Abstract
Cytokines IL-17 and IL-22 play pivotal roles in host defense against microbes and in the development of chronic inflammatory diseases. These cytokines are produced by cells that are often located in epithelial barriers, including subsets of T cells and innate lymphoid cells. In general, IL-17 and IL-22 can be characterized as important cytokines in the rapid response to infectious agents, both by recruiting neutrophils and by inducing the production of antimicrobial peptides. Although each cytokine induces an innate immune response in epithelial cells, their functional spectra are generally distinct: IL-17 mainly induces an inflammatory tissue response and is involved in the pathogenesis of several autoimmune diseases, whereas IL-22 is largely protective and regenerative. In this review, we compare IL-17 and IL-22, describing overlaps and differences in their cellular sources as well as their regulation, signaling, biological functions and roles during disease, with a focus on the contribution of these cytokines to the gut mucosal barrier during bacterial infection.
Keywords: IL-17, IL-22, mucosal immunity
The review highlights the pivotal roles of IL-17 and IL-22 in host defense against microbes.
INTRODUCTION
Leukocytes constitute the second largest class of cells found within the intestine, second only to epithelial cells (Artis 2008; Ma et al.2008). Within the intestinal mucosa, leukocyte types are segregated into two distinct anatomic areas: the lamina propria (LP) and the epithelium (Hooper and Macpherson 2010; Maynard et al.2012). The LP harbors adaptive immune cells such as T cells and B cells, as well as innate immune cells, including dendritic cells (DCs), macrophages and eosinophils. In contrast, specific subsets of cells called intraepithelial lymphocytes (IELs) are found associated with the epithelial layer, in particular at the basement membrane between enterocytes (Cheroutre, Lambolez and Mucida 2011). Gut IELs are almost exclusively T cells, as originally estimated based on histological sections (Darlington and Rogers 1966). In addition, CX3C-chemokine receptor 1 (CX3CR1)+ macrophages and DCs reside within the intestinal epithelial layer and have the capacity to sample antigens in the gut and promote appropriate T-cell responses (Bogunovic et al.2009; Schulz et al.2009).
The gastrointestinal (GI) tract is home to trillions of microbes, comprising thousands of species that reside within the lumen (Mowat and Agace 2014). These microbes, collectively referred to as the gut microbiota, vary in composition and number along the length of the GI tract (Mowat and Agace 2014). Although most intestinal microbes are beneficial and live symbiotically with the host, disruption of the epithelial barrier or invasion by pathogenic microbes elicits a complex immune response involving the intestinal epithelium and leukocytes. Restoration of epithelial integrity and eradication of invading microbes are orchestrated through the activity of cytokines and chemokines, which enable epithelial cells and leukocytes to communicate, and promote responses such as cell migration, differentiation, replication or activation of cell-intrinsic defenses (Matthews, Weight and Parkos 2014).
Intestinal cytokine responses comprise complex signaling networks, wherein cytokines regulate one another (Garlanda, Dinarello and Mantovani 2013; Manzanillo, Eidenschenk and Ouyang 2015). As such, disrupting one particular cytokine response can lead to different outcomes depending on the cell types and the intestinal regions involved, as well as on the gut microbiota. Recognizing the context of these signaling networks can help in understanding the various roles of intestinal cytokines under different conditions. In this review, we discuss recent findings on the interleukin (IL-) 17 and IL-22 pathways, focusing on their roles in intestinal homeostasis and in the host response to infection. Furthermore, we describe the various cell types that produce IL-17 and IL-22 during bacterial infection, and we discuss how these responses are activated and regulated by pattern recognition receptor (PRR)-dependent pathways. Finally, we examine the importance of IL-17 and IL-22 responses in mediating gut immunity to bacterial-induced colitis.
IL-17 and IL-22 are related, but distinct cytokines
Cytokines are secreted proteins that play an important role in intercellular communication. In addition to orchestrating the immune response to pathogens, cytokines also regulate wound healing, angiogenesis and physiological and pathological tissue reorganization (Zhu and Emerson 2002; Leoni et al.2015; Kreuger and Phillipson 2016). Cytokines elicit biological effects by binding to the extracellular moiety of specific transmembrane receptor proteins on the outer membrane of cells. This binding triggers a signaling pathway, which leads to functional changes in these cells. Cytokines are grouped into families based on similarities in genome location, gene structure, structure of the secreted protein and the receptor(s) the cytokine engages. IL-17 and IL-22 are leukocyte-derived cytokines that primarily impact epithelial cells in tissues such as the gut, the lung and the skin (Blaschitz and Raffatellu 2010; Qu et al.2013).
IL-17 belongs to the IL-17 cytokine family, whereas IL-22 is a member of the IL-10 cytokine family (Park et al.2005). The in vivo effector functions of IL-17 and IL-22 are crucial to maintaining mucosal immunity against specific pathogens and include induction of antimicrobial proteins, recruitment of neutrophils to sites of bacterial invasion and enhancement of mucosal barrier repair and maintenance by stimulating epithelial cell proliferation and tight junction protein production (Ye et al.2001a; Liang et al.2006; Aujla et al.2008; Raffatellu et al.2008; Zheng et al.2008; Conti et al.2009; Pickert et al.2009).
There are six members of the IL-17 family: IL-17A (commonly referred to as IL-17), IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25) and IL-17F (Kolls and Linden 2004; Iwakura et al.2011). These cytokines are often co-expressed and can form homo- and heterodimers. In 1995, IL-17 receptor A (IL-17RA) was identified as a new cytokine receptor for IL-17A and was later found to be part of a new cytokine receptor family (Yao et al.1995). The IL-17 receptor family now consists of five members (IL-17RA, RB, RC, RD and RE), all of which, like their ligands, share sequence homology (Aggarwal and Gurney 2002). IL-17RA is expressed on a wide range of tissues and cell types. Upon stimulation with IL-17, IL-17RA initiates the activation of downstream signaling pathways to induce the production of proinflammatory molecules. However, IL-17RA alone is insufficient to mediate IL-17 signaling. Further evaluation revealed that IL-17 signals through a heterodimeric receptor complex composed of IL-17RA and IL-17RC (Toy et al.2006; Rickel et al.2008; Song et al.2011). It is proposed that the binding of ligand to the first IL-17 receptor subunit alters the affinity and specificity of the second binding event, thereby promoting the formation of a heterodimeric, rather than a homodimeric, receptor complex (Ely, Fischer and Garcia 2009; Liu et al.2013). IL-17 receptors work through a pathway that depends on ACT1s (also referred to as CIKS) and activates NF-κB and MAP kinases for the induction of proinflammatory mediators such as IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), some chemokines (CXCL1, CXCL10, CCL2, CCL7, CCL20) and matrix metalloproteinase-3 (MMP3) and MMP13 (Fossiez et al.1996; Gaffen 2009).
IL-22 signals through a heterodimeric receptor comprised of the broadly expressed IL-10R2 subunit and the more restricted IL-22R1 subunit (Dumoutier, Louahed and Renauld 2000; Dumoutier et al.2000; Xie et al.2000). IL-22R1 is mainly expressed on intestinal and respiratory epithelial cells, on keratinocytes and on hepatocytes, but not on cells of hematopoietic origin (Dudakov, Hanash and van den Brink 2015). IL-22 binds first to IL-22R1, and then the IL-22–IL-22R1 complex binds IL-10R2 to propagate downstream signals (Logsdon et al.2002; Li et al.2004). IL-22 signals through the JAK-STAT pathway, inducing phosphorylation of the kinases JAK1 and TYK2, as well as of the transcription factors STAT1, STAT3 and STAT5 (Dumoutier, Louahed and Renauld 2000; Dumoutier et al.2000; Xie et al.2000; Pestka et al.2004). Triggering this signaling pathway allows IL-22 to induce the expression of various tissue-specific genes including those that encode proteins involved in tissue inflammation, immunosurveillance and homeostasis (Wolk et al.2004; Liang et al.2006, 2010; Zheng et al.2008). By eliciting various innate defense mechanisms from epithelial cells, IL-22 is essential for host defense at mucosal surfaces against extracellular pathogens such as bacteria and yeast (Zheng et al.2007, 2008; Aujla et al.2008; Sonnenberg et al.2012). In general, IL-22 acts to strengthen epithelial barrier functions and is involved in tissue homeostasis as well as in tissue repair and wound healing. However, excessive or prolonged production of IL-22 can cause pathology, such as psoriasis-like skin inflammation (Ma et al.2008).
IL-17 was initially found to be secreted by a subset of CD4+ T cells termed Th17 cells, which also secrete the cytokines IL-22 and IL-21 (Harrington et al.2005; Langrish et al.2005; Park et al.2005; Korn et al.2007; Zheng et al.2007). However, recent studies identified several other cell types that contribute to IL-17 and IL-22 production (Wolk et al.2002, 2011; Kondo et al.2009; Ortega et al.2009), including activated CD4+ T cells (Harrington et al.2005; Park et al.2005; Wolk et al.2011), CD8+ T cells (Kondo et al.2009; Ortega et al.2009), as well as various innate lymphoid cells (ILCs) such as natural killer (NK) cells (Hughes et al.2010; Pandya et al.2011), NKT cells (Rachitskaya et al.2008), lymphoid tissue inducer (LTi) cells (Cupedo et al.2009; Crellin et al.2010) and LTi-like cells (Luci et al.2009; Kim et al.2011) (Table 1). The role of these cells in secreting IL-17 and IL-22 is discussed in detail below.
Table 1.
Cell types that secrete IL-17 and/or IL-22.
| Cell type | Cytokine secreted | Transcription factor | Surface phenotype | Human/mouse studies | Ref |
|---|---|---|---|---|---|
| Th17 | IL-17, IL-22 | RORγt | CD4+, CCR4+, CCR6+, CXCR3−, CD161+, IL23R+ | Human and mouse studies | Cosmi et al. (2008), Kleinschek et al. (2009); Park et al. (2005) |
| Th22 | IL-22 | Unknown (AHR?) | CD4+, CCR4+, CCR6+, CCR10+ | Human | Eyerich et al. (2009); Trifari et al. (2009) |
| Tc22 | IL-22 | Unknown | CD4+, CD8+, IL-21R+ | Human | Nograles et al. (2009) |
| Tc17 | IL-17 | Unknown | CD8+, CD45RB+, CD38+, CD103+, CCR6+ | Mouse | Yen et al. (2009) |
| γδ cells | IL-17, IL-22 | AP1 | CD27−, CD28−, NKP80+, CD45RA+, CD158+ | Human and mouse | Peng et al. (2008), Riera-Sans and Behrens (2007), Shibata et al. (2008) |
| NKT | IL-17, IL-22 | ROR | CD3+, CD56+ | Mouse | Rachitskaya et al. (2008) |
| LTi | IL-17, IL-22 | RORC | CD3−, CD56−, CD161+, NKp44−, CD117+, CD127+ | Human | Cupedo et al. (2009) |
| NKp46+ | IL-17, IL-22 | RORC | CD3-, CD56+, NKp44+ NKp46+, NKG2D+, CD117+, CD127+ | Mouse | Satoh-Takayama et al. (2008) |
| NK22 | IL-22 | Unknown | CD3−, CD56+, NKp44+ CD117+, CD127+ | Mouse | Norian et al. (2009) |
Increasing evidence shows that IL-17 and IL-22 can be protective against infections, largely by the following two mechanisms. The first involves production of antimicrobial peptides, which is largely dependent on the synergistic action of IL-17 and IL-22 on epithelial cells (Wolk et al.2004; Liang et al.2006; Zheng et al.2008; Feng et al.2009). The second mechanism involves IL-17 and IL-22 inducing gut and lung epithelial cells to express chemokines that attract granulocytes, particularly neutrophils, to sites of infection (Albanesi et al.2000; Nograles et al.2008). Despite their protective role against extracellular pathogens, IL-17 and IL-22 may also be detrimental when they are not tightly regulated. Indeed, these cytokines contribute to the pathology observed in several autoimmune and chronic inflammatory diseases, including psoriasis, asthma and inflammatory bowel disease (Teunissen et al.1998; Andoh et al.2005; Chan et al.2006; Wolk et al.2007; McKinley et al.2008).
IL-17 and IL-22 are regulated by PRR-dependent pathways
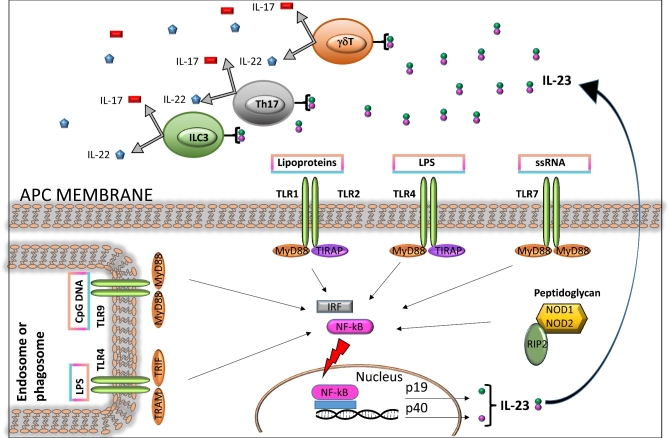
The innate immune system is crucial for controlling infectious agents. By using a wide range of innate immune sensors termed pattern recognition receptors (PRRs), the host recognizes general pathogen-associated molecular patterns (PAMPs), initiating a response that limits the pathogen and alerts the adaptive immune system (Fig. 1). Two of the most important PRR protein families are the membrane-bound Toll-like receptors (TLRs) and the cytosolic Nod-like receptors (NLRs). Mammals have many TLRs, each of which recognizes certain PAMPs. For example, TLR4 recognizes lipopolysaccharide, a major component of the Gram-negative bacterial cell wall (Lu, Yeh and Ohashi 2008). TLR2, which is also located on the cell membrane, recognizes bacterial surface lipoproteins (Kang et al.2009), whereas TLR9, which is located in the membrane of cellular organelles known as endosomes, recognizes non-methylated CpG sequences present in bacterial DNA (Latz et al.2004). When a TLR binds to its ligand, host-cell adaptor proteins, such as Myeloid differentiation primary response gene 88 (MyD88), associate with the cytoplasmic part of the receptor, and downstream signaling is initiated (Medzhitov et al.1998) (Fig. 1).
Figure 1.
Bacterium-induced signaling in antigen-presenting cells (APCs) leads to IL-23 production and subsequent activation of IL-17 and IL-22-producing cells. APCs, for instance DCs and macrophages, express PRRs on their surface, such as TLRs, and intracellular receptors in their cytosol, such as NLRs. These PRRs recognize conserved microbial-associated molecular motifs and PAMPs, including LPS, lipoproteins, and CpG oligodeoxynucleotides. When a TLR binds to its cognate PAMP, host-cell adaptor molecules such as MyD88, TIRAP and TRIF are recruited, and downstream signaling is initiated. The resulting activation of nuclear factor-κB (NF-κB) promotes transcription of a range of genes coding for proinflammatory cytokines, including IL-23, which trigger the production of IL-17 and IL-22 in cell subsets including Th17, γδ T and ILC3.
TLR-dependent pathways are important for host defense against bacterial pathogens (Charrel-Dennis et al.2008; Kawai and Akira 2011; Wang and Liu 2016), including pathways that control IL-17 and IL-22 responses (Abt et al.2016) (Fig. 1). For example, it was recently shown that TLR7 stimulation with the synthetic ligand resiquimod (R848) induces IL-23 expression followed by a burst of IL-22 secretion by ILCs, leading to expression of the antimicrobial lectin REG3γ and restoration of colonization resistance against vancomycin-resistant Enterococcus (VRE) (Abt et al.2016).
Interleukin-23 is a heterodimeric cytokine comprised of the subunits p19 and p40 (Hunter 2005), and is a crucial upstream regulator of IL-17 and IL-22 expression in vivo (Dubin and Kolls 2007; Godinez et al.2009; Gasse et al.2011; Cox et al.2012). In a mouse model of Klebsiella pneumoniae lung infection, bacterial stimulation of TLR4 on DCs results in IL-23 production (Happel et al.2003). IL-23, in turn, triggers rapid production of IL-17 and IL-22 by T cells (Ye et al.2001a,b; Happel et al.2005; Aujla et al.2008), which is required for efficient neutrophil recruitment in this model. Similarly, injection of Escherichia coli into the peritoneal cavity of naïve mice triggers IL-23 production in a TLR4-dependent manner, and the resulting secreted IL-17 largely originates from γδ T cells (Shibata et al.2007). TLR4 also modulates IL-23 expression in mouse bone marrow-derived conventional DCs during Salmonella enterica serovar Enteritidis infection (Siegemund et al.2007). In the presence of intestinal inflammation, IL-23 stimulates T cells in the murine intestinal mucosa to produce IL-17 and IL-22, promoting neutrophil recruitment in response to S. enterica serovar Typhimurium infection (Godinez et al.2009). The TLR/IL-23/IL-17 axis is also an important player in vaccine-induced protection, wherein TLR4 signaling is required for vaccine-induced immunity to Bordetella pertussis in mice (Higgins et al.2006). In this model, production of IL-23 and IL-1β by DCs contributed to Th17 cell differentiation, while increases in IL-17 levels led to enhanced bacterial clearance and macrophage bactericidal activity in a dose-dependent fashion (Higgins et al.2006).
All TLRs except TLR3 require MyD88 as an adaptor to mediate signal transduction (Gay et al.2014). MyD88 appears to be crucial for inducing maximal expression of IL-17 and IL-22 in the gut and the lung (Lebeis et al.2007; Gibson et al.2008; Zhang et al.2009; Keestra et al.2011). In the S. Typhimurium colitis model, Myd88−/− mice exhibit a delayed mucosal inflammatory response and blunted IL-17 and IL-22 levels when compared to wild-type controls (Keestra et al.2011). During gut infection with Citrobacter rodentium, Myd88−/− mice display increased mortality, intestinal pathology and systemic bacterial spread; whether this is associated with IL-17 and IL-22 responses has yet to be elucidated (Lebeis et al.2007; Gibson et al.2008). In the lung, Myd88−/− mice exhibit a reduced innate mucosal IL-17 response during infection with Chlamydia muridarum (Zhang et al.2009). Moreover, in a T-cell transfer model of colitis, Myd88−/− CD4+ T cells express lower levels of IL-17 than wild-type CD4+ T cells, indicating a direct role for MyD88 signaling in modulating T-cell effector function (Fukata et al.2008).
Whether MyD88-dependent regulation of T-cell-induced colitis is due to TLR signaling or IL-1 family cytokine signaling remains unclear. Fukata et al. (2008) showed that CD4+CD45Rbhigh T cells express significant levels of TLRs, suggesting a role for TLR signaling by T cells in Th17 differentiation and in the development of inflammatory bowel disease. On the other hand, Chang et al. (2013) demonstrated that MyD88 mediates IL-1 signaling in CD4+ T cells for the upregulation of IL-23R by IL-23, which expands committed Th17 cells via mTOR activation.
The cytosolic NLRs are another class of PRRs activated during enteric infections, recognizing bacterial PAMPs in the cystosol (Fig. 1) (Inohara and Nunez 2001). NLRs are classified into several subfamilies on the basis of their amino-terminal domain (Ting et al.2008), including the CARD-containing nucleotide-binding oligomerization domain proteins (e.g, NOD1, NOD2 and NLRC4), the pyrin domain-containing proteins (e.g. NLRP3) and the BIR-containing proteins (also known as NAIPs or BIRCs). NOD receptors recognize fragments of peptidoglycan; specifically, NOD1 recognizes γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP), a molecule produced by Gram-negative bacteria (Chamaillard et al.2003; Girardin et al.2003a,b), whereas NOD2 recognizes muramyl dipeptide (MDP), which is present in both Gram-positive and Gram-negative bacteria (Girardin et al.2003a,b). Several NLRs have been shown to have a role in inflammatory diarrhea. Mice deficient in Nod1 and Nod2 exhibit a delayed intestinal inflammatory response in colitis models employing S. Typhimurium or C. rodentium (Geddes et al.2011). In the cecal tissue of these models, when compared to wild-type mice, absence of both Nod1 and Nod2 results in reduced amounts of IL-17 and IL-22, as well as the antimicrobial proteins RegIIIγ and lipocalin 2 (Geddes et al.2011).
NLRP3 and NLRC4 trigger the formation of cytosolic protein complexes called inflammasomes, which in turn regulate the maturation of the cytokines IL-1β and IL-18 (Latz, Xiao and Stutz 2013). Inflammasomes are activated in response to a variety of signals that indicate injury to the host, including tissue damage, metabolic stress and infection. NLRP3 responds to a wide range of PAMPs and DAMPs, including bacterial messenger RNA, bacterial DNA:RNA hybrids, MDP, DNA and RNA viruses, fungi, protozoa, ATP, uric acid crystals, silica, aluminum hydroxide, asbestos and bee venom (Kanneganti et al.2006; Dostert et al.2008; Hornung et al.2008; Marina-Garcia et al.2008; Sander et al.2011; Franchi et al.2014; Kailasan Vanaja et al.2014; Sha et al.2014). The NLRC4 inflammasome is activated by flagellin (Franchi et al.2006; Miao et al.2006) and the inner rod proteins of type III secretion systems (Miao et al.2010). The inflammasome is a key regulator of immune responses in the gut and inflammasome-dependent IL-17 production has been implicated in protection from bacterial and fungal infection. IL-1β produced by NLRP3 inflammasome activity has been shown to mediate a protective Th17 response against the E. coli heat-labile enterotoxin in mice, indicating that NLRP3 activation could regulate IL-17 responses (Brereton et al.2011). Inflammasome-mediated IL-1β plays a critical role in promoting Ag-specific Th17 cells and in generating shielding immunity against B. pertussis infection (Dunne et al.2010). It has also been demonstrated that caspase-1 and ASC are important in the defense against Candida through IL-1β and IL-18 expression and consequent induction of antifungal Th1 and Th17 responses (van de Veerdonk et al.2011). Moreover, two recent studies (Levy et al.2015; Nowarski et al.2015) demonstrated that epithelial inflammasome activation and IL-18 secretion can control intestinal homeostasis or induce autoinflammation. ILC3 cells are triggered to secrete IL-22, regulating IL-18 expression in epithelial cells, in turn modulating homeostasis and inflammation. Finally, the IL-18 pathway seems to take part in the host's defense against C. rodentium infection downstream of IL-22 (Munoz et al.2015).
IL-17 and IL-22-producing cells in response to acute infection
T helper cells
During the immune response to infection, IL-17A/F and IL-22 are often produced at the same time and at high levels in inflamed tissues. In general, a broad variety of T cells and other leukocytes secrete IL-17 and/or IL-22 (Table 1). The most characterized IL-17/IL-22-secreting cells are T helper 17 (Th17) cells (Korn et al.2009). Th17 cells were first described as peripheral CD4+ T cells that differentiate into a distinct lineage in a GATA-3 and T-bet-independent fashion (Harrington et al.2005; Park et al.2005). This T-cell population is abundant at homeostasis in gut-associated tissues, particularly in the LP, and is defined by expression of the transcription factor RORγt (Ivanov et al.2006, 2008). Th17-cell differentiation depends on IL-6 and on transforming growth factor β (TGF-β) (Bettelli et al.2006; Ivanov et al.2006; Mangan et al.2006). Signal transduction downstream of IL-6 and TGF-β, including STAT3 activation downstream of the IL-6 receptor, induces expression of RORγt, which promotes transcription of Il17a and Il17f (Ivanov et al.2006; Harris et al.2007; Yang et al.2007).
In addition to IL-6 and TGFβ, commensal microbes are necessary for the development of Th17 cells (Atarashi et al.2008; Hall et al.2008), as Th17 cells are absent in germ-free mice (Ivanov et al.2006). Monoassociation of germ-free mice with commensal microbes of the class Clostridiales known as ‘Candidatus Arthromitus’ or ‘segmented filamentous bacteria’ (SFB) is sufficient to induce Th17-cell development (Gaboriau-Routhiau et al.2009; Ivanov et al.2009; Wu et al.2010). Colonization of mice with SFB results in rapid upregulation of serum amyloid A (SAA) proteins in intestinal epithelial cells, which is both necessary and sufficient to directly promote proliferation of RORγt+ CD4+ T cells and IL-17 production (Sano et al.2015). Moreover, IL-22 derived from group 3 innate lymphoid cells (ILC3) seems to potentiate expression of SAAs by intestinal epithelial cells (Atarashi et al.2015; Sano et al.2015). CD11c+ myeloid cells and adhesion-elicited epithelial cell production of reactive oxygen species also contribute to SAA-dependent production of Th17-promoting cytokines (Atarashi et al.2015).
TLR, inflammasome and dectin signaling each appear to be required for SFB-mediated Th17-cell development, suggesting that there may be redundant innate signals (Ivanov et al.2009). Additionally, a recent study suggests that intimate adhesion of SFB to the epithelium is important for Th17 development (Atarashi et al.2015). As SFB have not been identified in the human gut, it is not clear yet whether microbes contribute to Th17 development in humans. Nevertheless, Atarashi et al. (2015) showed that Th17 cells develop in mice upon infection with the human pathogen Enterohemorragic E. coli O157:H7, as well as after administration of a mixture of adherent human commensal strains belonging to different species, including Clostridium, Bifidobacterium, Ruminococcus and Bacteroides isolated from fecal samples of a patient with ulcerative colitis.
The Th17 response is a crucial arm of mucosal immunity against bacterial pathogens in the lung and the intestine. These cells mediate protection in a number of models of lung infection, including K. pneumoniae, Pseudomonas aeruginosa, Shigella flexneri and several Mycobacterium species (Khader et al.2007; Umemura et al.2007; Aujla et al.2008; Priebe et al.2008). In the GI tract, Th17 cells increase host resistance against Helicobacter pylori, C. rodentium and S. Typhimurium (Wu et al.2007; Raffatellu et al.2008; Ivanov et al.2009; Velin et al.2009; Sellge et al.2010). For instance, in a simian immunodeficiency virus infection model in macaques, depletion of gut CD4+ Th17 cells led to increased systemic dissemination of S. Typhimurium (Raffatellu et al.2008). Similarly, depletion of CD3+ T cells in the streptomycin-treated mouse model of S. Typhimurium colitis resulted in a blunted innate IL-17 response and an associated decrease in mucosal protection against the pathogen (Raffatellu et al.2008; Godinez et al.2009). A subset of LP CD4+ T cells that express IL-17 and IL-22 during the innate phase of infection with S. Typhimurium and C. rodentium has subsequently been identified and termed innate Th17 (iTh17) cells (Geddes et al.2011). Finally, two novel subsets of Th17 cells have recently been identified: a proinflammatory subset that produces GM-CSF (Codarri et al.2011; El-Behi et al.2011) and a regulatory subset that produces IL-10 (Esplugues et al.2011). The role of these cell subsets in response to infection is unknown.
Beyond the mucosa, SFB also trigger development of systemic Th17 cells, which can be detrimental; for example, by triggering spontaneous arthritis in a mouse model (Wu et al.2010). Similarly, monoassociation of mice with SFB exacerbates the course of experimental autoimmune encephalomyelitis (Lee et al.2011), a paralytic autoimmune disease induced by immunization with myelin protein and mediated by Th17 cells. Thus, depending on the setting, Th17 cells can be protective, but they can also initiate or exacerbate autoimmunity.
In addition to Th17 cells, a second T helper cell population was recently described as a major source of IL-22 in human tissue. Named Th22 cells, this population expresses the CCR4 and CCR10 chemokine receptors, and is enriched for during inflammatory skin diseases (Nograles et al.2008; Duhen et al.2009; Eyerich et al.2009; Trifari et al.2009). In contrast to Th17 cells, which are able to secrete both IL-17 and IL-22, Th22 cells do not secrete IL-17. A third group of adaptive immune cells that produce IL-17 and/or IL-22 are CD8+ T cells (Kondo et al.2009; Ortega et al.2009). Similar to Th17 cells, a population of CD8+ T cells produces IL-17, but it is unclear whether these cells coproduce IL-22 and whether they represent a functionally distinct subset (Tc17 cells) of CD8+ T cells (Shin et al.1999). Furthermore, there is evidence for a subgroup of CD8+ T cells that produces IL-22, but not IL-17 (Tc22 cells) (Nograles et al.2009). The discovery of these distinct lineages of leukocytes that produce different subsets of cytokines emphasizes that production of IL-17 and IL-22 is regulated by distinct processes, underlining the potential complexity of these responses in different models of infection and autoimmunity.
γδ T cells
γδ T cells are an innate immune cell population that plays important roles at the mucosal barrier. These cells are predominantly found in the intestinal epithelial lymphocyte compartment of the intestinal mucosa and they can be classified into an IFN-γ-producing subset or an IL-17 and IL-22-producing subset (Cua and Tato 2010). CD27− RORγt+ γδ T cells secrete IL-17 and/or IL-22 when stimulated with IL-23, IL-1β, or with a cocktail of phorbol 12-myristate-13-acetate (PMA) and ionomycin (Jensen et al.2008; Ribot et al.2009). Relatedly, IL-23 is sufficient to induce IL-17A production in γδ T cells purified from the peritoneum of infected mice (Shibata et al.2007). Of particular note, γδ T cells can rapidly produce IL-17A upon TLR and/or cytokine stimulation. Early production of IL-17A was observed during E. coli infection, where peritoneal IL-17A was found as early as 1 h after infection (maximal production at 6 h), and large amounts of neutrophils were recruited by 24 h post-infection (Shibata et al.2007). Similarly, IL-17A production by peritoneal γδ T cells is rapidly induced after injection of heat-killed mycobacteria (Martin et al.2009) or after sepsis due to cecal puncture (Flierl et al.2008).
In the skin, IL-17A-producing γδ T cells can promote the formation of abscesses and granulomas to help mediate containment of microbial infections (Cho et al.2010). In cutaneous Staphylococcus aureus infections, IL-17A-producing skin cells play a critical role in the immune response against this pathogen. In this setting, IL-17A helps to recruit neutrophils into skin abscesses, to limit the size of abscesses and to reduce the number of S. aureus bacteria contained in these abscesses (Cho et al.2010).
In Listeria monocytogenes infections, IL-17A-producing γδ T cells contribute to bacterial clearance by containing the bacteria within granulomas in the liver and by promoting the recruitment of neutrophils and other myeloid cells (Hamada et al.2008). In the absence of γδ T cells or IL-17A, bacterial numbers in the liver are more than 100-fold higher than in wild-type mice (Hamada et al.2008). Moreover, L. monocytogenes infection in γδ T-cell-deficient mice is associated with large inflammatory lesions in the liver with necrotic hepatocytes (Mombaerts et al.1993; Fu et al.1994) that are similar to those seen in IL-17A-deficient mice (Hamada et al.2008). IL-17RA and IL-23 are required to control L. monocytogenes systemic infections and γδ T cells are the principal source of IL-17A early in infection (Meeks et al.2009), although CD4−8− αβ T cells also produce IL-17A in the peritoneum (Riol-Blanco et al.2010).
γδ T cells can also be pathogenic. As with many T-cell populations, inappropriate or sustained production of a proinflammatory cytokine can have negative consequences. Several autoimmune disease models have identified IL-17A-producing γδ T cells as being important in disease progression. For example, in a model of collagen-induced arthritis, oligoclonal IL-17A-producing γδ T cells accumulate in the lymph nodes and joints of collagen-injected mice (Roark et al.2007). Depletion of Vγ4 γδ T cells greatly reduces disease severity consistent with a significant reduction in pathogenic anticollagen IgG2a (Roark et al.2007). Moreover, in different models of neuroinflammation, γδ T cells play an important role in early activation and recruitment of cells through the release of IL-17A. In experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis, γδ T cells producing IL-17A accumulate in the brain (Jensen et al.2008; Sutton et al.2009). Although γδ cells alone are not sufficient to reconstitute disease (αβ T cells are also required), γδ-deficient mice exhibit reduced EAE severity (Sutton et al.2009). In addition to producing IL-17A, γδ T cells also inhibit CD4+ Treg responses in EAE and reverse Treg suppression of αβ T cells in vitro (Petermann et al.2010).
The involvement of IL-17A-producing γδ T cells in models of microbial, autoimmune and inflammatory diseases underscores their importance in the initiation and resolution of acute inflammatory responses in a variety of settings. Despite their small numbers, γδ T cells can have large effects on the type of immune response and the disease outcome.
Innate lymphoid cells
The importance of a non-T-cell source for IL-22 was first demonstrated in a C. rodentium infection model, in which wild-type mice and mice that lack B and T cells (Rag2−/−) exhibited comparable IL-22 production and normal host defense during the early phase of infection (Zheng et al.2008). These newly identified cells were termed ILCs, then later categorized in human tonsils and intestinal LP as ILC3 based on their production of IL-17 and IL-22 (Montaldo et al.2014).
ILC3s can secrete IL-17 and IL-22 in response to IL-1β and/or IL-23 due to their constitutive expression of IL-1R and/or IL-23R (Spits and Cupedo 2012). ILCs, like B and T cells, are derived from a common lymphoid progenitor. However, they represent a very heterogeneous group of cells. All IL-17/IL-22-producing ILCs are dependent on RORγt for their development, including LTi cells (Takatori et al.2009), LTi-like ILCs (Buonocore et al.2010), NKp46+ ILCs (Satoh-Takayama et al.2008; Cella et al.2009; Luci et al.2009; Sanos et al.2009), invariant NKT cells (Doisne et al.2011) and mucosal-associated invariant T cells (Le Bourhis et al.2011). Unlike Th17 cells, antigenic priming is not required for activation of LTi, LTi-like, NKp46+ ILCs and probably γδ T cells; IL-23 stimulation is often sufficient for inducing IL-17 and IL-22 secretion by these cell types (Cua and Tato 2010; Cua and Sherlock 2011; Le Bourhis et al.2011).
The lineage of NKp46+ cells was first identified as a major source of IL-22 in the intestine (Satoh-Takayama et al.2008; Cella et al.2009; Luci et al.2009). These cells do not co-express IL-17, lack typical NK cell effector functions, and may or may not express NK1.1. Interestingly, some of these NKp46+ cells also express CD11c, an intergrin highly expressed by DCs. Surprisingly, mice deficient in NKp46 are still capable of secreting IL-22 and are fully resistant to C. rodentium infection (Satoh-Takayama et al.2009). In the spleen and in the mesenteric lymph nodes, another cell type, CD4+ LTi-like cells, produce IL-17 and IL-22 upon IL-23 stimulation (Takatori et al.2009; Sonnenberg et al.2011). LTi cells are also the building blocks of cryptopatches, which are lymphoid structures found in both the small and large intestine. During C. rodentium infection, LTi cells are major sources of IL-22 in the mesenteric lymph nodes (Sonnenberg et al.2011). In contrast to NKp46+ ILCs, the development of LTi cells is regulated by the lymphotoxin (LT) pathway (Eberl et al.2004; Satoh-Takayama et al.2011). LT is a member of the TNF core family and plays a critical role in regulation of mucosal immune responses (Fu and Chaplin 1999; Tumanov, Christiansen and Fu 2007) and contributes to effector immune responses (Ware 2005; Wang et al.2010). LT also regulates DCs and CD4+ T-cell homeostasis in the steady state, as well as determines the functions of these cells during pathogenic challenge (Lewis et al.2011; Upadhyay and Fu 2013).
Consistent with a role for LTi cells during C. rodentium infection, mice deficient in the LT pathway are also more susceptible (Spahn et al.2004; Ota et al.2011). Specifically, wild-type mice treated with either an anti-IL-22 antibody or with LTβRFc fusion protein show similar degrees of susceptibility to C. rodentium infection, suggesting a connection between the LT and IL-22 pathways. Indeed, IL-22 induction in the colon during C. rodentium infection is blocked when the LT pathway is deficient (Tumanov et al.2011). Taken together, these studies suggest a redundant role for different ILC subsets during C. rodentium infection, and the specific contribution of each cell type needs to be further elucidated.
In addition to the aforementioned cell types, a pathogenic population of Thy1+Sca1+ ILCs has been identified in the inflamed colon of mice in a H. hepaticus-induced colitis model (Buonocore et al.2010). These cells are negative for both NKp46 and CD4, but they are able to produce IL-17 and IL-22 upon stimulation with IL-23, and drive the development of colitis (Buonocore et al.2010).
NON-LYMPHOID SOURCES OF IL-17 AND IL-22
Other potential sources of IL-17 and IL-22 include neutrophils that are recruited to the site of infection (Zindl et al.2013; Taylor et al.2014; Lee et al.2015). In the dextran sodium sulfate colitis model, IL-22-producing neutrophils were shown to be protective (Zindl et al.2013). Intestinal pathology was alleviated in DSS-treated Il22−/− mice transplanted with neutrophils from wild-type mice, whereas neutrophils from Il22−/− mice had little effect on disease progression (Zindl et al.2013). During fungal infection, IL-17-producing neutrophils are recruited to the site of infection (Taylor et al.2014), but whether neutrophils produce IL-17 or not appears to be dependent on the infection model (Huppler et al.2015). Proposed mechanisms for activation of IL-17 and IL-22 production by neutrophils include stimulation with cytokines such as IL-6 and IL-23, activation of dectin-1 and 2, and through regulation of RORγt and aryl-hydrocarbon receptor (AhR) via activation of mTOR (Werner et al.2011; Zindl et al.2013; Taylor et al.2014; Chen et al.2016). Future studies are needed to further elucidate whether IL-17 and IL-22 production by neutrophils contributes to the host response to infection.
HOST DEFENSE AND PROTECTIVE FUNCTION OF IL-17 AND IL-22 DURING INTESTINAL INFECTION
In the gut mucosa, IL-17 and IL-22 have synergistic effects on the induction of antimicrobial proteins by GI epithelial cells, which have an important role in limiting dissemination of pathogens and of commensal bacteria that could penetrate a disrupted epithelial barrier (Zheng et al.2008; Ismail, Behrendt and Hooper 2009). IL-17 and IL-22 act in concert to orchestrate the mucosal barrier, but the biological effects of these cytokines differ in many aspects. IL-22 appears to be a novel type of immune mediator that increases the immune defenses of tissue cells, protects tissues from damage and enhances their regeneration, whereas IL-17A and IL-17F are typical proinflammatory mediators. Below we discuss the effects of IL-17 and IL-22 primarily in the context of gut infection.
IL-17
The most prominent function of IL-17 is the recruitment of neutrophils to the site of infection, which is necessary for clearing microorganisms (Ishigame et al.2009). IL-17 stimulates target cells to produce neutrophil chemoattractants such as the CXC chemokines IL-8 (CXCL8), CXCL1 and CXCL2, as well as growth factors, including G-CSF and GM-CSF (Fig. 2). Neutrophil recruitment is required for a rapid response (i.e. within 4–8 h) against bacterial and fungal pathogens such as Citrobacter rodentium, Salmonella Typhimurium and Candida albicans (Raffatellu et al.2008; Saijo et al.2010; Geddes et al.2011). IL-17 also synergizes with other cytokines, such as IL-1, IL-6 and TNF-α, which together promote activation of tissue infiltrating neutrophils to effectively eliminate extracellular pathogens (Awane et al.1999; Dong 2008). Mice infected with C. rodentium mount protective IL-17 responses, and disruption of IL-17 or its receptor leads to exacerbated bacterial burden and dissemination, increased disease susceptibility resulting from defective induction of CXC chemokines and impaired neutrophil recruitment (Happel et al.2005; Mangan et al.2006). Importantly, administration of recombinant IL-17 into IL-17-deficient mice infected with C. rodentium restored neutrophilia at sites of inoculation (Happel et al.2005), demonstrating the critical importance of the IL-23/IL-17 axis in host defense against extracellular bacterial infections. Neutrophils are also an important first line of mucosal defense against S. Typhimurium. In patients affected by primary neutrophil immunodeficiency (e.g. chronic granulomatous disease), S. Typhimurium often disseminates from the gut, resulting in bacteremia (Mouy et al.1989; Winkelstein et al.2000). In accordance with these observations, Il17ra−/− mice exhibit reduced neutrophil accumulation in the gut, and increased translocation of S. Typhimurium to the mesenteric lymph nodes and spleen (Raffatellu et al.2008).
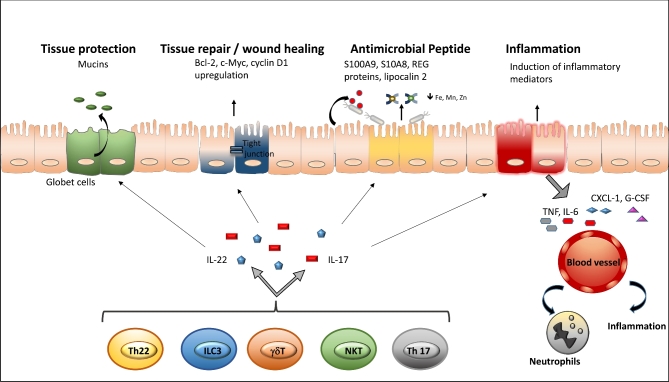
Figure 2.
Functions of IL-17 and IL-22 at epithelial surfaces. IL-17 and IL-22 are upregulated in response to infection with a variety of pathogens. These cytokines are produced by subsets of cells, including γδ T cells, ILC3 and Th17 cells, and elicit innate responses from mucosal epithelial cells. These responses include production of mucins by goblet cells, upregulation of genes involved in wound healing (e.g. Bcl-2, c-Myc, cyclin D1), the secretion of antimicrobial molecules (e.g. S100A8, S100A9, REG proteins, lipocalin-2) and the induction of proinflammatory mediators (e.g. TNF-α, IL-6, CXCL1, G-CSF) that contribute neutrophil recruitment at the site of infection. The functions of both cytokines are overlapping and in part synergistic. While IL-22 is more potent in tissue protection, repair and induction of antimicrobial peptides, IL-17 mediates stronger inflammatory responses by inducing other proinflammatory cytokines and the recruitment of neutrophils.
In addition to neutrophil recruitment, the IL-17 cytokine family targets epithelial cells to induce antimicrobial responses against extracellular pathogens and to promote tissue remodeling (Ouyang, Kolls and Zheng 2008). An important mechanism of host defense in the intestine against bacterial pathogens is the presence of tight junctions that maintain the integrity of the intestinal epithelium, thereby preventing bacterial translocation (Macdonald and Monteleone 2005). In the gut epithelium, IL-17 stimulates the secretion of claudin 1 and claudin 2, proteins that are involved in the formation of tight junctions, ultimately forming a large, interconnecting network and maintaining intestinal integrity (Moran et al.2009; Dungan and Mills 2011). Although IL-17 is largely considered proinflammatory, the cytokine may also promote restoration of the mucosal barrier and further control microbial translocation through the induction of tight junction proteins.
IL-22
The beneficial role of IL-22 during host defense has been studied in several models of intestinal infection, including C. rodentium (Ota et al.2011) and Clostridium difficile (Hasegawa et al.2014), as well as during infection with VRE, a gut commensal bacterium that causes bacteremia and endocarditis (Abt et al.2016).
The functions of IL-22 during host defense against pathogens can be summarized into three major categories (Fig. 2). First, by promoting epithelial proliferation, IL-22 helps to maintain and restore epithelial barrier function after infection. Second, IL-22, together with other cytokines such as IL-17 or TNF-α, induces the expression of antimicrobial proteins involved in host defense in the skin, the airways and the intestine. Some antimicrobial proteins induced by IL-22 include lipocalin-2 and calprotectin, which is a heterodimer of S100A8 and S100A9 (Behnsen et al.2014). These antimicrobial proteins sequester essential metal ions from pathogens (Corbin et al.2008; Liu et al.2012), a process known as nutritional immunity (Kehl-Fie and Skaar 2010). Therefore, IL-22-mediated induction of metal sequestration is an essential process in limiting growth and translocation of commensal microbes from the gut (Flo et al.2004; Berger et al.2006; Raffatellu et al.2009). IL-22 also promotes the production of inflammatory mediators such as IL-1β, SAA and LPS-binding protein (Wolk et al.2007; Dalmas et al.2014; Sano et al.2015). During Klebsiella pneumoniae infection, IL-22 is essential for the release of chemokines such as CXCL1, CXCL5 and CXCL9, as well as IL-6 and G-CSF from airway epithelial cells (Aujla et al.2008). Overall, the primary function of IL-22 is to promote mucosal barrier integrity, thus limiting bacterial replication and dissemination.
During C. rodentium infection, IL-22 stimulates the production of protective mucins from goblet cells (Sugimoto et al.2008; Turner, Stockinger and Helmby 2013) and induces the release of the C-type lectins REG3β and REG3γ from intestinal epithelial cells in the colon (Cash et al.2006; Zheng et al.2008). REG3γ is a soluble C-type lectin produced by Paneth cells and epithelial cells, which exerts its antimicrobial activity by interacting directly with the bacterial cell wall (Cash et al.2006). Mice lacking IL-17 and IL-22 have reduced amounts of antimicrobial peptides and are highly susceptible to C. rodentium infection; however, they can be rescued by treatment with recombinant REG3γ (Zheng et al.2008). Microbiota-derived LPS maintains basal expression of REG3γ in intestinal epithelial cells and Paneth cells. Indeed, REG3γ is not detected in germ-free mice (Cash et al.2006; Mukherjee et al.2014), and even short-term antibiotic treatment impairs its expression, rendering mice susceptible to VRE infection, a defect that can be reverted by oral administration of LPS (Brandl et al.2008). Similarly, flagellin from commensal bacteria sensed by TLR5 expressed on CD103+ CD11b+ DCs found in the LP contributes to the maintenance of REG3γ expression. Upon TLR5 activation, DCs produce IL-23, promoting IL-22 release by ILCs, and therefore REG3γ expression in intestinal epithelial cells (Kinnebrew et al.2010; Kinnebrew et al.2012). The importance of the IL-22-REG3γ axis against bacterial infections has been recently demonstrated by Abt et al. (2016). Briefly, these authors showed that Resiquimod (R848), a synthetic ligand for TLR-7 that stimulates antiviral innate immune defenses, induces IL-22 expression, restoring REG3γ and reestablishing colonization resistance against VRE in antibiotic-treated mice.
Another mechanism by which IL-22 controls opportunistic gut pathogens is by facilitating the binding of complement component C3 to bacteria (Hasegawa et al.2014). When the intestinal mucosa is infected with C. difficile, this organism is capable of translocating to other tissues, contributing to C. difficile-induced mortality. However, IL-22 induces an increase of C3 expression and secretion in the liver, which is mediated by the STAT3 pathway. This process leads to a substantial increase in systemic serum levels of C3, which in turn mediates enhanced opsonization of translocated commensals and pathogens, and their subsequent killing and clearance by neutrophils and macrophages (Hasegawa et al.2014).
Although IL-22 plays a beneficial role in many infection models, recent studies have shown that IL-22 production may also be detrimental to the host. For instance, during S. Typhimurium infection, IL-22 does not play a protective role, but is instead exploited by the pathogen to colonize the gut to high levels (Behnsen et al.2014). IL-22 favors S. Typhimurium colonization by inducing antimicrobial proteins that sequester metal ions. As the pathogen is able to overcome metal starvation, it outcompetes related commensals such as Escherichia coli (Behnsen et al.2014). During Helicobacter pylori infection, IL-22 also contributes to the development of gastric pathology. Both H. pylori-infected humans and mice exhibit an overabundance of IL-22 (Zhuang et al.2015), which stimulates gastric epithelial cells to secrete CXCL2. CXCL2 then recruits myeloid-derived suppressor cells that produce the proinflammatory proteins S100A8 and S100A9 and inhibit Th1 cell responses, thereby contributing to the development of H. pylori-associated gastritis (Zhuang et al.2015).
CONCLUSIONS
The intestinal tract is home to commensal bacteria and fungi that have coevolved with the mammalian host. In order to maintain intestinal homeostasis, the mucosal immune system must balance between an appropriate response to dangerous pathogens and an inappropriate response to commensal microbiota that breach the epithelial barrier. IL-17 and IL-22 have been shown to play a critical role in maintaining barrier homeostasis against intestinal pathogens and commensal bacteria. It is becoming increasingly apparent that bacterial pathogens trigger a rapid IL-17 and IL-22-dependent innate defense in the gut mucosa. In turn, intestinal production of these cytokines seems to be regulated homeostatically by interactions between the host and the intestinal microbiota (e.g. SFB).
IL-17 and IL-22 enhance basic innate barrier defenses at mucosal surfaces, such as antimicrobial peptide production and neutrophil recruitment, i.e. rapidly occurring events that precede adaptive immunity. Although the functional spectra of these cytokines overlap with regard to inducing an innate immune response in epithelial cells, they are generally different. IL-17 is largely proinflammatory and can be pathogenic, whereas IL-22 primarily exhibits regenerative and protective roles. Nevertheless, IL-22's proinflammatory effects might contribute to pathology, as could be the case in psoriasis (Zheng et al.2007) or IBD (Brand et al.2006). Altogether, cytokines IL-17 and IL-22 provide barrier integrity against extracellular pathogens by (i) instructing innate immune responses in tissue cells, (ii) inducing the recruitment of adaptive immune cells via epithelial-derived chemokines and (iii) inducing regeneration of epithelial surfaces after inflammation.
The IL-17 and IL-23 (i.e. the cytokine upstream of both IL-17 and IL-22) pathways are clinical targets for multiple inflammatory diseases. For example, the antibody ustekinumab, which neutralizes the p40 subunit of IL-23 and IL-12, is approved to treat psoriasis and is showing benefit in clinical trials for Crohn's disease (Sandborn et al.2008). Clinical data on the use of antibodies specific to the IL-23 p19 subunit are beginning to emerge in IBD and also show efficacy (Sandborn et al.2008). IL-23-specific antibodies are also highly efficacious in psoriasis, suggesting that the efficacy achieved with p40 inhibition is largely a result of IL-23 inhibition (Leonardi et al.2008; Papp et al.2008; Croft, Benedict and Ware 2013). Antibodies to IL-17A or IL-17RA are also highly effective in psoriasis (Papp et al.2012; Langley et al.2014), but surprisingly, they exacerbated Crohn's disease in a subset of patients (Hueber et al.2012). Thus, although IL-23 is important for production and maintenance of IL-17A and IL-17F, inhibition of IL-17A or IL-17RA in patients with Crohn's disease does not phenocopy the effects observed with a p40 or p19 inhibitor. As such, the mechanism(s) underlying the differential efficacy observed in Crohn's disease with inhibition of IL-17 versus IL-23 is an important unanswered question.
Acknowledgments
We would like to acknowledge the Raffatellu lab for helpful suggestions, and Sean-Paul Nuccio for his help with editing the manuscript.
Work in the MR lab is supported by Public Health Service Grants AI126277, AI121928, AI114625, AI105374, and DK058057. MR holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Conflict of interest.None declared.
REFERENCES
- Abt MC, Buffie CG, Susac B et al. TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant enterococcus. Sci Transl Med 2016;8:327ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukocyte Biol 2002;71:1–8 [PubMed] [Google Scholar]
- Albanesi C, Scarponi C, Cavani A et al. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol 2000;115:81–7 [DOI] [PubMed] [Google Scholar]
- Andoh A, Zhang Z, Inatomi O et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005;129:969–84 [DOI] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008;8:411–20 [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008;455:808–12 [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Ando M et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015;163:367–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 2008;14:275–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awane M, Andres PG, Li DJ et al. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol 1999;162:5337–44 [PubMed] [Google Scholar]
- Behnsen J, Jellbauer S, Wong CP et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 2014;40:262–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. P Natl Acad Sci USA 2006;103:1834–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–8 [DOI] [PubMed] [Google Scholar]
- Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol 2010;30:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J et al. Origin of the lamina propria dendritic cell network. Immunity 2009;31:513–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol-Gastr L 2006;290:G827–38 [DOI] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Mihu CN et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 2008;455:804–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton CF, Sutton CE, Ross PJ et al. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J Immunol 2011;186:5896–906 [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010;464:1371–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006;313:1126–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009;457:722–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamaillard M, Hashimoto M, Horie Y et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 2003;4:702–7 [DOI] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med 2006;203:2577–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Burkett PR, Borges CM et al. MyD88 is essential to sustain mTOR activation necessary to promote T helper 17 cell proliferation by linking IL-1 and IL-23 signaling. P Natl Acad Sci USA 2013;110:2270–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel-Dennis M, Latz E, Halmen KA et al. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe 2008;4:543–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Cao A, Yao S et al. mTOR mediates IL-23 induction of neutrophil IL-17 and IL-22 production. J Immunol 2016;196:4390–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 2011;11:445–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 2010;120:1762–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 2011;12:560–7 [DOI] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 2009;206:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Seeley EH, Raab A et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008;319:962–5 [DOI] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med 2008;205:1903–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ota N et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol 2012;5:99–109 [DOI] [PubMed] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD et al. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med 2010;207:281–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 2013;12:147–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock JP. Autoimmunity's collateral damage: Gut microbiota strikes 'back'. Nat Med 2011;17:1055–6 [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 2010;10:479–89 [DOI] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol 2009;10:66–74 [DOI] [PubMed] [Google Scholar]
- Dalmas E, Venteclef N, Caer C et al. T cell-derived IL-22 amplifies IL-1beta-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes 2014;63:1966–77 [DOI] [PubMed] [Google Scholar]
- Darlington D, Rogers AW. Epithelial lymphocytes in the small intestine of the mouse. J Anat 1966;100:813–30 [PMC free article] [PubMed] [Google Scholar]
- Doisne JM, Soulard V, Becourt C et al. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J Immunol 2011;186:662–6 [DOI] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 2008;8:337–48 [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol-Lung C 2007;292:L519–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 2015;33:747–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Geiger R, Jarrossay D et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 2009;10:857–63 [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 2000;164:1814–9 [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Van Roost E, Colau D et al. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. P Natl Acad Sci USA 2000;97:10144–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan LS, Mills KH. Caspase-1-processed IL-1 family cytokines play a vital role in driving innate IL-17. Cytokine 2011;56:126–32 [DOI] [PubMed] [Google Scholar]
- Dunne A, Ross PJ, Pospisilova E et al. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol 2010;185:1711–9 [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 2004;5:64–73 [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 2011;12:568–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol 2009;10:1245–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N et al. Control of TH17 cells occurs in the small intestine. Nature 2011;475:514–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009;119:3573–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JY, Rao GZ, Liu YP et al. Expression of CD1a and CD207 in condyloma acuminatum epidermis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2009;25:420–2 [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Gao H et al. Adverse functions of IL-17A in experimental sepsis. FASEB J 2008;22:2198–205 [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432:917–21 [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 1996;183:2593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 2006;7:576–82 [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol 2014;193:4214–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 1999;17:399–433 [DOI] [PubMed] [Google Scholar]
- Fu YX, Roark CE, Kelly K et al. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol 1994;153:3101–15 [PubMed] [Google Scholar]
- Fukata M, Breglio K, Chen A et al. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J Immunol 2008;180:1886–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009;31:677–89 [DOI] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 2009;9:556–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity 2013;39:1003–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasse P, Riteau N, Vacher R et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One 2011;6:e23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay NJ, Symmons MF, Gangloff M et al. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol 2014;14:546–58 [DOI] [PubMed] [Google Scholar]
- Geddes K, Rubino SJ, Magalhaes JG et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med 2011;17:837–44 [DOI] [PubMed] [Google Scholar]
- Gibson DL, Ma C, Bergstrom KS et al. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol 2008;10:618–31 [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Carneiro LA et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 2003a;300:1584–7 [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003b;278:8869–72 [DOI] [PubMed] [Google Scholar]
- Godinez I, Raffatellu M, Chu H et al. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun 2009;77:387–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 2008;29:637–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Umemura M, Shiono T et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol 2008;181:3456–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Dubin PJ, Zheng M et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 2005;202:761–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Zheng M, Young E et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol 2003;170:4432–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123–32 [DOI] [PubMed] [Google Scholar]
- Harris TJ, Grosso JF, Yen HR et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol 2007;179:4313–7 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Yada S, Liu MZ et al. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity 2014;41:620–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins SC, Jarnicki AG, Lavelle EC et al. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol 2006;177:7980–9 [DOI] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010;10:159–69 [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008;9:847–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W, Sands BE, Lewitzky S et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Becknell B, Freud AG et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 2010;32:803–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol 2005;5:521–31 [DOI] [PubMed] [Google Scholar]
- Huppler AR, Verma AH, Conti HR et al. Neutrophils do not express IL-17A in the context of acute oropharyngeal candidiasis. Pathogens 2015;4:559–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Nunez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene 2001;20:6473–81 [DOI] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009;30:108–19 [DOI] [PubMed] [Google Scholar]
- Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol 2009;182:3047–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008;4:337–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–33 [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S et al. Functional specialization of interleukin-17 family members. Immunity 2011;34:149–62 [DOI] [PubMed] [Google Scholar]
- Jensen KD, Su X, Shin S et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity 2008;29:90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailasan Vanaja S, Rathinam VA, Atianand MK et al. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. P Natl Acad Sci USA 2014;111:7765–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Nan X, Jin MS et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009;31:873–84 [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Ozoren N, Body-Malapel M et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 2006;440:233–6 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011;34:637–50 [DOI] [PubMed] [Google Scholar]
- Keestra AM, Godinez I, Xavier MN et al. Early MyD88-dependent induction of interleukin-17A expression during Salmonella colitis. Infect Immun 2011;79:3131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 2010;14:218–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007;8:369–77 [DOI] [PubMed] [Google Scholar]
- Kim S, Han S, Withers DR et al. CD117(+) CD3(-) CD56(-) OX40Lhigh cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur J Immunol 2011;41:1563–72 [DOI] [PubMed] [Google Scholar]
- Kinnebrew MA, Buffie CG, Diehl GE et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 2012;36:276–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew MA, Ubeda C, Zenewicz LA et al. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis 2010;201:534–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek MA, Boniface K, Sadekova S et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 2009;206:525–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004;21:467–76 [DOI] [PubMed] [Google Scholar]
- Kondo T, Takata H, Matsuki F et al. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol 2009;182:1794–8 [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007;448:484–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M et al. IL-17 and Th17 Cells. Annu Rev Immunol 2009;27:485–517 [DOI] [PubMed] [Google Scholar]
- Kreuger J, Phillipson M. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov 2016;15:125–42 [DOI] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. New Engl J Med 2014;371:326–38 [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005;201:233–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Schoenemeyer A, Visintin A et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004;5:190–8 [DOI] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 2013;13:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourhis L, Guerri L, Dusseaux M et al. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 2011;32:212–8 [DOI] [PubMed] [Google Scholar]
- Lebeis SL, Bommarius B, Parkos CA et al. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol 2007;179:566–77 [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y et al. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. P Natl Acad Sci USA 2011;108Suppl 14615–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Yang H, Yang JY et al. Interleukin-1 (IL-1) signaling in intestinal stromal cells controls KC/ CXCL1 secretion, which correlates with recruitment of IL-22- secreting neutrophils at early stages of Citrobacter rodentium infection. Infect Immun 2015;83:3257–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi CL, Kimball AB, Papp KA et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665–74 [DOI] [PubMed] [Google Scholar]
- Leoni G, Neumann PA, Sumagin R et al. Wound repair: role of immune-epithelial interactions. Mucosal Immunol 2015;8:959–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Thaiss CA, Zeevi D et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 2015;163:1428–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KL, Caton ML, Bogunovic M et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity 2011;35:780–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tomkinson KN, Tan XY et al. Temporal associations between interleukin 22 and the extracellular domains of IL-22R and IL-10R2. Int Immunopharmacol 2004;4:693–708 [DOI] [PubMed] [Google Scholar]
- Liang SC, Nickerson-Nutter C, Pittman DD et al. IL-22 induces an acute-phase response. J Immunol 2010;185:5531–8 [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006;203:2271–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Jellbauer S, Poe AJ et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 2012;11:227–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Song X, Chrunyk BA et al. Crystal structures of interleukin 17A and its complex with IL-17 receptor A. Nat Commun 2013;4:1888. [DOI] [PubMed] [Google Scholar]
- Logsdon NJ, Jones BC, Josephson K et al. Comparison of interleukin-22 and interleukin-10 soluble receptor complexes. J Interf Cytok Res 2002;22:1099–112 [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008;42:145–51 [DOI] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 2009;10:75–82 [DOI] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest 2008;118:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science 2005;307:1920–5 [DOI] [PubMed] [Google Scholar]
- McKinley L, Alcorn JF, Peterson A et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008;181:4089–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006;441:231–4 [DOI] [PubMed] [Google Scholar]
- Manzanillo P, Eidenschenk C, Ouyang W. Deciphering the crosstalk among IL-1 and IL-10 family cytokines in intestinal immunity. Trends Immunol 2015;36:471–8 [DOI] [PubMed] [Google Scholar]
- Marina-Garcia N, Franchi L, Kim YG et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol 2008;180:4050–7 [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ et al. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009;31:321–30 [DOI] [PubMed] [Google Scholar]
- Matthews JD, Weight CM, Parkos CA. Leukocyte-epithelial interactions and mucosal homeostasis. Toxicol Pathol 2014;42:91–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Elson CO, Hatton RD et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489:231–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998;2:253–8 [DOI] [PubMed] [Google Scholar]
- Meeks KD, Sieve AN, Kolls JK et al. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol 2009;183:8026–34 [DOI] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 2006;7:569–75 [DOI] [PubMed] [Google Scholar]
- Miao EA, Mao DP, Yudkovsky N et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. P Natl Acad Sci USA 2010;107:3076–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Arnoldi J, Russ F et al. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature 1993;365:53–6 [DOI] [PubMed] [Google Scholar]
- Montaldo E, Teixeira-Alves LG, Glatzer T et al. Human RORgammat(+)CD34(+) cells are lineage-specified progenitors of group 3 RORgammat(+) innate lymphoid cells. Immunity 2014;41:988–1000 [DOI] [PubMed] [Google Scholar]
- Moran EM, Mullan R, McCormick J et al. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther 2009;11:R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouy R, Fischer A, Vilmer E et al. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediat 1989;114:555–60 [DOI] [PubMed] [Google Scholar]
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol 2014;14:667–85 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Zheng H, Derebe MG et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014;505:103–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Eidenschenk C, Ota N et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity 2015;42:321–31 [DOI] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Brit J Dermatol 2008;159:1092–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Shemer A et al. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 2009;123:1244–52 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norian LA, Rodriguez PC, O'Mara LA et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res 2009;69:3086–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowarski R, Jackson R, Gagliani N et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 2015;163:1444–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega C, Fernandez AS, Carrillo JM et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukocyte Biol 2009;86:435–43 [DOI] [PubMed] [Google Scholar]
- Ota N, Wong K, Valdez PA et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol 2011;12:941–8 [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya AD, Al-Jaderi Z, Hoglund RA et al. Identification of human NK17/NK1 cells. PLoS One 2011;6:e26780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Lebwohl M et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008;371:1675–84 [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. New Engl J Med 2012;366:1181–9 [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng MY, Wang ZH, Yao CY et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol 2008;5:203–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004;22:929–79 [DOI] [PubMed] [Google Scholar]
- Petermann F, Rothhammer V, Claussen MC et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity 2010;33:351–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009;206:1465–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe GP, Walsh RL, Cederroth TA et al. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol 2008;181:4965–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu N, Xu M, Mizoguchi I et al. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin Dev Immunol 2013;2013:968549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachitskaya AV, Hansen AM, Horai R et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol 2008;180:5167–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 2009;5:476–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 2008;14:421–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 2009;10:427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickel EA, Siegel LA, Yoon BR et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol 2008;181:4299–310 [DOI] [PubMed] [Google Scholar]
- Riera-Sans L, Behrens A. Regulation of alphabeta/gammadelta T cell development by the activator protein 1 transcription factor c-Jun. J Immunol 2007;178:5690–700 [DOI] [PubMed] [Google Scholar]
- Riol-Blanco L, Lazarevic V, Awasthi A et al. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J Immunol 2010;184:1710–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA et al. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol 2007;179:5576–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010;32:681–91 [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Fedorak RN et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology 2008;135:1130–41 [DOI] [PubMed] [Google Scholar]
- Sander LE, Davis MJ, Boekschoten MV et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 2011;474:385–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Huang W, Hall JA et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 2015;163:381–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol 2009;10:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Dumoutier L, Lesjean-Pottier S et al. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J Immunol 2009;183:6579–87 [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Lesjean-Pottier S, Sawa S et al. Lymphotoxin-beta receptor-independent development of intestinal IL-22-producing NKp46+ innate lymphoid cells. Eur J Immunol 2011;41:780–6 [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008;29:958–70 [DOI] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 2009;206:3101–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellge G, Magalhaes JG, Konradt C et al. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J Immunol 2010;184:2076–85 [DOI] [PubMed] [Google Scholar]
- Sha W, Mitoma H, Hanabuchi S et al. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. P Natl Acad Sci USA 2014;111:16059–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H et al. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol 2007;178:4466–72 [DOI] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Nakamura R et al. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol 2008;181:5940–7 [DOI] [PubMed] [Google Scholar]
- Shin HC, Benbernou N, Esnault S et al. Expression of IL-17 in human memory CD45RO+ T lymphocytes and its regulation by protein kinase A pathway. Cytokine 1999;11:257–66 [DOI] [PubMed] [Google Scholar]
- Siegemund S, Schutze N, Freudenberg MA et al. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology 2007;212:739–50 [DOI] [PubMed] [Google Scholar]
- Song X, Zhu S, Shi P et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 2011;12:1151–8 [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 2012;336:1321–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM et al. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 2011;34:122–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn TW, Maaser C, Eckmann L et al. The lymphotoxin-beta receptor is critical for control of murine Citrobacter rodentium-induced colitis. Gastroenterology 2004;127:1463–73 [DOI] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol 2012;30:647–75 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Ogawa A, Mizoguchi E et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 2008;118:534–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31:331–41 [DOI] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 2009;206:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Roy S, Leal SM et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol 2014;15:143–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen MB, Koomen CW, de Waal Malefyt R et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol 1998;111:645–9 [DOI] [PubMed] [Google Scholar]
- Ting JP, Lovering RC, Alnemri ES et al. The NLR gene family: a standard nomenclature. Immunity 2008;28:285–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy D, Kugler D, Wolfson M et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol 2006;177:36–9 [DOI] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 2009;10:864–71 [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Christiansen PA, Fu YX. The role of lymphotoxin receptor signaling in diseases. Curr Mol Med 2007;7:567–78 [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Koroleva EP, Guo X et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 2011;10:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Stockinger B, Helmby H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog 2013;9:e1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 2007;178:3786–96 [DOI] [PubMed] [Google Scholar]
- Upadhyay V, Fu YX. Lymphotoxin signalling in immune homeostasis and the control of microorganisms. Nat Rev Immunol 2013;13:270–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Joosten LA, Shaw PJ et al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol 2011;41:2260–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velin D, Favre L, Bernasconi E et al. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology 2009;136:2237–46 e1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Koroleva EP, Kruglov AA et al. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 2010;32:403–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu L. The membrane protein of severe acute respiratory syndrome coronavirus functions as a novel cytosolic pathogen-associated molecular pattern to promote beta interferon induction via a Toll-like-receptor-related TRAF3-independent mechanism. MBio 2016;7:e01872–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 2005;23:787–819 [DOI] [PubMed] [Google Scholar]
- Werner JL, Gessner MA, Lilly LM et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun 2011;79:3966–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein JA, Marino MC, Johnston RB et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–69 [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Asadullah K et al. Cutting edge: immune cells as sources and targets of the IL-10 family members?. J Immunol 2002;168:5397–402 [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E et al. IL-22 increases the innate immunity of tissues. Immunity 2004;21:241–54 [DOI] [PubMed] [Google Scholar]
- Wolk K, Warszawska K, Hoeflich C et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol 2011;186:1228–39 [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Hoffmann U et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol 2007;178:5973–81 [DOI] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010;32:815–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Martin RJ, Rino JG et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect 2007;9:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie MH, Aggarwal S, Ho WH et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 2000;275:31335–9 [DOI] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 2007;282:9358–63 [DOI] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 1995;3:811–21 [DOI] [PubMed] [Google Scholar]
- Ye P, Garvey PB, Zhang P et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Resp Cell Mol 2001a;25:335–40 [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001b;194:519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HR, Harris TJ, Wada S et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol 2009;183:7161–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gao L, Lei L et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol 2009;183:1291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007;445:648–51 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008;14:282–9 [DOI] [PubMed] [Google Scholar]
- Zhu J, Emerson SG. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene 2002;21:3295–313 [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Cheng P, Liu XF et al. A pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritis. Gut 2015;64:1368–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindl CL, Lai JF, Lee YK et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. P Natl Acad Sci USA 2013;110:12768–73 [DOI] [PMC free article] [PubMed] [Google Scholar]