Abstract
Microglial NADPH oxidase (Nox2) plays a key role in chronic neuroinflammation and related dopaminergic neurodegeneration in Parkinson's disease (PD). However, the mechanisms behind Nox2 activation remain unclear. Here, we revealed the critical role of complement receptor 3 (CR3), a microglia-specific pattern recognition receptor, in Nox2 activation and subsequent dopaminergic neurodegeneration by using paraquat and maneb-induced PD model. Suppression or genetic deletion of CR3 impeded paraquat and maneb-induced activation of microglial Nox2, which was associated with attenuation of dopaminergic neurodegeneration. Mechanistic inquiry revealed that blocking CR3 reduced paraquat and maneb-induced membrane translocation of Nox2 cytosolic subunit p47phox, an essential step for Nox2 activation. Src and Erk (extracellular regulated protein kinases) were subsequently recognized as the downstream signals of CR3. Moreover, inhibition of Src or Erk impaired Nox2 activation in response to paraquat and maneb co-exposure. Finally, we found that CR3-deficient mice were more resistant to paraquat and maneb-induced Nox2 activation and nigral dopaminergic neurodegeneration as well as motor dysfunction than the wild type controls. Taken together, our results showed that CR3 regulated Nox2 activation and dopaminergic neurodegeneration through a Src-Erk-dependent pathway in a two pesticide-induced PD model, providing novel insights into the immune pathogenesis of PD.
Abbreviations: Aβ, β-amyloid; CNS, central nervous system; CR3, complement receptor 3; DAMPs, damage associated molecular patterns; DEPs, diesel exhaust particles; DHE, dihydroethidium; Erk, extracellular regulated protein kinases; HMGB1, high-mobility group box 1; Iba-1, ionized calcium binding adaptor molecule 1; iC3b, complement C3 fragment; CAM-1, intercellular adhesion molecule-1; LPS, lipopolysaccharide; MPP+, 1-methyl-4-phenyl-pyridium iodide; Nox2, NADPH oxidase; oxLDL, oxidized low-density lipoprotein; PAMPs, pathogen associated molecular patterns; PD, Parkinson's disease; PRRs, pattern recognition receptors (PRRs); SFKs, Src family kinases; SN, subsantia nigra; SOD, superoxide dismutase; TH, tyrosine hydroxylase
Keywords: CR3, Scavenger receptors, NADPH oxidase, Neuroinflammation, Parkinson's disease
Highlights
-
•
CR3, but not SRs is capable of mediating NOX2 activation in experimental PD.
-
•
CR3 regulates NOX2 activation via a Src-Erk-dependent manner.
-
•
Blocking CR3 ameliorates microglial activation and dopaminergic neurodegeneration.
1. Introduction
Parkinson's disease (PD) is the most common neurodegenerative movement disorder and affects more than 1.7% population over 65 years [1]. The pathological hallmark of PD is the progressive dopaminergic neurodegeneration in the substantia nigra (SN) coupled with inclusions known as Lewy bodies and Lewy neuritis [2]. Dopamine replacement intervention is still the gold standard therapy for PD, which provides temporary symptomatic relief but fails to stop disease progression. The therapeutic strategies aimed at arresting dopaminergic neurodegeneration are lacking due to the obscure of the mechanisms of PD. Therefore, elucidating the potential mechanisms of dopaminergic neurodegeneration and developing related therapeutic interventions are urgently needed.
Neuroinflammation mediated by glia cells, especially microglia, is a common feature shared by multiple neurodegenerative disorders including PD [3]. Activation of glial cells including microglia and astroglia and accumulation of proinflammatory factors are observed in the area of SN and striatum in PD patients and animal models [4], [5]. Experimental animals treated with inflammogen lipopolysaccharide (LPS) display progressive dopaminergic neurodegeneration and L-dopa-reversible motor impairments [6], [7], [8], [9]. Humans exposed to LPS also develop parkinsonian syndromes [10], supporting an etiologic role of neuroinflammation in PD. Although the exact mechanisms for neuroinflammation in PD remain unclear, recent studies revealed an essential role of NADPH oxidase (Nox2) in the initiation and maintenance of neuroinflammation. Nox2 is a superoxide-producing enzyme and is highly expressed in microglia [11]. We found that pharmacological inhibition or genetic deletion of Nox2 markedly reduced microglia-mediated neuroinflammation as well as dopaminergic neurodegeneration in multiple in vivo and in vitro PD models [8], [9], [12], [13], [14]. In addition, Nox2-derived H2O2 was further identified as a key mediator for the regulatory effects of microglia on astroglial activation in experimental models of PD [15]. Given the high level of Nox2 in the SN of PD patients compared with control subjects [16], elucidating the mechanisms of Nox2 activation is particularly important, which may help us to better understand the immune pathogenesis of PD and thus, develop novel therapeutic strategies.
Pattern recognition receptors (PRRs) play important roles in innate immune responses by recognizing and binding to pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs) [17]. Activation of PRRs induces the release of inflammatory cytokines that help remove pathogens or restore tissue homeostasis. However, chronic activation of these receptors can cause inflammatory disease [18]. Previous studies indicated that scavenger receptors (SRs) and complement receptor 3 (CR3, also called αMβ2 or CD11b/CD18), two members of PRR family [19], [20], are involved in regulating Nox2 activation. Genetic deletion or antibody neutralization of CD36, one of the extensively studied SRs, significantly decreased Nox2-generated superoxide induced by oxidized low-density lipoprotein (oxLDL) [21], [22] and β-amyloid (Aβ), the main component of senile plaques in Alzheimer's disease (AD) [23], [24], [25]. By contrast, CR3 was essential for LPS [26], high-mobility group box 1 (HMGB1) [27] and diesel exhaust particles [28]-induced Nox2 activation. However, whether SRs or CR3 is involved in Nox2 activation in PD remains unknown. In this study, by using a two pesticide (paraquat/maneb)-induced PD model, we investigated the role of SRs and CR3 in Nox2 activation and related dopaminergic neurodegeneration.
2. Materials and methods
2.1. BV2 microglial cells
The mouse microglia BV2 cell line was maintained as described previously [29]. Briefly, BV2 microglial cells were maintained at 37 °C in DMEM supplemented with 10% fetal bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin in a humidified incubator with 5% CO2 and 95% air. The cells were split or harvested every 3–5 days.
2.2. Primary cell cultures
Mesencephalic neuron-glia culture was prepared from the ventral mesencephalon of embryonic day 14 ± 0.5 SD rats according to previously published protocol [30]. The culture was maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air in minimum essential medium (MEM) containing 10% heat-inactivated fetal bovine serum (FBS), 10% heat-inactivated horse serum (HHS), 1 g/L glucose, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 µM nonessential amino acids, 50 U/ml penicillin, and 50 µg/ml streptomycin. Seven days after initial seeding, immunocytochemical analysis indicated that neuron-glia cultures consisted of 10% microglia, 50% astrocytes, 40% neurons, and 1% dopaminergic neurons.
Mixed-glia culture was prepared from whole brains of 1-d-old wild type (WT, C57BL/6J) and CR3-/- mice pups [30]. Disassociated brain cells were seeded onto 24- or 96-well plate and maintained in DMEM/F-12 medium supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. The medium was changed every 3 days.
2.3. Measurement of extracellular and intracellular superoxide
The production of extracellular superoxide was determined by measuring the superoxide dismutase (SOD)-inhibitable reduction of WST-1 as described previously [8], [14]. Briefly, microglial cells (1 × 105/well) were grown overnight in 96-well plates in DMEM/F12 medium containing 10% FBS and switched to phenol red–free HBSS (50 μl/well). Subsequently, 50 μl HBSS with and without SOD (50 U/ml) was added to each well along with 50 μl WST-1(1 mM) in HBSS and 50 μl vehicle or α-synuclein. The absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices). The difference between the absorbance in the presence and absence of SOD was considered to be the amount of produced superoxide.
The production of intracellular superoxide was measured by the fluorescence probe dihydroethidium (DHE, Invitrogen Life Technologies, Grand Island, NY, USA) as described previously [31]. Briefly, cells were incubated with paraquat and maneb overnight in 24-well plates, and then were incubated with 10 μM DHE for 30 mins at 37 °C. The fluorescence images were captured using a fluorescence microscopy (excitation 534 nm; emission 580 nm).
2.4. MTT assay
Culture media were removed 4 h before completion of the incubation time and then, 200 μl of 0.25 mg/ml MTT was added to each well. Additional 4 h later, the supernatants were removed and 200 μl DMSO was added to the wells, and the plates were shaken for 10 min. The absorbance was measured at 540 nm by a plate reader (Molecular Devices).
2.5. Immunocytochemistry
Immunocytochemistry was performed as described previously [8], [14]. Briefly, cells were fixed in 4% formaldehyde and followed by treatment with 1% hydrogen peroxide. After 20 mins of incubation in blocking solution (PBS containing 1% bovine serum albumin, 0.4% Triton X‐100 and 4% serum), cultures were incubated with rabbit antibody against tyrosine hydroxylase (TH, Chemicon, 1:5000) or ionized calcium binding adaptor molecule 1 (Iba1, Wako Chemicals, 1:5000) for 24 h at 4 °C and followed by biotinylated secondary antibody for 2 h at room temperature. Antibody binding was visualized using a Vectastain ABC Kit (Vector Laboratories, Inc) and diaminobenzidine substrate. TH-immunoreactive (THir) cells per well were counted. For each experiment, three wells per treatment condition were used, and results from three independent experiments were obtained.
2.6. Animal dosing
Combined paraquat (10 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) and maneb (30 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) were administrated (i.p) to WT and CR3-/- mice (Jackson laboratory) according to our previous report [12]. Mice in control group received an equivalent volume of 0.9% saline. Six weeks later, mice were euthanized and brains were dissected. Experiments were performed in accordance with the Animal Guideline of Dalian Medical University. Housing and breeding of animals were performed strictly with Dalian Medical University's Guide for the Care and Use of Laboratory Animals. All experimental protocols were approved by and in agreement with the Ethical Committee of Dalian Medical University.
2.7. Membrane extraction
The membrane fractions of microglia and midbrain tissue were prepared using the membrane protein extraction kit (Beyotime, Jiangsu, China). Briefly, microglia and midbrain tissue were lysed in lysis buffer A provided by the kit and then subjected to Dounce homogenization (20–25 St, tight pestle A). The lysates were centrifuged at 700×g for 10 mins; the supernatant was collected and centrifuged at 14,000×g for 30 mins. The pellets were suspended using extraction buffer B and incubated for 20 mins. After centrifugation at 14,000×g for 5 mins, the supernatant was used as membranous fraction.
2.8. Western blot
For western blot analysis, equal amounts of protein were separated by 4–12% Bis-Tris Nu-PAGE gel and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk and incubated with primary antibodies (1:1000) against p47phox, gp91phox, phosphorylated Src, total Src, phosphorylated Erk, total Erk, GAPDH and HRP-linked anti-rabbit or mouse IgG (1:3000) for 2 h. ECL reagents (Amersham Biosciences) were used as a detection system.
2.9. Immunohistochemistry
For immunohistochemistry, whole brains of mice were removed and processed for frozen sections as described previously [9], [32] and serially sectioned at 30 µm for systematic analysis. The boundary of SN was outlined under magnification of the 4 × objective as per the atlas. The sections encompassing the entire midbrain were immunoblocked with 4–10% NGS and then incubated with rabbit antibody to TH or Iba-1 for 24 h at 4 °C. Antibody binding was visualized using a Vectastain ABC Kit (Vector Laboratories, Inc) and diaminobenzidine substrate. The sections were mounted permanently with Permount. Coded slides were used to ensure unbiased counting of TH-positive (THir) neurons in every three serial section. The number of THir neurons was counted bilaterally using a Metamorph image analysis tool [33]. THir neuron counts were performed by two individuals blind to the treatment.
2.10. Statistical analysis
All values are expressed as the mean ± SEM. Differences among means were analyzed using one- or two-way ANOVA with treatment as the independent factors. When ANOVA showed significant differences, pairwise comparisons between means were tested by Newman–Keuls post hoc testing. In all analyses, a value of p < 0.05 was considered statistically significant.
3. Results
3.1. Paraquat and maneb co-exposure activates Nox2
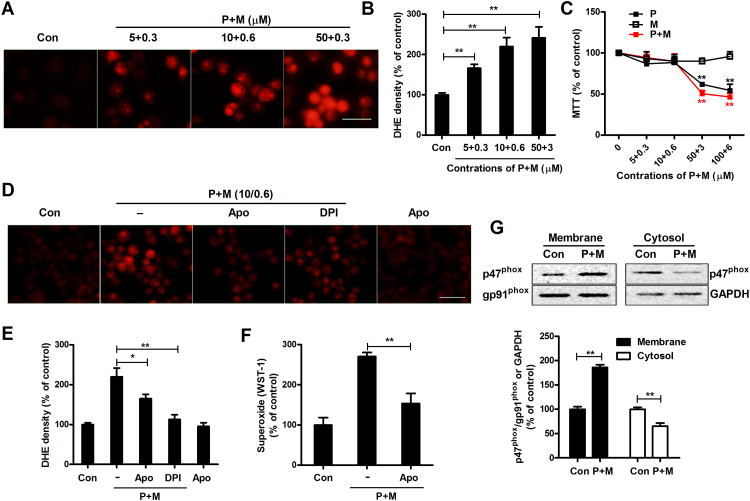
Nox2, a superoxide-producing enzyme, is composed of membrane (gp91phox, p22phox) and cytosolic (p47phox, p67phox, p40phox and Rac1/2) subunits. Membrane translocation of cytosolic subunits is necessary for the activation of Nox2 [34]. The effects of combined paraquat and maneb (referred to subsequently as P+M) on Nox2 were therefore examined by measuring the production of superoxide and membrane translocation of cytosolic subunit. In situ visualization of intracellular superoxide production was performed using DHE, a reactive oxygen species (ROS)-sensitive dye that can exhibit red fluorescence through interactions with superoxide and other free radicals. As shown in Fig. 1A, compared with vehicle controls, P+M exposure significantly increased the levels of red fluorescence in BV2 microglia in a concentration-dependent manner, indicating elevated superoxide production. Quantitative analysis supported the immunofluorescence observation by showing 66.1%, 119.9% and 141.5% increase of red fluorescence density in 5 + 0.3, 10 + 0.6 and 50 + 3 μM P+M-treated microglia, respectively, compared with vehicle controls (Fig. 1B). The dosage of paraquat or maneb was chosen based on previous report [35]. To rule out the possibility that the increase of superoxide production was attributed to nonspecific toxicity of P+M, we evaluated effects of P+M on the viability of microglia. P+M at concentrations of 10 + 0.6 μM and lower did not show significant toxicity (Fig. 1C). Therefore, 10 + 0.6 μM P+M was used in the subsequent experiments. P+M-induced increase of superoxide production was almost abolished by diphenyleneiodonium (DPI) and apocynin, two widely used Nox2 inhibitors, in microglia (Fig. 1D and E), indicating that Nox2 is the major source of superoxide stimulated by P+M. Nox2 activation increases both intracellular and extracellular superoxide [34]. The production of extracellular superoxide was further determined. Consistently, upregulated production of extracellular superoxide was also observed in P+M-treated microglia, which was significantly mitigated by Nox2 inhibitor, apocynin (Fig. 1F). In agreement with elevated superoxide production, exposed to P+M also induced membrane translocation of Nox2 cytosolic subunit, p47phox. Western blot analysis showed that compared with vehicle controls, the levels of p47phox in membrane fractions of P+M-treated microglia were significantly increased and coincidently, were significantly decreased in cytosolic fractions (Fig. 1G). No significant difference of p47phox expression in whole cell lysates prepared from vehicle and P+M-treated microglia was observed (Data not shown).
Fig. 1.
Paraquat and maneb co-exposure activates NOX2 in microglia. (A) The production of intracellular superoxide was assessed by DHE in BV2 microglial cells treated with different concentrations of P+M. The representative images of DHE oxidation were shown. (B) The density of red fluorescence of DHE oxidation was quantified. (C) Cell viability of BV2 microglial cells treated with different concentrations of P+M was detected using MTT assay. (D) BV2 microglial cells were pre-treated with apocynin or DPI, two widely used NOX2 inhibitors, and then P+M-induced production of intracellular superoxide was measured using DHE. (F) The density of red fluorescence of DHE oxidation was quantified. (F) P+M-induced production of extracellular superoxide was measured as described in “Materials and Methods”. (G) The membrane translocation of NOX2 cytosolic subunit, p47phox was detected after 15 mins of P+M stimulation by using Western blot and the density of blots was quantified. Gp91phox and GAPDH were used as internal membrane and cytosolic control, respectively. Results were expressed as a percentage of controls from three experiments performed in duplicate. *p < 0.05, **p < 0.01. Bar = 30 µm.
3.2. Blocking SRs fails to interfere with paraquat and maneb-induced Nox2 activation
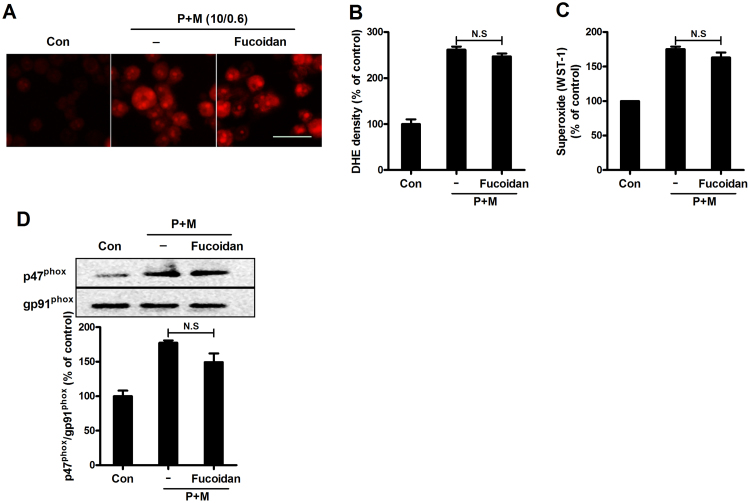
SRs are highly expressed in microglia [19]. To determine whether SRs are involved in P+M-induced Nox2 activation, fucoidan, a widely used inhibitor of SRs was used. As seen in Fig. 2A, a similar level of red DHE fluorescence was observed in P+M-treated microglia with or without fucoidan, indicating that fucoidan fails to block P+M-induced production of intracellular superoxide. Quantitative analysis of red fluorescence density supported our observation (Fig. 2B). Consistently, fucoidan also failed to interfere with production of extracellular superoxide and membrane translocation of p47phox induced by P+M (Fig. 2C and D), suggesting that SRs are not required for P+M-induced Nox2 activation.
Fig. 2.
SRs inhibitor fucoidan fails to interfere with paraquat and maneb-induced NOX2 activation. (A) The production of intracellular superoxide induced by P+M was assessed in BV2 microglial cells with or without fucoidan pre-treatment. (B) The density of red fluorescence of DHE oxidation was quantified. (C) P+M-induced production of extracellular superoxide was measured in the presence of fucoidan. (D) The effects of fucoidan on P+M-induced membrane translocation of p47phox was detected using Western blot and the density of blots was quantified. Results were expressed as a percentage of controls from three experiments performed in duplicate. N.S, not significant. Bar = 30 µm.
3.3. CR3 mediates Nox2 activation in response to paraquat and maneb co-exposure
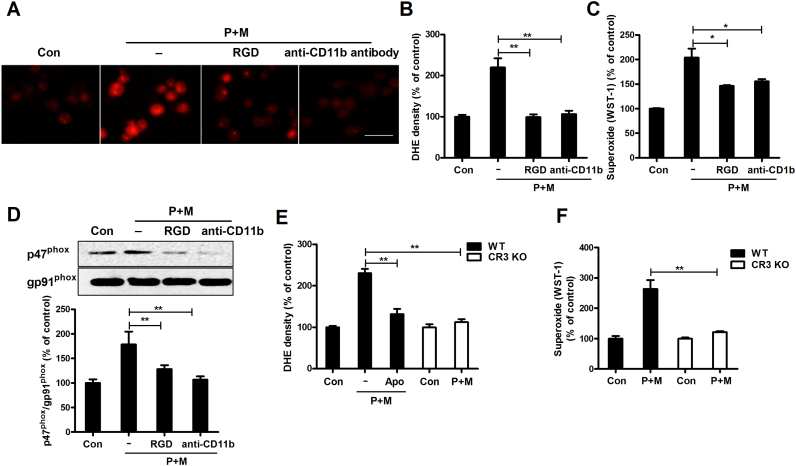
Next, we investigated the role of CR3 in P+M-induced Nox2 activation. Since CR3 belongs to a family known as integrins, RGD peptide, a cell adhesion motif that can bind to integrins, was initially used to block CR3. Interestingly, RGD treatment markedly reduced the red fluorescence of DHE oxidation in P+M-treated BV2 microglia (Fig. 3A and B). Consistently, P+M-induced production of extracellular superoxide and membrane translocation of p47phox were also mitigated by RGD peptide (Fig. 3C and D). In agreement with RGD peptide, antibody against CR3 subunit, CD11b also impeded P+M-induced production of both intracellular and extracellular superoxide as well as p47phox membrane translocation (Fig. 3A–D).
Fig. 3.
CR3 mediates paraquat and maneb-induced NOX2 activation. (A) BV2 microglial cells were pre-treated with RGD or anti-CD11b antibody and then P+M-induced production of intracellular superoxide was measured using DHE. (B) The density of red fluorescence of DHE oxidation was quantified. (C) P+M-induced production of extracellular superoxide was measured with or without RGD or anti-CD11b antibody pre-treatment. (D) The effects of RGD or anti-CD11b antibody on P+M-induced membrane translocation of p47phox was detected using Western blot and the density of blots was quantified. (E) P+M-induced production of intracellular superoxide was measured in mixed glial cultures prepared from WT and CR3-/- mice using DHE and the density of red fluorescence of DHE oxidation was quantified. (F) P+M-induced production of extracellular superoxide was measured in WT and CR3-/- mixed glial cultures. Results were expressed as a percentage of controls from three experiments performed in duplicate. *p < 0.05, **p < 0.01. Bar = 30 µm.
To further confirm the role of CR3 in P+M-induced Nox2 activation, primary mixed-glia cultures containing ~20% microglia were prepared from wild type (WT) and CR3-deficient mice. The reasons for using mixed-glia cells instead of purified microglia are as follows: 1) primarily enriched microglia are known to be short lived and become activated in the condition without the presence of either neurons or astrocytes; 2) it is difficult to harvest a sufficient amount of enriched primary microglia for experiments; 3) we previously reported that microglial Nox2 is the major source of superoxide production in mixed glial culture [8], [13]. As illustrated in Fig. 3E, P+M stimulated production of intracellular superoxide in WT mixed-glia cultures, which was markedly reduced by Nox2 inhibitor apocynin, indicating that P+M activate Nox2 in primary cultures. Interestingly, P+M-induced Nox2 activation was abrogated in CR3-/- cultures by showing a comparable level of DHE red fluorescence between P+M and vehicle control (Fig. 3E). The requirement of CR3 in P+M-induced activation of Nox2 was further confirmed by measuring extracellular superoxide. As shown in Fig. 3F, P+M induced production of extracellular superoxide in WT but not in CR3-/- cultures.
3.4. Src-Erk pathway is the downstream signal of CR3 for Nox2 activation
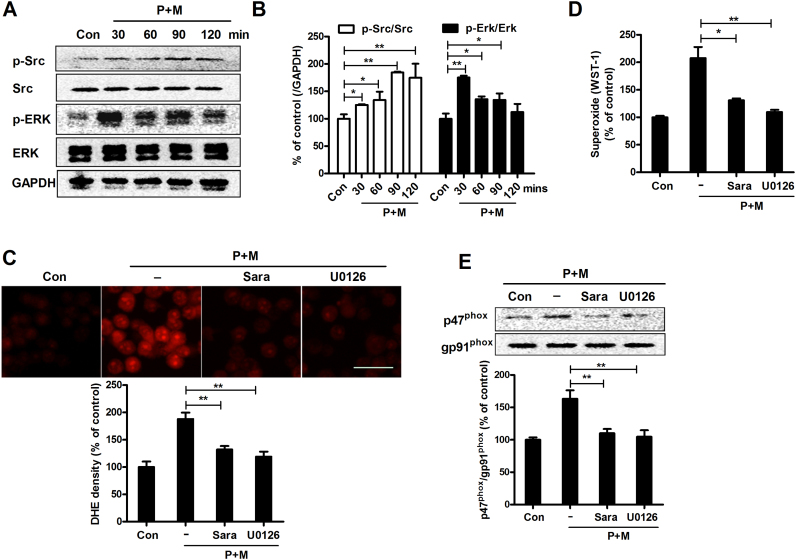
We subsequently explored the downstream signals of CR3 responsible for Nox2 activation. The kinase of Src is well-known to be a downstream target of CR3 [19]. Previous study revealed that Src is able to activate Erk [19], a kinase that can phosphorylate p47phox and then induce its membrane translocation [36]. To investigate whether Src and Erk underlay P+M-induced Nox2 activation, we firstly examined the activation of Src and Erk in P+M-treated microglia. As seen in Fig. 4A, compared with vehicle controls, P+M significantly elevated the levels of phosphorylated Src and Erk, indicating activation of both Src and Erk. Time-course study revealed that the activation of Src and Erk induced by P+M peaked at 90 and 30 mins after treatment, respectively (Fig. 4B).
Fig. 4.
Src and Erk, the downstream signals of CR3, mediate paraquat and maneb-induced NOX2 activation. (A) The levels of phosphorylated and total Src and Erk were determined in paraquat and maneb-treated BV2 microglia by Western blot using specific antibodies and the representative blots were shown. (B) The activation of Src and Erk was quantified by analysis of the blot density. (C) The effects of saracatinib (Src inhibitor) and U0126 (Erk inhibitor) on P+M-induced production of intracellular superoxide were measured using DHE and the density of red fluorescence was quantified. (D) The effects of saracatinib and U0126 on P+M-induced production of extracellular superoxide was measured. (E) The effects of saracatinib and U0126 on P+M-induced membrane translocation of p47phox was detected using Western blot and the density of blots was quantified. Results were expressed as a percentage of controls from three experiments performed in duplicate. *p < 0.05, **p < 0.01. Bar = 30 µm.
To determine whether the activation of Src and Erk contributes to Nox2 activation in response to P+M, BV2 microglial cells were treated with saracatinib (an inhibitor of Src) or U0126 (an inhibitor of Erk) prior to P+M stimulation. Interestingly, both saracatinib and U0126 blocked P+M-induced superoxide production (Fig. 4C and D). In agreement, P+M-induced membrane translocation of p47phox was also mitigated by saracatinib and U0126 (Fig. 4E), indicating the involvement of Src and Erk in P+M-induced Nox2 activation.
3.5. Antagonizing CR3 protects dopaminergic neurons against paraquat and maneb-induced degeneration in vitro
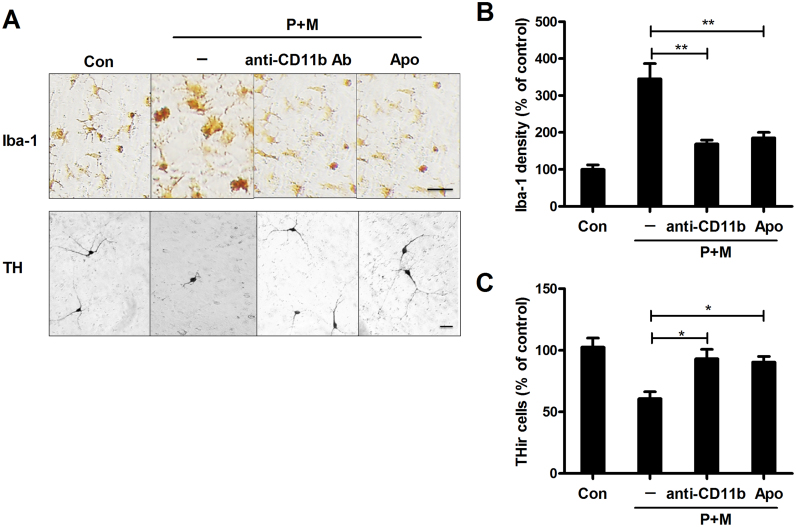
To investigate whether Nox2 inhibition by antagonizing CR3 is functional, we determined the effects of CR3 blocking on P+M-induced microglial activation and dopaminergic neurodegeneration in primary midbrain neuron-glia cultures. Immunostaining with antibody against Iba-1, a microglial marker, was performed to observe changes in microglial morphology. Fig. 5A depicted microglial activation in response to P+M when compared to vehicle controls. Activated microglial cells displayed larger cell bodies and irregular amoeboid morphology, as well as increased immunoreactivity to Iba-1 antibody. Interestingly, such activated microglia were not observed in P+M-treated cultures once CR3 was blocked by anti-CD11b antibody (Fig. 5A and B). Notably, the inhibitory effects of CR3 blocking against P+M-induced microglial activation were comparable with inactivation of Nox2 by apocynin (Fig. 5A and B).
Fig. 5.
Blocking CR3 alleviates paraquat and maneb-induced microglial activation and dopaminergic neurodegeneration. Rat mesencephalic neuron-glia cultures were pretreated with anti-CD11b antibody or apocynin for 30 min before the addition of P+M. (A) Microglia and dopaminergic neurons were stained with antibody against Iba-1 or TH, respectively, and the representative images were shown. (B) The density of Iba-1 was quantified. (C) The effects of anti-CD11b antibody or apocynin on P+M-induced dopaminergic neurodegeneration were assessed by THir neuron counts. Results were expressed as a percentage of controls from three experiments performed in duplicate. *p < 0.05, **p < 0.01. Bar = 50 µm.
Dopaminergic neuron was identified by immunostaining with antibody against TH, a marker for dopaminergic neuron. THir neuron counts showed that P+M treatment resulted in a significant loss of dopaminergic neurons in midbrain neuron-glia cultures, which was significantly reduced by antibody against CD11b (Fig. 5A and C). In addition to restoring the number of dopaminergic neuron, anti-CD11b antibody treatment also rescued the dopaminergic neurite retraction and degradation associated with P+M-mediated neurotoxicity (Fig. 5A). Taken together, these measurements clearly demonstrated the neuroprotective effects of blocking CR3.
3.6. Genetic deletion of CR3 attenuates paraquat and maneb-induced Nox2 activation and microglial activation in vivo
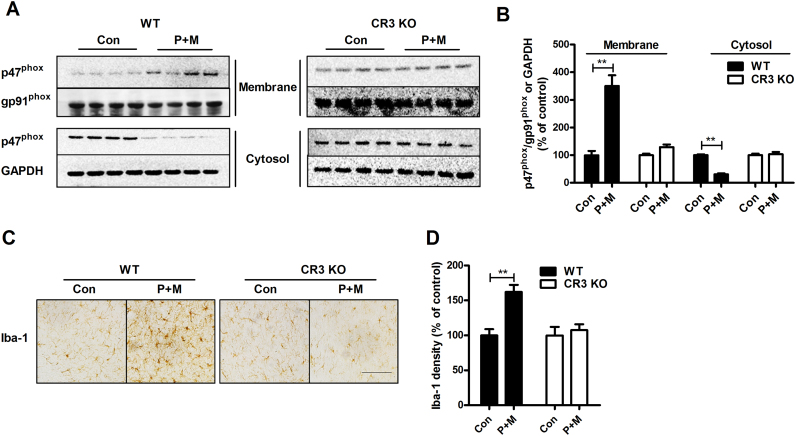
To verify the role of CR3 in P+M-induced Nox2 activation and dopaminergic neurodegeneration in vivo, P+M were administrated to CR3-deficient mice. Consistent with in vitro, compared with vehicle controls, P+M stimulation significantly increased the levels of p47phox in the membrane factions of midbrain tissues prepared from WT mice. Coincidentally, a reduced level of p47phox in the cytosol was observed (Fig. 6A and B), indicating p47phox membrane translocation and Nox2 activation. However, P+M-induced p47phox membrane translocation was not detected in CR3-/- mice (Fig. 6A and B).
Fig. 6.
CR3 deficiency attenuates paraquat and maneb-induced NOX2 activation and microglial activationin vivo. (A) WT and CR3-/- mice were treated with P+M for 1 h and then the membrane translocation of p47phox was determined in midbrain tissues using Western blot. (B) The density of blots was quantified. (C) WT and CR3-/- mice were treated with P+M for 6 weeks. Microglia in the SN of mice were stained using Iba-1 antibody and the representative images were shown. (D) The density of Iba-1 staining was quantified. n = 4–5. *p < 0.05, **p < 0.01. Bar = 100 µm.
Consistent with inactivation of Nox2, activated microglia characterized by a hypertrophied morphology and an elevated density of Iba-1 staining were observed in the SN of WT but not CR3-deficient mice (Fig. 6C). Quantitative analysis of Iba-1 density supported these morphological observations. Compared with vehicle controls, a 62% increase of Iba-1 density in the SN was observed in WT mice, which was reduced to 7.5% in CR3-/- mice (Fig. 6D).
3.7. CR3 deficiency ameliorates paraquat and maneb-induced dopaminergic neurodegeneration and motor deficits in mice
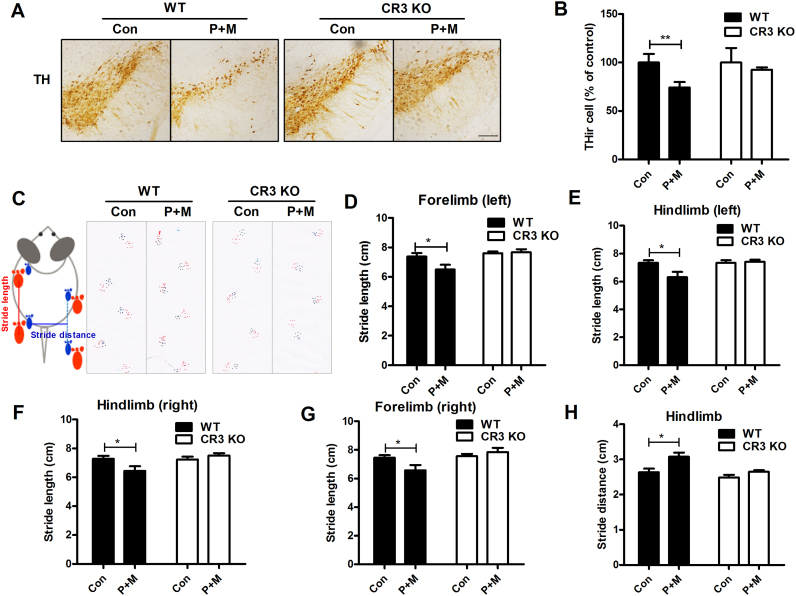
To determine whether the inactivation of Nox2 and neuroinflammation in P+M-treated CR3-/- mice was associated with attenuation of dopaminergic neurodegeneration, the number of dopaminergic neurons (THir) in the SN was counted. As shown in Fig. 7A, P+M treatment resulted in a significant decrease in nigral THir neurons in WT mice, which was consist with previous reports [33], [37]. The loss of nigral dopaminergic neurons was attenuated in CR3-/- mice. Quantitative analysis showed that exposure to P+M resulted in a 24% loss of nigral dopaminergic neurons in WT mice, which was reduced to a 7.5% loss in CR3-/- mice (Fig. 7B).
Fig. 7.
CR3 deficiency ameliorates paraquat and maneb-induced dopaminergic neurodegeneration and motor deficits in mice. (A) WT and CR3-/- mice were treated with P+M for 6 weeks. Dopamienrgic neurons in the SNpc of mice were stained using TH antibody and the representative images were shown. (B) The number of THir neurons was counted. (C) Gait analysis was performed in WT and CR3-/- mice and schematic illustration of gait analysis measurements of stride length and stride distance were shown. (D-G) The distance between subsequent limb placements (stride length) was measured. (H) The stride distance between hindlimb placements of the mice was detected. n = 6–9. *p < 0.05, **p < 0.01. Bar = 200 µm.
In addition to protecting dopaminergic neurons, CR3 deficiency potently ameliorated P+M-induced motor deficits. WT mice treated with P+M displayed shorter stride length and wider stride distance than vehicle controls (Fig. 7C–H), indicating gait abnormality. Interestingly, P+M-induced impairments of gait performance were not observed in CR3-/- mice as shown by similar levels of stride length and stride distance as vehicle control mice (Fig. 7C–H).
4. Discussion
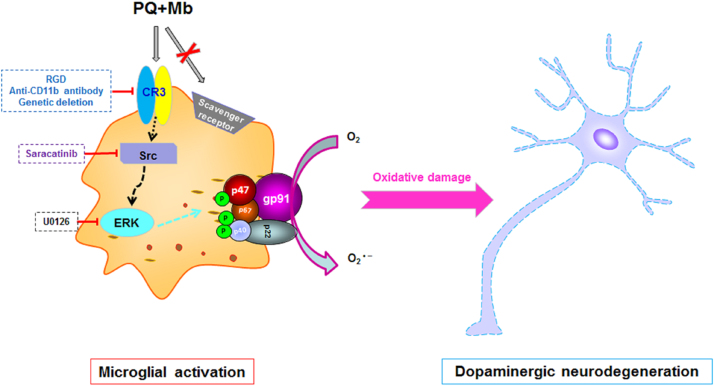
In this study, we demonstrated that CR3 mediated Nox2 activation and subsequent degeneration of dopaminergic neurons in a two pesticide-induced PD model (Fig. 8). Pharmacological inhibition or genetic deletion of CR3 attenuated P+M-induced superoxide production and p47phox membrane translocation, which was associated with protection of dopaminergic neurons in midbrain neuron-glia cultures. The involvement of CR3 in Nox2 activation and related dopaminergic neurodegeneration was also verified in vivo. This study pinpoints a major contributor that mediates Nox2 activation and chronic dopaminergic neurodegeneration, which may facilitate our understanding of the immune pathogenesis of PD.
Fig. 8.
Proposed model showing how CR3 mediates NOX2 activation and dopaminergic neurodegeneration in experimental PD. Consistent with previous report, activation of microglial NOX2 was observed in P+M-induced experimental model of PD. Pharmacological inhibition or genetic deletion of CR3 but not SRs attenuates P+M-induced superoxide production and p47phox membrane translocation, indicating a CR3-dependent activation of NOX2. Our results show that P+M induce activation of CR3 downstream Src and Erk, which can phosphorylate NOX2 cytosolic subunits including p47phox, resulting in their membrane translocation and subsequent NOX2 activation. Blocking CR3 significantly reduces NOX2-related microglial activation and dopaminergic neurodegeneration.
Nox2 is a membrane-bound, multi-subunit enzyme complex that transfers electrons across the plasma membrane from NADPH to molecular oxygen and generate superoxide [34]. Although Nox2 is critical for the integrity and the normal function of the central nervous system (CNS), both postmortem observations and preclinical experiments have implicated an important role of over-activated Nox2 in neurodegenerative diseases, such as PD [16], amyotrophic lateral sclerosis [38] and multiple sclerosis [39]. Nox2 activation is an early event during microglial activation and has been implicated as a major contributor to neuroinflammation-mediated oxidative stress and chronic neurodegeneration [40]. Additionally, Nox2-derived superoxide can also be served as a paracrine signal to control chronic microglial activation and cytotoxic factors production through various inflammatory signaling pathways, such as mitogen-activated protein kinase, nuclear factor-kappa B and protein kinase C [3], [8]. Inhibition of Nox2 gradually becomes a promising therapeutic strategy for neurodegenerative disorders [9], [41]. Since current inhibitors of Nox2 lack clinical use due to non-specificity and displays toxicity at regular used dose [42], elucidating the mechanisms behind Nox2 activation are particular attracting. A number of SRs, such as SR-A, SR-BI and CD36 has been identified in the cell surface of microglia [43], [44]. In AD, SR-A is upregulated and mediates adhesion and endocytosis of Aβ as well as secretion of ROS by microglia [44], [45]. The class B SR, CD36 mediates Nox2-derived free radical and tissue injury in cerebral ischemia [46], [47]. In contrast, in this study, we found that SRs were not required for the production of superoxide by Nox2 in response to P+M. Consistent with our findings, Levesque and colleague demonstrated that diesel exhaust particles (DEPs)-induced Nox2 activation and dopaminergic neurodegeneration in primary cultures are not affected by SRs inhibitor fucoidan [28]. Similarly, SRs-independent activation of Nox2 was also observed in Pei et al.'s study by using LPS as a stimulator [48]. Notably, since SRs fulfill multiple functions in microglia, we could not exclude the possibility that SRs might be involved in the toxic effects of P+M, although they appeared to be non-essential for P+M-induced Nox2 activation. SRs have recently been identified as phagocytic receptors [49]. In previous studies, SRs have been implicated in the internalization of both DEPs and LPS in microglia, although they are not involved in DEPs and LPS-induced Nox2 activation [28], [48]. Due to the technic limitation, whether SRs are involved in phagocytosis of paraquat and/or maneb by microglia was not investigated in the current study.
CR3 is abundantly expressed by phagocytes, including microglia and has been shown to mediate immune cell responses, such as adhesion, migration, phagocytosis, chemotaxis and cytotoxicity [50]. CR3 is both an adhesion molecular and a microglia-specific PRR. This receptor recognizes a wide variety of structurally unrelated molecules, such as complement C3 fragment (iC3b), intercellular adhesion molecule-1 (ICAM-1), fibrinogen, LPS, HMGB1 and double-stranded RNA to orchestrate the inflammatory response [27], [48], [51]. Gao et al. reported that HMGB1, a non-histone DNA binding protein, induced Nox2 activation in microglia through a CR3-dependent manner [27]. CR3 was also Nox2-dependently triggered long-term synaptic depression in the hippocampus that might contribute to memory impairments and synaptic disruptions in neuroinflammation-related brain disorders [52]. However, the role of CR3 in Nox2 activation in PD remained controversial. Hu et al. reported that CR3 deficiency attenuates Nox2-derived superoxide production induced by 1-methyl-4-phenyl-pyridium iodide (MPP+), a toxin often used to create PD model [53]. By contrast, in Kinugawa et al.'s study, MPP+-stimulated superoxide is not altered in CR3-/- cultures [54]. Here, we provided direct experimental evidence implicating the involvement of CR3 in Nox2 activation and dopaminergic neurodegeneration in P+M-induced PD models.
Mechanistically, the most critical question to address is how CR3 regulates Nox2 activation. The activation of Src family kinases (SFKs) is one of the earliest biochemical events in CR3 signaling response [55]. Src is one of the mostly studied SFKs and is highly expressed in microglia. It has been reported that Src is involved in regulating Nox2-derived ROS production. In the HT29 human colon carcinoma cell line, Src induced Nox2-mediated ROS production by activating the regulatory subunit small GTPase Rac1 [56]. Down-regulation of Src by siRNA reduced activation of Rac1 and therefore Nox2-generated ROS [56]. In human adherent blood eosinophils, CR3-mediated Nox2 activation was inhibited by inhibitor of the Src [57]. Consistent with these findings, our results showed that inhibition of Src also impeded P+M-induced Nox2 activation. Although the exact mechanisms of how Src regulates Nox2 remain unclear, Src is known to be able to phosphorylate Erk [58], a kinase that can induce phosphorylation of p47phox at Ser345 and subsequently result in its translocation to the cell membrane, leading to Nox2 activation [36]. In this study, P+M treatment induced activation of Erk. Moreover, inhibition of Erk abrogated Nox2 activation induced by P+M. Together, our findings lend strong credence to the conclusion that Src and Erk, two downstream signals of CR3, could be critical for the regulatory effects of CR3 on Nox2 activation.
In conclusion, our studies reveal a new regulatory function of CR3 in Nox2 activation and dopaminergic neurodegeneration by using paraquat and maneb-induced PD model. We also identified Src and Erk signals as potential novel signaling pathways that bridges CR3 and Nox2 activation. These results help to expand our understanding for the immune pathogenesis of PD. Our findings also suggest that CR3, especially its subunit CD11b, may be a promising target for the treatment of patients suffering from PD and related disorders.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81703264); “QiZhen” talent project of Dalian Medical University (No. 201122) and Liaoning BaiQianWan Talents Program (No. [2017]90).
Contributor Information
Huihua Li, Email: hhli1935@aliyun.com.
Qingshan Wang, Email: wangq4@126.com.
References
- 1.Achey M., Aldred J.L., Aljehani N., Bloem B.R., Biglan K.M., Chan P., Cubo E., Dorsey E.R., Goetz C.G., Guttman M., Hassan A., Khandhar S.M., Mari Z., Spindler M., Tanner C.M., van den Haak P., Walker R., Wilkinson J.R. The past, present, and future of telemedicine for Parkinson's disease. Mov. Disord. 2014;29(7):871–883. doi: 10.1002/mds.25903. [DOI] [PubMed] [Google Scholar]
- 2.Olanow C.W., Tatton W.G. Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 3.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 4.Phani S., Loike J.D., Przedborski S. Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism Relat. Disord. 2012;18(Suppl. 1):S207–S209. doi: 10.1016/S1353-8020(11)70064-5. [DOI] [PubMed] [Google Scholar]
- 5.McGeer P.L., Schwab C., Parent A., Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann. Neurol. 2003;54(5):599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Qin L., Wilson B., Wu X., Qian L., Granholm A.C., Crews F.T., Hong J.S. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29(5):864–870. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S., Knapp D.J., Crews F.T., Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q., Chu C.H., Qian L., Chen S.H., Wilson B., Oyarzabal E., Jiang L., Ali S., Robinson B., Kim H.C., Hong J.S. Substance P exacerbates dopaminergic neurodegeneration through neurokinin-1 receptor-independent activation of microglial NADPH oxidase. J. Neurosci. 2014;34(37):12490–12503. doi: 10.1523/JNEUROSCI.2238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q., Qian L., Chen S.H., Chu C.H., Wilson B., Oyarzabal E., Ali S., Robinson B., Rao D., Hong J.S. Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson's disease models. Brain. 2015;138(Pt 5):1247–1262. doi: 10.1093/brain/awv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niehaus I., Lange J.H. Endotoxin: is it an environmental factor in the cause of Parkinson's disease? Occup. Environ. Med. 2003;60(5):378. doi: 10.1136/oem.60.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma M.W., Wang J., Zhang Q., Wang R., Dhandapani K.M., Vadlamudi R.K., Brann D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017;12(1):7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou L., Zhang C., Wang K., Liu X., Wang H., Che Y., Sun F., Zhou X., Zhao X., Wang Q. Paraquat and maneb co-exposure induces noradrenergic locus coeruleus neurodegeneration through NADPH oxidase-mediated microglial activation. Toxicology. 2017;380:1–10. doi: 10.1016/j.tox.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L., Chen S.H., Chu C.H., Wang S.J., Oyarzabal E., Wilson B., Sanders V., Xie K., Wang Q., Hong J.S. A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia. 2015;63(6):1057–1072. doi: 10.1002/glia.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Chu C.H., Oyarzabal E., Jiang L., Chen S.H., Wilson B., Qian L., Hong J.S. Subpicomolar diphenyleneiodonium inhibits microglial NADPH oxidase with high specificity and shows great potential as a therapeutic agent for neurodegenerative diseases. Glia. 2014;62(12):2034–2043. doi: 10.1002/glia.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou L., Zhou X., Zhang C., Wang K., Liu X., Che Y., Sun F., Li H., Wang Q., Zhang D., Hong J.S. NADPH oxidase-derived H2O2 mediates the regulatory effects of microglia on astrogliosis in experimental models of Parkinson's disease. Redox Biol. 2017;12:162–170. doi: 10.1016/j.redox.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D.C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc. Natl. Acad. Sci. USA. 2003;100(10):6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen L.M., Chamberlain G., Sacre S. Pattern recognition receptors as potential therapeutic targets in inflammatory rheumatic disease. Arthritis Res. Ther. 2015;17:122. doi: 10.1186/s13075-015-0645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kigerl K.A., de Rivero Vaccari J.P., Dietrich W.D., Popovich P.G., Keane R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canton J., Neculai D., Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13(9):621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers M.R. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2(3):289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 21.Magwenzi S., Woodward C., Wraith K.S., Aburima A., Raslan Z., Jones H., McNeil C., Wheatcroft S., Yuldasheva N., Febbriao M., Kearney M., Naseem K.M. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood. 2015;125(17):2693–2703. doi: 10.1182/blood-2014-05-574491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Febbraio M., Reddy S.P., Yu D.Y., Yamamoto M., Silverstein R.L. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J. Clin. Investig. 2010;120(11):3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald D.R., Brunden K.R., Landreth G.E. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J. Neurosci. 1997;17(7):2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coraci I.S., Husemann J., Berman J.W., Hulette C., Dufour J.H., Campanella G.K., Luster A.D., Silverstein S.C., El-Khoury J.B. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 2002;160(1):101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkinson K., El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer's disease. Int. J. Alzheimers Dis. 2012;2012:489456. doi: 10.1155/2012/489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin L., Liu Y., Hong J.S., Crews F.T. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61(6):855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao H.M., Zhou H., Zhang F., Wilson B.C., Kam W., Hong J.S. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J. Neurosci. 2011;31(3):1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levesque S., Taetzsch T., Lull M.E., Johnson J.A., McGraw C., Block M.L. The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. J. Neurochem. 2013;125(5):756–765. doi: 10.1111/jnc.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L., Wu X., Wang S., Chen S.H., Zhou H., Wilson B., Jin C.Y., Lu R.B., Xie K., Wang Q., Hong J.S. Clozapine metabolites protect dopaminergic neurons through inhibition of microglial NADPH oxidase. J. Neuroinflamm. 2016;13(1):110. doi: 10.1186/s12974-016-0573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S.H., Oyarzabal E.A., Hong J.S. Preparation of rodent primary cultures for neuron-glia, mixed glia, enriched microglia, and reconstituted cultures with microglia. Methods Mol. Biol. 2013;1041:231–240. doi: 10.1007/978-1-62703-520-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evangelista-Vargas S., Santiani A. Detection of intracellular reactive oxygen species (superoxide anion and hydrogen peroxide) and lipid peroxidation during cryopreservation of alpaca spermatozoa. Reprod. Domest. Anim. 2017 doi: 10.1111/rda.12984. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W., Wang T., Qin L., Gao H.M., Wilson B., Ali S.F., Hong J.S., Liu B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase. FASEB J. 2004;18(3):589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A., Leinisch F., Kadiiska M.B., Corbett J., Mason R.P. Formation and implications of alpha-synuclein radical in maneb- and paraquat-induced models of Parkinson's disease. Mol. Neurobiol. 2016;53(5):2983–2994. doi: 10.1007/s12035-015-9179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4(3):181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 35.Caputi F.F., Carretta D., Lattanzio F., Palmisano M., Candeletti S., Romualdi P. Proteasome subunit and opioid receptor gene expression down-regulation induced by paraquat and maneb in human neuroblastoma SH-SY5Y cells. Environ. Toxicol. Pharmacol. 2015;40(3):895–900. doi: 10.1016/j.etap.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Qian L., Wei S.J., Zhang D., Hu X., Xu Z., Wilson B., El-Benna J., Hong J.S., Flood P.M. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J. Immunol. 2008;181(1):660–668. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiruchelvam M., Richfield E.K., Baggs R.B., Tank A.W., Cory-Slechta D.A. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J. Neurosci. 2000;20(24):9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D.C., Re D.B., Nagai M., Ischiropoulos H., Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc. Natl. Acad. Sci. USA. 2006;103(32):12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer M.T., Sharma R., Lim J.L., Haider L., Frischer J.M., Drexhage J., Mahad D., Bradl M., van Horssen J., Lassmann H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135(Pt 3):886–899. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu B., Gao H.M., Hong J.S. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ. Health Perspect. 2003;111(8):1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao H.M., Zhou H., Hong J.S. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci. 2012;33(6):295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aldieri E., Riganti C., Polimeni M., Gazzano E., Lussiana C., Campia I., Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr. Drug Metab. 2008;9(8):686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 43.Alarcon R., Fuenzalida C., Santibanez M., von Bernhardi R. Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J. Biol. Chem. 2005;280(34):30406–30415. doi: 10.1074/jbc.M414686200. [DOI] [PubMed] [Google Scholar]
- 44.Husemann J., Loike J.D., Anankov R., Febbraio M., Silverstein S.C. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 45.El Khoury J., Hickman S.E., Thomas C.A., Loike J.D., Silverstein S.C. Microglia, scavenger receptors, and the pathogenesis of Alzheimer's disease. Neurobiol. Aging. 1998;19(Suppl. 1):S81–S84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 46.Cho S., Park E.M., Febbraio M., Anrather J., Park L., Racchumi G., Silverstein R.L., Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J. Neurosci. 2005;25(10):2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genovese T., Mazzon E., Paterniti I., Esposito E., Bramanti P., Cuzzocrea S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011;1372:92–102. doi: 10.1016/j.brainres.2010.11.088. [DOI] [PubMed] [Google Scholar]
- 48.Pei Z., Pang H., Qian L., Yang S., Wang T., Zhang W., Wu X., Dallas S., Wilson B., Reece J.M., Miller D.S., Hong J.S., Block M.L. MAC1 mediates LPS-induced production of superoxide by microglia: the role of pattern recognition receptors in dopaminergic neurotoxicity. Glia. 2007;55(13):1362–1373. doi: 10.1002/glia.20545. [DOI] [PubMed] [Google Scholar]
- 49.Penberthy K.K., Ravichandran K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016;269(1):44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hajishengallis G., Wang M., Liang S., Shakhatreh M.A., James D., Nishiyama S., Yoshimura F., Demuth D.R. Subversion of innate immunity by periodontopathic bacteria via exploitation of complement receptor-3. Adv. Exp. Med. Biol. 2008;632:203–219. doi: 10.1007/978-0-387-78952-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou H., Liao J., Aloor J., Nie H., Wilson B.C., Fessler M.B., Gao H.M., Hong J.S. CD11b/CD18 (Mac-1) is a novel surface receptor for extracellular double-stranded RNA to mediate cellular inflammatory responses. J. Immunol. 2013;190(1):115–125. doi: 10.4049/jimmunol.1202136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J., Malik A., Choi H.B., Ko R.W., Dissing-Olesen L., MacVicar B.A. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82(1):195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 53.Hu X., Zhang D., Pang H., Caudle W.M., Li Y., Gao H., Liu Y., Qian L., Wilson B., Di Monte D.A., Ali S.F., Zhang J., Block M.L., Hong J.S. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. J. Immunol. 2008;181(10):7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinugawa K., Monnet Y., Bechade C., Alvarez-Fischer D., Hirsch E.C., Bessis A., Hunot S. DAP12 and CD11b contribute to the microglial-induced death of dopaminergic neurons in vitro but not in vivo in the MPTP mouse model of Parkinson's disease. J. Neuroinflamm. 2013;10:82. doi: 10.1186/1742-2094-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abram C.L., Lowell C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannoni E., Taddei M.L., Chiarugi P. Src redox regulation: again in the front line. Free Radic. Biol. Med. 2010;49(4):516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Lynch O.T., Giembycz M.A., Barnes P.J., Hellewell P.G., Lindsay M.A. 'Outside-in' signalling mechanisms underlying CD11b/CD18-mediated NADPH oxidase activation in human adherent blood eosinophils. Br. J. Pharmacol. 1999;128(6):1149–1158. doi: 10.1038/sj.bjp.0702892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowell C.A. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb. Perspect. Biol. 2011;3(3) doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]