Abstract
Therapeutic strategies that increase hydrogen sulfide (H2S) or nitric oxide (NO) are cytoprotective in various models of cardiovascular injury. However, the nature of interaction between H2S and NO in heart failure and the underlying mechanisms for the protective effects remain undefined. The present study tested the cardioprotective effect of ZYZ-803, a novel synthetic H2S-NO hybrid molecule that decomposed to release H2S and NO. ZYZ-803 dose dependently improved left ventricular remodeling and preserved left ventricular function in the setting of isoprenaline-induced heart failure. The cardioprotective effect of ZYZ-803 is significantly more potent than that of H2S and/or NO donor alone. ZYZ-803 stimulated the expression of cystathionine γ-lyase (CSE) for H2S generation and the activity of endothelial NO synthase (eNOS) for NO production. Blocking CSE and/or eNOS suppressed ZYZ-803-induced H2S and NO production and cardioprotection. ZYZ-803 increased vascular endothelial growth factor (VEGF) concentration and cyclic guanosine 5′-monophosphate (cGMP) level. Moreover, ZYZ-803 upregulated the endogenous antioxidants, glutathione peroxidase (GPx) and heme oxygenase 1 (HO-1). These findings indicate that H2S and NO cooperatively attenuates left ventricular remodeling and dysfunction during the development of heart failure through VEGF/cGMP pathway and ZYZ-803 provide expanding insight into strategies for treatment of heart failure.
Abbreviations: CBS, Cystathionine β-synthase; cGMP, Guanosine 5′-monophosphate; CSE, Cystathionine γ-lyase; EF, Ejection fraction; eNOS, Endothelial NO synthase; FS, Fractional shortening; GPx, Glutathione peroxidase; HO-1, Heme oxygenase 1; H2S, Hydrogen sulfide; L-NAME, L-NG-nitro arginine methyl ester; LVID, Left ventricular internal dimension diastole; LV Vol, Left ventricular volume; NO, Nitric oxide; PAG, DL-propargylglycine; PKG, Protein kinase G; ROS, Reactive oxygen species; SPRC, S-propargyl-cysteine; VASP, Vasodilator-stimulated phosphoprotein; VEGF, Vascular endothelial growth factor
Keywords: Hydrogen sulfide, Nitric oxide, Heart failure
Graphical abstract
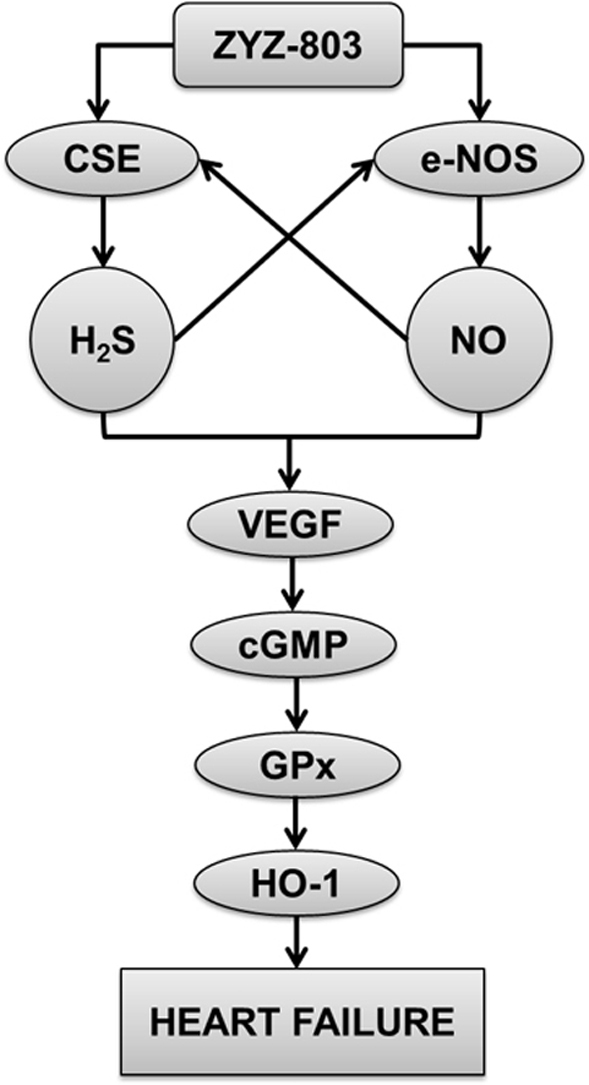
1. Introduction
Heart failure is a complicated disease caused by a variety of common stresses to the heart, such as hypertension, diabetes, myocardial infarction and so on. Heart failure remains to be the major health issue in the world [1], [2], [3]. Despite the development of conventional strategies and heart transplantation, the incidence of heart failure is rapidly rising [2]. Therefore, adjunct pharmacotherapies coincided with the standard means of care will be an effective way to protect against heart failure.
Hydrogen sulfide (H2S) and nitric oxide (NO) have been considered as toxic gases and environmental pollutants. More recently, they emerge as the essential physiological gaseous signaling molecules that can stimulate angiogenesis and vasorelaxation and protect ischemic heart diseases [4], [5], [6], [7] H2S is generated in mammalian tissues by l-cysteine metabolic enzymes, such as cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), or from other nonenzymatic pathways (e.g. 3-mercaptopyruvate sulfurtransferase). NO is synthesized from l-arginine by endothelial NO synthase under physiological status in the cardiovascular system [8], [9], [10].
H2S and NO have important roles in protecting against heart failure. First, The clinical evidence shows that the sulfur concentration in plasma is inversely proportional to the severity of congestive heart failure [11]. Exogenous administration of H2S or cardiac-specific CSE overexpression protects against acute myocardial ischemia-reperfusion injury and heart failure [12], [13], [14]. The clinical data also suggests that the expression of eNOS mRNA was changed in human heart failure [15], [16], [17]. Moreover, NO regulates cardiac excitation-contraction coupling in heart failure [18].
Growing evidence indicates that the potential couping between NO and H2S at different levels, such as biosynthesis and physiological response [19], [20]. However, the precise nature of these crosstalks and how they affect heart failure is not clear. Kondo et al. found that H2S protected against heart failure via up-regulation of eNOS activity [6]. Yong et al. showed that H2S reacted with NO to generate a new thiol sensitive molecule that could regulate heart function [21]. Therefore, a deeper understanding of the interactions between NO and H2S in treating heart failure is urgently needed, because it will enable the development of novel strategies to protect against heart failure.
Recently, our laboratory had developed a novel H2S-NO releasing molecule, named ZYZ-803, which significantly stimulated endothelial cell angiogenesis [22]. Angiogenesis is a key biological process characterized by the sprouting of new blood vessels from existing vessels, which reveals an effective therapy for the treatment of heart failure [23]. However, there is no evidence showing the interaction between H2S and NO to stimulate vessel formation in the processing of heart failure. Therefore, the goal of the present study was to investigate the therapeutic potential of ZYZ-803 and evaluate the biological function of interaction between H2S and NO on angiogenesis in the model of heart failure.
2. Methods and materials
2.1. Chemical synthesis
Briefly, ZYZ-803 was synthesized by the reaction of furoxan and 2-amino-3-propynylsulfanyl-propionic acid in the presence of Boc anhydride, which was described in our previous articles. Moreover, the final product was oleaginous solid and verified by 1H nuclear magnetic resonance spectroscopy [22].
2.2. Chemicals and antibodies
The following antibodies were used: anti-eNOS antibody, anti-p-eNOS antibody, anti-VASP antibody and anti-p-VASP antibody were purchased from Cell Signaling Technology. Anti-CSE antibody, anti-GPx antibody, anti-HO-1 antibody and anti-GAPDH antibody were purchased from Santa Cruze.
All drugs were purchased from Sigma-aldrich unless otherwise stated.
2.3. Animals
Male C57BL6/J mice, 6–8 weeks of age, were maintained on standard conditions and free to receive food and water. All animals were handled according to the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH Publications no. 8023, revised 1978). Experimental procedures were managed according to the local ethical committee of Fudan University. 7.5 mg/kg/day isoprenaline for 4 weeks was used to induce heart failure.
2.4. Echocardiography and cardiac parameters
Mice were anesthesia by isoflurane, and echocardiography was performed by Vevo770 with a 30 MHz transducer. Factional shortening (FS), ejection fraction (EF) were used to indicate the cardiac systolic function. Left-ventricular end-diastolic volume (LV Vold) and left-ventricular internal diameter-diastole (LVIDd) were showed as the mean values of the signals from 5 consecutive cardiac cycles.
2.5. Histopathological observation
Masson trichrome staining and hematoxylin-eosin (H&E) staining were used to evaluate the myocardial fibrosis. Briefly, 24 h after last administration of isoprenaline, the hearts were isolated and fixed in 10% formaldehyde solution. Every heart was embedded in paraffin and cut into 5 mm thickness sections. The sections were stained with Masson trichrome staining kit and hematoxylin-eosin staining kit (Yuanye Biotechnology) according to the manufacturer's protocol, and then photographed by the microscope.
2.6. Measurements of plasma H2S and NO concentrations
75 μL mice plasma was mixed with 250 μL zinc acetate (1%, w/v), 425 μL distilled water, 133 μL N-dimethyl-p-phenylenediamine sulfate (20 mmol/L, 7.2 mmol/L HCl), and 133 μL FeCl3 (30 mmol/L, 1.2 mmol/L HCl) for 10 min incubation at room temperature. 250 μL trichloroacetic acid (10%) was added and then centrifuged at 14,000 r/min, 5 min. The absorbance of samples at 670 nm was detected by the microplate reader.
The concentrarion of NO in plasma was detected with Griess Assay Kit (Beyotime Institute of Biotechnology) according to the manufacturer's protocol.
2.7. Western Blot analysis
Tissues were lyses in the lysis buffer in the presence of proteases inhibitors. Protein extracts were used for Western Blot as previously described. Briefly, protein were diluted in the sample buffer with β-mercaptoethanol and denatured at 95 °C for 10 min then, 30 μg protein were separated by SDS-PAGE and transferred to polyvinylidene fluoride membrane. Membranes were incubated with 5% non-fat milk in 0.1% Tween-20 for 1 h and probed with any primary antibodies 4 °C overnight. Following incubation with HRP-conjugated secondary antibody, immunoreactive proteins were detected with ECL.
2.8. VEGF assay and cGMP assay
The concentrations of VEGF and cGMP were measured by VEGF ELISA Kit (Boatman Biotechnology) and cGMP ELISA kit respectively (R&D Systems) according to the manufacturer's protocol.
2.9. Statistical analysis
All data were showed as mean ± SEM. Statistical analysis was performed using one-way ANOVA, and post hoc pairwise comparisons were performed using Prism Graph. A p value < 0.05 was considered significant difference.
3. Results
3.1. ZYZ-803 attenuated cardiac dysfunction after heart failure
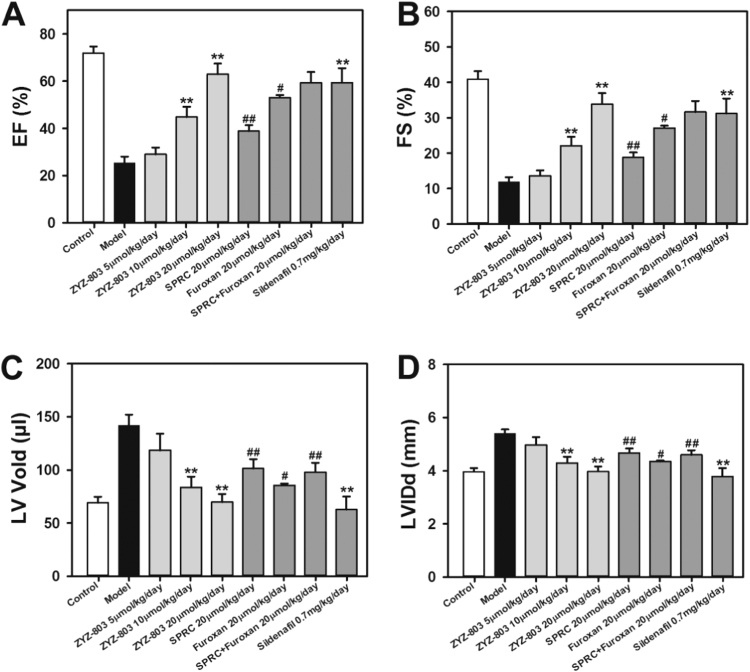
To determine the therapeutic potential of ZYZ-803 on heart failure, we performed heart failure procedure and daily administered ZYZ-803 (5–20 μmol/kg/day) orS-propargyl-cysteine (SPRC) (H2S donor) (20 μmol/kg/day) orfuroxan (NO donor) (20 μmol/kg/day), or SPRC + furoxan each (20 μmol/kg/day). Four weeks later, echocardiography revealed that heart failure decreased ejection fraction (EF) and fractional shortening (FS), and increased left ventricular volume (LV Vol) and left ventricular internal dimension diastole (LVID) in model group compared with that in control group (Fig. 1). However, administration of ZYZ-803 significantly attenuated these changes in a dose-dependent manner. Although SPRC and/or furoxan also improved cardiac function, this effect of SPRC and/or furoxan was much less potent than that of ZYZ-803 at the equimolar basis.
Fig. 1.
ZYZ-803 improved cardiac function in mice with heart failure. (A-D) The data showed changes of EF, FS, LV Vold and LVIDs in isoprenaline-induced heart failure mice with indicated treatments for 4 weeks. **p < 0.01 versus model group. #p < 0.05 and ##p < 0.01 versus corresponding ZYZ-803 group.
3.2. ZYZ-803 improved myocardial injury after heart failure
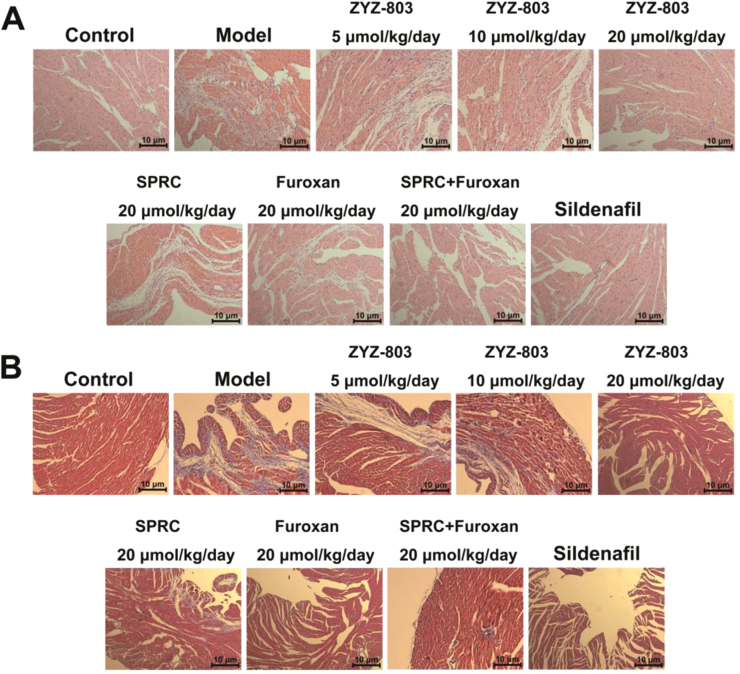
Histological analysis of H&E and Masson's Trichrome stained sections was to further assess the protective effect of ZYZ-803 against heart failure. As shown in Fig. 2, heart failure caused loss of myocardium and local necrosis compared with control group. What's more, extensive areas of fibrosis in hearts were seen in model group. Nevertheless, ZYZ-803 does-dependently protected against the myocardial injury. The areas of fibrosis in 20 μmol/kg/day ZYZ-803 treatment group were significantly less when compared with the SPRC and/or furoxan treatment group.
Fig. 2.
ZYZ-803 attenuated heart tissue injury after heart failure. (A) H&E staining of sections from left ventricular myocardium with indicated treatments for 4 weeks in isoprenaline-induced heart failure mice. (B) Masson trichrome staining of sections from left ventricular myocardium with indicated treatments for 4 weeks in isoprenaline-induced heart failure mice.
3.3. ZYZ-803 failed to attenuate the development of heart failure with PAG and/or L-NAME
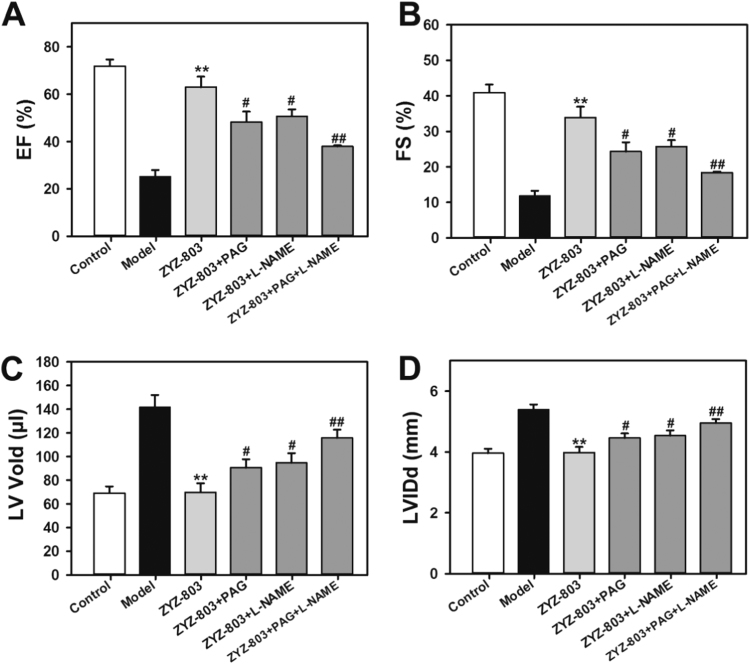
ZYZ-803 released H2S and NO through the promotion of CSE expression and eNOS activity. To determine the contribution of CSE and eNOS for ZYZ-803 to protect against heart failure, CSE inhibitor DL-propargylglycine (PAG) and eNOS inhibitor L-NG-nitro arginine methyl ester (L-NAME) were administered to mice. As shown in Fig. 3, PAG and/or L-NAME prevented the protective effects of ZYZ-803. In addition, compared with treatment with PAG or L-NAME alone, the inhibitory effect of PAG+L-NAME was much more significant.
Fig. 3.
PAG and L-NAME inhibited ZYZ-803-inducded cardioprotection in heart failure. (A-D) The data showed changes of EF, FS, LV Vold and LVIDs in isoprenaline-induced heart failure mice with indicated treatments for 4 weeks. **p < 0.01 versus model group. #p < 0.05 and ##p < 0.01 versus ZYZ-803 group.
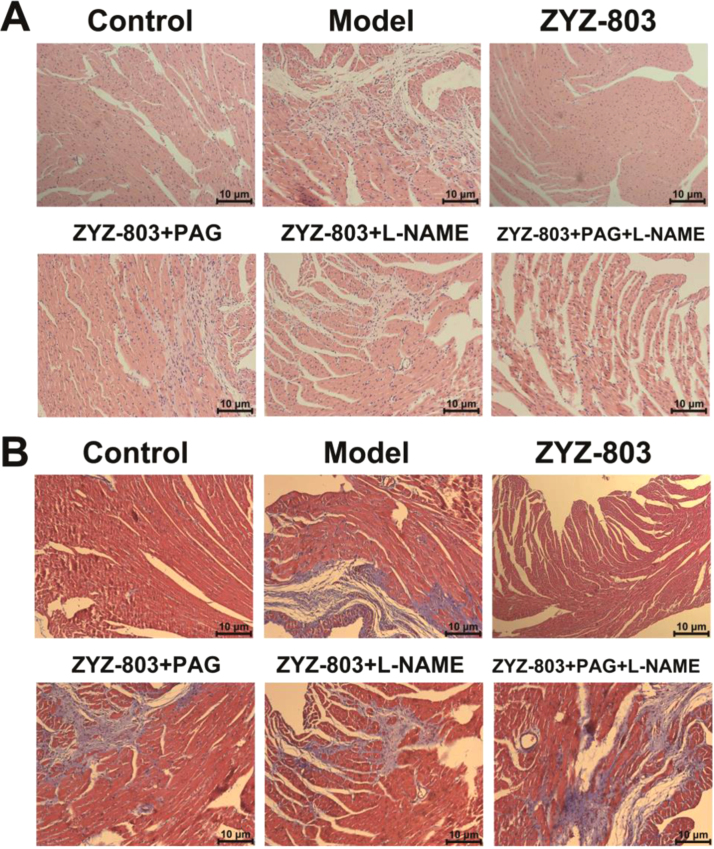
In line with echocardiography results, histological analysis of Hematoxylin-Eosin and Masson's Trichrome stained sections revealed that PAG and/or L-NAME treatment increased loss of myocardium and local necrosis compared with ZYZ-803-treated mice (Fig. 4).
Fig. 4.
ZYZ-803-improved heart tissue injury was attenuated by PAG and L-NAME. (A) H&E staining of sections from left ventricular myocardium with indicated treatments for 4 weeks in isoprenaline-induced heart failure mice. (B) Masson trichrome staining of sections from left ventricular myocardium with indicated treatments for 4 weeks in isoprenaline-induced heart failure mice.
3.4. ZYZ-803 increased H2S and NO concentrations after heart failure
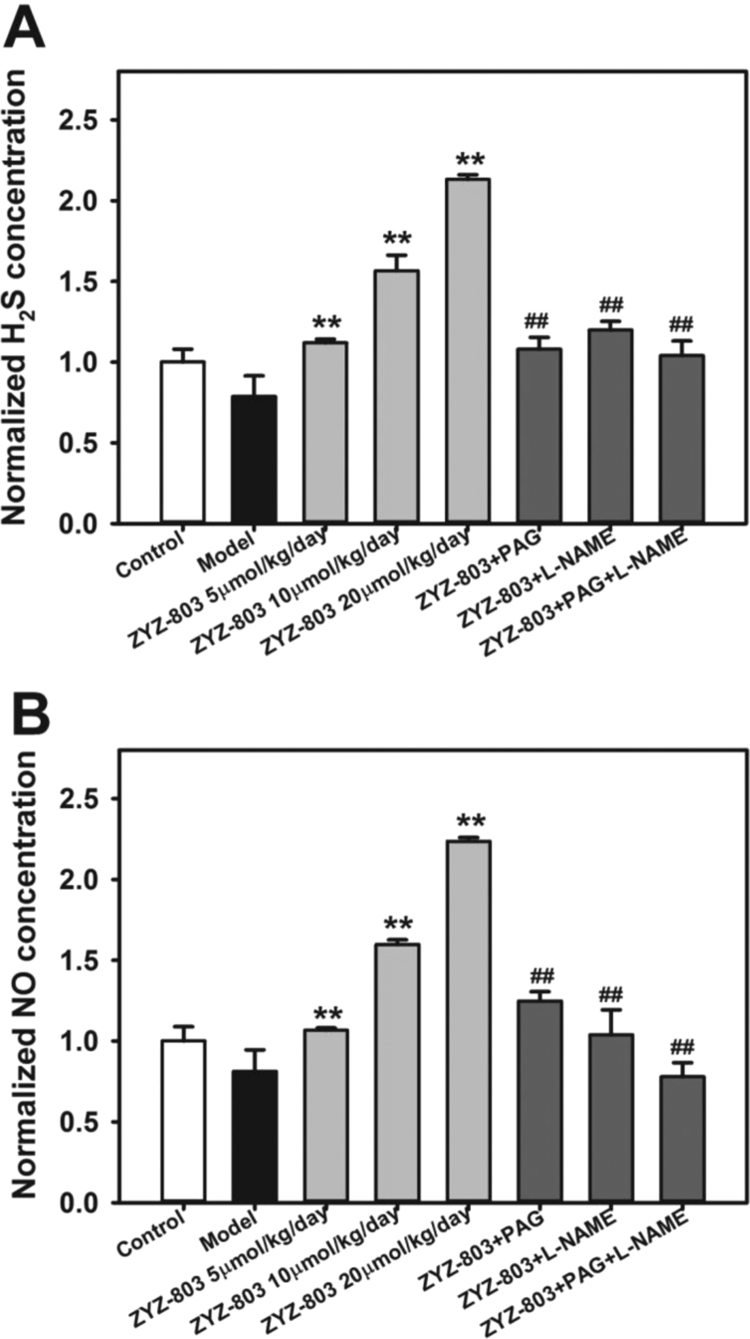
In order to further investigate whether ZYZ-803 released H2S and NO to protect against heart failure, we detected H2S and NO levels in plasma (Fig. 5). ZYZ-803 does-dependently increased H2S concentration. However, PAG or L-NAME prevented the increase in plasma H2S level, and the plasma H2S level was inhibited much more in PAG+L-NAME treated mice. Moreover, the similar results were found in plasma NO concentration.
Fig. 5.
ZYZ-803 increased H2S and NO concentrations in mice plasma. Concentration of plasma H2S level (A) and NO level (B) in isoprenaline-induced heart failure mice with indicated treatments for 4 weeks. **p < 0.01 versus model group. ##p < 0.01 versus ZYZ-803 group.
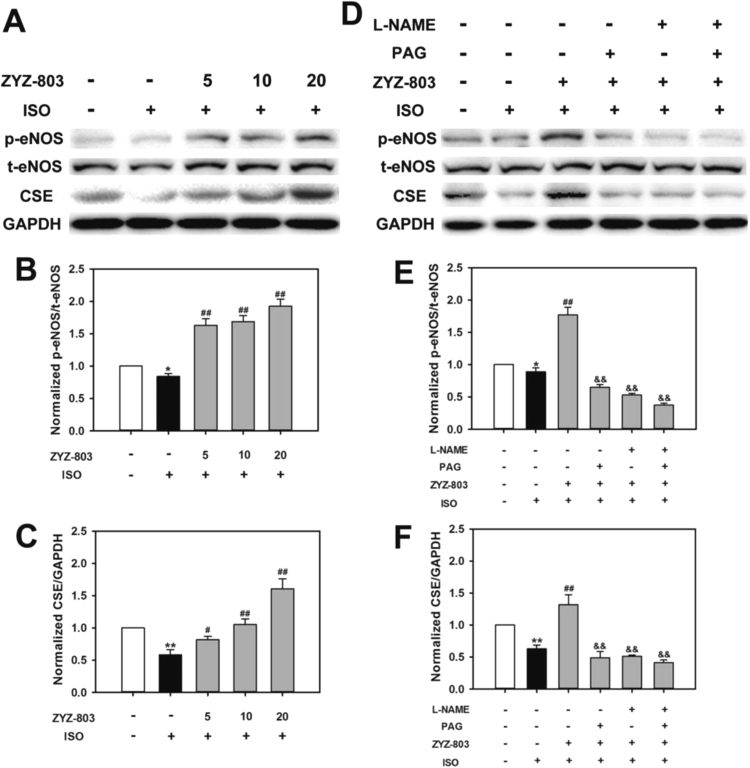
3.5. CSE expression and eNOS activity were mediated by ZYZ-803 after heart failure
As shown in Fig. 6, ZYZ-803 increased CSE expression and eNOS phosphorylation level in a dose-dependent manner after heart failure. However, PAG and/or L-NAME abolished these changes. In addition, we found that PAG not only attenuated ZYZ-803-induced CSE expression but also prevented ZYZ-803-induced eNOS phosphorylation level, and the similar result was observed in L-NAME treatment group. (Fig. 7)
Fig. 6.
ZYZ-803 increased CSE expression and eNOS activity after heart failure. Mice were induced with indicated treatments for 4 weeks. After that, lysate of heart tissues were subjected to SDS-PAGE, and immunoblotted with specific antibodies against eNOS, p-eNOS and CSE. (A-C) ZYZ-803 could increase CSE expression and eNOS activity dose-dependently. (D-F) The activity effect of ZYZ-803 on CSE expression and eNOS activity could be attenuated by PAG and L-NAME. *p < 0.05 and **p < 0.01 versus control group. #p < 0.05 and ##p < 0.01 versus isoprenaline group. &&p < 0.01 versus ZYZ-803 group.
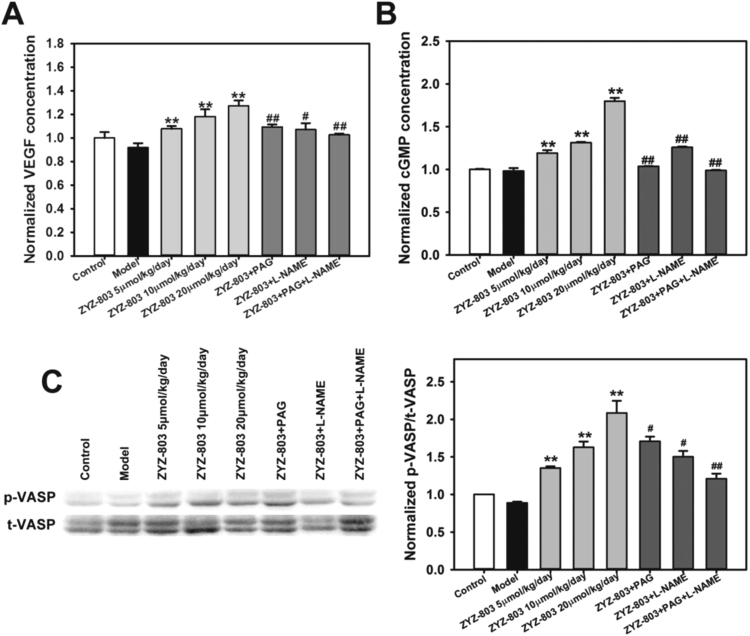
Fig. 7.
ZYZ-803 augmented myocardial angiogenic factors through VEGF/cGMP pathway after heart failure. VEGF concentrations (A), cGMP concentrations (B) and expression level of VASP and p-VASP (C) in isoprenaline-induced heart failure mice with indicated treatments for 4 weeks. **p < 0.01 versus model group. #p < 0.05 and ##p < 0.01 versus corresponding ZYZ-803 group.
3.6. ZYZ-803 augmented myocardial angiogenic factors after heart failure
Compared with vehicle-treated mice, ZYZ-803 increased vascular endothelial growth factor (VEGF) levels in a dose-dependent manner. However, PAG and/or L-NAME attenuated ZYZ-803-induced VEGF increase. Cyclic guanosine 5′-monophosphate (cGMP) is a downstream signaling molecule of VEGF, which plays a critical role in the regulation of heart failure [24]. ZYZ-803 dose-dependently elevated cGMP concentrations compared with Vehicle-treated group and these changes were abolished by PAG and/or L-NAME. The similar results were found in vasodilator-stimulated phosphoprotein (VASP) phosphorylation expressions, which is considered as the marker of cGMP downstream molecule protein kinase G (PKG) activity.
3.7. ZYZ-803 increased glutathione peroxidase and heme oxygenase 1 expressions after heart failure
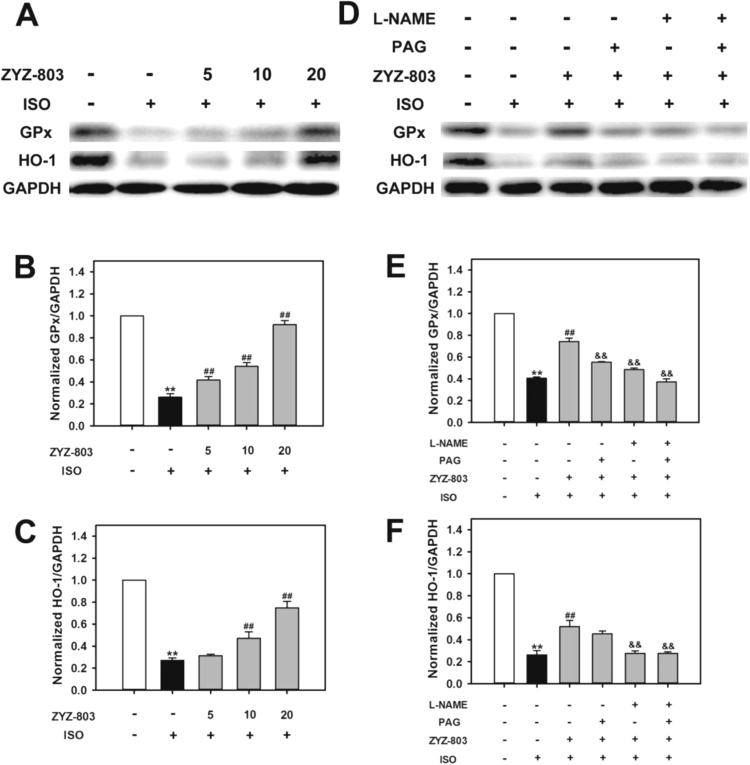
Glutathione peroxidase (GPx) and heme oxygenase 1 (HO-1) act as antioxidants protecting heart injury against reactive oxygen species (ROS) [25], [26]. As shown in Fig. 8, heart failure decreased GPx and HO-1 expressions compared with control group. However, administration of ZYZ-803 significantly promoted GPx and HO-1 expressions in a dose-dependent manner compared with model group. Furthermore, PAG and/or L-NAME prevented ZYZ-803-induced the increase of GPx and HO-1 expressions.
Fig. 8.
ZYZ-803 suppressed oxidative stress after heart failure. Mice were induced with indicated treatments for 4 weeks. After that, lysate of heart tissues were subjected to SDS-PAGE, and immunoblotted with specific antibodies against GPx and HO-1. (A-C) ZYZ-803 could increase GPx and HO-1 expression dose-dependently. (D-F) The activity effect of ZYZ-803 on GPx and HO-1 expressions could be attenuated by PAG and L-NAME. *p < 0.05 and **p < 0.01 versus control group. ##p < 0.01 versus isoprenaline(ISO) group. &&p < 0.01 versus ZYZ-803 group.
4. Discussion
Heart failure is often associated with ventricular remodeling, dysfunction, and decreased heart load, which is caused by many kinds of cardiovascular diseases. Isoprenaline is a β-adrenergic receptor agonist that can promote the generation of reactive oxygen species, apoptosis in cardiomyocytes and hypertrophy in the heart. It is initially considered adaptive that the left ventricular morphology changes after myocardial injury. However, heart failure occurs as a result of persistent pathological stimulation, such as increased vascular pressure and left ventricular remodeling [27]. There are many biological reactions during this process, such as oxidative stress, cell death and so on [28]. Although heart failure has long been paid attention to, many mechanisms remained still unknown. Therefore, it is important for drug design to explore the underlying mechanisms of heart failure. In the current study, we are the first to use a novel slow H2S-NO-releasing hybrid (ZYZ-803) to develop the therapeutic potential strategy and investigate the relationship between H2S and NO in heart failure. We found that H2S and NO cooperatively protected against heart failure via the VEGF/cGMP pathway. Due to synergistic effects, ZYZ-803 was much more potent than SPRC and/or furoxan, indicating a novel agent for cardiovascular protection.
It is well know that both NO and H2S play a critical role in the protection against heart failure. Johes et al. found that eNOS overexpression could attenuate congestive heart failure in mice [29]. Deficiency in eNOS resulted in heart failure and congenital septal defects during cardiac development, which is associated with increases of apoptosis in cardiomyocyte [30]. Moreover, H2S ameliorated oxidative and proteolytic stresses and protected the heart against adverse remodeling in chronic heart failure [31]. Givvimani et al. has shown that H2S could inhibit transition from compensatory hypertrophy to heart failure [32]. However, there is fewer report about the interaction between H2S and NO in heart failure.
It has been generally considered that H2S and NO operate their biological effects via district pathway. Recently there is growing evidence of crosstalk for the signaling pathways between the two gasotransmitters. For example, it has been shown that NO production was inhibited in the absence of CSE, while it could be increased by elevating CSE expression [33]. Ali et al. [34] and Whiteman and Moore [35] found that H2S might interact with NO to form a new nitrosothiol but without vasorelaxant activities. In the present study, we found that ZYZ-803 significantly attenuated ISO-induced cardiac dysfunctions via echocardiographic measurement. Moreover, ISO caused a large number of disappearance in cardiac myocytes and increased degree of fibrosis, but these pathological changes were inhibited by ZYZ-803. The cardioprotective effect of ZYZ-803 was much more potent than that of SPRC and/or furoxan, which indicated that H2S and NO cooperatively protected against heart failure.
Our previous results showed that ZYZ-803 stimulated CSE expression and eNOS activity to produce H2S and NO and no cytotoxic or proapoptotic effect in cells was observed [22]. In heart failure, we also found that CSE expression and eNOS activity were regulated by ZYZ-803. PAG and/or L-NAME significantly promoted cardioprotective effect of ZYZ-803 and the inhibitory effect of PAG + L-NAME was more obvious. It is further indicated that H2S and NO have a synergistic effect in heart failure. It has been reported that NO promoted CSE expression and increased H2S levels in vascular tissue and the vasorelaxant effect of H2S was inhibited by L-NAME [5], [36]. Hydrogen sulfide enhanced nitric oxide production with calcium-dependent activation of eNOS in endothelial cells [37]. After CSE knockout, the generation of NO was obviously suppressed, however, CSE overexpression could promoted NO production [33]. In line with these evidences, we found that PAG suppressed eNOS activity and NO concentration, while L-NAME reduced CSE expression and H2S level in heart failure. These data suggested that H2S and NO affected biological functions each other.
Angiogenesis was characterized by the sprouting of new blood vessels from existing vessels in a common physiological process [38]. Growing evidence suggests that angiogenesis has the therapeutic potential for heart failure. VEGF, a critical angiogenic cytokine, has an important role in vascular network growth, as evidenced by the results that the inhibition of VEGF signaling was crucial for the transition from compensatory hypertrophy to cardiac failure [39]. It has been shown that H2S and NO are strong accelerators of angiogenesis. For example, Cai et al. found that H2S stimulated angiogenesis through Akt activation [40]. NO promoted proliferation through endogenous basic fibroblastic growth factor in coronary venular endothelium [41]. H2S and NO were mutually dependent in the regulation of angiogenesis [4]. What's more, cGMP, the downstream molecular of VEGF, plays a critical role in angiogenesis [42]. In the current study, treatment of ZYZ-803 induced a dose-dependent increase in VEGF and cGMP concentrations, whereas the effect of ZYZ-803 was suppressed in the presence of PAG and/or L-NAME. VASP is determined as a substrate for cGMP-dependent protein kinase (PKG), which is an important regulator of angiogenesis via ERK and p38 [43]. The phosphorylation of VASP was regulated by ZYZ-803, but this effect was also inhibited by PAG and/or L-NAME. Based on the data above, ZYZ-803 caused CSE and eNOS activation and increased H2S and NO concentrations. These two gasotransmitters potentiated each other's production. Both of them increased VEGF level and cGMP concentration. Evoked by the increase in cGMP levels, PKG and downstream molecules, such as p38 and ERK, were activated to participate in the regulation of angiogenesis.
Considering the vital role of oxidative stress in isoprenaline-induced heart failure, we tested whether there was any effect of ZYZ-803 on GPx and HO-1. GPx is an antioxidant enzyme and functions in the detoxification of hydrogen peroxide. Mitochondrial ROS production and oxidative mtDNA damage were increased markedly during reoxygenation in GPx1(-/-) hearts [44]. HO-1 is an inducible stress-response protein that imparts antioxidant and antiapoptotic effects [45]. In the heart, HO-1 suppressed LV hypertrophy and remodeling via inhibiting the effects of prohypertrophic reactive oxygen species signals [46]. In the current study, treatment of ZYZ-803 promoted a dose-dependent increase in GPx and HO-1 concentrations, whereas the effect of ZYZ-803 was suppressed in the presence of PAG and/or L-NAME, which indicating that they contribute to the protective effect of ZYZ-803 in heart failure.
5. Conclusion
In summary, this study provided first evidence: (i) H2S and NO generation from ZYZ-803 interacted their biological functions each other in the process of heart failure and (ii) the synergistic effects of H2S and NO converge at VEGF/cGMP pathway to protect against heart failure. In addition, the current study indicates that ZYZ-803 upregulates the endogenous antioxidants, GPx and HO-1. These findings continue to support the emerging concept that H2S and NO are required to maintain the cellular homeostasis. Based on the current results, ZYZ-803 exhibits powerful therapeutic potential in cardioprotection, which sheds new light on further gaseous drugs design for the treatment of heart failure.
Acknowledgments
Thanks associate professor Wang Wang, who is from university of washington, for modifying the English.
This work was supported by the National Natural Science Foundation of China (Nos. 81703499, 81330080, 81402956, 81573421), Shanghai Committee of Science and Technology of China (No. 16431902000), Shanghai Chenguang Program (No. 14CG03), the Shanghai Sailing Program (No. 16YF1410400). A key laboratory program of the Education Committee of Shanghai Municipality (No. ZDSYS14005).
Acknowledgments
Conflict of interests
None of the authors has any conflict of interests to disclose.
Authors’ contribution
The contribution of each author was as follows: Yi Zhun Zhu, Dan Wu, and Qingxun Hu designed this study. Dan Wu, Qingxun Hu, Ying Xiong and Deqiu Zhu performed the experiments and Yicheng Mao gave technology supports. Yi Zhun Zhu, Dan Wu, and Qingxun Hu analyzed the data and wrote the paper. All authors read and approved the final paper. Dan Wu and Qingxun Hu contributed equally to this work.
Contributor Information
Yicheng Mao, Email: maoyc@fudan.edu.cn.
Yi Zhun Zhu, Email: yzzhu@must.edu.mo.
References
- 1.Mozaffarian E.J., Fau - Benjamin D., Benjamin Ej Fau - Go A.S., Go As Fau - Arnett D.K. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Foo R.S., Mani K Fau - Kitsis R.N., Kitsis R.N. Death begets failure in the heart. J. Clin. Investig. 2005;115(3):565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 4.Coletta C., Papapetropoulos A Fau - Erdelyi K., Erdelyi K Fau - Olah G. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA. 2012;109(23):9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu D., Hu Q., Ma F. Vasorelaxant effect of a new hydrogen sulfide-nitric oxide conjugated donor in isolated rat aortic rings through cGMP pathway. Oxid. Med. Cell. Longev. 2016;2016(1942–0994) doi: 10.1155/2016/7075682. (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo K., Bhushan S Fau - King A.L., King Al Fau - Prabhu S.D. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127(10):1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu D., Hu Q., Liu X. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide. 2015;46:204–212. doi: 10.1016/j.niox.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang R. Two's company, three'sa crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 9.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 2003;5(4):493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 10.Wu D., Hu Q., Zhu Y. Therapeutic application of hydrogen sulfide donors: the potential and challenges. Front. Med. 2016;10(1):18–27. doi: 10.1007/s11684-015-0427-6. [DOI] [PubMed] [Google Scholar]
- 11.Kovacic D., Glavnik N Fau - Marinsek M., Marinsek M Fau - Zagozen P. Total plasma sulfide in congestive heart failure. J. Card. Fail. 2012;18(7):541–548. doi: 10.1016/j.cardfail.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Elrod J.W., Calvert Jw Fau - Morrison J., Morrison J Fau - Doeller J.E. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA. 2007;104(39):15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvert J.W., Jha S Fau - Gundewar S., Gundewar S Fau - Elrod J.W. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009;105(4):365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvert J.W., Elston M Fau - Nicholson C.K., Nicholson Ck Fau - Gundewar S. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122(1):11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drexler H., Kastner S Fau - Strobel A., Strobel A Fau - Studer R. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J. Am. Coll. Cardiol. 1998;32(4):955–963. doi: 10.1016/s0735-1097(98)00336-2. [DOI] [PubMed] [Google Scholar]
- 16.Fukuchi M., Hussain Sn Fau - Giaid A., Giaid A. Heterogeneous expression and activity of endothelial and inducible nitric oxide synthases in end-stage human heart failure: their relation to lesion site and beta-adrenergic receptor therapy. Circulation. 1998;14(98):132–139. doi: 10.1161/01.cir.98.2.132. [DOI] [PubMed] [Google Scholar]
- 17.Stein B., Eschenhagen T Fau - Rudiger J., Rudiger J Fau - Scholz H. Increased expression of constitutive nitric oxide synthase III, but not inducible nitric oxide synthase II, in human heart failure. J. Am. Coll. Cardiol. 1998;32(5):1179–1186. doi: 10.1016/s0735-1097(98)00399-4. [DOI] [PubMed] [Google Scholar]
- 18.Simon J.N., Duglan D., Casadei B. Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J. Mol. Cell. Cardiol. 2014;73:80–91. doi: 10.1016/j.yjmcc.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Nagpure B.V., Bian J.S. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid. Med. Cell. Longev. 2016;2016(1942–0994):6904327. doi: 10.1155/2016/6904327. (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo Faro M.L., Fox B., Whatmore J.L. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47. doi: 10.1016/j.niox.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Yong Q.C., Cheong Jl Fau - Hua F., Hua F Fau - Deng L.-W. Regulation of heart function by endogenous gaseous mediators-crosstalk between nitric oxide and hydrogen sulfide. Antioxid. Redox Signal. 2011;14(11):2081–2091. doi: 10.1089/ars.2010.3572. [DOI] [PubMed] [Google Scholar]
- 22.Hu Q., Wu D., Ma F. Novel angiogenic activity and molecular mechanisms of ZYZ-803, a slow-releasing hydrogen sulfide-nitric oxide hybrid molecule. Antioxid. Redox Signal. 2016;25(8):498–514. doi: 10.1089/ars.2015.6607. [DOI] [PubMed] [Google Scholar]
- 23.Isner Jm Fau - Losordo D.W., Losordo D.W. Therapeutic angiogenesis for heart failure. Nat. Med. 1999;5(5):491–492. doi: 10.1038/8374. [DOI] [PubMed] [Google Scholar]
- 24.Senzaki H., Smith Cj Fau - Juang G.J., Juang Gj Fau - Isoda T. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2013;15(10):1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 25.Sun W.H., Liu F Fau - Chen Y., Chen Y Fau - Zhu Y.-C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012;421(2):164–169. doi: 10.1016/j.bbrc.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 26.Wu M.L., Ho Yc Fau - Yet S.-F., Yet S.F. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid. Redox Signal. 2011;15(7):1835–1846. doi: 10.1089/ars.2010.3726. [DOI] [PubMed] [Google Scholar]
- 27.Koitabashi N., Kass D.A. Reverse remodeling in heart failure--mechanisms and therapeutic opportunities. Nat. Rev. Cardiol. 2011;9(3):147–157. doi: 10.1038/nrcardio.2011.172. [DOI] [PubMed] [Google Scholar]
- 28.Maack C., Kartes T Fau - Kilter H., Kilter H Fau - Schafers H.-J. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108(13):1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 29.Jones S.P., Greer Jj Fau - van Haperen R., van Haperen R Fau - Duncker D.J. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc. Natl. Acad. Sci. USA. 2003;100(8):4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Q., Song W Fau - Lu X., Lu X Fau - Hamilton J.A. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation. 2002;106(7):873–879. doi: 10.1161/01.cir.0000024114.82981.ea. [DOI] [PubMed] [Google Scholar]
- 31.Mishra P.K., Tyagi N Fau - Sen U., Sen U Fau - Givvimani S. H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. Am. J. Physiol.-Heart Circ. Physiol. 2010;298(2):H451–H456. doi: 10.1152/ajpheart.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Givvimani S., Munjal C Fau - Gargoum R., Gargoum R Fau - Sen U. Hydrogen sulfide mitigates transition from compensatory hypertrophy to heart failure. J. Appl. Phycol. 2011;110(4):1093–1100. doi: 10.1152/japplphysiol.01064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altaany Z., Yang G Fau - Wang R., Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J. Cell. Mol. Med. 2013;17(7):879–888. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali M.Y., Ping Cy Fau - Mok Y.Y., Mok Yy Fau - Ling L. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006;149(6):625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiteman M., Moore P.K. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J. Cell. Mol. Med. 2009;13(3):488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W., Zhang J Fau - Lu Y., Lu Y Fau - Wang R. “The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kida M., Sugiyama T Fau - Yoshimoto T., Yoshimoto T Fau - Ogawa Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur. J. Pharm. Sci. 2013;48(1–2):211–251. doi: 10.1016/j.ejps.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P. Angiogenesis in life, disease and medicine. nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 39.Rengo G., Cannavo A Fau - Liccardo D., Liccardo D Fau - Zincarelli C. Vascular endothelial growth factor blockade prevents the beneficial effects of beta-blocker therapy on cardiac function, angiogenesis, and remodeling in heart failure. Circ. Heart Fail. 2013;6(6):1259–1267. doi: 10.1161/CIRCHEARTFAILURE.113.000329. [DOI] [PubMed] [Google Scholar]
- 40.Cai W.J., Wang Mj Fau - Moore P.K., Moore Pk Fau - Jin H.-M. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76(1):29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Ziche M., Parenti A Fau - Ledda F., Ledda F Fau - Dell'Era P. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ. Res. 1997;80(6):845–852. doi: 10.1161/01.res.80.6.845. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R., Wang L Fau - Zhang L., Zhang L Fau - Chen J. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ. Res. 2003;92(3):308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 43.Cook A.L., Haynes J.M. Phosphorylation of the PKG substrate, vasodilator-stimulated phosphoprotein (VASP), in human cultured prostatic stromal cells. Nitric Oxide. 2007;16(1):10–17. doi: 10.1016/j.niox.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Thu V.T., Kim Hk Fau - Ha S.H., Ha Sh Fau - Yoo J.-Y. Glutathione peroxidase 1 protects mitochondria against hypoxia/reoxygenation damage in mouse hearts. Pflug. Arch.-Eur. J. Physiol. 2010;460(1):55–68. doi: 10.1007/s00424-010-0811-7. [DOI] [PubMed] [Google Scholar]
- 45.Otterbein L.E., Choi A.M. Heme oxygenase: colors of defense against cellular stress. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000;279(6):L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 46.Wang G., Hamid T Fau - Keith R.J., Keith Rj Fau - Zhou G. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121(17):1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]