Abstract
The functioning of many natural and engineered environments is dependent on long distance electron transfer mediated through electrical currents. These currents have been observed in exoelectrogenic biofilms and it has been proposed that microbial biofilms can mediate electron transfer via electrical currents on the centimeter scale. However, direct evidence to confirm this hypothesis has not been demonstrated and the longest known electrical transfer distance for single species exoelectrogenic biofilms is limited to 100 μm. In the present study, biofilms were developed on electrodes with electrically non-conductive gaps from 50 μm to 1 mm and the in situ conductance of biofilms was evaluated over time. Results demonstrated that the exoelectrogenic mixed species biofilms in the present study possess the ability to transfer electrons through electrical currents over a distance of up to 1 mm, 10 times further than previously observed. Results indicate the possibility of interspecies interactions playing an important role in the spatial development of exoelectrogenic biofilms, suggesting that these biological networks might remain conductive even at longer distance. These findings have significant implications in regards to future optimization of microbial electrochemical systems.
Keywords: microbial fuel cell, biofilm, conductivity, exoelectrogenic bacteria, extracellular electron transfer
The exoelectrogenic biofilms in the present study possess the ability to transfer electrons through electrical currents over a distance of up to 1 mm, 10 times further than previously observed.
INTRODUCTION
The average bacterium is only ∼2 μm in length, yet electron transfer over distances that are of the order of 103–106-fold greater are essential to the metabolism of several microorganisms (Lovley 2011b; Reguera 2011, 2012). The efficiency with which electron transfer can be accomplished is not only critical to energy conservation within collections of cells but also vital to the performance of several microbial electrochemical systems (MESs) (Lovley 2011b; Malkin et al. 2014). Recently, the prevalence of electron transfer over extended distances via electrical currents has been recognized in several natural and engineered environments (Reguera 2011, 2012; Lovley 2012; Malvankar and Lovley 2014). Electrical currents offer clear temporal and efficiency advantages over the diffusion of small molecules in regards to electron transfer particularly in diffusion-limited environments like biofilms and anoxic sediments (Lovley 2011a,b, 2012). For this reason, it is not surprising that bacteria have evolved a diverse means of electrical conduction.
Exoelectrogenic bacteria, capable of transferring electrons extracellularly, have been well studied due to their biotechnological relevance and are ubiquitous in anoxic sediments and other anaerobic environmental systems (Logan 2009). The most prominent of these are Geobacter sulfurreducens and Shewanella oneidensis, used as model systems for electron transfer and recognized for their ability to conduct electrons through specialized appendages like microbial nanowires and c-type cytochromes (El-Naggar et al. 2010; Nakamura et al. 2010; Morita et al. 2011; Kato, Hashimoto and Watanabe 2012; Kato, Kazuhito and Kazuya 2012; Malvankar, Tuominen and Lovley 2012a,b; Shrestha and Rotaru 2014; Liu et al. 2015). Although no consensus has been reached on the mechanism of electron transfer along nanowires nor the function served by the semi-conductive minerals, they are central to the construction of conductive biofilm networks (Malvankar et al. 2011; Strycharz-Glaven et al. 2011; Liu et al. 2012; Snider et al. 2012; Kato, Hashimoto and Watanabe 2012; Malvankar, Tuominen and Lovley 2012b).
Another example of electron transfer through electrical currents has also been recognized in filamentous bacteria belonging to the family Desulfobulbaceae. In these bacteria, electrons are transferred through cable-like filaments in which a charged internal environment is enclosed in an insulative outer membrane (Pfeffer et al. 2012; Reguera 2012; Malkin et al. 2014; Larsen and Nielsen 2015; Risgaard-Petersen and Kristiansen 2015). The presence of these filaments putatively connects sulfide oxidation in the anoxic layer to oxygen reduction in the oxic layer separated by 1.4–1.8 cm, over 100-fold further than observations of biofilm-mediated electrical currents. These hypotheses were established on the observations of changed rates of oxygen consumption in sediment layers rather than direct measurement of electrical current and conductivity (Pfeffer et al. 2012; Malkin et al. 2014; Malvankar, King and Lovley 2014).
Electrical conductivity has been recognized as an important property in both single and mixed species exoelectrogenic biofilms (Malvankar et al. 2011, 2012; Malvankar, Tuominen and Lovley 2012a; Li et al. 2016). Electron transfer mediated by biofilm conductivity on a centimeter scale has been proposed as a means of efficient long-range transfer in natural and engineered environments, but has yet to be demonstrated. Single-species biofilms of G. sulfurreducens were the conduit, but the longest distance the conductive biofilm can span through a non-conductive gap was 100 μm, which is significantly less than that hypothesized in many environments (Malvankar et al. 2011, 2012). Interspecies interactions in mixed species communities can result in differential conductive behaviors and may lead to spatial distributions that promote extended electron transfer distances over those previously observed (Kouzuma, Kato and Watanabe 2015; Li et al. 2016).
To evaluate the conduction distance of exoelectrogenic mixed species biofilms, the conductance of such biofilms over non-conductive gaps with various widths from 50 μm to 1 mm was measured. Results demonstrate the ability of the conductive mixed species exoelectrogenic biofilms to transfer electrons via electrical currents on the millimeter scale, 10-fold greater than previously observed in any biofilms, with further expansion possible.
MATERIALS AND METHODS
Electrode preparation
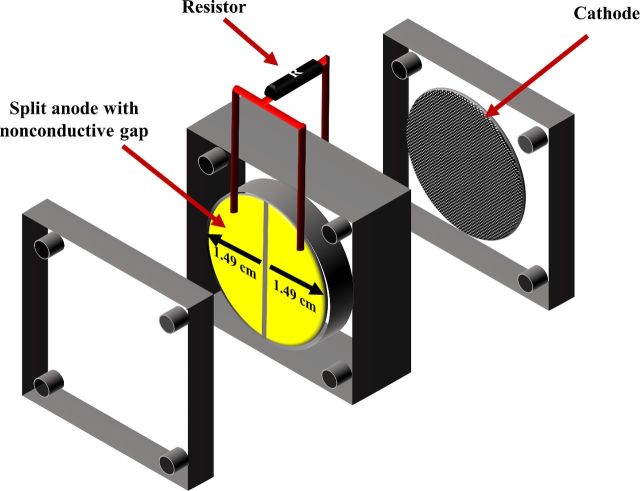
A split anode design was adapted from previous study to perform in situ measurement of biofilm conductivity (Malvankar et al. 2011). To construct split anode, a water resistant adhesive (Loctite, Düsseldorf, Germany) was applied to standard weighing paper (Schleicher & Schuell, Inc., Keene, NH, USA). Adhesive laden paper was then cut into a circle with area of 7 cm2, and an electrically conductive gold film with a thickness of 5 μm was applied onto the adhesive layer by a Cressington 108 Auto sputter coater (Cressington Scientific, Watford, UK). An ESI 5330 UV Laser machine (Electro Scientific Industries, Inc., Portland, OR, USA) was used to create non-conductive gap with various width (50, 150, 300 and 1000 μm) at the center of electrode. To ensure electrical separation of the two halves of the split anode, resistance measurements across the gap were conducted using a source meter (Model 2405; Keithley, USA). The gap was considered electrically separated if Rgap > 1010 Ω.
Microbial fuel cell design and operation
Single chamber air cathode microbial fuel cells (MFCs) with the gold-coated split anodes were used to develop biofilms on the anode surface. The procedure to fabricate carbon cloth/activated carbon cathode was developed similar to a previous study (Janicek, Fan and Liu 2015). The projected surface areas of anode and cathode were 7 cm2 and the total MFC liquid volume was 12 mL. To evaluate the possible distance of biofilm-mediated electron transfer, both halves or only one half of the split anode were connected to cathode (Fig. 1). MFCs labeled ‘MFC’ contained anodes in which both halves of the split anode were connected to cathode by resistor, while for MFCs labeled ‘SA-MFC’ only one half of the split anode was connected to cathode. Replicates were used (n = 3) to ensure the repeatability of experiments. Control reactors contained split electrode whose neither of the two halves was connected to cathode. Sufficient replicates were used (n = 3) to ensure the repeatability of experiments.
Figure 1.
Schematic of the MFC with both halves of the split anode connected by a resistor to the cathode.
Lab-maintained active MFC cultures enriched from active sludge of Corvallis Wastewater Treatment Plant (Corvallis, OR) were used as inoculum. This community has been demonstrated to maintain a relative consistent community composition over extended periods of operation and across reactor designs when fed with acetate (Lesnik and Liu 2014). Modified Geobacter medium (MGM) (pH 7) containing (per liter of solution) KCl, 0.13 g; NH4Cl, 0.31 g; NaH2PO4·H2O, 5.84 g; Na2HPO4·7H2O, 15.5 g; vitamin, 12.5 mL; and mineral 12.5 mL solution was used in all experiments. During the startup of MFCs, 30 mM of acetate with MGM buffer was used to avoid the substrate inhibition. When the current production became stable, the concentration of acetate was raised to 60 mM and maintained till the rest of the study. All cells were operated in fed-batch mode with external resistance decreased from 10 000 to 500 Ω. When voltages were under 5% of batch maximum, the media was removed and replaced with new media, to maintain optimal cell voltages around 0.3 V.
Two-electrode conductivity measurement
To evaluate in situ biofilm conductance, MFC anode was temporarily disconnected from cathode and was allowed to reach open circuit potential (460–470 mV versus Ag/AgCl). A voltage low enough to avoid the electrolysis of water or a self-heating effect was applied between two halves of a split anode (0–0.075 V) in steps of 0.025 V by using a source meter (Model 2405; Keithley, USA). For each voltage step, transient ionic current related to counter-ion diffusion was allowed to decline for more than 20 min until steady state was reached. Current of each voltage step was recorded every 30 s over a 3-min period. A slope was generated by plotting average currents against applied voltages and was also used in the calculation of biofilm conductance to avoid the inclusion of the current of acetate oxidation. Measurement of conductance was also performed in two types of control reactors. The first type of control reactors were inoculated with the same culture, but operated in open-circuit mode for over 30 days. The second type of control reactors were not inoculated and used to evaluate the background current.
Confocal laser scanning microscopy
Biofilm growth on the split electrode was examined by confocal laser scanning microscope (CLSM). When the measured conductance and maximum power of MFCs plateaued for 4 weeks, biofilms were considered to be mature. Electrodes from all reactors were then carefully taken out. A small piece of the electrodes was then gently cut and stained with LIVE/DEAD BacLight Bacterial Viaibility Kit (Molecular Probes, Eugene, OR, USA) following the instructions of manufacturer. Stained specimens were then examined with the Zeiss LSM 510 Meta confocal microscope with a 10× objective lens (Carl Zeiss AG, Oberkochen, Germany). ImageJ (1.49 d) was used to process images.
Statistical analysis
Correlation coefficient r was generated by using analysis package in Microsoft Excel and a correlation coefficient greater than 0.8 (n > 10) was considered as positively correlated and P < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
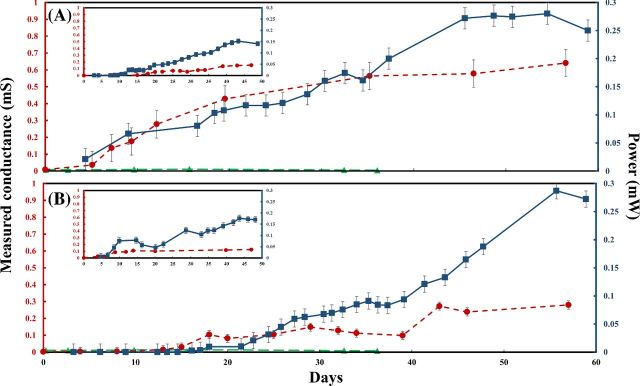
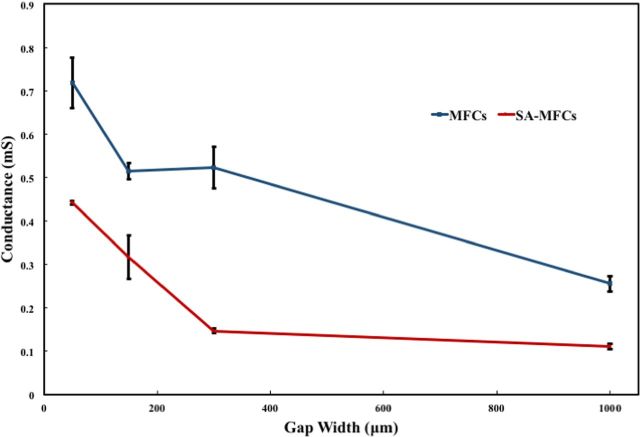
Following inoculation of the MFC reactors, measured conductance continually increased over time indicating growth of conductive biofilms across the gaps (Fig. 2). Conductance values of all gap width increased on the order of 100–300-fold from the initial average conductance value of 0.005 to 0.53 mS. The conductance of controls without inoculum was less, 0.003 mS. No increase in conductance was observed in controls without connection between anode and cathode. Starting from 150 μm, observed electron transfer distances that can be mediated by biofilms in present study are greater than previously reported for any exoelectrogenic biofilm (Malvankar et al. 2011; Malvankar, Tuominen and Lovley 2012a). When one side of the split anode was left unconnected during biofilm development in single-anode MFCs (SA-MFCs) (Table 1), similar results were yielded. Increases in conductance proceeded slower in SA-MFCs compared to MFCs, likely due to a single point of origin for exoelectrogenic growth (inserts of Fig. 2). Average biofilm conductance decreased as gap width increased (Fig. 3). This may be a result of lower overall concentrations of redox active sites across longer gaps, suggestive of redox concentration gradient-driven electron transfer mechanism previously observed in Geobacter sulfurreducens biofilms (Strycharz-Glaven et al. 2011). When the gap width increased, the ratio of exoeletrogenic to non-exoelectrogenic bacteria in gap area decreased possibly due to the increased hindrance of donating electron to active electrode, causing dilution to concentration of electroactive sites similar to previous observation in conductive polymer film (Ohsaka, Yamamoto and Oyama 1987). Further support of this hypothesis is that conductance of SA-MFCs was significantly lower than conductance of MFCs (P < 0.05).
Figure 2.
Measured conductance and power of MFCs over time (A) from MFCs with 300 μm non-conductive gap and (B) from MFCs with 1000 μm non-conductive gap. Inserts: measured conductance and power of SA-MFCs over time. Red dash line represents the measured conductance and blue solid line represents the power. Error bars indicate the standard error of individual measurements of several MFCs (n = 3).
Table 1.
Average power and biofilm thickness in MFCs and SA-MFCs reactors.
| MFCs | SA-MFCs | |||||||
|---|---|---|---|---|---|---|---|---|
| Gap width (μm) | 50 | 150 | 300 | 1000 | 50 | 150 | 300 | 1000 |
| Power (mW) | 0.19 ± 0.01 | 0.14 ± 0.02 | 0.25 ± 0.02 | 0.17 ± 0.00 | 0.11 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.00 | 0.14 ± 0.01 |
| Biofilm thickness (μm) | 38.4 ± 10.1 | 49.3 ± 11.7 | 60.3 ± 12.9 | 50.0 ± 10.3 | 52.2 ± 11.6 | 44.1 ± 7.4 | 39.5 ± 8.7 | 62.3 ± 17.7 |
Error bars indicate the standard error of individual measurements of several MFCs (n = 3).
Figure 3.
Conductance of MFCs and SA-MFCs over gap width after day 70. Error bars indicate the standard error of individual measurements of several MFCs (n = 3).
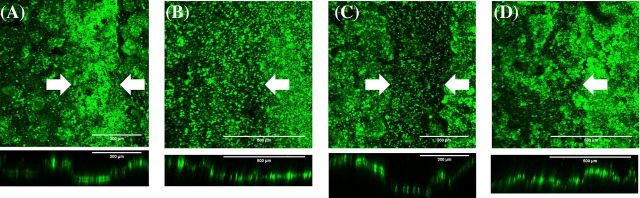
Biofilm growth across the non-conductive gaps was further confirmed using CLSM (Fig. 4). Average biofilm thickness across the non-conductive gaps was 50.1 ± 3.5 μm. Some growth was observed on electrode of control reactors that were inoculated but without a closed circuit. However, biofilm growth of these reactors was too sparse for thickness to be measured. While results of previous studies using pure cultures of G. sulfurreducens indicate that exoelectrogenic singles species biofilms were unable to extend more than 100 μm from active electrodes (Malvankar et al. 2011; Malvankar, Tuominen and Lovley 2012a), exoelectrogenic mixed species biofilms of present study were able to extend 1 mm. In mixed species environments, coadhesion or coaggregation could be initialized by various species and further expansion of the conductive matrix of the order of centimeters may even be possible (Ruhl et al. 2014).
Figure 4.
Top down CLSM images of anodes (A) from MFCs anode with 300 μm non-conductive gap, (B) from MFCs anode with 1000 μm non-conductive gap, (C) from SA-MFCs anode with 300 μm non-conductive gap and (D) from SA-MFCs anode with 1000 μm non-conductive gap. Arrows indicate the location of non-conductive gap. Frame size = 8.1 × 10−3 cm2.
Power generation was found to be positively correlated with conductance in both MFCs and SA-MFCs (r > 0.8) and statistically significant (P < 0.05). The correlation between conductivity and power is suggestive of an increase in biomass contact with an active electrode due to conductance enabling vertical stacking and longitudinal expansion. The establishment of conductivity in three dimensional space not only enables the transfer of electrons to the anode following oxidation of the substrate from superficial biomass distal from the anode surface, but also enables electron transfer from one half of the split anode to the other.
Conductivity is undoubtedly an important property of exoelectrogenic biofilms in relation to energy conservation during electron transfer (Malvankar, Tuominen and Lovley 2012a; Malvankar et al.2012). However, the connection between power output and conductivity of biofilms in mixed species settings remains unclear. Exoelectrogenic single species biofilms containing higher concentrations of redox proteins may possess higher measurable conductivity (Kato Marcus, Torres and Rittmann 2007). In environments limited by substrate diffusion and utilization, interspecies interactions that allow for increased biomass access to a limited respirable surface area and enhanced product removal by complimentary species may lead to the observed increases in performance in regards to power output.
The results of the present study provide evidence for biofilm-mediated electron transfer on the millimeter scale, 10 times greater than previously observed. In natural environments, conductive biofilms are likely an essential means of facilitating long-distance electron transfer that is the foundation of many biogeochemical processes (Kato, Hashimoto and Watanabe 2012; Kato, Kazuhito and Kazuya 2012; Reguera 2012). In engineered environments of MESs, interspecies interactions in exoelectrogenic mixed species biofilms may lead to the extension of the electroactive biomass compared to pure cultures, thereby allowing microbial communities to take advantage of limited electrode surface area. Such extension is beneficial in regards to optimizing the ratio of active biomass to electrode surface area and should be considered in future MES designs for various applications, such as wastewater treatment, bioremediation and energy production. Further, deciphering the conductive behavior of exoelectrogenic bacteria may lead to increased understanding of global carbon cycling in natural environments as well as opening up new applications, such as bioelectronics and biosensors, therefore warranting additional research.
Acknowledgments
The authors wish to acknowledge Anne-Marie Girard and the Confocal Microscopy Facility of the Center for Genome Research and Biocomputing (CGRB) and the Environmental and Health Sciences Center at Oregon State University for confocal support, Teresa Sawyer and the Electron Microscope facility at Oregon State University, and the equipment provided by Microproducts Breakthrough Institute.
FUNDING
This publication was made possible by grant CBET 0955124 and PFI 1312301 fromthe US National Science Foundation and in part by grant number 1S10RR107903-01 from the National Institutes of Health.
Conflict of interest. None declared.
REFERENCES
- El-Naggar MY, Wanger G, Leung KM, et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. P Natl Acad Sci USA. 2010;107:18127–31. doi: 10.1073/pnas.1004880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicek A, Fan Y, Liu H. Performance and stability of different cathode base materials for use in microbial fuel cells. J Power Sources. 2015;280:159–65. [Google Scholar]
- Kato Marcus A, Torres CI, Rittmann BE. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol Bioeng. 2007;98:1171–82. doi: 10.1002/bit.21533. [DOI] [PubMed] [Google Scholar]
- Kato S, Hashimoto K, Watanabe K. Microbial interspecies electron transfer via electric currents through conductive minerals. P Natl Acad Sci USA. 2012;109:10042–6. doi: 10.1073/pnas.1117592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kazuhito H, Kazuya W. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron‐oxide minerals. Environ Microbiol. 2012;14:1646–54. doi: 10.1111/j.1462-2920.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- Kouzuma A, Kato S, Watanabe K. Microbial interspecies interactions: recent findings in syntrophic consortia. Front Microbiol. 2015;6:477. doi: 10.3389/fmicb.2015.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Nielsen LP. Cable bacteria associated with long‐distance electron transport in New England salt marsh sediment. Environ Microbiol Rep. 2015;7:175–9. doi: 10.1111/1758-2229.12216. [DOI] [PubMed] [Google Scholar]
- Lesnik KL, Liu H. Establishing a core microbiome in acetate-fed microbial fuel cells. Appl Microbiol Biot. 2014;98:4187–96. doi: 10.1007/s00253-013-5502-9. [DOI] [PubMed] [Google Scholar]
- Li C, Lesnik KL, Fan Y, et al. Redox conductivity of current-producing mixed species biofilms. PLoS One. 2016;11:e0155247. doi: 10.1371/journal.pone.0155247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Rotaru A-E, Shrestha PM, et al. Magnetite compensates for the lack of a pilin‐associated c‐type cytochrome in extracellular electron exchange. Environ Microbiol. 2015;17:648–55. doi: 10.1111/1462-2920.12485. [DOI] [PubMed] [Google Scholar]
- Liu F, Rotaru AE, Shrestha PM, et al. Promoting direct interspecies electron transfer with activated carbon. Energ Environ Sci. 2012;5:8982–9. [Google Scholar]
- Logan B. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol. 2009;7:375–81. doi: 10.1038/nrmicro2113. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energ Environ Sci. 2011a;4:4896–906. [Google Scholar]
- Lovley DR. Reach out and touch someone: potential impact of DIET (direct interspecies energy transfer) on anaerobic biogeochemistry, bioremediation, and bioenergy. Rev Environ Sci Biotechnol. 2011b;10:101–5. [Google Scholar]
- Lovley DR. Electromicrobiology. Annu Rev Microbiol. 2012;66:391–409. doi: 10.1146/annurev-micro-092611-150104. [DOI] [PubMed] [Google Scholar]
- Malkin SY, Rao AMF, Seitaj D, et al. Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor. ISME J. 2014;8:1843–54. doi: 10.1038/ismej.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvankar NS, King GM, Lovley DR. Centimeter-long electron transport in marine sediments via conductive minerals. ISME J. 2014;9:527–31. doi: 10.1038/ismej.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvankar NS, Lau J, Nevin KP, et al. Electrical Conductivity in a Mixed species Biofilm. Appl Environ Microb. 2012;78:5967–71. doi: 10.1128/AEM.01803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvankar NS, Lovley RD. Microbial nanowires for bioenergy applications. Curr Opin Biotechnol. 2014;27:88–95. doi: 10.1016/j.copbio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Malvankar NS, Tuominen MT, Lovley DR. Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens. Energ Environ Sci. 2012a;5:8651–9. [Google Scholar]
- Malvankar NS, Tuominen MT, Lovley DR. Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells. Energ Environ Sci. 2012b;5:5790–7. [Google Scholar]
- Malvankar NS, Vargas M, Nevin KP, et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol. 2011;6:573–9. doi: 10.1038/nnano.2011.119. [DOI] [PubMed] [Google Scholar]
- Morita M, Malvankar N, Franks A, et al. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio. 2011;2:11. doi: 10.1128/mBio.00159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Okamoto A, Tajima N, et al. Biological iron-monosulfide production for efficient electricity harvesting from a deep-sea metal-reducing bacterium. Chembiochem. 2010;11:643–5. doi: 10.1002/cbic.200900775. [DOI] [PubMed] [Google Scholar]
- Ohsaka T, Yamamoto H, Oyama N. Thermodynamic parameters for charge-transfer reactions in pendant viologen polymers coated on graphite electrodes and at electrode/pendant viologen polymer film interfaces. J Phys Chem. 1987;91:3775–9. [Google Scholar]
- Pfeffer C, Larsen S, Song J, et al. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491:218–21. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- Reguera G. When microbial conversations get physical. Trends Microbiol. 2011;19:105–13. doi: 10.1016/j.tim.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G. Microbiology: bacterial power cords. Nature. 2012;491:201–2. doi: 10.1038/nature11638. [DOI] [PubMed] [Google Scholar]
- Risgaard-Petersen N, Kristiansen M. Cable bacteria in freshwater sediments. Appl Environ Microb. 2015;81:6003–11. doi: 10.1128/AEM.01064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Eidt A, Melzl H, et al. Probing microbial biofilm communities for coadhesion partners. Appl Environ Microb. 2014;80:6583–90. doi: 10.1128/AEM.01826-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha PM, Rotaru AE. Plugging in or going wireless: strategies for interspecies electron transfer. Front Microbiol. 2014;5:237. doi: 10.3389/fmicb.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider RM, Strycharz-Glaven SM, Tsoi SD, et al. Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. P Natl Acad Sci USA. 2012;109:15467–72. doi: 10.1073/pnas.1209829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strycharz-Glaven SM, Snider RM, Guiseppi-Elie A, et al. On the electrical conductivity of microbial nanowires and biofilms. Energ Environ Sci. 2011;4:4366–79. [Google Scholar]