus demonstrating a similar net benefit and a non-significant improvement in clinical usefulness (Fig. 3).
Figure 3.
Decision curve analyses for CHA2DS2-VASc and a modified CHA2DS2-VASc including soluble fibrin monomer complex.
DISCUSSION
In this study of AF patients treated with VKA anticoagulants, we found that SFMC was significantly and independently associated with adverse cardiovascular events, cardiovascular mortality, and all-cause mortality. Moreover, the addition of SFMC to the CHA2DS2-VASc score significantly improved its predictive value for adverse cardiovascular events and cardiovascular mortality, but DCA did not show an improvement in clinical usefulness.
The role of biomarkers for prognostic and risk stratification in various cardiovascular diseases has gained much interest in recent years.20 For example, sub-studies of the AF pivotal clinical trials have investigated the role of myocardial injury biomarkers such as cardiac troponins or N-terminal fragment B-type natriuretic peptide (NT-proBNP) in the prediction of adverse events.21–23 Nevertheless, the study of prothrombotic biomarkers in patients with AF, particularly those treated with oral anticoagulants or enrolled in clinical trials, is under debate, since these patients are carefully selected and followed up, whereas “real-world” AF patients tend to be older, with associated comorbidities and polypharmacy.24,25 Indeed, the performance of biomarker-based scores has been less useful in such real-world cohorts.26,27
The SFMC is produced by thrombin-mediated cleavage of fibrinogen in a hypercoagulable state, and thus could be considered as a pre-thrombotic marker.13 This biomarker is significantly elevated during the initial phase of thrombotic diseases such as MI,28,29 but it has also demonstrated utility for the diagnosis of DVT,30 and is a better indicator of DIC than the fibrin degradation product, D-dimer.12
Recent European guidelines have proposed the use of biomarkers to aid in risk stratification in AF.7 However, there are limited data on the prognostic use of SFMC as a biomarker of fibrin formation in AF. In a recent study investigating intracardiac hemostasis or fibrinolysis abnormalities in AF patients, the SFMC levels did not show significant changes compared with controls.31 In another study in patients with AF taking warfarin therapy, elevated SFMC levels during anticoagulant treatment were not useful as predictors of thromboembolic events.32
To our knowledge, this is the first large real-world study investigating the role of SFMC for risk stratification and prediction of adverse cardiovascular events and mortality in AF patients receiving VKA therapy. One of the strengths of the present study is the long, careful follow-up in an observational manner, with no loss to follow-up. Additionally, the fact that all patients who had not suffered acute cardiovascular disease in the previous 6 months were consecutively enrolled adds to the quality of our results. Nevertheless, despite the association of SFMC with cardiovascular events and mortality in AF, its addition to the CHA2DS2-VASc score did not improve clinical utility for the prediction of adverse cardiovascular events and cardiovascular mortality. Indeed, it remains unclear whether biomarkers may help refine risk assessment in AF, even when they show “statistical differences,” given that many studies are designed measuring baseline biomarkers and determining outcomes many years later, often in selected trial cohorts. In a recent study, we confirmed that von Willebrand factor (vWF) was associated with a significantly higher risk of cardiovascular events (including cardiovascular mortality), ischemic stroke and major bleeding. Even so, the inclusion of vWF in the CHA2DS2-VASc and HAS-BLED scores demonstrated clinical value similar to that of the original scores,33 as was also seen in the present study. CHA2DS2-VASc is a risk score based on clinical risk factors and characterized by its simplicity. Thus, the addition of biomarkers (often multiple) could enhance its predictive value, but at a cost of additional expense and the loss of simplicity and practicality that are so necessary in everyday clinical practice. Indeed, biomarkers are also subject to laboratory assay variability, and many are often not available in routine clinical laboratories. Therefore, the clinical usefulness and cost-effectiveness of routinely checking biomarkers in patients with AF must be investigated, given that the limited and questionable (and often marginal) improvement in risk stratification may not translate to an improvement in everyday decision-making for busy clinicians.
SFMC is a new biomarker that has not been extensively investigated in AF patients despite its clear relationship to thrombotic disorders. The present study has demonstrated that this biomarker was independently associated with higher risk of cardiovascular events, cardiovascular mortality and all-cause mortality in these patients. This is clinically relevant: while most studies investigating the complications of AF focus on the increased stroke risk associated with the disease, we now recognize that patients with AF are also at increased risk of non-stroke thrombotic events, such as ACS or heart failure.8,11,34,35 Prospective studies should be performed using real-world cohorts in order to validate our results and to clarify whether SFMC could differentiate cardiovascular risk (and not only stroke risk) assessment in AF.
Limitations
This study has some limitations to note. First, it was performed in a single center and comprised a white population. Second, all patients were clinically stable at entry; thus unstable AF patients, who are at a higher risk of adverse events, were not included. We selected patients with good anticoagulation control during the previous 6 months after entry to ensure that the impact of the biomarkers was not related to poor anticoagulation control. However, the long follow-up period under standard care makes our “real-world” population different from selected clinical trial cohorts where biomarkers have been investigated. In addition, our study was performed with patients taking VKA and not non-VKA oral anticoagulants (NOACs); thus our results cannot be translated to such a population. Our study was also performed in an outpatient anticoagulation clinic, where all patients seen were referred from other clinicians for initiation or management of anticoagulation. Therefore, we do not have data on patients not receiving anticoagulants. Indeed, most of our patients fulfilled the criteria for anticoagulation (i.e. CHA2DS2-VASc ≥2), and only 6% of our cohort had a CHA2DS2-VASc score of 0 or 1. Thus, we cannot perform analyses on patients not on anticoagulants or at very low stroke risk. Finally, the statistical analyses presented in this study were performed retrospectively, although our data set was collected in a prospective manner. Importantly, patients were carefully followed up, and all events (even very early ones) were recorded.
CONCLUSIONS
In AF patients treated with VKA anticoagulants, high SFMC levels were significantly associated with the risk of adverse cardiovascular events, cardiovascular mortality and all-cause mortality. The addition of SFMC to the CHA2DS2-VASc score improved its predictive performance for these adverse outcomes, but failed to show an improvement in clinical usefulness.
Funding
This work was supported by Instituto de Salud Carlos III (ISCIII), Fondo Europeo de Desarrollo Regional (FEDER) (research projects: PI13/00513 and P14/00253), Fundación Séneca (grant number: 19,245/PI/14) and Instituto Murciano de Investigación Biosanitaria (IMIB16/AP/01/06). José Miguel Rivera-Caravaca has received a grant from Sociedad Española de Trombosis y Hemostasia (grant for short international training stays 2016).
Compliance with Ethical Standards
Conflict of Interest
GYHL is a consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife and Daiichi-Sankyo; and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche and Daiichi-Sankyo. No fees are received personally.
All remaining authors declare that they do not have a conflict of interest.
References
- 1.Lopez-Cuenca A, Marin F, Roldan V, Gonzalez-Conejero R, Hernandez-Romero D, Valdes M, et al. Genetic polymorphisms and atrial fibrillation: Insights into the prothrombotic state and thromboembolic risk. Ann Med. 2010;42:562–575. doi: 10.3109/07853890.2010.507601. [DOI] [PubMed] [Google Scholar]
- 2.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373:155–166. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 3.Spronk HM, De Jong AM, Verheule S, De Boer HC, Maass AH, Lau DH, et al. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur Heart J. 2017;38:38–50. doi: 10.1093/eurheartj/ehw119. [DOI] [PubMed] [Google Scholar]
- 4.Mitusch R, Siemens HJ, Garbe M, Wagner T, Sheikhzadeh A, Diederich KW. Detection of a hypercoagulable state in nonvalvular atrial fibrillation and the effect of anticoagulant therapy. Thromb Haemost. 1996;75:219–223. doi: 10.1055/s-0038-1650247. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SR, Solymoss S, Flegel KM. Nonvalvular atrial fibrillation: evidence for a prothrombotic state. CMAJ. 1997;157:673–681. [PMC free article] [PubMed] [Google Scholar]
- 6.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.STR.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 8.Violi F, Soliman EZ, Pignatelli P, Pastori D. Atrial Fibrillation and Myocardial Infarction: A Systematic Review and Appraisal of Pathophysiologic Mechanisms. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed]
- 9.Polimeni L, Perri L, Saliola M, Basili S, Violi F. The risk of myocardial infarction in patients with atrial fibrillation: an unresolved issue. Intern Emerg Med. 2010;5:91–94. doi: 10.1007/s11739-010-0352-2. [DOI] [PubMed] [Google Scholar]
- 10.Goto S, Bhatt DL, Rother J, Alberts M, Hill MD, Ikeda Y, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863. doi: 10.1016/j.ahj.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Fauchier L, Villejoubert O, Clementy N, Bernard A, Pierre B, Angoulvant D, et al. Causes of deaths and influencing factors in patients with atrial fibrillation. Am J Med. 2016;129:1278–1287. doi: 10.1016/j.amjmed.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Pati HP, Tyagi S, Upadhyay AD, Saxena R. Evaluation of the Diagnostic Performance of Fibrin Monomer in Comparison to d-Dimer in Patients With Overt and Nonovert Disseminated Intravascular Coagulation. Clin Appl Thromb Hemost. 2017;23:460–465. doi: 10.1177/1076029615615959. [DOI] [PubMed] [Google Scholar]
- 13.Hou H, Ge Z, Ying P, Dai J, Shi D, Xu Z, et al. Biomarkers of deep venous thrombosis. J Thromb Thrombolysis. 2012;34:335–346. doi: 10.1007/s11239-012-0721-y. [DOI] [PubMed] [Google Scholar]
- 14.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 15.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72; discussion 207–12. [DOI] [PubMed]
- 18.Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. doi: 10.1186/1472-6947-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas MR, Lip GY. Novel Risk Markers and Risk Assessments for Cardiovascular Disease. Circ Res. 2017;120:133–149. doi: 10.1161/CIRCRESAHA.116.309955. [DOI] [PubMed] [Google Scholar]
- 21.Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, et al. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) J Am Coll Cardiol. 2013;61:2274–2284. doi: 10.1016/j.jacc.2012.11.082. [DOI] [PubMed] [Google Scholar]
- 22.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation. 2012;125:1605–1616. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]
- 23.Ruff CT, Giugliano RP, Braunwald E, Murphy SA, Brown K, Jarolim P, et al. Cardiovascular Biomarker Score and Clinical Outcomes in Patients With Atrial Fibrillation: A Subanalysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial. JAMA Cardiol. 2016;1:999–1006. doi: 10.1001/jamacardio.2016.3311. [DOI] [PubMed] [Google Scholar]
- 24.Danese E, Montagnana M, Cervellin G, Lippi G. Hypercoagulability, D-dimer and atrial fibrillation: an overview of biological and clinical evidence. Ann Med. 2014;46:364–371. doi: 10.3109/07853890.2014.912835. [DOI] [PubMed] [Google Scholar]
- 25.Freedman B, Lip GY. “Unreal world” or “real world” data in oral anticoagulant treatment of atrial fibrillation. Thromb Haemost. 2016;116:587–589. doi: 10.1160/TH16-08-0658. [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Caravaca JM, Roldán V, Esteve-Pastor MA, Valdés M, Vicente V, Lip GYH, et al. Long-Term Stroke Risk Prediction in Patients With Atrial Fibrillation: Comparison of the ABC-Stroke and CHA2DS2-VASc Scores. J Am Heart Assoc. 2017;6:e006490. doi: 10.1161/JAHA.117.006490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, Vicente V, Valdes M, Marin F, et al. Long-term bleeding risk prediction in ‘real world’ patients with atrial fibrillation: Comparison of the HAS-BLED and ABC-Bleeding risk scores. The Murcia Atrial Fibrillation Project. Thromb Haemost. 2017;117:1848–1858. doi: 10.1160/TH17-10-0710. [DOI] [PubMed] [Google Scholar]
- 28.Lee LV, Ewald GA, McKenzie CR, Eisenberg PR. The relationship of soluble fibrin and cross-linked fibrin degradation products to the clinical course of myocardial infarction. Arterioscler Thromb Vasc Biol. 1997;17:628–633. doi: 10.1161/01.ATV.17.4.628. [DOI] [PubMed] [Google Scholar]
- 29.Saigo M, Waters DD, Abe S, Biro S, Minagoe S, Maruyama I, et al. Soluble fibrin, C-reactive protein, fibrinogen, factor VII, antithrombin, proteins C and S, tissue factor, D-dimer, and prothrombin fragment 1 + 2 in men with acute myocardial infarction </=45 years of age. Am J Cardiol. 2004;94:1410–1413. doi: 10.1016/j.amjcard.2004.07.144. [DOI] [PubMed] [Google Scholar]
- 30.Dopsaj V, Bogavac-Stanojevic N, Vasic D, Vukosavljevic D, Martinovic J, Kotur-Stevuljevic J, et al. Excluding deep venous thrombosis in symptomatic outpatients: is fibrin monomer aid to D-dimer analysis? Blood Coagul Fibrinolysis. 2009;20:546–551. doi: 10.1097/MBC.0b013e32832e0605. [DOI] [PubMed] [Google Scholar]
- 31.Toth NK, Csanadi Z, Hajas O, Kiss A, Nagy-Balo E, Kovacs KB, et al. Intracardiac Hemostasis and Fibrinolysis Parameters in Patients with Atrial Fibrillation. Biomed Res Int. 2017;2017:3678017. doi: 10.1155/2017/3678017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadanaga T, Mitamura H. Soluble fibrin monomer complex levels during oral anticoagulant therapy do not predict subsequent thromboembolic events in patients with permanent atrial fibrillation. Int J Cardiol. 2013;168:578–580. doi: 10.1016/j.ijcard.2013.01.259. [DOI] [PubMed] [Google Scholar]
- 33.García-Fernández A, Roldán V, Rivera-Caravaca JM, Hernández-Romero D, Valdés M, Vicente V, et al. Does von Willebrand factor improve the predictive ability of current risk stratification scores in patients with atrial fibrillation? Sci Rep. 2017;7:41565. doi: 10.1038/srep41565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastori D, Farcomeni A, Poli D, Antonucci E, Angelico F, Del Ben M, et al. Cardiovascular risk stratification in patients with non-valvular atrial fibrillation: the 2MACE score. Intern Emerg Med. 2016;11:199–204. doi: 10.1007/s11739-015-1326-1. [DOI] [PubMed] [Google Scholar]
- 35.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]