Abstract
Non-alcoholic fatty liver disease (NAFLD) represents the most common chronic liver disease in industrialized countries. NAFLD progresses through the inflammatory phase of non-alcoholic steatohepatitis (NASH) to fibrosis and cirrhosis, with some cases developing liver failure or hepatocellular carcinoma (HCC). Liver biopsy remains the gold standard approach to a definitive diagnosis of NAFLD and the distinction between simple steatosis and NASH. The pathogenesis of NASH is still not clear. Several theories have been proposed ranging from the “Two Hit Theory” to the “Multiple Hit Theory”. However, the general consensus is that the gut microbiota, oxidative stress, and mitochondrial damage play key roles in the pathogenesis of NASH. The interaction between the gut epithelia and some commensal bacteria induces the rapid generation of reactive oxygen species (ROS). The main goal of any therapy addressing NASH is to reverse or prevent progression to liver fibrosis/cirrhosis. This problem represents the first “Achilles’ heel” of the new molecules being evaluated in most ongoing clinical trials. The second is the inability of these molecules to reach the mitochondria, the primary sites of energy production and ROS generation. Recently, a variety of non-pharmacological and pharmacological treatment approaches for NASH have been evaluated including vitamin E, the thiazolidinediones, and novel molecules related to NASH pathogenesis (including obeticholic acid and elafibranor). Recently, a new isoform of human manganese superoxide dismutase (MnSOD) was isolated and obtained in a synthetic recombinant form designated rMnSOD. This protein has been shown to be a powerful antioxidant capable of mediating ROS dismutation, penetrating biological barriers via its uncleaved leader peptide, and reducing portal hypertension and fibrosis in rats affected by liver cirrhosis. Based on these distinctive characteristics, it can be hypothesized that this novel recombinant protein (rMnSOD) potentially represents a new and highly efficient adjuvant therapy to counteract the progression from NASH to HCC.
Abbreviations: ATP, Adenosine 5c-triphosphate; ALT, Alanine aminotransferase; aa, Amino acid; ASK1, Apoptosis signal- regulating kinase 1; AST, Aspartate aminotransferase; BASH, Both alcoholic and non-alcoholic liver disease; CCR2/CCR5, C-C chemokine receptor types 2 and 5; CVC, Cenicriviroc; CASH, Chemotherapy-associated steatohepatitis; JNK, c-Jun N-terminal kinase; CD14, Cluster of differentiation 14; PGC-1α, Coactivator peroxisome proliferator-activated receptor-γ-1α; CS + WR, Cold storage and warm reperfusion; Cu/ZnSOD, Copper/zinc superoxide dismutase; DPP-4 inhibitor, Dipeptidyl peptidase 4 inhibitor; DASH, Drug-associated steatohepatitis; ER, Estrogen receptor; ecSOD, Extracellular Cu/ZnSOD; FXR, Farnesoid X receptor; FIAF, Fasting-induced adipose factor; FDA, Food and drug administration; FGF19, Fibroblast growth factor 19; FGF21, Fibroblast growth factor 21; FADH, Flavin adenine dinucleotide; FFA, Free fatty acids; γgt, Gamma-glutamyl transferase; GF, Germ-free; GIT, Gastrointestinal tract; GLP-1, Glucagon-like peptide-1; H. pilory, Helicobacter pylori; HSCs, Hepatic stellate cells; HVPG, Hepatic venous pressure gradient; HCC, Hepatocellular carcinoma; •OH, Hydroxyl free radicals; IL-10, Interleukin-10; IL-6, Interleukin-6; LPS, Lipopolysaccharide; LPB, Lipopolysaccharide-binding protein; LPL, Lipoprotein lipase; NN2211, Liraglutide; LOXL, Lysyl oxidase and lysyl oxidase-like; MDA, Malondialdehyde; MnSOD, Manganese superoxide dismutase; Mkt, Market; MS, Metabolic syndrome; H2, Molecular hydrogen; O2, Molecular oxygen; RG-125 AZD4076, N-acetylgalactosamine (GalNAc)-conjugated anti-miR-103/107 oligonucleotide; NAP, H. pylori-induced neutrophil-activating protein; NAS, NAFLD activity score; NKT, Natural killer T; NADH, Nicotinamide adenine dinucleotide; NO, Nitric oxide; NAFLD, Non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; NF-κB, Nuclear factor kappa; INT-747, Obeticholic acid (OCA); PNPLA3, Patatin-like phospholipase 3; PAMPs, Pathogen-associated molecular patterns (PAMPs); PPAR, Peroxisome proliferator-activated receptor; PIVENS, Pioglitazone versus Vitamin E versus Placebo; PASH, PNPLA3-associated steatohepatitis; HPC, Primary hepatic carcinoma; ROS, Reactive oxygen species; ETC, Respiratory electron transport chain; Saroglitazar-ZYH1, [(S)-α-ethoxy-4-{2-[2-methyl-5-(4-methylthio) phenyl)]-1H-pyrrol-1-yl]-ethoxy})-benzenepropanoic acid magnesium salt]; SSAO, Semicarbazide-sensitive amine oxidase; PXS-4728A, SSAO/VAP-1 inhibitor BI 1467335; SCD1, Stearoyl-coenzyme A desaturase 1; O2·–, Superoxide anion; SOD, Superoxide dismutase; TZDs, Thiazolidinediones; TLR, Toll-like receptor; TNF, Tumor necrosis factor; VAP-1, Vascular adhesion protein-1; VLX103, Venlafaxine-103
Keywords: Antioxidants, Drugs, Interventions, Manganese superoxide dismutase, Probiotics
1. Introduction
Fatty liver is an emerging medical problem. This condition is often discovered at the same time during ultrasound examinations performed for other purposes. Although the possible etiological factors have been investigated, in a high proportion of cases, the cause of the disease remains unidentified. These cases, defined as “metabolic syndrome” are often related to other diseases, or to other predictive parameters (hyperglycemia, dyslipidemia, high blood pressure, and abdominal obesity) and are classified as non-alcoholic fatty liver disease (NAFLD). This condition is one of the most common benign liver disorders in modern societies, and represents the first stage of a process that may evolve into an inflammatory phase defined as non-alcoholic steatohepatitis (NASH). Subsequently, this process can lead to fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC) with high rates of morbidity and mortality [1]. A schematic diagram of these processes is shown in Fig. 1. Over the years, several terms have been used to describe NASH, such as pseudo-alcoholic hepatitis, alcohol-like hepatitis, fatty liver hepatitis, steatonecrosis and diabetic hepatitis. More recently, abbreviations such as BASH (both alcoholic and non-alcoholic liver disease), DASH (drug-associated steatohepatitis), CASH (chemotherapy-associated steatohepatitis), and PASH (PNPLA3-associated steatohepatitis) have been adopted to distinguish the various etiologies [2]. In this review, we provide an updated overview of NAFLD/NASH and progression to cirrhosis/HCC, with a particular focus on the roles of the gut microbiota and oxidative stress, as well as the prospects for novel therapies.
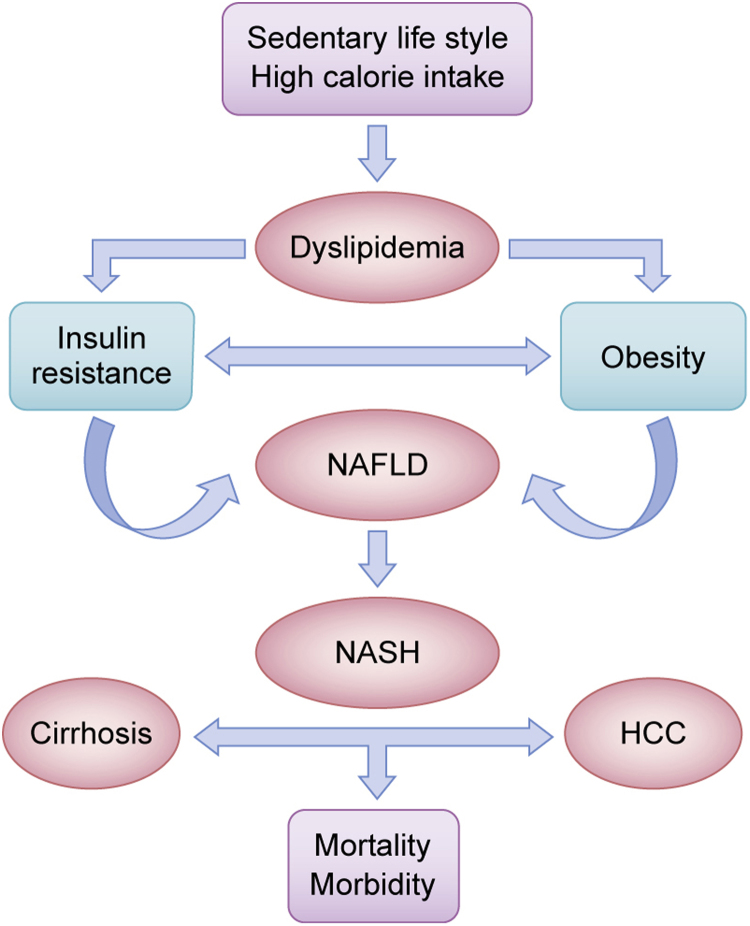
Fig. 1.
Schematic representation of progression from NAFLD/NASH to cirrhosis/HCC. The history of non-alcoholic fatty liver disease (NAFLD). This term assembles a wide spectrum of conditions ranging from dyslipidemia to steatohepatitis (NASH). Lifestyle factors, such as sedentary lifestyle and high calorie intake combined with insulin resistance and several others genetic and epigenetic factors induce the progression of NASH to cirrhosis and hepatocarcinoma (HCC) with its clinical consequences.
2. Worldwide incidence of NAFLD
The prevalence of NAFLD in the general population is approximately 6–35%, with a median incidence of 20% [2], [3]. Worldwide distribution, referred to a meta-analysis carried out with studies conducted between the years 2000–2015, is highly variable as shown in Table 1 [4], [5].
Table 1.
Worldwide incidence of NAFLD. The prevalence is highly variable although the distribution in North America and Asia is similar.
| Geographic location | Prevalence (%) |
|---|---|
| North America | 11–46 |
| South America | 27–35 |
| Europe | 4–49 |
| Asia | 15–44 |
| Saudi Arabia | 15–55 |
| Africa | 9–20 |
In the general population of Western countries, the incidence of NAFLD is 20–30%, with a prevalence of approximately 30% among adults in the United States (US) and 25% in Italy [6].
Patients with NAFLD have higher overall mortality than control patients [1], and most deaths are due to cardiovascular events [7]. Male sex, older age, increased waist circumference, low high-density lipoprotein levels, and the presence of metabolic syndrome are the independent predictors of mortality in patients with NAFLD [8]. In healthy men, increased serum alanine aminotransferase (ALT) levels, even within the reference range, are an independent predictor of NAFLD [9], although this parameter is not predictive of death. A reversal of an ALT/AST (aspartate aminotransferase) ratio of more than 1 is an index of advanced fibrosis [1]. Other predictors of NAFLD are insulin resistance (which can induce dyslipidemia and atherosclerosis), endothelial dysfunction, alteration of the left ventricular energy metabolism, stroke, and increased expression of inflammation mediators [10]. NAFLD is present in 80–90% of obese individuals, in 30–50% of diabetics and approximately 90% of patients with hyperlipidemia [6].
3. Worldwide incidence of NASH
Among the general population, the worldwide incidence of NASH is 5–7%, as reported in a study performed by Pereira K. et al. in 2015 [11], with 30–40% having raised liver enzymes and progressing from a chronic liver disease to cirrhosis and hepatocarcinoma. Among patients with NASH, 34–50% remain stable and 18–29% improve the histological aspect of their liver, while 26–37% progress to fibrosis, with 9–25% of these patients going on to develop cirrhosis [11], [12]. NASH is involved in approximately 30–40% of deaths caused by liver disease. In terms of progression to HCC, 0–0.5% of patients progress from simple hepatic steatosis, 0–2.8% from NASH and 40–62% from cirrhosis [11]. The risk factor of NAFLD/NASH is similar in all countries [6], [13]. The evolution of NASH to fibrosis/cirrhosis depends critically on the initial stage of inflammation [14]. Cirrhosis is not always present and it has been reported that 41.7% of patients develop HCC without showing cirrhosis [15], [16]. Among patients with NASH, 20% progress to cirrhosis over a 20-year period with increased risk of liver failure or HCC resulting in transplantation or death [17]. After hepatitis C virus, NASH has become the leading etiology of liver transplantation due to HCC in the US, with a four-fold increase reported over the period from 2002 to 2012 [18]. However, HCC is not necessarily associated with cirrhosis, but depends on metabolic diseases (diabetes, obesity, and insulin resistance) [19].
4. NAFLD/NASH: pathogenesis
A subset of patients with NAFLD develops NASH; however, the mechanism is poorly understood and the pathogenesis of NASH is unclear although the current scientific consensus accepts the concept of the “Multiple Hit Theory” [20] (Fig. 2) rather than the “Two Hit Theory” [21] (Fig. 3).
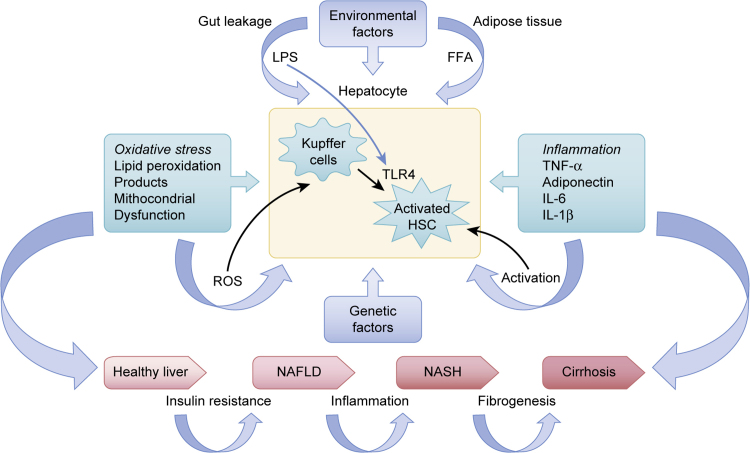
Fig. 2.
The “Multiple Hit Theory”. The “Multiple Hit Theory” that has now superseded the Two Hit Theory is based on the hypothesis that inflammation precedes steatosis in environmentally and genetically predisposed subjects. According to the “Multiple Hit Theory”, numerous insults act together to induce NAFLD. Such hits include insulin resistance, hormones secreted from the adipose tissue, nutritional factors, endotoxins (lipopolysaccharide) released by the gut microbiota, oxidative stress damage, and genetic and epigenetic factors. These insults act on liver parenchymal cells via toll-like receptors to drive the progression of NASH.
Fig. 3.
The “Two Hit Theory”. Schematic diagram of the “Two Hit Theory”. According to this now obsolete theory, fat accumulation (steatosis), increased levels of free fatty acids (FFAs), and insulin resistance represent the “first hit” in the progression of NAFLD, leading to sensitization of the hepatic tissues to further injuries caused by factors such as oxidative stress and proinflammatory adipokine release, which represent the “second hit”.
Until recently, hepatic steatosis [22] and oxidative stress [23] – the latter also caused by changes in the gut microbiota [24], [25] – were seen as a first and second causes of disease progression. The “first hit” (steatosis) induces fat accumulation in hepatocytes and increased hepatic uptake of free fatty acids (FFA). This situation is optimal for the “second hit” (oxidative stress) that leads to lipid peroxidation, proinflammatory molecule release and mitochondrial damage [26]. There is now a consensus on a multi-parallel hit theory [27] according to which – in many patients – inflammation rather than steatosis represents the first cause of NASH progressing to fibrosis [20], [28]. Tilg et al. [20] defined steatosis as a bystander phenomenon that follows inflammatory events and is determined by estimating the proportion of hepatocytes containing fat droplets [1]. Treatment with anti-tumor necrosis factor (TNF)-ɑ antibody induces an improvement of hepatic steatosis in ob/ob mice, which is a model of NAFLD [29]. A decreased number of Kupffer cells leads to a decrease in interleukin-10 (IL-10) release and probably, to subsequent hepatic steatosis [30]. The multiple hit hypothesis considers that multiple injuries act concomitantly in individuals who are genetically predisposed to NAFLD. In recent years, increasing credence is given to the assumption that the presence of genetic polymorphism of the patatin-like phospholipase 3 (PNPLA3) gene in NAFLD is essential to differentiate simple steatosis from NAFLD that progresses to NASH [31]. The protein encoded by this gene is a triacylglycerol lipase that mediates triacylglycerol hydrolysis in adipocytes and may be involved in the balance of energy usage/storage in adipocytes. The main pathogenic stages for NAFLD are insulin resistance [32], alterations in other regulated hormone sets that regulate carbohydrate and lipid metabolism (leptin, adiponectin) and increases in the levels of certain proinflammatory adipokines, such as TNF-α and interleukin-6 (IL-6) [33]. Visceral fat accumulation is the main consequence of insulin resistance that can be considered the key marker of NASH and is a significant risk factor. Adiponectin has anti-inflammatory and anti-diabetic properties [34]. TNF-α and IL-6 inhibit adiponectin but increase leptin levels that inhibit the anabolic pathways and increase metabolism. Adiponectin and leptin act antagonistically on liver fibrogenesis and inflammation [35], [36], [37]. However, reports of serum levels of adiponectin and the expression of its receptor are inconsistent; therefore, further investigations are necessary to clarify the function of these novel adipokines [38], [39].
5. The role of the gut microbiota
The gut microbiota comprises the set of microbial species (Bacteroides, Eubacterium, Peptococcaceae, Bifidobacterium, Escherichia coli, Streptococci, Staphylococci, Lactobacillus, Clostridium perfringens) as well as virions that inhabit the gastrointestinal tract. These organisms contribute to food digestion, produce vitamins, and modulate immunity [40]. The composition and distribution of the gut microbiota strains are influenced by various factors: age, health/disease status, environmental pH of the intestinal lumen, nutrition, and probiotics [41]. There is a link between microbiota and overweight, subclinical inflammation and insulin resistance (Fig. 4). Despite reduced food intake, a 60% increase in body fat content and insulin resistance has been produced by conventionalization of adult germ-free (GF) C57BL/6 mice with a normal microbiota harvested from the distal intestine of conventionally raised animals [25].
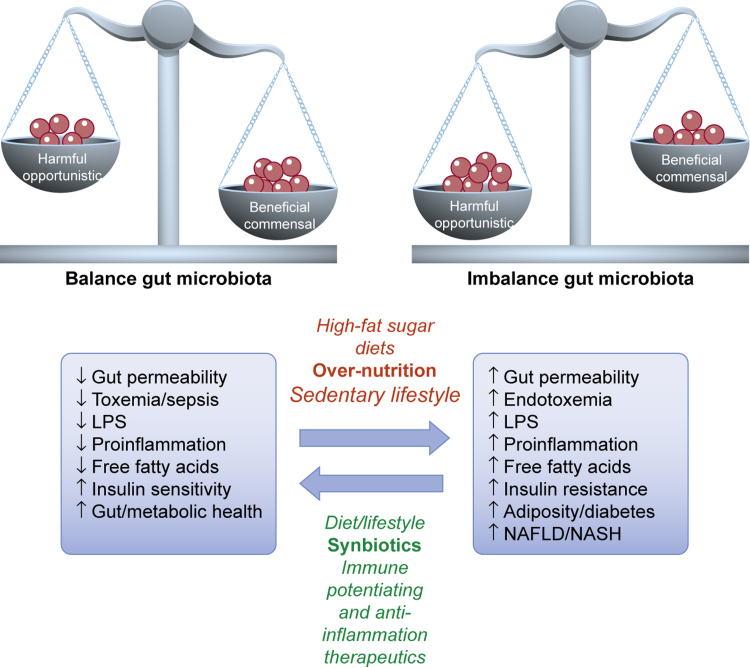
Fig. 4.
Importance of balanced gut microbiota, and consequences of gut dysbiosis. The gut bacteria may contribute to NAFLD via production of bacteria-derived toxins, e.g., LPS, production of pro-inflammatory cytokine, deposition of triglycerides in adipocytes and liver, causing insulin resistance, adiposity and diabetes.
The gut microbiota decreases the expression of fasting-induced adipose factor (Fiaf) [42], which is a suppressor of lipoprotein lipase (LPL), a key regulator of fatty acid release from triglyceride-rich lipoproteins in muscle, heart, and fat [43]. Increased cellular uptake of fatty acids and triglyceride accumulation are induced by increased adipocyte LPL activity. The gut microbiota releases pathogen-associated molecular patterns (PAMPs), which are Toll-like receptor (TLR) ligands [27]. In humans, TLR2, TLR4, and TLR9 are involved in the pathogenesis of NASH [44]. The gut microbiota and the derived endotoxins participate in the mechanism of insulin resistance via TRL signals, in particular through the interaction between lipopolysaccharide (LPS) and its ligand TLR4 (localized on the surfaces of monocytes, mast cells, B cells and gut epithelium) with the monocyte differentiation antigen CD14 system [24], [45], [46]. LPS, which is produced by intestinal Gram-negative bacteria, is a complex of polysaccharides and lipids. It is an active component of endotoxin and, when it is released by these bacteria, binds to a lipopolysaccharide-binding protein (LPB), CD14, and TRL4 and migrates into intestinal vessels [47]. A high-fat diet stimulates microbiota containing LPS, which is transported by chylomicrons [48], [49]. LPS translocation to extra-intestinal tissues is facilitated by downregulation of tight junction proteins (ZO-1 and occludin) leading to increased intestinal permeability [50]. When LPS binds the LPB complex and TRL4 associates with CD14 on Kupffer cells, an intracellular inflammatory cascade is triggered [51], which activates nuclear factor kappa (NF-κB) and its related pathway, inducing the production of proinflammatory cytokines, such as TNF-α, IL-1, and IL-6 [52]. This pathway is stimulated in the presence of NASH, and patients with this disease exhibit upregulated TNF-α gene expression and high plasma levels of LBP [53]. TLR4 can also be present on hepatic stellate cells (HSCs), which produce most of the extracellular matrix deposited by the fibrotic process associated with endotoxemia [54], [55]. HSCs respond to the activation of LPS through a TRL4-dependent pathway [55].
6. The role of oxidative stress
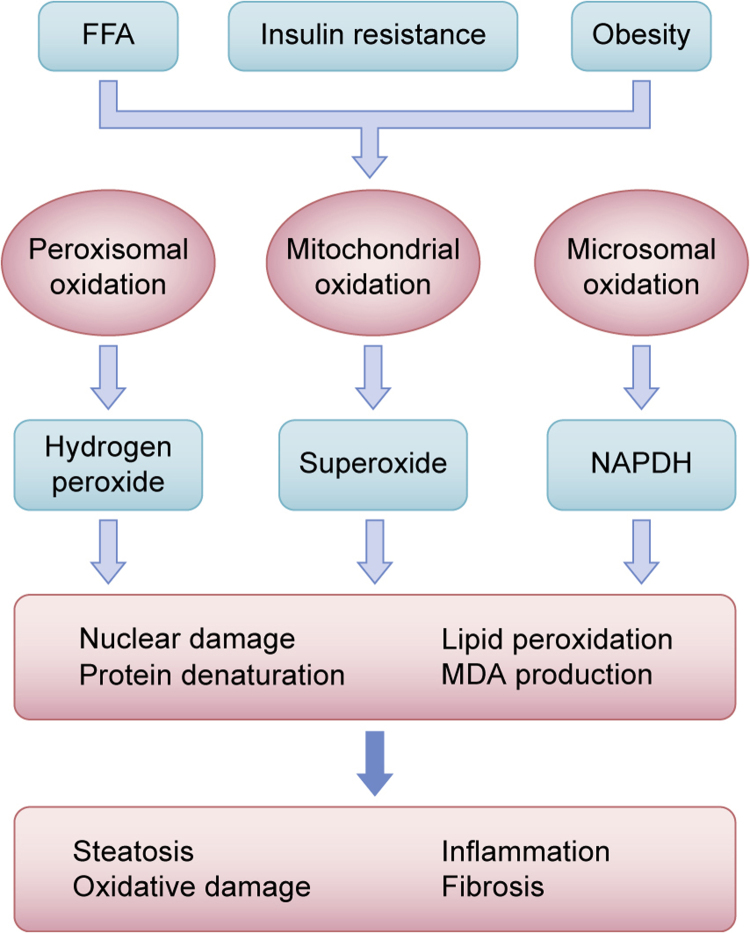
Many recent studies have shown the importance of oxidative stress in the progression of steatosis to steatohepatitis [56], [57], [58], [59]. Neish et al. demonstrated that the interaction between the gut epithelia and some groups of enteric commensal bacteria induces rapid generation of ROS within host cells [60]. Kountouras et al. hypothesized a significant possible linkage and a crucial role of Helicobacter pylori (H. pylori) in the pathophysiology of metabolic syndrome, in H.pilory–related extragastric diseases and neoplastic conditions. H. pylori, apart from classic microbiota, plays its pathogenic role by triggering a severe leukocyte infiltration of the tissue, releases a virulence factor (H. pylori-induced neutrophil-activating protein (NAP)) that stimulates neutrophils to produce ROS and secrete myeloperoxidase, chemokines and proinflammatory cytokines. H.pilory may also colonize gut itself and affect the pathogenesis of NAFLD determining a dysbiosis of gastrointestinal tract (GIT) microbiota [61], [62]. The intestinal microbiota produces endogenous ethanol contributing to the morphological and functional changes in the cells that constitute the intestinal barrier favoring the transit of endotoxins in the gut vessels [63]. Ethanol and its derived compounds (acetaldehyde and acetate) [64] induce the formation of ROS by HSC cells and Kupffer cells [65]. Together with LPS, ROS promote increased TLR4 gene expression [66]. Acetate is also a substrate for fatty acid synthesis; therefore, steatosis, elevated FFAs and ROS generation caused by mitochondrial dysfunction, lead to the production of inflammatory cytokines and ultimately, to liver injury [23], [27], [32], [67], [68]. Hyperinsulinemia blocks the mitochondrial oxidation of fatty acids, which therefore accumulate and are then partially metabolized by the peroxisomes [69] and microsomes, with the subsequent production of ROS and lipid peroxidation. The result is the formation of products, such as malondialdehyde (MDA) [70], which has a longer half-life than ROS, and can spread to other regions to generate oxidative stress. These phenomena are key factors in the development of NASH. The oxidative balance is crucial [71], and can collapse if the mitochondrial ability to control this balance is damaged under constant oxidative stress. Under these conditions, such as β oxidation, oxided cofactors (NAD+ and FAD) are converted into reduced cofactors (NADH and FADH2). Electrons are delivered to respiratory chain. The transfer of electrons to the mitochondrial respiratory chain (ETC) on one side and their outflow on the other, creates an imbalance that leads to an accumulation of ROS [27] (Fig. 5).
Fig. 5.
The role of oxidative stress. Elevated levels of free fatty acids (FFA), insulin resistance, and obesity are triggers for peroxisomal, mitochondrial and microsomal oxidation leading to the production of reactive oxygen species (ROS) and products such as malondialdehyde (MDA), which that has a longer half-life than ROS. These products mediate oxidation of reduced cofactors, nuclear damage, lipid peroxidation, protein denaturation, inflammation and fibrosis.
Normally, the superoxides (incompletely reduced forms of oxygen) are dismutated by superoxide dismutase (SOD) into hydrogen peroxide, which in turn is converted into non-toxic water by glutathione peroxidase and catalase [72]. At relatively low levels of these various antioxidant repair enzymes, hydrogen peroxide, which is generated by the Fenton reaction and mediated by iron levels, can induce fatty acid oxidation [73]. This leads to damage to the ETC and the mitochondrial DNA located near the inner membrane of the organelle, DNA mutations, and cellular apoptosis [74]. Elevated levels of iron, an inducer of oxidative stress, are observed in NASH, although its role in this disease remains to be fully elucidated [73]. Acute stress tends to upregulate mitochondrial biogenesis and function, while chronic stress tends to mediate the opposite effects. In response to multiple individual and environmental factors, mitochondria produce factors that affect cellular function, gene expression, and cellular senescence [75]. Hepatocyte apoptosis and senescence contribute to the activation of the inflammasome via a variety of intra- and inter-cellular signaling mechanisms that lead to fibrosis and may mediate disease progression [28]. The role of mitochondrial DNA in NASH differs from that in simple fatty liver. In fatty liver, increased mitochondrial DNA protects against inflammation and fibrosis, while these processes are exacerbated by the decreased levels associated with NASH. The transcription of PPARG Coactivator 1 Alpha (PPARGC1A) is the most important regulator of mitochondrial biogenesis [76], [77] and is involved in hepatic glucogenesis [78]. Recent data show that the gut microbiota regulate the metabolism of the major intracelluar antioxidant, glutathione, in the host organism [79]. Thus, lower levels of glutathione can contribute to oxidative stress [80], [81]. Several researchers have observed that glycine, which is necessary for glutathione synthesis, is consumed by the microbiota in the small intestine, resulting in a clear deficiency glutathione. This discovery may lead to the identification and commercialization of probiotics that replenish glycine and/or increase glutathione in the gut [82].
7. NAFLD/NASH: diagnosis
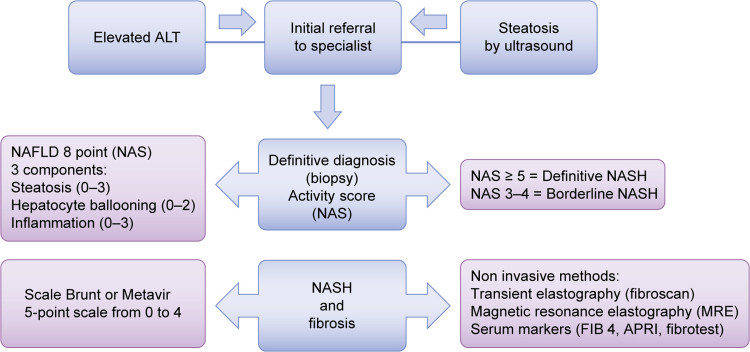
There are no biochemical parameters that allow accurate diagnosis of steatosis. NAFLD is often accompanied by moderate elevation of serum alanine aminotransferase (ALT), gamma-glutamyl transferase (γGT) [83] and an ALT/AST ratio < 1 [84], as well as levels of triglycerides and cholesterol that exceed the normal range. However, a significant number of subjects may present normal parameters of the liver while suffering from advanced forms of liver disease. Liver ultrasound has a high sensitivity (89%) and specificity (93%) for the diagnosis of steatosis, but not for the diagnosis of fibrosis (sensitivity 77%, specificity 89%) [84]. Moreover, while ultrasound is highly effective in determining the presence of fat in the liver parenchyma, it does not provide information regarding the likelihood of disease progression. Ultrasonography of the liver combined with serum transaminase levels is now a simple and efficient approach used to screen subjects at risk of hepatosteatosis [67], [85], [86]. The NAFLD activity score (NAS), which is a conventional system used to grade NAFLD diagnosis, is based on three histological features: steatosis (0–3), inflammation (0–3) and hepatocyte ballooning (0–2), with an overall score in the range from 0 to 8 [87]. NASH is divided into three different categories: non-NASH, borderline NASH (NAS 3–4) and definitive NASH (NAS ≥ 5) [88], while fibrosis is staged on a scale from 0 to 4 [87], [89] (Fig. 6).
Fig. 6.
NAFLD and NASH: diagnosis. The diagnosis of NAFLD in a patient with raised levels of alanine aminotransferase (ALT) and steatosis detected by ultrasound examination, requires histological confirmation. The NAFLD activity score (NAS) is an accepted system used to grade NAFLD diagnosis. The score is based on evaluation of three histological features: steatosis (0–3), hepatocyte ballooning (0–2) and inflammation (0–3), with an overall score in the range from 0 to 8. The diagnosis of NASH is divided into three different categories: non-NASH, borderline NASH (NAS 3–4) and definitive NASH (NAS ≥ 5). Fibrosis is staged on a scale from 0 to 4.
In conclusion, excluding all other causes of hepatosteatosis and in the absence of an intake of alcohol exceeding 20 g/day, a diagnosis of NAFLD/NASH can only be confirmed on the basis of liver biopsy and histological examination [88], [90], [91], [92]. It is important to stress that liver biopsy is an invasive procedure with rare, but severe, adverse events, including mortality. Transient elastography is a new, noninvasive, and economically attractive technique that measures tissue hardness, which is mainly due to the extent of fibrosis [93], [94]
8. Current therapies for NAFLD/NASH: unmet medical needs
The first step in developing a new therapy is to establish the goals as reversal or prevention of fibrosis/cirrhosis progression, improvement or maintenance of liver function or prevention of transplantation or death. Many different pharmacotherapeutic strategies have been considered, although the impact of these therapies has not been satisfactory. Lifestyle modifications (gradual weight loss with a low-fat and glycemic diet, exercise and a constant supply of fruit and vegetables) could be effective for NAFLD patients. Some authors report an improvement in steatosis following 5% weight loss [95], while others report that a mild weight loss of approximately 3% reduces steatosis, although weight loss of up to 10% is required for regression of fibrosis in NASH patients [96]. The use of probiotic bacteria reduces the risk of developing an overgrowth of intestinal bacteria by decreasing the levels of low-grade inflammation. Probiotic therapy on the other hand, has been suggested to counteract the development of NAFLD on various levels. Probiotic bacteria regulate liver inflammation by balancing the production of pro- and anti-inflammatory cytokines (TNF-α by downregulation of NF-κB activity), improving liver function, and reducing the content and β oxidation of hepatic fatty acids [29]. Studies of the intestinal microflora composition have shown that changes in its composition (e.g. reduced number of Bifidobacteria) induce an increase in plasma LPS levels with a consequent increase in proinflammatory cytokines [97]. Treatment with the probiotic Bifidobacteria has increased insulin sensitivity and reduced fat deposition in the liver [97]. A multi-species blend of probiotics (VSL#3) ameliorates insulin resistance and steatosis induced by a high-fat-diet, by increasing the frequency of hepatic natural killer T (NKT) cells [98]. These unconventional T cells express both T cell and killer cell receptors, regulate the production of pro-and anti-inflammatory cytokines [99] and stimulate TNF-ɑ levels resulting in increased insulin resistance according to the previously described mechanism [98].
9. Synbiotic in NASH/cirrhosis progression: what can we expect?
Synbiotics represent the combined use of probiotics and prebiotics [100]. Probiotics were defined by the World Health Organization in 2001 as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [101], [102]. In contrast to probiotics, prebiotics are not living organisms and stimulate the growth of beneficial bacteria in the gastrointestinal tract. In fact, prebiotics such as high-fiber foods, are consumed by and help the endogenous gut bacteria to survive and contribute to the maintenance of the general welfare of the organism [103]. Interest in this relatively inexpensive, non-pharmacological category, has grown over the years; however, is this enough to justify their use in the prevention/treatment of NASH/cirrhosis progression? The ability to modify the gut microbiota, to strengthen the intestinal barrier and to modulate the inflammatory response, according to the previously described mechanisms, is unquestioned [104]; nevertheless, definitive evidence of the efficacy of microbiome modulation in NASH/cirrhosis progression remains to be obtained. If on the one hand, there are several reports of the beneficial effects of some probiotics on hepatic damage in animal models, and on the other, the use of probiotics in experimental models of cirrhosis has provided discouraging results [105], [106]. The following objectives should be met in the prevention and therapy of the progression to NASH/cirrhosis: 1) improved hyperdynamic circulation in cirrhosis, caused by an increased resistance to flow due to portal hypertension and to altered hepatic vascular morphology of chronic liver disease; 2) hindrance of infection and; 3) prevention of hepatic encephalopathy [107], [108], [109], [110], [111], [112]. The inability to evaluate the quality of trials as well as the inherent heterogeneity and variety associated with such studies are major limitations that hinder clinical trials of probiotics. Probiotics are currently considered to be food products not as drugs, and are subject different government regulations. Consequently, it is difficult to interpret the results of numerous experimental and clinical studies. Furthermore, many of these studies are carried out on a small number of patients [113] and are not randomized or are associated with the use of prebiotics, which makes it difficult to assess the utility of probiotics [114]. The only studies in which the rationale has major scientific validity are those conducted with the probiotic, VSL#3 [29], which is a multi-species probiotic consisting of a proprietary combination of eight strains of bacteria (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus helveticus). The combination of several bacterial species and strains can act synergistically to generate a more marked effect than a single strain. VSL#3 was shown to decrease fibrosis in a mouse model of NASH [115] and to recover liver function by lowering oxidative damage in patients with various liver diseases, including cirrhosis [116]. On the other hand, in a randomized, double-blind placebo-controlled trial in patients with decompensated cirrhosis, VSL#3 failed to achieve a significant decrease in the hepatic venous pressure gradient (HVPG) [113]. However, it may have been an overambitious aim to decrease the HVPG in patients with cirrhosis and clinically significant portal hypertension by probiotic treatment alone. The use of probiotics has, therefore, recently been proposed as adjuvant therapy in addition to drugs [117], [118]. It is necessary to remember that although the use of probiotics was found to be safe in these studies, their long-term safety remains to be confirmed.
10. Pharmacological treatments
Currently approved drugs that are already in use for diabetes mellitus treatment have been considered for the treatment of NASH, since these conditions share the characteristics of obesity and insulin resistance. Among these drugs, metformin and thiazolidinediones (pioglitazone and rosiglitazone; PPAR-ɣ agonists) improve NASH both clinically and histologically [119], [120]. Metformin was reported to induce weight loss, while pioglitazone induced weight gain, with an increased risk of bladder cancer [121]. Rosiglitazone is no longer available in the European Union and other countries due to the reported risk of cardiovascular events [122]. Therefore, additional randomized controlled studies on a larger number of observed samples and for a greater period of observation are required to evaluate the long-term safety, efficacy and side-effects of these insulin-sensitizing agents. These drugs also appear to have additional antioxidant activity [123]. Vitamin E, one of the best known antioxidants, protects against mitochondrial damage [124] and is considered to be the first line treatment for NASH [125], despite its association with an increased risk of prostate cancer and hemorrhage [126]. Many authors reporting a histological improvement of liver NASH patients after bariatric surgery consider this approach to be feasible in obese patients [127], [128], although the data are not definitive, especially in cirrhotic patients, due to the absence of prospective randomized trials [129]. Several clinical studies have been conducted to evaluate new classes of drugs that target different molecules or mechanisms of action related to NASH. A list of these molecules categorized according to drug class (metabolic homeostasis, inflammation, oxidative stress, apoptosis, and fibrosis), target, mechanism of action, and phases of clinical trials is presented in Table 2.
Table 2.
List of drugs evaluated for non-alcoholic steatohepatitis (NASH) treatment. Several new molecules that are being evaluated in ongoing clinical trials for NASH treatment. These molecules target various metabolic pathways including metabolic homeostasis, inflammation, oxidative stress, apoptosis and fibrosis. Many are in Phase 2 or 3 although, with the exception of Sitagliptin and Evogliptin, there are currently no FDA-approved therapies for this disease.
| Drug class | Compound | Phase | Target//Mode-Of-Action (MOA) | Company | Refs. |
|---|---|---|---|---|---|
| Metabolic homeostasis | RG-125 (AZD4076) | Preclin. | microRNA-103/107 ("miR103/107")/insulin sensitivity and resistance | Regulus | [130] |
| Metabolic homeostasis | Saroglitazar (ZYH1) | 1 | PPAR/PPAR α/γ agonist | Zydus Cadila | [131] |
| Metabolic homeostasis | GS-9674 | 1 | FXR/FXR agonist | Gilead Sciences | [132] |
| Metabolic homeostasis | PF-05231023 | 1 | Fibroblast Growth Factor 21 (FGF21)/FGF21 analog | Pfizer | [133] |
| Metabolic homeostasis | IVA337 | 2 | PPAR/PPARɑ-δ and γ (PanPPAR) | Inventiva Pharma | [134] |
| Metabolic homeostasis | LJN-452 | 2 | FXR/FXR agonist | Novartis | [135] |
| Metabolic homeostasis | NGM-282 | 2 | Fibroblast Growth Factor 19 (FGF19)/FGF19 analog | NGM Biopharmaceutic | [136], [137] |
| Metabolic homeostasis | Aramchol | 2 | Stearoyl-coenzyme A desaturase 1 (SCD1)/SCD1 inhibitor | Galmed Pharmaceuticals | [138] |
| Metabolic homeostasis | LUM002 | 2 | ASBT/ASBT inhibitor | Lumena | [139], [140] |
| Metabolic homeostasis | Liraglutide (NN2211) | 2 | Glucagon-like peptide-1 (GLP-1) receptor/GLP-1 analog | Novo Nordisk | [141] |
| Metabolic homeostasis | Elafibranor (GFT505) | 3 | PPAR/PPARɑ-δ agonist | Genfit | [142] |
| Metabolic homeostasis | Rosiglitazone | 3 | PPAR//insulin sensitizer | GlaxoSmithKline | [122] |
| Metabolic homeostasis | Pioglitazone | 3 | PPAR//insulin sensitizer | Takeda Pharmaceutical | [120] |
| Metabolic homeostasis | Obeticholic acid (OCA) (INT-747) | 3 | FXR/FXR agonist | Intercept | [143], [144] |
| Inflammation | Cenicriviroc (CVC) | 2 | C-C chemokine receptor types 2 (CCR2) and 5/CCR2/CCR5 antagonist (CCR5) | Tobira Therapeutics | [145] |
| Inflammation | Venlafaxine-103(VLX103) | 2 | TNF/downregulation of proinflammatory cytokines | Verlix Pharma | [146] |
| Inflammation | Sitagliptin | Mkt | Dipeptidyl peptidase 4/(DPP-4 inhibitor) | Merck Sharp & Dohme | [147] |
| Inflammation | Evogliptin | Mkt | Dipeptidyl peptidase 4/(DPP-4 inhibitor) | Dong-A ST | [148] |
| Oxidative stress | PXS4728A | 1 | Semicarbazide-Sensitive Amine Oxidase (SSAO)/VAP-1 inhibitor | Boehringer Ingelheim | [149] |
| Oxidative stress | GS- 4997 | 2 | Apoptosis signal- regulating kinase 1 (ASK1)/ASK1 inhibitor | Gilead Sciences | [150] |
| Fibrosis | Simtuzumab (GS-6624ClinicalTrials.gov Identifier: NCT01672879 | 2 | Lysyl oxidase and lysyl oxidase- like (LOXL) enzymes)/lysyl oxidase and lysyl oxidase-like inhibitor | Gilead Sciences | [151] |
| Fibrosis | GR-MD-02 | 2 | Galectin- 3 protein/Galectin-3 protein inhibitor | Galectin Therapeutics | [152] |
| Apoptosis | Emricasan (IDN-6566) | 2 | Caspase/caspase inhibitor | Conatus Pharmaceuticals | [153] |
To date, many of these new molecules are being evaluated in randomized trials (Phases 1, 2, and 3), although there are no FDA-approved therapies for this disease [96], [154], with the exception of Sitagliptin and Evogliptin, that are in the market (Mkt) approval phase. The presence of fibrosis is the main obstacle to the success of these agents because these drugs do not exert a reductive effect on fibrosis [32], [96].
11. Antioxidant treatments in clinical trials
Oxidative stress is well established as a major factor contributing to progression from simple fatty liver to NASH. Ongoing clinical trials have shown improvements in several liver activities following anti-oxidative therapy, although the long-term effects remain to be clarified. One of these drugs that is currently under investigation (PXS-4728A) [155] is a selective inhibitor of vascular adhesion protein-1 (VAP-1), which is an enzyme expressed constitutively in the human hepatic endothelial cells and adipocytes. It induces liver inflammation, fibrosis, and cirrhosis [156] promoting leukocyte migration due to the generation of ROS such as aldehyde, ammonia and hydrogen peroxide. These products induce oxidative stress as a result of the conversion of hydrogen peroxide to hydroxyl free radicals (•OH). VAP-1 plays a significant role in the progression of NAFLD/NASH. Weston et al. [156] showed an increased hepatic expression of this enzyme in human NAFLD compared with that in controls. The novel molecule PXS-4728A inhibits this enzyme and might act as an anti-inflammatory and anti-fibrotic drug in NASH by reducing oxidative stress. PXS-4728A has good oral bioavailability and nanomolar potency; however, the trial is still in Phase 1 and therefore, the efficacy profile of this molecule remains to be determined. GS-4497 is a small molecule that inhibits apoptosis signal-regulating kinase 1 (ASK1). In response to oxidative stress, this redox-sensitive mitogen-activated protein kinase kinase kinase (MAPKKK) induces activation of MAPK kinases p38 and c-Jun N-terminal kinase (JNK), which stimulate the release of inflammatory molecules, the expression of cellular matrix genes (fibrosis) and promote cellular apoptosis and necrosis. GS-4497 competes with ATP in the ASK1 catalytic domain [150]. Lin et al. described an ongoing Phase 2 clinical trial evaluating the effects of this new molecule in patients with diabetic kidney disease. Gilead Sciences demonstrated an improvement in NASH patients with moderate or severe fibrosis following treatment with GS-4497 alone or in combination with the monoclonal antibody, Simtuzumab (Phase 2 trial). These results were presented during an abstract session at The 2016 Liver Meeting (Boston, MA).
Ohsawa et al. [157] identified molecular hydrogen (H2) as a powerful antioxidant. H2 prevents the reduction in the mitochondrial membrane potential and promotes cellular ATP synthesis. In vitro, H2 scavenges hydroxyl radicals, but not superoxide, hydrogen peroxide or nitric oxide (NO). NO is essential for blood vessel dilation, and protects against endothelial cell activation, suggesting that oxidative stress is necessary for survival [158]. Halliwell [159] described antioxidants as “any substance that, when present at low concentrations compared to those of an oxidizable substrate, significantly delays or prevents oxidation of that substrate”. Given the undisputed importance of ROS for the physiologic and pathophysiologic states of the organism, especially in the mitochondria, a basal level of ROS is essential. Most antioxidants cannot diffuse into the cytoplasm and penetrate the mitochondria, but are retained in the membranes (which is characteristic of their hydrophilic nature) and cannot overcome the biological barriers without specific carriers or receptors (this is typical of hydrophobic compounds such as vitamin E) [27]. One plausible explanation for the therapeutic failure of antioxidants is that they do not accumulate preferentially in the mitochondria, which is the major site of ROS generation.
12. Effects and side-effects of current clinical trials
Peroxisome proliferator activator receptors (PPARs) are a group of nuclear receptors expressed not only in the liver, but also in other tissues (heart, kidney, and adipose tissue). This non-specific expression limits the use of PPARs in the treatment of NASH and only one, the PPARα/δ agonist, Elafibranor, has been shown to regress the state of fibrosis: this drug is currently in a Phase 3 trial (GFT505) [142]. Obeticholic acid (OCA), which is a synthetic bile acid derivative that functions as an agonist of the intracellular bile acid receptor, farnesoid X receptor (FXR), is in an ongoing Phase 3 trial ((NCT02548351) showing a significant improvement in fibrosis score (35% vs. 19% of controls; P = .004). Pruritus has been reported as a reversible side-effect of treatment with this molecule, in addition to the emergence of cardiovascular events [143], [144]. At present, insulin sensitizers, such as pioglitazone, which are chemically classified as thiazolidinediones (TZDs), have been shown to be useful in the treatment of NASH. In a Phase 3 PIVENS trial (pioglitazone vs. vitamin E vs placebo for treatment of non-diabetic patients with NASH), pioglitazone and vitamin E were found to improve the histology of NASH but not fibrosis [125]. The use of this drug was associated with weight gain as a side-effect and increased risk of congestive heart failure. Furthermore, the continued usage of vitamin E might have a negative influence on patient mortality since the dose used in the clinical study (400–800 IU/day to 1000 IU/day) was much higher than the daily dose recommended by the FDA (22.4 IU) [26]. An inappropriate use of antioxidants may also be harmful because it would lead to a complete abolition of all cellular ROS. On the other hand, ROS overproduction leads to cell damage, aging and various diseases [160].
13. rMnSOD as a potential innovative antioxidant treatment for NASH
Humans are equipped with a group of antioxidant enzymes known as superoxide dismutases (SODs) that catalyze the dismutation of superoxide anion (O2·–) to hydrogen peroxide and molecular oxygen (O2), thereby preventing the accumulation of these activated oxygen species. Mitochondrial manganese (Mn) SOD (MnSOD-2), that is a mitochondrial enzyme, is characteristic of aerobic organisms and is composed of four homologous 24-kDa subunits. MnSOD-2, unlike cytoplasmic Copper/zinc superoxide dismutase (Cu/ZnSOD) and extracellular Cu/ZnSOD (ecSOD) [161], [162], [163], is synthesized in the cytoplasm and then transported to the mitochondrial matrix via its leader sequence, consisting of 24 amino acids. This peptide is subsequently cleaved, resulting in a mature and enzymatically active protein that plays a pivotal role within the cell [164]. Despite our knowledge of superoxides, clinical application of therapies based on superoxide-scavenging mechanisms, whether in the form of SOD as a drug, as SOD-mimetics, or as stoichiometric scavengers of the radical, is still far from being widespread. The use of SODs as drugs was met with several technical difficulties related to the chemical nature of these enzymes. Like any other enzyme used as a drug, SODs have a charge density and are characterized by a rapid renal clearance that negatively affects pharmacodynamics and pharmacokinetics. Fibrosis is a frequent consequence of bladder irradiation and clinical trials of SODs for treatment of bladder inflammation resulting from irradiation leading to fibrosis have been conducted. These are preliminary results that need to be confirmed. They propose to use SOD, not only before radiation treatment, as a protecting agent, but also after exposure, as therapeutic treatment for radiation damage [165], [166]. Furthermore, promising results have emerged from a study of SODs in kidney transplant patients [167]. It is noteworthy that the protective effects of MnSOD has already been widely documented [168], [169], [170], [171], although most of these studies were performed using the bovine protein. While SOD was found to protect organisms under ischemic conditions, the effects were less than satisfactory because of the bovine origin of the protein administered. Also, the protein was not considered injectable, as biological barriers resulted in its rapid inactivation. The same phenomenon was also observed following administration of constructs expressing human MnSOD genes bound to viral probes [172]. Only a few major pharmaceutical companies are reported to be conducting R&D (research and development) projects addressing oxidative stress using SOD-like drugs [173]. However, none of the commercially available SODs are able to enter cells. Moreover, these SODs are inactivated or excreted by the kidney [172].
Recently, a new isoform of human MnSOD was isolated and obtained in a synthetic recombinant form, designated rMnSOD. This isoform is distinguished by its ability to enter cells, its strong antioxidant and antitumor activities and its easy administration by injection [174], [175]. rMnSOD appears to be a very effective scavenger of both intra- and extracellular O2·– and improves pathological conditions associated with increased oxidative stress [161], [176]. Furthermore, rMnSOD shows good biodistribution in vivo, particularly in the liver [177], indicating the great potential of this molecule for correcting hepatic oxidative stress. Elevated oxidative stress in the cirrhotic liver is one of the mechanisms responsible for both liver fibrosis and reduced NO availability [178]. The authors demonstrated that chronic rMnSOD administration to cirrhotic animals decreases hepatic oxidative stress, improves intrahepatic vascular resistance, and decreases portal pressure mainly by ameliorating liver fibrosis. They showed a significant reduction in the hepatic oxidative stress generated experimentally through activation of the NADPH-oxidase system, confirming its biodistribution in the liver and the preservation of enzymatic activity. More significantly, a decrease in the elevated levels of O2·– and improved NO availability were observed. Based on these data, it can be speculated that rMnSOD might represent a novel therapeutic strategy for liver disease in which oxidative stress is a pathogenic factor. This study suggests that rMnSOD might be a useful agent in the treatment of patients with liver cirrhosis, which is a pathological condition that is also present in NASH [178]. Hide et al. [179] used primary cultured liver sinusoidal endothelial cells, liver grafts from healthy and steatotic rats, and human liver samples, to characterize the effects of rMnSOD on cold storage and warm reperfusion (CS + WR) injury incurred during liver transplantation that is partly mediated by oxidative stress and may lead to graft dysfunction. This novel form of the human manganese superoxide dismutase protects rat and human livers against ischemia and reperfusion injury by ameliorating oxidative stress, leading to global reduction in liver damage and microcirculatory derangements. The antioxidant capability of rMnSOD added to the cold storage solution was maintained and protected rat and human liver tissues against the damage caused by oxidative stress. Another “Achilles’ heel” associated with antioxidant therapies is the lack of cellular selectivity. Previously, Mancini et al. showed that rMnSOD displays a specific and selective cytotoxic activity against tumor cells expressing the estrogen receptor (ER) [180] and enters cells using its 24-amino acid (aa) leader peptide, which represents the rMnSOD molecular carrier [181]. Recently, Borrelli et al. demonstrated that six of the 24 amino acids penetrate cells through a particular “gate” represented by a specific amino acid sequence of the estrogen receptor (ER) [161]. These characteristics may provide an opportunity to formulate new drugs with specific and selective effects for use in therapy. ERs are present in the human liver, rendering this organ sensitive to estrogen, which binds to these receptors with high affinity to exert saturable and specific effects at low concentrations [182]. ER levels in males with chronic liver disease and patients with primary hepatic carcinoma (PHC) are twice the levels of normal males. Normal females have basal values that are approximately three times higher than those of control males [183]. Furthermore, variant estrogen receptors (ERs) were found to be expressed in at higher levels in male patients with chronic liver disease. Recently, several pieces of evidence have linked estrogenic signaling with metabolic syndrome (MS) and liver disease [184]. The beneficial effects of estrogen in counteracting some of the symptoms of MS are undeniable, though the underlying mechanisms remain largely unknown [185]. Cell penetration is an essential prerequisite for therapies [161] and poor permeability and selectivity of the cell membrane is an important obstacle to the development of drugs and therapeutic molecules [172], [186] because of the endothelium, which is the first physiological barrier between the blood and tissues. To deliver full-length molecules into a large number of cells, researchers are designing new nanotech strategies [187] based on the delivery of drugs via nanoparticles. These include adenoviral vectors to improve gene transfer into injured tissues, lipid-based nanostructures, micelles from amphiphilic block copolyphosphates, antibody-drug conjugates, and biodegradable polyester or polyethyleneimine nanoparticles approved for human use, as well as graphene oxide-based noncovalent nano supramolecular complexes [188], [189], [190], [191], [192], [193], [194]. Recently, we have used confocal microscopy analysis to obtain data (unpublished) demonstrating that rMnSOD penetrates biological barriers and localizes in mitochondria.
In conclusion, it has been confirmed that rMnSOD is: 1) a powerful antioxidant capable of dismutating ROS; 2) capable of penetrating biological barriers through its uncleaved leader peptide, and more specifically, through six of the 24 amino acids that constitute it and that are also responsible for its tranlocation into mitochondria; and 3) capable of reducing cirrhosis and portal hypertension in the liver. Thus, these distinguishing features implicate rMnSOD as a novel and highly effective adjuvant therapy that can significantly counteract the progression from NASH to hepatocarcinoma in the liver in rodent models.
14. Conclusions
As one of the major causes of liver disease worldwide, NAFLD can lead to fibrosis, cirrhosis, and even HCC. The current consensus on the mechanism underlying the pathogenesis of this heterogeneous disease is based on a multi-parallel hit theory according to which inflammation represents the first cause of NASH progressing to fibrosis. The gut microbiota or its products are thought to play a fundamental role in the etiology and progression of NASH. Several lines of evidence suggest that lipopolysaccharides are a prominent mediator of the both the metabolic and proinflammatory effects of the gut microbiota. Changes in lifestyle and the use of synbiotics (probiotics and prebiotics) have been evaluated for their effects on the progression of NASH, although no randomized studies have been conducted to date. The benefits of insulin sensitizers, such as TZDs, in the treatment of NASH have been demonstrated in a Phase 3 PIVENS trial in which pioglitazone and vitamin E were found to improve NASH histology, but not fibrosis. However, the application of this regimen was limited by the side-effects of weight gain and increased risk of congestive heart failure. Ongoing clinical trials of molecules that target different pathways, such as metabolic homeostasis, inflammation, oxidative stress and fibrosis, have shown improvements in several liver activities, although the long-term effects remain to be determined. Several lines of evidence have confirmed that oxidative stress is a major factor contributing to the progression from simple fatty liver to NASH. Based on previously reported studies, we propose to investigate a mutated form of the rMnSOD enzyme as a powerful antioxidant that is capable of reducing cirrhosis and portal hypertension in the liver, for its potential in the treatment of NASH.
Acknowledgements
The authors thank Lega Italiana di Napoli per la Lotta Contro i Tumori (LILT) and Ministero della Salute (Ricerca Corrente 2017) for the support.
Acknowledgments
Authors' contributions
AB conceived and wrote the manuscript. PB and FMT helped to draft the manuscript. AM, FMB, IDG and JLE revised the manuscript. All authors read and approved the final manuscript.
Declaration of interest
AM, IDG and JLE declare that they have potential competing interests because AM is co-founder of Leadhexa Biotechnologies Inc., IDG and JE are consultants to Leadhexa Biotechnologies Inc. The other authors declare that they have no competing interests. This manuscript has been read and approved by all the authors, and not submitted or under consider for publication elsewhere.
References
- 1.Paschos P., Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 2.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl. 1):S81–S84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Do A., Lim J.K. Epidemiology of nonalcoholic fatty liver disease: a primer. Clin. Liver Dis. 2016;7:106–108. doi: 10.1002/cld.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S., Scaglioni F., Marino M., Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig. Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 7.Misra V.L., Khashab M., Chalasani N. Nonalcoholic fatty liver disease and cardiovascular risk. Curr. Gastroenterol. Rep. 2009;11:50–55. doi: 10.1007/s11894-009-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazo M., Hernaez R., Eberhardt M.S., Bonekamp S., Kamel I., Guallar E., Koteish A., Brancati F.L., Clark J.M. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y., Ryu S., Sung E., Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin. Chem. 2007;53:686–692. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V., Bellentani S., Cortez-Pinto H., Day C., Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Pereira K., Salsamendi J., Casillas J. The global nonalcoholic fatty liver disease epidemic: what a radiologist needs to know. J. Clin. Imaging Sci. 2015;5:32. doi: 10.4103/2156-7514.157860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adami H.O., Chow W.H., Nyren O., Berne C., Linet M.S., Ekbom A., Wolk A., McLaughlin J.K., Fraumeni J.F., Jr. Excess risk of primary liver cancer in patients with diabetes mellitus. J. Natl. Cancer Inst. 1996;88:1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 13.Bedogni G., Miglioli L., Masutti F., Tiribelli C., Marchesini G., Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 14.Argo C.K., Northup P.G., Al-Osaimi A.M., Caldwell S.H. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J. Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Ertle J., Dechene A., Sowa J.P., Penndorf V., Herzer K., Kaiser G., Schlaak J.F., Gerken G., Syn W.K., Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 16.Chagas A.L., Kikuchi L.O., Oliveira C.P., Vezozzo D.C., Mello E.S., Oliveira A.C., Cella L.C., Herman P., Bachella T., Caldwell S.H., Alves V.A., Carrilho F.J. Does hepatocellular carcinoma in non-alcoholic steatohepatitis exist in cirrhotic and non-cirrhotic patients? Braz. J. Med. Biol. Res. 2009;42:958–962. doi: 10.1590/s0100-879x2009005000019. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal A.J., Banas C., Sargeant C., Luketic V.A., Sterling R.K., Stravitz R.T., Shiffman M.L., Heuman D., Coterrell A., Fisher R.A., Contos M.J., Mills A.S. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 18.Wong R.J., Cheung R., Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 19.Pagano G., Pacini G., Musso G., Gambino R., Mecca F., Depetris N., Cassader M., David E., Cavallo-Perin P., Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 21.Day C.P., James O.F. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 22.Gentile C.L., Pagliassotti M.J. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2008;19:567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumida Y., Niki E., Naito Y., Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic. Res. 2013;47:869–880. doi: 10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Shanab A., Quigley E.M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010;7:691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 25.Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Busafi S.A., Bhat M., Wong P., Ghali P., Deschenes M. Antioxidant therapy in nonalcoholic steatohepatitis. Hepat. Res. Treat. 2012;2012:947575. doi: 10.1155/2012/947575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takaki A., Kawai D., Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH) Int. J. Mol. Sci. 2013;14:20704–20728. doi: 10.3390/ijms141020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peverill W., Powell L.W., Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int. J. Mol. Sci. 2014;15:8591–8638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z., Yang S., Lin H., Huang J., Watkins P.A., Moser A.B., Desimone C., Song X.Y., Diehl A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 30.Clementi A.H., Gaudy A.M., van Rooijen N., Pierce R.H., Mooney R.A. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim. Biophys. Acta. 2009;1792:1062–1072. doi: 10.1016/j.bbadis.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin. Gastroenterol. Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 33.Carbone F., La Rocca C., Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94:2082–2088. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Polyzos S.A., Toulis K.A., Goulis D.G., Zavos C., Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60:313–326. doi: 10.1016/j.metabol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Wolf A.M., Wolf D., Rumpold H., Enrich B., Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 36.La Cava A., Alviggi C., Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J. Mol. Med. 2004;82:4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- 37.La Cava A., Matarese G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 38.Nannipieri M., Cecchetti F., Anselmino M., Mancini E., Marchetti G., Bonotti A., Baldi S., Solito B., Giannetti M., Pinchera A., Santini F., Ferrannini E. Pattern of expression of adiponectin receptors in human liver and its relation to nonalcoholic steatohepatitis. Obes. Surg. 2009;19:467–474. doi: 10.1007/s11695-008-9701-x. [DOI] [PubMed] [Google Scholar]
- 39.Ma H., Gomez V., Lu L., Yang X., Wu X., Xiao S.Y. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2009;24:233–237. doi: 10.1111/j.1440-1746.2008.05548.x. [DOI] [PubMed] [Google Scholar]
- 40.Bull M.J., Plummer N.T. Part 1: the human gut microbiome in health and disease. Integr. Med. 2014;13:17–22. [PMC free article] [PubMed] [Google Scholar]
- 41.Gratz S.W., Mykkanen H., El-Nezami H.S. Probiotics and gut health: a special focus on liver diseases. World J. Gastroenterol. 2010;16:403–410. doi: 10.3748/wjg.v16.i4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zak-Golab A., Olszanecka-Glinianowicz M., Kocelak P., Chudek J. The role of gut microbiota in the pathogenesis of obesity. Post. Hig. Med. Dosw. 2014;68:84–90. doi: 10.5604/17322693.1086419. (Online) [DOI] [PubMed] [Google Scholar]
- 43.Preiss-Landl K., Zimmermann R., Hammerle G., Zechner R. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr. Opin. Lipidol. 2002;13:471–481. doi: 10.1097/00041433-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Valentini M., Piermattei A., Di Sante G., Migliara G., Delogu G., Ria F. Immunomodulation by gut microbiota: role of toll-like receptor expressed by T cells. J. Immunol. Res. 2014;2014:586939. doi: 10.1155/2014/586939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J.J., Sears D.D. TLR4 and insulin resistance. Gastroenterol. Res. Pract. 2010;2010 doi: 10.1155/2010/212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmee E., Cousin B., Sulpice T., Chamontin B., Ferrieres J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 47.Neal M.D., Leaphart C., Levy R., Prince J., Billiar T.R., Watkins S., Li J., Cetin S., Ford H., Schreiber A., Hackam D.J. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Ghoshal S., Ward M., de Villiers W., Woodward J., Eckhardt E. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PLoS One. 2009;4:e8442. doi: 10.1371/journal.pone.0008442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vreugdenhil A.C., Rousseau C.H., Hartung T., Greve J.W., van't Veer C., Buurman W.A. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immunol. 2003;170:1399–1405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- 50.Brun P., Castagliuolo I., Di Leo V., Buda A., Pinzani M., Palu G., Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 51.Wright S.D., Ramos R.A., Tobias P.S., Ulevitch R.J., Mathison J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 52.Beutler B., Hoebe K., Du X., Ulevitch R.J. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 2003;74:479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz A.G., Casafont F., Crespo J., Cayon A., Mayorga M., Estebanez A., Fernadez-Escalante J.C., Pons-Romero F. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes. Surg. 2007;17:1374–1380. doi: 10.1007/s11695-007-9243-7. [DOI] [PubMed] [Google Scholar]
- 54.Brun P., Castagliuolo I., Pinzani M., Palu G., Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G571–G578. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 55.Paik Y.H., Schwabe R.F., Bataller R., Russo M.P., Jobin C., Brenner D.A. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 56.Albano E., Mottaran E., Occhino G., Reale E., Vidali M. Review article: role of oxidative stress in the progression of non-alcoholic steatosis. Aliment. Pharmacol. Ther. 2005;22(Suppl. 2):S71–S73. doi: 10.1111/j.1365-2036.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 57.Albano E., Mottaran E., Vidali M., Reale E., Saksena S., Occhino G., Burt A.D., Day C.P. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–993. doi: 10.1136/gut.2004.057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seki S., Kitada T., Yamada T., Sakaguchi H., Nakatani K., Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 59.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Jones R.M., Neish A.S. Redox signaling mediated by the gut microbiota. Free Radic. Biol. Med. 2017;105:41–47. doi: 10.1016/j.freeradbiomed.2016.10.495. [DOI] [PubMed] [Google Scholar]
- 61.Kountouras J., Boziki M., Polyzos S.A., Katsinelos P., Gavalas E., Zeglinas C., Tzivras D., Romiopoulos I., Giorgakis N., Anastasiadou K., Vardaka E., Kountouras C., Kazakos E., Xiromerisiou G., Dardiotis E., Deretzi G. Impact of reactive oxygen species generation on Helicobacter pylori-related extragastric diseases: a hypothesis. Free Radic. Res. 2017;51:73–79. doi: 10.1080/10715762.2016.1271122. [DOI] [PubMed] [Google Scholar]
- 62.Doulberis M., Kotronis G., Gialamprinou D., Kountouras J., Katsinelos P. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism. 2017;71:182–197. doi: 10.1016/j.metabol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Purohit V., Bode J.C., Bode C., Brenner D.A., Choudhry M.A., Hamilton F., Kang Y.J., Keshavarzian A., Rao R., Sartor R.B., Swanson C., Turner J.R. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Setshedi M., Wands J.R., Monte S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell. Longev. 2010;3:178–185. doi: 10.4161/oxim.3.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su G.L. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 66.Gustot T., Lemmers A., Moreno C., Nagy N., Quertinmont E., Nicaise C., Franchimont D., Louis H., Deviere J., Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 67.Wieckowska A., McCullough A.J., Feldstein A.E. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 68.Nassir F., Ibdah J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014;15:8713–8742. doi: 10.3390/ijms15058713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fritz R., Bol J., Hebling U., Angermuller S., Volkl A., Fahimi H.D., Mueller S. Compartment-dependent management of H(2)O(2) by peroxisomes. Free Radic. Biol. Med. 2007;42:1119–1129. doi: 10.1016/j.freeradbiomed.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 70.Rolo A.P., Teodoro J.S., Palmeira C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nelson J.E., Wilson L., Brunt E.M., Yeh M.M., Kleiner D.E., Unalp-Arida A., Kowdley K.V., Nonalcoholic Steatohepatitis Clinical Research N. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ricci C., Pastukh V., Leonard J., Turrens J., Wilson G., Schaffer D., Schaffer S.W., Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am. J. Physiol. Cell Physiol. 2008;294:C413–C422. doi: 10.1152/ajpcell.00362.2007. [DOI] [PubMed] [Google Scholar]
- 75.Picard M. Pathways to aging: the mitochondrion at the intersection of biological and psychosocial sciences. J. Aging Res. 2011;2011:814096. doi: 10.4061/2011/814096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scarpulla R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aharoni-Simon M., Hann-Obercyger M., Pen S., Madar Z., Tirosh O. Fatty liver is associated with impaired activity of PPARgamma-coactivator 1alpha (PGC1alpha) and mitochondrial biogenesis in mice. Lab. Invest. 2011;91:1018–1028. doi: 10.1038/labinvest.2011.55. [DOI] [PubMed] [Google Scholar]
- 78.Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C.R., Granner D.K., Newgard C.B., Spiegelman B.M. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 79.Mardinoglu A., Shoaie S., Bergentall M., Ghaffari P., Zhang C., Larsson E., Backhed F., Nielsen J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol. Syst. Biol. 2015;11:834. doi: 10.15252/msb.20156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kojer K., Bien M., Gangel H., Morgan B., Dick T.P., Riemer J. Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state. EMBO J. 2012;31:3169–3182. doi: 10.1038/emboj.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgan B., Ezerina D., Amoako T.N., Riemer J., Seedorf M., Dick T.P. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat. Chem. Biol. 2013;9:119–125. doi: 10.1038/nchembio.1142. [DOI] [PubMed] [Google Scholar]
- 82.Davila A.M., Blachier F., Gotteland M., Andriamihaja M., Benetti P.H., Sanz Y., Tome D. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013;68:95–107. doi: 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Sanyal D., Mukherjee P., Raychaudhuri M., Ghosh S., Mukherjee S., Chowdhury S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J. Endocrinol. Metab. 2015;19:597–601. doi: 10.4103/2230-8210.163172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grattagliano I., Portincasa P., Palmieri V.O., Palasciano G. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can. Fam. Phys. 2007;53:857–863. [PMC free article] [PubMed] [Google Scholar]
- 85.Portincasa P., Grattagliano I., Palmieri V.O., Palasciano G. The emerging problem of nonalcoholic steatohepatitis (NASH) Rom. J. Gastroenterol. 2005;14:43–51. [PubMed] [Google Scholar]
- 86.Portincasa P., Grattagliano I., Palmieri V.O., Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clin. Biochem. 2005;38:203–217. doi: 10.1016/j.clinbiochem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 87.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., Yeh M., McCullough A.J., Sanyal A.J., Nonalcoholic Steatohepatitis Clinical Research N. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 88.Brunt E.M., Kleiner D.E., Wilson L.A., Belt P., Neuschwander-Tetri B.A., Network N.C.R. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., Mills P.R., Keach J.C., Lafferty H.D., Stahler A., Haflidadottir S., Bendtsen F. liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. (e310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alkhouri N., Berk M., Yerian L., Lopez R., Chung Y.M., Zhang R., McIntyre T.M., Feldstein A.E., Hazen S.L. OxNASH score correlates with histologic features and severity of nonalcoholic fatty liver disease. Dig. Dis. Sci. 2014;59:1617–1624. doi: 10.1007/s10620-014-3031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V., Fontaine H., Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 92.Castera L., Vergniol J., Foucher J., Le Bail B., Chanteloup E., Haaser M., Darriet M., Couzigou P., De Ledinghen V. Prospective comparison of transient elastography, fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 93.van Katwyk S., Coyle D., Cooper C., Pussegoda K., Cameron C., Skidmore B., Brener S., Moher D., Thavorn K. Transient elastography for the diagnosis of liver fibrosis: a systematic review of economic evaluations. Liver Int. 2017;37:851–861. doi: 10.1111/liv.13260. [DOI] [PubMed] [Google Scholar]
- 94.Masuzaki R., Tateishi R., Yoshida H., Goto E., Sato T., Ohki T., Goto T., Yoshida H., Kanai F., Sugioka Y., Ikeda H., Shiina S., Kawabe T., Omata M. Comparison of liver biopsy and transient elastography based on clinical relevance. Can. J. Gastroenterol. 2008;22:753–757. doi: 10.1155/2008/306726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Musso G., Gambino R., Cassader M. Non-alcoholic fatty liver disease from pathogenesis to management: an update. Obes. Rev. 2010;11:430–445. doi: 10.1111/j.1467-789X.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- 96.Oseini A.M., Sanyal A.J. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37:97–103. doi: 10.1111/liv.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cani P.D., Delzenne N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 98.Ma X., Hua J., Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J. Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seki S., Habu Y., Kawamura T., Takeda K., Dobashi H., Ohkawa T., Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol. Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 100.Collins M.D., Gibson G.R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 101.Usami M., Miyoshi M., Yamashita H. Gut microbiota and host metabolism in liver cirrhosis. World J. Gastroenterol. 2015;21:11597–11608. doi: 10.3748/wjg.v21.i41.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanders M.E., Guarner F., Guerrant R., Holt P.R., Quigley E.M., Sartor R.B., Sherman P.M., Mayer E.A. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lata J., Jurankova J., Kopacova M., Vitek P. Probiotics in hepatology. World J. Gastroenterol. 2011;17:2890–2896. doi: 10.3748/wjg.v17.i24.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jonkers D., Stockbrugger R. Review article: probiotics in gastrointestinal and liver diseases. Aliment. Pharmacol. Ther. 2007;26(Suppl. 2):S133–S148. doi: 10.1111/j.1365-2036.2007.03480.x. [DOI] [PubMed] [Google Scholar]
- 106.Soriano G., Sanchez E., Guarner C., Schiffrin E.J. Lactobacillus johnsonii La1 without antioxidants does not decrease bacterial translocation in rats with carbon tetrachloride-induced cirrhosis. J. Hepatol. 2012;57:1395–1396. doi: 10.1016/j.jhep.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 107.Usami M., Miyoshi M., Kanbara Y., Aoyama M., Sakaki H., Shuno K., Hirata K., Takahashi M., Ueno K., Tabata S., Asahara T., Nomoto K. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J. Parenter. Enter. Nutr. 2011;35:317–328. doi: 10.1177/0148607110379813. [DOI] [PubMed] [Google Scholar]
- 108.Blendis L., Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol. Ther. 2001;89:221–231. doi: 10.1016/s0163-7258(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 109.Liu Q., Duan Z.P., Ha D.K., Bengmark S., Kurtovic J., Riordan S.M. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 110.Agrawal A., Sharma B.C., Sharma P., Sarin S.K. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am. J. Gastroenterol. 2012;107:1043–1050. doi: 10.1038/ajg.2012.113. [DOI] [PubMed] [Google Scholar]
- 111.Bajaj J.S., Saeian K., Christensen K.M., Hafeezullah M., Varma R.R., Franco J., Pleuss J.A., Krakower G., Hoffmann R.G., Binion D.G. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am. J. Gastroenterol. 2008;103:1707–1715. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 112.Malaguarnera M., Gargante M.P., Malaguarnera G., Salmeri M., Mastrojeni S., Rampello L., Pennisi G., Li Volti G., Galvano F. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur. J. Gastroenterol. Hepatol. 2010;22:199–206. doi: 10.1097/MEG.0b013e328330a8d3. [DOI] [PubMed] [Google Scholar]
- 113.Tandon P., Moncrief K., Madsen K., Arrieta M.C., Owen R.J., Bain V.G., Wong W.W., Ma M.M. Effects of probiotic therapy on portal pressure in patients with cirrhosis: a pilot study. Liver Int. 2009;29:1110–1115. doi: 10.1111/j.1478-3231.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 114.McGee R.G., Bakens A., Wiley K., Riordan S.M., Webster A.C. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst. Rev. 2011:CD008716. doi: 10.1002/14651858.CD008716.pub2. [DOI] [PubMed] [Google Scholar]
- 115.Velayudham A., Dolganiuc A., Ellis M., Petrasek J., Kodys K., Mandrekar P., Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Loguercio C., Federico A., Tuccillo C., Terracciano F., D'Auria M.V., De Simone C., Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J. Clin. Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 117.Gupta N., Kumar A., Sharma P., Garg V., Sharma B.C., Sarin S.K. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int. 2013;33:1148–1157. doi: 10.1111/liv.12172. [DOI] [PubMed] [Google Scholar]
- 118.Pande C., Kumar A., Sarin S.K. Addition of probiotics to norfloxacin does not improve efficacy in the prevention of spontaneous bacterial peritonitis: a double-blind placebo-controlled randomized-controlled trial. Eur. J. Gastroenterol. Hepatol. 2012;24:831–839. doi: 10.1097/MEG.0b013e3283537d61. [DOI] [PubMed] [Google Scholar]
- 119.Musso G., Cassader M., Rosina F., Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 120.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C., Tio F., Hardies J., Darland C., Musi N., Webb A., Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann. Intern. Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 121.Neumann A., Weill A., Ricordeau P., Fagot J.P., Alla F., Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953–1962. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bourg C.A., Phillips B.B. Rosiglitazone, myocardial ischemic risk, and recent regulatory actions. Ann. Pharmacother. 2012;46:282–289. doi: 10.1345/aph.1Q400. [DOI] [PubMed] [Google Scholar]
- 123.Molavi B., Rassouli N., Bagwe S., Rasouli N. A review of thiazolidinediones and metformin in the treatment of type 2 diabetes with focus on cardiovascular complications. Vasc. Health Risk Manag. 2007;3:967–973. [PMC free article] [PubMed] [Google Scholar]
- 124.Sokol R.J., McKim J.M., Jr, Goff M.C., Ruyle S.Z., Devereaux M.W., Han D., Packer L., Everson G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114:164–174. doi: 10.1016/s0016-5085(98)70644-4. [DOI] [PubMed] [Google Scholar]
- 125.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., Van Natta M., Clark J., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R., Nash C.R.N. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klein E.A., Thompson I.M., Jr., Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J., Minasian L.M., Ford L.G., Parnes H.L., Gaziano J.M., Karp D.D., Lieber M.M., Walther P.J., Klotz L., Parsons J.K., Chin J.L., Darke A.K., Lippman S.M., Goodman G.E., Meyskens F.L., Jr., Baker L.H. vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (select) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Freitas A.C., Campos A.C., Coelho J.C. The impact of bariatric surgery on nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:267–274. doi: 10.1097/MCO.0b013e3282fbd33f. [DOI] [PubMed] [Google Scholar]
- 128.Rabl C., Campos G.M. The impact of bariatric surgery on nonalcoholic steatohepatitis. Semin. Liver Dis. 2012;32:80–91. doi: 10.1055/s-0032-1306428. [DOI] [PubMed] [Google Scholar]
- 129.Lim J.K. The role of bariatric surgery in the management of nonalcoholic steatohepatitis. Gastroenterol. Hepatol. 2016;12:397–399. [PMC free article] [PubMed] [Google Scholar]
- 130.Gerhard G.S., DiStefano J.K. Micro RNAs in the development of non-alcoholic fatty liver disease. World J. Hepatol. 2015;7:226–234. doi: 10.4254/wjh.v7.i2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jani R.H., Kansagra K., Jain M.R., Patel H. Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARalpha agonist with moderate PPARgamma agonist activity in healthy human subjects. Clin. Drug Investig. 2013;33:809–816. doi: 10.1007/s40261-013-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liles J.T., Karnik S., Hambruch E., Kremoser C., Birkel M., Watkins W.J., Tumas D., Breckenridge D., French D. Fxr agonism by Gs-9674 decreases steatosis and fibrosis in a murine model of nash. J. Hepatol. 2016;64:S169. [Google Scholar]
- 133.Dong J.Q., Rossulek M., Somayaji V.R., Baltrukonis D., Liang Y., Hudson K., Hernandez-Illas M., Calle R.A. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br. J. Clin. Pharmacol. 2015;80:1051–1063. doi: 10.1111/bcp.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.G. Wettstein, C. Estivalet, J. Tessier, P.T. Boustugue, I. Jantzen, E. Defrene, F. Kupkowski, P. Faye, V. Adarbes, J.M. Germain, E. Vasseur, C. Robert, C. Fromond, P. Broqua, J.L. Junien, J.M. Luccarini, I. Konstantinova, The new generation Pan-Ppar agonist Iva337 protects the liver from metabolic disorders and fibrosis, J. Hepatol., 64, pp. S169–S170. [DOI] [PMC free article] [PubMed]
- 135.Hegade V.S., Speight R.A., Etherington R.E., Jones D.E. Novel bile acid therapeutics for the treatment of chronic liver diseases. Ther. Adv. Gastroenterol. 2016;9:376–391. doi: 10.1177/1756283X16630712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mousa H.S., Lleo A., Invernizzi P., Bowlus C.L., Gershwin M.E. Advances in pharmacotherapy for primary biliary cirrhosis. Expert Opin. Pharmacother. 2015;16:633–643. doi: 10.1517/14656566.2015.998650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu X., Ge H., Lemon B., Vonderfecht S., Weiszmann J., Hecht R., Gupte J., Hager T., Wang Z., Lindberg R., Li Y. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J. Biol. Chem. 2010;285:5165–5170. doi: 10.1074/jbc.M109.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Safadi R., Konikoff F.M., Mahamid M., Zelber-Sagi S., Halpern M., Gilat T., Oren R., Group F. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014;12:2085–2091. doi: 10.1016/j.cgh.2014.04.038. (e2081) [DOI] [PubMed] [Google Scholar]
- 139.Miethke A.G., Zhang W., Simmons J., Taylor A.E., Shi T., Shanmukhappa S.K., Karns R., White S., Jegga A.G., Lages C.S., Nkinin S., Keller B.T., Setchell K.D. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–523. doi: 10.1002/hep.27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rao A., Kosters A., Mells J.E., Zhang W., Setchell K.D., Amanso A.M., Wynn G.M., Xu T., Keller B.T., Yin H., Banton S., Jones D.P., Wu H., Dawson P.A., Karpen S.J. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016;8:357ra122. doi: 10.1126/scitranslmed.aaf4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R., Hazlehurst J.M., Guo K., L.t. team, Abouda G., Aldersley M.A., Stocken D., Gough S.C., Tomlinson J.W., Brown R.M., Hubscher S.G., Newsome P.N. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 142.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., Romero-Gomez M., Boursier J., Abdelmalek M., Caldwell S., Drenth J., Anstee Q.M., Hum D., Hanf R., Roudot A., Megnien S., Staels B., Sanyal A., Group G.-I.S. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159. doi: 10.1053/j.gastro.2016.01.038. (e1145) [DOI] [PubMed] [Google Scholar]
- 143.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F., Chalasani N., Dasarathy S., Diehl A.M., Hameed B., Kowdley K.V., McCullough A., Terrault N., Clark J.M., Tonascia J., Brunt E.M., Kleiner D.E., Doo E., Network N.C.R. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H.U., Kipnes M., Adorini L., Sciacca C.I., Clopton P., Castelloe E., Dillon P., Pruzanski M., Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. doi: 10.1053/j.gastro.2013.05.042. (e571) [DOI] [PubMed] [Google Scholar]
- 145.Friedman S., Sanyal A., Goodman Z., Lefebvre E., Gottwald M., Fischer L., Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR phase 2b study design. Contemp. Clin. Trials. 2016;47:356–365. doi: 10.1016/j.cct.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 146.Godoy A.L., Rocha A., da Silva Souza C., Lanchote V.L. Pharmacokinetics of venlafaxine enantiomers and their metabolites in psoriasis patients. J. Clin. Pharmacol. 2016;56:567–575. doi: 10.1002/jcph.630. [DOI] [PubMed] [Google Scholar]
- 147.Cui J., Philo L., Nguyen P., Hofflich H., Hernandez C., Bettencourt R., Richards L., Salotti J., Bhatt A., Hooker J., Haufe W., Hooker C., Brenner D.A., Sirlin C.B., Loomba R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J. Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McCormack P.L. Evogliptin: first global approval. Drugs. 2015;75:2045–2049. doi: 10.1007/s40265-015-0496-5. [DOI] [PubMed] [Google Scholar]
- 149.Schilter H.C., Collison A., Russo R.C., Foot J.S., Yow T.T., Vieira A.T., Tavares L.D., Mattes J., Teixeira M.M., Jarolimek W. Effects of an anti-inflammatory VAP-1/SSAO inhibitor, PXS-4728A, on pulmonary neutrophil migration. Respir. Res. 2015;16:42. doi: 10.1186/s12931-015-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin J.H., Zhang J.J., Lin S.L., Chertow G.M. Design of a phase 2 clinical trial of an ASK1 inhibitor, GS-4997, in patients with diabetic kidney disease. Nephron. 2015;129:29–33. doi: 10.1159/000369152. [DOI] [PubMed] [Google Scholar]
- 151.Hardy T., Anstee Q.M., Day C.P. Nonalcoholic fatty liver disease: new treatments. Curr. Opin. Gastroenterol. 2015;31:175–183. doi: 10.1097/MOG.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harrison S.A., Marri S.R., Chalasani N., Kohli R., Aronstein W., Thompson G.A., Irish W., Miles M.V., Xanthakos S.A., Lawitz E., Noureddin M., Schiano T.D., Siddiqui M., Sanyal A., Neuschwander-Tetri B.A., Traber P.G. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment. Pharmacol. Ther. 2016;44:1183–1198. doi: 10.1111/apt.13816. [DOI] [PubMed] [Google Scholar]
- 153.Barreyro F.J., Holod S., Finocchietto P.V., Camino A.M., Aquino J.B., Avagnina A., Carreras M.C., Poderoso J.J., Gores G.J. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35:953–966. doi: 10.1111/liv.12570. [DOI] [PubMed] [Google Scholar]
- 154.Filozof C., Goldstein B.J., Williams R.N., Sanyal A. Non-alcoholic steatohepatitis: limited available treatment options but promising drugs in development and recent progress towards a regulatory approval pathway. Drugs. 2015;75:1373–1392. doi: 10.1007/s40265-015-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.W. Jarolimek B. Charlton LP22: phase 1 results from PXS-4728A, a selective SSAO/VAP-1 inhibitor, for the treatment of non-alcoholic steatohepatitis J. Hepatol. 62 S274 S275 .
- 156.Weston C.J., Shepherd E.L., Claridge L.C., Rantakari P., Curbishley S.M., Tomlinson J.W., Hubscher S.G., Reynolds G.M., Aalto K., Anstee Q.M., Jalkanen S., Salmi M., Smith D.J., Day C.P., Adams D.H. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J. Clin. Invest. 2015;125:501–520. doi: 10.1172/JCI73722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 158.Liao J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Invest. 2013;123:540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Halliwell B. Antioxidants: the basics–what they are and how to evaluate them. Adv. Pharmacol. 1997;38:3–20. doi: 10.1016/s1054-3589(08)60976-x. [DOI] [PubMed] [Google Scholar]
- 160.Domann F.E. Aberrant free radical biology is a unifying theme in the etiology and pathogenesis of major human diseases. Int. J. Mol. Sci. 2013;14:8491–8495. doi: 10.3390/ijms14048491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Borrelli A., Schiattarella A., Mancini R., Pica A., Pollio M.L., Ruggiero M.G., Bonelli P., De Luca V., Tuccillo F.M., Capasso C., Gori E., Sanseverino M., Carpentieri A., Birolo L., Pucci P., Rommelaere J., Mancini A. A new hexapeptide from the leader peptide of rMnSOD enters cells through the oestrogen receptor to deliver therapeutic molecules. Sci. Rep. 2016;6:18691. doi: 10.1038/srep18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wan X.S., Devalaraja M.N., St Clair D.K. Molecular structure and organization of the human manganese superoxide dismutase gene. DNA Cell Biol. 1994;13:1127–1136. doi: 10.1089/dna.1994.13.1127. [DOI] [PubMed] [Google Scholar]
- 163.Folz R.J., Guan J., Seldin M.F., Oury T.D., Enghild J.J., Crapo J.D. Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am. J. Respir. Cell Mol. Biol. 1997;17:393–403. doi: 10.1165/ajrcmb.17.4.2826. [DOI] [PubMed] [Google Scholar]
- 164.Wispe J.R., Clark J.C., Burhans M.S., Kropp K.E., Korfhagen T.R., Whitsett J.A. Synthesis and processing of the precursor for human mangano-superoxide dismutase. Biochim. Biophys. Acta. 1989;994:30–36. doi: 10.1016/0167-4838(89)90058-7. [DOI] [PubMed] [Google Scholar]
- 165.Marberger H., Huber W., Bartsch G., Schulte T., Swoboda P. Orgotein: a new antiinflammatory metalloprotein drug evaluation of clinical efficacy and safety in inflammatory conditions of the urinary tract. Int. Urol. Nephrol. 1974;6:61–74. doi: 10.1007/BF02081999. [DOI] [PubMed] [Google Scholar]
- 166.Emerit J., Michelson A.M., Robert H.G., Chomette G., Guerin R.A., Blondon J., Bertrand M. Superoxide dismutase treatment of 2 cases of radiation-induced sclerosis. Sem. Hop. 1983;59:277–281. [PubMed] [Google Scholar]
- 167.Land W., Schneeberger H., Schleibner S., Illner W.D., Abendroth D., Rutili G., Arfors K.E., Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57:211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 168.Brazier M.W., Doctrow S.R., Masters C.L., Collins S.J. A manganese-superoxide dismutase/catalase mimetic extends survival in a mouse model of human prion disease. Free Radic. Biol. Med. 2008;45:184–192. doi: 10.1016/j.freeradbiomed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 169.Dhar S.K., St Clair D.K. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012;52:2209–2222. doi: 10.1016/j.freeradbiomed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 170.Weydert C., Roling B., Liu J., Hinkhouse M.M., Ritchie J.M., Oberley L.W., Cullen J.J. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol. Cancer Ther. 2003;2:361–369. [PubMed] [Google Scholar]
- 171.Oberley L.W. Mechanism of the tumor suppressive effect of MnSOD overexpression. Biomed. Pharmacother. 2005;59:143–148. doi: 10.1016/j.biopha.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 172.Borrelli A., Schiattarella A., Bonelli P., Tuccillo F.M., Buonaguro F.M., Mancini A. The functional role of MnSOD as a biomarker of human diseases and therapeutic potential of a new isoform of a human recombinant MnSOD. Biomed. Res. Int. 2014;2014:476789. doi: 10.1155/2014/476789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McCord J.M., Edeas M.A. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed. Pharmacother. 2005;59:139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 174.Mancini A., Borrelli A., Schiattarella A., Fasano S., Occhiello A., Pica A., Sehr P., Tommasino M., Nuesch J.P., Rommelaere J. Tumor suppressive activity of a variant isoform of manganese superoxide dismutase released by a human liposarcoma cell line. Int. J. Cancer. 2006;119:932–943. doi: 10.1002/ijc.21904. [DOI] [PubMed] [Google Scholar]
- 175.Mancini A., Borrelli A., Formisano S., Masucci M.T., Maffeo A., Perla G., De Martino L., Bevilacqua N., Botti G., Maggino T. Establishment and growth regulation of a novel ovarian cancer cell line from a poorly-differentiated adenocarcinoma: proposal for a new treatment. Eur. J. Gynaecol. Oncol. 1999;20:45–52. [PubMed] [Google Scholar]
- 176.Pisani A., Sabbatini M., Riccio E., Rossano R., Andreucci M., Capasso C., De Luca V., Carginale V., Bizzarri M., Borrelli A., Schiattarella A., Santangelo M., Mancini A. Effect of a recombinant manganese superoxide dismutase on prevention of contrast-induced acute kidney injury. Clin. Exp. Nephrol. 2014;18:424–431. doi: 10.1007/s10157-013-0828-2. [DOI] [PubMed] [Google Scholar]
- 177.Guillaume M., Rodriguez-Vilarrupla A., Gracia-Sancho J., Rosado E., Mancini A., Bosch J., Garcia-Pagan J.C. Recombinant human manganese superoxide dismutase reduces liver fibrosis and portal pressure in CCl4-cirrhotic rats. J. Hepatol. 2013;58:240–246. doi: 10.1016/j.jhep.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 178.Gracia-Sancho J., Lavina B., Rodriguez-Vilarrupla A., Garcia-Caldero H., Fernandez M., Bosch J., Garcia-Pagan J.C. Increased oxidative stress in cirrhotic rat livers: a potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008;47:1248–1256. doi: 10.1002/hep.22166. [DOI] [PubMed] [Google Scholar]
- 179.Hide D., Ortega-Ribera M., Fernandez-Iglesias A., Fondevila C., Salvado M.J., Arola L., Garcia-Pagan J.C., Mancini A., Bosch J., Gracia-Sancho J. A novel form of the human manganese superoxide dismutase protects rat and human livers undergoing ischaemia and reperfusion injury. Clin. Sci. 2014;127:527–537. doi: 10.1042/CS20140125. [DOI] [PubMed] [Google Scholar]
- 180.Mancini A., Borrelli A., Schiattarella A., Aloj L., Aurilio M., Morelli F., Pica A., Occhiello A., Lorizio R., Mancini R., Sica A., Mazzarella L., Sica F., Grieco P., Novellino E., Pagnozzi D., Pucci P., Rommelaere J. Biophysical and biochemical characterization of a liposarcoma-derived recombinant MnSOD protein acting as an anticancer agent. Int. J. Cancer. 2008;123:2684–2695. doi: 10.1002/ijc.23791. [DOI] [PubMed] [Google Scholar]
- 181.Borrelli A., Schiattarella A., Mancini R., Morelli F., Capasso C., De Luca V., Gori E., Mancini A. The leader peptide of a human rec. MnSOD as molecular carrier which delivers high amounts of Cisplatin into tumor cells inducing a fast apoptosis in vitro. Int. J. Cancer. 2011;128:453–459. doi: 10.1002/ijc.25334. [DOI] [PubMed] [Google Scholar]
- 182.Porter L.E., Elm M.S., Van Thiel D.H., Eagon P.K. Hepatic estrogen receptor in human liver disease. Gastroenterology. 1987;92:735–745. doi: 10.1016/0016-5085(87)90026-6. [DOI] [PubMed] [Google Scholar]
- 183.Melegari M., Scaglioni P.P., Mao G.P., Pasquinelli C., Baldini G. Estrogen receptors in the human liver. Medicina. 1990;10:154–157. [PubMed] [Google Scholar]
- 184.Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63–69. doi: 10.1034/j.1600-0676.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 185.Matic M., Bryzgalova G., Gao H., Antonson P., Humire P., Omoto Y., Portwood N., Pramfalk C., Efendic S., Berggren P.O., Gustafsson J.A., Dahlman-Wright K. Estrogen signalling and the metabolic syndrome: targeting the hepatic estrogen receptor alpha action. PLoS One. 2013;8:e57458. doi: 10.1371/journal.pone.0057458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pan H., Marsh J.N., Christenson E.T., Soman N.R., Ivashyna O., Lanza G.M., Schlesinger P.H., Wickline S.A. Postformulation peptide drug loading of nanostructures. Methods Enzymol. 2012;508:17–39. doi: 10.1016/B978-0-12-391860-4.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Egusquiaguirre S.P., Igartua M., Hernandez R.M., Pedraz J.L. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin. Transl. Oncol. 2012;14:83–93. doi: 10.1007/s12094-012-0766-6. [DOI] [PubMed] [Google Scholar]
- 188.Paulo C.S., Pires das Neves R., Ferreira L.S. Nanoparticles for intracellular-targeted drug delivery. Nanotechnology. 2011;22:494002. doi: 10.1088/0957-4484/22/49/494002. [DOI] [PubMed] [Google Scholar]
- 189.Hogg R.T., Thorpe P., Gerard R.D. Retargeting adenoviral vectors to improve gene transfer into tumors. Cancer Gene Ther. 2011;18:275–287. doi: 10.1038/cgt.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaneda Y. Update on non-viral delivery methods for cancer therapy: possibilities of a drug delivery system with anticancer activities beyond delivery as a new therapeutic tool. Expert Opin. Drug Deliv. 2010;7:1079–1093. doi: 10.1517/17425247.2010.510511. [DOI] [PubMed] [Google Scholar]
- 191.Namiki Y., Fuchigami T., Tada N., Kawamura R., Matsunuma S., Kitamoto Y., Nakagawa M. Nanomedicine for cancer: lipid-based nanostructures for drug delivery and monitoring. Acc. Chem. Res. 2011;44:1080–1093. doi: 10.1021/ar200011r. [DOI] [PubMed] [Google Scholar]
- 192.Zhai X., Huang W., Liu J., Pang Y., Zhu X., Zhou Y., Yan D. Micelles from amphiphilic block copolyphosphates for drug delivery. Macromol. Biosci. 2011;11:1603–1610. doi: 10.1002/mabi.201100163. [DOI] [PubMed] [Google Scholar]
- 193.Pasquetto M.V., Vecchia L., Covini D., Digilio R., Scotti C. Targeted drug delivery using immunoconjugates: principles and applications. J. Immunother. 2011;34:611–628. doi: 10.1097/CJI.0b013e318234ecf5. [DOI] [PubMed] [Google Scholar]
- 194.Liu K., Wang X., Fan W., Zhu Q., Yang J., Gao J., Gao S. Degradable polyethylenimine derivate coupled to a bifunctional peptide R13 as a new gene-delivery vector. Int. J. Nanomed. 2012;7:1149–1162. doi: 10.2147/IJN.S28819. [DOI] [PMC free article] [PubMed] [Google Scholar]