Abstract
N-acetylcysteine and bupropion are two promising candidate medications for treatment of substance use disorder. The effects of N-acetylcysteine or bupropion on methamphetamine self-administration of female rats are not well understood. To fill this gap, this study assessed the effects of N-acetylcysteine (0, 30, 60, or 120 mg/kg) and bupropion (0, 10, 30, and 60 mg/kg) on methamphetamine self-administration of female rats across the natural estrous cycle. Following a completed dose-response curve, responding for methamphetamine self-administration was extinguished and the effects of N-acetylcysteine or bupropion on methamphetamine-triggered reinstatement was evaluated in separate experiments. N-acetylcysteine did not decrease responding maintained by methamphetamine or methamphetamine-triggered reinstatement. Bupropion significantly decreased methamphetamine self-administration and methamphetamine-triggered reinstatement in female rats with highest dose (60 mg/kg) also significantly decreasing general chamber activity. In a companion experiment, testing the effect of bupropion on responding maintained by sucrose, we confirmed non-specificity of bupropion’s effects as bupropion also decreased responding for sucrose. Considered together, our findings suggest that while N-acetylcysteine has considerable promise for treatment of cocaine dependence it may not generalize to other stimulants like methamphetamine. Furthermore, although bupropion has been shown to effectively decrease methamphetamine self-administration, and presently methamphetamine-triggered reinstatement, its locomotor and reward suppressing effects warrant further investigation including both sexes.
Keywords: methamphetamine, N-acetylcysteine, N-acetyl-L-cysteine, bupropion, drug self-administration, female rat
1. Introduction
United Nations Office on Drugs and Crime estimates that there are 0.3 to 1.1% of amphetamine-type stimulants users worldwide (13.8–53.8 millions; Burns, 2014). In the US alone, methamphetamine use places a significant economic and societal burden, costing an estimated 23 billion dollars annually (Nicosia et al., 2009). Despite a slight global downward trends in methamphetamine use, approximately 7–8% decrease from 2009–2013, methamphetamine use is increasing in parts of North America and Europe. Although many users desire to quit using methamphetamine, majority of users are not able to do so due to lack of efficacious treatment strategies (Burns, 2014; Nicosia et al., 2009; Substance Abuse and Mental Health Services Administration, 2014).
The primary model of drug taking in the rat is the intravenous drug self-administration. This model has a high degree of face validity as drugs that are abused by humans are generally self-administered by laboratory animals (Brady et al., 1987; Yokel, 1987). In addition, this method models two key features of addiction –namely, drug seeking and drug relapse (Stewart, 2008). Surprisingly, few preclinical studies have examined methamphetamine self-administration patterns in female rats with a focus on examining drugs that could be used to treat methamphetamine use disorder.
Although many pharmacological agents have been identified as potential medications for methamphetamine dependence (Karila et al., 2010), two pharmacological agents that have received a great deal of attention for their suggested therapeutic efficacy include N-acetylcysteine (NAC) and bupropion (Berk et al., 2013; Karila et al., 2010). NAC is a cystine prodrug that increases activity of the cystine-glutamate antiporter system xc− thereby, restoring extracellular glutamate levels following repeated drug use (Moussawi et al., 2011; Nocito Echevarria et al., 2017). NAC also regulates dopamine release from presynaptic terminals through it’s enhancement of extracellular glutamate which activates metabotropic glutamate receptors (mGluR2/3; Baker et al., 2002; Nocito Echevarria et al., 2017). The promise of NAC as a therapeutic agent is supported by studies showing that NAC rescues methamphetamine-induced reductions in dopamine and DA transporter levels in the striatum (Fukami et al., 2004; Hashimoto et al., 2004). In addition, Fukami et al. (2004) reported that NAC significantly attenuates methamphetamine-induced hyperlocomotion and behavioral sensitization in male rats. The effects of NAC are not restricted to methamphetamine as previous studies have shown that NAC significantly decreases nicotine self-administration and secondly, significantly attenuates cue-induced reinstatement of both nicotine and cocaine responding in male rats (Amen et al., 2011; Kupchik et al., 2012; Ramirez-Niño et al., 2013; Reichel et al., 2011). Furthermore, clinical studies have shown that NAC normalizes elevated glutamate levels in the dorsal anterior cingulated cortex of cocaine-dependent patients and significantly reduces cocaine craving in cocaine-dependent subjects following an experimenter delivered intravenous injection of cocaine (Amen et al., 2011; Schmaal et al., 2012).
Bupropion is an atypical antidepressant with stimulant properties that inhibits the reuptake of dopamine and norepinephrine - thereby restoring monoamine levels following chronic drug use (for review see Foley et al., 2006; Karila et al., 2010). Thus, bupropion may act to ameliorate withdrawal symptoms (e.g., depression) and cognitive impairments during the early part of methamphetamine abstinence (Karila et al., 2010; Newton et al., 2006). Previous studies supporting the use of bupropion as a candidate medication for methamphetamine dependence include findings showing that bupropion decreases methamphetamine self-administration in rats during the acquisition and maintenance phases of drug taking (Reichel et al., 2008, 2011). Clinical studies have found that bupropion is well-tolerated by study participants that receive an intravenous methamphetamine infusion (15 or 30 mg) and that bupropion fails to potentiate methamphetamine’s cardiovascular effects (Newton et al., 2005). In addition, bupropion significantly reduces methamphetamine’s acute subjective effects and cue-induced craving in methamphetamine-dependent individuals while increasing the duration of abstinence in male participants described as having “mild-to-moderate” methamphetamine dependence (Elkashef et al., 2008; Newton et al., 2006).
There is little research in female rats attempting to elucidate behavioral and pharmacological mechanisms associated with methamphetamine self-administration. To fill this gap, this study assessed the effects of NAC and bupropion on methamphetamine self-administration in female rats across the natural estrous cycle. Following a completed dose-response curve, responding for methamphetamine self-administration was extinguished and the effects of NAC and bupropion on methamphetamine-triggered reinstatement was evaluated in separate experiments. To determine the specificity of the bupropion effect on methamphetamine self-administration, a third experiment examined the effect of bupropion on sucrose-maintained responding.
2. Materials and methods
2.1. Subjects
Experimentally naïve, female, Sprague-Dawleys rats (Harlan, Indianapolis, IN) that were 63–70 days of age upon arrival were used in this study. Rats were individually housed in clear polycarbonate tubs (48.3 × 26.7 × 20.3 cm) lined with cellulose bedding (Harlan Laboratories, Indianapolis, IN). The colony was maintained on a 12-hr light/12-hr dark illumination cycle with lights on from 0600 to 1800 hours and maintained at a constant temperature and humidity. All behavioral testing occurred during the light phase, between 0900 and 1600 hours. Rats had free access to water except during behavioral testing. Rat chow was restricted to maintain the rats at 90% of their free-feeding bodyweight in Experiments 1 and 2. In Experiment 3, rats were maintained at 85% of their free-feeding weight. All experiments described in this paper were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee. Upon arrival to the animal facility, all rats received a 7-day acclimation period and were handled for three days (i.e., approximately 2 min/day) before initiation of experimental testing.
2.2. Apparatus
Behavioral testing was conducted in commercially available chambers (ENV-008CT; Med Associates, Inc., St. Albans, VT) enclosed in sound- and light-attenuating cubicles equipped with an exhaust fan. Each conditioning chamber had aluminum sidewalls, metal rod floors with polycarbonate front, back, and ceiling. A recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) was centered on one of the sidewalls. A dipper arm, when raised, provided access to 0.1 ml of 26% (w/v) sucrose solution in the receptacle. Access to the dipper was monitored by an infrared beam mounted 1.2 cm into the receptacle and 3 cm above the chamber floor. A second infrared emitter/detector unit that monitored general activity levels was located 4 cm above the grid floor bisecting the chamber into two halves. The number of times that the photocell beam was interrupted during a session served as a measure of general activity levels. Two retractable levers (147nN of force was required for micro-switch closure and the recording of a lever press) were located on each side of the receptacle. A white cue-light (2.54 cm diameter; 28 V, 100 mA) was positioned 7 cm above each lever and a house light (two white 28V, 100 mA lamps) was positioned 10 cm above the conditioning chamber. Each chamber was equipped with a metal arm and a spring leash (C313C; Plastics One; Roanoke, VA) attached to a liquid swivel. Tygon tubing AAQ04103 (VWR, West Chester, PA) extended through the spring leash and connected to a 5 ml syringe mounted on an infusion pump (Med Associates, PMH-100VS) located outside the sound-attenuating cubicle. Programmable events (e.g., sucrose delivery) and data collection were performed with the Med Associates interface/software system.
2.3. Drugs
D-methamphetamine hydrochloride and NAC were purchased from Sigma Chemical (St. Louis, MO) and were dissolved in sterile 0.9% physiological saline. NAC was pH adjusted with NaOH to 7.2 ± 0.2. Bupropion hydrochloride was purchased from Toronto Research Chemicals (Toronto, Canada) and dissolved in sterile water. Methamphetamine was administered intravenously (IV) at a volume of 35.74 μl per infusion. NAC and bupropion were administered intraperitoneally (IP; 1ml/kg injection volume). Methamphetamine, bupropion, and NAC doses were adapted from previous studies (Reichel et al., 2008, 2011, 2009).
2.4. Surgical Procedures
Rats were anesthetized with a ketamine (100 mg/ml) and xylazine (10 mg/ml) mixture (IM; Midwest Veterinary Supply, Lincoln, NE). A polyurethane catheter (RJVR-23; Strategic Applications Inc., Lake Villa, IL) with rounded tip and double suture beads (one secured internally and other externally) was then implanted into the right external jugular vein. The other end of the catheter was subcutaneously placed around the shoulder and exited below the scapula via polycarbonate back-mount access port (313-000BM; Plastics One Inc., Roanoke, VA). Immediately after the surgery, catheters were flushed with 0.2 ml of streptokinase (2 mg/ml; Sigma) diluted in sterile heparinized saline (30 U/ml; Midwest Veterinary Supply, Lincoln, NE). Atipamezole hydrochloride (0.5 mg/kg; IM; Sigma), diluted in saline, was used to terminate anesthesia. To manage post-surgical pain, buprenorphine hydrochloride (0.1 mg/kg; Sigma) was administered subcutaneously immediately following surgery and during the first three day of surgical recovery. Rats were given seven days to recover from surgery before starting the experiment. During recovery, rats had ad libitum access to food for the first 4 days and then gradually food restricted to 90%. Catheters were flushed twice daily (morning and late afternoon) with heparinized saline (30 U/ml). Catheter patency was assessed with a 0.05 ml IV infusion of xylazine (20 mg/ml) at the end of methamphetamine self-administration phase or when patency loss was suspected. This concentration of xylazine produces clear motor ataxia within 5 sec of intravenous infusion if the catheter is patent (Charntikov et al., 2015, 2013). Only rats with patent catheters were included in the data analysis.
2.5. Vaginal Smears
Vaginal smears were taken daily during the methamphetamine self-administration sessions (excluding all sessions during a period of time when the dose-response to NAC or bupropion was acquired). Vaginal smears were taken by inserting a medicine dropper filled with 0.9% physiological saline into the vagina of a female rat and then aspirating a small amount of vaginal fluid (Marcondes et al., 2002). The vaginal fluid contained cells that were then examined under a microscope (100X objective) and a photograph of the smear was taken and saved to a computer hard drive for later identification. More than 50% of the smear had to contain a specific cell type to be classified using the following criteria: a) diestrus - containing predominantly leukocytes, b) proestrus - containing predominantly well-developed nucleated epithelial cells, c) estrus - containing predominantly well-cornified epithelial cells, and d) metestrus - containing the same proportion of leukocytes, cornified, and nucleated epithelial cells (Marcondes et al., 2002). Two raters blind to the stage of the estrous cycle rated a subset of vaginal smears and a Cohen’s Kapa test was performed to assess inter-rater reliability (inter-rater reliability was satisfactory with κ = 0.72). Data from one of the raters, chosen at random, was used for final analysis.
2.6. Experiment 1: The effect of NAC on methamphetamine self-administration
2.6.1. Preliminary training
Rats were first trained to lever press over 3 daily sessions. At the start of each session, the house-light was turned on and a randomly selected lever (right or left) was inserted. A lever press or lapse of 15 s resulted in sucrose delivery (4-s access), lever retraction, and commencement of a timeout (average=60 s; range=30 to 89 s). Following the timeout, a randomly selected lever was inserted with the condition that the same lever could not be presented more than twice in a row. This protocol was repeated for 60 sucrose deliveries. Sessions lasted 65 to 80 min depending on individual performance. Training continued until a lever press was made on at least 80% of the lever insertions for two consecutive days (i.e., 3 to 5 sessions). After rats met this criterion, they were surgically implanted with an intravenous catheter as described earlier. Following recovery, lever press training continued as described above but the response contingency was changed to a variable ratio (VR3) schedule of reinforcement where on average every third response was followed by delivery of 0.1 ml of sucrose (range=1 to 6 presses). At least 80% of the 60 available sucrose deliveries had to be earned to move to the self-administration phase; this occurred after 3 to 5 sessions. This protocol ensures high rates of responding, yet both levers have similar reinforcement history so as to avoid any potential bias of differential lever press training in later phases.
2.6.2. Methamphetamine self-administration
At the start of each 60 min daily self-administration session, catheters were flushed with 0.2 ml of heparinized saline. The start of each session was signaled by illumination of a house light and the insertion of both levers; the active lever was randomly assigned for each rat. Meeting a schedule requirement resulted in illumination of the cue light above the active lever for 1 sec, infusion of methamphetamine (0.05 mg/kg/infusion) for 1 sec, and retraction of both levers for 1 min. Pressing the inactive lever had no programmed consequences. The schedule of reinforcement was increased automatically from an FR1 (3 days) to an FR3 (2 days) and finally an FR5 for the duration of the self-administration phase.
2.6.3. Dose-response testing
NAC testing commenced following the stabilization of methamphetamine self-administration responding on an FR5 (10 daily FR5 sessions). On the day of the test, rats were injected with NAC (0, 30, 60, or 120 mg/kg, IP) 2.5 hrs prior to the methamphetamine self-administration session. All doses of NAC were administered in a randomized order for each rat. At least two methamphetamine self-administration retraining days separated each test day. To advance to the next test dose each rat was required to reach 80% of responding on the active lever compared to the day preceding very first dose-response test. Following the acquisition of dose-response phase all rats were allowed to self-administer methamphetamine for additional 9 days.
2.6.4. Extinction
Rats then underwent 12 daily extinction training sessions. Extinction training was identical to self-administration sessions except that methamphetamine was never available. Extinction was considered to have occurred when a rat’s responding fell below 50% of the responding that occurred on the last three days of the FR5 methamphetamine self-administration sessions. All rats reached this criterion after 12 extinction sessions.
2.6.5. Reinstatement
Daily reinstatement sessions were 60 min and identical to the extinction sessions except that 2.5 hrs prior to each reinstatement session, rats were injected with a pseudo-randomly selected dose of NAC (0, 30, 60, or 120 mg/kg, IP) and 5 min prior to extinction session rats were injected with methamphetamine (0.25 mg/kg, IP). There were no intervening extinction sessions between these tests.
2.7. Experiment 2: The effect of bupropion on methamphetamine self-administration
This experiment was identical to Experiment 1 except that, instead of NAC, we tested the effect of bupropion (0, 10, 30, or 60 mg/kg; 10 min prior to test sessions; IP). Another exception was that after completing an assessment of bupropion on methamphetamine-induced reinstatement we added one more test day where we assessed whether bupropion alone (30 mg/kg; IP) evoked reinstatement.
2.8. Experiment 3: The effect of bupropion on sucrose-maintained responding
All rats first completed the preliminary training as previously described in Experiment 1. Following the completion of training, rats did not undergo catheter surgery but rather remained in their home cages for the 7 days to mimic the surgical recovery time used in the IV self-administration experiments. The procedures used for the sucrose experiment were identical to those described above for the methamphetamine self-administration sessions except that active lever presses resulted in 4-s access to a 0.1 ml sucrose solution rather than an infusion of methamphetamine. Bupropion testing was identical to Experiment 2 except that reinstatement tests did not include bupropion alone test and that sucrose evoked reinstatement consisted of 10 non-contingent sucrose presentations over 2 min prior to the extinction session (8 seconds on average between free sucrose presentations with range of 4–12 seconds).
2.9. Statistical Analyses
Analyses were performed in R 3.4.0 (R Core Team, 2017) using {stats} package for t-tests and {nlme} package for all analyses associated with linear mixed-effects modeling (Pinheiro et al., 2017).
2.9.1. Dependent measures
Responding on the active lever was used as a primary dependent measure throughout the study. To measure general chamber activity, we used the number of center beam breaks throughout all the sessions.
2.9.2. Methamphetamine or sucrose self-administration
To examine behavior during methamphetamine or sucrose self-administration, we analyzed active and inactive lever responding on the last five training sessions (FR5) using linear mixed-effects modeling with nested random effects (Laird and Ware, 1982). The use of linear mixed-effect modeling provides a number of advantages when compared to ANOVAs. For example, this analysis does not require the assumption that the relation between the covariate and the outcome is the same across the groups and thus does not require meeting the assumption of homogeneity. Furthermore, unlike ANOVA, linear mixed-effects modeling does not assume that the different cases of data were independent and hence can model relations between different outcomes which may be inter-related. Finally, linear mixed-effects modeling is more robust in dealing with missing data or unequal group sizes which is often the case in preclinical animal models. With these advantages, acquisition of methamphetamine or sucrose self-administration was analyzed by building a model with a maximum likelihood fit from a baseline that does not include any predictors other than an intercept. The model was built by first adding predictor 1 (Session), then predictor 2 (Lever - Active or Inactive), and finally an interaction between predictor 1 and 2 (Session × Lever). The predictor or an interaction was declared significant when its addition improved the model by accounting for significantly more variance (the fit was examined using Likelihood Ratio test of fixed effects; p < 0.05). Pairwise comparisons were performed using estimates from the model or using paired t-tests where appropriate.
2.9.3. The effect of NAC or bupropion on methamphetamine or sucrose self-administration
To assess the effect of NAC or bupropion on methamphetamine or sucrose self-administration, we used linear mixed-effects modeling, described earlier, with active lever responding as a dependent measure while Dose, Activity, and Dose by Activity interaction served as predictors (Charntikov et al., 2017).
2.9.4. Methamphetamine, bupropion, or sucrose induced reinstatement of active lever presses
To assess the effect of methamphetamine, bupropion, or free sucrose deliveries on reinstatement of active lever responding, we compared responding on the last extinction session to the responding evoked by methamphetamine alone (0.25 mg/kg; 0 dose of NAC or bupropion), by bupropion alone (30 mg/kg; Experiment 2), or by presentation of free sucrose deliveries using paired t-tests.
2.9.5. The effect of NAC or bupropion on methamphetamine or sucrose-induced reinstatement
To assess the effect of NAC or bupropion on methamphetamine- or sucrose-triggered reinstatement, we used the modeling approach described earlier with Dose, Activity, and Dose × Activity interaction as predictors. The summaries of the model were used to estimate and report contrasts.
3. Results
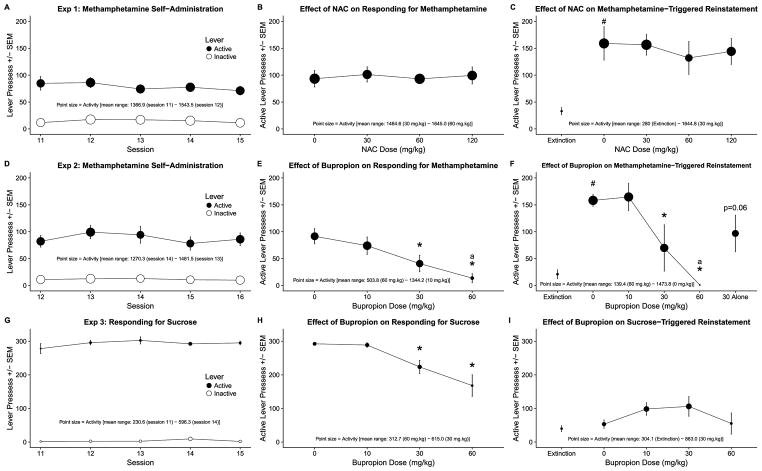
All data are visualized in Figure 1. This combined visualization approach facilitates visual comparisons within and between experiments. This approach is especially valuable considering that each panel represents a multivariable data - active lever responding on the y axis and general chamber activity as a size of each data point from each major stage of each experiment. Y-axis are equalized for each experiment and all point sizes are normalized across all experiments for accuracy and ease of data exploration. Figure caption for Figure 1 contains all information about number of rats for each phase of each experiment to aid with assessment of effects and variance represented as standard error of the means for each data point. Decrease in number of subjects in the later phases of Experiments 1 and 2 indicates removal of rats from statistical analyses due to patency loss during that particular phase.
Figure 1.
Panels A–C show (A; n=8) methamphetamine self-administration, (B; n=8) the effect of NAC on active lever responding for methamphetamine, (C; n=6) the effect of NAC on methamphetamine-triggered reinstatement of active lever responding, and activity for each aforementioned phase, visualized using point size, from Experiment 1. Panels D–F show (D; n=14) methamphetamine self-administration, (E; n=10) the effect of bupropion on active lever responding for methamphetamine, (F; n=5) the effect of bupropion on methamphetamine-triggered reinstatement of active lever responding, and activity for each aforementioned phase, visualized using point size, from Experiment 2. Panels G–I show (G; n=7) responding for sucrose, (H; n=7) the effect of bupropion on active lever responding for sucrose, (I; n=7) the effect of bupropion on sucrose-triggered reinstatement of active lever responding, and activity for each aforementioned phase, visualized using point size, from Experiment 2. #Indicates reinstatement effect - significant difference from active lever responding on the last extinction session. *Indicates significant difference from responding following pretreatment with 0 mg/kg. aIndicates significant difference in general chamber activity in comparison to activity after pretreatment with 0 mg/kg dose. p=0.06Indicates p value of t-test comparing responding on the active lever following injection of 30 mg/kg bupropion alone to responding on the last extinction session. p<0.05.
3.1. Experiment 1
3.1.1. Methamphetamine self-administration
Lever responding significantly differed depending on the Lever (active or inactive; χ2(2) = 125.20, p < 0.0001) but not Session. There was no Lever × Session interaction (for the essential statistical output related to Experiment 1 see Table 1). Over the sessions, responding on the active lever was significantly higher than responding on the inactive levers (b = −64.15, t(39) = −18.44, p < 0.0001).
Table 1.
Statistical output from Experiment 1.
| Model | df | AIC | BIC | logLik | Test | L.Ratio | p-value | |
|---|---|---|---|---|---|---|---|---|
| Acquisition of methamphetamine self-administration | ||||||||
|
| ||||||||
| Baseline Model | 1 | 6 | 821.06 | 835.36 | −404.53 | |||
| Adding Session Factor | 2 | 7 | 822.63 | 839.31 | −404.32 | 1 vs 2 | 0.43 | 0.51 |
| Adding Lever Factor | 3 | 8 | 699.43 | 718.48 | −341.71 | 2 vs 3 | 125.21 | 0.00 |
| Adding Session × Lever | 4 | 9 | 699.51 | 720.95 | −340.76 | 3 vs 4 | 1.92 | 0.17 |
|
| ||||||||
| The effect of NAC on responding for methamphetamine | ||||||||
|
| ||||||||
| Baseline Model | 1 | 5 | 321.66 | 328.99 | −155.83 | |||
| Adding Dose Factor | 2 | 8 | 327.03 | 338.76 | −155.52 | 1 vs 2 | 0.63 | 0.89 |
| Adding Activity Factor | 3 | 9 | 328.54 | 341.73 | −155.27 | 2 vs 3 | 0.49 | 0.48 |
| Adding Dose × Activity | 4 | 12 | 321.23 | 338.82 | −148.62 | 3 vs 4 | 13.30 | 0.00 |
|
| ||||||||
| The effect of NAC on methamphetamine-triggered reinstatement | ||||||||
|
| ||||||||
| Baseline Model | 1 | 5.00 | 259.53 | 265.42 | −124.76 | |||
| Adding Dose Factor | 2 | 8.00 | 262.67 | 272.09 | −123.33 | 1 vs 2 | 2.86 | 0.41 |
| Adding Activity Factor | 3 | 9.00 | 261.42 | 272.02 | −121.71 | 2 vs 3 | 3.25 | 0.07 |
| Adding Dose × Activity | 4 | 12.00 | 264.08 | 278.22 | −120.04 | 3 vs 4 | 3.34 | 0.34 |
3.1.2. The effect of NAC on responding for methamphetamine
Active lever responding did not differ depending on the dose of NAC or general chamber activity. There was a significant Dose × Activity interaction (χ2(7) = 13.30, p < 0.01). Despite the interaction, there was no differences in active lever responding or general chamber activity across the dose range.
3.1.3. Methamphetamine-triggered reinstatement
Methamphetamine (0.25 mg/kg; I1P) significantly increased active lever responding in extinction when compared to responding on the last extinction session (t(5) = −5.04, p < 0.01; Figure 1C - compare Extinction to 0 mg/kg dose).
3.1.4. The effect of NAC on methamphetamine-triggered reinstatement
Active lever responding did not differ depending on Dose or Activity across the dose range (Figure 1C). There was no Dose × Activity interaction.
3.2. Experiment 2
3.2.1. Methamphetamine self-administration
Lever responding significantly differed depending on the Lever (active or inactive; χ2(2) = 140.40, p < 0.0001) but not Session. There was no Lever × Session interaction (for the essential statistical output related to Experiment 2 see Table 2). Over the sessions, responding on the active levers was significantly higher than responding on the inactive levers (b = −76.51, t(69) = −15.88, p < 0.0001; Figure 1D).
Table 2.
Statistical output from Experiment 2.
| Model | df | AIC | BIC | logLik | Test | L.Ratio | p-value | |
|---|---|---|---|---|---|---|---|---|
| Acquisition of methamphetamine self-administration | ||||||||
|
| ||||||||
| Baseline Model | 1 | 6 | 1509.63 | 1527.28 | −748.82 | |||
| Adding Session Factor | 2 | 7 | 1511.54 | 1532.13 | −748.77 | 1 vs 2 | 0.09 | 0.77 |
| Adding Lever Factor | 3 | 8 | 1373.14 | 1396.67 | −678.57 | 2 vs 3 | 140.40 | 0.00 |
| Adding Session × Lever | 4 | 9 | 1375.06 | 1401.54 | −678.53 | 3 vs 4 | 0.08 | 0.78 |
|
| ||||||||
| The effect estrous on responding for methamphetamine (prior to dose-effect phase) | ||||||||
|
| ||||||||
| Baseline Model | 1 | 4 | 1372.54 | 1384.31 | −682.27 | |||
| Adding Estrous Factor | 2 | 5 | 1373.56 | 1388.27 | −681.78 | 1 vs 2 | 0.98 | 0.32 |
|
| ||||||||
| The effect of bupropion on responding for methamphetamine | ||||||||
|
| ||||||||
| Baseline Model | 1 | 5 | 436.99 | 445.43 | −213.49 | |||
| Adding Dose Factor | 2 | 8 | 419.57 | 433.08 | −201.79 | 1 vs 2 | 23.42 | 0.00 |
| Adding Activity Factor | 3 | 9 | 407.78 | 422.98 | −194.89 | 2 vs 3 | 13.79 | 0.00 |
| Adding Dose × Activity | 4 | 12 | 410.90 | 431.17 | −193.45 | 3 vs 4 | 2.88 | 0.41 |
|
| ||||||||
| The effect estrous on responding for methamphetamine (after dose-effect phase) | ||||||||
|
| ||||||||
| Baseline Model | 1 | 4 | 614.79 | 623.43 | −303.40 | |||
| Adding Estrous Factor | 2 | 5 | 616.65 | 627.44 | −303.32 | 1 vs 2 | 0.15 | 0.70 |
|
| ||||||||
| The effect of bupropion on methamphetamine-triggered reinstatement | ||||||||
|
| ||||||||
| Baseline Model | 1 | 5 | 301.20 | 307.29 | −145.60 | |||
| Adding Dose Factor | 2 | 9 | 289.33 | 300.30 | −135.67 | 1 vs 2 | 19.87 | 0.00 |
| Adding Activity Factor | 3 | 10 | 288.57 | 300.76 | −134.29 | 2 vs 3 | 2.76 | 0.10 |
| Adding Dose × Activity | 4 | 14 | 291.73 | 308.80 | −131.87 | 3 vs 4 | 4.84 | 0.30 |
3.2.2. The effect of bupropion on responding for methamphetamine
Active lever responding significantly differed depending on the Dose (χ2(3) = 23.41, p < 0.0001) and Activity (χ2(4) = 13.79, p < 0.001). There was no Dose × Activity interaction. Active lever responding following pretreatment with 30 or 60 mg/kg doses of bupropion was significantly lower than after pretreatment with saline (0 mg/kg; Figure 1E). Activity after pretreatment with 60 mg/kg was significantly lower than after pretreatment with 0 mg/kg but comparable to that of stimulant free rats in Experiment 3 (compare with Figure 1G).
3.2.3. Methamphetamine-triggered reinstatement
Methamphetamine (0.25 mg/kg; IP) significantly increased active lever responding in extinction when compared to responding on the last extinction session (t(4) = −11.58, p < 0.001; Figure 1F - compare Extinction to 0 mg/kg dose).
3.2.4. The effect of bupropion on methamphetamine-triggered reinstatement
Active lever responding significantly differed depending on the bupropion Dose (χ2(4) = 19.86, p < 0.001) but not the Activity. There was no Dose × Activity interaction. Pretreatment with 30 or 60 mg/kg bupropion doses significantly decreased methamphetamine-triggered reinstatement (Figure 1F). Although our statistical analysis showed no significant effect of Actiivty on active lever responding, there was a significant decrease in activity following pretreatment with 60 mg/kg. We followed-up this effect by analyzing general chamber activity alone during the reinstatement and confirmed that activity significantly varied over the range of doses (χ2(4) = 24.43, p < 0.0001) and that activity following 60 mg/kg pretreatment was significantly lower than after pretreatment with 0 mg/kg (Figure 1F).
3.2.5. Bupropion-triggered reinstatement of responding on the active Lever
Bupropion (30 mg/kg) alone increased active lever responding in extinction when compared to the responding on the last extinction session but this effect did not reach significance although it was trending towards it (t(4) = −2.5, p = 0.06; Figure 1F - compare responding on the last Extinction session to responding evoked by 30 mg/kg of bupropion alone).
3.2.6. The effect of estrous on methamphetamine self-administration
Active lever responding for methamphetamine, during 10 FR5 sessions prior to dose-effect acquisition and during 8 sessions after dose-effect acquisition, did not significantly differ depending on the estrous phase.
3.3. Experiment 3
3.3.1. Responding for sucrose
Lever responding significantly differed depending on the Lever (χ2(2) = 304.00, p < 0.0001) but not Session. There was no Lever × Session interaction (for the essential statistical output related to Experiment 3 see Table 3). Over the sessions, responding on the active levers was significantly higher than responding on the inactive lever (b = −289.14, t(69) = −75.59, p < 0.0001; Figure 1G).
Table 3.
Statistical output from Experiment 3.
| Experiment 1 | Model | df | AIC | BIC | logLik | Test | L.Ratio | p-value |
|---|---|---|---|---|---|---|---|---|
| Acquisition of responding for sucrose | ||||||||
|
| ||||||||
| Baseline Model | 1 | 6 | 908.00 | 921.49 | −448.00 | |||
| Adding Session Factor | 2 | 7 | 909.98 | 925.72 | −447.99 | 1 vs 2 | 0.02 | 0.88 |
| Adding Lever Factor | 3 | 8 | 607.97 | 625.96 | −295.98 | 2 vs 3 | 304.01 | 0.00 |
| Adding Session × Lever | 4 | 9 | 609.24 | 629.47 | −295.62 | 3 vs 4 | 0.73 | 0.39 |
|
| ||||||||
| The effect estrous on responding for sucrose (prior to dose-effect phase) | ||||||||
|
| ||||||||
| Baseline Model | 1 | 4 | 680.39 | 689.38 | −336.19 | |||
| Adding Estrous Factor | 2 | 5 | 679.52 | 690.76 | −334.76 | 1 vs 2 | 2.87 | 0.09 |
|
| ||||||||
| The effect of bupropion on responding for sucrose | ||||||||
|
| ||||||||
| Baseline Model | 1 | 5 | 327.00 | 333.66 | −158.50 | |||
| Adding Dose Factor | 2 | 8 | 310.57 | 321.23 | −147.29 | 1 vs 2 | 22.42 | 0.00 |
| Adding Activity Factor | 3 | 9 | 311.46 | 323.45 | −146.73 | 2 vs 3 | 1.12 | 0.29 |
| Adding Dose × Activity | 4 | 12 | 315.87 | 331.85 | −145.93 | 3 vs 4 | 1.59 | 0.66 |
|
| ||||||||
| The effect estrous on responding for sucrose (after dose-effect phase) | ||||||||
|
| ||||||||
| Baseline Model | 1 | 4.00 | 344.65 | 352.75 | −168.33 | |||
| Adding Estrous Factor | 2 | 5.00 | 346.46 | 356.58 | −168.23 | 1 vs 2 | 0.20 | 0.66 |
|
| ||||||||
| The effect of bupropion on sucrose-triggered reinstatement | ||||||||
|
| ||||||||
| Baseline Model | 1 | 5 | 319.18 | 325.84 | −154.59 | |||
| Adding Dose Factor | 2 | 8 | 318.40 | 329.06 | −151.20 | 1 vs 2 | 6.78 | 0.08 |
| Adding Activity Factor | 3 | 9 | 318.71 | 330.70 | −150.35 | 2 vs 3 | 1.69 | 0.19 |
| Adding Dose × Activity | 4 | 12 | 313.15 | 329.13 | −144.57 | 3 vs 4 | 11.56 | 0.01 |
3.3.2. The effect of bupropion on responding for sucrose
Active lever responding significantly differed depending on the bupropion Dose (χ2(3) = 22.42, p < 0.0001) but not the Activity and there was no Dose × Activity interaction. Active lever responding following pretreatment with 30 or 60 mg/kg doses of bupropion was significantly lower than after pretreatment with saline (0 mg/kg; Figure 1H).
3.3.3. Sucrose-triggered reinstatement
Free sucrose deliveries prior to test session did not significantly increase active lever responding in extinction when compared to the responding on the last extinction session (Figure 1I - compare Extinction to 0 mg/kg dose).
3.3.4. The effect of bupropion on sucrose-triggered reinstatement
Active lever responding did not significantly differed depending on the bupropion Dose or chamber Activity but there was significant Dose × Activity interaction (χ2(7) = 11.56, p < 0.01). Neither bupropion dose significantly affected active lever responding (Figure 1I).
3.3.5. The effect of estrous on responding for sucrose
Active lever responding for sucrose, during 10 FR5 sessions prior to dose-effect tests and during 7 FR5 sessions after dose-effect tests, did not significantly differ depending on the estrous phase.
4. Discussion
The main objective of this study was to assess the effect of NAC and bupropion on methamphetamine self-administration and methamphetamine-triggered reinstatement of freely cycling female rats. In the present study, we found that NAC, a precursor of amino acid cysteine, did not have a significant effect on methamphetamine self-administration or methamphetamine-triggered reinstatement. On the other hand, bupropion, an atypical antidepressant with stimulant properties, attenuated responding reinforced by methamphetamine as well as methamphetamine-triggered reinstatement. To assess bupropion’s specificity for methamphetamine, we tested bupropion’s effects in a parallel study with sucrose as a reinforcer. To this end, bupropion significantly attenuated responding for sucrose but did not have an effect on responding during sucrose-triggered reinstatement phase -likely because free sucrose presentations failed to reinstate active lever responding. Finally, responding maintained by methamphetamine or sucrose did not differ across different phases of the estrous cycle.
NAC is a is a derivative of cysteine and is converted into cysteine within the brain through oxidization process (McClure et al., 2014; Murray, J Lacoste, et al., 2012). Earlier reports show that NAC can reduce cocaine seeking during self-administration phase, without affecting reinforcing value of cocaine (Murray, Everitt, et al., 2012), can decrease lever pressing during extinction of cocaine self-administration (LaRowe and Kalivas, 2013), and can also decrease reinstatement of cocaine seeking following extinction of self-administration behavior (Baker et al., 2003; Madayag et al., 2007; Moran et al., 2005; for review see Nocito Echevarria et al., 2017; Reichel et al., 2011). In the current study, we extended this work by assessing the effectiveness of NAC in decreasing established responding for methamphetamine and by testing its impact on methamphetamine-triggered reinstatement of well extinguished behavior. We found that NAC had no effect on responding for methamphetamine. This finding corroborates previous reports from cocaine self-administration literature. For example, NAC pretreatment does not affect cocaine taking under fixed (FR1 or FR5) or progressive ratio schedules of reinforcement (Ducret et al., 2016; Murray, Everitt, et al., 2012). In addition, we also found that NAC had no effect on methamphetamine-triggered reinstatement of well extinguished responding for methamphetamine. Specifically, in Experiment 1, pretreatment with 0.25 mg/kg of methamphetamine significantly increased responding on the active lever in extinction when compared to the responding in late stage of extinction. Pretreatment with NAC prior to methamphetamine-triggered reinstatement tests did not increased or decreased magnitude of the reinstatement likely indicating key differences in glutamatergic activity associated with these drugs (Cruickshank and Dyer, 2009; Ernst and Chang, 2008; Kalivas and Volkow, 2011). As mentioned earlier, a number of previous reports from cocaine self-administration literature show that pretreatment with NAC decreases reinstatement of cocaine seeking behavior (Baker et al., 2003; Madayag et al., 2007; Moran et al., 2005; for review see Nocito Echevarria et al., 2017; Reichel et al., 2011). Although we were not able to corroborate these reports from cocaine self-administration literature, we also were not able to find any other reports demonstrating any effect of NAC within a context of methamphetamine self-administration. Thus, our findings provide an important initial account of NAC effects on self-administration and reinstatement behaviors associated with methamphetamine drug taking.
Bupropion is a dopamine and norepinephrine reuptake inhibitor approved as an antidepressant and smoking cessation treatment. Previous reports show that bupropion attenuates responding for D-amphetamine and methamphetamine in adult male rats (Rauhut et al., 2003; Reichel et al., 2008, 2009). We extend these findings to include the first report using female rats accompanied with detailed analysis of estrous and activity data. In the present study, higher doses of bupropion (30 and 60 mg/kg) significantly decreased responding for methamphetamine. It is important to note that estrous was an unlikely factor in this effect because estrous did not significantly affect responding maintained by methamphetamine prior to determination of- or following after bupropion dose response curve. Furthermore, although general chamber activity varied over the range of bupropion doses, it is unclear whether decrease in locomotion following a bupropion pretreatment is indicative of bupropion’s ability to block methamphetamine’s hyperlocomotor effects or whether this represents a summation of hyperlocomotor effects between the two stimulants - methamphetamine and bupropion culminating in stereotyped behavior often associated with a decrease in motor activity (Battisti et al., 1999), especially as measured herein as chamber crossings. Because we do not have direct evidence, mainly assessment of stereotyped behavior, to support either position, we can only speculate about the nature of this effect. We speculate that this decrease in locomotion likely represents a summation of hyperlocomotor properties of these drugs. This suggestion is partly supported by the fact that bupropion alone induced hyperlocomotion in our study: compare activity following pretreatment with 30 mg/kg of bupropion alone, visualized in Figure 1F, to methamphetamine-induced activity visualized in Figures 1A,D, and to stimulant free activity visualized in Figure 1G. This effect of bupropion on locomotion is also consistent with a number of previous reports (Mori et al., 2013; Sidhpura et al., 2007; Wilkinson and Bevins, 2007). Overall, our findings in females are consistent with previous studies using male rats, however, we extend previous reports by showing for the first time that female rats are more sensitive to locomotor effects associated with combination of methampthetamine and bupropion (compared to Reichel et al., 2008, 2009).
The effect of bupropion on methamphetamine-triggered reinstatement has not been examined in the past. For the first time, we show that in addition to decreasing methamphetamine self-administration bupropion also attenuates magnitude of methamphetamine-triggered reinstatement following prolonged extinction. We found that the higher doses of bupropion (30 and 60 mg/kg) significantly attenuated methamphetamine-triggered reinstatement; the effect after 30 mg/kg dose was not associated with a significant decrease in general chamber activity. This finding corroborates the locomotor supressing effects of bupropion from the self-administration phase of this study and indicates a pattern of sensitivity to higher doses of bupropion when combined with methamphetamine (see Figures 1E and F). Although activity during reinstatement following pretreatment with 60 mg/kg of bupropion was significantly lower in comparison to activity evoked by methamphetamine (0.25 mg) or bupropion alone (30 mg/kg), it was comparable to activity of stimulant free rats observed in Experiment 1 (compare to activity visualized in Figures 1G–I). Our findings from the reinstatement tests do suggest that bupropion may have therapeutic potential for relapse prevention associated with methamphetamine use.
We found that bupropion dose dependently reduced responding for both methamphetamine and sucrose. This non-specificity in bupropion’s effect suggests that the mechanism of action for bupropion likely involves generalized effect on the reward system and likely affects a wide variety of appetitive stimuli. This assumption is supported by previous reports showing that bupropion increases extracellular dopamine in the nucleus accumbens - a major reward center and a part of a major neural reward circuit (Ascher et al., 1995; Nomikos et al., 1992). Thus, it is possible that a) bupropion’s rewarding effects summate with other reinforcers resulting in decreased motivation and responding to obtain them and/or b) bupropion “overshadows” the detection of other appetitive stimuli which also results in decreased responding for those stimuli. Support for the latter claim can be found in Reichel et al., (2008) study where repeated pretreatment with bupropion interfered with acquisition of discrimination between active and inactive lever of rats self-administering methamphetamine. Furthermore, an alternative or contributing factor that decreases responding for food reinforcers may be the appetite suppressing effects of bupropion (Anderson et al., 2002; Audrain-McGovern and Benowitz, 2011; Gadde and Xiong, 2007; Klonoff and Greenway, 2008; Plodkowski et al., 2009). Although bupropion has not been approved for treatment of obesity there are a number of clinical trials that have shown its potential in weight reduction (for review see Gadde and Xiong, 2007). With these effects in mind, future studies should take in consideration the effects of bupropion on reinforcing, motivational, and appetite suppressing effects when trying to assess its potential therapeutic effect for drug cessation.
This study was designed to test the effectiveness of two candidate treatments in the preclinical model of methamphetamine use and relapse using female rats. For the first time, we show the effect of NAC within a context of methamphetamine self-administration. Specifically, NAC did not decrease responding maintained by methamphetamine or methamphetamine-triggered reinstatement of well extinguished active lever responding. Our study also extends previous reports showing the ability of bupropion to decrease methamphetamine self-administration by observing this effect in female rats with notable difference in sensitivity to the locomotor effects of the highest dose (60 mg/kg). We also for the first time show that burpopion dose-dependently attenuated methamphetamine-triggered reinstatement with similar sensitivity to locomotor effects at the highest dose. Notably, bupropion at 30 mg/kg triggered reinstatement of methamphetamine seeking (p=0.06). Importantly, this effect, at least in this experimental preparation, suggests that bupropion shares stimulus properties with methamphetamine or is methamphetamine like from the stimulus generalization perspective (Wilkinson et al., 2009). Finally, with a companion experiment, testing the effect of bupropoion on responding maintained by sucrose, we confirm non-specificity of bupropion’s effects that likely generalizes to other appetitive rewards. Taken together, our findings suggest that while NAC has considerable promise for treatment of cocaine dependence it may not generalize to other stimulants like methamphetamine. Furthermore, although bupropion has been shown to effectively decrease methamphetamine self-administration, and presently methamphetamine-triggered reinstatement, its locomotor and reward suppressing effects warrant further investigation including both sexes.
Highlights.
Female menstrual cycle did not affect methamphetamine self-administration
NAC did not affect methamphetamine self-administration or reinstatement
Bupropion attenuated methamphetamine self-administration and reinstatement
Locomotion decreased when higher doses of bupropion were combined with methamphetamine
Acknowledgments
6. Funding
This work was supported by the NIH Pre-Doctoral Fellowship [DA034449]. RA Bevins was partially supported by DA034389 while preparing this manuscript for publication. S Charntikov was partially supported by GM113131 (CIBBR, P20) while preparing this manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:871–8. doi: 10.1038/npp.2010.226. https://doi.org/10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR Enhances Weight Loss: A 48-Week Double-Blind, Placebo- Controlled Trial. Obesity Research. 2002;10:633–641. doi: 10.1038/oby.2002.86. https://doi.org/10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- Ascher JA, Cole JO, Colin J-N, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E. Bupropion: a review of its mechanism of antidepressant activity. The Journal of clinical psychiatry. 1995 [PubMed] [Google Scholar]
- Audrain-McGovern J, Benowitz NL. Cigarette Smoking, Nicotine, and Body Weight. Clinical Pharmacology & Therapeutics. 2011;90:164–168. doi: 10.1038/clpt.2011.105. https://doi.org/10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker Da, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003;6:743–9. doi: 10.1038/nn1069. https://doi.org/10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW. The Origin and Neuronal Function of In Vivo Nonsynaptic Glutamate. Journal of Neuroscience. 2002:22. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti JJ, Chang CH, Uretsky NJ, Wallace LJ. Sensitization of stereotyped behavior to amphetamine is context and response dependent. Pharmacology Biochemistry and Behavior. 1999;63:263–269. doi: 10.1016/s0091-3057(98)00259-7. https://doi.org/10.1016/S0091-3057(98)00259-7. [DOI] [PubMed] [Google Scholar]
- Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends in Pharmacological Sciences. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. https://doi.org/10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Brady JV, Griffiths RR, Hienz RD, Ator NA, Lukas SE, Lamb RJ. Assessing Drugs for Abuse Liability and Dependence Potential in Laboratory Primates. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer; New York, New York, NY: 1987. pp. 45–85. https://doi.org/10.1007/978-1-4612-4812-5_3. [Google Scholar]
- Burns L. World Drug Report 2013 By United Nations Office on Drugs and Crime New York: United Nations, 2013ISBN: 978-92-1-056168-6, 151ppabcxyzppGrey literature. Drug and Alcohol Review. 2014;33:216–216. https://doi.org/10.1111/dar.12110. [Google Scholar]
- Charntikov S, Falco A, Fink K, Dwoskin L, Bevins R. The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats. Neuropharmacology. 2017;113:354–366. doi: 10.1016/j.neuropharm.2016.10.014. https://doi.org/10.1016/j.neuropharm.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Thapa I, Bastola DR, Bevins RA, Pendyala G. Ibudilast reverses the decrease in the synaptic signaling protein phosphatidylethanolamine-binding protein 1 (PEBP1) produced by chronic methamphetamine intake in rats. Drug and Alcohol Dependence. 2015;152:15–23. doi: 10.1016/j.drugalcdep.2015.04.012. https://doi.org/10.1016/j.drugalcdep.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, Fleckenstein AE, Hu G, Li M, Bevins RA. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology. 2013;75:138–144. doi: 10.1016/j.neuropharm.2013.07.019. https://doi.org/10.1016/j.neuropharm.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. https://doi.org/10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Ducret E, Puaud M, Lacoste J, Belin-Rauscent A, Fouyssac M, Dugast E, Murray JE, Everitt BJ, Houeto JL, Belin D. N-acetylcysteine Facilitates Self-Imposed Abstinence After Escalation of Cocaine Intake. Biological Psychiatry. 2016;80:226–234. doi: 10.1016/j.biopsych.2015.09.019. https://doi.org/10.1016/j.biopsych.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. https://doi.org/10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of Brain Glutamate Plus Glutamine during Abstinence from Chronic Methamphetamine Use. Journal of Neuroimmune Pharmacology. 2008;3:165–172. doi: 10.1007/s11481-008-9108-4. https://doi.org/10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert Review of Neurotherapeutics. 2006;6:1249–1265. doi: 10.1586/14737175.6.9.1249. https://doi.org/10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyll-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Research. 2004;1016:90–95. doi: 10.1016/j.brainres.2004.04.072. https://doi.org/10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Xiong GL. No Title. 2007;7:17–24. doi: 10.1586/14737175.7.1.17. https://doi.org/10.1586/14737175.7.1.17. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, Iyo M. Protective Effects of N-acetyl-L-cysteine on the Reduction of Dopamine Transporters in the Striatum of Monkeys Treated with Methamphetamine. Neuropsychopharmacology. 2004;29:2018–2023. doi: 10.1038/sj.npp.1300512. https://doi.org/10.1038/sj.npp.1300512. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Volkow N. New medications for drug addiction hiding in glutamatergic neuroplasticity. Molecular Psychiatry. 2011;1646:974–986. doi: 10.1038/mp.2011.46. https://doi.org/10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. British Journal of Clinical Pharmacology. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. https://doi.org/10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonoff DC, Greenway F. Drugs in the Pipeline for the Obesity Market. Journal of Diabetes Science and Technology. 2008;2:913–918. doi: 10.1177/193229680800200525. https://doi.org/10.1177/193229680800200525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The Effect of N-Acetylcysteine in the Nucleus Accumbens on Neurotransmission and Relapse to Cocaine. Biological Psychiatry. 2012;71:978–986. doi: 10.1016/j.biopsych.2011.10.024. https://doi.org/10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-Effects Models for Longitudinal Data. Biometrics. 1982;38:963. https://doi.org/10.2307/2529876. [PubMed] [Google Scholar]
- LaRowe SD, Kalivas PW. The Role of N-Acetylcysteine in Inhibiting Responding During Extinction in Rats Trained to Self-Administer Cocaine. The Open Addiction Journal. 2013;3:88–91. doi: 10.2174/1874941001003010088. https://doi.org/10.2174/1874941001003010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker Da. Repeated N-Acetylcysteine Administration Alters Plasticity-Dependent Effects of Cocaine. Journal of Neuroscience. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. https://doi.org/10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian journal of biology = Revista brasleira de biologia. 2002;62:609–14. doi: 10.1590/s1519-69842002000400008. https://doi.org/10.1590/S1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs. 2014;28:95–106. doi: 10.1007/s40263-014-0142-x. https://doi.org/10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/Glutamate Exchange Regulates Metabotropic Glutamate Receptor Presynaptic Inhibition of Excitatory Transmission and Vulnerability to Cocaine Seeking. Journal of Neuroscience. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. https://doi.org/10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Shibasaki M, Ogawa Y, Hokazono M, Wang TC, Rahmadi M, Suzuki T. Comparison of the behavioral effects of bupropion and psychostimulants. European Journal of Pharmacology. 2013;718:370–375. doi: 10.1016/j.ejphar.2013.07.046. https://doi.org/10.1016/j.ejphar.2013.07.046. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences. 2011;108:385–390. doi: 10.1073/pnas.1011265108. https://doi.org/10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Everitt BJ, Belin D. N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addiction biology. 2012;17:437–40. doi: 10.1111/j.1369-1600.2011.00330.x. https://doi.org/10.1111/j.1369-1600.2011.00330.x. [DOI] [PubMed] [Google Scholar]
- Murray JE, Lacoste J, Belin D, Belin D. Addictions: From Pathophysiology to Treatment. InTech; 2012. N-Acetylcysteine as a Treatment for Addiction; pp. 335–380. [Google Scholar]
- Newton TF, Roache JD, De La Garza R2, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R, TFN, JDR, RDLG, TF, CLW, S-HL, AE, NC Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology. 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. https://doi.org/10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza RII, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion Reduces Methamphetamine-Induced Subjective Effects and Cue-Induced Craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. https://doi.org/10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. 2009 The economic cost of methamphetamine use in the United States. 2005. [Google Scholar]
- Nocito Echevarria MA, Andrade Reis T, Ruffo Capatti G, Siciliano Soares V, da Silveira DX, Fidalgo TM. N-acetylcysteine for treating cocaine addiction - A systematic review. Psychiatry research. 2017;251:197–203. doi: 10.1016/j.psychres.2017.02.024. https://doi.org/10.1016/j.psychres.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology. 1992 [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D R CoreTeam. {nlme}: Linear and Nonlinear Mixed Effects Models. 2017. [Google Scholar]
- Plodkowski RA, Nguyen Q, Sundaram U, Nguyen L, Chau DL, St Jeor S. Bupropion and naltrexone: a review of their use individually and in combination for the treatment of obesity. Expert Opinion on Pharmacotherapy. 2009;10:1069–1081. doi: 10.1517/14656560902775750. https://doi.org/10.1517/14656560902775750. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [Google Scholar]
- Ramirez-Niño AM, D’Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225:473–482. doi: 10.1007/s00213-012-2837-3. https://doi.org/10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. https://doi.org/10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacology Biochemistry and Behavior. 2008;89:463–472. doi: 10.1016/j.pbb.2008.02.002. https://doi.org/10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther. 2011;337:487–493. doi: 10.1124/jpet.111.179317. https://doi.org/10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug and Alcohol Dependence. 2009;100:54–62. doi: 10.1016/j.drugalcdep.2008.09.006. https://doi.org/10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:2143–52. doi: 10.1038/npp.2012.66. https://doi.org/10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Redfern P, Rowley H, Heal D, Wonnacott S. Comparison of the effects of bupropion and nicotine on locomotor activation and dopamine release in vivo. Biochemical Pharmacology. 2007;74:1292–1298. doi: 10.1016/j.bcp.2007.06.025. https://doi.org/10.1016/j.bcp.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Stewart J. Psychological and neural mechanisms of relapse. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3147–3158. doi: 10.1098/rstb.2008.0084. https://doi.org/10.1098/rstb.2008.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. pp. 1–143. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. https://doi.org/NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- Wilkinson JL, Bevins RA. Bupropion hydrochloride produces conditioned hyperactivity in rats. Physiology & Behavior. 2007;90:790–796. doi: 10.1016/j.physbeh.2007.01.003. https://doi.org/10.1016/j.physbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Li C, Bevins RA. Pavlovian drug discrimination with bupropion as a feature positive occasion setter: Substitution by methamphetamine and nicotine, but not cocaine. Addiction Biology. 2009;14:165–173. doi: 10.1111/j.1369-1600.2008.00141.x. https://doi.org/10.1111/j.1369-1600.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA. Intravenous Self-Administration: Response Rates, the Effects of Pharmacological Challenges, and Drug Preference. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer; New York, New York, NY: 1987. pp. 1–33. https://doi.org/10.1007/978-1-4612-4812-5_1. [Google Scholar]