Abstract
Physiological responses that occur during infection are most often thought of in terms of effectors of microbial destruction through the execution of resistance mechanisms, due to a direct action of the microbe, or are maladaptive consequences of host–pathogen interplay. However, an examination of the cellular and organ-level consequences of one such response, thermoregulation that leads to fever or hypothermia, reveals that these actions cannot be readily explained within the traditional paradigms of microbial killing or maladaptive consequences of host–pathogen interactions. In this review, the concept of disease tolerance is applied to thermoregulation during infection, inflammation and trauma, and we discuss the physiological consequences of thermoregulation during disease including tissue susceptibility to damage, inflammation, behavior and toxin neutralization.
Keywords: tolerance, fever, hypothermia
In this review, the concept of disease tolerance is applied to thermoregulation during infection, inflammation and trauma, and the authors discuss the physiological consequences of thermoregulation during disease including tissue susceptibility to damage, inflammation, behavior and toxin neutralization.
INTRODUCTION
Perhaps because our view of host defenses is shaped by the importance of anti-microbial strategies, we assume that any physiological change that occurs during an infection does so to support the immunological response is a direct action of microbial products, or is simply a maladaptive consequence of the immune or other host response. Thermoregulation during infection is a prime example of this. Infection elicits changes in the thermoregulatory strategies of a host that occurs through the integration of signals from the immune, metabolic and neural systems to change the thermal regulatory set point and change body temperature (Kluger 1980). In many cases, a higher body temperature is achieved—a response known as fever or hyperthermia. However, with certain infection types, particularly during sepsis or severe systemic inflammation, a lower body temperature is selected for a response known as hypothermia. In certain organisms, such as songbirds, a single infection type can cause circadian-associated patterns of hypothermia and fever (Skold-Chiriac et al.2015). Infection-associated changes in body temperature had long been viewed as an undesirable consequence of host–pathogen interplay. However, because of the widespread occurrence of fever and hypothermia among many different animals in the context of many different types of infectious diseases, and because of the costs to the host associated with thermoregulatory changes, we support the idea that thermoregulation is likely an evolved mechanism that has adaptive value for the host in fighting infections (Kluger 1980, 1986). This initial proposal made by Kluger in the 1970s only considered that the fever response is beneficial for host defenses (Kluger, Ringler and Anver 1975). However, we suggest that both fever and hypothermic response are evolved strategies of the host to optimize host defenses during infection and non-infectious diseases in which thermoregulatory strategies of the host are employed. Indeed, there is evidence to suggest that both fever and hypothermic responses are beneficial for host health in the context of an array of diseases. In pioneering studies using the ectotherm Dipsosaurus dorsalis, which uses behavioral thermoregulation to generate environmental fevers, Kluger demonstrated that iguanas infected with the pathogen Aeromonas hydrophila that were prevented from seeking warmer environments showed significantly increased mortality compared to infected iguanas that were allowed to generate fevers (Kluger, Ringler and Anver 1975; Bernheim and Kluger 1976). The snail, Lymnaca stagnalis, exhibited behavioral hypothermia when infected with the trematode species Diplostomum pseudospathaceum or Plagiorchis elegans. Achieving behavioral hypothermia increased longevity in infected snails compared to those that were maintained at ambient temperatures during infection (Zbikowska 2005).
Similar benefits for infection-associated thermoregulation in endotherms have also been established. Treatment of Pasteurella multocida-infected rabbits with an antipyretic was associated with greater mortality than infected rabbits that were allowed to develop fevers (Vaughn, Veale and Cooper 1980). In humans, the therapeutic use of fevers demonstrates the importance of fever in host defenses. Plasmodium, the causative agent of malaria, causes cyclical fevers due to the rupture of erythocytic stage schizonts. Wagner-Jauregg discovered that upon infection of neurosyphilus, patients with Plasmodium infection developed very high fevers and were cured of the neurosyphilis (Karamanou et al.2013). In the zebra finch, injection with the immune elicitor lipopolysaccharide (LPS) induces a fever response at night but a hypothermic response during the day (Skold-Chiriac et al.2015). These day-to-night differences in body temperature may indicate that there is a tradeoff between the benefit of fever and the possibility of overheating. During the night, when body temperature is lower, the birds are able to achieve a fever to promote host defense but during the day, when body temperatures are normally higher, the birds induce a hypothermic response to offset the costs of fever and prevent overheating. Thus, an additional thermoregulatory strategy may be to use both fever and hypothermic responses in complimentary ways over the course of a single infection to offset the potential maladaptive effects they may have on host health.
Why infection-induced alterations in thermoregulatory strategies are beneficial for the host in combatting infections and the mechanisms by which these protective responses occur remain unknown. The widespread assumption is that thermoregulation protects the host by having a negative impact on pathogen fitness. For example, pathogens may be less able to replicate if the host body temperatures are above or below the optimal temperature for the pathogen. During fever, iron, which is used by many microbes, is sequestered by host tissues (Hacker, Rothenburg and Kluger 1981; Zinchuk and Borisiuk 1997). Thus, changes in body temperature may make the host a less hospitable niche for microbes, yet pathogens vary in their temperature preferences and dependency on nutrient utilization. It has also been proposed that thermoregulation is important for shaping the immune response by optimizing resistance mechanisms and inducing the remobilization of energy stores to fuel microbial killing mechanisms (Hart 1988; Evans, Repasky and Fisher 2015). However, cellular and physiological evidence suggest that temperature-associated defense against infections cannot be readily explained only with the traditional paradigms of microbial killing mechanisms (Table 1).
Table 1.
Contribution of thermoregulation to resistance and tolerance defenses. This table summarizes in vitro and in vivo observations of how temperature influences host defense. This table also summarizes data describing how temperature sensitive factors influence various processes and the observed or predicted effects this would have on resistance and tolerance.
| Observation | Thermal sensitivity | Apparent effects on host defense strategies/pathogen fitness | Reference |
|---|---|---|---|
| Aeromonas hydrophila-infected iguanas that seek warmer environments were better able to survive infection | Heat sensitive | Undetermined | Kluger, Ringler and Anver (1975); Bernheim and Kluger (1976) |
| Trematode-infected snails that seek colder environments were better able to survive infection | Cold sensitive | Tolerance | Zbikowska (2005) |
| Blocking fever in Pasteurella multocida infected rabbits increased mortality | Heat sensitive | Undetermined | Vaughn, Veale and Cooper (1980) |
| LPS challenged zebra finches develop cyclical hypothermia and fever responses | Heat and cold sensitive | Tolerance | Karamanou et al. (2013) |
| Elevated or reduced temperatures impair pathogen growth | Heat and cold sensitive | Impair pathogen fitness | Carmichael, Barnes and Percy (1969); MacKowiak et al. (1981) |
| Sequestration of iron by host | Heat sensitive | Impair pathogen fitness | Hacker, Rothenburg and Kluger (1981); Zinchuk and Borisiuk (1997) |
| Ancient Greeks used hypothermia to treat hemorrhage | Cold sensitive | Tolerance | Diller and Zhu (2009) |
| Hibernating animals are less susceptible to ischemic tissue damage | Cold sensitive | Tolerance | Dave et al. (2012) |
| Honeybees develop a brood-comb fever by exerting movement in response to a colonial infection with the fungus Ascosphaera apis | Heat sensitive | Impair pathogen fitness | Starks, Blackie and Seeley (2000) |
| In LPS-treated human monocytes, HSF-1 inhibits transcription of genes encoding proinflammatory cytokines | Heat sensitive | Tolerance | Cahill et al. (1996) |
| Listeria monocytogenes infected hsf-1-/- mice exhibited significantly higher levels of serum TNF-α and IFN-γ and rapidly succumbed to infection without a significant difference in liver and spleen Listeria burdens | Heat sensitive |
Tolerance | Murapa et al. (2011) |
| Heat exposure prevented transcriptional upregulation of proinflammatory cytokines by preventing the release of the damage associated molecular pattern, HMGB1 | Heat sensitive | Tolerance | Fiuza et al. (2003); Lee and Repasky (2012) |
| In a mouse model of arthritis, mice exposed to higher temperatures had reduced joint damage | Heat sensitive | Tolerance | Lee et al. (2015) |
| IL-1b causes insulin resistance and the resulting impairment in glucose uptake can limit further IL-1b transcription and ROS production. | Heat sensitive | Tolerance | Jager et al. (2007); Wen et al. (2011); Benetti et al. (2013) |
| HSP32 detoxifies free heme during malaria and sepsis | Heat sensitive | Tolerance | Larsen et al. (2010); Ferreira et al. (2011) |
| Alkaline phosphatase detoxifies LPS | Heat sensitive | Tolerance | Koyama et al. (2002); Beumer et al. (2003); Tuin et al. (2006) |
| HSP72 inhibits proteotoxicity | Heat sensitive | Tolerance | Mayer and Bukau (2005) |
| Hypothermia induces expression of cold shock proteins that activate the UPR | Cold sensitive | Tolerance | Rzechorzek et al. (2015) |
| HSP72 maintains cardiac function during sepsis | Heat sensitive | Tolerance | Robert et al. (2014) |
| Shift from carbohydrate to lipid metabolism during hibernation and hypothermia limiting ischemic injury | Cold sensitive | Tolerance | Wong (1983); Drew et al. (2007); Suozzi, Malatesta and Zancanaro (2009); Hindle et al. (2011); Darwazeh and Yan (2013); Xu et al. (2013); Quinones et al. (2014) |
| Hypothermia improved coagulopathy in septic patients | Cold sensitive | Tolerance | Johansen et al. (2015) |
| Cold temperatures delay initiation of thrombus formation and speed of clot formation | Cold sensitive | Tolerance | Valeri et al. (1987); Patt, McCroskey and Moore (1988); Michelson et al. (1994); Ruzicka et al. (2012) |
| Endotherms and ectotherms lower body temperatures under hypoxic conditions | Cold sensitive | Tolerance | Hicks and Wood (1985); Dupre and Owen (1992) |
| Survival of hypoxic animals increased when put at lower temperatures | Cold sensitive | Tolerance | Gollan and Aono (1973); Hicks and Wood (1985); Gordon (2001) |
| Hypothermia protects from reperfusion tissue injury | Cold sensitive | Tolerance | Ning et al. (2007); Lampe and Becker (2011) |
| Reduced sensitivity of fibrosarcoma cells to TNFα-mediated cell lysis | Heat sensitive | Tolerance | Gromkowski, Yagi and Janeway (1989) |
| Incubation of HeLa cells at mild hyperthermic temperatures rendered them resistant to apoptosis when incubated at lethal hyperthermic temperatures | Heat sensitive | Tolerance | Bettaieb and Averill-Bates (2008) |
| HSP32-deficient mice infected with Plasmodium had increased liver damage caused by increased sensitivity of hepatocytes to TNF-mediated apoptosis | Heat sensitive | Tolerance | Seixas et al. (2009) |
| HSP70 and HSP90 inhibit apoptosome formation | Heat sensitive | Tolerance | Beere et al. (2000); Pandey et al. (2000); Saleh et al. (2000) |
| HSP60 affects caspase-3 activation to inhibit apoptosis | Heat sensitive | Tolerance | Xanthoudakis et al. (1999) |
| Hypothermia has been shown to alleviate pump dysregulation and help maintain intracellular Ca2+ homeostasis and may prevent cell death | Cold sensitive | Tolerance | Siesjo et al. (1989); Hall (1997) |
| Cold temperatures delay apoptosis in response to stress by delaying cytochrome c release | Cold sensitive | Tolerance | Goldstein et al. (2000) |
| Heat can directly activate Bax and Bak to induce cytochrome c release | Heat sensitive | Tolerance | Pagliari et al. (2005) |
| HSP90 is required for the induction of necroptosis | Heat sensitive | Tolerance | Jacobsen et al. (2016); Zhao et al. (2016) |
| Increased phagocytosis by macrophages | Heat sensitive | Resistance | Evans, Repasky and Fisher (2015) |
| Increased expression and release of cytokines | Heat sensitive | Resistance or tolerance | Evans, Repasky and Fisher (2015) |
| Hypothermia protected rats from endotoxemia | Cold sensitive | Trade-offs in resistance and tolerance | Liu et al. (2012) |
| Increased neutrophil release, infiltration and microbial killing | Heat sensitive | Resistance | Evans, Repasky and Fisher (2015) |
| Enhanced lymphocyte trafficking and proliferation in response to antigens | Heat sensitive | Resistance | Hart (1988) |
| Increased antibody synthesis | Heat sensitive | Resistance | Hart (1988) |
Infectious diseases cause significant physiological damage to the host. In addition to executing resistance mechanisms to kill microbes, a host must deal with the collateral damage that occurs during host–pathogen interplay in order to survive. Ecologists have long recognized that genetic variation in disease susceptibility exists in plants that can be dissociated from the plants ability to kill a pathogen or pest. This variation is due to a distinct defense strategy encoded by plants, called ‘tolerance’ that promotes plant fitness in the presence of a given level of pathogen or herbivore. In recent years, the concept of tolerance defenses has been introduced into the field of animal immunology (Raberg, Sim and Read 2007; Ayres and Schneider 2008, 2009, 2012; Ayres, Freitag and Schneider 2008; Ayres, Trinidad and Vance 2012; Medzhitov, Schneider and Soares 2012; Schieber et al.2015). In this context, tolerance is a defense strategy that minimizes the physiological damage that occurs during infection without having a negative impact on microbial fitness. We propose that thermoregulatory mechanisms that both increase and decrease body temperature are adaptive strategies of the host to promote tolerance defenses and survival following infections. There is evidence that fever and heat shock induce cyto- and tissue-protective responses that will work to limit tissue susceptibility to damage.
In addition to fever, the beneficial effects of hypothermia in defense against tissue damage have long been recognized. For example, the ancient Greeks used hypothermia for treating various conditions including hemorrhaging (Diller and Zhu 2009). Hibernating animals are less susceptible to damage induced by ischemia due to their low body temperatures causing reduced oxygen consumption, cardiac function and metabolism rendering tissues less susceptible to damage (Dave et al.2012). In current medical practices, hypothermia is used for its cyto- and tissue-protective effects in the context of trauma in which the metabolic and inflammatory states of the body and microenvironments change frequently making tissues and cells vulnerable to damage, compromising the normal function of organs (Andresen et al.2015; Saigal et al.2015; Usach, Sakopoulos and Razavi 2015; Alkabie and Boileau 2016). Behavioral experiments demonstrated that animals that chose to move to colder environments under conditions in which tissue homeostasis is compromised are healthier than those that do not develop behavioral hypothermia (Romanovsky et al.2005; Almeida et al.2006b). In infections, hypothermia is most often associated with advanced stage septic patients and those with severe systemic inflammation, whom are vulnerable to similar forms of cellular and physiological damage as trauma patients (Perman et al.2014; Chisholm et al.2016; Kohlhauer et al.2015). Hypothermia may be viewed as a host's final effort to limit physiological damage during these disease states but over longer periods of time become maladaptive to the host as is the case with prolonged hyperthermic responses.
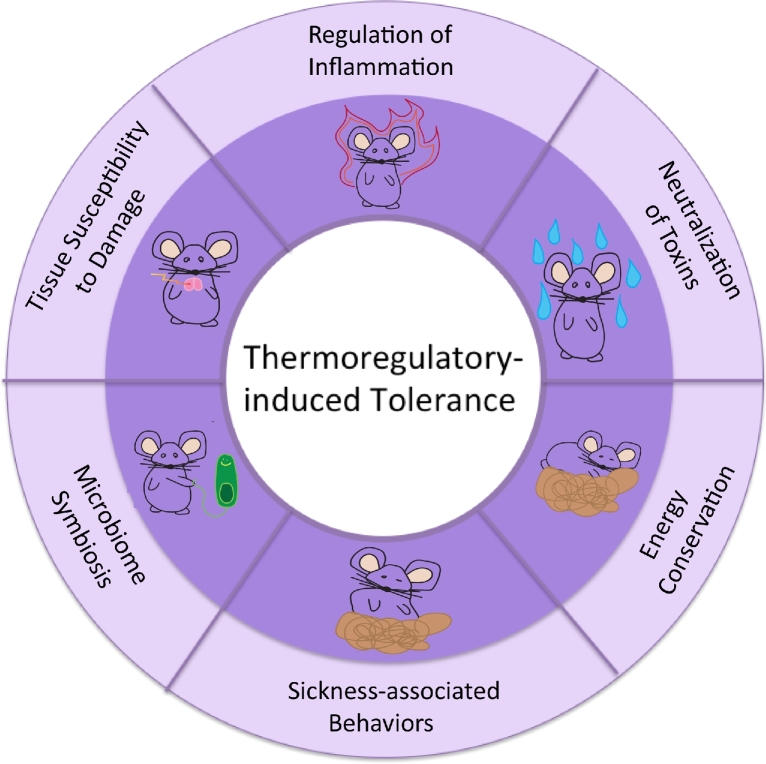
The function of thermoregulation during infections is almost inevitably defined in terms of microbial killing mechanisms. Evidence shows, however, that several effects of temperature cannot be readily explained within the paradigm of resistance defenses. In this review, we apply the conceptual framework of tolerance to thermoregulation in animals to illustrate how the physiological responses that occur during hyperthermia and hypothermia can promote health and survival during infectious diseases in animals (Fig. 2).
Figure 2.
Thermoregulatory induced tolerance mechanisms.
THERMOREGULATORY MECHANISMS IN ANIMALS
Initiation of fever in endotherms occurs upon central and peripheral recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors on innate immune cells including macrophages and dendritic cells (Jounai et al.2012). In addition, multiple cell types in the brain including endothelial cells, microglial cells and neurons can respond to PAMPs (Rivest 2009; Hernangomez et al.2014). The fever response has been best characterized in the context of LPS challenge and we will focus our discussion around these studies. Activation of Toll-like receptor 4 (TLR4) by LPS centrally and peripherally induces the production of host-derived pyrogens including IL-1β, TNF-α and IL-6. These cytokines circulate through the lymphatic system and signal to the brain (Kluger et al.1995; Romanovsky, Steiner and Matsumura 2006; Zhang et al.2008; Yamawaki et al.2010; Nakano et al.2015; Poon et al.2015). Il-1β has been long thought to be the primary pyrogen responsible for fever induction; however, accumulating evidence using genetic knockout and neutralization studies as well as clinical data now suggests that IL-6 is the essential mediator for the febrile response (Hart 1988; Evans, Repasky and Fisher 2015). The febrile response is due to these systemic and locally produced cytokines acting on the hypothalamus in the brain to change the thermoregulatory set point. This set point is raised so that what was once a thermal neutral temperature is now subjectively cold to the host. The host reaches a new thermal equilibrium by a number of mechanisms. Among these are brown adipose tissue thermogenesis driven by noradrenaline release and increasing metabolism to induce shivering to produce heat. Vasoconstriction is also induced to reduce blood flow to peripheral fevers preventing heat loss (Almeida et al.2006a; Nakamura and Morrison 2011). Endotherms will also exhibit behavioral changes including seeking warmer environments and curling up to reduce the amount of body surface exposed to the environment to minimize heat loss. The mechanism by which endogenous pyrogenic cytokines induce the hypothalamus to raise the thermogenic set point involves their induction of cyclooxygenase 2 (COX2) to induce the conversion of arachidonic acid into prostaglandin E2 (PGE2). Peripheral synthesis of COX2 and PGE2 is important for initiation of fever. In a LPS fever model, recognition of LPS by TLR4 on lung and hepaptic hematopoietic cells induces transcriptional upregulation of COX2 leading to production of PGE2 to signal to the brain (Steiner et al.2006). Injection of a cyclooxygenase inhibitor in the preoptic area (POA) has shown inconsistent results, but seems to mainly attenuate activation of median preoptic and arcuate hypothalamus suggesting that there may also be a central mechanism that regulates COX2 activity in fever induction (Nadjar et al.2010). PGE2 binds to the PGE2 receptors (EP), on thermoregulatory neurons in the hypothalamus (Oka et al.2000; Nakamura et al.2002; Oka 2004; Lazarus et al.2007). Signals are then sent through neurons to the dorsomedial hypothalamic nucleus to elicit sympathetic thermogenesis in peripheral effector organs (Nakamura et al.2005). The cytokine receptor activator of NF-kB, TNFSF11, found on astrocytes in the hypothalamus, also activates COX2 and PGE2 production to activate EP3 (Hanada et al.2009). Other EP receptors including EP1 and EP4 may contribute to different phases of fever suggesting functional redundancy of EP receptors in fever induction (Oka et al.2000). Drugs that suppress fever including salicylic acid do so through the inhibition of prostaglandin synthesis (Vane and Botting 2003). Antipyretics are non-specific and can also influence resistance mechanisms. This must be considered when examining how such drugs promote host defenses during infection.
Recent evidence suggests that the microbiota, the trillions of beneficial microbes that colonize the body surfaces exposed to the environment, can also regulate hyperthermic responses. Cold temperatures can induce ecological perturbations in the intestinal microbiota composition that are apparently adaptive for the host. These ecological changes are associated with intestinal tissue remodeling and changes in adipose tissue physiology that cause white adipose tissue to become beige, which has thermogenic properties similar to brown adipose tissue, and cause a rise in body temperature (Chevalier et al.2015). This is a complex relationship in that environmental conditions cause a host response that perturbs the microbiota, which in turn influences host physiology to return to homeostasis.
While ectotherms such as amphibians, reptiles and insects do not generate endogenous pyrogenic chemical responses, they can generate fevers through behavioral regulation of body temperature during infections. For example, ectotherms will move to warmer environments in order to exogenously induce fever in response to an LPS injection and this movement is required to increase survival during challenge (Vaughn, Bernheim and Kluger 1974). Honeybees develop a brood-comb fever by exerting movement in response to a colonial infection with the fungus Ascosphaera apis. This pathogen is heat sensitive and in this case it seems that this social fever response promotes host defense by creating a less hospitable niche for the pathogen (Starks, Blackie and Seeley 2000). Thus, the recognition of infections and microbial-derived products in ectotherms appears to induce behavioral changes that result in thermoregulatory responses to promote host survival by promoting mechanisms that can have either a negative or neutral/positive impact on pathogen fitness.
There is also evidence to suggest that in addition to endotherms, ectotherms use PGE2 in order to induce behavioral fevers. Treatment of the toad, Bufo paracnemis, with the COX inhibitor indomethacin rendered the toads unable to produce fevers in response to an LPS challenge (Bicego et al.2002). Histamine, hemoglobin, corticosterine and temperature-sensitive transient receptor potential channels have also presented as possible factors in mediating ectothermal fever (Hutchison and Spriestersbach 1986; Wiggins and Frappell 2000; Preest and Cree 2008; Saper, Romanovsky and Scammell 2012). Thermoregulatory behavior requires a complex neuronal circuitry of initiating, organizing, performing and controlling motor actions. Our current understanding of the neural pathways regulating behavioral thermoregulation is limited, and neural pathways controlling thermoregulatory behaviors differ from those controlling autonomic thermoeffectors, making work in these areas more complex (Nagashima et al.2000; Flouris 2011). Recent studies involving neural thermal stimulation have provided evidence as to which central thermosensors are involved with behavioral thermoregulation; these include the medulla oblongata, pons, midbrain, and amygdala, and the orbitofrontal, insular and somatosensory cortex. Further studies in the toad B. paracnemis have demonstrated that lesions in the POA of the hypothalamus prevented toads from developing a febrile response triggered by LPS challenge, although regular thermoregulatory abilities were not affected. These data suggest that the POA may be important in fever generation in ectotherms (Bicego and Branco 2002).
Hypothermia or anapyrexia has been most studied as a clinical therapeutic and less focus has been placed on mechanistically understanding the induction of the cryogenic response triggered by infection and immune stimulation. Studies of hypoxia-induced anapyrexia, which may serve as a strategy to avoid severe hypoxia by reducing metabolic demands, have unraveled some mechanisms that may apply to other forms of hypothermia. Centrally, the glutamatergic action of the lateral POA of the hypothalamus has been implicated in the control of hypoxia-induced hypothermia in endotherms. This appears to be mediated by the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and ionotropic GABA receptors in the rostral parapyramidal region of the medulla oblongata, as neurons directly project between these parts of the brain (Yoshida et al.2009; Osaka 2014). Connecting the brainstem to the rest of the brain, the medulla oblongata contains respiratory, cardiac and vasomotor centers, positioning itself as a reasonable culprit of thermoregulatory action. While endogenous regulation of the hypothermic response involves the POA, the behavioral responses that mediate hypothermia appear to involve the neuronal bodies located in the dorsomedial nucleus and neural fibers passing through the paraventricular nucleus of the hypothalamus (Almeida et al.2006a).
In certain contexts, proinflammatory cytokines that typically act as pyrogens can also act as cryogens. For example, the proinflammatory cytokines IL-1β, IL-6 and TNF-α have been shown to act as cryogens in animal models of infection and systemic inflammation. In a mouse model of antibiotic-induced dysbiosis and intestinal injury, administration of broad spectrum antibiotics induced the expansion of a multi-antibiotic-resistant Escherichia coli strain. Upon disruption of the intestinal barrier with the sulfated polysaccharide dextran sulfate sodium (DSS), this E. coli translocated to extraintestinal tissues causing a sepsis-like disease characterized by multi-organ dysfunction and hypothermia. The authors found that this hypothermic response was dependent on activation of a component of the innate immune system called the NLRC4 inflammasome by this E. coli strain, which resulted in an overly exuberant response mediated by IL-1β (Ayres, Trinidad and Vance 2012). The hypothermic response seen in this model and many other mouse infection models is likely due to the animals being thermally stressed. While pyrogenic infections cause fever at thermoneutrality and above, subneutral temperatures elicit a hypothermic response in response to similar agents in animals (Ivanov et al.2003). Most animal vivariums that conduct research house their mice at subthermal temperatures (∼19°C–26°C; Speakman and Keijer 2012), despite knowledge that the rodent's thermal neutral zone is ∼30°C, causing thermal stress (Watkinson and Gordon 1993; Swoap et al.2008). Due to this, biological studies of infectious diseases and in general have been in the context of hypothermia. This hypothermic rather than hyperthermic response may result from various inflammatory stimuli including proinflammatory cytokines, differences in pathogen load, route/site of administration, ambient temperatures, circadian timing and other factors that influence host temperature differently when animals are under thermal neutral and thermal stressed conditions (Nomoto 1996; Szelenyi et al.2004). With this in mind, it is important to recognize what we can learn from these ‘mistakes’ and use them as observations for relevant studies.
Other cryogens potentially exist and take part in LPS-induced hypothermia, including the cytokines IL-10 and IFNγ (Leon 2004). 3-Iodothyronamine (T1AM), an endogenous derivative of thyroid hormone, induces robust hypothermia in mice and rhesus monkeys, possibly related to hypothermic neuroprotective effects during stroke (Scanlan et al.2004; Panas et al.2010). Hypothalamic cysteinyl-LT (CysLT), an arachidonate metabolism product, 5-lipoxygenase and epoxygenase-derived eicosanoids have also been indicated as promoters of anapyrexia. This may suggest that LPS-induced hypothermia may be mediated by leukotrienes (Paul, Fraifeld and Kaplanski 1999; Kozak and Fraifeld 2004). A few cryogens have been identified in the context of hypoxic-induced hypothermia. Nitric oxide in the POA is a potential promoter of hypothermia induced by hypoxia (Steiner and Branco 2003). Simultaneous increases in the levels of cyclic adenosine monophosphate (cAMP) and cGMP in the POA may play a role in hypoxia-induced hypothermia due to increases in production and/or release of serotonin and nitric oxide (Steiner and Branco 2003). An intracellular cascade of adenylate cyclase, protein kinase A and cAMP caused by high levels of hydrogen sulfide in the POA may play an essential role in the occurrence of the hypothermia in response to hypoxia (Kwiatkoski et al.2012). It appears that carbon monoxide may downregulate hypoxia-induced hypothermia, as studies using heme-oxygenase (CO-synthesizing enzyme) inhibition showed that hypothermia was attenuated (Paro et al.2002). There have been indications of human cryogens, but the specific identity of these molecules remains to be determined (Shido et al.2004; Shido and Sugimoto 2011).
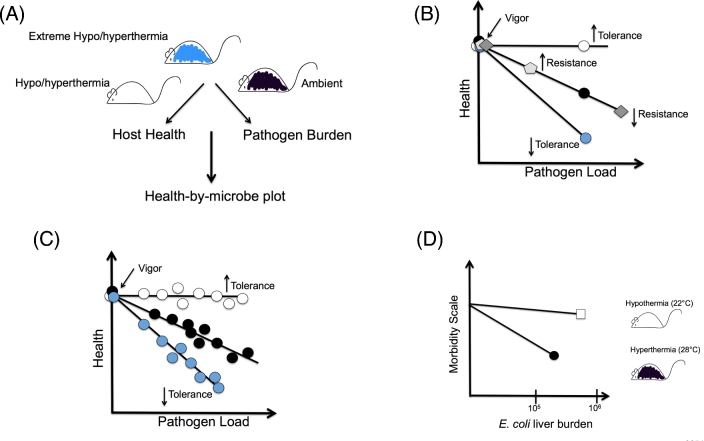
Methods by which tolerance can be measured have been described in several recent reviews (Raberg, Sim and Read 2007; Ayres and Schneider 2008, 2011). The relative contributions of resistance and tolerance to host defense against infection can be distinguished in any host microbe system by examining the relationship between host health and pathogen levels. Using these parameters, and assuming health of the host when uninfected is equivalent between different host populations (vigor), a dose response curve can be generated to determine how host health changes as microbial levels change. Changes in health as microbe levels change would shift the host along the diagonal and indicate differences in resistance (Fig. 1 ). Changes in health without a change in microbe levels would shift the curve along the y-axis and would indicate differences in tolerance (Fig. 1). The more tolerant a host, the shallower the slope of the dose response curve would be. This method can be used to determine how different factors including environmental and genetic factors can influence host defenses. For example, mice infected with pathogen A that differ in the ability to thermally regulate their bodies may differ in how the shift within this health-by-microbe space with respect to each other, revealing how temperature regulates resistance and tolerance in the context of pathogen A infection. Ayres and Schneider (2008, 2012) reported the point tolerance method, which uses microbial levels at a single defined time point to reveal how a single mutation in a component of the fly immune response can impact resistance and tolerance in response to different infection challenges (Fig. 1). Using a mouse malaria model, Raberg, Sim and Read (2007) reported that range tolerance, which measures host health at various pathogen doses, can reveal variations in resistance and tolerance (Fig. 1). Both models are based on the assumption that these relationships are linear and have been useful at identifying environmental, microbial and genetic factors that influence tolerance defenses. These relationships however are likely more complex and further experimentation and data points, for example, measuring pathogen burden over the course of the infection will reveal how differences in host populations influence resistance and tolerance at different stages of the infection.
Figure 1.
Measuring the effects of thermoregulation on resistance and tolerance. (A) Host health and pathogen burdens of infected host populations that differ in their thermoregulatory responses are measured. Using these values, a health-by-pathogen plot is generated in (B) and (C) to determine how these host populations move in space with respect to each other. (B) Point tolerance. The relationship between host health and pathogen levels at a defined time point is examined. Shifts along the y-axis indicate health is changing as microbe levels are changing and indicate changes in resistance. Shifts along the y-axis indicate changes in tolerance. The steeper the slope the more tolerant the host. Adapted from Ayres and Schneider, (2008, 2012). (C) Range tolerance. The relationship between host health and different levels of pathogen are examined. Adapted from Raberg, Sim and Read (2007). (D) Wisteria rats were infected intraperitoneally with a clinical E. coli isolate and placed in either hyperthermic or hypothermic conditions. Morbidity and E. coli burdens in livers at 5 h post infection were measured. Shown are the data represented as point tolerance. Animals at 28°C have an increase in resistance. On the other hand, animals at ambient temperature that were allowed to develop hypothermia had lessened morbidity as characterized by decreased lung inflammation and neutrophil infiltration. Decreased tolerance is represented by a less steep slope. Data from Liu et al. (2012).
CATABOLISM AND ENERGETIC COSTS OF THERMOREGULATION
Fever responses caused by many forms of insult including sepsis induce a hypermetabolic state in the host (Frankenfield et al.1997). Induction of a hypermetabolic state increases heat production to raise and maintain body temperature at the new elevated thermoregulatory set point. Studies in humans have suggested that the induction of fever causes a 30%–50% increase in metabolism and the average percent increase in metabolism for every 1°C of fever is ∼13% (Mackowiak and Plaisance 1998; Kluger et al.1998; Kluger 2002). Consequences of the hypermetabolic response during fever are muscle proteolysis and a negative nitrogen balance in the host that results clinically in the wasting of energetic body tissues. While anabolic responses occur during infection, the magnitude of catabolic responses tends to be greater and results in the depletion of body tissues. The function of muscle wasting is not understood but possibly its function may serve to generate a source of amino acids that are mobilized to other parts of the body that are used for the anabolic requirements of the host response. Catabolism of body tissues does not become evident until the fever has been fully reached. Consistent with the notion that active thermoregulation during infection is beneficial for host defenses, muscle catabolism should also be beneficial for the host. Given that there are costs to mounting resistance responses (Iseri and Klasing 2014), the current hypothesis is that this muscle wasting occurs to fuel the resistance response. However, we propose that muscle catabolism is also necessary to fuel tolerance defense mechanisms that are induced by fever (Fig. 2). For example, defense responses that negatively regulate inflammatory responses and the induction of cytoprotective and tissue repair pathways are also energetically costly (Ayres and Schneider 2011).
The pathophysiology of muscle wasting is complex and incompletely understood. Factors including severity of the primary disease, proinflammatory cytokines such as TNFα, IL-1β and IL-6, severity of the anorexic response, hormones, metabolism and pathogen factors are all believed to be the main drivers of skeletal muscle catabolism that can lead to the induction of atrophy-dependent programs in muscle (Beutler et al.1985; Goodman 1991, 1994; Zamir et al.1992; Costelli et al.1993). The intestinal microbiota is also a critical regulator of skeletal muscle wasting in response to infectious and inflammatory diseases (Schieber et al.2015). Colony born C57Bl/6 mice were protected from muscle wasting caused by DSS-induced intestinal injury compared to mice from Jackson Labs. Colony born mice harbored an Escherichia coli O21:H+ strain in the intestine that was absent in the mice from Jackson Labs. According to Koch's postulates, administration of this commensal to Jackson mice resulted in protection from wasting induced by intestinal injury. This protection could be extended to oral infection with the pathogen Salmonella Typhimurium and lung infection caused by Burkholderia thailandensis. Escherichia coli O21:H+ mediates its protective effect by preventing infection/inflammation-induced systemic drop in circulating levels of IGF-1 in an NLC4 inflammasome-dependent manner without impacting pathogen infection levels indicating that this E. coli promotes tolerance. This protection was associated with E. coli O21:H+ occupying a new niche—white adipose tissue—during challenged states. In this case, muscle wasting appears to be maladaptive to the host by reducing host tolerance (Schieber et al.2015). Clearly, this is inconsistent with the idea that wasting provides resources that enhance both resistance and tolerance mechanisms, as mentioned above. One possible explanation is that these studies were done in animals housed under thermally stressed conditions and that wasting in the absence of a febrile response is maladaptive. The effects of muscle wasting on tolerance defenses are likely to be complex and context dependent and will require further investigation.
REGULATION OF INFLAMMATION
The most obvious way to promote tolerance would be to regulate the degree and duration of an inflammatory response during an infection. In doing so, the energetic costs associated with mounting an immune response would be minimized to avoid excessive and potentially pathogenic skeletal muscle catabolism. Furthermore, the potential tissue damage caused by the inflammatory response would be reduced. Elevated body temperatures induce the expression of heat shock factor 1 (HSF-1), which is involved in the acute heat response in mammals to activate expression of cytoprotective genes (Hasday and Singh 2000). HSF-1 has been demonstrated to act as a negative transcriptional regulator of inflammatory responses. In LPS-treated human monocytes, HSF-1 inhibits transcription of genes encoding proinflammatory cytokines (Cahill et al.1996). Consistent with this, when hsf-1−/− mice were infected with Listeria monocytogenes, they exhibited significantly higher levels of serum TNFα and IFNγ and rapidly succumbed to infection without a significant difference in liver and spleen Listeria burdens (Murapa et al.2011). Neutralization of TNFα promoted survival of infected animals deficient for HSF-1 demonstrating that inhibition of the inflammatory response by HSF-1 promotes tolerance (Murapa et al.2011). Heat exposure appears to act as a negative regulator of inflammation in activated macrophages and this is dependent on HSF-1 transcriptional repressive behavior. Heat exposure also prevents transcriptional upregulation of proinflammatory cytokines by preventing the release of the damage-associated molecular pattern, HMGB1, reducing transcript stability and the recruitment of NF-kB to promoter regions of cytokine genes (Fiuza et al.2003; Lee and Repasky 2012). In a mouse model of arthritis, mice exposed to higher temperatures had reduced joint damage associated with lower levels of circulating levels of TNFα and increased levels of the anti-inflammatory cytokine IL-10 in the joints (Lee et al.2015). Downregulation of inflammation may also occur through the metabolic effects caused by the fever response. For example, IL-1β causes insulin resistance, and the resulting impairment in glucose uptake can limit further IL-1β transcription and reactive oxygen species (ROS) production (Jager et al.2007; Wen et al.2011; Benetti et al.2013).
Hypothermia can also reduce inflammation to promote tolerance. For example, Wistar rats infected with a clinical Escherichia coli isolate that were maintained under hypothermic conditions displayed less endotoxemia, organ dysfunction and lung neutrophil recruitment despite higher liver bacterial burden (Fig. 1) (Liu et al.2012). Thus, shifts in the thermostatic set point of a host appear to provide a negative feedback mechanism that downregulates proinflammatory cytokine production to prevent excessive tissue damage.
SICKNESS-INDUCED BEHAVIORS
The fever response in animals and humans is accompanied by stereotypical behavioral modifications including anorexia, sleep disturbances, social withdrawal and grooming disturbances that are known as sickness-induced behaviors (Hart 1988) (Fig. 2). These behaviors appear to be highly conserved as they are found in both invertebrates and vertebrates. Similar to fever, the general assumption regarding these sickness-associated behaviors is that they are maladaptive consequences of the host response to the infection. However, because these behaviors are ubiquitous among animal species during infection it is likely that these are evolved behavioral adaptations to increase the chance of survival during infection (Hart 1988; Ayres and Schneider 2009, 2012; Ayres 2013). Hart (1988) proposed that the main purpose of these behaviors is to facilitate the febrile response to better promote resistance defenses. While there is evidence to suggest that resistance defenses are influenced by sickness-induced behaviors (Hart 1988; Morag et al.1998; Ayres and Schneider 2009), we propose that fever induces tolerance defenses in part by promoting these behaviors. Given the energetic costs of fever, the induction of anorexia and the reduced consumption of food during infection seem counterintuitive. Foraging and capture of food is energetically demanding and a reduced motivation to eat may promote tolerance by conserving energy stores for host defense and minimize wasting pathology. Calorically restricted states induce stress responses in animals that may reduce the susceptibility of tissues to damage during infection (Koella and Sorense 2002; Partridge, Piper and Mair 2005; Burger et al.2007; Kristan 2007; Libert et al.2008; Mair and Dillin 2008; Ayres and Schneider 2009). Consistent with this, in Drosophila, mutations in the gustatory receptor Gr28b render flies constitutively anorexic. When infected with Salmonella Typhimurium, these flies have enhanced tolerance. Similarly, when flies are calorically restricted they have increased tolerance to infection compared to flies fed a standard diet (Ayres and Schneider 2009). These same parameters, however, rendered flies more susceptible to infection with Listeria monocytogenes due to resistance defects (Ayres, Freitag and Schneider 2008). Thus, the effects of sickness-induced anorexia on resistance and tolerance defenses will be context dependent.
Infected animals typically exhibit sleepiness or fatigue and have a tendency to sleep during periods that they would otherwise be awake. Increasing sleep may be an additional means to conserve energy for the host defense response. Disruptions in sleep during infection lead to mortality in animal studies, for example, sleep deprived mice were more susceptible to infection with Plasmodium (Lungato et al.2015). In humans, workers are more susceptible to infection (Pietroiusti et al.2006). The circadian timing system in animals regulates the wake-sleep cycle and synchronizes biological processes and is important for host defense against infections (Shirasu-Hiza et al.2007; Stone et al.2012; Allen et al.2016). In ectotherms, both light and external temperatures regulate the circadian clocks (Garrity et al.2010; Romeijn et al.2012; Villamizar et al.2012; Fan, Stuart-Fox and Cadena 2014). In endotherms, peripheral tissues have independent clocks that are regulated by the master clock in the brain called the suprachiasmatic nucleus (SCN) in the hypothalamus. The master clock is regulated by light-dark cycles and is resistant to temperature changes. The peripheral clocks respond to temperature changes (Reppert and Weaver 2002; Buhr, Yoo and Takahashi 2010). It appears that the SCN drives core body temperature rhythms that are sensed by the peripheral clocks. This suggests that fever and hypothermic responses may induce clock-dependent responses in peripheral tissues that influence host defenses.
Reduced grooming and social withdrawal are additional sickness-associated behaviors in animals. Similar to sleep and changes in feeding, reduced grooming may serve as an energy conservation mechanism. Social withdrawal likely serves a beneficial function for a group rather than an individual. Workers of the ant Temnothorax unifasciatus infected with the fungal pathogen Metarhizium anisopliae leave their social network prior to death (Heinze and Walter 2010). In this context, social withdrawal would protect the group due to avoidance defenses. The exact function of social withdrawal and the extent to which it and other sickness-induced behaviors influences tolerance defenses and how this relates to temperature requires further exploration.
NEUTRALIZATION OF TOXINS
Infection-induced tissue damage can release host-derived toxic compounds that must be dealt with to minimize further tissue damage. Mechanisms that do so would operate as tolerance mechanisms because they target the toxin rather than the pathogen (Fig. 2). For example, infection with Plasmodium, the causative agent of malaria, causes hemolysis freeing host-derived hemoglobin into circulation. Under inflammatory conditions, free hemoglobin is oxidized resulting in the release of its prosthetic heme groups into circulation, which is toxic to the host. Heat shock protein 32 (HSP-32, also known as heme oxygenase-1 HO-1) is induced during Plasmodium infection and in response to heat shock and catalyzes the degradation of free heme resulting in carbon monoxide, labile iron and biliverden (Seixas et al.2009). Individuals hemizygous for the sickle cell mutation contain a point mutation in the beta chain of hemoglobin and have elevated basal levels of free heme and HSP32 expression and are less susceptible to Plasmodium infection (Ferreira et al.2011). Using a transgenic mouse model hemizygous for the human sickle cell trait, HbS, investigators found that animals had increased circulating levels of free heme, which activated HSP32 expression via the Nrf2 transcription factor. Evidence suggests that the CO produced during the heme detoxification reaction by HSP32 prevented further release of cell-free hemoglobin into circulation during infection and the pathogenic effects of CD8+ T cells in cerebral malaria (Ferreira et al.2011). This protection conferred by the HbS trait did so without influencing Plasmodium levels; thus, the detoxification mechanism of HSP32 promotes tolerance of malaria infection. In a mouse cecal ligation and puncture sepsis model, HSP32 was required for survival without an apparent difference in systemic bacterial levels (Larsen et al.2010). A caveat of this model however is that the authors only measured culturable levels of bacteria which only represents a small fraction of the members of the intestinal microbiota.
In addition to host-derived toxic compounds, mechanisms that neutralize microbial-derived toxins will promote tolerance of the host. Although we have evolved mechanisms to use LPS as an elicitor of tissue protective responses, systemic recognition of LPS by TLR4 leads to severe systemic inflammation. Alkaline phosphatases have been shown to modify LPS by dephosphorylating the lipid A moiety, which confers LPS toxicity (Koyama et al.2002; Beumer et al.2003; Tuin et al.2006). Mild heat shock (39oC–41oC) has been shown to induce expression of alkaline phosphatase (Shui and Scutt 2001), as well as enhance enzymatic activity (Trieb, Blahovec and Kubista 2007). In a zebrafish model, intestinal alkaline phosphatase was required to prevent intestinal inflammation and pathology caused by the microbiota (Bates et al.2007). Therefore, temperature regulation of alkaline phosphatase may represent a host-encoded strategy to promote tolerance by detoxification of microbial products.
TISSUE SUSCEPTIBILITY TO DAMAGE
Tissue susceptibility to damage during an infection is largely dependent on a cell's ability to mount an appropriate compensatory stress response that will contribute to tolerance defenses (Fig. 2). In the absence of such responses, cells and tissues become vulnerable to host–pathogen interplay. For example, the host response to an infection places heavy demands on a cell's protein synthesis machinery and can lead to the accumulation of damaged proteins as well as misfolded and unfolded proteins that leads to proteotoxicity. In Drosophila, pcmt, which encodes an L-isoaspartyl methyltransferase that is important for the damaged protein repair response, is required for tolerance of infection with the lethal intracellular bacterium Listeria monocytogenes (Ayres, Freitag and Schneider 2008). In Caenorhabditis elegans, the transcription factor xbp-1 that is a critical component of the unfolded protein response (UPR) promotes tolerance in worms challenged with the pathogen Pseudomonas, by limiting the accumulation of unfolded protein species (Richardson, Kooistra and Kim 2010). Changes in body temperature are sensed and trigger activation of stress response transcriptional programs, which include protection from proteotoxicity. In human cortical neurons, hypothermia induces the expression of cold shock proteins and endoplasmic reticulum stress leading to activation of the UPR. This can lead to cross-protection against oxidative stress in neurons (Rzechorzek et al.2015). HSF-1 induces expression of a variety of genes including HSP-72, which inhibits proteotoxicity by facilitating the proper folding of newly translated or misfolded proteins (Mayer and Bukau 2005). In a variety of septic models, mice deficient for HSF-1 presented with impaired cardiac contraction and relaxation that was associated with increased production of immune effectors in cardiomyocytes, suggesting that HSF-1 may promote tolerance by maintaining cardiac function during sepsis (Robert et al.2014). In a forward genetic ENU screen in mice, Kcnj8mydy/mydy mice have a null allele for Kir6.1 which encodes an ATP-sensitive potassium channel, and are hypersusceptible to challenges with LPS and MCMV infection without differences in viral titers compared to challenged wild-type mice. During infection, this channel maintains coronary homeostasis and prevents coronary artery vasoconstriction induced by the inflammatory response to maintain cardiac tonicity and prevent death (Croker et al.2007).
HSF-1 likely promotes tolerance via mechanisms independent of the protein stress response and downregulation of inflammation. In another study, T cells isolated from hsf-1−/− mice exhibit defective proliferation at febrile temperatures and this was due to an inability of HSF-1-deficient mice to restore ROS levels after heat challenge (Murapa et al.2007). Thus, regulation of cellular stress responses by body temperature may represent a general tolerance mechanism that is protective against an array of infections.
Cellular and tissue health is dependent on proper circulation of the body's blood supply to provide oxygen and nutrients to the tissue. In the clinical setting of sepsis and in severe inflammatory disorders, dysregulated activation of the coagulation cascade and the inhibition of fibrinolysis can lead to disseminated intravascular coagulation that ultimately can cause multi-organ failure due to hypoxia caused by perturbations in the microcirculation (Levi, van der Poll and Schultz 2012). This causes ischemia of the tissue due to the limited amount of oxygen available for cellular aerobic respiration leading to a reduction in ATP and phosphocreatine levels and a switch in intracellular metabolism to anaerobic glycolysis. In anaerobic glycolysis, glucose is transformed into lactate generating 2 ATP molecules per glucose molecule. The resulting accumulation of hydrogen ions and lactate leads to the production of lactic acid and a drop in extracellular and intracellular pH, a condition called lactic acidosis. Patients who develop severe sepsis or septic shock have elevated circulating levels of lactic acid and lactic acidosis, and this is a marker of the severity of the infection. Early lactate normalization within the first six hours of resuscitation is a strong predictor of sepsis survival (Puskarich et al.2013). In hibernating mammals, the internal thermostat is lowered and tissues function at lower temperatures. There is a shift from carbohydrate to lipid metabolism during hibernation limiting anaerobic metabolism and ischemic injury (Suozzi, Malatesta and Zancanaro 2009; Hindle et al.2011; Xu et al.2013; Quinones et al.2014). In the hypothermic brain, metabolism also shifts from glucose to lipid utilization (Wong 1983; Drew et al.2007; Darwazeh and Yan 2013). Thus, changing substrate utilization induced by temperature changes may represent a tolerance mechanism to prevent lactate build-up. An obvious mechanism to prevent tissue hypoxia in a septic patient would be to prevent dysregulation in the coagulation pathway to maintain blood flow to tissues. In a randomized controlled trial, mild hypothermia induction improved coagulopathy in septic patients (Johansen et al.2015). In experimental studies, temperatures below 33°C reduce the synthesis and kinetics of clotting enzymes and plasminogen activator inhibitors as well as delay the initiation of thrombus formation and speed of clot formation (Valeri et al.1987; Patt, McCroskey and Moore 1988; Michelson et al.1994; Ruzicka et al.2012), suggesting that cold temperatures may provide a defense strategy against severe coagulopathy.
Once hypoxia occurs, however, the body must employ mechanisms to combat this stress. Increasing cardiac output and ventilation are possible defense strategies; however, these methods are energetically costly to the critically ill host. An alternative mechanism is to induce hypothermia to reduce the oxygen demand of tissues. Consistent with this, physiological mechanisms that promote hypothermia are induced in mammals during hypoxic conditions. Typically endotherms produce a thermogenic response when ambient temperatures are below thermoneutrality. However, in hypoxic rodents, the thermogenic response to cold temperatures is depressed (Tattersall and Milsom 2003; McAllen 2009). Furthermore, there is decreased heat production in hypoxic animals due to allocation of blood supply away from brown adipose tissue (McAllen 2009). Thus, hypoxic mammals appear to reduce the temperature threshold at which they begin their shivering response to generate heat for thermoneutrality. Both ectotherms and endotherms including lizards, mice and rats chose to maintain lower body temperatures under hypoxic conditions through behavioral thermoregulation (Hicks and Wood 1985; Dupre and Owen 1992). The discovery that animals exhibit behavioral hypothermia in response to hypoxia provides support that hypothermia likely provides physiological protection under oxygen stressed conditions. Indeed, survival studies demonstrate that hypothermia induces a number of physiological benefits under oxygen limiting conditions. Survival of hypoxic mice was increased when animals were maintained at 35°C and reduced when housed at 40°C (Gordon 2001). Hypoxic lizards that were allowed to seek cold temperatures exhibited 100% survival. By contrast, 100% of hypoxic lizards that were not allowed to seek cold temperatures died (Hicks and Wood 1985). Rabbits that are anemic exhibited increased survival when maintained at hypothermic conditions presumably due to cold temperatures shifting the oxyhemoglobin dissociation curve to the left influencing oxygen accessibility (Gollan and Aono 1973). Thus, the induction of hypothermia in response to hypoxia may promote tolerance by reducing the oxygen demand by tissues, altering the affinity of hemoglobin for oxygen and avoiding the energetic costs that are associated with increasing cardiac output and ventilation.
After ischemic episodes, restoration of circulation to tissues can be detrimental to tissue health and result in reperfusion injury. Cellular metabolites accumulate during ischemia that become oxidized upon the reintroduction of molecular oxygen to the tissues when blood flow is reestablished resulting in the accumulation of ROS. This activates an inflammatory response in the tissue that leads to tissue damage and death. Evidence from therapeutic hypothermia studies suggests that cold body temperatures can prevent reperfusion induced tissue injury. This protection is likely due to the fact that hypothermia reduces the levels of free radicals and stabilizes cell membranes, cellular swelling and edema (Ning et al.2007; Lampe and Becker 2011).
A wide variety of pathogens can cause tissue damage by induction of host cell death either by direct activation of host cell death machinery or indirectly through the host response to the infection. The dysregulated physiological responses that occur in severe systemic inflammatory conditions and sepsis, such as ischemia and reperfusion, cause cell death and lead to multi-organ failure. The cytokine TNFα is a central mediator of inflammation and induction of the febrile response (Jiang et al.1999). TNFα has also cytolytic effects via the extrinsic apoptosis pathway involving activation of caspase-8 downstream of TNF receptor signaling (Micheau and Tschopp 2003; Walczak 2013). An important component of the heat shock response induced by fever is to promote stress responses that will enable a cell to survive during an inflammatory response. Janeway and colleagues showed that heat shock of fibrosacoma cells reduced sensitivity to TNFα-mediated lysis (Gromkowski, Yagi and Janeway 1989). Furthermore, incubation of TNFα secreting T lymphocytes at elevated temperatures reduced their secretion of TNFα and their cytolytic capabilities (Gromkowski, Yagi and Janeway 1989). Consistent with this, incubation of HeLa cells at a mild hyperthermic temperature of 40°C promoted thermotolerance and conferred protection from apoptosis induced by lethal hyperthermia (42°C –45°C). Protection was associated with the increased expression of various HSPs including HSP 27, 32, 60, 72 and 90 (Bettaieb and Averill-Bates 2008). In a mouse model of non-cerebral malaria, mice deficient for HSP32 (Hmox1−/−) succumbed to infection due to hepatic failure despite having equivalent levels of plasmodium as infected wild-type mice. The heme detoxification conferred by HO-1 was required to prevent sensitization of hepatocytes to TNF-mediated apoptosis (Seixas et al.2009). Thus, inhibition of apoptosis by HO-1 promotes tolerance of malaria by preventing hepatic failure and death. In addition to controlling sensitivity to TNF, HSPs have been shown to influence downstream signaling upon activation of the death receptor (Meng et al.1999; Hasday and Singh 2000; Johnson and Fleshner 2006). TNFα can also induce necrosis in some cell types (Morgan, Kim and Liu 2008). HSP90 has been reported to determine whether a cell will face an apoptotic or necrotic death fate (Fulda et al.2010).
HSPs also influence the intrinsic apoptosis pathway involving the release of cytochrome c from the mitochondria in response to cell death signals. Cytochrome c binds to Apaf-1, inducing oligomerization and recruitment of procaspase-9 resulting in the formation of the apoptosome and activation of caspase-9, which then activates caspase-3. This results in freeing of caspase-9 from the apoptosome and is then replaced with another caspase-9 molecule. HSP70 and HSP90 have also been observed to inhibit apoptosome formation (Beere et al.2000; Pandey et al.2000; Saleh et al.2000). HSP-60 primarily resides in the mitochondrial matrix and has been shown to exert its antiapoptotic effects by influencing caspase-3 activation (Xanthoudakis et al.1999). Various HSPs have also been demonstrated to block apoptosis by manipulating processes downstream of caspase activation (Creagh, Sheehan and Cotter 2000). During anaerobic glycolysis, the resulting acidosis leads to the influx of Ca2+ into cells and an inhibition of membrane-bound pumps and channels that are normally responsible for maintaining intracellular calcium homeostasis. Mitochondrial dysfunction may result from the excess intracellular Ca2+ leading to cell death via the intrinsic apoptotic pathway. Hypothermia has been shown to alleviate pump dysregulation and help maintain intracellular Ca2+ homeostasis and may prevent cell death (Siesjo et al.1989; Hall 1997). This effect is likely indirect as studies have shown that, while not affecting apoptotic caspase activation, cold temperatures delay apoptosis in response to stress by delaying cytochrome c release (Goldstein et al.2000).
Heat and response proteins associated with heat have also been shown to promote various types of cell death. Permeabilization of the mitochondrial membrane is dependent on the activation and oligomerization of multidomain Bcl2-family proteins Bax and Bak. Heat can directly activate Bax and Bak to induce cytochrome c release (Pagliari et al.2005). Necroptosis, a programmed form of necrosis type cell death, has important roles in host defense against viral infections and inflammation. Emerging evidence suggests that HSP90 is required for the induction of necroptosis (Jacobsen et al.2016; Zhao et al.2016). Thus, body temperature changes during infection likely directly or indirectly orchestrates the optimal balance of pro- and anti-cell death regulator mechanisms that likely influence host tolerance.
OPEN QUESTIONS AND FUTURE PERSPECTIVES
It is clear from experimental and clinical studies that thermoregulation has broad beneficial effects for host defense against infectious diseases and beyond (Table 1, Fig. 2). However, we know very little about the mechanisms by which thermoregulation—both hypothermia and hypothermia influence host defenses. The field of thermoregulation in general and in the context of host defense needs to be reignited. We propose an integration of the conceptual framework of resistance and tolerance into these studies will shed light on our understanding of the function of fever and hypothermic responses in surviving infectious diseases and beyond.
FUNDING
This work was supported by NIH grant R01AI114929 (JSA), the NOMIS Foundation, the Searle Scholar Foundation (JSA), the Ray Thomas Edward Foundation (JSA).
Conflict of interest. None declared.
REFERENCES
- Alkabie S, Boileau AJ. The role of therapeutic hypothermia after traumatic spinal cord injury-a systematic review. World Neurosurg 2016;86:432–49 [DOI] [PubMed] [Google Scholar]
- Allen VW, O'connor RM, Ulgherait M et al. Period-regulated feeding behavior and TOR signaling modulate survival of infection. Curr Biol 2016;26:184–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, et al. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS One 2006a;1:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, et al. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci 2006b;23:3359–67 [DOI] [PubMed] [Google Scholar]
- Andresen M, et al. Therapeutic hypothermia for acute brain injuries. Scand J Trauma Resusc Emerg Med 2015;23:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JS. Inflammasome-microbiota interplay in host physiologies. Cell Host Microbe 2013;14:491–7 [DOI] [PubMed] [Google Scholar]
- Ayres JS, Freitag N, Schneider DS. Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics 2008;178:1807–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol 2008;6:2764–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol 2009;7:e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol 2011;30:271–94 [DOI] [PubMed] [Google Scholar]
- Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol 2012;30:271–94 [DOI] [PubMed] [Google Scholar]
- Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med 2012;18:799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JM, et al. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007;2:371–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2000;2:469–75 [DOI] [PubMed] [Google Scholar]
- Benetti E, et al. The NLRP3 Inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediat Inflamm 2013;2013:678627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim HA, Kluger MJ. Fever and antipyresis in the lizard Dipsosaurus dorsalis. Am J Physiol 1976;231:198–203 [DOI] [PubMed] [Google Scholar]
- Bettaieb A, Averill-Bates DA. Thermotolerance induced at a fever temperature of 40 degrees C protects cells against hyperthermia-induced apoptosis mediated by death receptor signalling. Biochem Cell Biol 2008;86:521–38 [DOI] [PubMed] [Google Scholar]
- Beumer C, et al. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther 2003;307:737–44 [DOI] [PubMed] [Google Scholar]
- Beutler B, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 1985;316:552–4 [DOI] [PubMed] [Google Scholar]
- Bicego KC, Branco LG. Discrete electrolytic lesion of the preoptic area prevents LPS-induced behavioral fever in toads. J Exp Biol 2002;205:3513–8 [DOI] [PubMed] [Google Scholar]
- Bicego KC, et al. Indomethacin impairs LPS-induced behavioral fever in toads. J Appl Physiol (1985) 2002;93:512–6 [DOI] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010;330:379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JM, et al. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell 2007;6:63–71 [DOI] [PubMed] [Google Scholar]
- Cahill CM, et al. Transcriptional repression of the prointerleukin 1beta gene by heat shock factor 1. J Biol Chem 1996;271:24874–9 [PubMed] [Google Scholar]
- Carmichael LE, Barnes FD, Percy DH. Temperature as a factor in resistance of young puppies to canine herpesvirus. J Infect Dis 1969;120:669–78 [DOI] [PubMed] [Google Scholar]
- Chevalier C, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell 2015;163:1360–74 [DOI] [PubMed] [Google Scholar]
- Chisholm KI, et al. Hypothermia protects brain mitochondrial function from hypoxemia in a murine model of sepsis. J Cerebr Blood F Met 2016;36:1955–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costelli P, et al. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 1993;92:2783–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagh EM, Sheehan D, Cotter TG. Heat shock proteins–modulators of apoptosis in tumour cells. Leukemia 2000;14:1161–73 [DOI] [PubMed] [Google Scholar]
- Croker B, et al. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat Genet 2007;39:1453–60 [DOI] [PubMed] [Google Scholar]
- Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries: positive or negative effects. Neural Regen Res 2013;8:2677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, et al. Neuroprotection: lessons from hibernators. Comp Biochem Phys B 2012;162:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller KR, Zhu L. Hypothermia therapy for brain injury. Annu Rev Biomed Eng 2009;11:135–62 [DOI] [PubMed] [Google Scholar]
- Drew KL, et al. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem 2007;102:1713–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre RK, Owen TL. Behavioral thermoregulation by hypoxic rats. J Exp Zool 1992;262:230–5 [DOI] [PubMed] [Google Scholar]
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015;15:335–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Stuart-Fox D, Cadena V. Cyclic colour change in the bearded dragon Pogona vitticeps under different photoperiods. PLoS One 2014;9:e111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, et al. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 2011;145:398–409 [DOI] [PubMed] [Google Scholar]
- Fiuza C, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003;101:2652–60 [DOI] [PubMed] [Google Scholar]
- Flouris AD. Functional architecture of behavioural thermoregulation. Eur J Appl Physiol 2011;111:1–8 [DOI] [PubMed] [Google Scholar]
- Frankenfield DC, et al. Relative association of fever and injury with hypermetabolism in critically ill patients. Injury 1997;28:617–21 [DOI] [PubMed] [Google Scholar]
- Fulda S, et al. Cellular stress responses: cell survival and cell death. Int J Cell Biol 2010;2010:214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity PA, et al. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Gene Dev 2010;24:2365–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JC, et al. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2000;2:156–62 [DOI] [PubMed] [Google Scholar]
- Gollan F, Aono M. The effect of temperature on asanguineous rabbits. Cryobiology 1973;10:321–7 [DOI] [PubMed] [Google Scholar]
- Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol 1991;260:E727–30 [DOI] [PubMed] [Google Scholar]
- Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. P Soc Exp Biol Med 1994;205:182–5 [DOI] [PubMed] [Google Scholar]
- Gordon CJ. The therapeutic potential of regulated hypothermia. Emerg Med J 2001;18:81–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkowski SH, Yagi J, Janeway CA Jr. Elevated temperature regulates tumor necrosis factor-mediated immune killing. Eur J Immunol 1989;19:1709–14 [DOI] [PubMed] [Google Scholar]
- Hacker MR, Rothenburg BA, Kluger MJ. Plasma iron, copper, and zinc in lizard Dipsosaurus dorsalis: effects of bacteria injection. Am J Physiol 1981;240:R272–5 [DOI] [PubMed] [Google Scholar]
- Hall ED. Brain attack. Acute therapeutic interventions. Free radical scavengers and antioxidants. Neurosurg Clin N Am 1997;8:195–206 [PubMed] [Google Scholar]
- Hanada R, et al. Central control of fever and female body temperature by RANKL/RANK. Nature 2009;462:505–9 [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 1988;12:123–37 [DOI] [PubMed] [Google Scholar]
- Hasday JD, Singh IS. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperon 2000;5:471–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze J, Walter B. Moribund ants leave their nests to die in social isolation. Curr Biol 2010;20:249–52 [DOI] [PubMed] [Google Scholar]
- Hernangomez M, et al. Brain innate immunity in the regulation of neuroinflammation: therapeutic strategies by modulating CD200-CD200R interaction involve the cannabinoid system. Curr Pharm Des 2014;20:4707–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JW, Wood SC. Temperature regulation in lizards: effects of hypoxia. Am J Physiol 1985;248:R595–600 [DOI] [PubMed] [Google Scholar]
- Hindle AG, et al. Skeletal muscle proteomics: carbohydrate metabolism oscillates with seasonal and torpor-arousal physiology of hibernation. Am J Physiol-Reg I 2011;301:R1440–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison VH, Spriestersbach KK. Histamine and histamine receptors: behavioral thermoregulation in the salamander Necturus maculosus. Comp Biochem Phys C 1986;85:199–206 [DOI] [PubMed] [Google Scholar]
- Iseri VJ, Klasing KC. Changes in the amount of lysine in protective proteins and immune cells after a systemic response to dead Escherichia coli: implications for the nutritional costs of immunity. Integr Comp Biol 2014;54:922–30 [DOI] [PubMed] [Google Scholar]
- Ivanov AI, et al. Platelet-activating factor: a previously unrecognized mediator of fever. J Physiol 2003;553:221–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AV, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis 2016;7:e2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager J, et al. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007;148:241–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, et al. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol 1999;276:R1653–60 [DOI] [PubMed] [Google Scholar]
- Johansen ME, et al. Mild induced hypothermia: effects on sepsis-related coagulopathy–results from a randomized controlled trial. Thromb Res 2015;135:175–82 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukocyte Biol 2006;79:425–34 [DOI] [PubMed] [Google Scholar]
- Jounai N, et al. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol 2012;2:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanou M, et al. Julius Wagner-Jauregg (1857–1940): introducing fever therapy in the treatment of neurosyphilis. Psychiatriki 2013;24:208–12 [PubMed] [Google Scholar]
- Kluger MJ. Fever. Pediatrics 1980;66:720–4 [PubMed] [Google Scholar]
- Kluger MJ. Is fever beneficial?. Yale J Biol Med 1986;59:89–95 [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ. Fever in acute disease–beneficial or harmful?. Wien Klin Wochenschr 2002;114:73–5 [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science 1975;188:166–8 [PubMed] [Google Scholar]
- Kluger MJ, et al. Cytokines and fever. Neuroimmunomodulation 1995;2:216–23 [DOI] [PubMed] [Google Scholar]
- Kluger MJ, et al. Role of fever in disease. Ann NY Acad Sci 1998;856:224–33 [DOI] [PubMed] [Google Scholar]
- Koella JC, Sorense FL. Effect of adult nutrition on the melanization immune response of the malaria vector Anopheles stephensi. Med Vet Entomol 2002;16:316–20 [DOI] [PubMed] [Google Scholar]
- Kohlhauer M, et al. Hypothermic total liquid ventilation is highly protective through cerebral hemodynamic preservation and sepsis-like mitigation after asphyxial cardiac arrest. Crit Care Med 2015;43:e420–30 [DOI] [PubMed] [Google Scholar]
- Koyama I, et al. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin Biochem 2002;35:455–61 [DOI] [PubMed] [Google Scholar]
- Kozak W, Fraifeld V. Non-prostaglandin eicosanoids in fever and anapyrexia. Front Biosci 2004;9:3339–55 [DOI] [PubMed] [Google Scholar]
- Kristan DM. Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell 2007;6:817–25 [DOI] [PubMed] [Google Scholar]
- Kwiatkoski M, et al. Hydrogen sulfide as a cryogenic mediator of hypoxia-induced anapyrexia. Neuroscience 2012;201:146–56 [DOI] [PubMed] [Google Scholar]
- Lampe JW, Becker LB. State of the art in therapeutic hypothermia. Annu Rev Med 2011;62:79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2010;2:51ra71. [DOI] [PubMed] [Google Scholar]
- Lazarus M, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 2007;10:1131–3 [DOI] [PubMed] [Google Scholar]
- Lee CT, Repasky EA. Opposing roles for heat and heat shock proteins in macrophage functions during inflammation: a function of cell activation state?. Front Immunol 2012;3:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, et al. Defining immunological impact and therapeutic benefit of mild heating in a murine model of arthritis. PLoS One 2015;10:e0120327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR. Hypothermia in systemic inflammation: role of cytokines. Front Biosci 2004;9:1877–88 [DOI] [PubMed] [Google Scholar]
- Levi M, van der Poll T, Schultz M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin Immunopathol 2012;34:167–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, et al. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol Immunol 2008;45:810–7 [DOI] [PubMed] [Google Scholar]
- Liu E, et al. Naturally occurring hypothermia is more advantageous than fever in severe forms of lipopolysaccharide- and Escherichia coli-induced systemic inflammation. Am J Physiol-Reg I 2012;302:R1372–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungato L, et al. Paradoxical sleep deprivation impairs mouse survival after infection with malaria parasites. Malar J 2015;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM. Short of air? Cool it!. J Physiol 2009;587:5009–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak PA, Plaisance KI. Benefits and risks of antipyretic therapy. Ann N Y Acad Sci 1998;6:214–23 [DOI] [PubMed] [Google Scholar]
- Mackowiak PA, Ruderman E, Martin EM et al. Effects of physiologic variations in temperature on the rate of antibiotic-induced bacterial killing. Am J Clin Pathol 1981;76:57–62 [DOI] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem 2008;77:727–54 [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 2005;62:670–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 2012;335:936–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, et al. Inhibition of myocardial TNF-alpha production by heat shock. A potential mechanism of stress-induced cardioprotection against postischemic dysfunction. Ann N Y Acad Sci 1999;874:69–82 [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003;114:181–90 [DOI] [PubMed] [Google Scholar]
- Michelson AD, et al. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost 1994;71:633–40 [PubMed] [Google Scholar]
- Morag M, et al. Influence of socioeconomic status on behavioral, emotional and cognitive effects of rubella vaccination: a prospective, double blind study. Psychoneuroendocrinology 1998;23:337–51 [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Kim YS, Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res 2008;18:343–9 [DOI] [PubMed] [Google Scholar]
- Murapa P, et al. Physiological fever temperature induces a protective stress response in T lymphocytes mediated by heat shock factor-1 (HSF1). J Immunol 2007;179:8305–12 [DOI] [PubMed] [Google Scholar]
- Murapa P, et al. Heat shock factor 1 protects mice from rapid death during Listeria monocytogenes infection by regulating expression of tumor necrosis factor alpha during fever. Infect Immun 2011;79:177–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjar A, et al. Brain cyclooxygenase-2 mediates interleukin-1-induced cellular activation in preoptic and arcuate hypothalamus, but not sickness symptoms. Neurobiol Dis 2010;39:393–401 [DOI] [PubMed] [Google Scholar]
- Nagashima K, et al. Neuronal circuitries involved in thermoregulation. Auton Neurosci 2000;85:18–25 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol 2011;589:3641–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, et al. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 2002;22:4600–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, et al. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci 2005;22:3137–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, et al. Astrocytic TLR4 expression and LPS-induced nuclear translocation of STAT3 in the sensory circumventricular organs of adult mouse brain. J Neuroimmunol 2015;278:144–58 [DOI] [PubMed] [Google Scholar]
- Ning XH, et al. Moderate hypothermia (30 degrees C) maintains myocardial integrity and modifies response of cell survival proteins after reperfusion. Am J Physiol-Heart C 2007;293:H2119–28 [DOI] [PubMed] [Google Scholar]
- Nomoto S. Diurnal variations in fever induced by intravenous LPS injection in pigeons. Pflugers Arch 1996;431:987–9 [DOI] [PubMed] [Google Scholar]
- Oka T. Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors. Front Biosci 2004;9:3046–57 [DOI] [PubMed] [Google Scholar]
- Oka T, et al. Relationship of EP(1-4) prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J Comp Neurol 2000;428:20–32 [DOI] [PubMed] [Google Scholar]
- Osaka T. Hypoxia-induced hypothermia mediated by GABA in the rostral parapyramidal area of the medulla oblongata. Neuroscience 2014;267:46–56 [DOI] [PubMed] [Google Scholar]
- Pagliari LJ, et al. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. P Natl Acad Sci USA 2005;102:17975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas HN, et al. Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res 2010;88:1962–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J 2000;19:4310–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro FM, et al. Central heme oxygenase-carbon monoxide pathway in the control of breathing under normoxia and hypoxia. Resp Physiol Neurobi 2002;130:151–60 [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev 2005;126:938–50 [DOI] [PubMed] [Google Scholar]
- Patt A, McCroskey BL, Moore EE. Hypothermia-induced coagulopathies in trauma. Surg Clin North Am 1988;68:775–85 [DOI] [PubMed] [Google Scholar]
- Paul L, Fraifeld V, Kaplanski J. Evidence supporting involvement of leukotrienes in LPS-induced hypothermia in mice. Am J Physiol 1999;276:R52–8 [DOI] [PubMed] [Google Scholar]
- Perman SM, et al. Clinical applications of targeted temperature management. Chest 2014;145:386–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietroiusti A, et al. Shift work increases the frequency of duodenal ulcer in H pylori infected workers. Occup Environ Med 2006;63:773–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon DC, et al. Sickness: from the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci Biobehav R 2015;57:30–45 [DOI] [PubMed] [Google Scholar]
- Preest MR, Cree A. Corticosterone treatment has subtle effects on thermoregulatory behavior and raises metabolic rate in the New Zealand common gecko, Hoplodactylus maculatus. Physiol Biochem Zool 2008;81:641–50 [DOI] [PubMed] [Google Scholar]
- Puskarich MA, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013;143:1548–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones QJ, et al. Organ protective mechanisms common to extremes of physiology: a window through hibernation biology. Integr Comp Biol 2014;54:497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007;318:812–4 [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002;418:935–41 [DOI] [PubMed] [Google Scholar]
- Richardson CE, Kooistra T, Kim DH. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 2010;463:1092–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol 2009;9:429–39 [DOI] [PubMed] [Google Scholar]
- Robert CB, Maass DL, White DJ et al. Deficiency in heat shock factor 1 (HSF-1) expression exacerbates sepsis-induced inflammation and cardiac dysfunction. SOJ Surjery 2014;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Steiner AA, Matsumura K. Cells that trigger fever. Cell Cycle 2006;5:2195–7 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, et al. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 2005;10:2193–216 [DOI] [PubMed] [Google Scholar]
- Romeijn N, et al. Sleep, vigilance, and thermosensitivity. Pflugers Arch 2012;463:169–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka J, et al. Hypothermic anticoagulation: testing individual responses to graded severe hypothermia with thromboelastography. Blood Coagul Fibrin 2012;23:285–9 [DOI] [PubMed] [Google Scholar]
- Rzechorzek NM, et al. Hypothermic preconditioning of human cortical neurons requires proteostatic priming. EBioMedicine 2015;2:528–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, et al. Targeted temperature management: current evidence and practices in critical care. Indian J Crit Care Med 2015;19:537–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, et al. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2000;2:476–83 [DOI] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci 2012;15:1088–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan TS, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med 2004;10:638–42 [DOI] [PubMed] [Google Scholar]
- Schieber AM, et al. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science 2015;350:558–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas E, et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. P Natl Acad Sci USA 2009;106:15837–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shido O, Sugimoto N. Possible human endogenous cryogens. Curr Protein Pept Sc 2011;12:288–92 [DOI] [PubMed] [Google Scholar]
- Shido O, et al. Endogenous cryogens existing in the blood of a hypothermic patient. Jpn J Physiol 2004;54:449–56 [DOI] [PubMed] [Google Scholar]
- Shirasu-Hiza MM, et al. Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr Biol 2007;17:R353–5 [DOI] [PubMed] [Google Scholar]
- Shui C, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res 2001;16:731–41 [DOI] [PubMed] [Google Scholar]
- Siesjo BK, et al. Calcium, excitotoxins, and neuronal death in the brain. Ann N Y Acad Sci 1989;568:234–51 [DOI] [PubMed] [Google Scholar]
- Skold-Chiriac S, et al. Body temperature changes during simulated bacterial infection in a songbird: fever at night and hypothermia during the day. J Exp Biol 2015;218:2961–9 [DOI] [PubMed] [Google Scholar]
- Speakman JR, Keijer J. Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab 2012;2:5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starks PT, Blackie CA, Seeley TD. Fever in honeybee colonies. Naturwissenschaften 2000;87:229–31 [DOI] [PubMed] [Google Scholar]
- Steiner AA, Branco LG. Fever and anapyrexia in systemic inflammation: intracellular signaling by cyclic nucleotides. Front Biosci 2003;8:s1398–408 [DOI] [PubMed] [Google Scholar]
- Steiner AA, et al. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood 2006;107:4000–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EF, et al. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog 2012;8:e1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suozzi A, Malatesta M, Zancanaro C. Subcellular distribution of key enzymes of lipid metabolism during the euthermia-hibernation-arousal cycle. J Anat 2009;214:956–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, et al. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol-Heart C 2008;294:H1581–8 [DOI] [PubMed] [Google Scholar]
- Szelenyi Z, et al. Cholecystokinin: possible mediator of fever and hypothermia. Front Biosci 2004;9:301–8 [DOI] [PubMed] [Google Scholar]
- Tattersall GJ, Milsom WK. Transient peripheral warming accompanies the hypoxic metabolic response in the golden-mantled ground squirrel. J Exp Biol 2003;206:33–42 [DOI] [PubMed] [Google Scholar]
- Trieb K, Blahovec H, Kubista B. Effects of hyperthermia on heat shock protein expression, alkaline phosphatase activity and proliferation in human osteosarcoma cells. Cell Biochem Funct 2007;25:669–72 [DOI] [PubMed] [Google Scholar]
- Tuin A, et al. On the role and fate of LPS-dephosphorylating activity in the rat liver. Am J Physiol-Gastr L 2006;290:G377–85 [DOI] [PubMed] [Google Scholar]
- Usach I, Sakopoulos A, Razavi H. Complete resolution of postoperative hemiparesis following carotid endarterectomy with therapeutic hypothermia: a case study and literature review. Ann Vasc Surg 2015;29:1319.e15–7 [DOI] [PubMed] [Google Scholar]
- Valeri CR, et al. Hypothermia-induced reversible platelet dysfunction. Ann Surg 1987;205:175–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 2003;110:255–8 [DOI] [PubMed] [Google Scholar]
- Vaughn LK, Bernheim HA, Kluger MJ. Fever in the lizard Dipsosaurus dorsalis. Nature 1974;252:473–4 [DOI] [PubMed] [Google Scholar]
- Vaughn LK, Veale WL, Cooper KE. Antipyresis: its effect on mortality rate of bacterially infected rabbits. Brain Res Bull 1980;5:69–73 [DOI] [PubMed] [Google Scholar]
- Villamizar N, et al. Impact of daily thermocycles on hatching rhythms, larval performance and sex differentiation of zebrafish. PLoS One 2012;7:e52153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol 2013;5:a008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson WP, Gordon CJ. Caveats regarding the use of the laboratory rat as a model for acute toxicological studies: modulation of the toxic response via physiological and behavioral mechanisms. Toxicology 1993;81:15–31 [DOI] [PubMed] [Google Scholar]
- Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins PR, Frappell PB. The influence of haemoglobin on behavioural thermoregulation and oxygen consumption in Daphnia carinata. Physiol Biochem Zool 2000;73:153–60 [DOI] [PubMed] [Google Scholar]
- Wong KC. Physiology and pharmacology of hypothermia. West J Med 1983;138:227–32 [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S, et al. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J 1999;18:2049–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, et al. Molecular signatures of mammalian hibernation: comparisons with alternative phenotypes. BMC Genomics 2013;14:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki Y, et al. MyD88 plays a key role in LPS-induced Stat3 activation in the hypothalamus. Am J Physiol-Reg I 2010;298:R403–10 [DOI] [PubMed] [Google Scholar]
- Yoshida K, et al. Parallel preoptic pathways for thermoregulation. J Neurosci 2009;29:11954–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir O, et al. Muscle protein breakdown during endotoxemia in rats and after treatment with interleukin-1 receptor antagonist (IL-1ra). Ann Surg 1992;216:381–5, discussion 86–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbikowska Elzbieta. Do larvae of trichobilharzia szidati and echinostoma revolutum generate behavioral fever in lymnaea stagnalis individuals?. Parasitol Res 2005;97:68–72 [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Localized inflammation in peripheral tissue signals the CNS for sickness response in the absence of interleukin-1 and cyclooxygenase-2 in the blood and brain. Neuroscience 2008;157:895–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XM, et al. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis 2016;7:e2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk V, Borisiuk MV. Fe(2+)-initiated chemiluminescence in rats with high hemoglobin-oxygen affinity during fever. J Physiol Pharmacol 1997;48:111–7 [PubMed] [Google Scholar]