Abstract
HIV-1 and Zika virus (ZIKV) represent RNA viruses with neurotropic characteristics. Infected individuals suffer neurocognitive disorders aggravated by environmental toxins, including drugs of abuse such as cocaine, exacerbating HIV-associated neurocognitive disorders through a combination of astrogliosis, oxidative stress and innate immune signaling; however, little is known about how cocaine impacts the progression of ZIKV neural perturbations. Impaired innate immune signaling is characterized by weakened antiviral activation of interferon signaling and alterations in inflammatory signaling, factors contributing to cognitive sequela associated with cocaine in HIV- 1/ZIKV infection. We employed cellular/molecular biology techniques to test if cocaine suppresses the efficacy of astrocytes to initiate a Type 1 interferon response to HIV-1/ZIKV, in vitro. We found cocaine activated antiviral signaling pathways and type I interferon in the absence of inflammation. Cocaine pre-exposure suppressed antiviral responses to HIV-1/ZIKV, triggering antiviral signaling and phosphorylation of interferon regulatory transcription factor 3 to stimulate type I interferon gene transcription. Our data indicate that oxidative stress is a major driver of cocaine-mediated astrocyte antiviral immune responses. Although astrocyte antiviral signaling is activated following detection of foreign pathogenic material, oxidative stress and increased cytosolic double-stranded DNA (dsDNA) can drive antiviral signaling via stimulation of pattern recognition receptors. Pretreatment with the glial modulators propentofylline (PPF) or pioglitazone (PIO) reversed cocaine-mediated attenuation of astrocyte responses to HIV-1/ZIKV. Both PPF/PIO protected against cocaine-mediated generation of reactive oxygen species (ROS), increased dsDNA, antiviral signaling pathways and increased type I interferon, indicating that cocaine induces astrocyte type I interferon signaling in the absence of virus and oxidative stress is a major driver of cocaine-mediated astrocyte antiviral immunity. Lastly, PPF and PIO have therapeutic potential to ameliorate cocaine-mediated dysregulation of astrocyte antiviral immunity possibly via a myriad of protective actions including decreases in reactive phenotype and damaging immune factors.
Keywords: Astrocytes, Cocaine, Type I interferon, Oxidative stress, HIV-1, Zika virus
Graphical abstract
1. INTRODUCTION
Cocaine use disorder (CUD) continues to be a significant public health problem, with rising cocaine- related overdose deaths associated with the opioid epidemic (McCall Jones et al., 2017). Cocaine is a powerful psychostimulant that acts within the central nervous system (CNS) to evoke addictive properties (for reviews) (Cunningham and Anastasio, 2014; Koob and Volkow, 2016) and its actions in the CNS contributes to the neuropathological signature of human immunodeficiency virus (HIV)-1 and the transmission and progression of acquired immunodeficiency syndrome (AIDS) (for review) (Tyagi et al., 2016). Cocaine also increases the severity of viral-mediated neurotoxicity (Fiala et al., 2005; Yao et al., 2009) which culminates in higher viral loads, neurotransmitter dysregulation, oxidative stress and neuroinflammation (Ersche et al., 2013; Swepson et al., 2016; Yao et al., 2009). This intersection of the public health crisis of CUD and HIV/AIDS premises our need to uncover the mechanisms through which cocaine perpetuates and/or regulates CNS pathological outcomes of viral infections.
The CNS is comprised of neurons and several other cell types, including astrocytes. Astrocyte dysfunction is recognized as a pathology leading to neurodegeneration and is a common feature of cocaine use and viral CNS infections (Cao et al., 2016; Daniels et al., 2017; Hamel et al., 2017; Scofield et al., 2016). Astrocytes respond to infiltrating pathogens and foreign substances by releasing proinflammatory cytokines and chemokines, impacting blood brain barrier integrity (Burns et al., 2012; Cisneros and Ghorpade, 2014; Gorina et al., 2011; Mamik and Ghorpade, 2016). Astrocytes are reactive upon cocaine exposure, partly through signaling of innate immune receptors (Lacagnina et al., 2017; Lee et al., 2016; Periyasamy et al., 2016). Cocaine increases interleukin (IL)-1β in the mesocorticostriatal circuit, a function involved in the addictive properties of cocaine (Cearley et al., 2011; Northcutt et al., 2015). Cocaine also induces autophagy through a nitric oxide-mediated pathway, which is thought to stimulate inflammatory and antiviral neuroprotective responses. Cocaine has a low affinity for sigma 1 receptors (Sharkey et al., 1988), which are also postulated to mediate neuroinflammation and neurogeneration during cocaine exposure (Cai et al., 2017; Guha et al., 2016). Although cocaine activates host pattern recognition receptors (PRRs), toll-like receptor 2 (TLR2) and toll-like receptor 4 (TLR4)/lymphocyte antigen 96 (MD-2) signaling complexes to modulate neuroinflammatory cytokines (Northcutt et al., 2015; Periyasamy et al., 2016), less studied is its role in regulating the protective CNS responses to viral threat, specifically activation of antiviral type I interferon signaling. Cocaine-mediated regulation, activation and/or overstimulation of host innate immune pathways may reveal mechanisms by which cocaine exacerbates virus-mediated neurotoxicity.
Immune responses are activated by cytosolic mammalian PRRs include retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated protein 5 (MDA-5) and cyclic GMP-AMP synthase (cGAS), which are canonically activated by pathogen genetic material (Honda and Taniguchi, 2006). Signaling events within these pathways ultimately result in interferon regulatory transcription factor 3/7 (IRF3/7) and nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) translocation to the nucleus for transcriptional activation of type I interferon and proinflammatory cytokine genes (Baker et al., 2017; Honda and Taniguchi, 2006) The impact of cocaine on activating NF-κB signaling and increasing neuroinflammation has been demonstrated specifically in microglia, the resident macrophage of the CNS which, when stimulated, elicit a cascade of cytokines, chemokines and neurotoxic stimuli (Brown et al., 2017; Buch et al., 2012; Sajja et al., 2016). Furthermore, cocaine- mediated activation of c-jun N-teminal kinase (JNK), p38, extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinases (MAPK), ultimately culminates in NF-κB signaling and increased neuroinflammation (Yao et al., 2009). Interestingly, TBK1-IRF3 signaling and induction of type I interferon is less understood. As microglia, astrocytes can serve as immunocompetent cells, producing both pro- and anti-inflammatory signaling, and cocaine may elicit both immunostimulatory and immunosuppressive responses as dictated by the extent of cocaine exposure in astrocytes (Bik et al., 2008; Cui et al., 2014). We postulated that cocaine exposure triggers innate immune signaling in astrocytes and results in attenuated antiviral responses upon treatment with two different model viruses, a retrovirus (HIV-1) and a single stranded RNA virus (Zika, ZIKV).
We tested the effects of cocaine on antiviral responses in cultured normal human astrocytes (NHA) alone or upon exposure to HIV-1 or ZIKV. Employing molecular biology techniques, we tested the hypothesis that cocaine attenuates the ability of astrocytes to initiate a robust type I interferon response to invading viruses. Our data suggest that cocaine use triggers CNS immune responses that compromise responses to subsequent viral pathogen exposure. We have also identified the efficacy of glial modulators [i.e., propentofylline (PPF), pioglitazone (PIO)] to ameliorate cocaine- induced dysregulation of antiviral immune responses in astrocytes. Interestingly, the glial modulators PPF and PIO differ with regard to their initial biological interaction with cells, yet directly regulate glial activity via several mechanisms, including decreasing glial activation and reactive phenotypes, improving mitochondrial activity and reducing oxidative stress, decreasing the production of damaging immune factors and increasing astrocyte-mediated glutamate clearance (Cooper et al., 2012; Jones et al., 2016; Swanson et al., 2011; Sweitzer and De Leo, 2011). Lastly, a growing body of evidence suggests that glial modulators, including PPF and PIO, are promising potential therapeutics for attenuating the behavioral effects of abused drugs (Cooper et al., 2012; Sweitzer and De Leo, 2011).
2. MATERIALS AND METHODS
2.1 Cell culture and activation of human astrocytes
Clonetics normal human astrocytes (NHA), sex of the primary cells unknown, were maintained in DMEM:F12 supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin (Lonza Walkersville, Inc. Walkersville, MD, cat # CC-2565). Astrocyte preparations are routinely of >99% purity, as described from parent company. Cocaine was obtained from the National Institute on Drug Abuse (NIDA, Research Triangle Park, NC) and was dissolved in phosphate buffer saline solution prior to dilutions in complete astrocyte media for in vitro treatments (0.05, 0.1, 1.0, 10.0 μM), +/− antioxidants, propentofylline (PPF, 10 μM, Sigma-Aldrich Inc., St. Louis, MO) or pioglitazone (PIO, 1 μM, Watson Pharmaceuticals), HIV-1JR-CSF (p24 10 ng/mL, University of California in Los Angeles Center for AIDS Research Virology Core Lab) or Zika virus (ZIKV, 0.125 MOI, World Reference Center for Emerging Viruses and Arboviruses; NIH grant R24AI120942) on astrocytes that reached a final confluency of ~85-90%. HIV-1JR-CSF has been demonstrated to infect human astrocytes (Eugenin and Berman, 2007; Pužar Dominkuš et al., 2017). All treatment were performed at 37°C and 5% CO2.
2.2 RNA extraction and gene expression analyses
NHA were seeded at 2.0 × 106 cells/well in a 6- well plate in 2 mL of growth media. Following 24 hr post-plating, astrocytes were treated with cocaine concentrations ranging from 0-10 μM for 24 hr, in parallel to untreated NHA as the control. DNase- treated total RNA was isolated from untreated and treated cells 24 hr post-treatment using TRIzol Reagent (Invitrogen Corporation, Carlsbad, CA), followed by RNAaqueous Mini and RNeasy-Free DNase Set (QIAGEN Inc., Valencia, CA) according to manufacturer’s instructions. First strand cDNA was synthesized using iScript cDNA synthesis kit from BioRad (cat # 172-5037). BioRad, SYBR Green qPCR commercially available primers were used to measure interleukin (IL)6 (Cat #10025637; Assay ID: qHsaCID0020314), IL8 (Cat #10025637; Assay ID: qHsaCED0046633), interferon (IFN)β1 (Cat #10025637, Assay ID: qHsaCED0046851), IFNα1 (Cat #10025637; Assay ID: qHsaCED0048248), interferon stimulated gene (ISG)15 (Cat #10025637; Assay ID: qHsaCED0001967) and Beta-actin (β-actin) (Cat #10025636; Assay ID: qHsaCED0036269) mRNA levels. β-actin, a ubiquitously expressed housekeeping gene, was used as an internal normalizing control. RT2PCR Profiler™ PCR Array Human Type I interferon Response was used to quantify changes in 68 Type I interferon genes from two independent experiments (n=2, two arrays, BioRad CFX96, Cat #PAHS-016ZD-2, SABiosciences, Frederick, MD). The 20 μL reactions were carried out at 48°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60° C for 1 min in 96-well optical, real-time PCR plates using Bio-Rad CFX96 system. Transcripts were quantified by the comparative ΔΔCT method, and represented as the average fold-change to control, from two independent experiments. The data are represented as a heat map, identifying the genes that were up- or down-regulated by two-fold or more.
2.3 Viability assays
NHA were plated in 48-well tissue culture plates at a density of 0.15 × 106 cells/well and allowed to recover, post-plating, for 24 hr prior to cocaine treatment as described in section 2.1. Following 24 hr treatment, metabolic activity, lactate dehydrogenase (LDH) release and cytosolic double stranded DNA (dsDNA) were measured. The 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay, a colorimetric assay for measurement of metabolic activity, was performed at appropriate time points. Briefly, five percent MTT reagent in astrocyte medium was added to astrocytes and incubated for 20-45 min at 37°C. The MTT solution was removed and crystals were dissolved in DMSO for 15 min with gentle agitation. The absorbance of the DMSO/crystal solution was assayed at 490 nm in a Bio-Tek ELx808 Absorbance microplate reader and analyzed by the Gen5 2.06 software (BioTek Instruments, Inc., Winooski, VT). Cytotoxicity by LDH release was quantified using the cytotoxicity detection kit (Roche Diagnostics, Indianapolis, IN, USA) according to manufacturer’s instructions. Cytosolic dsDNA fragmentation was assayed using the dsDNA ELISA (Roche Diagnostics, Indianapolis, IN, USA) according to manufacturer’s instructions. To measure cell proliferation, a 5-bromo-2′-deoxyridine (BrdU) assay was utilized (cat# 6813, Cell Signaling). Briefly, NHA were plated at 0.05 × 106 cells/well in a 96-well tissue culture plate in 100 μL of growth media. Cocaine and the BrdU solution were supplemented in the media 24 hr post plating. Following 24 hr treatment, the BrdU assay was performed, as per manufacturer’s instructions.
2.4 Intracellular ROS measurements
Intracellular reactive oxygen species (ROS) levels were assayed using a commercially available homogenous, fast and sensitive bioluminescent ROS-Glo™ H2O2 assay that measures the levels of hydrogen peroxide (H2O2) directly in cell culture media or from lysed cells (Promega Corp., Madison, WI). Adherent monolayers of astrocytes were cultured in 96-well tissue culture plates at 0.05 × 106 cells/well. PPF (10 μM) or PIO (1 μM) was administered 10 min prior to cocaine (0-10 μM) exposure. Astrocytes were directly lysed in the tissue culture plate using the commercially available Glo-Lysis buffer as described by manufacturer. Cell lysates were diluted to a final cell concentration of approximately 1,000 cells/μL in dilution buffer and transferred to white opaque flat bottom 96-well assay plates at approximately 5,000 cells/reaction to be further assayed (Corning Life Sciences Inc., Tewksbury, MA). ROS levels were assayed using GloMax 96- well Microplate Luminometer (Promega).
2.5 Immunofluorescence
Cultured, untreated and treated, astrocytes were fixed after 24 hr treatment with 1:1 treatment of acetone:methanol solution for 20 min at −20°C and blocked with blocking buffer (2% BSA in 1X PBS containing 0.1% Triton X-100) for 1 hr. Cells were then incubated with primary antibodies specific to phosphorylated IRF3 (1:1000, rabbit polyclonal, Cell Signaling, cat # 29047), IRF3 (1:1000, rabbit polyclonal, Cell Signaling, cat # 11904) and GFAP (1:1000, chicken polyclonal, cat 829401) in blocking buffer overnight at 4°C, washed and incubated with Alexa Fluor secondary antibodies, anti-rabbit (488 nm, green) and anti-chicken (594 nm, red) (1:100, Life Technologies). Nuclei were visualized with DAPI (1:1000, Life Technologies). Micrographs were obtained on an Olympus IX71 inverted fluorescent microscope at room temperature. Images represent composite astrocyte images.
2.6 Quantification of innate immune proteins by ELISA
Cultured astrocytes were plated in a 48- well tissue culture plate at a density of 0.15 × 106 cells/well and allowed to recover for 24 hr prior to cocaine and HIV-1JR-CSF treatments as described in section 2.1. IL6 (cat #HS600B R & D Systems, Minneapolis, MN), IL8 (cat # D8000C, R & D Systems) and IFNβ1 (cat #41410-1, R & D Systems) were assayed from the supernatants by sandwich ELISA according to manufacturer’s instructions.
2.7 Statistical Analysis
Statistical analysis were carried using GraphPad Prism V6.0 (GraphPad Software, La Jolla, CA, USA). One-way or two-way analysis of variance (ANOVA) and Tukey’s post- hoc analysis for multiple comparisons were performed as described in the legends. Significance was set at p<0.05 and data represent +/− SEM. Representative graphs are shown from a minimum of three independent astrocyte experiments each tested and analyzed in a minimum of triplicate determinations.
3. RESULTS
3.1 Cocaine increases Type I interferon genes
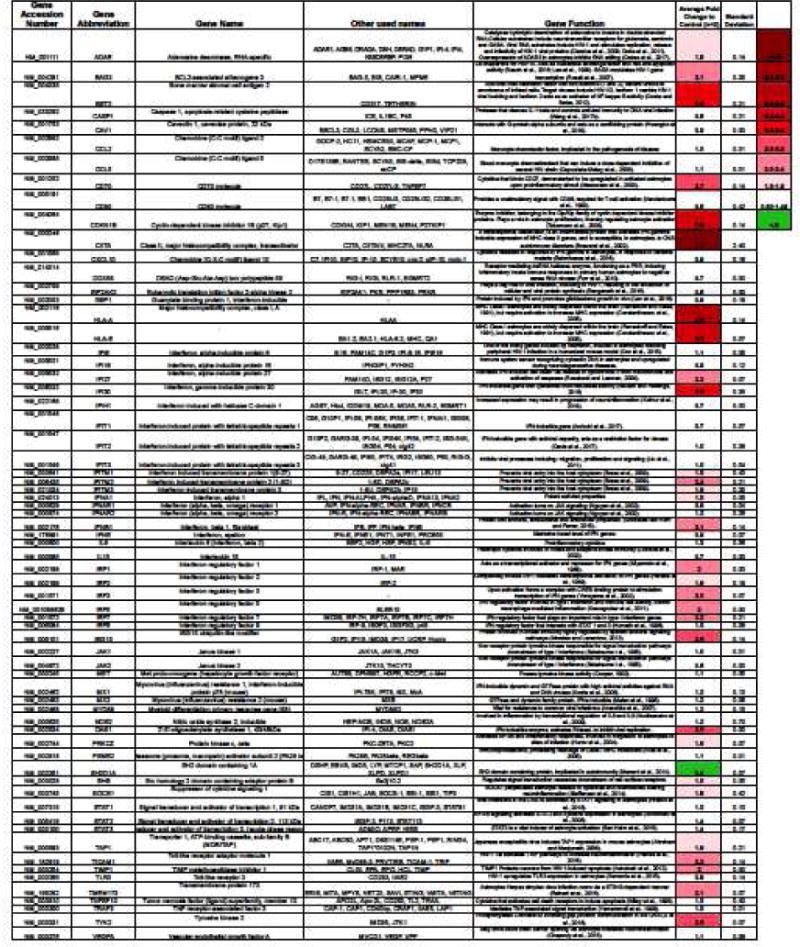
Cocaine is reported to initiate immunomodulatory characteristics in the periphery and the CNS (Cearley et al., 2011; Northcutt et al., 2015) however, little is known about its role in regulating type I interferon responses. We measured gene expression changes in NHA using a commercially available RT2PCR Profiler™ PCR Array Human Type I interferon Response (SABiosciences, Cat #PAHS-016ZD-2), following 24 hr cocaine treatment (0.1 μM) (Table 1). We found that cocaine upregulated 21 genes by 2-fold or higher and downregulated one gene by 2-fold or lower. All other genes we unchanged. Statistical analyses were not conducted. Amongst the genes upregulated, the literature suggests major roles for CD70 molecule (Meeuwsen et al., 2003), CDKN1B (Tabernero et al., 2006), CIITA (Brawand et al., 2002), HLA-A, HLA-E (Ransohoff and Estes, 1991) and STING (Reinert et al., 2016) in astrocyte activation and responses to viral infection, replication and production. Some genes (e.g., ADAR, BAG3, BST2, TICAM1, TIMP1) are specifically demonstrated to be involved in HIV-1 pathogenesis (Clerzius et al., 2009; Doria et al., 2011, Rosati et al., 2007, Cocka and Bates, 2012, Planes et al., 2016, Ashutosh et al., 2012) and a majority are classified specifically as IFN-inducible genes (Morales and Lenschow, 2013, Horvath et al., 1996, Krausgruber et al., 2011, Yoneyama et al., 2002, Harada et al., 1989, Miyamoto et al., 1988, Lodolce et al., 2002, Nguyen et al., 2002, Liu et al., 2011, Davis et al., 2017, Ambuhl et al., 2017, Rausch and Hastings, 2015, Roseback and Leaman, 2008) (Table 1). These data illustrate that a 24 hr cocaine exposure has a profound impact on gene expression in NHA.
Table 1. Changes in Type I Interferon genes mediated by cocaine.
Normal human astrocytes (NHA) were treated with escalating concentration of cocaine (0-10 μM) (Cooper et al., 2012; Jones et al., 2016; Swanson et al., 2011; Sweitzer and De Leo, 2011) in parallel to untreated astrocytes for 24 hr. The RT2PCR Profiler™ PCR Array Human Type I interferon Response was loaded with 100 ng/μL of cDNA. Gene targets from the Type I interferon array are included on the table, labeled by gene Accession number, gene abbreviation, gene name, other names used and their functions. Gene functions are described in the context of astrocytes or HIV-1 infection, when known. Two independent experiments were conducted and the average fold change for each gene following treatment with cocaine is described as fold change relative to control, from two independent experiments. The heat map describes the degree of fold change down or up.
|
3.2 Cocaine alters neuroinflammatory and antiviral gene expression in NHA
To further elucidate the impact of cocaine on the regulation of NHA neuroinflammatory and antiviral genes, we assayed gene expression levels by the quantitative RT-PCR following 24-hr exposure to cocaine. RNA was assayed from the same samples for IL6 (Fig. 1A), IL8 (Fig. 1B), IFNβ (Fig. 1C) and ISG15 (Fig. 1D). While the proinflammatory cytokine IL6 was unaffected (Fig. 1A), cocaine-evoked increases in IL8 (10 μM; ***p<0.001, Fig. 1B), IFNβ1 (0.1 μM, 1 μM and 10 μM; *p<0.05, **p<0.01, ***p<0.001, Fig. 1C) and ISG15 (all concentrations; **p<0.01, ***p<0.001, Fig. 1D) were observed. Thus, these data demonstrate that cocaine exposure did not increase proinflammatory cytokine, IL6 and IL8 expression as robustly as type I interferon genes (above).
Figure 1. Cocaine regulates innate immune responses in a dose dependent manner in astrocytes.
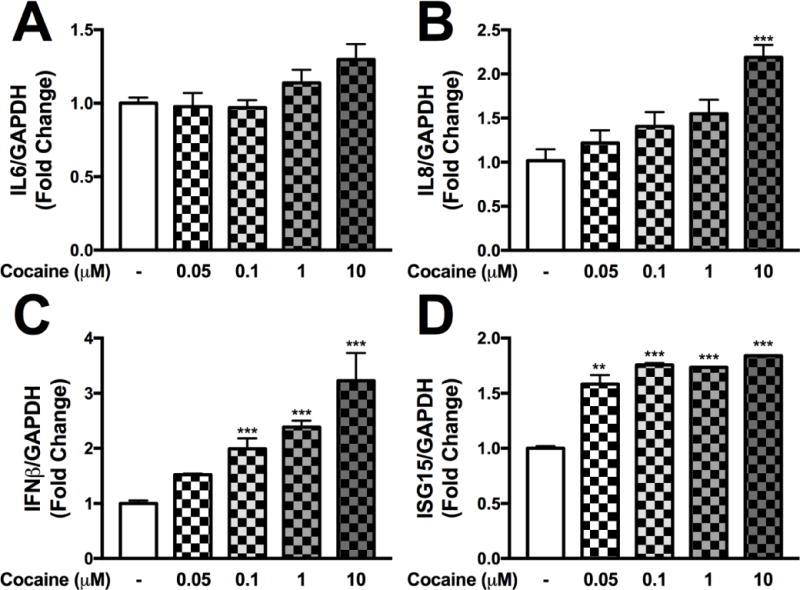
Normal human astrocytes (NHA) were treated with increasing doses of cocaine (0-10 μM) in parallel to untreated NHA for 24 hr. IL6 (A), IL8 (B), IFNβ1 (C) and ISG15 (D) mRNA levels were analyzed following cocaine treatments. Statistical analyses were performed using GraphPad Prism V6.0 with one-way ANOVA and Tukey’s post-hoc analysis for multiple comparisons. p values <0.05 were considered statistically significant and data represent means +/− SEM. Representative graphs are shown from a minimum of three independent astrocyte experiments each tested and analyzed in a minimum of triplicate technical determinations. (**p<0.01, ***p<0.001)
3.3 Cocaine does not affect astrocyte viability or proliferation
To evaluate the impact of cocaine on astrocyte viability we quantified changes in metabolic activity in three assays: [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] tetrazolium (MTT) reduction assay (Fig. 2A), cytotoxicity by LDH release (Fig. 2B) and proliferation by BrDU incorporation (Fig. 2C). Cocaine exposure did not alter MTT, LDH activity or BrDU incorporation (Fig. 2A–C). These data suggest that 24-hr cocaine treatment did not result in impaired cellular metabolic activity of NHA.
Figure 2. Cocaine does not alter astrocyte viability or proliferation.
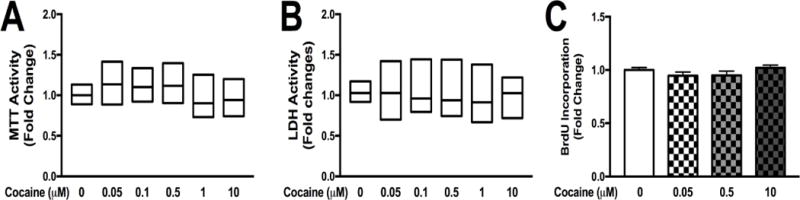
NHA were treated with escalating does of cocaine (0-10 μM), for 24 hr. Viability and proliferation assays were performed and changes were quantified in MTT (A), LDH (B) and BrdU incorporation (C). Statistical analyses were performed using GraphPad Prism V6.0 with one-way ANOVA and Tukey’s post-hoc analysis for multiple comparisons. p values <0.05 were considered statistically significant and data represent means +/− SEM. Representative graphs are shown from a minimum of three independent astrocyte experiments each tested and analyzed in a minimum of triplicate technical determinations. (*p<0.05, **p<0.01, ***p<0.001)
3.4 Cocaine-mediated oxidative stress and toxicity is reduced with PPF and PIO
We determined that cocaine did not alter astrocyte viability (3.3), however, cocaine result in cellular toxicity, such as cellular apoptosis or necrosis (Lepsch et al., 2015; Schiavone et al., 2016). We measured intracellular levels of dsDNA fragmentation (Fig. 3A) in NHA as a measure of intracellular astrocyte toxicity following escalating concentrations of cocaine (0 – 10 μM). A concentration- dependent increase in intracellular fragmented dsDNA was observed between 0.1 – 10 μM of cocaine (*p<0.05, **p<0.01, respectively, Fig. 3A), suggesting increased intracellular levels of self-DNA. Cocaine has previously been noted to increase extracellular and intracellular ROS levels in cultured cells and tissues (Samikkannu et al., 2016; Schiavone et al., 2016; Womersley and Uys, 2016). We quantified levels of intracellular ROS levels using a commercially available ROS-Glo assay following escalating concentrations of cocaine (Fig. 3B). Cocaine significantly increased intracellular levels of ROS in a concentration-dependent manner with an EC50 of ~73 nM (Fig. 3B). To determine if PPF or PIO prevents cocaine-mediated intracellular dsDNA fragmentation (Fig. 3C) or ROS levels (Fig. 3D), astrocytes were treated with PPF or PIO 10 min prior to treatment with cocaine. As previously determined, 0.1 μM of cocaine significantly increased cytosolic dsDNA fragmentation (**p<0.01, Fig. 3C); PPF and PIO significantly reduced dsDNA fragmentation (**p<0.01, Fig. 3C). Cocaine (0.1 μM), a concentration that approximated the EC50 value to evoke elevated ROS levels, significantly increased intracellular levels of ROS (***p<0.001, Fig.3D and F), an effect that was significantly reduced in the presence of PPF or PIO (**p<0.01, ***p<0.001, Fig. 3D). Neither PPF nor PIO alone significantly altered intracellular levels of dsDNA fragmentation or ROS (Fig. 3C and D). These data suggest that PPF and PIO pretreatment are sufficient to decrease cocaine-mediated cytotoxicity and oxidative stress.
Figure 3. Antioxidants, PPF and PIO, reduce cocaine-mediated DNA fragmentation and oxidative stress.
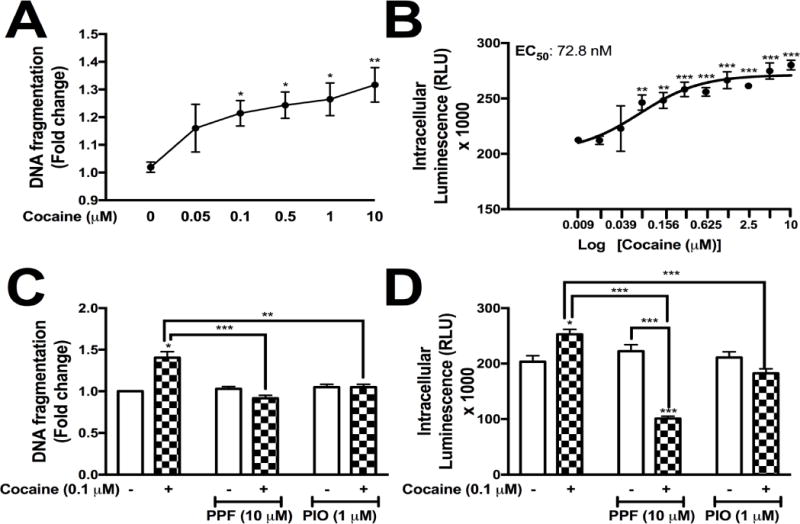
Normal human astrocytes (NHA) were treated with increasing doses of cocaine (0- 10 μM) in parallel to untreated NHA for 24 hr. DNA fragmentation was measured at all cocaine concentrations (A). Cocaine-induced oxidative stress was measured using the ROS-glo assay (Promega) in a dose-dependent manner (0-10 μM, EC50 = 72.8 nM, B). Astrocytes were treated with cocaine (0.1 μM) +/− PPF (10 μM) or PIO (1 μM), and changes in DNA fragmentation (C) and intracellular reactive oxygen species (D) were quantified at 24 hr. Statistical analyses were performed using GraphPad Prism V6.0 with two-way ANOVA and Tukey’s post-hoc analysis for multiple comparisons. p values <0.05 were considered statistically significant and data represent means +/− SEM. Representative graphs are shown from a minimum of three independent astrocyte experiments each tested and analyzed in a minimum of technical triplicate determinations. (*p<0.05, **p<0.01, ***p<0.001)
3.5 Cocaine activates nuclear translocation of IRF3 that is reduced with PPF or PIO
Canonical antiviral signaling pathways include activation of PRRs, such as RIG-I/MDA-5, TLRs and cGAS (Honda and Taniguchi, 2006; Kato et al., 2017). Downstream activation of PRRs results in phosphorylation of TBK1 and IRF3, resulting in translocation of transcription factor, IRF3 to the nucleus (Kato et al., 2017). To evaluate whether cocaine-induced oxidative stress activates nuclear translocation of IRF3, astrocytes were pretreated with PPF (10 μM) or PIO (1 μM) for 10 min, followed by cocaine (0.1 μM) stimulation for 24 hr. Astrocytes were fixed and immunostained with antibodies against IRF3 (green), pIRF3 (green) or GFAP (red) (Fig. 4A–E). In control astrocytes, total IRF3 was distributed intracellularly (Fig. 4A). Trace amounts of phosphorylated IRF3 were detected in control astrocytes (Fig. 4B). Upon cocaine treatment, pIRF3 was expressed robustly in the nuclei of astrocytes (Fig. 4C). PPF or PIO cotreatment blocked phosphorylation and nuclear localization of pIRF3 (Fig. 4D–E). Thus, these data demonstrate cocaine and HIV-1 activates canonical antiviral signaling that can be prevented with PPF and PIO.
Figure 4. Cocaine-mediated phosphorylation and nuclear localization of pIRF3 is reduced with antioxidants, PPF or PIO cotreatments.
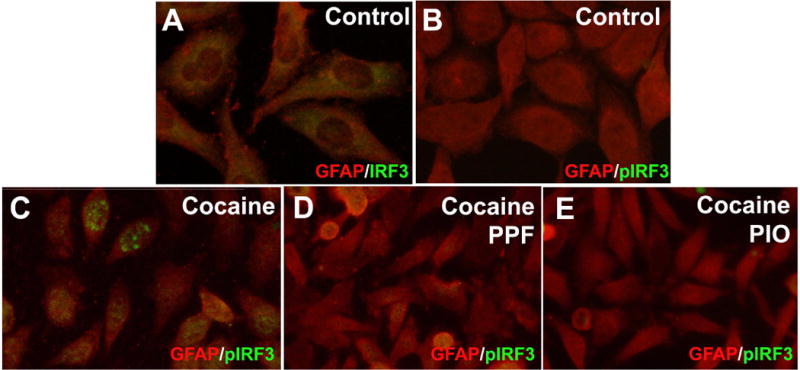
NHA were treated with cocaine (0.1 μM) in the presence or absence of PPF (10 μM) or PIO (1 μM). Astrocytes treated with cocaine +/− PPF or PIO were fixed and fluorescently labeled with antibodies specific to IRF3 (green, A), pIRF3 (green, B-E) or GFAP (red) (C-E). Representative donors chosen from multiple biological replicates were tested, each analyzed in a minimum of triplicate determinations.
3.6 HIV-1 and cocaine-induced oxidative stress increases astrocyte antiviral gene expression
Phosphorylation of IRF3/7 and translocation to the nucleus results in binding to Interferon stimulated gene promoter regions and activation of type I interferon gene transcription (Kato et al., 2017). Therefore, we evaluated HIV-1- and cocaine-mediated transcriptional changes in ISG15, IFNβ1 and IFNα1 mRNAs in the presence or absence of PPF (10 μM) or PIO (1 μM) pretreatment. Cocaine significantly increased ISG15 (**p<0.01, Fig. 5A) that was reduced during cotreatment with PPF or PIO (**p<0.01, ***p<0.001, respectively, Fig. 5A). Cocaine alone also significantly increased both IFNβ1 and IFNα1 (***p<0.001, Figs. 5B and C). PPF and PIO reduced cocaine-evoked IFNβ1 levels (***p<0.001, Fig. 5B). Although PPF alone induced IFNα1 expression (**p<0.01, Fig. 5C), coadministration of either PPF or PIO with cocaine significantly lowered IFNα1 levels (*p<0.05, **p<0.01, respectively, Fig. 5C). HIV-1 alone significantly reduced ISG15 mRNA levels (***p<0.001, Fig. 5D). ISG15 mRNA levels following HIV-1 plus cocaine treatment was significantly lower than control (*p<0.05, Fig. 5D); however, was significantly higher than HIV-1 alone (**p<0.01, Fig. 5E). PPF or PIO did not change HIV-1-mediated decreases in ISG15 expression (Fig. 5D). HIV-1 treatments alone significantly increased IFNβ1 and IFNα1 levels (***p<0.001, Fig. 5E and F) that were significantly reduced with coadministration of cocaine, PPF or PIO (***p<0.001, Fig. 5E and F). We show that PPF and PIO are sufficient in reducing both cocaine and HIV-1-mediated increases in type I interferon.
Figure 5. Cocaine-induced ROS regulates astrocytes innate immune gene expression, alone or in combination with HIV-1.
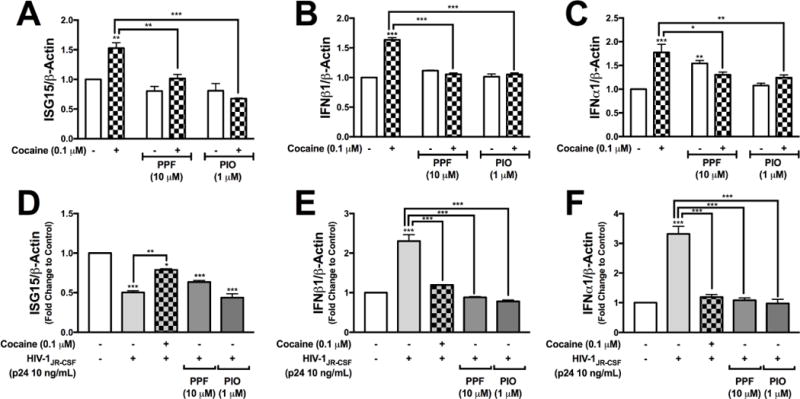
NHA were treated with cocaine (0.1 μM) in the presence or absence of PPF (10 μM) or PIO (1 μM) (A-C). NHA were treated with HIV-1JR-CSF (p24 10 ng/mL) alone or in combination with cocaine (0.1 μM), PPF (10 μM) or PIO (1 μM) (D-F). At 24 hr post treatment, RNA was collected and assayed for ISG15 (A and D), IFNβ1 (B and E) and IFNα1 (C and F). Statistical analyses were performed using GraphPad Prism V6.0 with two-way ANOVA (A-C) or one-way ANOVA (D-F) and Tukey’s post-hoc analysis for multiple comparisons. p values <0.05 were considered statistically significant and data represent means +/− SEM. Representative graphs are shown from a minimum of three independent astrocyte experiments each tested and analyzed in a minimum of triplicate technical determinations. (*p<0.05, **p<0.01, ***p<0.001).
3.7 Cocaine differentially regulates viral-mediated changes in proinflammatory cytokines and antiviral genes
Cocaine-induced changes of astrocyte innate immune responses are vital in understanding the how cocaine modulates the ability of astrocytes to initiate an immune response to infiltrating viruses identified to have neurotropic characteristics. We have shown that cocaine reduces HIV-1-induced IFNβ1 and IFNα1 mRNA levels (Fig. 5E and 5F); here, we evaluated the impact of cocaine on HIV-1- and ZIKV-induced IL6 and IL8 levels (Fig. 6A–D), in parallel to ZIKV-induced alterations in IFNβ1 and IFNα1 expression (Fig. 6E and 6F). Gene expression levels were quantified for IL6 (Fig. 6A and 6C), IL8 (Fig. 6B and 6D), IFNα1 (Fig. 6E) and IFNβ1 (Fig. 6F). Neither HIV-1JR-CSF nor cocaine altered IL6 mRNA levels (Fig. 6A). Pretreatment with cocaine followed by HIV-1JR-CSF treatment, significantly increased IL6 mRNA levels compared to HIV-1 or cocaine alone (***p<0.001, Fig. 6A). HIV-1JR-CSF, alone or following cocaine stimulation, significantly reduced IL8 levels compared to control (*p<0.05, **p<0.01, Fig. 6B). Cocaine pretreatments combined with HIV-1 significantly lowered IL8 levels compared to cocaine alone (***p<0.001, Fig. 6B). ZIKV alone or following cocaine pretreatment significantly increased IL6 (***p<0.001, Fig. 6C). Combined treatments of ZIKV and cocaine significantly increased IL6 levels compared to ZIKV or cocaine alone (***p<0.001, Fig. 6C). ZIKV alone significantly increased IL8 (***p<0.001, Fig. 6D). Interestingly, pretreatment of cocaine, followed by ZIKV significantly reduced IL8 levels back to baseline (***p<0.001, Fig. 6D). As expected, cocaine alone increased IFNα1 and IFNβ1 levels (*p<0.05, Fig. 6E and 6F). In parallel, ZIKV increased IFNα1 and IFNβ1 levels (***p<0.001, Fig. 6E and 6F). Importantly, cocaine pretreatment significantly reduced ZIKV-mediated IFNα1 and IFNβ1 mRNA levels (***p<0.001, Fig. 6E and 6F), suggesting that cocaine preexposure modulates intracellular pathways dictating antiviral responses prior to viral treatments. Combined treatment with ZIKV and cocaine did not alter astrocyte metabolic activity as measured by MTT (Fig. 6G) nor cytotoxicity as measured by LDH release (Fig. 6H). These data demonstrate that changes measured in IL6, IL8, IFNβ1 or IFNα1 are not a reflection of cellular toxicity, but are true representation of RNA expression.
Figure 6. Cocaine differentially regulates viral-induced antiviral and proinflammatory immune expression in astrocytes.
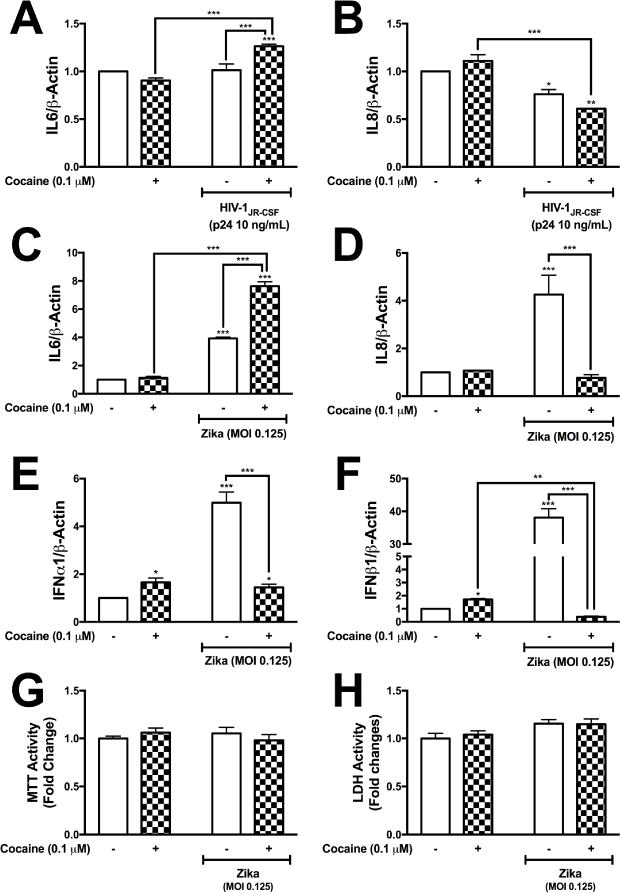
NHA were pretreated with cocaine (0.1 μM) followed by HIV-1JR-CSF (p24 10 ng/mL) or ZIKV (0.125 MOI) in parallel to control. Following 24 hr treatment, RNA was collected and assayed for IL6 (A and C), IL8 (B and D), IFNα1 (E) and IFNβ1 (F). Changes in astrocyte viability were evaluated at 24 hr post cocaine, ZIKV or cotreatments with cocaine and ZIKV by measuring MTT activity (G) and LDH release (H). Statistical analyses were performed using GraphPad Prism V6.0 with two-way ANOVA and Tukey’s post-hoc analysis for multiple comparisons. p values <0.05 were considered statistically significant and data represent means +/− SEM. Representative graphs are shown from a minimum of three independent astrocyte experiments each tested and analyzed in a minimum of triplicate technical determinations. (*p<0.05, **p<0.01, ***p<0.001)
3.8 Cocaine alters astrocytes immune responses to HIV-1 and ZIKV
To evaluate whether changes in gene expression are reflected at the protein level, astrocytes were treated with cocaine prior to HIV-1 or ZIKV treatments. Supernatants were collected and assayed for IL6 (Fig. 7A), IL8 (Fig. 7B) and IFNβ1 (Fig. 7C). As demonstrated at the mRNA level, cocaine alone did not change IL6 or IL8 protein levels (Fig. 7A and 7B). Cocaine significantly increased IFNβ1 levels (**p<0.05, Fig. 7C). HIV-1 and ZIKV significantly increased IL6 (**p<0.01, Fig. 7A), IL8 (***p<0.001, Fig. 7B) and IFNβ1 (***p<0.001, Fig. 7C). Combined exposure to cocaine plus HIV-1 or ZIKV resulted in significantly higher protein levels of IL6 (***p<0.001, Fig. 7A) and IL8 (***p<0.001, Fig. 7B) when compared to control or cocaine alone. Cocaine combined with HIV-1 exacerbated HIV-1 elevations in IL6 protein levels compared to HIV-1 alone (***p<0.001, Fig. 7A). Interestingly, cocaine exposure followed by HIV-1 or ZIKV treatment attenuated the ability of astrocytes to initiate as robust release of IL8 (***p<0.001, Fig. 7B) or IFNβ1 (***p<0.001, Fig. 7C). These data suggest cocaine treatments alter viral-induced inflammatory and type I interferon levels.
Figure 7. Cocaine increases IL6 and attenuates IL8 and IFNβ1 levels induced by HIV-1 and ZIKV in astrocytes.
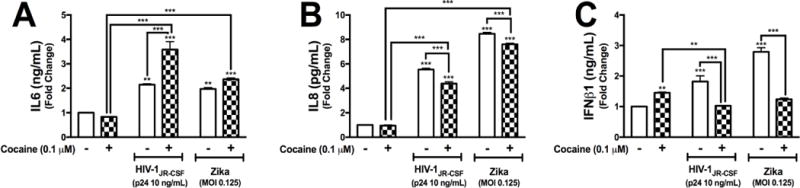
NHA were pretreated with cocaine (0.1 μM) followed by HIV-1JR-CSF (p24 10 ng/mL) or ZIKV (0.125 MOI) in parallel to control. Following 24 hr treatments, supernatant were collected and IL6 (A), IL8 (B) and IFNβ1 (C) protein levels were assayed by ELISAs. Statistical analyses were performed using GraphPad Prism V6.0 with two-way ANOVA and Tukey’s post-hoc analysis for multiple comparisons. p values <0.05 were considered statistically significant and data represent means +/− SEM. Representative graphs are shown from a minimum of three independent astrocyte experiments each tested and analyzed in a minimum of triplicate technical determinations. (*p<0.05, **p<0.01, ***p<0.001)
4.0 DISCUSSION
We provide multiple lines of evidence to demonstrate that cocaine exposure initiates innate immune responses via oxidative stress and decreases the ability of astrocytes to initiate a robust type I interferon response to HIV-1 or ZIKV. We found that 24 hrs of cocaine exposure significantly increased expression of innate immune response genes and increased intracellular generation of ROS; outcomes significantly reduced by the glial modulators PPF and PIO. Together, these data support the hypothesis that oxidative stress resulting from cocaine exposure modulates astrocyte-mediated innate immune signaling. We also demonstrated that cocaine does not alter cell viability, but does significantly increase intracellular levels of fragmented dsDNA, which itself may serve to activate innate immune responses; PPF and PIO exhibited efficacy to suppress this outcome as well. Furthermore, PPF and PIO prevented cocaine-induced transcriptional upregulation of type I interferon signaling and canonical intracellular activation and phosphorylation of TBK1 and IRF3/7. Lastly, cocaine attenuated astrocyte type I interferon response to exposure to retrovirus (HIV-1) or single stranded RNA virus (Zika, ZIKV), whereas treatment with PFF and PIO reduced cocaine or HIV-1 mediated increases in type I interferon. Thus, we propose that cocaine triggers CNS immune pathways that compromise the biological response to subsequent viral pathogen exposure. Lastly, we have identified potential clinical interventions to attenuate both cocaine and viral-mediated innate immune responses in astrocytes, by targeting increased reactive oxygen species and cytotoxicity.
Cocaine alters viral-mediated neuroinflammatory gene expression in human astrocytes while attenuating viral-induced IFNβ1 and IFNα1 gene expression in parallel to IFNβ1 protein production. Taken together, cocaine regulation of antiviral signaling in human astrocytes is highly significant in potentially dictating the balance of viral-induced activation of astrocyte innate immune responses, therefore, having larger implications in innate immune responses to CNS viral infections during cocaine use. These observations are critical in evaluating the molecular mechanisms of cocaine mediated activation and attenuation of type I interferon.
Astrocyte dysfunction is increasingly recognized as a pathology leading to neuroinflammation and neurodegeneration (Borgmann and Ghorpade, 2015; Cisneros and Ghorpade, 2012). Astrogliosis is a common feature of cocaine abuse and CNS viral infections resulting in homeostatic imbalances in the brain microenvironment. In this study we confirm that cocaine activates astrocyte antiviral immune responses via increased oxidative stress. Oxidative stress is implicated in the pathology and development of cocaine use disorder and is a relevant player in the activation of NF-κB signaling within the NAc, frontal cortex and hippocampus (Dietrich et al., 2005; Jang et al., 2015; Muriach et al., 2010). Not surprisingly, cocaine increased ROS in human astrocyte that was significantly lower in astrocytes pretreated with PPF or PIO. PPF, a xanthine derivative and glial modulator, has both neuroprotective and antioxidant characteristics with clinical significance for treatment of Alzheimer’s disease, schizophrenia and multiple sclerosis (Banati et al., 1994; Bondan et al., 2014; Sweitzer and De Leo, 2011). PIO, belongs to the thiazolinedione class of insulin sensitizer drugs that is prescribed for type 2 diabetes. PIO selectively stimulates the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) and the outer mitochondrial membrane protein, mitoNEET (Wang et al., 2017). PIO rapidly effects mitochondrial energy metabolism by inhibiting the electron transfer capacity of mitoNEET (Wang et al., 2017). PIO, as a PPARγ agonist, attenuates cocaine cue reactivity (Miller et al., 2016) prompting its evaluation in a pilot clinical trial (Schmitz et al., 2017). In a placebo controlled, pilot clinical trial targeting non-treatment seeking subjects diagnosed with cocaine use disorder, PIO reduced self-reported cocaine craving and improved brain white matter integrity, indicating that PIO ameliorated cocaine CNS toxicity (Schmitz et al., 2017). Overall, glial modulators, including PPF and PIO, directly regulate glial activity and can improve mitochondrial activity and reduce oxidative stress in parallel to decreasing the production of damaging immune factors (Cooper et al., 2012; Jones et al., 2016; Swanson et al., 2011; Sweitzer and De Leo, 2011). This conclusion is supported by our findings that PPF and PIO significantly reduced cocaine-mediated type I interferon gene expression and ROS levels. Additionally, PPF or PIO reduced several innate immune genes and canonical signaling proteins involved in intracellular antiviral signaling cascades back to baseline levels. Thus, further clinical studies are warranted to investigate these drugs as therapies with the potential to limit immune dysfunction in CUD.
Chronic cocaine exposure, exemplified in humans with CUD and animal models of the disorder, elicits behavioral and CNS structural changes that exacerbate the severity of CNS viral infections and neurological sequelae. However, the role of cocaine in activating an antiviral immune response has remained largely unexplored. Nonetheless, clinical studies have shown that cocaine dependent individuals have chronic immune system changes measured by alterations in peripheral TNFα, IL-10, IL-6 and IL1-Rα levels (Fox et al., 2012; Moreira et al., 2016). The immunosuppressive nature of cocaine may be via the neuroendocrine or autonomic nervous system (Marasco et al., 2014). Cocaine exposure results in neuroinflammation and release of TNF, IL1β, IL-6 and CCL2 in the CNS (Guo et al., 2015). Recently, cocaine has been shown to increase the activating capacity of TLR4 thereby increasing neuroinflammation (Periyasamy et al., 2017). Additionally, cocaine binds to sigma 1 receptors at physiologically relevant concentrations of 2-7 μM (for review) (Yasui and Su, 2016), which has been suggested to activate neuroinflammatory pathways (Liao et al., 2016). Interestingly, cocaine may stimulate expression of HIV via sigma 1 receptors (Gekker et al., 2006). Moreover, cocaine increases expression of chemokine receptors (CCR5) and co-receptors (CXCR4) in human peripheral blood mononuclear cells that result in increased HIV replication and can be blocked with a sigma 1 selective antagonist, BD1047 (Roth et al., 2005). We found that cocaine mediates translocation of IRF3 in the absence of NF-κB activation, suggesting that cocaine is selectively activating TBK1-IRF3 signaling. Several mechanisms may dictate this selective activation. For example, mitochondrial antiviral signaling (MAVS) protein is regulated via cleavage, aggregation, oxidative stress and changes in mitochondrial dynamics that differentially result in IRF3 and NF-κB signaling (Horner et al., 2015; Koshiba et al., 2011; Seth et al., 2005; Shao et al., 2016; Vazquez and Horner, 2015; Yoneyama et al., 2016). MAVS function often results in simultaneous activation of IRF3 and NF-κB; however, differential activation can occur. MAVS interaction with TNF receptor- associated factors (TRAF)2/6, along with TNFR1-associated death domain protein results in canonical NF-κB signaling and increased proinflammatory cytokine expression (Vazquez and Horner, 2015). MAVS interaction with TRAF3 and the stimulator of IFN genes (STING) at the endoplasmic reticulum (ER) interface activates IRF3 signaling (Liu et al., 2017). Interestingly, the canonical DNA PRR, cGAS, is aberrantly activated by self-DNA, released during cytotoxic events (Chen et al., 2016). Alternatively, cocaine-mediated increases in dsDNA, during cocaine-mediated toxicity, may be sufficient to activate the cGAS-STING pathway, thereby increasing TBK1-IRF3 intracellular signaling and increased type I interferon gene transcription. Cocaine-mediated activation or regulation of these PRR proteins may play a central role in driving antiviral gene expression in human astrocytes in the absence of viral pathogens.
Astrocytes are non-productively infected by HIV-1 (Chauhan and Khandkar, 2015) and are described to serve as reservoirs for latent HIV-1 infection (Gray et al., 2014) (Cisneros and Ghorpade, 2014), which may be why astrocytes respond to a lesser immunological severity when compared to ZIKV. We did find that NHA do express the HIV-1 coreceptor CCR5 (data not shown), but we did not specifically determine whether astrocytes were infected with HIV-1JR-CSF. We show cocaine alters HIV-1- and ZIKV-mediated innate immunity. HIV-1 entry and uncoating exposes ssRNA that may be recognized by endosomal TLR7 or RIG-I and/or MDA-5 and downstream activation of MAVS protein or STING protein (Cohen et al., 2015; Giraldo et al., 2016; Meulendyke et al., 2014). Reverse transcription produces ssDNA and dsDNA that may be recognized by protein kinase R (PKR), an interferon-induced, double stranded RNA-activated kinase or cGAS (Sunita et al., 2015; Vermeire et al., 2016; Vivarini Ade et al., 2015). Likewise, ZIKV, an ssRNA virus may be recognized by TLR3 or activate the cGAS-STING and RIG-I/MDA-5-MAVS innate immune pathways (Da Costa et al., 2017; Lindqvist et al., 2016). Taken together, cocaine may directly result in attenuated astrocyte antiviral responses to ensuing CNS viral infection possibly through activation of the cGAS-STING pathways.
The ability of cocaine to induce IFNβ/α levels alone, while attenuating viral-mediated type I interferon response, may occur via regulation and activation of several PRRs as discussed above; however, cocaine indirectly results in epigenetic modifications (Hayase, 2017; Kenny, 2014). Amongst these mechanisms is cocaine-mediated modulation of microRNAs (miRNAs), resulting in regulation of gene expression at the posttranscriptional level (Hayase, 2017). Type I interferon in the CNS is a central regulator of innate antiviral responses and may be subjected to regulatory fine- tuning. Interestingly, several miRNAs are identified to positively and negatively regulate type I interferon levels to retroviruses and flaviviruses including miR-15, -22, -26a, -34a, -145, -146a, -517 and let-7d (Pilakka-Kanthikeel and Nair, 2015; Smith et al., 2017; Tsetsarkin et al., 2017; Wan et al., 2016). Likewise, drugs of abuse are identified to regulate several miRNAs including miR-146a, -212, - 132, -181a, -124, -133b, -134, -22, let-7d, -21, -335, -146a, -133, -23, -190, -29a some of which overlap with miRNAs regulating type I interferon(Cheng et al., 2017). Interestingly, miR-22, is upregulated in the CNS by cocaine (Chen et al., 2013) and directly targets MAVS (Wan et al., 2016), a vital intracellular PRRs, which positively regulates type I interferon production. Likewise, let-7d is suppressed during chronic cocaine use in the NAc (Chandrasekar and Dreyer, 2011) and cocaine decreases miR-155 and -146a (Fiala et al., 2005; Kocerha et al., 2009; Li et al., 2002). Specifically, miR-155 promotes type I IFN signaling to suppress viral replication (Wang et al., 2010). miR-146a and -155 are demonstrated to exert anti-inflammatory mechanisms by downregulating both IL6 and IL8 in human endothelial cells (Pfeiffer et al., 2017). Therefore, several direct and indirect mechanisms mediated by cocaine in human astrocytes may serve as regulatory checkpoints in attenuating viral-mediated antiviral responses within the CNS.
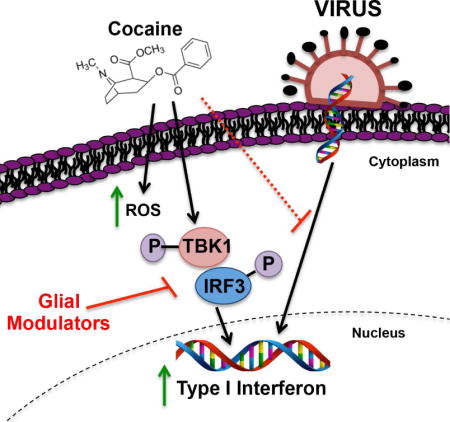
To our knowledge, little to no information is available regarding cocaine-mediated activation of antiviral processes. Schematic 1 illustrates cocaine activates signal transduction pathways to increase type I interferon via oxidative stress. Furthermore, cocaine attenuates HIV-1- and ZIKV- induced type I interferon. We propose that cocaine triggers type I interferon, which potentially has larger bearing on the ability of astrocytes to initiate an immune response to neurotoxic viruses.
Schematic 1. Cocaine induces oxidative stress and type I interferon responses that lead to the attenuation of viral-mediated innate immune responses.
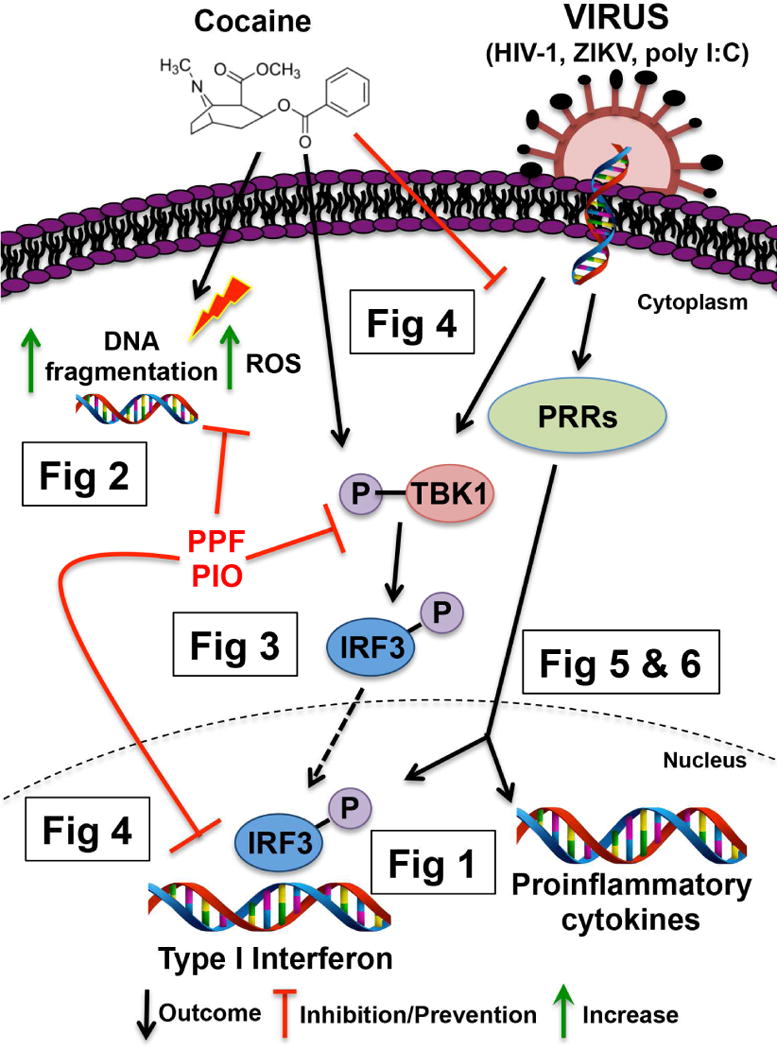
We show in normal human astrocytes (NHA) that cocaine treatment increased type I interferon responses in a concentration-dependent manner (Fig 1). In parallel to increases in type I interferon, cocaine increases intracellular DNA fragmentation and ROS levels that are reduced with antioxidants PPF and PIO (Fig 2). Likewise, antiviral signaling pathway, TBK1/IRF3, is activated by cocaine and HIV-1, but can be prevented with PPF and PIO (Fig 3). Phosphorylation and translocation of IRF3 to the nucleus results in type I interferon gene transcription, including ISG15, IFNα1 and IFNβ1, which are increased upon cocaine, but reduced with PPF and PIO, exposure (Fig 4). Moreover, HIV-1 increased antiviral genes which were also reduced upon cocaine pretreatment; the glial modulators PPF and PIO reduced either cocaine or HIV-1-mediated increases in type I interferon back to baseline levels (Fig 4). Lastly, at the RNA and protein levels, we demonstrated that cocaine pretreatment reduced the ability of HIV-1 or ZIKV to initiate robust type I interferon responses when compared to virus alone (Fig 5 and 6). Thus, our work demonstrates that preventing cocaine-mediated increases in type I interferon by targeting oxidative stress and cytotoxicity with glial modulators, PPF or PIO, may recover viral-mediated increases in astrocyte antiviral responses, allowing for astrocytes to initiate a stronger innate immune response to infiltrating viruses.
5.0 CONCLUSIONS
Increasing evidence suggests that neuroimmune mechanisms contribute to the symptomology seen in CUD, having larger implications for the comorbidity of drug abuse and CNS viral infection. Cocaine-mediated oxidative stress and neuroinflammation initiate antiviral responses with ensuing quenched response to viral pathogens. The antioxidant and anti-inflammatory characteristics of PPF and PIO, to control astrocyte oxidative stress and type I interferon response induced by cocaine, have larger implications for these FDA-approved drugs in treating cocaine neurotoxicity and abstinence relapse of cocaine-use disorder.
HIGHLIGHTS.
Cocaine increased levels of reactive oxygen species in normal human astrocytes.
Cocaine increased astrocyte interferon β (IFNβ) levels and activated type I interferon signaling.
Cocaine predisposes dysregulated innate immune responses to HIV-1 and ZIKV
Glial activators attenuated cocaine-mediated astrocyte innate immune signaling.
Acknowledgments
We appreciate the assistance of the members of our laboratories as well as Mark and Janice Endsley for providing technical and scientific support. I would like to acknowledge Cheng Huang, Ph.D., who assisted with manuscript editing and Natasha Bukreyeva for experimental and cell culture support and Robert G. Fox for laboratory assistance. We would also like to acknowledge UCLA CFAR Virology Core Lab at the UCLA AIDS Institute for preparation of HIV-1JR-CSF stocks; NIH grant 5P30AI028697 and the World Reference Center for Emerging Viruses and Arboviruses for supplying ZIKV stocks (NIH grant R24AI120942).
FUNDING SOURCES
This research was supported by a postdoctoral fellowship from the National Institute on Drug Abuse (IEC; NIDA T32 DA07287), NIDA F32 DA045445 (IEC), NIDA P50 DA033935 (KAC), the McManus Charitable Trust (KTD) and the UTMB Institute for Human Infection and Immunity (IHII).
ABBREVIATIONS
- CARD
caspase recruitment domain
- CCL2
chemokine (C-C motif) ligand 2
- cGAS
cyclic guanosine monophosphate-adenosine monophosphate synthase
- CIITA
class II Major Histocompatibility Complex Transactivator
- CNS
central nervous system
- CUD
cocaine use disorder
- ER
endoplasmic reticulum
- GFAP
glial fibrillary acidic protein
- HIV
human immunodeficiency virus
- IFNα/β
interferon α/β
- IL1-Rα
interleukin 1 receptor α
- IL1β/6/8/10
interleukin 1β/6/8/10
- IRF3/7
interferon regulatory transcription factor 3/7
- ISG15
interferon stimulating gene 15
- LDH
lactate dehydrogenase
- MAVS
mitochondrial antiviral signaling protein
- MD2
myeloid differentiation factor 2
- MDA5
melanoma differentiation-associated protein 5
- MTT
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAc
nucleus accumbens
- NF-κB
nuclear-factor kappa light-chain-enhancer of activated B cells
- NHA
normal human astrocytes
- OAS1
2′–5′-oligoadenylate synthetase 1
- PIO
pioglitazone
- PKR
protein kinase R PPARγ: peroxisome proliferator-activated receptor γ
- PPF
propentofylline
- PRR
pattern recognition receptor
- RIG-I
retinoic acid-inducible gene I
- ROS
reactive oxygen species
- STING
stimulator of interferon genes
- TBK1
serine/threonine-protein kinase 1
- TIMP1
tissue inhibitor of metallopeptidases
- TLR2/4/7
toll-like receptor 2/4/7
- TNFα
tumor necrosis factor α
- TNFR1
tumor necrosis factor receptor 1
- TRAF
TNF receptor associated factor
- TYK2
tyrosine kinase 2
- VTA
ventral tegmental area
- ZIKV
Zika virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest to report.
References
- Baker PJ, De Nardo D, Moghaddas F, Tran LS, Bachem A, Nguyen T, Hayman T, Tye H, Vince JE, Bedoui S, et al. Posttranslational Modification as a Critical Determinant of Cytoplasmic Innate Immune Recognition. Physiological reviews. 2017;97:1165–1209. doi: 10.1152/physrev.00026.2016. [DOI] [PubMed] [Google Scholar]
- Banati RB, Schubert P, Rothe G, Gehrmann J, Rudolphi K, Valet G, Kreutzberg GW. Modulation of intracellular formation of reactive oxygen intermediates in peritoneal macrophages and microglia/brain macrophages by propentofylline. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1994;14:145–149. doi: 10.1038/jcbfm.1994.19. [DOI] [PubMed] [Google Scholar]
- Bik W, Skwarlo-Sonta K, Szelagiewicz J, Wolinska-Witort E, Chmielowska M, Martynska L, Baranowska-Bik A, Baranowska B. Involvement of the cocaine-amphetamine regulated transcript peptide (CART 55-102) in the modulation of rat immune cell activity. Neuro endocrinology letters. 2008;29:359–365. [PubMed] [Google Scholar]
- Bondan EF, Martins Mde F, Menezes Baliellas DE, Monteiro Gimenez CF, Castro Poppe S, Martha Bernardi M. Effects of propentofylline on CNS remyelination in the rat brainstem. Microscopy research and technique. 2014;77:23–30. doi: 10.1002/jemt.22308. [DOI] [PubMed] [Google Scholar]
- Borgmann K, Ghorpade A. HIV-1, methamphetamine and astrocytes at neuroinflammatory Crossroads. Frontiers in microbiology. 2015;6:1143. doi: 10.3389/fmicb.2015.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KT, Levis SC, O’Neill CE, Northcutt AL, Fabisiak TJ, Watkins LR, Bachtell RK. Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain, behavior, and immunity. 2017 doi: 10.1016/j.bbi.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Mathias-Costa B, Singh V, Seth P, Wang J, Su TP. Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Current HIV research. 2012;10:425–428. doi: 10.2174/157016212802138823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SA, Lee Archer R, Chavis JA, Tull CA, Hensley LL, Drew PD. Mitoxantrone repression of astrocyte activation: relevance to multiple sclerosis. Brain Res. 2012;1473:236–241. doi: 10.1016/j.brainres.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Yang L, Niu F, Liao K, Buch S. Role of Sigma-1 Receptor in Cocaine Abuse and Neurodegenerative Disease. Advances in experimental medicine and biology. 2017;964:163–175. doi: 10.1007/978-3-319-50174-1_12. [DOI] [PubMed] [Google Scholar]
- Cao L, Walker MP, Vaidya NK, Fu M, Kumar S, Kumar A. Cocaine-Mediated Autophagy in Astrocytes Involves Sigma 1 Receptor, PI3K, mTOR, Atg5/7, Beclin-1 and Induces Type II Programed Cell Death. Molecular neurobiology. 2016;53:4417–4430. doi: 10.1007/s12035-015-9377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Blindheim K, Sorg BA, Krueger JM, Churchill L. Acute cocaine increases interleukin-1beta mRNA and immunoreactive cells in the cortex and nucleus accumbens. Neurochemical research. 2011;36:686–692. doi: 10.1007/s11064-011-0410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:1149–1164. doi: 10.1038/npp.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Khandkar M. Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: a fiery path to its destination. Microbial pathogenesis. 2015;78:1–6. doi: 10.1016/j.micpath.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Liu H, Guan X. Changes in microRNA expression profile in hippocampus during the acquisition and extinction of cocaine-induced conditioned place preference in rats. Journal of biomedical science. 2013;20:96. doi: 10.1186/1423-0127-20-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nature immunology. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- Cheng J, Liao Y, Xiao L, Wu R, Zhao S, Chen H, Hou B, Zhang X, Liang C, Xu Y, et al. Autophagy regulates MAVS signaling activation in a phosphorylation-dependent manner in microglia. Cell Death Differ. 2017;24:276–287. doi: 10.1038/cdd.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Current HIV research. 2012;10:392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology. 2014;85:499–507. doi: 10.1016/j.neuropharm.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen KW, Dugast AS, Alter G, McElrath MJ, Stamatatos L. HIV-1 single-stranded RNA induces CXCL13 secretion in human monocytes via TLR7 activation and plasmacytoid dendritic cell- derived type I IFN. J Immunol. 2015;194:2769–2775. doi: 10.4049/jimmunol.1400952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Jones JD, Comer SD. Glial modulators: a novel pharmacological approach to altering the behavioral effects of abused substances. Expert opinion on investigational drugs. 2012;21:169–178. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Shurtleff D, Harris RA. Neuroimmune mechanisms of alcohol and drug addiction. International review of neurobiology. 2014;118:1–12. doi: 10.1016/B978-0-12-801284-0.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76(Pt B):460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa A, Garza E, Graham JB, Swarts JL, Soerens AG, Gale M, Lund JM. Extrinsic MAVS signaling is critical for Treg maintenance of Foxp3 expression following acute flavivirus infection. Sci Rep. 2017;7:40720. doi: 10.1038/srep40720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BP, Jujjavarapu H, Durrant DM, Williams JL, Green RR, White JP, Lazear HM, Gale M, Diamond MS, Klein RS. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J Clin Invest. 2017;127:843–856. doi: 10.1172/JCI88720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich JB, Mangeol A, Revel MO, Burgun C, Aunis D, Zwiller J. Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology. 2005;48:965–974. doi: 10.1016/j.neuropharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing? Molecular psychiatry. 2013;18:134–135. doi: 10.1038/mp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, Yang W, Zhang J, Popik W, Singer E, et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. Journal of neurovirology. 2005;11:281–291. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R. Immune system inflammation in cocaine dependent individuals: implications for medications development. Human psychopharmacology. 2012;27:156–166. doi: 10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. International immunopharmacology. 2006;6:1029–1033. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Giraldo DM, Hernandez JC, Urcuqui-Inchima S. HIV-1-derived single-stranded RNA acts as activator of human neutrophils. Immunologic research. 2016;64:1185–1194. doi: 10.1007/s12026-016-8876-9. [DOI] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88- dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- Gray LR, Roche M, Flynn JK, Wesselingh SL, Gorry PR, Churchill MJ. Is the central nervous system a reservoir of HIV-1? Current opinion in HIV and AIDS. 2014;9:552–558. doi: 10.1097/COH.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P, Harraz MM, Snyder SH. Cocaine elicits autophagic cytotoxicity via a nitric oxide- GAPDH signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:1417–1422. doi: 10.1073/pnas.1524860113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy. 2015;11:995–1009. doi: 10.1080/15548627.2015.1052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R, Ferraris P, Wichit S, Diop F, Talignani L, Pompon J, Garcia D, Liégeois F, Sall AA, Yssel H, et al. African and Asian Zika virus strains differentially induce early antiviral responses in primary human astrocytes. Infect Genet Evol. 2017;49:134–137. doi: 10.1016/j.meegid.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Hayase T. Epigenetic mechanisms associated with addiction-related behavioural effects of nicotine and/or cocaine: implication of the endocannabinoid system. Behavioural pharmacology. 2017 doi: 10.1097/FBP.0000000000000326. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nature reviews Immunology. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Horner SM, Wilkins C, Badil S, Iskarpatyoti J, Gale M. Proteomic analysis of mitochondrial-associated ER membranes (MAM) during RNA virus infection reveals dynamic changes in protein and organelle trafficking. PLoS One. 2015;10:e0117963. doi: 10.1371/journal.pone.0117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang EY, Ryu YH, Lee BH, Chang SC, Yeo MJ, Kim SH, Folsom RJ, Schilaty ND, Kim KJ, Yang CH, et al. Involvement of reactive oxygen species in cocaine-taking behaviors in rats. Addiction biology. 2015;20:663–675. doi: 10.1111/adb.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay JM, Mogali S, Metz VE, Ciccocioppo R, Comer SD. The effects of pioglitazone, a PPARgamma receptor agonist, on the abuse liability of oxycodone among nondependent opioid users. Physiology & behavior. 2016;159:33–39. doi: 10.1016/j.physbeh.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Omura H, Ishitani R, Nureki O. Cyclic GMP-AMP as an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Annual review of biochemistry. 2017;86:541–566. doi: 10.1146/annurev-biochem-061516-044813. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues in clinical neuroscience. 2014;16:335–344. doi: 10.31887/DCNS.2014.16.3/pkenny. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3507–3512. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, Bilbo SD. Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2017;42:156–177. doi: 10.1038/npp.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Boeshore KL, Wu C, Becker KG, Errico SL, Mash DC, Freed WJ. Cocaine promotes primary human astrocyte proliferation via JNK-dependent up-regulation of cyclin A2. Restorative neurology and neuroscience. 2016;34:965–976. doi: 10.3233/RNN-160676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepsch LB, Planeta CS, Scavone C. Cocaine Causes Apoptotic Death in Rat Mesencephalon and Striatum Primary Cultures. BioMed research international. 2015;2015:750752. doi: 10.1155/2015/750752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. The Journal of infectious diseases. 2002;185:118–122. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation. 2016;13:33. doi: 10.1186/s12974-016-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist R, Mundt F, Gilthorpe JD, Wolfel S, Gekara NO, Kroger A, Overby AK. Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. Journal of neuroinflammation. 2016;13:277. doi: 10.1186/s12974-016-0748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lin R, Olagnier D. RIGulation of STING expression: at the crossroads of viral RNA and DNA sensing pathways. Inflamm Cell Signal. 2017;4:e1491. doi: 10.14800/ics.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamik MK, Ghorpade A. CXCL8 as a Potential Therapeutic Target for HIV-Associated Neurocognitive Disorders. Curr Drug Targets. 2016;17:111–121. doi: 10.2174/1389450116666150626124544. [DOI] [PubMed] [Google Scholar]
- Marasco CC, Goodwin CR, Winder DG, Schramm-Sapyta NL, McLean JA, Wikswo JP. Systems-level view of cocaine addiction: the interconnection of the immune and nervous systems. Exp Biol Med (Maywood) 2014;239:1433–1442. doi: 10.1177/1535370214537747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall Jones C, Baldwin GT, Compton WM. Recent Increases in Cocaine-Related Overdose Deaths and the Role of Opioids. American journal of public health. 2017;107:430–432. doi: 10.2105/AJPH.2016.303627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulendyke KA, Croteau JD, Zink MC. HIV life cycle, innate immunity and autophagy in the central nervous system. Current opinion in HIV and AIDS. 2014;9:565–571. doi: 10.1097/COH.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Schnell MJ, Rall GF. Keeping it in check: chronic viral infection and antiviral immunity in the brain. Nat Rev Neurosci. 2016;17:766–776. doi: 10.1038/nrn.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FP, Medeiros JR, Lhullier AC, Souza LD, Jansen K, Portela LV, Lara DR, da Silva RA, Wiener CD, Oses JP. Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug and alcohol dependence. 2016;158:181–185. doi: 10.1016/j.drugalcdep.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Muriach M, Lopez-Pedrajas R, Barcia JM, Sanchez-Villarejo MV, Almansa I, Romero FJ. Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. Journal of neurochemistry. 2010;114:675–684. doi: 10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, et al. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Molecular psychiatry. 2015;20:1525–1537. doi: 10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy P, Guo ML, Buch S. Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy. 2016;12:1310–1329. doi: 10.1080/15548627.2016.1183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo ML, Buch S. Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Molecular neurobiology. 2017 doi: 10.1007/s12035-017-0584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer D, Rossmanith E, Lang I, Falkenhagen D. miR-146a, miR-146b, and miR-155 increase expression of IL-6 and IL-8 and support HSP10 in an In vitro sepsis model. PLoS One. 2017;12:e0179850. doi: 10.1371/journal.pone.0179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilakka-Kanthikeel S, Nair MP. Interaction of drugs of abuse and microRNA with HIV: a brief review. Frontiers in microbiology. 2015;6:967. doi: 10.3389/fmicb.2015.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pužar Dominkuš P, Ferdin J, Plemenitaš A, Peterlin BM, Lenassi M. Nef is secreted in exosomes from Nef.GFP-expressing and HIV-1-infected human astrocytes. J Neurovirol. 2017;23:713–724. doi: 10.1007/s13365-017-0552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. Journal of leukocyte biology. 2005;78:1198–1203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- Sajja RK, Rahman S, Cucullo L. Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:539–554. doi: 10.1177/0271678X15616978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samikkannu T, Atluri VS, Nair MP. HIV and Cocaine Impact Glial Metabolism: Energy Sensor AMP-activated protein kinase Role in Mitochondrial Biogenesis and Epigenetic Remodeling. Sci Rep. 2016;6:31784. doi: 10.1038/srep31784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone S, Neri M, Mhillaj E, Pomara C, Trabace L, Turillazzi E. The role of the NADPH oxidase derived brain oxidative stress in the cocaine-related death associated with excited delirium: A literature review. Toxicology letters. 2016;258:29–35. doi: 10.1016/j.toxlet.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Green CE, Hasan KM, Vincent J, Suchting R, Weaver MF, Moeller FG, Narayana PA, Cunningham KA, Dineley KT, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: a double-blind randomized controlled pilot trial. Addiction. 2017 doi: 10.1111/add.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, Boger HA, Kalivas PW, Reissner KJ. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biological psychiatry. 2016;80:207–215. doi: 10.1016/j.biopsych.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Shao WH, Shu DH, Zhen Y, Hilliard B, Priest SO, Cesaroni M, Ting JP, Cohen PL. Prion-like Aggregation of Mitochondrial Antiviral Signaling Protein in Lupus Patients Is Associated With Increased Levels of Type I Interferon. Arthritis Rheumatol. 2016;68:2697–2707. doi: 10.1002/art.39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Jeng S, McWeeney SK, Hirsch AJ. A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection. J Virol. 2017;91 doi: 10.1128/JVI.02388-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunita S, Schwartz SL, Conn GL. The Regulatory and Kinase Domains but Not the Interdomain Linker Determine Human Double-stranded RNA-activated Kinase (PKR) Sensitivity to Inhibition by Viral Non-coding RNAs. J Biol Chem. 2015;290:28156–28165. doi: 10.1074/jbc.M115.679738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, Kemnitz JW, Johnson JA, Emborg ME. The PPAR-gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. Journal of neuroinflammation. 2011;8:91. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer S, De Leo J. Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handbook of experimental pharmacology. 2011:235–250. doi: 10.1007/978-3-642-13443-2_8. [DOI] [PubMed] [Google Scholar]
- Swepson C, Ranjan A, Balasubramaniam M, Pandhare J, Dash C. Cocaine Enhances HIV-1 Transcription in Macrophages by Inducing p38 MAPK Phosphorylation. Frontiers in microbiology. 2016;7:823. doi: 10.3389/fmicb.2016.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin KA, Liu G, Volkova E, Pletnev AG. Synergistic Internal Ribosome Entry Site/MicroRNA-Based Approach for Flavivirus Attenuation and Live Vaccine Development. mBio. 2017;8 doi: 10.1128/mBio.02326-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi M, Weber J, Bukrinsky M, Simon GL. The effects of cocaine on HIV transcription. J Neurovirol. 2016;22:261–274. doi: 10.1007/s13365-015-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez C, Horner SM. MAVS Coordination of Antiviral Innate Immunity. J Virol. 2015;89:6974–6977. doi: 10.1128/JVI.01918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire J, Roesch F, Sauter D, Rua R, Hotter D, Van Nuffel A, Vanderstraeten H, Naessens E, Iannucci V, Landi A, et al. HIV Triggers a cGAS-Dependent, Vpu- and Vpr-Regulated Type I Interferon Response in CD4+ T Cells. Cell reports. 2016;17:413–424. doi: 10.1016/j.celrep.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Vivarini Ade C, Pereira Rde M, Barreto-de-Souza V, Temerozo JR, Soares DC, Saraiva EM, Saliba AM, Bou-Habib DC, Lopes UG. HIV-1 Tat protein enhances the intracellular growth of Leishmania amazonensis via the ds-RNA induced protein PKR. Sci Rep. 2015;5:16777. doi: 10.1038/srep16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Ashraf U, Ye J, Duan X, Zohaib A, Wang W, Chen Z, Zhu B, Li Y, Chen H, et al. MicroRNA-22 negatively regulates poly(I:C)-triggered type I interferon and inflammatory cytokine production via targeting mitochondrial antiviral signaling protein (MAVS) Oncotarget. 2016;7:76667–76683. doi: 10.18632/oncotarget.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X. Inducible microRNA- 155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- Wang Y, Landry AP, Ding H. The mitochondrial outer membrane protein mitoNEET is a redox enzyme catalyzing electron transfer from FMNH2 to oxygen or ubiquinone. J Biol Chem. 2017;292:10061–10067. doi: 10.1074/jbc.M117.789800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womersley JS, Uys JD. S-Glutathionylation and Redox Protein Signaling in Drug Addiction. Progress in molecular biology and translational science. 2016;137:87–121. doi: 10.1016/bs.pmbts.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Allen JE, Zhu X, Callen S, Buch S. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. Journal of neurovirology. 2009;15:164–175. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Su TP. Potential Molecular Mechanisms on the Role of the Sigma-1 Receptor in the Action of Cocaine and Methamphetamine. J Drug Alcohol Res. 2016;5 doi: 10.4303/jdar/235970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Jogi M, Onomoto K. Regulation of antiviral innate immune signaling by stress-induced RNA granules. J Biochem. 2016;159:279–286. doi: 10.1093/jb/mvv122. [DOI] [PMC free article] [PubMed] [Google Scholar]


