Abstract
Monocytes/macrophages are thought to be recruited to the renal interstitium during calcium oxalate (CaOx) kidney stone disease for crystal clearance. Mitochondria play an important role in monocyte function during the immune response. We recently determined that monocytes in patients with CaOx kidney stones have decreased mitochondrial function compared to healthy subjects. The objective of this study was to determine whether oxalate, a major constituent found in CaOx kidney stones, alters cell viability, mitochondrial function, and redox homeostasis in THP-1 cells, a human derived monocyte cell line. THP-1 cells were treated with varying concentrations of CaOx crystals (insoluble form) or sodium oxalate (NaOx; soluble form) for 24 h. In addition, the effect of calcium phosphate (CaP) and cystine crystals was tested. CaOx crystals decreased cell viability and induced mitochondrial dysfunction and redox imbalance in THP-1 cells compared to control cells. However, NaOx only caused mitochondrial damage and redox imbalance in THP-1 cells. In contrast, both CaP and cystine crystals did not affect THP-1 cells. Separate experiments showed that elevated oxalate also induced mitochondrial dysfunction in primary monocytes from healthy subjects. These findings suggest that oxalate may play an important role in monocyte mitochondrial dysfunction in CaOx kidney stone disease.
Abbreviations: ATP, adenosine triphosphate; CaOx, calcium oxalate; CaP, calcium phosphate; ECAR, extracellular acidification rate; GSH, glutathione; GSSG, glutathione disulfide; MnSOD, manganese superoxide dismutase; NaOx, sodium oxalate; OCR, oxygen consumption rate
Keywords: Mitochondria, Monocytes, Calcium oxalate, Sodium oxalate, Kidney stones, MnSOD
Graphical abstract
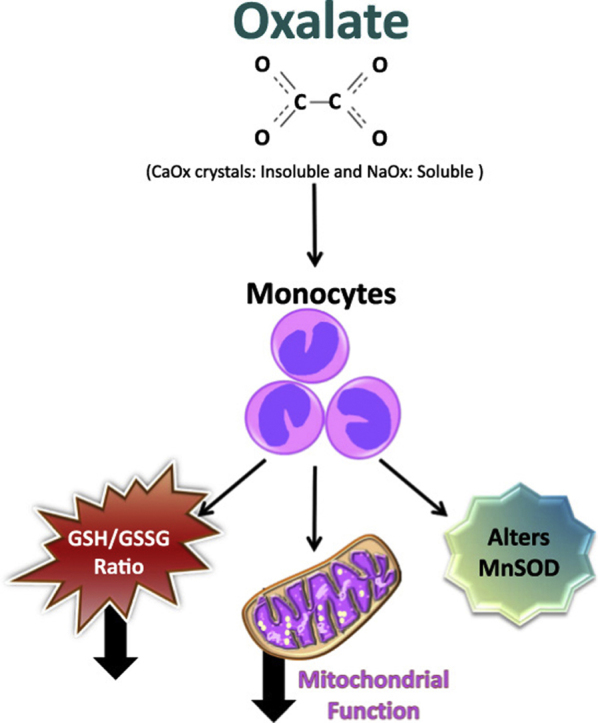
Highlights
-
•
Oxalate is a major constituent of calcium oxalate (CaOx) kidney stones and can be found in either soluble or insoluble forms.
-
•
CaOx crystals are required for CaOx kidney stone formation.
-
•
Monocytes/macrophages play an important role in crystal clearance.
-
•
Oxalate causes mitochondrial dysfunction and disrupts redox homeostasis in monocytes.
1. Introduction
Kidney stones are one of the most common urological conditions that affects approximately 9% of the population in the United States [1]. The recurrence rate within the first 5 years of having a stone event is between 35% and 50% [2]. Unfortunately, the etiology of stone formation is a complex process, which is not well defined. Thus, there is a crucial need to dissect mechanisms that contribute to stone formation with an aim to identify potential therapeutic targets for intervention. Several lines of evidence have identified lifestyle factors [1], [3] and genetics [4] as contributors to stone formation. Kidney stones form by a process of crystallization, growth, and accumulation in the renal epithelium involving mineral and organic substances such as calcium, magnesium, phosphate, and oxalate [5], [6].
The most common type of kidney stone is comprised of calcium oxalate (CaOx). Oxalate is derived from dietary sources (e.g. plant and plant-derived foods) and can be synthesized by the body and is excreted in the urine [3], [7]. Oxalate also exists in soluble and insoluble forms. CaOx crystals form when the urine becomes supersaturated with calcium and oxalate. Several studies have reported that crystals and oxalate stimulate inflammatory responses, including monocyte chemoattractant protein-1 (MCP-1) release in renal epithelial cells [8], [9], [10], [11], [12]. MCP-1 plays an important role in monocyte/macrophage recruitment and activation, and has been shown to be elevated in the urine and renal tissue of patients with kidney stones [13], [14].
Monocytes are derived from myeloid progenitor cells and are key players in the innate immune system. They are important for fighting infections and responding to inflammation. Their ability to carry out their physiological functions depends on oxidative phosphorylation/mitochondrial function [15]. Mitochondria are critical for regulating intracellular signaling via formation of reactive oxygen species (ROS) [16]. However, excessive levels of ROS can damage the cell, disrupt mitochondrial function and stimulate a cascade of events leading to further ROS generation and inflammation. Crystals and oxalate have been reported to generate ROS in renal cells [17]. In addition, it has been reported that human macrophages exposed to CaOx crystals release inflammatory cytokines and chemokines [18]. Thus, monocytes recruited to sites of inflammation and injury within the kidney may have compromised mitochondria due to the pro-inflammatory and pro-oxidative environment.
We have previously determined that patients with CaOx kidney stones have decreased mitochondrial function in their circulating monocytes compared to healthy subjects [19]. A potential candidate responsible for suppressing mitochondrial function in monocytes is oxalate. We have previously shown that healthy subjects that consume a high dietary oxalate load have elevated urinary oxalate [7]. It is possible that high oxalate levels may stimulate crystal formation and elicit an immune response. How oxalate affects monocytes that respond to these signals has not been elucidated and warrants further investigation. Identifying and defining such processes may provide insights into mechanism of kidney stone formation. Here, we investigated whether elevated oxalate (soluble and insoluble forms) alters cell viability, mitochondrial function, and redox imbalance in monocytes. In addition, we assessed whether other types of kidney stone precursors (i.e. calcium phosphate (CaP) and cystine crystals) would negatively affect mitochondrial function and redox imbalance in monocytes. The results from this study suggest that oxalate may impact monocyte mitochondrial function during CaOx kidney stone disease.
2. Materials and methods
2.1. Reagents
The following reagents were purchased from Sigma-Aldrich (St. Louis, MO): Calcium oxalate (CaOx), calcium phosphate (CaP), cystine, sodium oxalate (NaOx), oligomycin, FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone), antimycin A, Triton X-100, Trypan Blue solution, triethanolamine (TEA), 5-sulfosalicylic acid (5-SA), 2-metlyl-5-vinylpyridine (MVP), DTNB (Ellman's Reagent) and diethylenetriamine pentaacetate (DTPA). All other reagents or kits used are noted elsewhere.
2.1.1. Cell culture and viability assessment
THP-1 cells (TIB202), a human monocyte derived cell line, were obtained from the American Type Culture Collection (Manassas, VA). THP-1 cells were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum and 2-mercaptoethanol (0.05 mM) in T-75 flasks. For all experiments, cultured THP-1 cells were treated with CaOx, Cystine or CaP crystals (50, 100, 200, 500, 1000 µg/ml) or NaOx (0.1, 0.5, 1, 1.5, and 2 mM) and incubated at 37 °C in 5% CO2 for 24 h. Following treatment, cell viability was determined by the Trypan Blue exclusion assay. In brief, cells were treated with 0.4% Trypan Blue (1:1 dilution) and counted using the Countess Automated Cell Counter (Thermo Fisher Scientific Inc., Waltham, MA).
2.1.2. Primary human monocytes
Written informed consent was obtained from all study participants following UAB Institutional Review Board approval. Blood samples were collected from healthy subjects (n = 10; 32.0 ± 3.3 years of age) to isolate monocytes as previously described [20]. In brief, blood was separated on Ficoll-density gradients and the mononuclear cell fraction was collected. Monocytes were isolated from the mononuclear cell layer using CD14+ magnetic antibodies and magnetic bead separation (Miltenyi Biotec Inc., San Diego, CA). Cells were counted using the Bio-Rad TC20 Automated Cell Counter (Bio-Rad, Hercules, CA). Monocytes were subsequently exposed to either CaOx crystals (50 µg/ml) or NaOx (0.1 mM) for 40 min prior to analysis.
2.1.3. Cellular bioenergetics analysis
Mitochondrial function was assessed using the Seahorse XF96e Analyzer (Agilent Technologies, Santa Clara, CA). Following treatment, 150,000 cells per well were seeded on Cell-Tak coated Seahorse plates. Cells were equilibrated in XF media prior to measuring the mitochondrial oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). The mitochondrial stress test was implemented as previously described [20]. Oligomycin (0.5 µg/ml), FCCP (0.6 µM), and antimycin A (10 µM) injections defined the following parameters: basal OCR, ATP-linked OCR, proton leak OCR, maximal OCR, reserve capacity, and non-mitochondrial OCR [21]. In addition, the oligo-sensitive ECAR was determined.
2.1.4. Determination of GSH and GSSG levels
Reduced glutathione (GSH), oxidized glutathione (GSSG), and total glutathione levels were determined in THP-1 cells following treatment. Glutathione was determined based on modifications of the Tietze recycling assay [22]. In brief, cells were lysed in lysis buffer containing 0.1% Triton X-100 in PBS buffer, pH 7.4, containing 10 µM DTPA. Cell lysates were treated with triethanolamine (TEA), 2-methyl-5-vinylpyridine (MVP), and 5% 5-sulfosalicylic acid (5-SA) to measure GSSG or 5-SA alone to measure GSH based on adapted methods from Anderson and Neufer [23], [24]. Glutathione was determined based on the reduction of DTNB (Ellman's Reagent) at 412 nm in the Diabetes Research Center BioAnalytical Redox Biology Core (DK 079626) using a Synergy-2 Multimode plate reader (Biotek, Winooski, VT). Samples were normalized to cellular protein.
2.1.5. Western blotting
Following treatment, THP-1 cells were lysed in 25 mM HEPES buffer containing 0.1% Triton X-100 with protease and phosphate inhibitors. Protein concentrations were quantified using the Bradford protein assay (Thermo Fisher Scientific). Protein extracts (15 µg) were separated on 12% polyacrylamide precast gels (Bio-Rad) at 180 V for 45 min before being transferred to PVDF membrane using Trans-Blot Turbo (Bio-Rad) 24 V for 7 min. Membranes were blocked with 5% milk solution in 1X Tris buffered saline with 0.001% Tween 20 (1XTBST) for 1 h at room temperature and probed for manganese superoxide dismutase (MnSOD) antibody (Millipore, Billerica, MA) at 1:1000 dilution overnight at 4 °C. The next day, membranes were washed 3 times for 10 min each with 1XTBST. Membranes were then incubated with anti-rabbit secondary horse radish peroxidase-conjugated antibody (Abcam, Cambridge, UK) at 1:10,000 dilution for 1 h at room temperature. The membranes were washed again with 1XTBST and incubated with Luminata Forte Chemiluminescence (Millipore) for the detection of horseradish peroxidase activity. The bands were detected and analyzed using ImageQuant LAS 4000 imager and software (GE Healthcare Life Sciences, Marlborough, MA). Membranes were prepared for re-blotting by using ReBlot Plus Strong Solution (Millipore) for 15 min at room temperature and then blocked with 5% milk in 1XTBST for 1 h at room temperature. After washing with 1XTBST, membranes were subsequently incubated with GAPDH antibody (1:5000) overnight at 4 °C. The membranes were washed with 1XTBST and incubated with HRP anti-rabbit secondary antibody (Abcam) (1:10,000) for 1 h at room temperature prior to imaging as detailed above. Densitometry analysis was performed using the Image-J software package. Protein levels were normalized using GAPDH as a loading control.
2.1.6. Quantitative real time (qRT-PCR) analysis
To assess gene expression, total RNA from cells was isolated using Maxwell 16 LEV simplyRNA Cells kit (Promega; Madison, WI). Genomic DNA contamination was removed and cDNA was synthesized from half of a microgram of RNA using QuantiTect Reverse Transcription Kit (QIAGEN; Hilden, Germany). PowerUP SYBR Green Master Mix (ThermoFisher Scientific, Grand Island, NY) was used for quantitative RT-PCR along with primers for human MnSOD and GAPDH. Reactions were performed in duplicates and specificity was monitored using melting curve analysis after cycling. Primers were purchased from Eurofins MWG Operon (Ebersberg, Germany) and used to detect the specific genes listed: Human GAPDH forward 5′-CTCCTGTTCGACAGTCAGCC-3′ reverse 5′-TGGAATTTGCCATGGGTGGA’-3 and Human MnSOD forward 5′-GTTGGGGTTGGCTTGGTTTC-’3 and reverse 5′-CATAAAGAGCTTAACATACTCAGCA-3′. GAPDH was used as an internal control and the ∆∆ Ct Method [25], [26] was used to quantify relative mRNA expression. Results were expressed as fold change over untreated controls.
2.1.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism (La Jolla, CA, USA). All data reported as mean ± SEM and n = 3 or more determinations. Data were analyzed by one-way analysis of variance (ANOVA) or paired t-tests. A p < 0.05 was considered statistically significant.
3. Results
3.1. Oxalate decreases cell viability in THP-1 cells
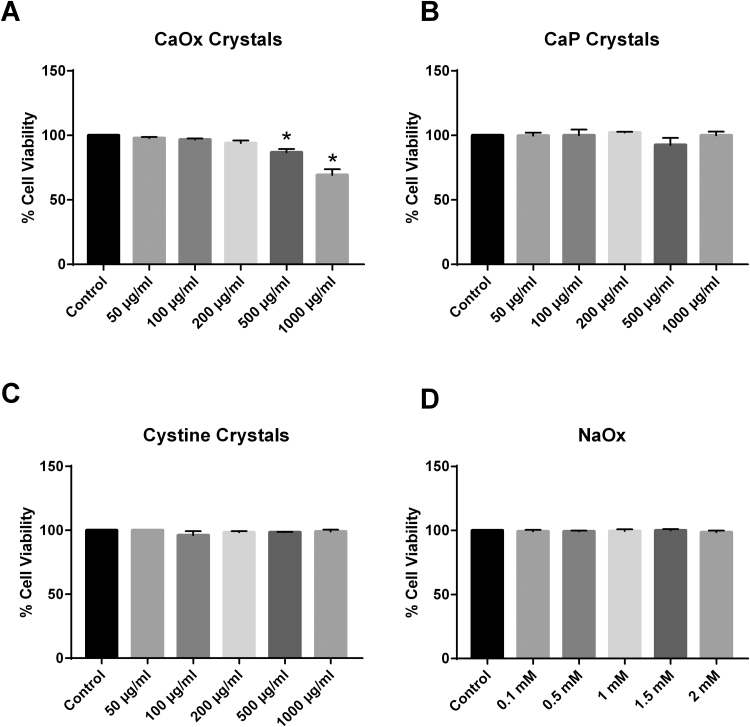
The Trypan Blue exclusion assay was utilized to assess cell viability in THP-1 cells treated with increasing concentrations of either CaOx, CaP, or cystine crystals or NaOx for 24 h. As shown in Fig. 1A, lower concentrations of CaOx crystals (insoluble oxalate) did not change cell viability. However, there was a significant decrease in cell viability at higher doses. In particular, 500 μg/ml of CaOx crystals significantly decreased cell viability to 85%. This was further exacerbated to 68% viability when cells were treated with 1000 μg/ml of CaOx crystals. However, treatment with CaP, cystine crystals or NaOx (soluble oxalate) did not alter THP-1 cell viability at any of the concentrations tested (Fig. 1B–D).
Fig. 1.
The effect of (A) calcium oxalate (CaOx) crystals, (B) calcium phosphate (CaP) crystals, (C) cystine crystals, and (D) sodium oxalate (NaOx) on THP-1 cell viability. Results are means ± SEM; n = 3–5 individual experiments. *p < 0.05 compared to untreated monocytes.
3.2. Oxalate alters mitochondrial function in THP-1 cells
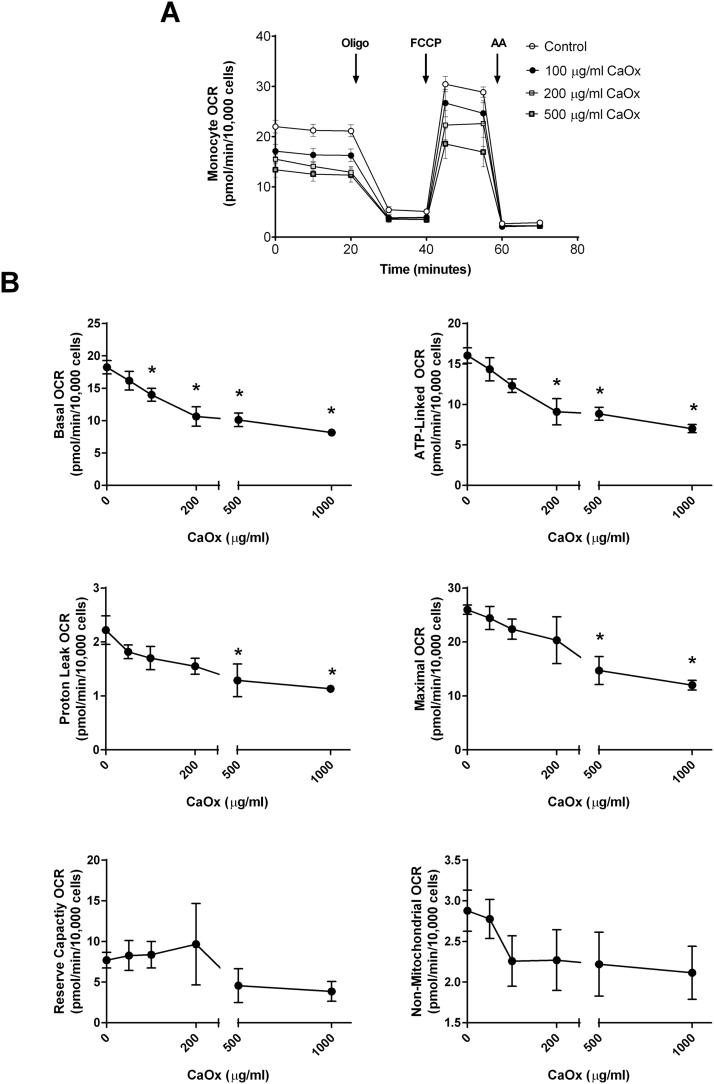
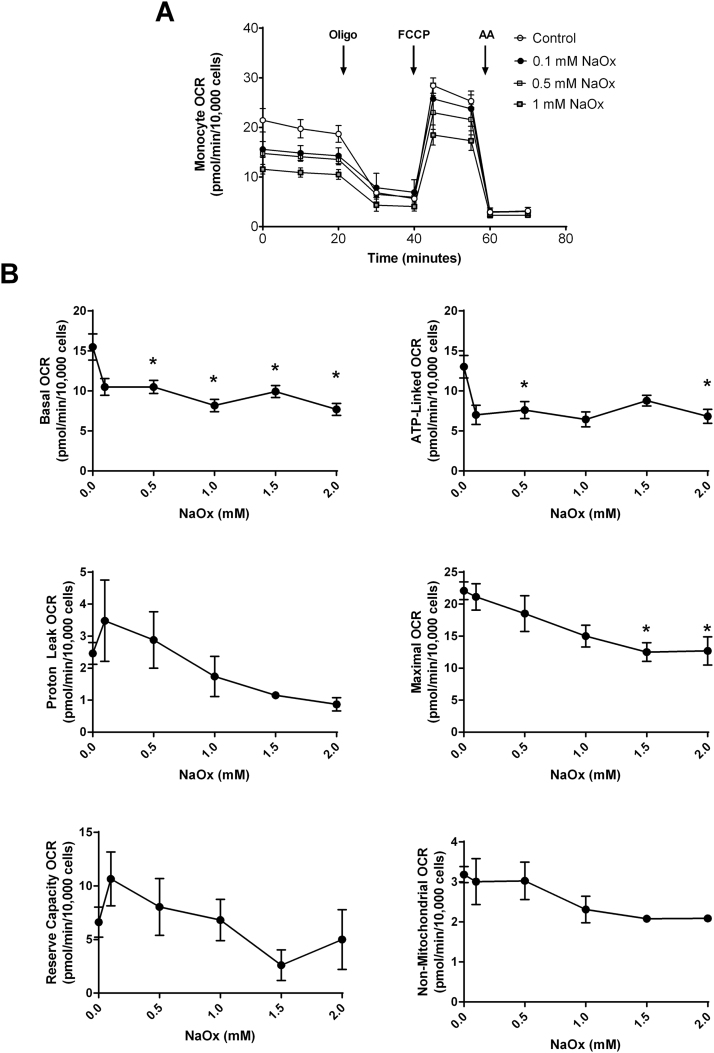
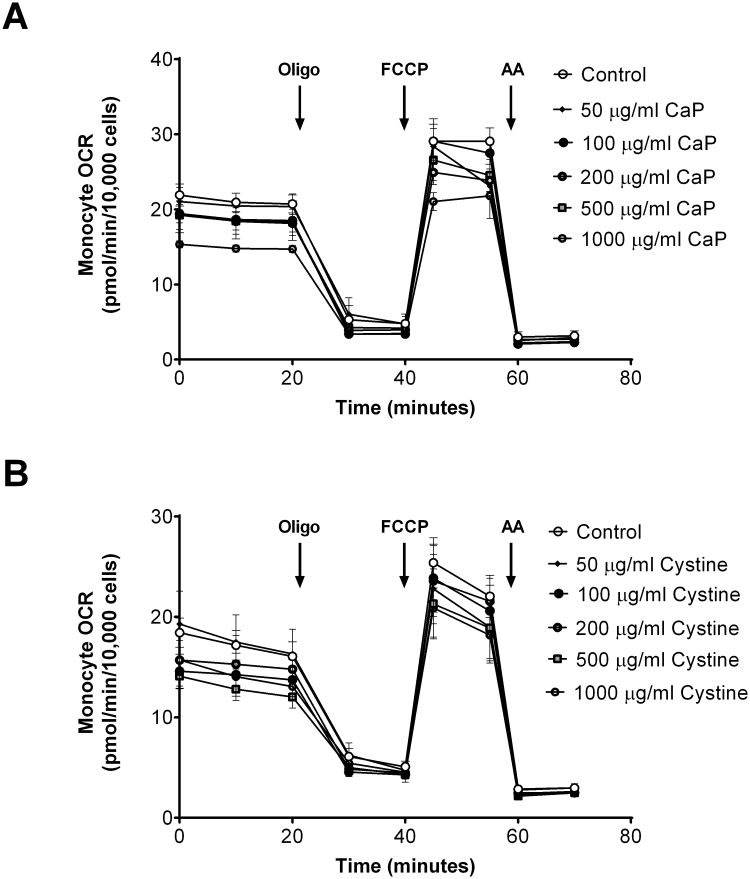
To determine whether oxalate negatively impacts monocyte mitochondrial function, THP-1 cells were treated with various concentrations of CaOx, CaP, and cystine crystals or NaOx for 24 h prior to assessing mitochondrial function and glycolysis. As shown in Fig. 2A, treating THP-1 cells with increasing concentrations of CaOx crystals decreased mitochondrial function in a dose-dependent fashion compared to THP-1 cells not exposed to crystals. In particular, basal OCR significantly declined in cells treated with 100 μg/ml or higher concentrations of CaOx crystals (Fig. 2B). ATP-linked OCR showed a progressive decline in a dose dependent manner with CaOx crystal treatment. Both proton leak and maximal OCR decreased when cells were treated with the highest concentrations of CaOx crystals (500 and 1000 μg/ml) (Fig. 2B). However, reserve capacity and non-mitochondrial OCR was not affected. Interestingly, both CaP and cystine crystals did not alter mitochondrial function in THP-1 cells at any of the concentrations examined (Supplementary Fig. 1). However, NaOx caused a dose dependent decrease in mitochondrial function (Fig. 3A). Basal OCR significantly decreased when cells were treated with 0.5 mM or higher concentrations of NaOx (Fig. 3B). ATP-linked OCR was inhibited only at 0.5 and 2 mM concentrations. Maximal OCR was significantly decreased at higher concentrations (1.5 and 2 mM NaOx) (Fig. 3B). The remaining mitochondrial parameters were not affected in NaOx treated cells. The oligo-sensitive ECAR was not different in any of the cells treated with CaOx, CaP, or cystine crystals or NaOx (data not shown).
Fig. 2.
The effect of calcium oxalate (CaOx) crystals on monocyte mitochondrial function. Cells were pretreated with CaOx crystals (0, 50, 100, 200, 500, 1000 µg/ml) for 24 h prior to being seeded on Seahorse XF96 plates. Mitochondrial function was determined using the “Mito Stress Test” inhibitors: oligomycin (Oligo), FCCP, and antimycin A (AA). The effect of CaOx crystals on (A) mitochondrial OCR and (B) individual parameters in THP-1 cells. Results are means ± SEM; n = 5–6 replicates per group; n = 3–5 individual experiments. *p < 0.05 compared to untreated monocytes.
Fig. 3.
The effect of sodium oxalate (NaOx) on monocyte mitochondrial function. Cells were pretreated with NaOx (0, 0.1, 0.5, 1, 1.5, and 2 mM) for 24 h prior to being seeded on Seahorse XF96 plates. Mitochondrial function was determined using the “Mito Stress Test” inhibitors: oligomycin (Oligo), FCCP, and antimycin A (AA). The effect of NaOx on (A) mitochondrial OCR and (B) individual parameters in THP-1 cells. Results are means ± SEM; n = 5–6 replicates per group; n = 3–5 individual experiments. *p < 0.05 compared to untreated monocytes.
3.3. Oxalate alters manganese superoxide dismutase (MnSOD) and Glutathione levels in THP-1 cells
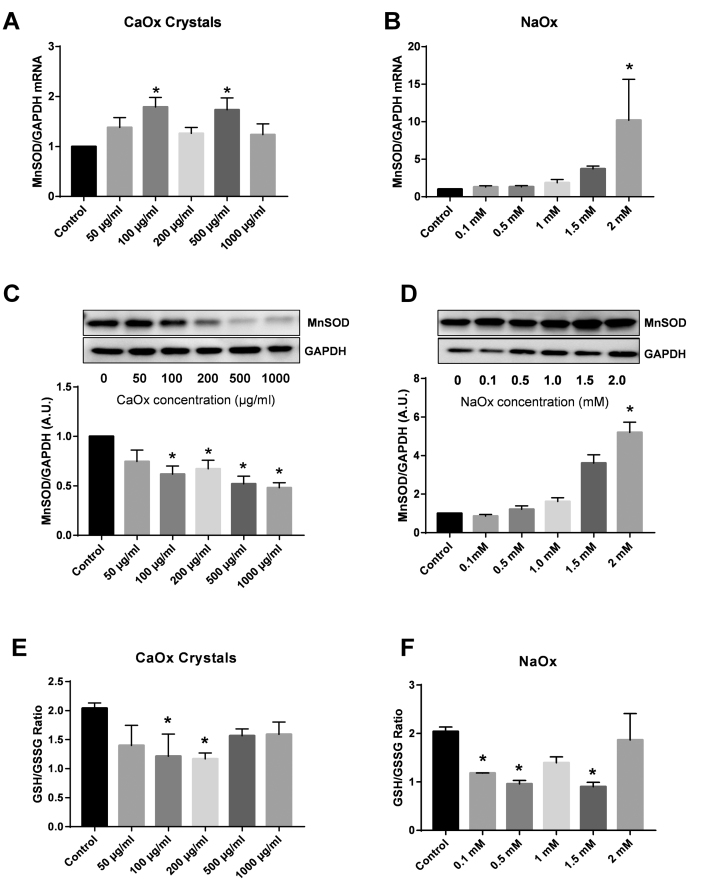
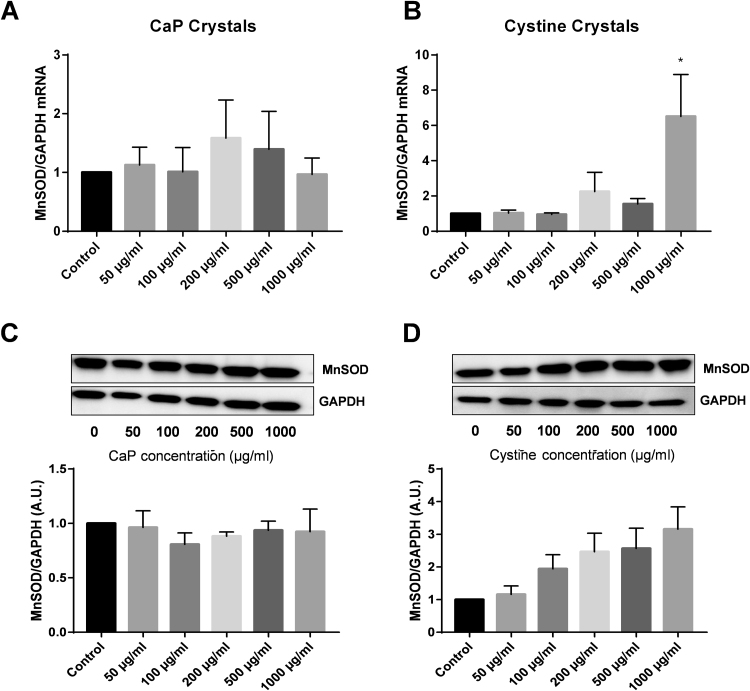
Manganese superoxide dismutase (MnSOD) is a mitochondrial antioxidant that detoxifies superoxide and reduces oxidative stress within the mitochondria. The effect of CaOx crystals and NaOx treatment (24 h) on MnSOD gene expression and protein levels were determined via qRT-PCR and western blotting, respectively (Fig. 4). THP-1 cells exposed to CaOx crystals had increased MnSOD mRNA levels starting at the 100 μg/ml concentration (Fig. 4A). NaOx treatment elicited different responses in THP-1 cells (Fig. 4B). NaOx concentrations between 0.1 mM and 1.5 mM did not alter MnSOD gene expression compared to control cells (Fig. 4B). MnSOD protein levels were significantly decreased in a dose dependent fashion starting at 100 μg/ml in cells treated with CaOx crystals (Fig. 4C). NaOx concentrations between 0.1 mM and 1.5 mM did not alter MnSOD protein levels compared to control cells (Fig. 4D). Only 2 mM NaOx significantly increased both MnSOD gene expression and protein levels in THP-1 cells (Fig. 4B and D). MnSOD did not change significantly following treatment with CaP or cystine crystals (Supplementary Fig. 2). The highest concentration of cystine crystals significantly increased MnSOD mRNA levels but not protein expression (Supplementary Fig. 2).
Fig. 4.
The effect of calcium oxalate (CaOx) crystals and sodium oxalate (NaOx) on MnSOD gene expression and protein levels and glutathione levels in THP-1 cells. (A and B) Real-time PCR was performed to determine MnSOD and GAPDH expression in THP-1 cells treated with CaOx crystals or NaOx. MnSOD was normalized to GAPDH (housekeeping gene). Relative fold change was calculated using the ΔΔCT method relative to control samples. (C and D) Representative western blot of MnSOD (25 kDa) and GAPDH (37 kDa) protein levels in THP-1 cells treated with CaOx crystals or NaOx. (E and F) GSH (reduced glutathione)/GSSG (oxidized glutathione) ratio in THP-1 cells treated with CaOx crystals or NaOx. Results are means ± SEM; n = 3–5 individual experiments. *p < 0.05 compared to untreated monocytes.
Reduced glutathione (GSH) is an antioxidant that maintains redox balance within the cell. Intracellular GSH and oxidized glutathione (GSSG) levels were determined using modifications of the Tiezte assay [22]. As shown in Fig. 4E, low concentrations of CaOx crystals (100 and 200 µg/ml) caused a significant decrease in the GSH/GSSG ratio (a surrogate for redox homeostasis within cells) in THP-1 cells. However, the GSH/GSSG ratio in cells treated with higher concentrations was similar to control cells. Additionally, NaOx concentrations of 0.1, 0.5, and 1.5 mM significantly decreased the GSH/GSSG ratio in THP-1 cells (Fig. 4F).
3.4. Oxalate alters mitochondrial function and glycolysis in primary monocytes
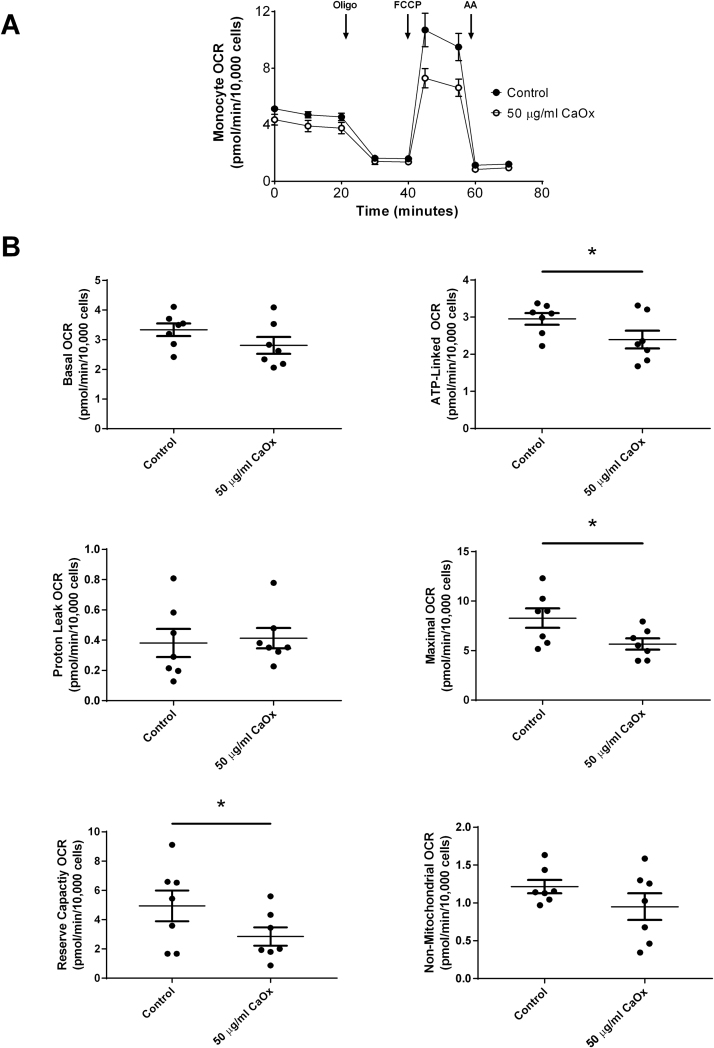
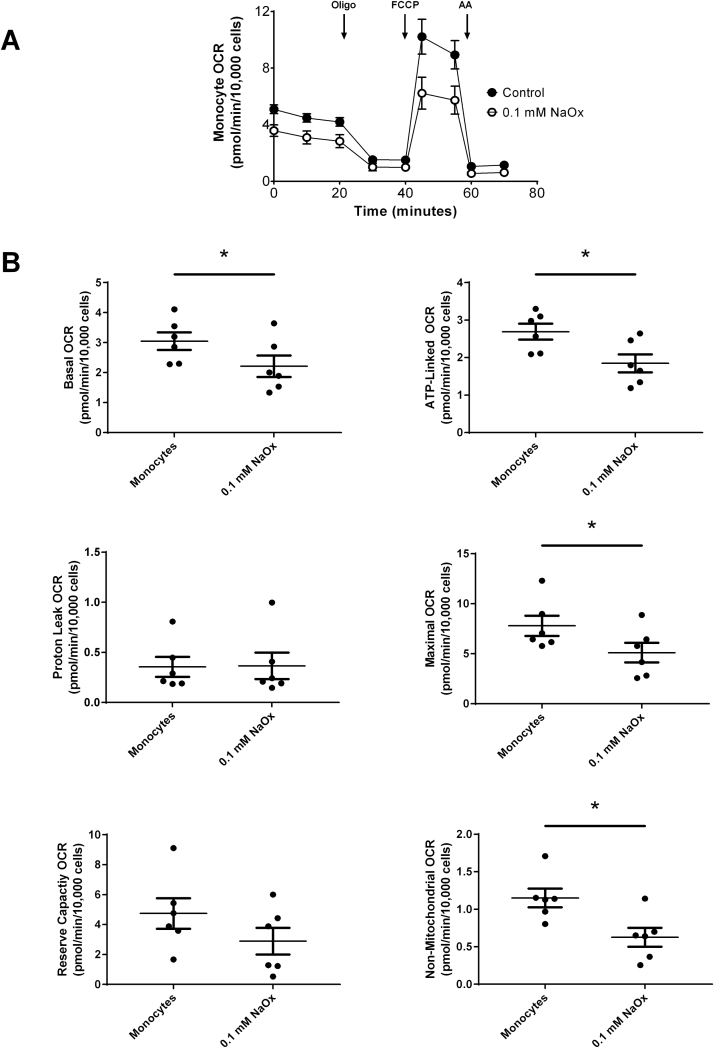
Primary monocytes from healthy subjects were treated with 50 μg/ml CaOx (crystalline oxalate) or 0.1 mM NaOx (soluble oxalate) for 40 min prior to assessing mitochondrial function. As shown in Fig. 5A, CaOx crystals caused a decrease in mitochondrial function in primary monocytes compared to monocytes not treated with CaOx crystals. Specifically, CaOx crystals significantly decreased ATP-linked and maximal OCR, and reserve capacity (Fig. 5B). There was no difference in basal, proton leak, and non-mitochondrial OCR. The oligo-sensitive ECAR was not affected in primary monocytes treated with CaOx crystals (data not shown). Treating primary monocytes with NaOx also affected mitochondrial function as shown in Fig. 6A. However, these responses were different than those observed in CaOx treated monocytes. Basal, ATP-linked, maximal OCR and non-mitochondrial OCR were significantly decreased (Fig. 6B); whereas, proton leak and reserve capacity were not affected. Only 0.1 mM NaOx significantly decreased the oligo-sensitive ECAR in primary monocytes (p = 0.0104) (data not shown).
Fig. 5.
The effect of calcium oxalate (CaOx) crystals on mitochondrial function in primary monocytes. Monocytes were treated with or without CaOx crystals (50 µg/ml) for 40 min prior to assessing mitochondrial function using the “Mito Stress Test” inhibitors: oligomycin (Oligo), FCCP, and antimycin A (AA). The effect of CaOx crystals on (A) mitochondrial OCR and (B) individual parameters in primary monocytes. Results are means ± SEM; n = 5–6 replicates per group; n = 7 healthy subjects.
Fig. 6.
The effect of sodium oxalate (NaOx) on mitochondrial function in primary monocytes. Monocytes were treated with or without NaOx (0.1 mM) for 40 min prior to assessing mitochondrial function using the “Mito Stress Test” inhibitors: oligomycin (Oligo), FCCP, and antimycin A (AA). The effect of NaOx on (A) mitochondrial OCR and (B) individual parameters in primary monocytes. Results are means ± SEM; n = 5–6 replicates per group; n = 6 healthy subjects.
4. Discussion
Mitochondrial function is important in regulating energy production and redox signaling in monocytes [15]. We have previously reported that monocyte mitochondrial function is impaired in patients with CaOx kidney stone disease [19]. The objective of this study was to identify whether oxalate in its soluble or crystalline form could decrease monocyte mitochondrial function. CaOx crystals, the crystalline form of oxalate, form in the nephron when urine becomes supersaturated with calcium and oxalate. A large source of oxalate is from dietary sources and is linked to stone formation [3]. CaOx crystals have been observed in the renal cortex of experimental animals exposed to lithogenic agents and in individuals with Primary Hyperoxaluria [27], [28]. In addition, CaOx crystals stimulate the production of chemokines such as MCP-1 by renal epithelial cells [10], [11]. Macrophages are likely to be involved in crystal clearance and if not properly cleared, could lead to increased inflammation, oxidative stress, and tissue injury. CaOx crystals have been shown to induce innate inflammatory pathways in dendritic cells [29] and to induce changes in mitochondrial proteins involved in metabolism in a human monocytic cell line [30]. Thus, we investigated whether CaOx crystals could affect mitochondrial function and disturb redox balance in monocytes.
We treated THP-1 cells, a human derived monocyte cell line, with various crystals (CaOx, CaP, and cystine) known to be precursors to stone formation. The concentrations used in this study are consistent with others who have studied the physiological relevance of oxalate in cell culture models [8], [30]. We determined that only CaOx crystals (insoluble oxalate) significantly decreased cell viability; whereas, the other crystal types did not affect cell viability. These findings imply oxalate may be a significant contributor to this pathology. Therefore, we exposed THP-1 cells to NaOx, the soluble form of oxalate. NaOx was used as a surrogate for soluble oxalate and was included to further understand the influence of oxalate on monocytes. We determined that NaOx did not alter monocyte cell viability. However, the impact of soluble oxalate on mitochondrial function and redox status was significant.
It is well known that oxidative stress and inflammation can trigger mitochondrial damage and cell death. It is critical for mitochondrial function to be intact in monocytes/macrophages in order to regulate inflammation [31]. Oxalate has been reported to disrupt mitochondrial proteins and to increase oxidative stress in renal proximal tubular cells [9], [17], [32]. Consistent with these findings, we determined for the first time to our knowledge that oxalate (CaOx crystals and NaOx) negatively affects mitochondrial function and changes redox status in monocytes. In particular, CaOx crystals disrupted basal, ATP-linked, proton leak and maximal OCR in THP-1 cells in a dose dependent fashion. These findings suggest that oxalate may interfere with substrate availability or directly at the electron transport level by damaging mitochondrial proteins. NaOx also disrupted monocyte mitochondrial function but not to the same extent as CaOx crystals. In particular, NaOx caused a significant decrease in basal, ATP-linked, and maximal OCR in monocytes. It is logical to conclude from the data that elevated oxalate levels could independently impact monocyte health and function in human subjects. Monocytes exposed to oxalate mediated injury in the circulation could be sub-optimal prior to entering tissues and differentiating into macrophages. We propose that cells previously exposed to oxalate in the circulation are further injured once they enter the kidney and are exposed to CaOx crystals.
A recent study by Zhang et al. demonstrated that oxalate disrupts mitochondrial membrane potential and induces 3,4-Methylenedioxyamphetamine (MDA) release in a rat renal proximal tubule cell line and this is prevented with a mitochondrial targeted antioxidant [33]. We evaluated MnSOD and glutathione to determine whether oxalate could affect these antioxidant proteins. We determined that MnSOD gene expression was significantly elevated after treatment with two concentrations (100 and 500 µg/ml) of CaOx. However, MnSOD protein levels were significantly decreased in THP-1 cells treated with CaOx crystals in a dose dependent manner suggesting these cells are exposed to a highly oxidative environment and that MnSOD could be post-translationally modified. In contrast, increasing concentrations of NaOx did not modify MnSOD gene expression or protein levels. However, the highest concentration of NaOx (2 mM) significantly elevated MnSOD mRNA and protein levels. Interestingly, these alterations in MnSOD levels are oxalate specific and both CaP and cystine crystals did not significantly change MnSOD protein levels. These data imply oxalate may stimulate a pro-oxidative environment in monocytes. To further understand this phenomenon, we examined the GSH/GSSG ratio, a surrogate for redox status within cells, in THP-1 cells. We found both CaOx crystals and NaOx caused THP-1 cells to be more oxidized than control, untreated cells, suggesting oxalate causes a more oxidative state in monocytes.
To test whether oxalate would have any effect on human monocytes, primary monocytes from healthy subjects were exposed to low doses of CaOx crystals and NaOx. Consistent with our findings in THP-1 cells, we determined that oxalate disrupted mitochondrial function in primary monocytes within a short period of time (40 min). These results suggest that interaction of oxalate with primary monocytes may alter monocyte/macrophage function in the circulation and within the kidney. Kusmartsev et al. recently reported that human monocytes differentiated into macrophages stimulate inflammatory responses following CaOx crystal exposure and that these cells may play an important role in crystal clearance [18]. It is possible that macrophage differentiation and crystal clearance could be disrupted or cell death may occur in cases were monocytes are exposed to elevated levels of oxalate. The long term effect of some of these events could compromise the immune system over time in patients with kidney stones and/or predispose them to recurring stones. One potential source for such an event is the consumption of oxalate-rich meals. It is likely that oxalate-rich meals that induce CaOx crystalluria could cause inflammation and monocyte mitochondrial dysfunction in patients and would be accentuated in patients with hypercalciuria and/or hyperoxaluria. We have previously determined that oxalate levels are increased in the urine and circulation of human subjects following a dietary oxalate load [7], [34]. Thus, it would be of interest to determine whether a single dietary oxalate load could impact monocytes in human subjects.
Our findings suggest that the interaction of monocytes with oxalate both in the soluble and insoluble form injures mitochondria and disturbs redox homeostasis. It is likely that once these cells enter a pro-inflammatory environment within the kidney, the cells are exposed to a secondary insult of CaOx crystals and/or the pro-inflammatory environment. As a result this may alter monocyte/macrophage cellular integrity and metabolic activity. These findings suggest that oxalate may be a major contributor to mitochondrial dysfunction observed in our patient population. Additional studies are currently underway to evaluate monocyte/macrophage function and whether dietary oxalate alters mitochondrial function in monocytes using an animal model and human subjects.
Acknowledgements
Research reported in this publication was supported by the UAB Faculty Development Grant Program, Office of the Provost (TM) and the NIH under Award nos. P30 DK079337, P30 DK079626, 5K12GM088010 (OA), R01 DK054468 (RH), and K01DK106284 (TM). The authors thank Ms. Jennifer Williams for technical support.
Acknowledgments
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.12.003.
Appendix A. Supplementary materials
Fig. S1.
The effect of (A) calcium phosphate (CaP) and (B) cystine crystals on monocyte mitochondrial function. Cells were pretreated with CaP or cystine crystals (0, 50, 100, 200, 500, 1000 µg/ml) for 24 h prior to being seeded on Seahorse XF96 plates. Mitochondrial function was determined using the “Mito Stress Test” inhibitors: oligomycin (Oligo), FCCP, and antimycin A (AA). Results are means ± SEM; n = 5–6 replicates per group; n = 3–5 individual experiments.
Fig. S2.
The effect of calcium phosphate (CaP) and cystine crystals on MnSOD gene expression and protein levels in THP-1 cells. (A and B) Real-time PCR was performed to determine MnSOD and GAPDH expression in THP-1 cells treated with CaP or cystine crystals. MnSOD was normalized to GAPDH (housekeeping gene). Relative fold change was calculated using the DDCT method relative to control samples. (C and D) Representative western blot of MnSOD (25 kDa) and GAPDH (37 kDa) protein levels in THP-1 cells treated with CaP or cystine crystals. Results are means ± SEM; n = 3–5 individual experiments. *p < 0.05 compared to untreated monocytes.
References
- 1.Scales C.D., Smith A.C., Hanley J.M., Saigal C.S. Project UDiA. Prevalence of kidney stones in the United States. Eur. Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink H.A., Wilt T.J., Eidman K.E. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann. Intern. Med. 2013;158:535–543. doi: 10.7326/0003-4819-158-7-201304020-00005. [DOI] [PubMed] [Google Scholar]
- 3.Holmes R.P., Assimos D.G. The impact of dietary oxalate on kidney stone formation. Urol. Res. 2004;32:311–316. doi: 10.1007/s00240-004-0437-3. [DOI] [PubMed] [Google Scholar]
- 4.Goodman H.O., Brommage R., Assimos D.G., Holmes R.P. Genes in idiopathic calcium oxalate stone disease. World J. Urol. 1997;15:186–194. doi: 10.1007/BF02201856. [DOI] [PubMed] [Google Scholar]
- 5.Khan S.R. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol. Res. 1995;23:71–79. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- 6.Lieske J.C., Huang E., Toback F.G. Regulation of renal epithelial cell affinity for calcium oxalate monohydrate crystals. Am. J. Physiol. Ren. Physiol. 2000;278:F130–F137. doi: 10.1152/ajprenal.2000.278.1.F130. [DOI] [PubMed] [Google Scholar]
- 7.Holmes R.P., Ambrosius W.T., Assimos D.G. Dietary oxalate loads and renal oxalate handling. J. Urol. 2005;174:943–947. doi: 10.1097/01.ju.0000169476.85935.e2. (discussion 7) [DOI] [PubMed] [Google Scholar]
- 8.Koul S., Khandrika L., Pshak T.J. Oxalate upregulates expression of IL-2Rbeta and activates IL-2R signaling in HK-2 cells, a line of human renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2014;306:F1039–F1046. doi: 10.1152/ajprenal.00462.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang M.Y., Chaturvedi L.S., Koul S., Koul H.K. Oxalate stimulates IL-6 production in HK-2 cells, a line of human renal proximal tubular epithelial cells. Kidney Int. 2005;68:497–503. doi: 10.1111/j.1523-1755.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 10.Umekawa T., Chegini N., Khan S.R. Oxalate ions and calcium oxalate crystals stimulate MCP-1 expression by renal epithelial cells. Kidney Int. 2002;61:105–112. doi: 10.1046/j.1523-1755.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 11.Umekawa T., Tsuji H., Uemura H., Khan S.R. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int. 2009;104:115–120. doi: 10.1111/j.1464-410X.2009.08374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z., Wang T., Yang J. Calcium oxalate monohydrate crystals stimulate monocyte chemoattractant protein-1 and transforming growth factor beta1 expression in human renal epithelial cells. Mol. Med. Rep. 2012;5:1241–1244. doi: 10.3892/mmr.2012.813. [DOI] [PubMed] [Google Scholar]
- 13.Rhee E., Santiago L., Park E., Lad P., Bellman G.C. Urinary IL-6 is elevated in patients with urolithiasis. J. Urol. 1998;160:2284–2288. doi: 10.1097/00005392-199812010-00101. [DOI] [PubMed] [Google Scholar]
- 14.Boonla C., Hunapathed C., Bovornpadungkitti S. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int. 2008;101:1170–1177. doi: 10.1111/j.1464-410X.2008.07461.x. [DOI] [PubMed] [Google Scholar]
- 15.Ravi S., Mitchell T., Kramer P.A., Chacko B., Darley-Usmar V.M. Mitochondria in monocytes and macrophages-implications for translational and basic research. Int. J. Biochem. Cell Biol. 2014;53:202–207. doi: 10.1016/j.biocel.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan S.R. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 2014;3:256–276. doi: 10.3978/j.issn.2223-4683.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusmartsev S., Dominguez-Gutierrez P.R., Canales B.K., Bird V.G., Vieweg J., Khan S.R. Calcium oxalate stone fragment and crystal phagocytosis by human macrophages. J. Urol. 2016;195:1–9. doi: 10.1016/j.juro.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams J., Holmes R.P., Assimos D.G., Mitchell T. Monocyte mitochondrial function in calcium oxalate stone formers. Urology. 2016;93(224):e1–e6. doi: 10.1016/j.urology.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer P.A., Chacko B.K., Ravi S., Johnson M.S., Mitchell T., Darley-Usmar V.M. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J. Vis. Exp.: JoVE. 2014;85:1–9. doi: 10.3791/51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill B.G., Benavides G.A., Lancaster J.R., Jr. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 23.Anderson E.J., Lustig M.E., Boyle K.E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 25.Adedoyin O., Boddu R., Traylor A.M. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2017:2017. doi: 10.1152/ajprenal.00044.2017. (ajprenal00044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Lieske J.C., Spargo B.H., Toback F.G. Endocytosis of calcium oxalate crystals and proliferation of renal tubular epithelial cells in a patient with type 1 primary hyperoxaluria. J. Urol. 1992;148:1517–1519. doi: 10.1016/s0022-5347(17)36954-9. [DOI] [PubMed] [Google Scholar]
- 28.de Water R., Noordermeer C., van der Kwast T.H. Calcium oxalate nephrolithiasis: effect of renal crystal deposition on the cellular composition of the renal interstitium. Am. J. Kidney Dis. 1999;33:761–771. doi: 10.1016/s0272-6386(99)70231-3. [DOI] [PubMed] [Google Scholar]
- 29.Mulay S.R., Kulkarni O.P., Rupanagudi K.V. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Investig. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singhto N., Sintiprungrat K., Sinchaikul S., Chen S.T., Thongboonkerd V. Proteome changes in human monocytes upon interaction with calcium oxalate monohydrate crystals. J. Proteome Res. 2010;9:3980–3988. doi: 10.1021/pr100174a. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Armada M.J., Riveiro-Naveira R.R., Vaamonde-Garcia C., Valcarcel-Ares M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Chaiyarit S., Thongboonkerd V. Changes in mitochondrial proteome of renal tubular cells induced by calcium oxalate monohydrate crystal adhesion and internalization are related to mitochondrial dysfunction. J. Proteome Res. 2012;11:3269–3280. doi: 10.1021/pr300018c. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J., Wang Q., Xu C. MitoTEMPO prevents oxalate induced injury in NRK-52E cells via inhibiting mitochondrial dysfunction and modulating oxidative stress. Oxid. Med. Cell Longev. 2017;2017 doi: 10.1155/2017/7528090. (7528090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes R.P., Goodman H.O., Assimos D.G. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001;59:270–276. doi: 10.1046/j.1523-1755.2001.00488.x. [DOI] [PubMed] [Google Scholar]