Abstract
Nitrite represents an endocrine reserve of bioavailable nitric oxide (NO) that mediates a number of physiological responses including conferral of cytoprotection after ischemia/reperfusion (I/R). It has long been known that nitrite can react with non-heme iron to form dinitrosyliron complexes (DNIC). However, it remains unclear how quickly nitrite-dependent DNIC form in vivo, whether formation kinetics differ from that of NO-dependent DNIC, and whether DNIC play a role in the cytoprotective effects of nitrite. Here we demonstrate that chronic but not acute nitrite supplementation increases DNIC concentration in the liver and kidney of mice. Although DNIC have been purported to have antioxidant properties, we show that the accumulation of DNIC in vivo is not associated with nitrite-dependent cytoprotection after hepatic I/R. Further, our data in an isolated mitochondrial model of anoxia/reoxygenation show that while NO and nitrite demonstrate similar S-nitrosothiol formation kinetics, DNIC formation is significantly greater with NO and associated with mitochondrial dysfunction as well as inhibition of aconitase activity. These data are the first to directly compare mitochondrial DNIC formation by NO and nitrite. This study suggests that nitrite-dependent DNIC formation is a physiological consequence of dietary nitrite. The data presented herein implicate mitochondrial DNIC formation as a potential mechanism underlying the differential cytoprotective effects of nitrite and NO after I/R, and suggest that DNIC formation is potentially responsible for the cytotoxic effects observed at high NO concentrations.
Abbreviations: DNIC, Dinitrosyliron Complexes; NO, Nitric Oxide; I/R, Ischemia/Reperfusion; SNO, S-nitrosothiol
Keywords: Nitrite, Nitric oxide, Dinitrosyliron complexes, Ischemia, Mitochondria
Graphical abstract
Highlights
-
•
Dietary nitrite results in DNIC formation in many tissues, most notably the liver.
-
•
Nitrite-dependent DNIC accumulate within the mitochondrion.
-
•
NO generates greater DNIC formation in the mitochondrion than nitrite.
-
•
At high concentrations of NO DNIC formation is associated with mitochondrial injury.
1. Introduction
Nitrite (NO2-) is a dietary constituent and intrinsic signaling molecule that mediates a number of physiological and cytoprotective effects. It is now accepted that nitrite represents an endocrine reservoir of nitric oxide (NO) in blood and tissues which can be reduced to bioavailable NO through a reaction that is catalyzed by a host of heme and molybdenum containing proteins in hypoxic conditions [1], [2], [3], [4], [5], [6]. Nitric oxide generated via nitrite reduction activates canonical soluble guanylate cyclase signaling or propagates further anhydrase chemistry resulting in the S-nitrosation of protein targets to mediate biologic effects [7], [8], [9]. While the majority of nitrite's effects are dependent on its reduction to NO, several studies have demonstrated NO independent actions of nitrite [10], [11], [12], [13], [14], [15]. However, the chemistry underlying this signaling remains less clear.
Dinitrosyliron complexes (DNIC) have been reported to be the most abundant NO-derived adduct in the cell [16]. These complexes, comprised of non-heme iron, two nitrosyl groups and thiol-containing ligands, are formed rapidly in the presence of low concentrations of free NO and persist in the cell long after the disappearance of NO production [16], [17]. While nitrite is known to form DNIC, the formation of this species by nitrite versus NO has not been compared [18], [19], [20]. Further, DNIC have been reported to have both cytoprotective and cytotoxic roles physiologically, but it remains unclear whether DNIC formation contributes to biological effects mediated by nitrite [16], [18], [21], [22], [23], [24].
One of the most reproducible and potent effects of nitrite is its ability to confer cytoprotection after ischemia/reperfusion (I/R) in cellular models and a number of organs including the heart, brain, kidney, lung and liver [25], [26], [27], [28], [29], [30]. Mechanistically, this protection is dependent on the reduction of nitrite and the subsequent S-nitrosation of a critical cysteine residue in complex I of the mitochondrion. This modification leads to inhibition of complex I enzymatic activity, resulting in attenuation of reperfusion mitochondrial oxidant production, preventing downstream apoptosis [31], [32]. Notably, recent studies have implicated the formation of mitochondrial DNIC in nitrite-mediated protection after I/R [18]. Separate studies have shown that DNIC formation is potentiated during prolonged cellular hypoxia and that DNIC can be converted to S-nitrosothiols (SNO) [18], [21], [33], [34]. Thus, we sought to determine whether nitrite-dependent DNIC formation in mitochondria contributes to nitrite-mediated protection after I/R.
Our data confirm that DNIC are formed by chronic dietary nitrite supplementation but show that this formation is not associated with the protective effect of nitrite. We compare NO and nitrite-dependent DNIC formation in isolated mitochondria and demonstrate that DNIC formation occurs concomitant with NO-induced mitochondrial damage. These results demonstrate that nitrite-dependent DNIC formation is physiologically relevant and highlight a mechanistic difference between NO and nitrite mediated signaling. This is discussed in the context of the pathological role of DNIC and its implication for nitrite versus NO-dependent mitochondrial regulation and hypoxic signaling.
2. Methods
2.1. Chemicals
All chemicals were purchased from Sigma-Aldrich unless otherwise noted.
2.2. Animals
All animal studies were performed in accordance with and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. For nitrite supplementation studies, C57/Bl6 mice (8–10 week old males) were maintained on a standard diet and autoclaved water. Water was supplemented with 0, 0.3, 1.5, or 3.0 g/L of sodium nitrite and mice were allowed to drink ad libitum. Mitochondria were isolated from male Sprague Dawley rats (250–500 g).
2.3. Hepatic I/R
Liver I/R was performed as previously described [32]. Briefly, after nitrite supplementation, mice were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (8 mg/kg). A midline laparotomy incision was performed, and mice were heparinized (100 μg/kg). The hepatic artery and portal vein were completely clamped to cause ischemia of the left lateral and median lobes of the liver. The liver was kept moist with normal saline for 45 min, after which the clamp was removed to reperfuse the liver. Mice were sutured, and plasma collected 5 h later for measurement of alanine transaminase levels.
2.4. DNIC measurement
Tissue and mitochondrial samples were collected and immediately flash frozen in EPR tubes. All samples were shipped between labs on dry ice and stored in liquid nitrogen. Measurements were performed on a Bruker X-band EMX Plus EPR spectrometer. All samples were read in liquid nitrogen and were not thawed at any point before measurement. Dinitrosyliron complexes (DNIC) were detected at g = 2.03. Settings were as follows: centerfield 3335.25 G, microwave frequency 9.460544 GHz, modulation amplitude 10 G, 200 G scan range, 90-s scan time, one scan. For quantification, amplitude of signal from 2.04 to 2.02 was compared with amplitude of a standard curve generated with synthetic diglutathione DNIC as described previously [16], [23].
2.5. S-nitrosothiol and iron-nitrosyl measurement
S-nitrosothiol concentration was measured by tri-iodide based reductive chemiluminescence using a Nitric Oxide Analyzer (Sievers) as previously described [32], [35]. Briefly, each sample was divided and treated with either acidified sulfanilamide (16% in 2 M HCl) or with mercuric chloride. Each treated sample was injected into tri-iodide and the area under the curve measured, and concentration quantified using a standard curve of known concentrations. SNO concentration was the calculated as the difference between the acid sulfanilamide and mercuric chloride treated samples. Iron-nitrosyl concentration was measured by injecting untreated samples into potassium ferricyanide (0.1 M). NO released from the Fe-NO was measured by chemiluminescence [35].
2.6. Mitochondrial Isolation
Rat liver mitochondria were isolated by differential centrifugation as previously described [32], [35].
2.7. In vitro anoxia/reoxygenation
The in vitro anoxia/reoxygenation protocol was performed as previously described [32]. Briefly, mitochondria were suspended in respiration buffer(120 mM KCl, 25 mM sucrose, 10 mM Hepes, 1 mM EGTA, 1 mM KH2PO4, 5 mM MgCl2) in a stirred, sealed chamber fit with a Clark-type oxygen electrode (Instech Corp.) connected to a data recording device (DATAQ Systems). State 3 respiration was initiated and mitochondria were allowed to consume oxygen until the chamber became anoxic. Nitrite or NO was added to the chamber once it reached anoxia. The mitochondria were left in anoxia for 30 min, after which the mitochondria were centrifuged and resuspended in oxygenated buffer containing fresh substrate and ADP, and allowed to respire once again. Post-anoxic respiratory rate was expressed as a percentage of pre-anoxic rate and called recovery of respiration.
2.8. Aconitase activity
Mitochondria were lysed by three cycles of freeze/thaw, and aconitase activity was measured by spectrophotometrically monitoring the formation of NADPH at 340 nm using the Bioxytech Aconitase-340 kit (Oxis Research).
2.8.1. Statistics
Values are expressed as means ± SEM of at least 3 independent experiments. Single comparisons were tested for significance using a two-tailed Student's t-test. Multiple comparisons were made using ANOVA followed by the Bonferroni post hoc test. P<0.05 was considered significant.
3. Results
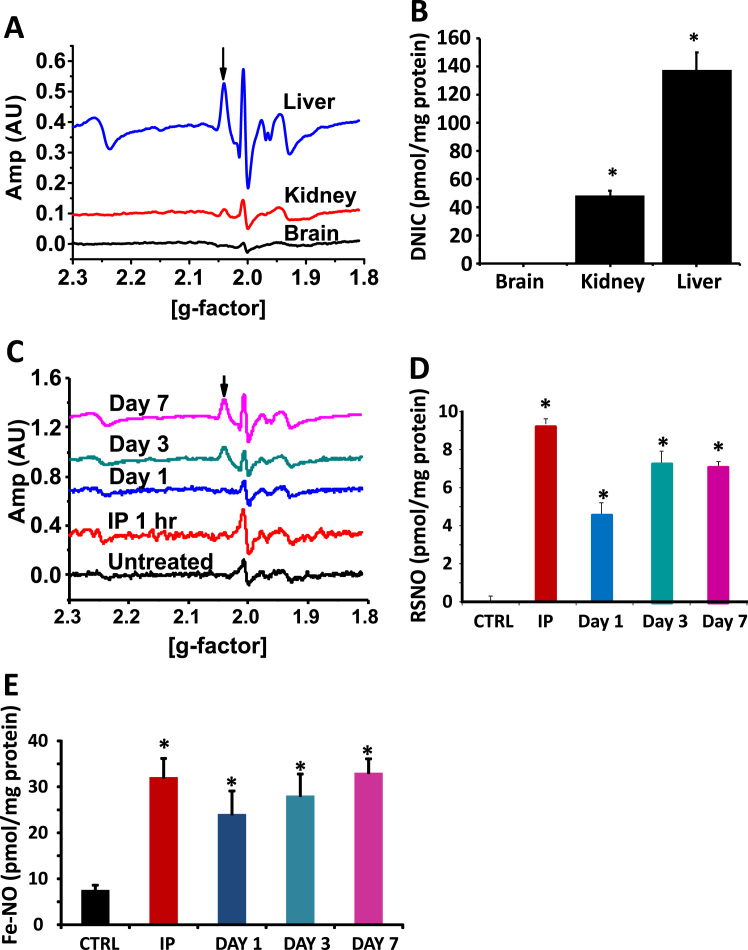
In the first series of experiments, we tested whether oral nitrite administration resulted in DNIC formation in vivo. Mice were fed nitrite supplemented water (3 g/L) ad libitum for seven days and DNIC levels were measured in the heart, liver, kidney and brain. A significant DNIC signal was detected by EPR in kidney tissue, while no signal was measured in the brain (Fig. 1A-B). The greatest concentration of DNIC was measured in the liver and thus, we decided to focus our studies on this organ. DNIC signal was undetectable in any organ from mice not supplemented with nitrite (data shown for liver in Fig. 1C).
Fig. 1.
Oral nitrite supplementation results in DNIC formation. (A) Representative EPR traces demonstrating DNIC formation (denoted with an arrow) in the liver, kidney and brain of mice after oral nitrite supplementation on day 3. (B) Quantification of DNIC from several traces such as those shown in (A). (C) Representative EPR traces demonstrating DNIC formation in the liver of mice supplemented with nitrite (3 g/L) for 0,1,3, or 7 days or after one hour of an intraperitoneal nitrite (260 µg/kg) injection. (D) S-nitrosothiol and (E)heme-nitrosyl concentrations in the liver of mice supplemented as described in panel C. All values are mean ± SEM. n = 5; *p < 0.01.
Measurement of the time course of liver DNIC formation confirmed prior studies demonstrating that DNIC were detectable only after three or more days of nitrite supplementation [19], [36] (Fig. 1C). Since NO-dependent DNIC formation has been shown to be rapid in cell culture systems [16], we administered one intraperitoneal injection of nitrite (260 µg/kg) and measured liver DNIC one hour later. This dose of nitrite acutely increased plasma nitrite levels to ~12 µM, however DNIC were not detectable at this time point (Fig. 1C). Notably, concentrations of S-nitrosated (Fig. 1D) and iron-nitrosylated (Fig. 1E) proteins were increased after the IP injection, demonstrating that nitrite was metabolized in the liver. S-nitrosothiols and iron-nitrosyls were also present in the absence of DNIC after one day of oral nitrite supplementation and their concentration plateaued after 3 days of supplementation, concomitant with the appearance of DNIC (Fig. 1C-E).
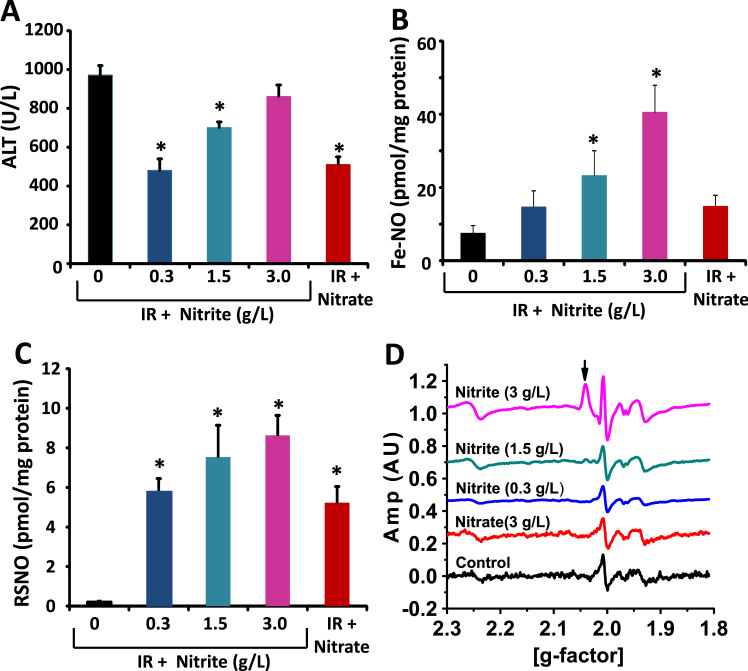
We and others have previously demonstrated that nitrite-mediated cytoprotection after ischemia/reperfusion (I/R) is dependent on S-nitrosation of mitochondrial complex I [31], [32]. Prior studies have also suggested that mitochondrial DNIC formation is protective in ex vivo models of hypoxia-reoxygenation [18], and that DNIC can be converted to SNO [33], [34]. Thus, we next sought to determine whether nitrite-dependent DNIC formation was associated with S-nitrosation and nitrite-dependent cytoprotection after I/R. Mice were placed on nitrite supplemented water (0–3 g/L) for three days and then subjected to hepatic artery/portal vein ligation as a model of hepatic I/R. As expected, supplementation with nitrite increased plasma nitrite levels from 103 ± 16 nM (untreated group) to 360 ± 43 nM, 567 ± 40 nM, and 780 ± 48 nM in the mice supplemented with 0.3, 1.5, and 3 g/L nitrite respectively. Consistent with prior studies [37], measurement of plasma alanine aminotransferase (ALT) levels six hours after I/R showed that 0.3 and 1.5 g/L of nitrite mediated significant liver protection after I/R, while no protection was observed with nitrite at the highest dose (3 g/L) (Fig. 2A). Measurement of NO adducts in the liver showed a dose dependent increase in iron-nitrosyl concentration (Fig. 2B) with increasing nitrite as well as increased liver SNO (Fig. 2C). However, DNIC was detectable only at the highest dose of nitrite (Fig. 2D). These data demonstrate that tissue SNO and Fe-NO accumulates at lower concentrations of nitrite than DNIC, and that DNIC accumulation is not significant at cytoprotective doses of nitrite in vivo.
Fig. 2.
DNIC formation is not associated with nitrite-dependent attenuation of hepatic I/R injury. Mice were orally supplemented with nitrite (0–3 g/L in the water) or nitrite (3 g/L) and then subjected to hepatic I/R. (A) Plasma alanine aminotransferase levels 6 h after I/R. (B-C) Concentration of (B) Fe-NO and (C) RSNO in the liver tissue 6 h after I/R. (D) Representative EPR traces showing DNIC formation (denoted with arrow) in the liver 6 h after I/R. All values are mean ± SEM. n = 5; *p < 0.01.
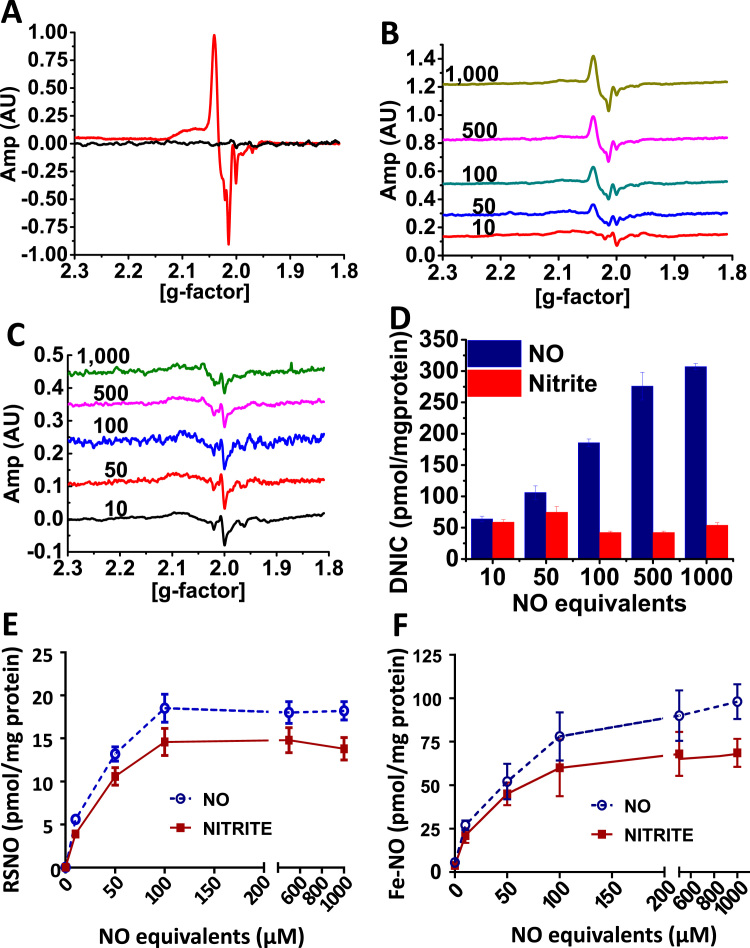
Given the essential role of the mitochondrion in the mechanism of nitrite-mediated protection after I/R and the prior evidence of in vitro mitochondrial DNIC formation during anoxia [18], [22], we examined whether dietary nitrite supplementation resulted in mitochondrial DNIC formation. Fractionation of liver tissue from nitrite supplemented (3 g/L) mice showed that significant DNIC was formed in the mitochondrial compartment (Fig. 3A). To more directly probe the role of mitochondrial DNIC formation, we utilized isolated mitochondria to compare DNIC formation by nitrite and NO and its effect on mitochondrial protection in hypoxia. Isolated liver mitochondria were treated in anoxic conditions with equal equivalents of either nitrite (0–1 mM) or NO (donor DEANONOate; DEANO; 0–500 µM). Notably, NO and nitrite showed distinctly different profiles of DNIC formation. DNIC levels were similar between DEANO and nitrite at low concentrations of NO equivalents (10 and 50 µM). At increasing concentrations, however, the levels of mitochondrial DNIC remained constant for nitrite but continually increased in response to NO (Fig. 3B-D). Both NO and nitrite-dependent mitochondrial SNO (Fig. 3E) and Fe-NO (Fig. 3F) levels showed a concentration dependent increase demonstrating that DNIC formation was not limited by the lack of nitrite to NO conversion in the mitochondrion.
Fig. 3.
NO forms DNIC more efficiently than nitrite in the mitochondrion. (A) A representative EPR trace showing the DNIC signal in liver mitochondria from mice supplemented (red) or not (black) with nitrite (3 g/L in the water). (B-C) Representative EPR traces demonstrating DNIC levels in isolated rat liver mitochondria treated with (B) DEANONOate (0–500 µM) or (C) nitrite (0–1 mM) in anoxic conditions. (D) Quantitation of DNIC from traces similar to those shown in (B-C). (E-F) Concentrations of (E) S-nitrosothiol and (F) Fe-NO in the same livers as (B-C). All values are mean ± SEM. n = 5; *p < 0.01.
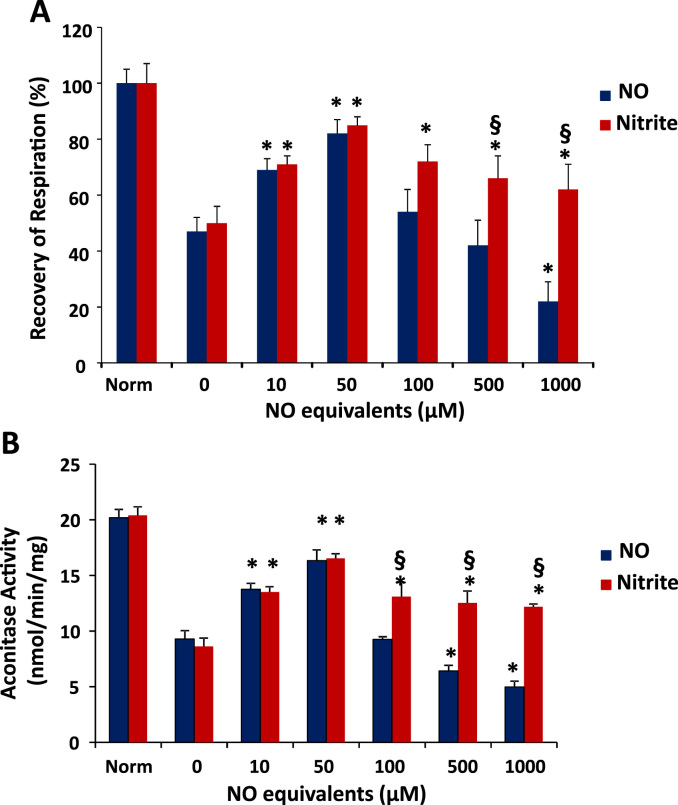
To test whether mitochondrial DNIC formation was associated with mitochondrial protection, we subjected the isolated mitochondria to an in vitro assay of anoxia/reoxygenation which has previously been shown to mimic mitochondrial damage observed during tissue ischemia/reperfusion [32]. In this assay, mitochondrial respiration is decreased after anoxia due to oxidative damage, and greater recovery of respiration after an anoxic period is indicative of mitochondrial protection. Fig. 4A demonstrates that treatment with either nitrite or NO during the anoxic period mediated a biphasic protection curve. Both molecules were significantly protective at low concentrations (10–50 µM NO equivalents). However, while NO lost its protective effect at 100 µM NO equivalents and induced damage at higher concentrations, nitrite maintained protection at 100 µM and did not cause damage at any concentration (Fig. 4A). Notably, the lack of protection at 100 µM NO equivalents coincides with the plateau in SNO formation by both molecules (Fig. 3E), and the damage induced by DEANO is concomitant with its significant increase in DNIC formation compared to nitrite (Fig. 3D).
Fig. 4.
High concentrations of nitrite and NO have differing effects on mitochondrial protection. (A) Recovery of respiration and (B) aconitase activity of isolated mitochondria subjected to anoxia/reoxygenation in the presence of either DEANONOate (0–500 µM) or nitrite (0–1 mM) compared to mitochondria that were not subjected to anoxia/reoxygenation (Norm). All values are mean ± SEM. n = 7; *p < 0.01 versus corresponding 0 µM treatment. §p < 0.01 versus same NO equivalents.
Mitochondrial aconitase is an iron-sulfur protein that is a major target for oxidation, resulting in the loss of its activity. Additionally, its iron centers have previously been identified as a potential target for DNIC formation [20], [22]. Measurement of aconitase activity in the isolated mitochondria subjected to anoxia/reoxygenation showed that aconitase activity was significantly decreased after anoxia/reoxygenation (Fig. 4B), consistent with prior reports [32]. Low concentrations of both nitrite and NO attenuated the loss in aconitase activity, potentially due to decreased oxidative stress. While higher concentrations of nitrite also prevented anoxia-induced loss of aconitase activity, concentrations of DEANO above 250 µM decreased aconitase activity (Fig. 4B).
4. Discussion
The main findings of this study are that 1) dietary nitrite supplementation results in hepatic mitochondrial DNIC formation in vivo, 2) NO and nitrite mediate differential profiles of mitochondrial DNIC formation in vitro and 3) high concentrations of NO-dependent DNIC formation are associated with the potentiation of anoxic mitochondrial damage.
Our data demonstrate that DNIC form in vivo predominantly in the liver and only with higher concentrations (3 g/L) or longer duration (3 days) of nitrite supplementation. These data are very consistent with prior studies by Vanin and colleagues who measured ~10nmol DNIC/g of liver tissue in rats after administering 0.3% nitrite for three days in the drinking water [19]. While prior studies have shown that the formation of DNIC in isolated tissues and cells is rapid [16], [18], [38], our in vivo model demonstrated accumulation of SNO and Fe-NO much earlier than DNIC. In vitro studies demonstrate that DNIC contribute to SNO formation through transnitrosation reactions [21], [33], [34]. It is possible that nitrite forms tissue DNIC which are rapidly converted to SNO, such that DNIC do not accumulate at detectable levels (by EPR) until targets for S-nitrosation are saturated. Alternatively, the converse is possible in which SNO form first and subsequently participate in DNIC assembly by directly contributing the NO and thiol ligands or liberating free NO that reacts with iron [39]. Notably, Asanuma and colleagues have shown more rapid DNIC formation that appears in 2–8 h after nitrite/nitrate supplementation. However, this signal is detected in the acidic environment of the gastric tissue, which potentially lends itself to greater nitrite/nitrate reduction than other tissues [20]. While we did not measure DNIC levels in the gut in this study, the results presented here further highlight the necessity to consider the role of DNIC formation in nitrite signaling. This is particularly important given that 30–40% of endogenous tissue nitrite is derived from dietary intake of nitrite and nitrate, and that oral nitrite/nitrate supplementation is being explored as a strategy for a number of pathologies including hypertension and atherosclerosis [40], [41], [42]. Relevant to the use of nitrite/nitrate as a potential therapeutic, the plasma nitrite concentrations achieved in the present study (~0.3–0.6 µM), particularly at the lower supplementation groups (0.3 and 1.5 g/L nitrite) are within the range that has been shown to protect against I/R injury in other animal models [25], [27], [28], [29], [32], [43] as well as lower blood pressure [42], [44], attenuate platelet aggregation in response to stimuli [42], improve vascular function [41], and increase exercise efficiency [45], [46] in humans.
While prior studies have demonstrated nitrite-dependent DNIC formation in whole tissues in vitro and in vivo [18], [19], the subcellular localization of DNIC within the tissue has not been explored. Here we show for the first time that significant levels of DNIC accumulate within the mitochondrion in vivo after dietary nitrite supplementation (Fig. 3A). Interestingly, prior studies have suggested that oral nitrite supplementation leads to DNIC formation predominantly in the extracellular compartment [19]. While mechanisms of cellular DNIC transport have been reported [24], it remains unknown whether the mitochondrion can import pre-formed DNIC in intact cells or tissue. However, our data demonstrating nitrite-dependent DNIC formation in isolated mitochondria are consistent with de novo DNIC formation within the organelle rather than import. While the exact proportion of tissue DNIC that accumulates within the mitochondrion is unclear, it is well established that the organelle contains a pool of free iron that potentially contributes to DNIC formation [18], [22]. Further, nitrite has been shown to enter the mitochondrion, where it can be reduced to NO by a number of proteins including cytochrome c or cytochrome c oxidase [47], [48]. Indeed our data demonstrating the formation of SNO and Fe-NO show that DNIC formation is concomitant with reduction of nitrite. Regardless of the mechanism of formation, the data presented herein establish the mitochondria as a physiological target of DNIC action.
It is intriguing that though nitrite and NO mediate similar levels of other NO-dependent protein modifications (SNO and Fe-NO), NO generates significantly more DNIC than nitrite. Levels of SNO and Fe-NO begin to plateau around 100 µM NO equivalents. In contrast, DNIC concentration increases dose dependently with increasing NO donor treatment while no change in DNIC is observed with changing nitrite concentrations. The lower levels of DNIC formation by nitrite versus NO is likely due to the exhaustion of nitrite reductase proteins, leading to the inability to convert increasing concentrations of nitrite to NO in order to form DNIC. While further kinetic study is required to determine why no concentration dependent increase in nitrite-dependent DNIC formation is observed, it is interesting to speculate that perhaps while the mitochondrial chelatable iron pool is a greater or equivalent target to heme and thiol containing proteins for NO, nitrite selectively modifies proteins that reduce nitrite or are in close proximity to nitrite reducing proteins before reacting with free iron.
DNIC have been associated with both cytoprotective and pathogenic effects in cell and animal systems. Cytoprotective effects of the species have predominantly been reported to be due its antioxidant capabilities. For example, Li and colleagues demonstrated that NO-dependent DNIC formation attenuated free-iron catalyzed Fenton chemistry in an in vitro model of cellular anoxia [21]. Dungel and colleagues demonstrated a similar protective phenomenon by nitrite-dependent DNIC formation in an anoxic isolated mitochondrial system supplemented with free iron [18]. In contrast, other reports have associated DNIC formation with cytotoxicity and pathogenesis. Mechanistically, cytotoxicity has been attributed to the disruption of iron containing enzymes, such as the iron-sulfur protein aconitase [16], [20], [22], [36]. Our data could be consistent with both a protective and toxic effect of DNIC. Importantly, Hickok and colleagues demonstrated in a murine macrophage model that DNIC formation becomes cytotoxic only once it exceeds the concentration of the chelatable iron pool [16]. This paradigm is likely applicable to the data presented here. In the case of nitrite and lower concentrations of NO (<100 µM DEANO), DNIC formation may contribute to mitochondrial protection after anoxia/reoxygenation through an antioxidant mechanism. However, in the case of the NO donor, once greater levels of DNIC formation are achieved (>100 µM NO treatment), aconitase activity begins to decrease (consistent with disruption of iron-sulfur centers) and injury is potentiated. Notably, while nitrite-dependent mitochondrial protection peaks at 50 µM NO equivalents, higher concentrations of nitrite neither disrupt aconitase activity nor injure the mitochondrion. Similarly, in the in vivo hepatic I/R model, nitrite-derived Fe-NO and RSNO are associated with protection only in the absence of DNIC (<3 g/L; Fig. 2). While these data suggest that DNIC potentiate mitochondrial injury only once the free iron pool is exhausted, measurement of the mitochondrial chelatable iron pool will be required to test this concept fully.
In summary, our in vivo data confirm that dietary nitrite supplementation generates DNIC in the liver and demonstrates a measurable proportion of these DNIC are localized within the mitochondrion. In vitro studies show that at the mitochondrial level NO generates greater concentrations of DNIC than nitrite, and high DNIC concentration is associated with the loss of aconitase activity and potentiation of anoxia-induced mitochondrial injury. These data demonstrate another significant difference between nitrite and NO signaling and highlight the importance of considering DNIC formation in the context of nitrite-dependent signaling and therapeutics.
Conflict of interest
None.
Funding
This work was supported by the Hemophilia Center of Western Pennsylvania (SS), R01-GM113816-01 (SS) and American Heart Association grant 16GRNT27740024 (SS), GM0852321S1ORR027848-01A1 (DDT).
Contributor Information
Douglas D. Thomas, Email: ddthomas@uic.edu.
Sruti Shiva, Email: sss43@pitt.edu.
References
- 1.Khambata R.S., Ghosh S.M., Ahluwalia A. "Repurposing" of xanthine oxidoreductase as a nitrite reductase: a new paradigm for therapeutic targeting in hypertension. Antioxid. Redox Signal. 2015;23:340–353. doi: 10.1089/ars.2015.6254. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg J.O., Gladwin M.T., Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 3.Omar S.A., Webb A.J., Lundberg J.O., Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J. Int. Med. 2016;279:315–336. doi: 10.1111/joim.12441. [DOI] [PubMed] [Google Scholar]
- 4.Shiva S. Nitrite: a physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 2013;1:40–44. doi: 10.1016/j.redox.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zweier J.L., Li H., Samouilov A., Liu X. Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric Oxide: Biol. Chem. 2010;22:83–90. doi: 10.1016/j.niox.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tejero J., Gladwin M.T. The globin superfamily: functions in nitric oxide formation and decay. Biol. Chem. 2014;395:631–639. doi: 10.1515/hsz-2013-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu S. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 8.Gladwin M.T., Grubina R., Doyle M.P. The new chemical biology of nitrite reactions with hemoglobin: r-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc. Chem. Res. 2009;42:157–167. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 9.Roche C.J., Cassera M.B., Dantsker D., Hirsch R.E., Friedman J.M. Generating S-nitrosothiols from hemoglobin: mechanisms, conformational dependence, and physiological relevance. J. Biol. Chem. 2013;288:22408–22425. doi: 10.1074/jbc.M113.482679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryan N.S. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat. Chem. Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 11.Kamga Pride C. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc. Res. 2014;101:57–68. doi: 10.1093/cvr/cvt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mo L. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radic. Biol. Med. 2012;53:1440–1450. doi: 10.1016/j.freeradbiomed.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Totzeck M. Nitrite circumvents canonical cGMP signaling to enhance proliferation of myocyte precursor cells. Mol. Cell. Biochem. 2015;401:175–183. doi: 10.1007/s11010-014-2305-y. [DOI] [PubMed] [Google Scholar]
- 14.Wang L., Frizzell S.A., Zhao X., Gladwin M.T. Normoxic cyclic GMP-independent oxidative signaling by nitrite enhances airway epithelial cell proliferation and wound healing. Nitric Oxide: Biol. Chem. 2012;26:203–210. doi: 10.1016/j.niox.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M. nitrite-mediated renal vasodilatation is increased during ischemic conditions via cGMP-independent signaling. Free Radic. Biol. Med. 2015;84:154–160. doi: 10.1016/j.freeradbiomed.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Hickok J.R. Dinitrosyliron complexes are the most abundant nitric oxide-derived cellular adduct: biological parameters of assembly and disappearance. Free Radic. Biol. Med. 2011;51:1558–1566. doi: 10.1016/j.freeradbiomed.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira J.C., Iretskii A.V., Han R.M., Ford P.C. Dinitrosyl iron complexes with cysteine. Kinetics studies of the formation and reactions of DNICs in aqueous solution. J. Am. Chem. Soc. 2015;137:328–336. doi: 10.1021/ja510393q. [DOI] [PubMed] [Google Scholar]
- 18.Dungel P., Perlinger M., Weidinger A., Redl H., Kozlov A.V. The cytoprotective effect of nitrite is based on the formation of dinitrosyl iron complexes. Free Radic. Biol. Med. 2015;89:300–310. doi: 10.1016/j.freeradbiomed.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Vanin A.F., Kiladze S.V., Kubrina L.N. [Factors influencing formation of dinitrosyl complexes of non-heme iron in the organs of animals in vivo] Biofizika. 1977;22:850–855. [PubMed] [Google Scholar]
- 20.Asanuma K. Fe-S cluster proteins are intracellular targets for nitric oxide generated luminally at the gastro-oesophageal junction. Nitric Oxide: Biol. Chem. 2007;16:395–402. doi: 10.1016/j.niox.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Li Q. Nitrosothiol formation and protection against Fenton chemistry by nitric oxide-induced dinitrosyliron complex formation from anoxia-initiated cellular chelatable iron increase. J. Biol. Chem. 2014;289:19917–19927. doi: 10.1074/jbc.M114.569764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shumaev K.B. [Formation of dinitrosyl iron complexes in cardiac mitochondria] Biofizika. 2010;55:460–466. [PubMed] [Google Scholar]
- 23.Hickok J.R., Sahni S., Mikhed Y., Bonini M.G., Thomas D.D. Nitric oxide suppresses tumor cell migration through N-Myc downstream-regulated gene-1 (NDRG1) expression: role of chelatable iron. J. Biol. Chem. 2011;286:41413–41424. doi: 10.1074/jbc.M111.287052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lok H.C. Glutathione S-transferase and MRP1 form an integrated system involved in the storage and transport of dinitrosyl-dithiolato iron complexes in cells. Free Radic. Biol. Med. 2014;75:14–29. doi: 10.1016/j.freeradbiomed.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Bryan N.S. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dezfulian C., Raat N., Shiva S., Gladwin M.T. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc. Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duranski M.R. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendgen-Cotta U.B. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. (0801336105)(pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung K.H. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. (doi:01.STR.0000245116.40163.1c)(pii)(, 10.1161/01.STR.0000245116.40163.1c) [DOI] [PubMed] [Google Scholar]
- 30.de Lima Portella R., Lynn Bickta J., Shiva S. Nitrite confers preconditioning and cytoprotection after ischemia/reperfusion injury through the modulation of mitochondrial function. Antioxid. Redox Signal. 2015;23:307–327. doi: 10.1089/ars.2015.6260. [DOI] [PubMed] [Google Scholar]
- 31.Chouchani E.T. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiva S. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosworth C.A., Toledo J.C., Jr, Zmijewski J.W., Li Q., Lancaster J.R., Jr. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. USA. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boese M., Mordvintcev P.I., Vanin A.F., Busse R., Mulsch A. S-nitrosation of serum albumin by dinitrosyl-iron complex. J. Biol. Chem. 1995;270:29244–29249. doi: 10.1074/jbc.270.49.29244. [DOI] [PubMed] [Google Scholar]
- 35.Curtis E., Hsu L.L., Noguchi A.C., Geary L., Shiva S. Oxygen regulates tissue nitrite metabolism. Antioxid. Redox Signal. 2012;17:951–961. doi: 10.1089/ars.2011.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanin A.F., Kubrina L.N., Lisovskaia I.L., Malenkova I.V., Chetverikov A.G. [Endogenous nitrosyl complexes of heme and non-heme iron in microorganisms and animal tissues] Biofizika. 1971;16:650–656. [PubMed] [Google Scholar]
- 37.Raat N.J. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic. Biol. Med. 2009;47:510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanin A.F., Kubrina L.N., Kiladze S.V., Burbaev D. [Factors influencing formation of dinitrosyl complexes of non-heme iron in vitro preparations of mouse liver and yeasts] Biofizika. 1977;22:646–650. [PubMed] [Google Scholar]
- 39.Hickok J.R., Vasudevan D., Thatcher G.R., Thomas D.D. Is S-nitrosocysteine a true surrogate for nitric oxide? Antioxid. Redox Signal. 2012;17:962–968. doi: 10.1089/ars.2012.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapil V. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velmurugan S. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb A.J. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dezfulian C. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapil V., Khambata R.S., Robertson A., Caulfield M.J., Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen F.J. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 47.Castello P.R., David P.S., McClure T., Crook Z., Poyton R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Basu S. Nitrite reductase activity of cytochrome c. J. Biol. Chem. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]