Abstract
Intervertebral disc degeneration is considered a major cause of back pain that places a heavy burden on society, both because of its effect on the physiology of individuals and its consequences on the world economy. During the past few decades, research findings in the pre-clinical setting have led to a significant increase in the understanding of intervertebral disc degeneration, although many aspects of the disease remain unclear. The goal of this review is to summarize existing animal models for disc degeneration studies and the difficulties that are associated with the use of such models. A firm understanding of the cellular and molecular events that ensue as a result of injuries, as well as environmental factors, could be instrumental in the development of targeted therapies for the treatment of intervertebral disc degeneration.
Keywords: disc degeneration, animal model, intervertebral disc, annulus injury, low back pain
Low back and leg pain is a common musculoskeletal disorder, and affects 70–85% of people at some time in their life (Andersson, 1999; Vos et al., 2012). Intervertebral disc degeneration (IVD) is the main cause of back pain (Adams and Hutton, 1983; Vos et al., 2012). Due to aging of the population, disc degeneration is increasing exponentially (Urban et al., 2003). Despite tremendous efforts in disc research, current treatment strategies are limited and focus on alleviating pain and delaying the time to surgery. The main reason is the complicated nature of the disease: a wide range of etiology, vague definition, unclear pathology, lack of a good animal model, and unclear underlying cellular mechanisms. Furthermore, the anatomical differences between the disc of humans and of laboratory animals further hinder progress in identifying an ideal target for the treatment of disc degeneration. Although animal models help us understand disc biology, disease progression and the quest for therapeutic remedy, the interpretation of information obtained from animal studies should be cautious in extrapolating to humans, as there are many differences between species (detailed information please refer to excellent reviews (Lotz and Ulrich, 2006; Alini et al., 2008; Daly et al., 2016)). The intent of this review is to update knowledge of existing animal models and discuss some of their limitations, but not a metadata analysis.
1. Intervertebral disc anatomy
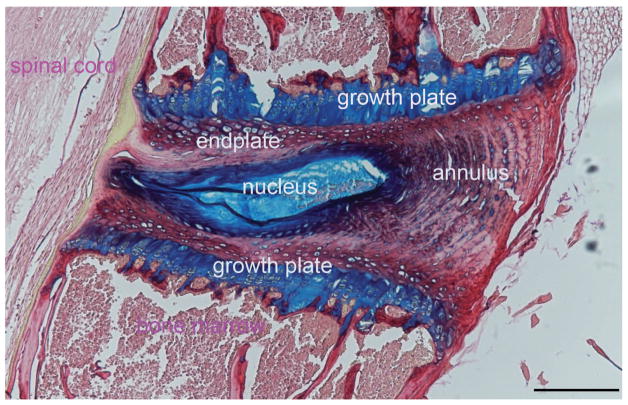
The IVD is composed of a gelatinous nucleus pulposus (NP) in the center, surrounded by the fibrocartilaginous annulus fibrosus (AF), and inferior and superior cartilage endplates (Fig. 1). The NP is highly hydrated and rich in proteoglycans that resist compressive force. The AF is a concentric lamellar structure that resists tensile strain. The endplate functions as a center for the transfer of nutrients and waste through the vasculature of the vertebral bone (Nachemson et al., 1970). Each compartment of the IVD is comprised of its own cells to maintain disc homeostasis: NP cells are chondrocyte-like, present at a low density and interspersed in a proteoglycan-rich matrix, making up 50% of the wet weight, and because of that, 80% of the wet weight of the NP is water ( Maroudas et al., 1975). Cells in the AF are fibroblast-like and are aligned parallel to the collagen fibers; while cells in the inner AF are more oval compared to the outer AF. The cartilage of the endplates is a thin layer (<1mm) of hyaline cartilage (Sah et al., 1989).
Figure 1.
The general view of a healthy disc is an avascular and aneural structure with few blood vessels, and with some sensory and sympathetic perivascular nerve fibers in the outer lamellae of the annulus (Ashton et al., 1994; Virri et al., 1996; Palmgren et al., 1996)). The small vessels are located in the canals of endplates and in the outer layer of the AF. The aneural nature of the inner disc may be due to the matrix of dense collagen that prevents vessels from passing through the annulus (Gruber et al., 2005).
2. Intervertebral disc degeneration
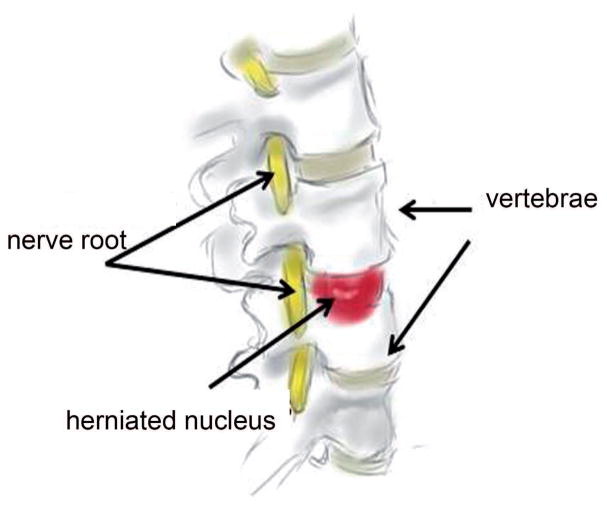
During aging and early disc degeneration, the most significant change in the disc is loss of proteoglycan leading to diminished hydration. The boundary between NP and AF is blurred. With less proteoglycan, NP becomes more fibrotic with irregular bifurcating and interdigitating lamellae. Clefts and fissures are often found in the discs, especially in the nucleus. Nerve fibers and vessels increase with the degeneration (Urban et al., 2003). Apoptotic and necrotic cells are seen in the inner part of the disc (Gruber et al., 1998). The biochemical activity of catabolic enzymes, including cathepsins and MMPs increases in degenerated discs (Yang and Li, 2009; Jin et al., 2013, Vo et al., 2013a). In addition, the distribution of collagen types shows significant changes. Eventually, disc degeneration leads to a loss of load bearing function, a diminution in disc height that together lead to a tendency towards bulging and disc herniation (Fig. 2).
Figure 2.
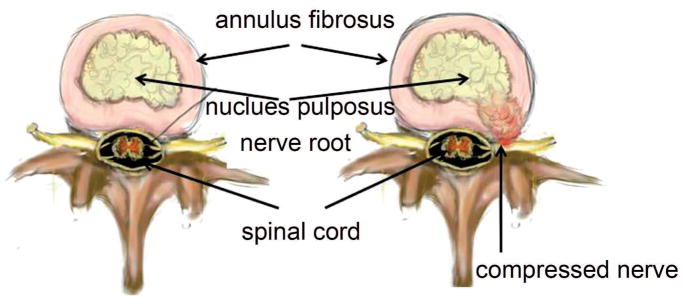
The most common condition in patients seen by a spine surgeon is a posteriorly or postero-laterally herniated or prolapsed IVD that is pressing on the nerve roots in the spinal canal (Fig. 3). The herniated nucleus exacerbates the symptoms further by inducing local sterile inflammation that sensitizes the nerve root thereby causing pain. The mechanisms that are the basis for disc degeneration and the connections between disc degeneration and painful symptoms are still a mystery, therefore animal models can serve as important tools for further investigation. However, most existing animal models may not cause nerve compression or neuropathic pain.
Figure 3.
3. Animal models of the disease
Animal models are important pre-clinical tools for biomedical research. Ideally, the most appropriate animal model for a specific disease is to artificially develop a condition that replicates the human condition. Due to the complicated etiology and pathogenesis of disc degeneration, developing a suitable animal model is challenging because it should 1) share features that are similar to disc degeneration in humans; 2) be reliable, and reproducible, as well as cost and labor efficient. There are 4 major categories among the animal models that are available (Table 1): genetic predisposition, mechanical loading, structural disruption including annulus/nucleus injury, chemical digestion, and endplate injury, and radicular pain.
Table 1.
current existing animal model for disc degeneration.
1) Genetic predisposition
The animal model that exhibits age dependent changes in the disc shares many features with human IVD disease. The sand rat is a well characterized spontaneous disc degeneration model. Gruber and coworkers (Gruber et al., 2002; 2009; 2011; 2014; Tapp et al., 2008) found significant age related degenerative changes. Radiographic signs of degeneration were evident in the animals at the age of 2 months; wedging, narrowing, irregular disc margins, cell death, and endplate calcification were the most common degenerative changes in the older animals. The males of this species showed a higher incidence of wedging and endplate calcification than the females at 2–6 months, however, such differences between males and females disappeared by 12 months of age.
A spontaneous mutation of the BDL strain of mice leads to the Kyphoscoliotic (KY) condition; the mice show postural muscle atrophy during post-natal growth (Mason and Palfrey, 1984; Blanco et al., 2001). A hereditary form of the KY BDL strain exhibited changes in thickness of the thoracic-lumbar vertebrae and structural changes in the cervical discs. In newborn ky/ky mice, both the vertebrae and the IVDs are normal, however, small changes in the shape of the disc and the ratio of NP/AF occur 9 days after birth. In the adult mice, disc degeneration, including disc herniation could be seen both anteriorly and posteriorly, with destruction of the vertebral endplate and cystic lesions affecting the spinal cord.
A mutation in GDF5 mice showed signs of disc degeneration (Li et al., 2004) and GDF5 overexpression rescued the disease (Liang et al., 2010). Mutations of GDF5 protein lead to skeletal malformations in human, such as brachydactyly type A2, brachydactyly type C, Du Pan Syndrome, and Grebe type chondrodysplasia (Jin and Li, 2013).
Advances in gene knockout and knockin technology provide important opportunities for the addition of new animal models for the investigation of a host of diseases. Select transgenic mouse strains have been shown to be valuable for studying disc degeneration (Vo et al., 2013a).
Millecamps (Millecamps et al., 2011; 2012; 2015; Miyagi et al., 2014) described a SPARC (secreted protein, acidic, rich in cysteine) transgenic mouse strain that developed age-dependent disc degeneration with increasing severity. These mice exhibited low tolerance to axial stretching, hind paw hypersensitivity to cold, and impaired motor activity, as well as increased innervation of discs accompanied with upregulation of CGRP and neuropeptide-Y in the dorsal root ganglia (DRG) and the spinal cord dorsal horn.
Bedore et al (Bedore et al., 2013) reported a decrease in aggrecan and collagen II, but an increase in collagen I, in the NP of a notochord specific CCN2 knockout mouse, which, also showed a reduction of CCN1 and CCN3 expression. I IL-1 receptor antagonist (IL-1rn) knockout mice showed alterations in catabolic and anabolic metabolism, including the loss of proteoglycan and collagen structure that were accompanied by an increase in the expression of matrix degrading enzymes (Phillips et al., 2013). This finding is consistent with the role of IL-1 in the progression of disc degeneration and suggests that IL-1 is a possible target for the therapeutic treatment of disc degeneration.
The β-catenin conditional activation mice (Wang et al., 2012a), breeding Col2a1-CreER(T2) and β-catenin(fx(Ex3)/fx(Ex3)) transgenic mice, showed extensive osteophyte formation, upregulation of mmp13, adamts4, and adamts5 genes. Furthermore, deletion of mmp13 or adamts5 expression in these animals rescued them from disc degeneration suggesting that β-catenin signaling may be involved in the pathogenesis of degenerative disease.
GDF8, a member of the transforming growth factor β superfamily, functions as a negative regulator of skeletal muscle growth. The muscle mass of GDF8 knockout mouse is double that of the WT mouse. Hamrick found that GDF8 deficient mice showed signs of disc degeneration, and staining with Toluidine blue showed loss of proteoglycan in the endplate and inner AF areas that were associated with endplate ossification (McPherron and Lee, 1997). Such observations suggest that muscle mass and bone mass are associated with disc degeneration.
Large animals can also serve as experimental models for the study of spontaneous disc degeneration. Bergknut et al (Bergknut et al., 2012) compared the discs of chondrodystrophic and non-chondrodystrophic (with notochordal cells) dogs. Signs of disc degeneration were seen in the younger (<1-year-old) chondrodystrophic and the 5–7-year-old nonchondrodystrophic dogs. Cho et al (Cho et al., 2011) observed that the number of disc cells and anabolic metabolism decreased during the aging of pig, and these changes were associated with an increase in MMP-1 expression. The results suggest that changes in IVD with age in dogs and pigs are similar to the changes seen with age in humans.
Recent studies (Valentine et al., 2006; Stolworthy et al., 2015) have proposed the use of alpacas as a potential large animal model for further studies of disc degeneration. These animals not only share similar spinal posture, disc size, and biomechanical flexibility to humans but also develop features of disc degeneration as they age: the incidence rate and severity of lower cervical discs increases in the older animals. These studies suggest that alpacas, and closely related animal species, may serve as a large animal model for future investigations on disc degeneration of the cervical spine.
Other factors contribute to disc degeneration also. Wang (Wang et al., 2012b) reported degenerative changes of the disc in mice with chronic tobacco smoke. Smoke induced cellular senescence, reduction in proteoglycan synthesis as well as total proteoglycan content, with an associated elevation of MMP activity, increased vertebral endplate porosity and bone loss. Finally, changes in cell metabolism and ossification in the endplate appear in the mouse model of arthrosclerosis (ApoE mice) (Zhang et al., 2013) as well as the diabetic mouse model (Fields et al., 2015).
2) Mechanical force
Biomechanics plays a crucial role in disc homeostasis. Mechanical loading is one of the contributing factors for disc degeneration; notably, the risk of disc degeneration is increased in manual laborers such as carpenters and drivers of machinery (Luoma et al., 2000). Exercises, such as walking and jogging have some beneficial effects on maintaining a healthy disc (Iatridis et al., 1999a; Saamanen et al., 1993). Altering the mechanical environment of the spine causes morphologic and biochemical changes in the disc; these changes are similar to early disc disease in human IVD (Cassidy et al., 1989; Puustjarvi et al., 1993).
Kroeber et al (Kroeber et al., 2002) applied axial load to the lumbar spine of rabbits using a custom made external loading device. After 14 and 28 days of loading the discs exhibited a typical degenerative phenotype: narrowed disc space, disorganized annulus, an increase in dead cells both in the annulus and endplates. These changes were irreversible after 28 days of unloading. In a rat tail disc model, dynamic loading for two weeks at a physiological level led to a decrease in the height and angular compliance of the disc, and increased angular laxity (Ching et al., 2003). The cellular response to dynamic loading was different between the AF and the NP. Kim et al observed changes in COMP and type II collagen gene expression following one hour of dynamic compression of the rat tail disc (Kim et al., 2011). MacLean et al observed elevated anabolic gene expression in the nucleus at 0.01 Hz but an increase in catabolic genes at 1 Hz. However, all loading frequencies caused a significant upregulation of the mRNA for catabolic factors (Maclean et al., 2004).
Static compression induced degenerative changes in disc composition and mechanical properties, as well as a decrease in the number of cells in proportion to the loading frequency and magnitude (Lotz et al., 1998; 2000; Iatridis et al., 1999b; Hutton et al., 2000). After static compression in a rat tail disc model, MMP-3 was upregulated at 7 days and increased with the loading duration. The same group later showed that the number of NP and AF cells decreased as the time of loading increased. Starting at day 7, notochordal cells decreased and this was accompanied by an increase in apoptotic cells. Upregulation of all MMPs and ADAMT-4 was observed in this static compression model together with a decrease in aggrecan and type II collagen (Hirata et al., 2014; Yurube et al., 2010; 2012; 2014). These and other observations (Vo et al., 2013b; Kuo et al., 2014 ) suggest that static compression induces cell death and mild disc degeneration. In a rat tail disc model, Yan et al. reported that in a rat tail disc model, static compression changed the mRNA expression of integrins as well as their downstream signaling. In addition, compression on 1-level disc induced severe disc degeneration and remodeling (Yan et al., 2016).
Inflammation was also induced in the compression model. Comparing the expression of inflammatory mediators and neuropeptide in DRGs between rat disc dynamic compression and injury models, Miyagi (Miyagi et al., 2011) demonstrated that inflammatory mediators (TNFa, IL-1, IL6) and the neuropeptides were elevated only transiently in the injury group but persisted in the compression group up to 8 weeks.
More recently, Sakai et al studied the effect of constant compression in a GFP bone marrow chimeric mouse by looping the tail and aspirating NP tissue, and found a limited number of GFP positive cells in the IVD (Sakai et al., 2015). This study suggested that the bone marrow cells are recruited during the degenerative process in this compression model. However, it is not clear whether the bone marrow cells migrate to the IVD after the compression has induced structural changes in the disc.
Mechanical loading also induces disc degeneration in large animals. Hutton (Hutton et al., 2000) attached coil springs to the dog lumbar IVDs for 16 and 27 weeks. The most pronounced alteration was seen in the matrix of the NP but not in AF; the matrix showed an increase in collagen type I accompanied with a decrease in proteoglycan and collagen type II.
Results from mechanical loading in all of the above mentioned animal models lead to changes in disc matrix constituents, enzyme activity, the fate of cells, and gene expression after both static and dynamic loading, the degree of degeneration is dependent on the magnitude, duration, and frequency of loading.
3) Structural disruption
surgical procedure and enzyme digestion have been widely used to induce disc degeneration in rodents and large animals. This mainly includes injuries to NP, AF, and endplates,
Nucleus or Annular injury
Many different disc injury techniques have been investigated to induce disc degeneration; these include nucleotomy, full and partial disc puncture through a ventral approach, and percutaneous puncture of the annulus.
Varying degrees of nucleotomy have been performed to induce disc degeneration. Omlor et al (Omlor et al., 2009) removed 10% of NP volume with a 16G biopsy cannula in a minipig disc degeneration model. Changes in disc height and matrix components were seen 3 weeks after nucleotomy. Kim et al (Kim et al. 2011) removed the NP from rat discs with a microsurgical drill leading to the loss of proteoglycan and disc height at 9 weeks, which was associated with sustained hyperalgesia. A comparison of needle aspiration with a diode laser (Lucas et al., 2012) demonstrated that histologically, laser induced disc degeneration is similar to the spontaneous and progressive disc degeneration of needle approach.
Puncturing the annulus with a needle has become a preferred method for disc degeneration studies because it is straightforward and relatively reproducible. Masuda (Masuda et al., 2005) compared the annular stab to a needle puncture model in rabbits. Following the stab, disc height shrank at 2 weeks with no further progression. Ulrich (Ulrich et al., 2007) compared the inflammatory response to single and triple-stab disc injury. In discs stabbed multiple times, the NP was replaced with collagen and elevated pro-inflammatory cytokines, while the inflammation in a single stab was transient and localized to the wound. This study suggested that repeated injury accelerated disc degeneration and was associated with an inflammatory response. Cunha et al (Cunha et al., 2017) compared two different gauge needles in a rat tail disc injury model. Both radiographical and histological observations showed that a 21G needle induced more severe disc degeneration compared to a 25G needle. The incidence of disc herniation is also proportional to needle size, moreover, the number of macrophages that infiltrate the disc as well as apoptotic cells are commensurate to the volume of the herniated tissue. After needle puncture, the mouse tail disc showed a decrease in GAG content and mRNA expression of aggrecan at 12 weeks, this was accompanied by an increase in fibronectin and collagen I (Yang et al., 2009). Similar to the rabbit annular puncture model, the fibrocartilaginouse phenotype was observed in the degenerated discs. Moss et al has reported (Moss et al., 2013) details of surgical techniques used in an annular puncture model of rabbits.
In addition to changes in the structure and biochemical composition, the biomechanics of disc is also impacted by needle size as well as the depth of the injury. Michalek et al (Michalek et al., 2010) reported that the torsional properties were directly related to the disruption of fibers, while the compression stiffness was not impacted by needle size or loading. Elliott (Elliott et al., 2008) evaluated disc mechanics after injection of PBS with a 27G or 33G needle in rats, or, a 27G needle in sheep. Disc biomechanics changed after injection compared to the pre-injury with a large diameter needle, while the small needles had no impact on the mechanics. The severity of disc changes depended on the needle to disc height ratio: a ratio over 40% induced disc changes in all the studies.
To minimize the variation in technical parameters, percutaneous annular injury under fluoroscopic or CT guidance was investigated in several laboratories. After surgery, gradual progression of disc degeneration, including changes in the structure and matrix components, was confirmed by MRI, histology, biochemistry, and gene expression profiling (Kwon, 2013; Li et al., 2014; Kim et al., 2015). In one study, Issy (Issy et al., 2015) inserted the needle tip crossing the NP to the contralateral AF, and then rotated it 360° twice. Radiology and histology confirmed disc degeneration between 7 and 30 days after injury. Zhou et al (Zhou et al., 2013) created a rabbit disc degeneration model guided by CT. Keorochana (Keorochana et al., 2010) compared disc degeneration induced by three different sized needles inserted percutaneously into rat caudal discs. The severity of disc degeneration increased with increasing needle size. Proteoglycan and aggrecan decreased over time. By contrast to other studies, Sox-9 and collagen II positive cells were increased in the pericellular area within the NP and at the junction between NP and AF.
The minimally invasive procedure was also tested in large animal models. Xi et al (Xi et al., 2013) found progressive and mild disc degeneration in Rhesus monkeys by percutaneous puncture of the disc. Similarly, Yoon et al (Yoon et al., 2008) punctured the lumbar spine of mini pigs under fluoroscopy. Using the uninjured discs as controls, early degeneration of the injured discs was seen at 5 weeks as MRI and histology confirmed progressive degeneration following injury to the annulus.
This method offers a minimally invasive approach for the study of disc degeneration, however injury to blood vessels and nerves may be difficult to control at the site and depth of entry especially in the small rodents.
Chemical induction
Biochemical reagents have been used to induce animal models for disc degeneration. Sugimura (Sugimura et al., 1996) compared different enzymes on the IVD of monkeys. The study showed that both chondroitinase ABC and chymopapain induced degenerative changes but the former was less toxic than chymopapain to the non-cartilage tissue. Norcross et al (Norcross et al., 2003) injected chondroitinase ABC into tail discs of rat, and observed significant loss of disc height, as well as proteoglycan and NP cells. However, stiffness of the NP was increased in the chondroitinase ABC group compared to the control. Another group (Boxberger et al., 2008) reported similar results confirming that enzymatic digestion with chondroitinase ABC induced early signs of disc degeneration in a rat lumbar spine. In a goat model (Hoogendoorn et al., 2008), injection of Chondroitinase ABC induced long-term, mild disc degeneration within 18 weeks. The degeneration did not recover during the 26 week follow up period.
Injection of other reagents has also been used to induce disc degeneration. Anderson et al (Anderson et al., 2005) demonstrated that addition of the N-terminal fibronectin fragment to rabbit disc led to osteophyte formation, a progressive loss of normal disc structure, and a reduction in anabolic metabolism. Later, the same group reported similar findings in NP explant cultures and NP cells in alginate culture. Oegema et al showed that the fibronectin fragments increased in the degenerated human discs, and may further enhance the degradation of matrix (Oegema et al., 2000). Lee et al (Lee et al., 2009) evaluated disc degeneration following the injection of incomplete Freund’s adjuvant (CFA) into rat IVD. The CFA rat showed an increase in hind paw withdrawal response, progressive disc degeneration, and expression of specific pain-related transmitters and mediators. Zhou et al (Zhou et al., 2007) assessed disc degeneration by percutaneously injecting 5-bromodeoxyuridine (BrdU) into the IVD of sheep and found progressive structural changes in the disc up to 14 weeks.
Chemically induced disc degeneration involves the injection of materials into the disc space, consequently, the results may be confounded by the process of inserting the needle that is used to deliver the enzyme, reagent or growth factor, therefore, needle size and depth of injury need to be controlled carefully. Peeters et al (Peeters et al., 2015) used a slow release system for goat disc regeneration by conjugating BMP2/BMP7 to a fibrin/hyaluronic acid hydrogel.
Endplate injury
The endplate is an important constituent that maintains disc structure. Yuan et al (Yuan et al., 2015) reported a rat ischemic sub-endplate induced disc degeneration model developed by injecting absolute alcohol, intradiscally, into the rat tail. Changes were seen in disc height and bone sclerosis. NP cells first changed from a vacuolar cell type to chondrocyte-like cells and then to fibrocartilaginous cells. Disorganized lamellae appeared first followed by fibrosis and rupture of the AF. The growth plate in the endplate regressed and eventually disappeared. Wei et al (Wei et al., 2015a; 2015b,) reported percutaneous injection of pingyangmycin (in rabbit) or bleomycin (in rhesus monkey) into the subchondral bone adjacent to the disc using CT guidance. After surgery, progressive narrowing of the disc space and loss of MRI signal were observed accompanied with osteogenesis in the endplate. An elevation in catabolic and reduction in anabolic gene expression were also detected.
In a dog model, disc degeneration was induced by perforating the NP via the vertebral endplate. The ensuing degeneration was confirmed by MRI, histology and biochemistry (Hutton et al., 2004). In mature dogs, the endplate channels were sealed by placing bone cement by way of the vertebrae, and, subsequent to this treatment, there was no detectable difference between the condition of the control and experimental discs with respect to disc space, gross morphology and an increase in proteoglycan content. In contrast, Kang et al (Kang et al., 2014) detected severe disc degeneration in immature pigs when the endplate was blocked with bone cement, which represents a more severe model than the stab-induced AF injury controls. Not surprisingly, MRI showed abnormal nutritional diffusion patterns in the discs after the endplate had been blocked.
While the studies that involve injuring the endplate showed promising results, details of structural changes to the endplate and consequent effects on nutrient transport between the vertebrae and discs need to be explored further.
4) Radicular pain
when disc herniation occurs, the herniated NP not only compresses the nerve roots but also induces inflammatory responses, which may play a crucial role in the radicular pain. Researchers have applied autologous NP to spinal nerve roots in rodents to mimic radicular pain in human (Kallakuri et al., 2005; Omarker and Myers, 1998; Olmarker, 2011; Kim et al., 2011; de Souza Grava et al., 2012; Cuellar et al., 2013). However, this procedure is technically demanding, predisposes to iatrogenic neural deficit, and uses a posterior laminectomy approach generating confounding bony pain.
4. Conclusion and perspective
Due to the unique position and complexity of the IVD and the consequent degenerative changes with age, it has been proven difficult to create an ideal animal model for disc degeneration that replicates the disease in humans. In addition, differences in anatomy between animal and human posture, disc size, cell type, and loading further complicate the quest for an ideal animal model. Notochord cells disappear with maturity at 10 years old in humans, representing an important step of disc degeneration. By contrast to the human disc, notochord cells are retained in the majority of animal species (Daly et al., 2016), which manifests as a difference between human and animal models. Animals with spontaneous disc degeneration as well as transgenic mice are good candidates for helping us understand the disease despite their limitations. Chemical induction may be an adequate approach for mild disc degeneration, although dosage of reagents and the depth of injection need to be optimized. The mechanical force model has been shown to be suitable for progressive disc degeneration but loading time and frequency need to be standardized. Needle puncture has become a popular means for modeling disc degeneration due to reproducibility and the relatively short time for induction of degenerative changes, but it is not an appropriate model for early intervention. It is noteworthy that most of these studies have focused on the changes in disc structure and biochemistry but neglected the natural progressive process of disc degeneration and symptomatic radiculopathy. Moreover, a posterior or posterolateral approach to the disc space is not suitable for open surgery because the neural components and bony structures are located posterior to the disc. Thus, a posterior approach has a higher chance of injuring a neural component than an anterior approach. A new animal model is in needed to reflect the disease in human: disc degeneration and the associated pain.
A recent study compared the hyperalgesia response in rat between annular puncture and inflammatory cytokine injection. The authors (Lai et al., 2016) showed that the rate and severity of disc degeneration are proportional to the degree of injury to the annulus, and the ensuing behavioral response to pain is commensurate with the loss of disc height and the inflammatory state of the disc. Annular puncture with injection of an inflammatory cytokine might be a potential model for disc degeneration. In another study, disc degeneration was induced in rat L4/5 discs by puncturing with a 0.4 mm needle anteriorly or posteriorly. Inflammatory cytokines were detected in the NP cells. Mechanical allodynia was observed with the posterior approach from day 1 up to 21 days but not in the anterior approach (Li et al., 2014; Liu et al., 2014). These studies suggest that the inflammation and disc herniation cause behavioral changes in response to pain. Future investigations may benefit from the development of models in large animals, combined with studies using models that exhibit spontaneous mutations as well as transgenic animal models.
Acknowledgments
we thank the financial supports from NIAMS R01AR064792 and North America Spine Society to XL.
Footnotes
Conflict of interests: we have no conflict of interests.
References
- Adams MA, Hutton WC. The effect of fatigue on the lumbar intervertebral disc. J Bone Joint Surg Br. 1983;65:199–203. doi: 10.1302/0301-620X.65B2.6826631. [DOI] [PubMed] [Google Scholar]
- Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2005;30:1242–1246. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Ashton IK, Walsh DA, Polak JM, Eisenstein SM. Substance P in intervertebral discs. binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994;65:635–639. doi: 10.3109/17453679408994620. [DOI] [PubMed] [Google Scholar]
- Bedore J, Sha W, McCann MR, Liu S, Leask A, Seguin CA. Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis Rheum. 2013;65:2634–2644. doi: 10.1002/art.38075. [DOI] [PubMed] [Google Scholar]
- Bergknut N, Rutges JP, Kranenburg HJ, Smolders LA, Hagman R, Smidt HJ, Lagerstedt AS, Penning LC, Voorhout G, Hazewinkel HA, Grinwis GC, Creemers LB, Meij BP, Dhert WJ. The dog as an animal model for intervertebral disc degeneration? Spine (Phila Pa 1976) 2012;37:351–358. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- Blanco G, Coulton GR, Biggin A, Grainge C, Moss J, Barrett M, Berquin A, Maréchal G, Skynner M, van Mier P, Nikitopoulou A, Kraus M, Ponting CP, Mason RM, Brown SD. The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum Mol Genet. 2001;10:9–16. doi: 10.1093/hmg/10.1.9. [DOI] [PubMed] [Google Scholar]
- Boxberger JI, Auerbach JD, Sen S, Elliott DM. An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine (Phila Pa 1976) 2008;33:146–154. doi: 10.1097/BRS.0b013e31816054f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- Ching CT, Chow DH, Yao FY, Holmes AD. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: An in vivo study in a rat tail model. Clin Biomech (Bristol, Avon) 2003;18:182–189. doi: 10.1016/s0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Cho H, Park SH, Lee S, Kang M, Hasty KA, Kim SJ. Snapshot of degenerative aging of porcine intervertebral disc: A model to unravel the molecular mechanisms. Exp Mol Med. 2011;43:334–340. doi: 10.3858/emm.2011.43.6.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar JM, Borges PM, Cuellar VG, Yoo A, Scuderi GJ, Yeomans DC. Cytokine expression in the epidural space: a model of noncompressive disc herniation-induced inflammation. Spine. 2013;38:17–23. doi: 10.1097/BRS.0b013e3182604baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Lamas S, Goncalves RM, Barbosa MA. Joint analysis of IVD herniation and degeneration by rat caudal needle puncture model. J Orthop Res. 2017;35:258–268. doi: 10.1002/jor.23114. [DOI] [PubMed] [Google Scholar]
- Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016 doi: 10.1155/2016/5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Grava AL, Ferrari LF, Defino HL. Cytokine inhibition and time-related influence of inflammatory stimuli on the hyperalgesia induced by the nucleus pulposus. Eur Spine J. 2012;21:537–545. doi: 10.1007/s00586-011-2027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine (Phila Pa 1976) 2008;33:588–596. doi: 10.1097/BRS.0b013e318166e0a2. [DOI] [PubMed] [Google Scholar]
- Fields AJ, Berg-Johansen B, Metz LN, Miller S, La B, Liebenberg EC, Coughlin DG, Graham JL, Stanhope KL, Havel PJ, Lotz JC. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCD-T2DM rat model of type 2 diabetes. J Orthop Res. 2015;33:738–746. doi: 10.1002/jor.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. comparison of surgical specimens with normal controls. Spine (Phila Pa 1976) 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr Autologous intervertebral disc cell implantation: A model using psammomys obesus, the sand rat. Spine (Phila Pa 1976) 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Ashraf N, Kilburn J, Williams C, Norton HJ, Gordon BE, Hanley EN., Jr Vertebral endplate architecture and vascularization: Application of micro-computerized tomography, a vascular tracer, and immunocytochemistry in analyses of disc degeneration in the aging sand rat. Spine (Phila Pa 1976) 2005;30:2593–600. doi: 10.1097/01.brs.0000187877.30149.83. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Gordon B, Williams C, Ingram JA, Norton HJ, Hanley EN., Jr A new small animal model for the study of spine fusion in the sand rat: Pilot studies. Lab Anim. 2009;43:272–277. doi: 10.1258/la.2008.008055. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Norris RA, Kern MJ, Hoelscher GL, Ingram JA, Zinchenko N, Hanley EN., Jr Periostin is expressed by cells of the human and sand rat intervertebral discs. Biotech Histochem. 2011;86:199–206. doi: 10.3109/10520291003722774. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Phillips R, Ingram JA, Norton HJ, Hanley EN., Jr Spontaneous age-related cervical disc degeneration in the sand rat. Clin Orthop Relat Res. 2014;472:1936–1942. doi: 10.1007/s11999-014-3497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Yurube T, Kakutani K, Maeno K, Takada T, Yamamoto J, Kurakawa T, Akisue T, Kuroda R, Kurosaka M, Nishida K. A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell phenotype. J Orthop Res. 2014;32:455–463. doi: 10.1002/jor.22533. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn RJ, Helder MN, Kroeze RJ, Bank RA, Smit TH, Wuisman PI. Reproducible long-term disc degeneration in a large animal model. Spine (Phila Pa 1976) 2008;33:949–954. doi: 10.1097/BRS.0b013e31816c90f0. [DOI] [PubMed] [Google Scholar]
- Hutton WC, Ganey TM, Elmer WA, Kozlowska E, Ugbo JL, Doh ES, Whitesides TE., Jr Does long-term compressive loading on the intervertebral disc cause degeneration? Spine (Phila Pa 1976) 2000;25:2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- Hutton WC, Murakami H, Li J, Elmer WA, Yoon ST, Minamide A, Akamaru T, Tomita K. The effect of blocking a nutritional pathway to the intervertebral disc in the dog model. J Spinal Disord Tech. 2004;17:53–63. doi: 10.1097/00024720-200402000-00011. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Kumar S, Foster RJ, Weidenbaum M, Mow VC. Shear mechanical properties of human lumbar annulus fibrosus. J Orthop Res. 1999;17:732–737. doi: 10.1002/jor.1100170517. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine (Phila Pa 1976) 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- Issy AC, Castania V, Silveira JW, Nogueira-Barbosa MH, Salmon CE, Del-Bel E, Defino HL. Does a small size needle puncture cause intervertebral disc changes? Acta Cir Bras. 2015;30:574–579. doi: 10.1590/S0102-865020150080000009. [DOI] [PubMed] [Google Scholar]
- Jin L, Shimmer AL, Li X. The challenge and advancement of annulus fibrosus tissue engineering. Eur Spine J. 2013;22:1090–1100. doi: 10.1007/s00586-013-2663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Li X. Growth differentiation factor 5 regulation in bone regeneration. Curr Pharm Des. 2013;19:3364–3373. doi: 10.2174/1381612811319190003. [DOI] [PubMed] [Google Scholar]
- Kang R, Li H, Ringgaard S, Rickers K, Sun H, Chen M, Xie L, Bünger C. Interference in the endplate nutritional pathway causes intervertebral disc degeneration in an immature porcine model. Int Orthop. 2014;38:1011–1017. doi: 10.1007/s00264-014-2319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallakuri S, Takebayashi T, Ozaktay AC, Chen C, Yang S, Wooley PH, Cavanaugh JM. The effects of epidural application of allografted nucleus pulposus in rats on cytokine expression, limb withdrawal and nerve root discharge. Eur Spine J. 2005;14:956–964. doi: 10.1007/s00586-004-0773-6. [DOI] [PubMed] [Google Scholar]
- Keorochana G, Johnson JS, Taghavi CE, Liao JC, Lee KB, Yoo JH, Ngo SS, Wang JC. The effect of needle size inducing degeneration in the rat caudal disc: Evaluation using radiograph, magnetic resonance imaging, histology, and immunohistochemistry. Spine J. 2010;10:1014–1023. doi: 10.1016/j.spinee.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kroin JS, Li X, An HS, Buvanendran A, Yan D, Tuman KJ, van Wijnen AJ, Chen D, Im HJ. The rat intervertebral disk degeneration pain model: Relationships between biological and structural alterations and pain. Arthritis Res Ther. 2011;13:R165. doi: 10.1186/ar3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Park SM, Cho YW, Jung YJ, Lee DG, Jang SH, Park HW, Hwang SJ, Ahn SH. Changes in expression of mRNA for interleukin-8 and effects of interleukin-8 receptor inhibitor in the spinal dorsal horn in a rat model of lumbar disc herniation. Spine. 2011;36:2139–2146. doi: 10.1097/BRS.0b013e31821945a3. [DOI] [PubMed] [Google Scholar]
- Kim DW, Chun HJ, Lee SK. Percutaneous needle puncture technique to create a rabbit model with traumatic degenerative disk disease. World Neurosurg. 2015;84:438–445. doi: 10.1016/j.wneu.2015.03.066. [DOI] [PubMed] [Google Scholar]
- Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, Richter W. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine (Phila Pa 1976) 2002;27:2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- Kuo YJ, Wu LC, Sun JS, Chen MH, Sun MG, Tsuang YH. Mechanical stress-induced apoptosis of nucleus pulposus cells: An in vitro and in vivo rat model. J Orthop Sci. 2014;19:313–322. doi: 10.1007/s00776-013-0510-2. [DOI] [PubMed] [Google Scholar]
- Kwon YJ. A minimally invasive rabbit model of progressive and reproducible disc degeneration confirmed by radiology, gene expression, and histology. J Korean Neurosurg Soc. 2013;53:323–330. doi: 10.3340/jkns.2013.53.6.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Moon A, Purmessur D, Skovrlj B, Laudier DM, Winkelstein BA, Cho SK, Hecht AC, Iatridis JC. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in-vivo. Spine J. 2016;16:420–431. doi: 10.1016/j.spinee.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kim BJ, Lim EJ, Back SK, Lee JH, Yu SW, Hong SH, Kim JH, Lee SH, Jung WW, Sul D, Na HS. Complete freund’s adjuvant-induced intervertebral discitis as an animal model for discogenic low back pain. Anesth Analg. 2009;109:1287–1296. doi: 10.1213/ane.0b013e3181b31f39. [DOI] [PubMed] [Google Scholar]
- Li D, Yang H, Huang Y, Wu Y, Sun T, Li X. Lumbar intervertebral disc puncture under C-arm fluoroscopy: A new rat model of lumbar intervertebral disc degeneration. Exp Anim. 2014;63:227–234. doi: 10.1538/expanim.63.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu H, Yang H, Wang J, Wang H, Zhang K, Zheng Z. Both expression of cytokines and posterior annulus fibrosus rupture are essential for pain behavior changes induced by degenerative intervertebral disc: An experimental study in rats. J Orthop Res. 2014;32:262–272. doi: 10.1002/jor.22494. [DOI] [PubMed] [Google Scholar]
- Liu H, Li S, Wang J, Wang T, Yang H, Li Z, Li X, Zheng Z. An analysis of spinopelvic sagittal alignment after lumbar lordosis reconstruction for degenerative spinal diseases: How much balance can be obtained? Spine (Phila Pa 1976) 2014;39:B52–59. doi: 10.1097/BRS.0000000000000500. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine (Phila Pa 1976) 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine (Phila Pa 1976) 2000;25:1477–1483. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006;88(Suppl 2):76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine (Phila Pa 1976) 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- Liang H, Ma SY, Feng G, Shen FH, Li X. Therapeutic effects of adenovirus-mediated growth and differentiation factor-5 in a mice disc degeneration model induced by annulus needle puncture. Spine J. 2010;10:32–41. doi: 10.1016/j.spinee.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas O, Hamel O, Blanchais A, Lesoeur J, Abadie J, Fellah BH, Fusellier M, Gauthier O, Bord E, Grimandi G, Vinatier C, Guicheux J, Clouet J. Laser-treated nucleus pulposus as an innovative model of intervertebral disc degeneration. Exp Biol Med (Maywood) 2012;237:1359–1367. doi: 10.1258/ebm.2012.012049. [DOI] [PubMed] [Google Scholar]
- Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- Mason RM, Palfrey AJ. Intervertebral disc degeneration in adult mice with hereditary kyphoscoliosis. J Orthop Res. 1984;2:333–338. doi: 10.1002/jor.1100020405. [DOI] [PubMed] [Google Scholar]
- Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: Correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010;10:1098–1105. doi: 10.1016/j.spinee.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps M, Tajerian M, Sage EH, Stone LS. Behavioral signs of chronic back pain in the SPARC-null mouse. Spine (Phila Pa 1976) 2011;36:95–102. doi: 10.1097/BRS.0b013e3181cd9d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps M, Tajerian M, Naso L, Sage EH, Stone LS. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC-null mice. Pain. 2012;153:1167–1179. doi: 10.1016/j.pain.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Czerminski JT, Mathieu AP, Stone LS. Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. Spine J. 2015;15:2524–2537. doi: 10.1016/j.spinee.2015.08.055. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Ishikawa T, Orita S, Eguchi Y, Kamoda H, Arai G, Suzuki M, Inoue G, Aoki Y, Toyone T, Takahashi K, Ohtori S. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: Pathomechanism of chronic diskogenic low back pain. Spine (Phila Pa 1976) 2011;36:2260–2266. doi: 10.1097/BRS.0b013e31820e68c7. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Millecamps M, Danco AT, Ohtori S, Takahashi K, Stone LS. ISSLS prize winner: Increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976) 2014;39:1345–1354. doi: 10.1097/BRS.0000000000000334. [DOI] [PubMed] [Google Scholar]
- Moss IL, Zhang Y, Shi P, Chee A, Piel MJ, An HS. Retroperitoneal approach to the intervertebral disc for the annular puncture model of intervertebral disc degeneration in the rabbit. Spine J. 2013;13:229–234. doi: 10.1016/j.spinee.2012.02.028. [DOI] [PubMed] [Google Scholar]
- Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41:589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- Norcross JP, Lester GE, Weinhold P, Dahners LE. An in vivo model of degenerative disc disease. J Orthop Res. 2003;21:183–188. doi: 10.1016/S0736-0266(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Oegema TRJr, Johnson SL, Aguiar DJ, Ogilvie JW. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine (Phila Pa 1976) 2000;25:2742–2747. doi: 10.1097/00007632-200011010-00005. [DOI] [PubMed] [Google Scholar]
- Omarker K, Myers RR. Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain. 1998;78:99–105. doi: 10.1016/S0304-3959(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Olmarker K. Combination of two cytokine inhibitors reduces nucleus pulposus-induced nerve injury more than using each inhibitor separately. Open Orthop J. 2011;5:151–153. doi: 10.2174/1874325001105010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlor GW, Nerlich AG, Wilke HJ, Pfeiffer M, Lorenz H, Schaaf-Keim M, Bertram H, Richter W, Carstens C, Guehring T. A new porcine in vivo animal model of disc degeneration: Response of anulus fibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine (Phila Pa 1976) 2009;34:2730–2739. doi: 10.1097/BRS.0b013e3181b723c9. [DOI] [PubMed] [Google Scholar]
- Palmgren T, Gronblad M, Virri J, Seitsalo S, Ruuskanen M, Karaharju E. Immunohistochemical demonstration of sensory and autonomic nerve terminals in herniated lumbar disc tissue. Spine (Phila Pa 1976) 1996;21:1301–1306. doi: 10.1097/00007632-199606010-00004. [DOI] [PubMed] [Google Scholar]
- Peeters M, Detiger SE, Karfeld-Sulzer LS, Smit TH, Yayon A, Weber FE, Helder MN. BMP-2 and BMP-2/7 heterodimers conjugated to a fibrin/hyaluronic acid hydrogel in a large animal model of mild intervertebral disc degeneration. Biores Open Access. 2015;4:398–406. doi: 10.1089/biores.2015.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KL, Jordan-Mahy N, Nicklin MJ, Le Maitre CL. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann Rheum Dis. 2013;72:1860–1867. doi: 10.1136/annrheumdis-2012-202266. [DOI] [PubMed] [Google Scholar]
- Puustjarvi K, Lammi MJ, Kiviranta I, Helminen HJ, Tammi M. Flat bed scanner in the quantitative assay of 35SO4-incorporation by X-ray film autoradiography of intervertebral disc sections. Histochemistry. 1993;99:67–73. doi: 10.1007/BF00268023. [DOI] [PubMed] [Google Scholar]
- Saamanen AM, Puustjarvi K, Ilves K, Lammi M, Kiviranta I, Jurvelin J, Helminen HJ, Tammi M. Effect of running exercise on proteoglycans and collagen content in the intervertebral disc of young dogs. Int J Sports Med. 1993;14:48–51. doi: 10.1055/s-2007-1021145. [DOI] [PubMed] [Google Scholar]
- Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Sakai D, Nishimura K, Tanaka M, Nakajima D, Grad S, Alini M, Kawada H, Ando K, Mochida J. Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: A pilot study. Spine J. 2015;15:1356–1365. doi: 10.1016/j.spinee.2013.07.491. [DOI] [PubMed] [Google Scholar]
- Stolworthy DK, Bowden AE, Roeder BL, Robinson TF, Holland JG, Christensen SL, Beatty AM, Bridgewater LC, Eggett DL, Wendel JD, Stieger-Vanegas SM, Taylor MD. MRI evaluation of spontaneous intervertebral disc degeneration in the alpaca cervical spine. J Orthop Res. 2015;33:1776–1783. doi: 10.1002/jor.22968. [DOI] [PubMed] [Google Scholar]
- Sugimura T, Kato F, Mimatsu K, Takenaka O, Iwata H. Experimental chemonucleolysis with chondroitinase ABC in monkeys. Spine (Phila Pa 1976) 1996;21:161–165. doi: 10.1097/00007632-199601150-00001. [DOI] [PubMed] [Google Scholar]
- Tapp H, Deepe R, Ingram JA, Kuremsky M, Hanley EN, Jr, Gruber HE. Adipose-derived mesenchymal stem cells from the sand rat: Transforming growth factor beta and 3D co-culture with human disc cells stimulate proteoglycan and collagen type I rich extracellular matrix. Arthritis Res Ther. 2008;10:R89. doi: 10.1186/ar2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: Repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 2007;32:2812–2819. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine BA, Saulez MN, Cebra CK, Fischer KA. Compressive myelopathy due to intervertebral disk extrusion in a llama (lama glama) J Vet Diagn Invest. 2006;18:126–129. doi: 10.1177/104063870601800122. [DOI] [PubMed] [Google Scholar]
- Virri J, Gronblad M, Savikko J, Palmgren T, Seitsalo S, Ruuskanen M, Karaharju E. Prevalence, morphology, and topography of blood vessels in herniated disc tissue. A comparative immunocytochemical study. Spine (Phila Pa 1976) 1996;21:1856–1863. doi: 10.1097/00007632-199608150-00004. [DOI] [PubMed] [Google Scholar]
- Vo N, Niedernhofer LJ, Nasto LA, Jacobs L, Robbins PD, Kang J, Evans CH. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013;31:831–837. doi: 10.1002/jor.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–341. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Nasto LA, Roughley P, Leme AS, Houghton AM, Usas A, Sowa G, Lee J, Niedernhofer L, Shapiro S, Kang J, Vo N. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20:896–905. doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tang D, Shu B, Wang B, Jin H, Hao S, Dresser KA, Shen J, Im HJ, Sampson ER, Rubery PT, Zuscik MJ, Schwarz EM, O’Keefe RJ, Wang Y, Chen D. Conditional activation of beta-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012;64:2611–2623. doi: 10.1002/art.34469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhong R, Pan X, Khaleel M, Hammoud A, Zhou Z, Liu S, Sun H, Zhao Y, Zou X, Jiang B, Zhuang W, Chen N, Chen Y. Computed tomography guided subendplate injection of pingyangmycin for A novel rabbit model of slowly progressive disc degeneration. Spine J. 2015 doi: 10.1016/j.spinee.2015.04.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wei F, Zhong R, Wang L, Zhou Z, Pan X, Cui S, Sun H, Zou X, Gao M, Jiang B, Chen W, Zhuang W, Sun H, Liu S. Pingyangmycin-induced in vivo lumbar disc degeneration model of rhesus monkeys. Spine (Phila Pa 1976) 2015;40:E199–210. doi: 10.1097/BRS.0000000000000736. [DOI] [PubMed] [Google Scholar]
- Xi Y, Kong J, Liu Y, Wang Z, Ren S, Diao Z, Hu Y. Minimally invasive induction of an early lumbar disc degeneration model in rhesus monkeys. Spine (Phila Pa 1976) 2013;38:E579–E586. doi: 10.1097/BRS.0b013e31828b695b. [DOI] [PubMed] [Google Scholar]
- Yan Z, Pan Y, Wang S, Cheng M, Kong H, Sun C, Hu K, Chen T, Dong Q, Chen J. Static Compression Induces ECM Remodeling and Integrin α2β1 Expression and Signaling in a Rat Tail Caudal Intervertebral Disc Degeneration Model. Spine (Phila Pa 1976) 2016 doi: 10.1097/BRS.0000000000001856. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Yang F, Leung V, Luk K, Chan D, Cheung K. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218:113–121. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- Yang X, Li X. Nucleus pulposus tissue engineering: A brief review. Eur Spine J. 2009;18:1564–1572. doi: 10.1007/s00586-009-1092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Miyazaki M, Hong SW, Tow B, Morishita Y, Hu M, Ahn SJ, Wang JC. A porcine model of intervertebral disc degeneration induced by annular injury characterized with magnetic resonance imaging and histopathological findings. laboratory investigation. J Neurosurg Spine. 2008;8:450–457. doi: 10.3171/SPI/2008/8/5/450. [DOI] [PubMed] [Google Scholar]
- Yuan W, Che W, Jiang YQ, Yuan FL, Wang HR, Zheng GL, Li XL, Dong J. Establishment of intervertebral disc degeneration model induced by ischemic sub-endplate in rat tail. Spine J. 2015;15:1050–1059. doi: 10.1016/j.spinee.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Yurube T, Takada T, Suzuki T, Kakutani K, Maeno K, Doita M, Kurosaka M, Nishida K. Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther. 2012;14:R51. doi: 10.1186/ar3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurube T, Nishida K, Suzuki T, Kaneyama S, Zhang Z, Kakutani K, Maeno K, Takada T, Fujii M, Kurosaka M, Doita M. Matrix metalloproteinase (MMP)-3 gene up-regulation in a rat tail compression loading-induced disc degeneration model. J Orthop Res. 2010;28:1026–1032. doi: 10.1002/jor.21116. [DOI] [PubMed] [Google Scholar]
- Yurube T, Hirata H, Kakutani K, Maeno K, Takada T, Zhang Z, Takayama K, Matsushita T, Kuroda R, Kurosaka M, Nishida K. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther. 2014;16:R31. doi: 10.1186/ar4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Jin L, Reames DL, Shen FH, Shimer AL, Li X. Intervertebral disc degeneration and ectopic bone formation in apolipoprotein E knockout mice. J Orthop Res. 2013;31:210–217. doi: 10.1002/jor.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Hou S, Shang W, Wu W, Cheng Y, Mei F, Peng B. A new in vivo animal model to create intervertebral disc degeneration characterized by MRI, radiography, CT/discogram, biochemistry, and histology. Spine (Phila Pa 1976) 2007;32:864–872. doi: 10.1097/01.brs.0000259835.31062.3d. [DOI] [PubMed] [Google Scholar]
- Zhou RP, Zhang ZM, Wang L, Huang MJ, Zheng XC, Cui YN, Yin M, Wang XK, Yao NZ, Chen TY, Chen J, Bai XC, Jin DD. Establishing a disc degeneration model using computed tomography-guided percutaneous puncture technique in the rabbit. J Surg Res. 2013;18:e65–74. doi: 10.1016/j.jss.2012.07.027. [DOI] [PubMed] [Google Scholar]