Abstract
Background
Fatigue is a common and debilitating feature of multiple sclerosis (MS) that remains without reliably effective treatment. Transcranial direct current stimulation (tDCS) is a promising option for fatigue reduction. We developed a telerehabilitation protocol that delivers tDCS to participants at home using specially designed equipment and real-time supervision (remotely supervised transcranial direct current stimulation (RS-tDCS)).
Objective
To evaluate whether tDCS can reduce fatigue in individuals with MS.
Methods
Dorsolateral prefrontal cortex left anodal tDCS was administered using a RS-tDCS protocol, paired with 20 minutes of cognitive training. Here, two studies are considered. Study 1 delivered 10 open-label tDCS treatments (1.5 mA; n = 15) compared to a cognitive training only condition (n = 20). Study 2 was a randomized trial of active (2.0 mA, n = 15) or sham (n = 12) delivered for 20 sessions. Fatigue was assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS)—Fatigue Short Form.
Results and conclusion
In Study 1, there was modest fatigue reduction in the active group (−2.5 ± 7.4 vs −0.2 ± 5.3, p = 0.30, Cohen’s d = −0.35). However, in Study 2 there was statistically significant reduction for the active group (−5.6 ± 8.9 vs 0.9 ± 1.9, p = 0.02, Cohen’s d = −0.71). tDCS is a potential treatment for MS-related fatigue.
Keywords: Fatigue, multiple sclerosis, telerehabilitation, transcranial direct current stimulation, tDCS, tES
Introduction
Multiple sclerosis (MS) is a common progressive neurologic disorder in adults of working age marked by episodes of neuroinflammation and demyelination.1 Fatigue has consistently been shown to be the most common MS symptom; 75% or more of patients report it as among their most disabling MS problem,2,3 and 55% indicate it to be their worst.4 However, despite its frequency, fatigue remains poorly understood. Fatigue in MS is neither consistently linked to disease severity as measured by the Expanded Disability Status Scale (EDSS5) nor disease duration, although it is generally found to be worse in individuals with the secondary progressive subtype.2,6 Fatigue is distinct from sleepiness, and fails to improve with adequate sleep.7 Multiple factors are thought to contribute to fatigue8,9 with no specific biomarker or etiology yet confirmed.10
A wide variety of therapies have been tested to reduce fatigue in MS, but unfortunately, none have been consistently effective.10 Fatigue may improve with disease-modifying therapy, but there is no evidence of consistent benefit.11 No symptomatic medication, including large trials of modafinil,12 amantadine and pemoline,13 has been found to be reliably effective.4 Behavior-based management programs (e.g. cognitive behavioral therapy, mindfulness-based interventions),14 exercise programs,15,16 and comprehensive strategies to manage MS fatigue17,18 have demonstrated modest benefit but are costly in terms of clinician and patient time and are not widely available.
Transcranial direct current stimulation (tDCS) is a relatively recent therapeutic development that utilizes low-amplitude direct currents to induce changes in cortical excitability. tDCS has promising potential therapeutic applications that are without evidence of detrimental physiological or behavioral side-effects.19,20 Although various non-invasive neuromodulation technologies are available (e.g. transcranial magnetic stimulation), tDCS has unique advantages compared to other stimulation methods such as its ease of use, lower cost, and greater safety and tolerability.21 Initial studies have found tDCS to be effective for many different uses in healthy participants as well as in a range of clinical conditions.20,22–25
Small preliminary studies have found that tDCS may be a promising treatment for MS fatigue, all using sham-controlled crossover designs, with between 10 and 25 participants and five tDCS treatment sessions, using either a motor, sensory, or dorsolateral prefrontal cortex (DLPFC) montage.26,27 The most recent study by Chalah et al.27 demonstrated that the DLPFC (left anodal) when compared the posterior parietal cortex led to the most fatigue-specific improvements. In addition, DLPFC tDCS has demonstrated benefit for fatigue in post-polio syndrome28 and Parkinson’s disease29 (with improvement specific to fatigue vs daytime sleepiness).
While these initial findings indicate promise, crossover designs for the study of fatigue are difficult to interpret given that it is not clear that fatigue treatment can be washed out between treatment options. Conclusions are also limited due to the small sample sizes and low number of tDCS treatments administered. Given that there is a cumulative benefit with repeated administration,20,30 we have carefully designed a remotely supervised tDCS protocol (“RS-tDCS”) to enable larger study designs.31,32 This protocol extends access to participants to complete their sessions from home while maintaining the standards of clinic-based treatment through the use of real-time videoconferencing during the treatment administrations. In addition, the use of the home delivery of treatment can offer a bridge to real-world clinical use. We have demonstrated that this protocol can be used to treat individuals with a wide range of symptoms and levels of disability and have been able to achieve rapid recruitment with high levels of compliance.32
We tested whether tDCS can reduce fatigue in MS patients using remotely administered tDCS in two separate cohorts of MS patients. These studies include an open-label trial of 10 sessions delivered over 2 weeks (Study 1) and a subsequent randomized sham-controlled trial of 20 sessions delivered over 4 weeks (Study 2). If tDCS can reduce fatigue burden for people with MS, it may be possible to implement a tDCS therapy for symptomatic management of fatigue.
Methods
Participants were recruited for two separate feasibility studies for the development of the RS-tDCS protocol. Both studies conformed to the guidelines set by the Declaration of Helsinki, and all study procedures were approved by Stony Brook University Institutional Review Board (Study 1) and New York University Institutional Review Board (Study 2). Written, informed consent was obtained for all participants. Participation was compensated after completion of study visits.
Eligibility criteria for both studies were purposefully broad to assess the feasibility of the RS-tDCS protocol. Participants received neurological examination including EDSS5 prior to screening. As participants enrolled with the purpose of assisting with the development of our methods, they were not specifically recruited on the basis of any symptom including fatigue. Eligibility criteria included a definite diagnosis of MS (all subtypes—required to be in remission if a relapsing–remitting subtype), aged 18–70 years, without history of brain trauma, seizures, or uncontrolled migraine headaches, and be physically, visually, and cognitively competent enough to perform study procedures. Participants were outside of at least a 1-month window of steroid treatment and/or clinical relapse. We used the Symbol Digit Modalities Test (SDMT) to screen for cognitive competence with a z score ≤ −3.0 indicating exclusion from the study. Additonally, if their disability was greater than an EDSS score of 6.5 (i.e. they required assistance with the device application due to upper extrmity deficits), participants were required to enroll in the study with a healthcare proxy (usually a spouse caregiver). All participants were evaluated by a study clinician to ensure eligibility and screened for any major health concern that would result in exclusion from the study (i.e. severe psychiatric diagnoses, cardiac disease).
RS-tDCS protocol
The RS-tDCS protocol includes a baseline in-clinic visit, remote tDCS sessions completed at the participant’s home, and a follow-up in-clinic visit. According to convention,33 sessions were 20 minutes in duration and administered daily, 5 days per week. During the 20-minute stimulation period, participants completed cognitive training games targeting processing speed and working memory.34
The baseline visit consisted of cognitive testing and the completion of symptom inventories. Participants were then trained on the operation of the tDCS device and then completed a 60-second tolerability test. Next, they completed the first tDCS session in clinic. Participants were then sent home with a study kit for at-home use (laptop computer with mouse and charger, tDCS device and headset, sponges, and extra saline solution).
Each remote session is self-administered with real-time guidance from a study technician; subjects are supervised at all times during sessions via videoconferencing software. Extensive safety measures are taken including strict and well-defined study stop criteria and a device customized for remote use to prevent adverse events and misuse of the device.
Safety and tolerability were measured daily by assessing both experiences of minor adverse events and pain ratings. Following each session, participants reported and rated any adverse events. Pain ratings (using a visual analog scale, rating 1 for minimal to 10 for most severe) were measured before, during, and after each session. Any experience of pain or other adverse event above an intensity rating of 7 resulted in study discontinuation. At study end, participants returned for a final visit with repeat administration of the baseline cognitive tests and symptom inventories.
Equipment
The Soterix mini-Clinical Trials tDCS device was used for all sessions.35 This device is customized specially for remote sessions. The device is fully programmable with a large button keypad for easy use. It records session data (connection quality, time of session, etc.) for all sessions. The device also has built-in safety functions including an automatic abort feature which ends the session if electrode contact is lost. The device is locked to the participant until they receive a one-time use “dose code” that unlocks the device for the programmed dose given to the participant, preventing misuse.
The headset is optimized for stimulation of the DLPFC (left anodal).36 We use 5 × 5 cm2 sponge electrodes that easily snap into the headset. Overall, the headset and electrode sponges are designed to ensure easy and reliable headset placement.
Finally, all participants receive a standardized study laptop (HP Stream 13) to use for the cognitive games and connection to study technician.
Study 1
MS participants were recruited between the dates of March 2015 and February 2016 at Stony Brook Medicine Hospital. Participants enrolled for open-label tDCS received 10 sessions of 20 minute × 1.5 mA stimulation. Control participants were recruited separately. They completed the same remote procedures including the cognitive training games for 10 sessions × 20 minutes but did not have any tDCS device or headset.
Study 2
Participants with MS were recruited between January 2016 and September 2016 at the MS Care Center at New York University Langone Medical Center as part of an ongoing, actively recruiting, randomized, double-blinded, controlled clinical trial using RS-tDCS. Participants completed 20 sessions of active tDCS or sham tDCS, each session lasting 20 minutes. Participants randomized into the active group received 2.0 mA stimulation (1.5 mA if they could not tolerate 2.0 mA during the tolerability test at baseline). A member of the study staff who was not involved with baseline, follow-up, or daily sessions was specified to prepare the study device according to the randomization scheme to ensure blinding of study technician and participant. Randomization was achieved via blocked stratifaction using block sizes of 4 and 6, and participants were stratified by their prescreening EDSS and SDMT scores. Sham was delivered following the conventional method of a ramp up to 2.0 mA and back down during the first and last minutes of the session.20
Study measures
Fatigue Severity Scale
The Fatigue Severity Scale (FSS) includes nine items assessing fatigue in the past week with scores ranging from 9 to 63 with higher scores indicating worse fatigue.37 Baseline level of fatigue was characterized at baseline by the FSS with scores >36 indicating the presence of clinically significant fatigue.
Patient-Reported Outcomes Measurement Information System—fatigue short form
The Patient-Reported Outcomes Measurement Information System (PROMIS)—fatigue short form served as this study’s primary outcome for fatigue change and includes eight items rated according to severity of fatigue experienced over the past 7 days.38 Scores range from 8 to 40 with higher scores indicating worse fatigue. It was administered at baseline and study end (after 10 or 20 sessions for Studies 1 and 2, respectively), with change scores representing treatment response.
Visual analog fatigue ratings
Before and after each tDCS session, participants were asked to rate their fatigue on a visual analog scale from 0 (no fatigue) to 10 (severe, worst fatigue). Visual analog scales were set as laptop backgrounds for the participant to easily refer. This was used as a secondary outcome for fatigue response.
Beck Depression Inventory–Fast Screen
Baseline severity of depression was characterized at baseline by the Beck Depression Inventory (BDI) –Fast Screen categories.39 The BDI stratifies patients into four categories of depression severity: 1—minimal, 2— mild, 3—moderate, 4—severe.
Analyses
Fatigue outcomes were measured by change in PROMIS Fatigue ratings from baseline to study end (10 or 20 treatment sessions for Studies 1 and 2, respectively), compared between the treatment groups using two-tailed independent sample t-tests and within-subject, paired sample t-tests. In addition, daily fatigue ratings were measured by the visual analog ratings across the daily treatment sessions. Analysis was completed using IBM SPSS Statistics 23. An intent to treat analysis was not used as both studies were designed as feasibility studies with broad recruitment criteria.
Results
Sample characteristics
Recruitment details for both Studies 1 and 2 are shown in Figure 1. Demographic and clinical characteristics are described in Table 1. There were a greater proportion of participants with progressive subtypes in the active vs comparison condition of Study 1, but otherwise, the groups were well-matched for both Study 1 and 2.
Figure 1.
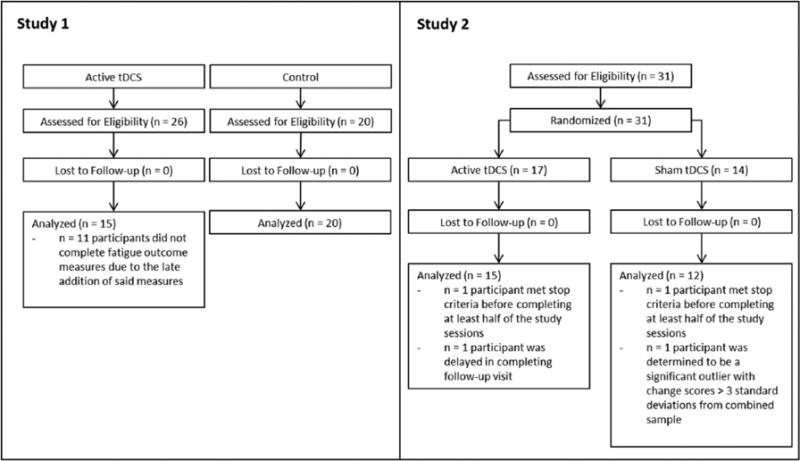
CONSORT flow diagram for Studies 1 and 2 shown. Study 2 was randomized upon enrollment, while Study 1 had active and control conditions recruited separately.
Table 1.
Demographic and clinical characteristics.
| Value | Study 1
|
Study 2
|
||||
|---|---|---|---|---|---|---|
| Active (n = 15) |
Control (n = 20) |
p value | Active (n = 15) |
Sham (n = 12) |
p value | |
| Age (mean ± SD) | 53.4 ± 8.0 | 51.0 ± 12.7 | 0.50 | 44.8 ± 16.2 | 43.4 ± 16.2 | 0.83 |
| Gender (% female) | 66 | 65 | 1.00 | 53 | 67 | 0.70 |
| Race (% African American) | 13 | 15 | 0.80 | 33 | 33 | 1.00 |
| Ethnicity (% Hispanic) | 0 | 10 | 0.50 | 14 | 12 | 0.99 |
| Handedness (% right-handed) | 92 | 95 | 0.69 | 73 | 92 | 0.43 |
| Years education (mean ± SD) | 15.9 ± 2.6 | 14.8 ± 2.0 | 0.19 | 15.5 ± 2.0 | 16.1 ± 1.8 | 0.41 |
| Years with MS (mean ± SD) | 15.6 ± 8.9 | 15.7 ± 9.6 | 0.99 | 15.8 ± 9.4 | 13.3 ± 11.3 | 0.51 |
| Diagnosis (% RRMS) | 20 | 75 | 0.002 | 40 | 58 | 0.45 |
| EDSS (median and range) | 6.0 (1.0–8.0) | 4.0a (2.0–7.0) | 0.18 | 6.0 (0.0–7.0) | 3.5 (0.0–8.5) | 0.89 |
| Baseline FSS (% clinical fatigue) | 66 | 75 | 0.71 | 50 | 76 | 0.15 |
| Baseline PROMIS Fatigue (mean ± SD) | 26.9 ± 7.6 | 23.85 ± 5.8 | 0.19 | 26.6 ± 9.0 | 22.9 ± 7.9 | 0.30 |
| Baseline BDI category (% Category 1) | 50 | 65 | 0.15 | 50 | 64 | 0.84 |
SD: standard deviation; MS: multiple sclerosis; RRMS: relapsing–remitting multiple sclerosis; EDSS: Expanded Disability Status Scale; FSS: Fatigue Severity Scale; PROMIS: Patient-Reported Outcomes Measurement Information System; BDI: Beck Depression Inventory.
n = 8 due to missing EDSS data for Study 1 Controls.
Primary outcome—change in fatigue following tDCS
Our primary outcome was change in PROMIS Fatigue scores from baseline to treatment end. In Study 1, there was modest fatigue reduction in the active group (−2.5 ± 7.4 vs −0.2 ± 5.3, p = 0.30, Cohen’s d = −0.35). However, in Study 2 there was a statistically significant reduction for the active group (−5.6 ± 8.9 vs 0.9 ± 1.9, p = 0.02, Cohen’s d = −0.71).These data are shown in Figure 2.
Figure 2.
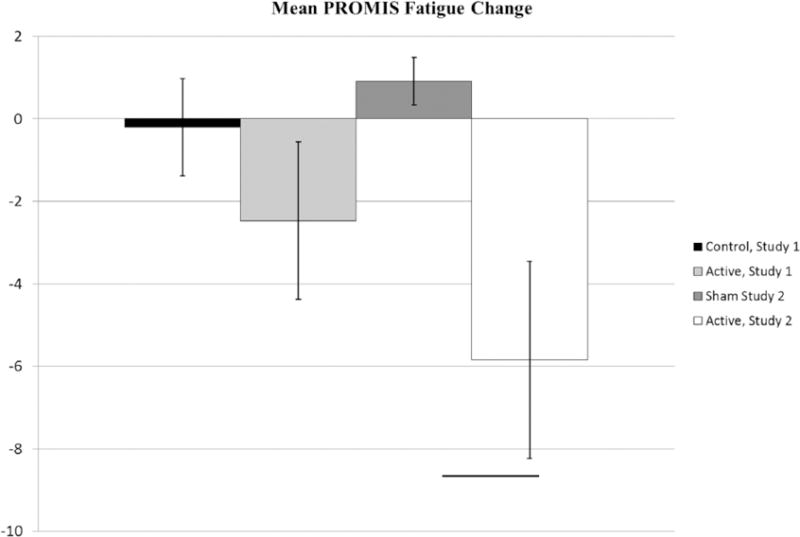
Mean change in PROMIS Fatigue score is shown. Error bars represent standard error of the mean.
*significance at p < 0.05 as determined by a two sample t-test.
The magnitude of within-participant change was tested using paired samples t-tests, as shown in Table 2, with benefit in actively treated groups. The effect is moderate and not statistically significant in the active group of Study 1, with a strong and significant effect is observed in the active group of Study 2.
Table 2.
Within-subject fatigue responses (PROMIS Fatigue Scale).
| Value | Study 1
|
Study 2
|
||
|---|---|---|---|---|
| Active | Control | Active | Sham | |
| Pre-treatment meana | 26.9 ± 7.6 | 23.9 ± 5.8 | 26.6 ± 9.2 | 22.9 ± 7.9 |
| Post-treatment meana | 24.4 ± 6.3 | 23.7 ± 7.1 | 21.0 ± 6.4 | 23.8 ± 8.4 |
| Mean change | −2.5 | −0.2 | −5.6 | 0.9 |
| p value | 0.22 | 0.87 | 0.04 | 0.15 |
| Cohen’s d | −0.35 | −0.03 | −0.71 | 0.11 |
PROMIS: Patient-Reported Outcomes Measurement Information System.
Pre-/post-treatment values display mean ± standard deviation.
As shown in Figure 1 and Table 2, the increase from to 10 to 20 sessions leads to a notable decrease in fatigue, supporting a hypothesized cumulative benefit of tDCS sessions.
Responder rates
Clinically meaningful change on the PROMIS Fatigue scale can be defined by a reduction in 8 or more points.38 Using this definition, in Study 1, there were 3/15 (20%) responders in the active group vs 2/20 (10%) responders in the control group; χ2 test p = 0.63. In Study 2, there were 5/13 (36%) responders in the active group and 0/11 (0%) responders in the sham group; χ2 test p = 0.046.
Baseline fatigue severity and response
For the groups treated with active tDCS, baseline fatigue severity was associated with magnitude of response. Pearson’s correlation coefficients were calculated to assess the relationship between baseline FSS score and change in PROMIS Fatigue. Study 1 active group Pearson’s r = −0.48, p = 0.07; control group Pearson’s r = 0.23, p = 0.34. Study 2 active group Pearson’s r = −0.67, p = 0.01; sham group Pearson’s r = 0.04, p = 0.91.
Secondary outcome: acute fatigue response following tDCS
We next analyzed the acute daily effects of tDCS by comparing fatigue ratings before and after each treatment sessions and across treatment sessions. These analyses were completed only on Study 2 because of the randomized sham-controlled design and findings of significant benefit following the 20 treatment sessions.
First, we averaged each participant’s pre-session fatigue and post-session fatigue based on the 1–10 visual analog rating immediately before and following each session. Paired t-tests were run revealing a significant within-subject effect for active participants (mean change in rating after the session = −0.64, p = 0.01) and no significant effect for sham participants (mean change = −0.09, p = 0.18). These data are shown in Figure 3.
Figure 3.
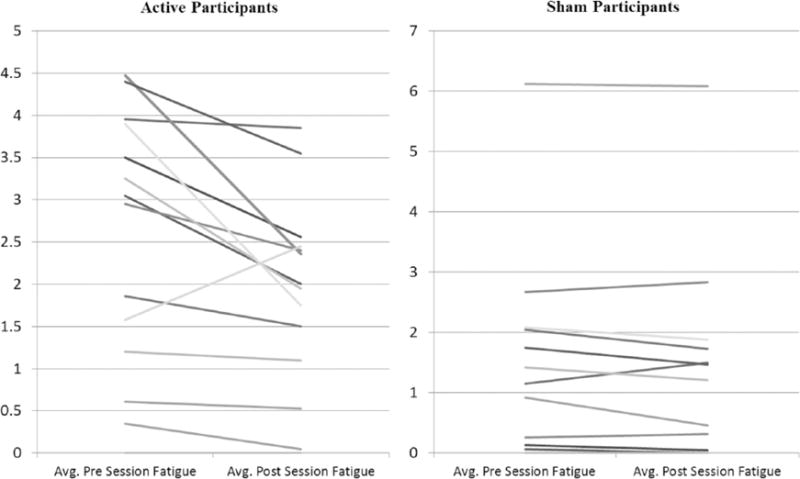
Data for average daily fatigue are shown. Data shown are only from Study 2. Each line represents the average change in daily fatigue for a participant. *significance at p < 0.05 as determined by a paired sample t-test.
Adverse events
In Study 2, n = 4 participants were excluded from analysis (see Figure 1). Of these four, n = 2 participants were excluded from analysis as they had too little RS-tDCS sessions completed. As mentioned previously, any participants who did not complete more than eight sessions were excluded from analyses. Both excluded participants reported pain above a 6 on the visual analog scale in reference to pain associated with the tDCS device which met the RS-tDCS protocol’s stop criteria and led to suspension of all further tDCS sessions for both participants before they could reach the number of sessions necessary to warrant inclusion in analyses. Frequency of common side effects of tDCS33 was recorded as well, shown in Table 3.
Table 3.
tDCS side effect frequency.
| Side effect | Active frequency (%) | Sham frequency (%) |
|---|---|---|
| Tingling | 43 | 46 |
| Itching | 21 | 10 |
| Burning sensation | 23 | 24 |
| Headache | 0 | 0 |
| Head pain or pressure | 2 | 4 |
| Dizziness | <1 | 0 |
| Forgetfulness | 0 | <1 |
| Difficulty concentrating | 4 | 1 |
| Blurred vision | <1 | 0 |
| Facial muscle twitching | <1 | 2 |
| Nausea | 0 | 1 |
| Difficulty breathing | 0 | 0 |
tDCS: transcranial direct current stimulation.
Blinding
We used the standard approach to sham blinding through an initial and end session 60-second period of stimulation. We asked participants to guess their assigned condition at study end. Across study participants, 17/24 participants who gave a response guessed their condition correctly. There was no significant difference in outcome between those participants who guessed correctly vs incorrectly in either condition.
Discussion
We found that 20 sessions of tDCS using a left anodal DLPFC montage combined with computer-based cognitive training lead to significant reduction in MS-related fatigue. The reduction in fatigue is greater following 20 sessions × 2.0 mA (Study 2) vs 10 sessions × 1.5 (Study 1), suggesting that longer treatment periods and higher stimulation intensity are of greater benefit. A significant and moderate reduction of fatigue was observed in the active group that received 20 sessions × 2.0 mA tDCS, relative to the sham group (Study 2), and this effect was greater than the reductions in fatigue experienced by participants who received 10 sessions × 1.5 mA, relative to the control group (Study 1). This effect seems to be both cumulative (more robust effect across repeated treatment sessions as shown in our primary outcome measure) and acute (as shown in daily reported fatigue). Further, correlations inidcate that participants with higher levels of fatigue undergoing tDCS may have the greatest benefit.
In comparison to prior pharmaceutical trials of fatigue treatments, our RS-tDCS protocol proves to be an especially promising treatments for MS fatigue. For example, trials of modafinil for fatigue management have shown mixed results with some trials failing to show a statistically significant effect on fatigue, and despite successful drug trials, no treatment is in routine clinical use.4,10 While more studies are needed, these data present very promising signals for RS-tDCS as a non-pharmacological treatment for MS fatigue.
Importantly, tDCS was delivered to participants at home through remote supervision in a telerehabilitation protocol, referred to as RS-tDCS. Home treatment overcomes many barriers to access, including time and travel limitations, and allows access to even those with more severe disabilities. Treating patients in their homes offers a practical, low cost, and real-world option for fatigue management that can be made widely available. It is promising that we have positive results similar to clinic-based tDCS trials for fatigue26,27 that supports future use of our RS-tDCS protocol.
There are several limitations to the current studies that we report. While our sample sizes are larger than most tDCS studies, there is still a need for a greater sample size in a large-scale trial. For both studies, participants were not specifically recruited for fatigue treatment, and they included participants who did not meet full criteria for clinically severe fatigue. However, given that the prevalence of fatigue is a near universal symptom of MS,2 and in our study 67% of the entire sample (Studies 1 and 2) met criteria for clinically significant fatigue, these results are important for guiding treatment. Another limitation is that Study 1 was open-label, and the control group did not receive sham treatment. However, in Study 2 using a more rigorous randomized sham-controlled design, a significant benefit was found. An additional question is the role of the computer-based cognitive training paired with stimulation in our protocol. tDCS is theorized to function via plasticity mechanisms in the brain, meaning the training tDCS is paired with which is believed to be important to the benefits found.20 We included cognitive training to separately determine whether tDCS can lead to enhanced cognitive training outcomes.34 The cognitive training may uniquely contribute to benefits for fatigue, for instance, by stimulating the regions underlying alertness and activation.36 However, the contribution of cognitive training is controlled for both Study 1 and Study 2 control conditions.
There are distinct differences in design and participant cohort that limit conclusions that can be drawn in comparing the two studies. Participant recruitment was not controlled for disease subtype, resulting in a disproportionate amount of participants with the progressive subtype in Study 1. While it is possible that this skewed results for Study 1, the same demographic imbalance is not present in Study 2 where an even stronger effect is seen. Whether MS subtype has a significant effect on treatment is unknown as our subtype cohorts were not large enough to reliably analyze for an effect. Another aspect to consider is the importance of amperage (1.5 vs 2.0 mA) and number of sessions (10 vs 20 sessions). While work is still being done to assess the relative importance of amperage and session number, it has been found that 1.5–4.0 mA is effective and cumulative sessions (preferably at least 10) are needed for long-lasting effect.20,33
These findings fully support the need for a larger scale clinical trial. Future studies in MS should be careful to address the different disease subtypes and general heterogeneity of the disease when designing the trial. Further study into the optimization of dosing parameters would also be helpful but not necessary for larger scale clinical trials.
Acknowledgments
The authors would like to thank Margaret Kasschau, Ariana Frontario, Natalie Pawlak, and William Pau for their efforts in participant recruitment and data collection for the study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: By the Lourie Foundation, Inc. and the National MS Society Pilot Grant (PP-1411-02021).
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CUNY has patents with M.B. as inventor. M.B is an advisor for and has equity in Soterix Medical. CUNY has patents with A.D. as inventor. A.D. is an employee and has equity in Soterix Medical.
Contributor Information
Leigh E Charvet, Department of Neurology, NYU Langone Medical Center, New York, NY, USA.
Bryan Dobbs, Department of Neurology, NYU Langone Medical Center, New York, NY, USA.
Michael T Shaw, Department of Neurology, NYU Langone Medical Center, New York, NY, USA.
Marom Bikson, Department of Biomedical Engineering, The City University of New York, New York, NY, USA.
Abhishek Datta, Soterix Medical, New York, NY, USA.
Lauren B Krupp, Department of Neurology, NYU Langone Medical Center, New York, NY, USA.
References
- 1.Poser CM, Brinar VV. The accuracy of prevalence rates of multiple sclerosis: A critical review. Neuroepidemiology. 2007;29(3–4):150–155. doi: 10.1159/000111576. [DOI] [PubMed] [Google Scholar]
- 2.Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367–368. doi: 10.1191/135248506ms1373ed. [DOI] [PubMed] [Google Scholar]
- 3.Lerdal A, Celius EG, Krupp L, et al. A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol. 2007;14(12):1338–1343. doi: 10.1111/j.1468-1331.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 4.Khan F, Amatya B, Galea M. Management of fatigue in persons with multiple sclerosis. Front Neurol. 2014;5:177. doi: 10.3389/fneur.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 6.Ghajarzadeh M, Jalilian R, Eskandari G, et al. Fatigue in multiple sclerosis: Relationship with disease duration, physical disability, disease pattern, age and sex. Acta Neurol Belg. 2013;113(4):411–414. doi: 10.1007/s13760-013-0198-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen MY, Wang EK, Jeng YJ. Adequate sleep among adolescents is positively associated with health status and health-related behaviors. BMC Public Health. 2006;6:59. doi: 10.1186/1471-2458-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kos D, Kerckhofs E, Nagels G, et al. Origin of fatigue in multiple sclerosis: Review of the literature. Neurorehabil Neural Repair. 2008;22(1):91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- 9.Genova HM, Rajagopalan V, Deluca J, et al. Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS ONE. 2013;8(11):e78811. doi: 10.1371/journal.pone.0078811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charvet L, Serafin D, Krupp LB. Fatigue in multiple sclerosis. Fatigue. 2014;2(1):3–13. [Google Scholar]
- 11.Kraft G, Bamer A, McMullen K, et al. Multiple sclerosis patients taking disease modifying treatments endorse less severe symptoms and improved quality of life compared with patients not on treatment. Neurology. 2012;78(Suppl. 1):P07.094. [Google Scholar]
- 12.Lange R, Volkmer M, Heesen C, et al. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009;256(4):645–650. doi: 10.1007/s00415-009-0152-7. [DOI] [PubMed] [Google Scholar]
- 13.Krupp LB, Coyle PK, Doscher C, et al. Fatigue therapy in multiple sclerosis: Results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology. 1995;45(11):1956–1961. doi: 10.1212/wnl.45.11.1956. [DOI] [PubMed] [Google Scholar]
- 14.Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: Exercise, education, and medication. Mult Scler Int. 2014;2014:798285. doi: 10.1155/2014/798285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fragoso YD, Santana DL, Pinto RC. The positive effects of a physical activity program for multiple sclerosis patients with fatigue. Neurorehabilitation. 2008;23(2):153–157. [PubMed] [Google Scholar]
- 16.Vore ME, Elgelid S, Bolger S, et al. Impact of a 10-week individualized exercise program on physical function and fatigue of people with multiple sclerosis: A pilot study. Int J MS Care. 2011;13(3):121–126. doi: 10.7224/1537-2073-13.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugos CL, Copperman LF, Fuller BE, et al. Clinical trial of a formal group fatigue program in multiple sclerosis. Mult Scler. 2010;16(6):724–732. doi: 10.1177/1352458510364536. [DOI] [PubMed] [Google Scholar]
- 18.Finlayson M, Preissner K, Cho C. Outcome moderators of a fatigue management program for people with multiple sclerosis. Am J Occup Ther. 2012;66(2):187–197. doi: 10.5014/ajot.2012.003160. [DOI] [PubMed] [Google Scholar]
- 19.Fregni F, Nitsche MA, Loo CK, et al. Regulatory Considerations for the Clinical and Research Use of Transcranial Direct Current Stimulation (tDCS): Review and recommendations from an expert panel. Clin Res Regul Aff. 2015;32(1):22–35. doi: 10.3109/10601333.2015.980944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunoni AR, Nitsche MA, Bolognini N, et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012;5(3):175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitsche MA, Boggio PS, Fregni F, et al. Treatment of depression with transcranial direct current stimulation (tDCS): A review. Exp Neurol. 2009;219(1):14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Borckardt JJ, Bikson M, Frohman H, et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012;13(2):112–120. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Doruk D, Gray Z, Bravo GL, et al. Effects of tDCS on executive function in Parkinson’s disease. Neurosci Lett. 2014;582:27–31. doi: 10.1016/j.neulet.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 24.Vigod S, Dennis CL, Daskalakis Z, et al. Transcranial direct current stimulation (tDCS) for treatment of major depression during pregnancy: Study protocol for a pilot randomized controlled trial. Trials. 2014;15:366. doi: 10.1186/1745-6215-15-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–578. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiote C, Goldschmidt T, Timaus C, et al. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor Neurol Neurosci. 2014;32(3):423–436. doi: 10.3233/RNN-130372. [DOI] [PubMed] [Google Scholar]
- 27.Chalah MA, Riachi N, Ahdab R, et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J Neurol Sci. 2017;372:131–137. doi: 10.1016/j.jns.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Acler M, Bocci T, Valenti D, et al. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor Neurol Neurosci. 2013;31(5):661–668. doi: 10.3233/RNN-130321. [DOI] [PubMed] [Google Scholar]
- 29.Forogh B, Rafiei M, Arbabi A, et al. Repeated sessions of transcranial direct current stimulation evaluation on fatigue and daytime sleepiness in Parkinson’s disease. Neurol Sci. 2017;38:249–254. doi: 10.1007/s10072-016-2748-x. [DOI] [PubMed] [Google Scholar]
- 30.Shiozawa P, Fregni F, Bensenor IM, et al. Transcranial direct current stimulation for major depression: An updated systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(9):1443–1452. doi: 10.1017/S1461145714000418. [DOI] [PubMed] [Google Scholar]
- 31.Charvet LE, Kasschau M, Datta A, et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: Guidelines for technology and protocols. Front Syst Neurosci. 2015;9:26. doi: 10.3389/fnsys.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasschau M, Sherman K, Haider L, et al. A protocol for the use of remotely-supervised transcranial direct current stimulation (tDCS) in multiple sclerosis (MS) J Vis Exp. 2015;106:e53542. doi: 10.3791/53542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2017;10:983–985. doi: 10.1016/j.brs.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Charvet L, Shaw M, Dobbs B, et al. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodulation. doi: 10.1111/ner.12583. Epub ahead of print 22 February 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medical S. Transcranial direct current stimulation (Soterix Medical tDCS details) http://soterixmedical.com/tdcs (accessed 18 May 2017)
- 36.Seibt O, Brunoni AR, Huang Y, et al. The pursuit of DLPFC: Non-neuronavigated methods to target the left dorsolateral pre-frontal cortex with symmetric bicephalic transcranial direct current stimulation (tDCS) Brain Stimul. 2015;8(3):590–602. doi: 10.1016/j.brs.2015.01.401. [DOI] [PubMed] [Google Scholar]
- 37.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 38.Jensen RE, Moinpour CM, Potosky AL, et al. Responsiveness of 8 Patient-Reported Outcomes Measurement Information System (PROMIS) measures in a large, community-based cancer study cohort. Cancer. 2017;123:327–335. doi: 10.1002/cncr.30354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]


