Abstract
Huntington disease (HD) is a progressive autosomal dominant neurodegenerative disorder in which patients typically present with uncontrolled involuntary movements and subsequent cognitive decline. In 1993, a CAG trinucleotide repeat expansion in the coding region of the huntingtin (HTT) gene was identified as the cause of this disorder. This extended CAG repeat results in production of HTT protein with an expanded polyglutamine tract, leading to pathogenic HTT protein conformers that are resistant to protein turnover, culminating in cellular toxicity and neurodegeneration. Research into the mechanistic basis of HD has highlighted a role for bioenergetics abnormalities stemming from mitochondrial dysfunction, and for synaptic defects, including impaired neurotransmission and excitotoxicity. Interference with transcription regulation may underlie the mitochondrial dysfunction. Current therapies for HD are directed at treating symptoms, as there are no disease-modifying therapies. Commonly prescribed drugs for involuntary movement control include tetrabenazine, a potent and selective inhibitor of vesicular monoamine transporter 2 that depletes synaptic monoamines, and olanzapine, an atypical neuroleptic that blocks the dopamine D2 receptor. Various drugs are used to treat non-motor features. The HD therapeutic pipeline is robust, as numerous efforts are underway to identify disease-modifying treatments, with some small compounds and biological agents moving into clinical trials. Especially encouraging are dosage reduction strategies, including antisense oligonucleotides, and molecules directed at transcription dysregulation. Given the depth and breadth of current HD drug development efforts, there is reason to believe that disease-modifying therapies for HD will emerge, and this achievement will have profound implications for the entire neurotherapeutics field.
Introduction
Huntington disease (HD) is a progressive autosomal dominant neurodegenerative disorder in which patients display motor and cognitive impairment (Table I) [Nance 1997]. HD is a chronic disorder with a relentlessly progressive clinical course, culminating in death typically 15 to 25 years after symptom onset. Most HD patients present in the 4th or 5th decade of life with a combination of motor, affective, and cognitive deficits, together with sleep disturbances and weight loss. HD patients typically display gradual onset of an involuntary movement disorder in which occasional adventitious movements develop into rhythmic uncontrolled motions. This appearance of rhythmic uncontrolled movements led clinicians to describe the disorder as Huntington chorea to emphasize the “dance-like” appearance of these movements. With progression of the involuntary movement disorder, the clinical course is then characterized by cognitive decline, exhibited by an inability to perform sequenced tasks, suggesting a decline in executive function [Paulsen 2011]. Cognitive decline is inexorably progressive and represents the most debilitating aspect of the disease course, leading patients to no longer be able to perform the activities of daily living, resulting in the need for institutional care [Paulsen 2011]. While most HD patients present with motor involvement, a subset of HD patients (∼15%) initially develop clinically significant psychiatric disease and thus present with a psychiatric diagnosis prior to onset of movement disorder [Paulsen 2011]. The genetic basis of HD was identified in 1993, and facilitated “presymptomatic” genetic testing of individuals at risk as well as a laboratory method for accurate confirmation of the clinical diagnosis [Huntington's and others 1993]. Symptomatic HD patients are diagnosed based upon the presence of involuntary adventitious movements (Table 1), in combination with a positive genetic test, and often, but not always, in the context of a positive family history of HD [Reilmann and others 2014].
Table I.
HD Diagnostic Criteria
| Genetically Confirmed | NOT Genetically Confirmed | |
|---|---|---|
| Presymptomatic |
|
Clinically At-Risk |
| Prodromal HD |
|
Clinically Prodromal HD |
| Manifest HD |
|
Clinically Manifest (requires Motor DCL = 4 and cognitive changes) |
DCL = diagnostic confidence level; TFC = Total Functional Capacity scale
While HD is designated as a rare or orphan disease, it is actually one of the most common monogenic inherited disorders known to humankind, with a frequency of ∼1 in 8,000 in individuals of Caucasian European ancestry [Dayalu and Albin 2015]. HD also occurs in individuals of various racial and population groups, though at a much lower frequency than in Caucasian Europeans, with exceptions due to founder effects, where the disease gene was introduced to isolated populations (e.g. Venezuelans living along the shores of Lake Maracaibo). Current estimates place the prevalence of HD in the USA at 35,000 – 40,000 affected individuals [Dayalu and Albin 2015].
Extensive neuropathology studies of HD have been performed, and have led to a rating scale of 0 (presymptomatic) to 4 (severe) based upon the degree and extent of neurodegenerative findings at autopsy [Vonsattel and others 1985]. Most striking is an overall loss of brain volume in HD patients, with different brain regions showing different rates of neuron loss [Nopoulos and others 2010; Squitieri and others 2009]. Significant cerebral cortex degeneration and atrophy occurs, especially in the striatum, while cerebellar, thalamic, and spinal cord neuron populations are spared [Ross and others 1997]. Of particular note is that only certain populations of neurons degenerate and certain non-neural cell types, in particular skeletal muscle and adipose tissue, are affected, providing an explanation for the clinical constellation of symptoms in HD [Dayalu and Albin 2015]. HD neuropathology is notable for the selective vulnerability of the medium-sized spiny neurons (MSNs) of the striatum in the basal ganglia region of the midbrain [Dayalu and Albin 2015]. The basal ganglia plays a key role in the control of involuntary movement. Striatal neurons in the putamen projecting to the substantia nigra are often the first to degenerate, and their demise can even be documented in presymptomatic HD patients [Albin and others 1992; Reiner and others 1988]. Cortical pyramidal neurons that project to the putamen in the striatum degenerate as well; hence, the death of MSNs and the cortical neurons that project to them represents the neuropathological hallmark of HD (for an anatomical diagram, please see [Calabresi and others 2014]). Although the basal ganglia is viewed as the predominant arbiter of movement control, the suite of functions under basal ganglia control is not completely understood, with some theories proposing a contribution to cognitive executive system control [Montoya and others 2006], as well as regulation of the motor circuit [Crossman 2000]. In addition to profound neurodegeneration in HD, recent studies over the last decade have uncovered evidence for glial dysfunction in HD, with astrogliosis, manifested as an abnormal increase in astrocytes, and activation of microglia (the brain's immune cells) being prominent features of HD neuropathology [Lobsiger and Cleveland 2007].
HD was originally considered as only a movement disorder with chorea as the defining feature, and while this is often the case, HD is now understood to be more complex, with a range of non-motor issues. Determining when a HD patient is symptomatic can be challenging, as subtle cognitive and psychiatric changes are being uncovered with more sensitive methods of evaluation [Stout and others 2011]. To address this issue, a number of longitudinal observational studies have been conducted in order to better define disease onset (PREDICT-HD [Paulsen and others 2014]; TRACK-HD [Tabrizi and others 2013]). These studies are also providing insights into the phenotype range of HD, as the clinical natural history can greatly vary over time in an individual patient. Indeed, the challenge for managing HD patients is to manage a variety of clinical problems simultaneously. As discussed below, widespread changes in diverse cellular and molecular processes, including neurotransmission, BDNF signaling, energy metabolism, and neuroinflammation, occur in HD and likely account for the clinical features of this disease (Figures 1 and 2).
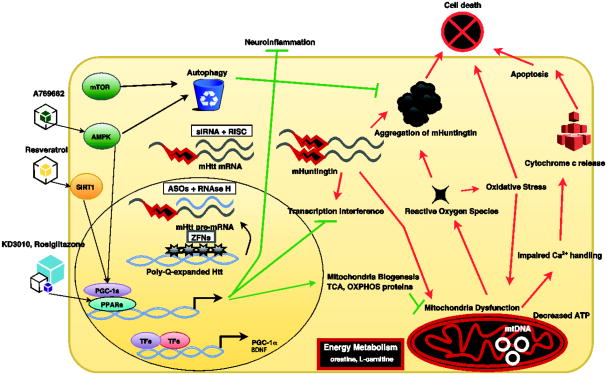
Figure 1. Huntington's Disease therapies directed at intracellular processes.
, A schematic illustration showing various intracellular processes that are perturbed by mutant huntingtin protein and thus high priority targets for therapy development. Note that some therapies are designed to work in the nucleus, others in the cytosol, and some at specific organelles, including especially mitochondria. AMPK (AMP-activated protein kinase); SIRT1 (Sirtuin 1); mTOR (mechanistic target of rapamycin); PPARs (peroxisome proliferator-activated receptors); PPARGC1A (PPAR gamma co-activator 1-alpha); OXPHOS (oxidative phosphorylation); TCA (tricarboxylic acid cycle); siRNA (small interfering RNA); RISC (RNA induced silencing complex); ASO (antisense oligonucleotide); ZFPs (zinc finger proteins); TFs (transcription factors); mtDNA (mitochondrial DNA).
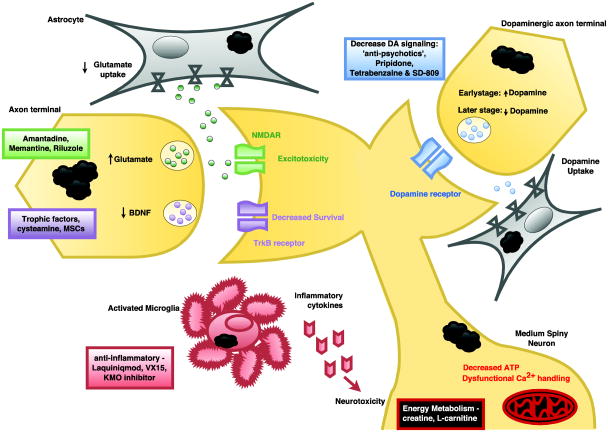
Figure 2. Huntington's Disease therapies that target extracellular pathways.
, Diagram of neurons, astrocyte, and microglia, highlighting treatments that act on extracellular processes and events at the cell surface membrane, including glutamatergic signaling, dopaminergic signaling, trophic factor release and action, inflammation, and calcium flux.
Underlying Mechanisms
In 1993, a CAG trinucleotide repeat expansion mutation in the coding region of the huntingtin (HTT) gene was identified as the cause of HD [Huntington's and others 1993]. As observed in other CAG – polyglutamine (polyQ) repeat diseases, HTT glutamine tracts that exceed a certain length threshold (about 37 repeats for HD) adopt a novel pathogenic confirmation, yielding conformers that are resistant to the normal processes of protein turnover, culminating in cellular toxicity and neurodegeneration [La Spada and Taylor 2010]. The CAG repeat size ranges established in HD are as follows: less than 35 CAGs is normal; more than 40 CAGs yields disease within the typical human lifespan; and 36 – 39 CAGs are indeterminate, as penetrance is reduced and may not manifest as clinical disease. The length of the mutant HTT polyQ expansion inversely correlates with the age of disease onset and rate of disease progression in HD patients. Furthermore, expanded CAG repeats in HTT are genetically unstable, leading to further expansion instead of reproducing an exact copy of the repeat, causing the number of repeats to change in successive generations, such that a parent with a so-called “intermediate” allele (27 – 35 CAGs), or “reduced penetrance” allele (36 – 39 CAGs) may transmit a CAG repeat that produces fully penetrant HD [Walker 2007]. This increase in the number of CAG repeats in successive generations accounts for the clinical phenomenon of anticipation, which is defined as an earlier age of disease onset and more rapid progression of disease in the children and grandchildren of affected HD patients [Walker 2007]. However, despite knowing the genetic basis of HD, the pathophysiological pathways leading to cellular dysfunction and neuronal cell loss are still being elucidated. Consequently, development of meaningful therapies for HD has not been straightforward. Indeed, there are no available disease-modifying therapies; hence, HD patients are currently managed with a wide range of symptomatic treatment modalities.
Over the last three decades, hundreds of research groups have devoted their efforts to understanding the cellular and molecular basis of HD. For a comprehensive discussion of this body of work, we refer the reader to these reviews [Imarisio and others 2008; Labbadia and Morimoto 2013; McFarland and Cha 2011; Zuccato and others 2010]. For the purposes of therapeutic strategies and treatment development, two key observations have turned out to be especially important: 1) HD patients consistently display bioenergetics abnormalities, likely stemming from mitochondrial dysfunction (reviewed in [Oliveira 2010]; and 2) Synaptic neurotransmission defects and excitotoxicity underlie degeneration of neural circuits, and thus are the defining features of the HD pathogenic cascade (reviewed in [Hardingham and Bading 2010].
Bioenergetics and mitochondrial abnormalities
Neurons have enormous demands for continued mitochondrial production of high-energy compounds, such as ATP [Oliveira 2010], and for the buffering of Ca2+ ions due to their susceptibility to excitotoxicity [Hardingham and Bading 2010]. Mitochondria functionalities: the negative mitochondria membrane potential, the ability to generate ATP, the production of reactive oxygen species, and the buffering of calcium ions (Ca2+) are interlinked. Changes in one will affect the others. These functions are also affected by the size and structure of the mitochondria – for example, mitochondria can form long calcium conduits in the dendrites of neurons to attenuate excitotoxicity [Popov and others 2005]. In 1993, one group reported that chronic administration of a mitochondrial toxin, 3-nitopropionic acid, resulted in a selective loss of MSNs in the striatum [Beal and others 1993]. This finding, which was corroborated by a wide range of studies in cell culture models, mice, and human HD patients (reviewed in [Lin and Beal 2006]), suggested that mitochondrial dysfunction may underlie HD pathogenesis and account for cell-type specificity in this disorder. At the same time, it was shown that the mutant htt protein must localize to the nucleus to produce disease [Saudou and others 1998], and an extensive literature then emerged, suggesting that amino-terminal fragments of mutant htt protein interfere with gene transcription to initiate the HD pathogenic cascade (reviewed in [Riley and Orr 2006]). We, and others, have linked the mitochondrial dysfunction and metabolic deficits in HD to transcription dysregulation of peroxisome proliferator-activated receptor (PPAR) gamma coactivator-1 alpha (PPARGC1A), a co-activator of the PPARs (α, β, γ) that is the key regulatory node in a network of transcriptional programs culminating in enhanced mitochondrial energy production through oxidative phosphorylation [Cui and others 2006; Lin and others 2004; Weydt and others 2006]. The importance of PGC-1α for HD pathogenesis is underscored by the observation that Ppargc1a over-expression in HD mice is sufficient to rescue motor phenotypes, prevent accumulation of misfolded htt protein in CNS through increased autophagy, and reduce neurodegeneration [Tsunemi and others 2012]. Additionally, the expression of dominant-negative PPARδ in the CNS of mice induced neurodegeneration in the cortex and striatum, and multiple motor symptoms, recapitulating aspects of HD [Dickey and others 2016]. Hence, nuclear transcription dysregulation and metabolic dysfunction are key molecular events in HD pathogenesis (Figure 1), and thus “high priority” targets for therapy development.
Synaptic neurotransmission defects and excitotoxicity
More than two decades ago, a model of basal ganglia organization and circuit pathway function was proposed in which cerebral cortex activity modulates physical movement through a set of distinct neuronal pathways originating in the striatum and projecting to the substantia nigra [Smith and others 1998]. In one pathway (the so-called “direct” pathway), activation of the striatum by the cerebral cortex resulted in striatal inhibitory neurotransmitter release onto substantia nigra, which sends inhibitory neuron projections to the thalamus. By inhibiting the substantia nigra inhibitory outflow tracts, activation of the direct pathway was posited to promote locomotion. In the other pathway (the so-called “indirect” pathway), activation of the striatum by the cerebral cortex resulted in striatal inhibitory neurotransmitter release onto the global pallidus pars externa, which sends inhibitory neuron projections to the subthalamic nucleus, which in turn sends activating neuron projections to the substantia nigra, whose inhibition of the thalamus results in reduced locomotion and movement [Walker 2007]. Furthermore, in addition to this anatomical pathway distinction, it was noted that the MSNs of the striatum could be distinguished by the presence of dopamine D1 or D2 receptors, such that D1 receptor-containing MSNs project to the substantia nigra via the direct pathway, while D2 receptor-containing MSNs project to the substantia nigra via the indirect pathway [Calabresi and others 2014], providing a molecular basis for our understanding of the HD synaptic pathophysiology that accounts for loss of involuntary movement control in affected patients.
Biphasic changes in dopamine neurotransmission in HD were initially revealed by neurochemical, electrophysiological, and behavioral studies in both patients and mouse models [Cepeda and others 2014]. In the early stages of disease, dopamine neurotransmission is increased, leading to hyperkinetic movements that can be alleviated by depleting dopamine stores (Figure 2). Altered dopamine neurotransmission through D2 receptors and the indirect pathway would thus increase glutamatergic signaling, producing the excitotoxicity seen early in disease [Cepeda and others 2014]. In contrast, in later stages, dopamine deficits produce hypokinesia that must be treated by increasing dopamine action. Cortical brain-derived neurotrophic factor (BDNF) production is required for the cortico-striatal synapse and for the survival of GABA-ergic striatal MSNs that are preferentially lost in HD [Zuccato and Cattaneo 2007]. Studies of HD postmortem patient samples have confirmed a dramatic loss of BDNF in the striatum, which likely contributes to the initial clinical manifestations of the disease [Zuccato and Cattaneo 2007]. Hence, ameliorating this deficit could be a useful therapeutic approach.
The current landscape and philosophy for treating patients with HD
At the time of writing this review, we still do not have any disease-modifying therapies to treat the underlying pathophysiology of HD. Rather, treatment is purely symptomatic, typically combining a variety of medications with speech, occupational, and/or physical therapy. While some medications have been specifically tested for efficacy in HD, many have been validated in other diseases to treat symptoms that also occur in HD. Most HD patients are prescribed multiple drugs to treat their array of symptoms, but as symptoms can and do evolve with disease progression, the use of a particular drug must be re-evaluated over time. For example, rigidity and bradykinesia can be problematic upon initial disease onset, while chorea and adventitial movements predominate, as HD progresses.
Compounds that primarily target motor symptoms
Uncontrolled movements in HD are believed to stem from over-activation of the dopaminergic D2 indirect pathway circuits in the basal ganglia [Schwab and others 2015]. Thus, neuroleptic agents are typically used to block receptors or deplete presynaptic dopamine to treat these motor aspects of HD [Schwab and others 2015]. This has become the standard of care based upon years of clinical experience (though there is little in the way of clinical trial data to support use of the most prescribed medications). A variety of other drugs can also be employed, and these compounds modulate glutamatergic neurotransmission, BDNF-Trk B signaling, or inflammation. In the United Kingdom, medications that bind the dopamine D2 receptor to block dopamine activation are preferred [Priller and others 2008]. Recently, tetrabenazine has emerged as an efficacious anti-chorea medication based upon clinical trial experience, and it was the first FDA-approved medication for HD. Deutetrabenazine was recently approved as the second. All other treatments are prescribed “off-label”.
1) Modulation of dopaminergic signaling
Tetrabenazine (TBZ), developed by Lundbeck pharmaceuticals (Copenhagen, Denmark), was FDA-approved in 2008 to suppress the involuntary movements classically observed in HD patients. TBZ is potent, selective, reversible, and short-acting; it is an inhibitor of the Vesicular Monoamine Transporter 2 (VMAT2) and thus depletes synaptic monoamines [Jankovic and Clarence-Smith 2011]. Unfortunately, TBZ has a serious side effect – it can trigger or worsen depression and other psychiatric conditions in some HD patients. TBZ can also interact with a subset of anti-depressant drugs, due to its effect on dopaminergic signaling.
Clinical experience with TBZ in HD dates back to 1988, where initial evidence for efficacy was suggested [Jankovic and Orman 1988]. However, it was not until 2006 that a landmark clinical trial by the Huntington Study Group, using the Unified Huntington's Disease Rating Scale (UHDRS), documented a decrease in total maximal chorea (TMC), baseline Clinical Global Impression (CGI) severity, and baseline chorea score, at a dose of 100 mg TBZ / day [HuntingtonStudyGroup 2006]. However, TBZ proved to be deleterious for other outcome measures, including the UHDRS Functional Checklist, the 17-item Hamilton Depression scale, the Epworth Sleepiness Scale, and the Stroop word reading test. Moreover, there were a number of adverse events (akathisia, suicide, intracranial hemorrhage, and depression) that prevented a subset of patients from completing the trial. Nonetheless, most clinicians who care for HD patients will attempt TBZ at dosages of up to 100 mg / day, and this clinical experience has confirmed that TBZ can quite effectively lessen chorea in ambulatory patients. A follow-up study examined the safety and efficacy of TBZ for both short-term and long-term control of chorea in HD, and found that mean UHDRS-TMC scores decreased markedly during the first 10 weeks of TBZ treatment, remained lower than baseline throughout 80 weeks, and returned to baseline levels after TBZ discontinuation [Frank 2009]. Nevertheless, TBZ-treated patients again performed worse in the UHDRS Functional Checklist, and serious adverse events occurred exclusively in the treatment group. Due to concern over serious side effects, TBZ must be dosed individually.
SD-809 (Deutetrabenazine)
Owned by Teva Pharmaceuticals (Petah Tikva, Israel), SD-809 is a drug with the same mechanism of action as TBZ. SD-809 (deutetrabenazine) is a novel molecule containing deuterium, which attenuates metabolism of TBZ and its break-down products, thereby increasing active metabolite half-life, and may therefore permit stable systemic exposure, while preserving pharmacological activity. In a Phase 3 study of 90 HD patients, SD-809 reduced chorea at 12 weeks compared to placebo [Frank and others 2016]. HD patients could safely switch from TBZ to SD-809 with continued control of chorea [Frank and others 2016]. In 2017, Teva received FDA approval for SD-809.
In addition to TBZ, a number of commonly used anti-psychotic medications, which bind the dopamine D2 receptor to block dopamine activation, exhibit the well-known side effect of suppressing voluntary movements, resulting in an inability to initiate motor actions or perform motor skills. As such, they can be used to treat chorea in HD. Haloperidol and chlorpromazine, “traditional” anti-psychotics, bind very tightly, and dissociate slowly from the dopamine receptor. Newer compounds (olanzapine, sulpiride, amisulpride, risperidone, and clozapine) transiently occupy D2 receptors, slowing dopamine neurotransmission to a lower rate, thereby sparing effects on cognition. However, these compounds can also worsen dystonia and muscle rigidity if the D1 direct pathway receptors are activated. As these drugs cause drowsiness due to blocking of the D2 receptors, physicians prescribe such anti-psychotics to HD patients to take at bedtime as a concomitant treatment for insomnia.
A survey of prescribing practice across Europe identified olanzapine, a second-generation atypical neuroleptic, as the most commonly used drug to manage chorea in HD. This is particularly true in the United Kingdom, where approximately 55% of all patients with HD are given this drug [Priller and others 2008]. Given the popularity of olanzapine, the absence of any robust scientific evidence to demonstrate its efficacy is somewhat surprising. In fact, evidence from two small open-label studies has shown that low doses of olanzapine, similar to those commonly prescribed clinically (5 mg), are ineffective at managing the motor aspects of HD [Paleacu and others 2002; Squitieri and others 2001]. However, a case report indicated that olanzapine at 5 mg/day, when used in combination with sodium valproate, was helpful in reducing chorea and improving the gait disturbance in two patients with advanced HD [Grove and others 2000]. Furthermore, a high dose of olanzapine (30 mg) virtually eradicated disabling chorea in a 30 year-old patient with advanced HD, allowing her to live independently [Bonelli and others 2002]. Olanzapine is often preferred to other anti-psychotics, because it is well tolerated and is associated with weight gain and sedation, which can help counteract the weight loss and sleep abnormalities commonly seen in HD [Goodman and others 2008]. Olanzapine is particularly indicated in patients with troublesome chorea, as this hyperkinetic movement disorder is often accompanied by “hyperkinetic” behavioral syndrome – with irritability, mood swings, poor sleep and impulsive behavior. Since it is taken once a day at night, this facilitates compliance. Many physicians start with olanzapine, and if it is not effective, then prescribe TBZ.
Other dopamine receptor antagonists, such as sulpiride, amisulpiride, and risperidone, can also be used clinically in HD, but are selected less often, as the data for their efficacy is minimal [Quinn and Marsden 1984; Reveley and others 1996]. While a series of studies in the late 1970's and 1980's found that haloperidol can be effective at suppressing chorea [Barr and others 1988; Gimenez-Roldan and Mateo 1989; Koller and Trimble 1985; Leonard and others 1975; Saran and others 1980], this drug is not commonly used due to the high frequency of side effects, including sedation, dystonia, akathisia, Parkinsonism, and tardive dyskinesia [Casey 1991; Grohmann and others 1990], which can be especially problematic for HD patients. A number of other anti-psychotics, including tiapride, clozapine, and transdihydrolisuride, have been evaluated in small series of HD patients, but none have demonstrated convincing results to warrant further consideration [Deroover and others 1984; Roos and others 1982; Stocchi and others 1989; van Vugt and others 1997].
2) Modulation of glutamatergic signaling
Beyond the dopaminergic system, other commonly used drugs in HD target glutamate neurotransmission. There is some evidence that amantadine, a glutamate antagonist, is effective at reducing chorea at a dose of 400 mg/day [Verhagen Metman and others 2002], but not at lower doses [O'Suilleabhain and Dewey 2003]. Unfortunately, this drug has also been shown to worsen irritability and aggression in HD patients [Stewart 1987], which can exacerbate the behavioral problems frequently found in this population of patients and thereby negatively impact quality of life [Banaszkiewicz and others 2012; Ho and others 2009; Ready and others 2008]. In 2002, a double-blind, placebo-controlled study examined the efficacy and safety of amantadine in 24 HD patients, and found that amantadine therapy was associated with a median reduction of 36% in extremity chorea score at rest versus 0% with placebo, with mean improvement of 56% for the 10 patients with the highest drug plasma levels [Verhagen Metman and others 2002]. Furthermore, amantadine was generally safe and well tolerated, and no deleterious effects on cognitive function were noted. Additional clinical studies of amantadine have been performed, and one trial documented reduced dyskinesia scores in treated patients [Lucetti and others 2003], while the other trial failed to detect any improvement in mean chorea score in patients receiving drug therapy [O'Suilleabhain and Dewey 2003]. In this latter study, a meta-analysis of amantadine therapy as a treatment for HD was conducted, and concluded that amantadine does not significantly reduce chorea in HD patients.
A number of other inhibitors of glutamatergic neurotransmission have been considered and evaluated in HD, including riluzole, which blocks a subset of sodium channels [Song and others 1997], and directly inhibits kainate and N-methyl-D-aspartate (NMDA) glutamate receptors [Debono and others 1993], ketamine and remacemide, which are NMDA receptor blockers, and lamotrigine, which inhibits glutamate release. For riluzole, a dosage of 200 mg/day showed efficacy for the selected primary outcome measure (change in total chorea score-UHDRS), but not when neuroleptic-treated patients were removed from analysis [HuntingtonStudyGroup 2003]. However, a safety issue was raised due to a persistent and significant elevation of the liver enzymes ALT and AST in the 200 mg/day riluzole group, which curtailed further investigation. 379 HD patients then completed a 3-year, randomized, placebo-controlled study of riluzole at 50 mg twice daily, but no intergroup difference in primary outcome was demonstrated [Landwehrmeyer and others 2007]. Thus, efficacy for riluzole, as well as for these other NMDA inhibitors, as a treatment for HD has not been attained.
Treatment algorithm for chorea
In summary, the choice for treating significant chorea in HD is typically between TBZ and olanzapine. Clinical evidence favors TBZ, but its side effects of depression and drowsiness are relative contra-indications in some HD patients. Furthermore, as TBZ is now approved for the treatment of HD in the USA, it costs more than $2000 per month at the starting dose of 25 mg/day, compared with about $45 per month, before it was licensed [Murphy and others 2012]. TBZ is typically prescribed in divided doses and thus must be taken several times a day, which can reduce compliance. For these reasons, many clinicians favor olanzapine as the initial choice in HD as olanzapine can be given once a day, typically at night, when its sedative side effects can counter insomnia. Other beneficial effects (mood stabilization and weight gain) of olanzapine make it an even more attractive first line therapy for HD, even though definitive clinical trial data confirming its utility in HD is still lacking. Hence, a reasonable algorithm is to initiate therapy with olanzapine, with TBZ as the second line therapy, if olanzapine fails to yield a satisfactory response. Selection of another drug from the various other options discussed above should only be pursued after both olanzapine and TBZ have failed to elicit symptomatic benefit.
Compounds that target bioenergetics pathways
1) Coenzyme Q10
Coenzyme Q10 is a component of the electron transport chain, and it acts as a reducing agent to transfer electrons to promote aerobic respiration and scavenge toxic reactive oxidative species generated during the process of oxidative phosphorylation in the mitochondria. Coenzyme Q10 has been studied extensively in different HD mouse models, and while initial experimental reports indicated that coenzyme Q10 extended survival, improved motor behavior, and countered molecular pathology in HD [Matthews and others 1998; Schilling and others 2001; Smith and others 2006], a more recent large-scale HD mouse study found no significant benefit [Menalled and others 2010]. Given the initial encouraging mouse data, a double-blind, randomized controlled trial of 347 early symptomatic HD patients commenced, and coenzyme Q10-treated patients showed a trend toward slowing in total functional capacity (TFC) decline (13%) over 30 months, but this did not achieve significance (p=0.15) [HuntingtonStudyGroup 2001]. A double-blind, placebo-controlled, randomized study of 609 early symptomatic HD subjects followed, but this study was terminated, as interim futility analysis predicted a very low likelihood for a positive outcome [McGarry and others 2017]. These studies indicate that coenzyme Q10 is likely ineffective in HD.
2) Creatine
Creatine is a naturally occurring compound that promotes energy production in cells by recycling adenosine diphosphate (ADP) to ATP via donation of phosphate groups. Considerable attention has focused on creatine as a potential therapy for HD, given its safety and presumed ability to favor a positive energy balance. Three double-blind placebo-controlled clinical trials of creatine were initially performed, and while two creatine clinical trials found no difference between placebo and treatment groups [Hersch and others 2006], [Verbessem and others 2003], another trial of 64 HD patients in which subjects were randomly allocated to 15 g twice daily or placebo for a 6-month double-blind phase, followed by a 12-month open-label extension, did demonstrate treatment-dependent slowing of cortical and striatal atrophy, but without improvement in performance on memory and cognitive tests [Rosas and others 2014]. To resolve these contradictory outcomes, the Huntington Study Group initiated the Phase 3 “CREST-E” clinical trial of 40 g/day of creatine, but a futility analysis part-way through the trial predicted that creatine would not show clinical benefit and thus the study was halted [Hersch and others 2017]. In conclusion, the clinical trial experience with creatine indicates that creatine conferred minimal, if any improvement, to HD patients.
3) Fatty acids, ethyl-EPA, carnitine, and free radical scavenger
A variety of other therapies targeting bioenergetics have been attempted in HD patients. Unsaturated fatty acids (at 8 g/day) were evaluated in a small HD cohort and yielded a significant reduction in dyskinesia, but without any beneficial effects on functional performance or other efficacy measures [Vaddadi and others 2002]. Three trials have studied the effects of ethyl-eicosapentaenoic acid (ethyl-EPA), a derivative of omega-3 fatty acid, and found no benefit in the primary outcome measure [Ferreira and others 2015; HuntingtonStudyGroup 2008; Puri and others 2005]. In another randomized double-blind placebo-controlled trial, no difference was noted on the Total Motor Score (TMS) or any measures of function, cognition, or global impression [HuntingtonStudyGroup 2008]. A small trial of acetyl-L-carnitine, 45mg/kg / day, to assess a potential acute effect on HD, found no evidence for efficacy for all treatment measures [Goety and others 1990]. The free-radical scavenger OPC-14117 also yielded no difference between treatment and placebo groups on defined outcome measures in a HD clinical trial [HuntingtonStudyGroup 1998].
Managing non-motor complications (I): cognitive impairment
One of the most troubling features of HD is cognitive dysfunction. With the advent of more sensitive neuropsychological testing modalities, it has become clear that mild cognitive impairment occurs early, often predating the onset of motor symptoms. Despite being functionally disabling, cognitive defects in HD remain refractory to all available therapies [Beglinger and others 2010]. One potentially promising approach is to use drugs shown to counter cognitive impairment in other neurodegenerative disorders, such as cholinesterase inhibitors. The rationale for the use of cholinesterase inhibitors to boost cognition is based upon numerous post-mortem and biopsy studies of dementia patients in whom reduced acetylcholine synthesis, decreased acetylcholine release, and loss of nicotinic CNS receptors were documented [Benzi and Moretti 1998]. Of the candidate drugs from this class of agents, interest has been drawn to rivastigmine, which has shown some benefit on motor performance in treated HD patients together with a trend toward improvement in functional disability and cognitive impairment [de Tommaso and others 2007]. In a follow-up placebo-controlled trial however, treatment with rivastigmine for 6 months had no statistically significant effect on cognition [Sesok and others 2014]. Evaluation of donepezil, another drug of the same class, also yielded no improvement in cognitive performance in a Phase 3 clinical trial [Cubo and others 2006]. Other drug classes once considered as candidate therapies for HD cognitive decline include selective serotonin reuptake inhibitors (citalopram) and norepinephrine reuptake inhibitors (atomoxetine) to boost CNS neurotransmission, but despite promising evidence in preclinical studies, clinical trials failed to show any improvement in cognitive performance in early-stage HD patients [Beglinger and others 2014; Beglinger and others 2009]. An alternative approach to directly treating the cognitive impairment in HD is to increase the alertness of the patient, which may in turn have an indirect beneficial effect on cognition, with drugs such as modafinil, a wakefulness-promoting agent, but this approach was ineffective [Blackwell and others 2008]. In light of the dismal experience with drugs tested to treat cognitive impairment in HD, it should come as no surprise that the cognitive problems experienced by HD patients tend to go untreated. However, physicians caring for HD patients need to ensure that any cognitive deficit is not being exacerbated by the side effects of other prescribed medications, or that cognitive issues in HD patients are not secondary to depression or an affective disorder, which can be treated with drugs directed specifically at these conditions.
Managing non-motor complications (II): Depression, irritability, and anxiety
Psychiatric disturbances experienced in HD remain poorly understood. Depression, irritability, and anxiety are commonly reported by HD patients and their caregivers [van Duijn and others 2007], and the rate of suicide in HD carriers is 4-6× higher than in the general population [Schoenfeld and others 1984]. The incidence of such psychiatric disturbances in HD is both variable and transient, and often does not track with disease progression [Zappacosta and others 1996]. The ability to treat psychiatric illness in HD can be transformative, as it is often much more disabling than the motor features, and can present earlier. As such, many agents have been attempted, but the empirical evidence supporting their use, or establishing the superiority of any one of them is lacking. Of course, one useful approach is to address the often complex and dysfunctional social situation by insuring that social work and other social services are in place to support the patient and his/her caregivers.
Depression is the most commonly treated problem in HD, with evidence from case studies indicating that patients are generally responsive to antidepressant medications, including mirtazapine [Bonelli 2003], fluoxetine (a SSRI) [Patel and others 1996], and monoamine oxidase inhibitors [Ford 1986]. In a review of international prescribing practices for treating the behavioral symptoms of HD [Groves and others 2011], antipsychotic drugs were the most common class of agents used to treat patients with severe aggressive behaviors, whereas selective serotonin reuptake inhibitors were typically used to treat mild-to-moderate irritability [Groves and others 2011] and obsessive-compulsive behaviors [Anderson and others 2011]. There is some anecdotal evidence to support the use of haloperidol [Candelise 1976], olanzapine [Paleacu and others 2002], risperidone [Dallocchio and others 1999], and quetiapine [Alpay and Koroshetz 2006] for aggressive outbursts and irritability in HD. In addition, in case studies, both lamotrigine [Shen 2008] and lithium [Danivas and others 2013] can positively affect mood swings, but no larger studies have been conducted to confirm their potential utility. Many other drugs have been deployed to treat the behavioral features of HD, such as mood stabilizers, particularly carbamazepine (GABA / serotonin-releasing agent) for irritability; olanzapine in combination with sodium valproate and lamotrigine; fluoxetine; paroxetine; citalopram; and benzodiazepines (clonazepam, lorazepam). In summary, several agents have been used for the treatment of affective disorder in HD, although the limited trial experience does not indicate that any one is superior.
Managing non-motor complications (III): Insomnia and weight loss
There is an increasing realization that HD has many more features than the classic triad of motor, cognitive, and psychiatric illness. Problems with weight loss and sleep disturbance are common in HD, and can exacerbate the neurological problems. Indeed, insomnia can lead to worsening cognition, mood disturbance, and apathy [Lazar and others 2015]. Similarly, weight loss can worsen gait and the motor features of HD. As these aspects of HD are greatly understudied, their treatment strategies are predicated upon the experience in more common neurodegenerative diseases, without empirical evidence of efficacy in HD. For example, weight loss is managed through a combination of dietary supplements and the use of olanzapine, because of its well-documented side effect of weight gain [Chen and others 2011]. Similarly, the side effect profile of a drug is often the approach to remedy insomnia in HD patients, with use of olanzapine, amitriptyline, trazodone, or mirtazapine at bed-time. Among drugs used specifically to treat insomnia in HD, commonly used sedatives include zopiclone, zolpidem or zaleplon.
Other compounds
Baclofen and benzodiazapines
Although loss of MSNs in the striatum is one of the main pathological hallmarks of HD, few drugs are known to target this neurotransmitter system. Baclofen, which can act as agonist at GABAB receptors to block mono-and-polysynaptic reflexes by acting as an inhibitory neurotransmitter, blocking the release of excitatory transmitters, significantly reduced chorea in small open-label trials, [Anden and others 1973; Paulson 1976] although the results from a larger placebo-controlled study did not corroborate this finding [Shoulson and others 1989]. Benzodiazapines, such as clonazepam and diazepam, enhance the effect of GABA at the GABAA receptor, leading to an increase in GABA's inhibitory effects and resultant central nervous system depression. There is some evidence that these drugs have a positive effect on motor signs, but this has only been reported in a few case studies [Farrell and Hofmann 1968; Peiris and others 1976; Stewart 1988]. Clonazepam may worsen the cognitive side effects of HD and cause drowsiness. It also has a high risk of dependence and abuse.
Cannabinoids
CB1 cannabinoid receptors, which mediate the hypokinetic motor effects of certain cannabinoids, are significantly reduced in the basal ganglia in HD [Pazos and others 2008]. Cannabinoids may also protect striatal neurons from death by reducing neuroinflammation via CB2 receptor activation, and by normalizing glutamate levels to limit excitotoxicity via CB1 receptor activation [Pazos and others 2008]. Cannabinoids, such as nabilone, have been proposed as a treatment for chorea, and although they are typically only used in HD patients who seem resistant to all other approaches, there is some indication of efficacy in small, placebo-controlled crossover trials [Curtis and others 2009; Curtis and Rickards 2006]. In a small trial of cannabidiol (CBD), non-significant changes were found in chorea severity and in functional capacity. In a variety of other tests measuring motor function, cognition, and psychological distress (Symptom Checklist-90-Revised), no difference was found between treatment and placebo [Consroe and others 1991]. A trial of nabiximols, an extract of cannabis with an equimolecular combination of delta-9-tetrahydrocannabinol (THC) and CBD, yielded no differences on motor, cognitive, behavioral, and functional scores as compared to placebo during a 12-week treatment period [Lopez-Sendon Moreno and others 2016].
Donepezil
Donepezil binds and reversibly inactivates cholinesterases, inhibiting hydrolysis of acetylcholine, thereby increasing acetylcholine concentrations at cholinergic synapses. A 12-week randomized double-blind placebo-controlled trial of donepezil failed to show significant improvement for the primary outcome, chorea, using the UHDRS [Cubo and others 2006]. Similar results were observed for selected secondary outcomes, and donepezil did not significantly improve cognitive symptoms in a small open-label study of HD [Fernandez and others 2000].
Treatments under development: Assessing the HD therapeutic pipeline
Shared challenges in the design, conduct, and interpretation of clinical trials in rare neurodegenerative disorders include diagnosis, patient selection, endpoint selection, and a lack of biomarkers. Hence, awareness of these pitfalls and developing strategies to address them is vital. Studies should be designed to include patients at a similar stage in their disease progression, which for HD is typically a 15 to 25-year course, but can vary because of differences in age of onset and rate of progression due to the clinical phenomenon of anticipation, increasing the difficulty of obtaining a large sample size. The National Institute for Neurological Disorders and Stroke (NINDS) solicited a group of HD clinicians and researchers to make recommendations for common data elements (CDE) in HD clinical trials and research [NINDS 2017]. The UHDRS Motor Exam is a core recommendation from the NINDS HD CDE group for motor testing, while both the Stroop Test and the Symbol Digit Modalities Test are recommended as core measures for cognitive evaluation. To assess functional limitations, the Total Functional Capacity scale, the Independence Scale, and the Functional Assessment Scale of the UHDRS are recommended. Since it may take three years for detectable changes to occur in scores, scientists are desperately searching for biomarkers that can be used as shorter-term indicators of potential therapeutic efficacy. Progress has been made in identifying potential biomarkers, but work is ongoing [Mastrokolias and others 2015; Ross and others 2014].
As HD is a rare disorder affecting approximately 1 in 8,000 individuals in the USA [Bonelli and Wenning 2006], conducting placebo-controlled clinical trials is challenging because patient recruitment is difficult due to a shortage of HD individuals not already enrolled in other studies or trials, and support from industry has traditionally been unattainable, since the financial return for an orphan disease therapy is expected to be modest. However, in recent years, biopharma has reversed course for Mendelian diseases that are not exceedingly rare, as such disorders offer the advantage of definitive diagnostic (genetic) testing and a patient population in which the pathogenic process is likely to be very similar across the disease group. Furthermore, due to collaborative data repositories such as the Huntington Study Group (www.huntingtonstudygroup.org) and the European Huntington's Disease Network (www.eurohd.net), large numbers of HD patients have been enrolled into central databases with extensive clinical information, permitting recruitment of patients of varying severity at all stages of the disease process. These factors have convinced industry sponsors that placebo-controlled trials of promising therapy candidates are appealing for HD. Here we describe some of the most exciting emerging therapies being developed as “disease-modifying” treatments for HD (Figure 1 and Figure 2).
Trophic Factor Supplementation
Brain-Derived Neurotrophic Factor (BDNF) promotes neuron survival and synaptic plasticity, and is markedly reduced in HD [Shannon and Fraint 2015] (Figure 2). RE1-Silencing Transcription factor (REST), also known as Neuron-Restrictive Silencer Factor (NRSF), acts as a transcriptional repressor, and its principal target is BDNF. Ischemia (reduced blood profusion of tissues; decreased nutrient and oxygen supply) induces REST transcription and nuclear accumulation, leading to the epigenetic repression of neuronal genes leading to cell death [Noh and others 2012]. Mutant huntingtin fails to bind REST in the cytosol, allowing REST to enter the nucleus and carry out its repression regime as above [Zuccato and Cattaneo 2007]. Reduction in BDNF in the striatum correlates with symptom onset and increased disease severity in HD transgenic mice. Interestingly, BDNF knock-out mice recapitulate the striatal atrophy phenotype in HD, further supporting the hypothesis that reduced trophic factor function in the striatum is a key contributor to HD neurodegeneration [Ciammola and others 2007; Zuccato and others 2001]. Treatment of HD model mice with BDNF delivered by osmotic pump has been shown to improve neurological dysfunction and survival [Giampa and others 2013]. Current research efforts are focused upon the development of effective delivery methods, such as the use of viral vectors, including adeno-associated virus (AAV), to target BDNF to striatal neurons [Kells and others 2008].
Cysteamine (RP103)
Cysteamine is a FDA-approved drug therapy for nephropathic cystinosis. Cysteamine is the reduced form of cystamine, and through its interaction with heat-shock protein chaperones, is believed to stimulate the release of BDNF in the brain [Borrell-Pages and others 2006]. A variety of preclinical trials in mice have established that cysteamine is neuroprotective in HD [Bailey and Johnson 2006; Dedeoglu and others 2002; Fox and others 2004; Van Raamsdonk and others 2005]; however, clinical trials in human HD patients have yielded mixed results, failing to improve outcome measures in one case [Prundean and others 2015], but yielding significantly slower progression of TMS in a sub-group of HD patients who were not concurrently on TBZ, and revealing a trend toward slower progression in the overall study group [Corp. 2015].
Mesenchymal stem cells (MSCs)
Despite success in preclinical trials, AAV delivery of BDNF in the clinic has proven difficult due to host immunogenicity and limited biodistribution [Murlidharan and others 2016]. As the beneficial effects of BNDF are potentiated by noggin [Benraiss and others 2012], a factor secreted by mesenchymal stem cells (MSCs) [Diefenderfer and others 2003], and as MSCs may release additional trophic factors, considerable efforts are being devoted to the evaluation of MSC delivery to HD patients. Initial clinical trial experience has shown that MSCs are well tolerated by HD patients, and enthusiasm for MSC therapy has been buoyed by findings in an amyotrophic lateral sclerosis (ALS) trial, in which treated patients displayed improvements in breathing and delayed motor decline compared to placebo-treated patients [Petrou and others 2016]. A future clinical trial to demonstrate safety of intra-striatal injection transplantation of MSCs is being planned to treat HD patients screened in a recent observational study (i.e. PRE-CELL; ClinicalTrials.gov identifier: NCT01937923).
Dosage reduction
As the expression of the toxic huntingtin protein drives all subsequent disease pathology in HD, a very attractive therapeutic paradigm is to prevent the expression of the mutant gene product. One approach to stop expression of a gene product is to target the messenger RNA for destruction or inhibit protein translation, a strategy known as “gene silencing”. As interrupting the pathogenic cascade at its earliest step has great potential to dramatically alter disease course, interventions intended to reduce huntingtin gene expression are now considered among the most promising emerging therapies [Garriga-Canut and others 2012; Magen and Hornstein 2014]. There are three major approaches that are under investigation to reduce mutant huntingtin expression: RNA interference (RNAi), antisense oligonucleotides (ASOs), and zinc finger proteins (ZFPs) (Figure 1).
RNA interference (RNAi)
In RNAi (reviewed and diagrammed in [Novina and Sharp 2004]), a so-called “short interfering RNA” (siRNA) is introduced into cells, and it provides a substrate that is processed by the RNA-induced silencing complex (RISC), which is the cellular machinery that normally mediates the action of microRNAs (miRNAs) – the endogenous RNAs that turn off gene expression. As delivery of naked double-stranded RNA is challenging, siRNA sequences can be encoded as short hairpin RNAs (shRNAs) that are expressed from viral vectors, such as AAV. While AAV delivery of shRNAs was found to be a robust and highly successful method for treating mouse models of polyglutamine disease [Xia and others 2004], further studies demonstrated that high-level expression of exogenous shRNAs may cause toxicity [Grimm and others 2006], prompting the development of artificial miRNA vectors, packaged into AAV, where huntingtin-targeting shRNAs are instead embedded in the sequence of a miRNA so that the processing of the siRNA follows the full endogenous Drosha-Dicer pathway [Boudreau and others 2009]. At present, various methods for siRNA delivery are being pursued, ranging from siRNAs in nanoparticles to shRNA viral vectors and artificial miRNA viral vectors. In the case of viral vectors, delivery will require a neurosurgical procedure of stereotactic injection into specific brain regions in HD patient subjects. In addition to viral vectors, exosomes [Alvarez-Erviti and others 2011], cholesterol conjugation [DiFiglia and others 2007], convection-enhanced delivery, and novel conjugates of single-stranded siRNA compounds [Lima and others 2012; Yu and others 2012] represent cutting-edge approaches that are also under development.
Antisense oligonucleotides (ASOs)
ASOs are modified single-stranded DNA molecules, and upon binding a target RNA, the bound RNA is either degraded by RNAse H (reviewed and diagrammed in [Lu and Yang 2012; Martinez and others 2013]), or protein translation of the bound RNA is inhibited. In either case, the production of protein product from the target mRNA is typically reduced by greater than one-half. ASO dosage reduction can be “non-allele specific”, i.e., both the disease allele and the normal allele mRNAs are targeted, or “allele-specific”, i.e., the disease allele is selectively targeted, usually because of a single nucleotide polymorphism that is in linkage with the disease allele. While allele-specific targeting of mutant huntingtin (htt) would be ideal, numerous studies in HD mouse models have established the safety and efficacy of non-allele specific ASOs [Boudreau and others 2009; Kordasiewicz and others 2012]. Because of dramatic successes in preclinical trial work in mice, ASO therapy is being advanced into human HD patients. Ionis Pharmaceuticals (Carlsbad, CA) and Roche (Indianapolis, IN) have completed enrollment for a randomized, double-blind, placebo-controlled Phase 1/2A clinical trial in early stage HD patients in Europe and Canada to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of ascending doses of an investigational ASO, IONIS-HTTRx (ClinicalTrials.gov identifier: NCT02519036). Each patient will receive four doses of IONIS-HTTRx or placebo by an intrathecal (spinal) injection, with doses four weeks apart for 13 weeks. The estimated study completion date is November 2017. IONIS-HTTRx is the first therapy to enter clinical development that is designed to treat the underlying cause of this fatal disease, and it has been granted Orphan Drug Designation by the FDA and the European Medicines Agency (EMA). As an ASO, IONIS-HTTRx binds to HTT RNA, promoting its destruction and decreasing the amount of HTT protein to cause toxicity. Research studies with IONIS-HTTRx in animal models of HD have been promising [Skotte and others 2014; Stanek and others 2013]. BioMarin Pharmaceutical (San Rafael, CA) is undertaking a similar approach to the Ionis-Roche ASO strategy [Inc. 2017]. Their PRO289 program targets CAG repeats in the HTT gene and has been shown to reduce levels of expanded HTT protein in fibroblast cultures derived from HD patients.
Zinc Finger Proteins
Zinc finger proteins (ZFPs) are transcription factor DNA-recognition motifs that can be designed to allow selective binding to specific DNA sequences, and when fused to a transcription repressor domain, can be used to repress protein production of a designated target [Maeder and Gersbach 2016]. By acting at the level of the DNA, production of mutant HTT RNA would be suppressed. Preliminary data indicate successful selective repression of mutant htt and amelioration of motor manifestations in HD mice [Garriga-Canut and others 2012]., but this approach still has the delivery and distribution hurdles of other viral vector methods. Sangamo BioSciences (Richmond, CA) is focused on developing ZFPs for treating HD patients [Sangamo 2012].
Modulation of Synaptic Function and Excitotoxicity
HD pathogenesis has been shown to involve alterations in cortico-striatal circuits that prevent maintenance of robust neurotransmission. At the same time, sustained over-activation at synapses, known as excitotoxicity, is hypothesized to contribute to the neural dysfunction and neuron cell death in HD [Matthews and others 1998]. A variety of therapies are thus being developed to promote synaptic function and to counter excitotoxicity (Figure 1).
PDE10A Inhibitor
Impairment of cyclic adenosine monophosphate (cAMP) signaling [Gines and others 2003] and dysregulation of gene transcription mediated by the cAMP response element (CRE) [Sugars and others 2004] have been implicated in HD. Phosphodiesterase (PDE) 10A is almost exclusively expressed in the striatum, and its activity is linked to the synaptic function of the striatal MSNs whose death is a prominent feature of HD [Coskran and others 2006]. PDE10A regulates cAMP, synaptic plasticity, and response to cortical stimulation [Threlfell and others 2009; Threlfell and West 2013]. PDE10A expression in early pre-manifest HD is decreased in striatum and pallidum, and increased in motor thalamic nuclei, compared to matched healthy controls. PDE10A inhibition with TP10 ameliorated motor deficits, reduced striatal atrophy and BDNF levels in a mouse model of HD [Giampa and others 2010].
Deep Brain Stimulation
Deep brain stimulation (DBS) is a surgical procedure wherein a neurostimulator is implanted into the midbrain to block abnormal neuronal electric activity and thereby treat abnormal involuntary movements in patients. DBS has been employed as a treatment for Parkinson disease, where it continues to be used. Several small case reports have reported improvement in chorea in HD patients, with either a trend or significant increase in dystonia and bradykinesia [Beste and others 2015; Delorme and others 2016; Gonzalez and others 2014; Gruber and others 2014]. In a longer term follow-up, one HD patient showed benefit for the first two years, however, from years 2 to 5, the patient had a steep decline in overall motor function [Lopez-Sendon Moreno and others 2014]. A Phase 2 prospective, randomized study to evaluate pallidal DBS in HD patients is currently underway: ClinicalTrials.gov Identifier: NCT02535884. Another safety and efficacy study of DBS in HD has not yet started recruiting: ClinicalTrials.gov Identifier: NCT02263430.
Kynurenine 3-monooxygenase (KMO) Inhibitors
The earliest HD rodent models were generated by intra-striatal injection of the excitotoxic NMDA agonist quinolinic acid (QA) [Schwarcz and others 1983]. QA is an endogenous metabolite produced by the degradation of tryptophan by the kynurenine pathway. The enzyme kynurenine 3-monooxygenase (KMO) is a key branch point in this pathway, and its activity determines the balance of QA and the neuroprotective metabolites, kynurenic acid (KA) and kynurenine. In the CNS, the kynurenine pathway is confined to microglial cells [Zadori and others 2009]. QA levels are increased and KA levels decreased in post-mortem HD patient brain [Beal and others 1992; Guidetti and others 2004]. The metabolites kynurenine and kynurenic acid may modulate synaptic plasticity and have neuroprotective and anti-inflammatory roles in HD models [Zadori and others 2009; Zwilling and others 2011]. Zwilling and colleagues treated HD model mice with a KMO inhibitor compound, JM6, and reported improved survival, reduced synapse loss, and a decrease in microglial activation [Zwilling and others 2011]. Neither JM6 nor its metabolites cross the blood–brain barrier, suggesting that its beneficial effects are mediated by peripheral KMO inhibition, producing beneficial effects for the CNS via the transit of an intermediate compound, possibly kynurenine [Zwilling and others 2011]. Subsequent work by Beconi and colleagues questioned if JM6 is a KMO inhibitor, suggesting that the observed effects were likely attributable to contamination by the known KMO inhibitor Ro 61-8048 [Sigma-Aldrich] [Beconi and others 2012b]. A novel peripherally acting KMO inhibitor, CHDI-340246, has been reported to increase levels of kynurenine and KA in HD rodent models and in the cerebrospinal fluid of non-human primates, and it can restore several electrophysiological alterations in mouse models of HD, both acutely and after chronic administration [Beaumont and others 2016]. However, using a comprehensive panel of behavioral tests, chronic dosing of this KMO inhibitor did not significantly modify behavioral phenotypes or natural progression in HD mouse models.
Transcription dysregulation and impaired bioenergetics function
HDAC Inhibitors
With the aim of correcting transcription dysregulation through chromatin modification, histone deacetylase (HDAC) inhibitors have been under study as a treatment for HD for a number of years. HDAC inhibitors prevent the removal of acetyl groups from histones, allowing the DNA to be accessible to transcription factors and permitting gene transcription. The non-selective HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) was shown to ameliorate the motor phenotype in R6/2 mice [Hockly and others 2003]. A reappraisal of SAHA in HD model mice revealed that it reduced HDAC4 levels by promoting its degradation [Mielcarek and others 2011]. HDAC4 inhibition thus emerged as a therapy focus in HD, and potent, selective small-molecule inhibitors of HDAC4 are under development [Burli and others 2013]. The basis for therapeutic benefit from HDAC4 inhibition remains unclear, however, as crossing R6/2 mice onto a HDAC4 null background ameliorated neuropathology, synaptic function, motor read-outs, and lifespan, but did not improve the transcription dysregulation [Mielcarek and others 2011]. Studies of a HDAC inhibitor 4b, which also targets HDAC1 and HDAC3, yielded beneficial effects in HD mice [Jia and others 2012], but this positive result could not be replicated in a follow-up preclinical trial by another group [Beconi and others 2012a]. Recent attention has turned to yet another HDAC inhibitor – RGFP966, which inhibits HDAC3, and showed some benefit as a treatment for HD in a preclinical trial [Jia and others 2016]. Thus, targeting HDACs as a treatment for HD remains under active investigation in mouse models, but is yet to yield a consistent enough set of results to warrant further evaluation in human patients.
Nuclear receptor transcription factors: PPARγ and PPARδ
The mitochondrial dysfunction and metabolic deficits in HD have been linked to transcription dysregulation of peroxisome proliferator-activated receptor (PPAR) gamma coactivator-1 alpha (PPARGC1A), a transcription factor that, through its co-activation of the PPARs (α,δ,γ), positively regulates a network of transcription programs that culminate in mitochondrial biogenesis and enhanced energy production through oxidative phosphorylation [Cui and others 2006; Lin and others 2004; Weydt and others 2006]. The importance of PGC-1α for HD pathogenesis is underscored by the observation that PGC-1α over-expression in HD mice is sufficient to rescue motor phenotypes, prevent accumulation of misfolded htt protein in CNS through increased autophagy, and reduce neurodegeneration [Tsunemi and others 2012]. Although strong evidence for impaired function of PGC-1α in HD has been documented by a number of independent groups, PGC-1α is less than ideal as a target for therapy development, because it regulates a vast array of processes through its interactions with many different nuclear receptor transcription factors. Thus, once PGC-1α was implicated in HD pathogenesis, a number of investigators evaluated PPARγ agonist therapies, including rosiglitazone, since PPARγ compounds had already been developed for human use for type II diabetes. While numerous studies found that PPARγ agonist treatment can ameliorate disease phenotypes in HD mice [Chiang and others 2010; Chiang and others 2012; Jin and others 2013], the beneficial effects of PPARγ agonist therapy in HD likely stem from improved metabolic function, based upon its actions in peripheral tissues, the hypothalamus, and non-neural cells of the CNS (i.e. microglia), as PPARγ is not expressed in midbrain or cortical neurons. Furthermore, safety concerns exist with PPARγ, as PPARγ agonists have been linked to adverse cardiovascular outcomes, including congestive heart failure [Graham and others 2010; Nissen and Wolski 2007].
To delineate the basis for PGC-1α transcription dysregulation, we performed an unbiased screen of transcription factors that physically interact with htt protein and identified PPARδ as an interactor [Dickey and others 2016]. This discovery led us to consider the role of PPARδ in the CNS and in HD, especially when we found that PPARδ is highly expressed in cortical and striatal neurons [Dickey and others 2016], consistent with earlier work that showed its expression in CNS to exceed that of skeletal muscle [Bookout and others 2006; Higashiyama and others 2007], where PPARδ agonist treatment and over-expression had elicited profound physiological effects [Evans and Mangelsdorf 2014]. We showed that PPARδ transactivation ameliorated mitochondrial dysfunction and improved cell survival in neurons from HD mice, and when we directed the expression of dominant-negative PPARδ to the CNS of mice, we observed motor defects, neurodegeneration in the cortex and striatum, and transcription interference, which accurately recapitulated key aspects of HD observed in human patients [Dickey and others 2016]. To determine if PPARδ agonist therapy might hold promise as a treatment for HD, we repurposed a drug known as KD3010, which had been developed as a treatment for diabetes and metabolic syndrome, and was found to be safe in humans in a Phase 1b clinical trial. Importantly, KD3010 crosses the blood-brain barrier and activates PPARδ in the brain, resulting in approximately 2-fold up-regulation of PPARδ target genes [Dickey and others 2016]. In a rigorous preclinical trial in HD N171-82Q mice (an aggressive HD model), we found that KD3010 improved motor function, neurodegeneration, and survival. We also confirmed the therapeutic utility of KD3010 in MSNs derived from HD patient pluripotent stem cells [Dickey and others 2016]. Further development of KD3010 as a therapy candidate for HD is underway, and plans for a Phase 2 clinical trial are being formulated.
Pridopidine
Pridopidine is a relatively new drug, originally developed to stabilize dopamine in the CNS; however, further analysis suggests that it can up-regulate the expression of BDNF and activate the PI3-kinase / AKT pathway, as well as other pathways thought to promote neuronal plasticity and survival, thereby eliciting therapeutic benefit in HD mice [Geva and others 2016]. Results of two large, randomized, placebo-controlled safety / efficacy trials of pridopidine were recently published, which indicated that the drug is generally well tolerated, but did not produce significant improvement in the primary motor endpoint, though a trend toward amelioration of certain motor outcomes was noted [de Yebenes and others 2011; HuntingtonStudyGroup 2013]. In light of these clinical trial data, regulatory agencies have requested further testing. Pridopidine will thus now be evaluated in another Phase 2 clinical trial to test the safety and tolerability of higher doses, and ascertain whether pridopidine may achieve a more marked symptomatic benefit at higher doses ClinicalTrials.gov Identifier: NCT02494778.
Immune modulation and neuroinflammation targeted therapies
Laquinimod
Prior work has shown that mutant huntingtin protein promotes aberrant activation of the nuclear factor kappa B (NFkB) pathway in monocytes and microglia, culminating in immune system over-activation in HD [Trager and others 2014]. Laquinimod reduces activation of the NFkB pathway in astrocytes [Bruck and others 2012], and may additionally boost BDNF levels [Aharoni and others 2012], thereby modulating immune cell lineages in the periphery and CNS [Ehrnhoefer and others 2016]. Experience with laquinimod in HD has been disappointing, as use of its highest dose in an ongoing HD clinical trial was discontinued, but a Phase 2 study of its safety and efficacy in HD is proceeding, with expected completion in late 2018 ClinicalTrials.gov Identifier: NCT02215616. The primary endpoint in this study is change from baseline in the UHDRS-TMS after 12 months of treatment.
VX15
VX15 is a humanized IgG4 monoclonal antibody that binds to and blocks the activity of semaphorin 4D protein, a molecule that induces inflammation in the brain of patients with HD [Southwell and others 2015]. Anti-semaphorin 4D immunotherapy ameliorates neuropathology and some cognitive impairment in HD mice [Southwell and others 2015]. Reducing brain inflammation may retard the progression of motor and cognitive defects in HD. To fully assess the therapeutic potential of VX15, a Phase 2 clinical trial (SIGNAL) is being organized to assess safety and efficacy of VX15 in at-risk HD individuals (ClinicalTrials.gov Identifier: NCT02481674). SIGNAL is a randomized, double-blind, placebo-controlled trial seeking to enroll 84 patients, who are either early in the progression of their disease or not yet diagnosed. VX15 will be administered via monthly intravenous infusion, and estimated completion date is 2020.
Caspase 6 inhibitor
ED11 is a novel caspase-6 inhibitor peptide based on the huntingtin caspase-6 cleavage site, fused with a cell-penetrating sequence. The peptide reduces caspase 6-mediated proteolytic cleavage of mutant huntingtin, which is expected to blunt mutant huntingtin toxicity. Continuous subcutaneous administration of the peptide protected pre-symptomatic BAC-HD mice from motor deficits and behavioral abnormalities. Moreover, administration of the peptide in an advanced disease state yielded partial recovery of motor performance, and alleviated depression-related behavior and cognitive deficits in full-length expanded polyQ tract htt transgenic mice, a longer-term model [Aharony and others 2015].
Synopsis
The HD therapeutic pipeline is clearly robust and diverse, with compounds targeting divergent aspects of disease pathophysiology (Figure 1 and Figure 2), and agents ranging from small molecules to biologicals, where delivery is achieved through direct injection with viral vectors or via instillation into cerebrospinal fluid to permit widespread distribution through the CNS. The vast number of ongoing and planned clinical trials of HD is also a strong testament to the vibrancy and vitality of therapy development for a disorder, which is classified as rare, based upon accepted criteria. While HD is a rare disease, its pathophysiology overlaps with much more common neurodegenerative disorders, such as Parkinson disease, suggesting that therapeutic efficacy in HD may highlight an approach or particular compound with promise for Parkinson disease and related disorders. Certainly, the advance of therapy development in HD has been aided by the existence of highly representative mouse models and availability of pluripotent stem cells from human patients, where differentiation into the neurons of interest is possible for cross-validation of treatments found to be efficacious in HD mice. While dosage reduction appears to be the most powerful therapeutic strategy under development, there is no reason to believe that combination of diverse therapies will ultimately be the standard of care for HD patients in the future. It is our belief that disease-modifying therapies for HD will soon be realized, and that HD patient management will convert from the use of drugs that are solely intended to treat symptoms to agents that will dramatically retard disease progression, or perhaps even prevent disease onset in the first place. Attaining this level of therapeutic efficacy for HD will be a wonderful development for the entire neurodegenerative disease field and for those committed to the neurotherapeutics endeavor.
Acknowledgments
Our research on Huntington disease is supported by funding from the N.I.H. (R01 NS065874 to A.R.L.)
References
- Aharoni R, Saada R, Eilam R, Hayardeny L, Sela M, Arnon R. Oral treatment with laquinimod augments regulatory T-cells and brain-derived neurotrophic factor expression and reduces injury in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2012;251(1-2):14–24. doi: 10.1016/j.jneuroim.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Aharony I, Ehrnhoefer DE, Shruster A, Qiu X, Franciosi S, Hayden MR, Offen D. A Huntingtin-based peptide inhibitor of caspase-6 provides protection from mutant Huntingtin-induced motor and behavioral deficits. Hum Mol Genet. 2015;24(9):2604–2614. doi: 10.1093/hmg/ddv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Reiner A, Anderson KD, Dure LSt, Handelin B, Balfour R, Whetsell WO, Jr, Penney JB, Young AB. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington's disease. Ann Neurol. 1992;31(4):425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington's disease. Psychosomatics. 2006;47(1):70–72. doi: 10.1176/appi.psy.47.1.70. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Anden NE, Dalen P, Johansson B. Baclofen and lithium in Huntington's chorea. Lancet. 1973;2(7820):93. doi: 10.1016/s0140-6736(73)93285-6. [DOI] [PubMed] [Google Scholar]
- Anderson K, Craufurd D, Edmondson MC, Goodman N, Groves M, van Duijn E, van Kammen DP, Goodman L. An International Survey-based Algorithm for the Pharmacologic Treatment of Obsessive-Compulsive Behaviors in Huntington's Disease. PLoS Curr. 2011;3 doi: 10.1371/currents.RRN1261. Rrn1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging. 2006;27(6):871–879. doi: 10.1016/j.neurobiolaging.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Banaszkiewicz K, Sitek EJ, Rudzinska M, Soltan W, Slawek J, Szczudlik A. Huntington's disease from the patient, caregiver and physician's perspectives: three sides of the same coin? J Neural Transm (Vienna) 2012;119(11):1361–1365. doi: 10.1007/s00702-012-0787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AN, Fischer JH, Koller WC, Spunt AL, Singhal A. Serum haloperidol concentration and choreiform movements in Huntington's disease. Neurology. 1988;38(1):84–88. doi: 10.1212/wnl.38.1.84. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13(10):4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Matson WR, Storey E, Milbury P, Ryan EA, Ogawa T, Bird ED. Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J Neurol Sci. 1992;108(1):80–87. doi: 10.1016/0022-510x(92)90191-m. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Mrzljak L, Dijkman U, Freije R, Heins M, Rassoulpour A, Tombaugh G, Gelman S, Bradaia A, Steidl E, Gleyzes M, Heikkinen T, Lehtimaki K, Puolivali J, Kontkanen O, Javier RM, Neagoe I, Deisemann H, Winkler D, Ebneth A, Khetarpal V, Toledo-Sherman L, Dominguez C, Park LC, Munoz-Sanjuan I. The novel KMO inhibitor CHDI-340246 leads to a restoration of electrophysiological alterations in mouse models of Huntington's disease. Exp Neurol. 2016;282:99–118. doi: 10.1016/j.expneurol.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Beconi M, Aziz O, Matthews K, Moumne L, O'Connell C, Yates D, Clifton S, Pett H, Vann J, Crowley L, Haughan AF, Smith DL, Woodman B, Bates GP, Brookfield F, Burli RW, McAllister G, Dominguez C, Munoz-Sanjuan I, Beaumont V. Oral administration of the pimelic diphenylamide HDAC inhibitor HDACi 4b is unsuitable for chronic inhibition of HDAC activity in the CNS in vivo. PLoS One. 2012a;7(9):e44498. doi: 10.1371/journal.pone.0044498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beconi MG, Yates D, Lyons K, Matthews K, Clifton S, Mead T, Prime M, Winkler D, O'Connell C, Walter D, Toledo-Sherman L, Munoz-Sanjuan I, Dominguez C. Metabolism and pharmacokinetics of JM6 in mice: JM6 is not a prodrug for Ro-61-8048. Drug Metab Dispos. 2012b;40(12):2297–2306. doi: 10.1124/dmd.112.046532. [DOI] [PubMed] [Google Scholar]
- Beglinger LJ, Adams WH, Langbehn D, Fiedorowicz JG, Jorge R, Biglan K, Caviness J, Olson B, Robinson RG, Kieburtz K, Paulsen JS. Results of the citalopram to enhance cognition in Huntington disease trial. Mov Disord. 2014;29(3):401–405. doi: 10.1002/mds.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger LJ, Adams WH, Paulson H, Fiedorowicz JG, Langbehn DR, Duff K, Leserman A, Paulsen JS. Randomized controlled trial of atomoxetine for cognitive dysfunction in early Huntington disease. J Clin Psychopharmacol. 2009;29(5):484–487. doi: 10.1097/JCP.0b013e3181b2ac0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger LJ, O'Rourke JJ, Wang C, Langbehn DR, Duff K, Paulsen JS. Earliest functional declines in Huntington disease. Psychiatry Res. 2010;178(2):414–418. doi: 10.1016/j.psychres.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Bruel-Jungerman E, Lu G, Economides AN, Davidson B, Goldman SA. Sustained induction of neuronal addition to the adult rat neostriatum by AAV4-delivered noggin and BDNF. Gene Ther. 2012;19(5):483–493. doi: 10.1038/gt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi G, Moretti A. Is there a rationale for the use of acetylcholinesterase inhibitors in the therapy of Alzheimer's disease? Eur J Pharmacol. 1998;346(1):1–13. doi: 10.1016/s0014-2999(98)00093-4. [DOI] [PubMed] [Google Scholar]
- Beste C, Muckschel M, Elben S, C JH, McIntyre CC, Saft C, Vesper J, Schnitzler A, Wojtecki L. Behavioral and neurophysiological evidence for the enhancement of cognitive control under dorsal pallidal deep brain stimulation in Huntington's disease. Brain Struct Funct. 2015;220(4):2441–2448. doi: 10.1007/s00429-014-0805-x. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Paterson NS, Barker RA, Robbins TW, Sahakian BJ. The effects of modafinil on mood and cognition in Huntington's disease. Psychopharmacology (Berl) 2008;199(1):29–36. doi: 10.1007/s00213-008-1068-0. [DOI] [PubMed] [Google Scholar]
- Bonelli RM. Mirtazapine in suicidal Huntington's disease. Ann Pharmacother. 2003;37(3):452. doi: 10.1345/aph.1C352. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Niederwieser G, Tribl GG, Koltringer P. High-dose olanzapine in Huntington's disease. Int Clin Psychopharmacol. 2002;17(2):91–93. doi: 10.1097/00004850-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Wenning GK. Pharmacological management of Huntington's disease: an evidence-based review. Curr Pharm Des. 2006;12(21):2701–2720. doi: 10.2174/138161206777698693. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pages M, Canals JM, Cordelieres FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Neri C, Alberch J, Brouillet E, Saudou F, Humbert S. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116(5):1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau RL, Martins I, Davidson BL. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther. 2009;17(1):169–175. doi: 10.1038/mt.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck W, Pfortner R, Pham T, Zhang J, Hayardeny L, Piryatinsky V, Hanisch UK, Regen T, van Rossum D, Brakelmann L, Hagemeier K, Kuhlmann T, Stadelmann C, John GR, Kramann N, Wegner C. Reduced astrocytic NF-kappaB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012;124(3):411–424. doi: 10.1007/s00401-012-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burli RW, Luckhurst CA, Aziz O, Matthews KL, Yates D, Lyons KA, Beconi M, McAllister G, Breccia P, Stott AJ, Penrose SD, Wall M, Lamers M, Leonard P, Muller I, Richardson CM, Jarvis R, Stones L, Hughes S, Wishart G, Haughan AF, O'Connell C, Mead T, McNeil H, Vann J, Mangette J, Maillard M, Beaumont V, Munoz-Sanjuan I, Dominguez C. Design, synthesis, and biological evaluation of potent and selective class IIa histone deacetylase (HDAC) inhibitors as a potential therapy for Huntington's disease. J Med Chem. 2013;56(24):9934–9954. doi: 10.1021/jm4011884. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nature Neuroscience. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- Candelise L. [Haloperidol, reserpine, l-dopa and amantidine in the treatment of Huntington chorea (author's transl)] Riv Patol Nerv Ment. 1976;96(1):54–62. [PubMed] [Google Scholar]
- Casey DE. Neuroleptic drug-induced extrapyramidal syndromes and tardive dyskinesia. Schizophr Res. 1991;4(2):109–120. doi: 10.1016/0920-9964(91)90029-q. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Murphy KP, Parent M, Levine MS. The role of dopamine in Huntington's disease. Prog Brain Res. 2014;211:235–254. doi: 10.1016/B978-0-444-63425-2.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VC, Wang TN, Lu ML, Chou JY, Ju PC, Wu JY, Lin ZR, Ji TT, Chou CE, Lee CT, Lai TJ. Weight gain and ghrelin level after olanzapine monotherapy. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):632–635. doi: 10.1016/j.pnpbp.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Chen CM, Lee MR, Chen HW, Chen HM, Wu YS, Hung CH, Kang JJ, Chang CP, Chang C, Wu YR, Tsai YS, Chern Y. Modulation of energy deficiency in Huntington's disease via activation of the peroxisome proliferator-activated receptor gamma. Hum Mol Genet. 2010;19(20):4043–4058. doi: 10.1093/hmg/ddq322. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Chern Y, Huang RN. PPARgamma rescue of the mitochondrial dysfunction in Huntington's disease. Neurobiol Dis. 2012;45(1):322–328. doi: 10.1016/j.nbd.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Ciammola A, Sassone J, Cannella M, Calza S, Poletti B, Frati L, Squitieri F, Silani V. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington's disease patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144b(4):574–577. doi: 10.1002/ajmg.b.30501. [DOI] [PubMed] [Google Scholar]
- Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991;40(3):701–708. doi: 10.1016/0091-3057(91)90386-g. [DOI] [PubMed] [Google Scholar]
- Corp. RP. Raptor Plans to Advance RP103 in a Registration Study in Huntington's Disease Based on Favorable Treatment Effects at 36 Months in CYST-HD Trial. @PRNewswire 2015 [Google Scholar]
- Coskran TM, Morton D, Menniti FS, Adamowicz WO, Kleiman RJ, Ryan AM, Strick CA, Schmidt CJ, Stephenson DT. Immunohistochemical localization of phosphodiesterase 10A in multiple mammalian species. J Histochem Cytochem. 2006;54(11):1205–1213. doi: 10.1369/jhc.6A6930.2006. [DOI] [PubMed] [Google Scholar]
- Crossman AR. Functional anatomy of movement disorders. J Anat. 2000;196(Pt 4):519–525. doi: 10.1046/j.1469-7580.2000.19640519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubo E, Shannon KM, Tracy D, Jaglin JA, Bernard BA, Wuu J, Leurgans SE. Effect of donepezil on motor and cognitive function in Huntington disease. Neurology. 2006;67(7):1268–1271. doi: 10.1212/01.wnl.0000238106.10423.00. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127(1):59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Curtis A, Mitchell I, Patel S, Ives N, Rickards H. A pilot study using nabilone for symptomatic treatment in Huntington's disease. Mov Disord. 2009;24(15):2254–2259. doi: 10.1002/mds.22809. [DOI] [PubMed] [Google Scholar]
- Curtis A, Rickards H. Nabilone could treat chorea and irritability in Huntington's disease. J Neuropsychiatry Clin Neurosci United States. 2006:553–554. doi: 10.1176/jnp.2006.18.4.553. [DOI] [PubMed] [Google Scholar]
- Dallocchio C, Buffa C, Tinelli C, Mazzarello P. Effectiveness of risperidone in Huntington chorea patients. J Clin Psychopharmacol. 1999;19(1):101–103. doi: 10.1097/00004714-199902000-00020. [DOI] [PubMed] [Google Scholar]
- Danivas V, Moily NS, Thimmaiah R, Muralidharan K, Purushotham M, Muthane U, Jain S. Off label use of lithium in the treatment of Huntington's disease: A case series. Indian J Psychiatry. 2013;55(1):81–83. doi: 10.4103/0019-5545.105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayalu P, Albin RL. Huntington disease: pathogenesis and treatment. Neurol Clin. 2015;33(1):101–114. doi: 10.1016/j.ncl.2014.09.003. [DOI] [PubMed] [Google Scholar]
- de Tommaso M, Difruscolo O, Sciruicchio V, Specchio N, Livrea P. Two years' follow-up of rivastigmine treatment in Huntington disease. Clin Neuropharmacol. 2007;30(1):43–46. doi: 10.1097/01.wnf.0000240945.44370.f0. [DOI] [PubMed] [Google Scholar]
- de Yebenes JG, Landwehrmeyer B, Squitieri F, Reilmann R, Rosser A, Barker RA, Saft C, Magnet MK, Sword A, Rembratt A, Tedroff J. Pridopidine for the treatment of motor function in patients with Huntington's disease (MermaiHD): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011;10(12):1049–1057. doi: 10.1016/S1474-4422(11)70233-2. [DOI] [PubMed] [Google Scholar]
- Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235(2-3):283–289. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22(20):8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme C, Rogers A, Lau B, Francisque H, Welter ML, Fernandez Vidal S, Yelnik J, Durr A, Grabli D, Karachi C. Deep brain stimulation of the internal pallidum in Huntington's disease patients: clinical outcome and neuronal firing patterns. J Neurol. 2016;263(2):290–298. doi: 10.1007/s00415-015-7968-0. [DOI] [PubMed] [Google Scholar]
- Deroover J, Baro F, Bourguignon RP, Smets P. Tiapride versus placebo: a double-blind comparative study in the management of Huntington's chorea. Curr Med Res Opin. 1984;9(5):329–338. doi: 10.1185/03007998409109601. [DOI] [PubMed] [Google Scholar]
- Dickey AS, Pineda VV, Tsunemi T, Liu PP, Miranda HC, Gilmore-Hall SK, Lomas N, Sampat KR, Buttgereit A, Torres MJ, Flores AL, Arreola M, Arbez N, Akimov SS, Gaasterland T, Lazarowski ER, Ross CA, Yeo GW, Sopher BL, Magnuson GK, Pinkerton AB, Masliah E, La Spada AR. PPAR-delta is repressed in Huntington's disease, is required for normal neuronal function and can be targeted therapeutically. Nat Med. 2016;22(1):37–45. doi: 10.1038/nm.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenderfer DL, Osyczka AM, Reilly GC, Leboy PS. BMP responsiveness in human mesenchymal stem cells. Connect Tissue Res 44 Suppl. 2003;1:305–311. [PubMed] [Google Scholar]
- DiFiglia M, Sena-Esteves M, Chase K, Sapp E, Pfister E, Sass M, Yoder J, Reeves P, Pandey RK, Rajeev KG, Manoharan M, Sah DW, Zamore PD, Aronin N. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci U S A. 2007;104(43):17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Caron NS, Deng Y, Qiu X, Tsang M, Hayden MR. Laquinimod decreases Bax expression and reduces caspase-6 activation in neurons. Exp Neurol. 2016;283(Pt A):121–128. doi: 10.1016/j.expneurol.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157(1):255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell DF, Hofmann WW. A quantitative evaluation of the effect of diazepam in Huntington's chorea. Arch Phys Med Rehabil. 1968;49(10):586–591. [PubMed] [Google Scholar]
- Fernandez HH, Friedman JH, Grace J, Beason-Hazen S. Donepezil for Huntington's disease. Mov Disord. 2000;15(1):173–176. doi: 10.1002/1531-8257(200001)15:1<173::aid-mds1032>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Ferreira JJ, Rosser A, Craufurd D, Squitieri F, Mallard N, Landwehrmeyer B. Ethyl-eicosapentaenoic acid treatment in Huntington's disease: A placebo-controlled clinical trial. Mov Disord. 2015;30(10):1426–1429. doi: 10.1002/mds.26308. [DOI] [PubMed] [Google Scholar]
- Ford MF. Treatment of depression in Huntington's disease with monoamine oxidase inhibitors. Br J Psychiatry. 1986;149:654–656. doi: 10.1192/bjp.149.5.654. [DOI] [PubMed] [Google Scholar]
- Fox JH, Barber DS, Singh B, Zucker B, Swindell MK, Norflus F, Buzescu R, Chopra R, Ferrante RJ, Kazantsev A, Hersch SM. Cystamine increases L-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J Neurochem. 2004;91(2):413–422. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- Frank S. Tetrabenazine as anti-chorea therapy in Huntington disease: an open-label continuation study. Huntington Study Group/TETRA-HD Investigators. BMC Neurol. 2009;9:62. doi: 10.1186/1471-2377-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Testa CM, Stamler D, Kayson E, Davis C, Edmondson MC, Kinel S, Leavitt B, Oakes D, O'Neill C, Vaughan C, Goldstein J, Herzog M, Snively V, Whaley J, Wong C, Suter G, Jankovic J, Jimenez-Shahed J, Hunter C, Claassen DO, Roman OC, Sung V, Smith J, Janicki S, Clouse R, Saint-Hilaire M, Hohler A, Turpin D, James RC, Rodriguez R, Rizer K, Anderson KE, Heller H, Carlson A, Criswell S, Racette BA, Revilla FJ, Nucifora F, Jr, Margolis RL, Ong M, Mendis T, Mendis N, Singer C, Quesada M, Paulsen JS, Brashers-Krug T, Miller A, Kerr J, Dubinsky RM, Gray C, Factor SA, Sperin E, Molho E, Eglow M, Evans S, Kumar R, Reeves C, Samii A, Chouinard S, Beland M, Scott BL, Hickey PT, Esmail S, Fung WL, Gibbons C, Qi L, Colcher A, Hackmyer C, McGarry A, Klos K, Gudesblatt M, Fafard L, Graffitti L, Schneider DP, Dhall R, Wojcieszek JM, LaFaver K, Duker A, Neefus E, Wilson-Perez H, Shprecher D, Wall P, Blindauer KA, Wheeler L, Boyd JT, Houston E, Farbman ES, Agarwal P, Eberly SW, Watts A, Tariot PN, Feigin A, Beck C, Orme C, Edicola J, Christopher E. Effect of Deutetrabenazine on Chorea Among Patients With Huntington Disease: A Randomized Clinical Trial. Jama. 2016;316(1):40–50. doi: 10.1001/jama.2016.8655. [DOI] [PubMed] [Google Scholar]
- Garriga-Canut M, Agustin-Pavon C, Herrmann F, Sanchez A, Dierssen M, Fillat C, Isalan M. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc Natl Acad Sci U S A. 2012;109(45):E3136–3145. doi: 10.1073/pnas.1206506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva M, Kusko R, Soares H, Fowler KD, Birnberg T, Barash S, Wagner AM, Fine T, Lysaght A, Weiner B, Cha Y, Kolitz S, Towfic F, Orbach A, Laufer R, Zeskind B, Grossman I, Hayden MR. Pridopidine activates neuroprotective pathways impaired in Huntington Disease. Hum Mol Genet. 2016;25(18):3975–3987. doi: 10.1093/hmg/ddw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampa C, Laurenti D, Anzilotti S, Bernardi G, Menniti FS, Fusco FR. Inhibition of the striatal specific phosphodiesterase PDE10A ameliorates striatal and cortical pathology in R6/2 mouse model of Huntington's disease. PLoS One. 2010;5(10):e13417. doi: 10.1371/journal.pone.0013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampa C, Montagna E, Dato C, Melone MA, Bernardi G, Fusco FR. Systemic delivery of recombinant brain derived neurotrophic factor (BDNF) in the R6/2 mouse model of Huntington's disease. PLoS One. 2013;8(5):e64037. doi: 10.1371/journal.pone.0064037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Roldan S, Mateo D. Huntington disease: tetrabenazine compared to haloperidol in the reduction of involuntary movements. Neurologia. 1989;4(8):282–287. [PubMed] [Google Scholar]
- Gines S, Seong IS, Fossale E, Ivanova E, Trettel F, Gusella JF, Wheeler VC, Persichetti F, MacDonald ME. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington's disease knock-in mice. Hum Mol Genet. 2003;12(5):497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- Goety CG, Tanner CM, Cohen JA, Thelen JA, Carroll VS, Klawans HL, Fariello RG. L-acetyl-carnitine in Huntington's disease: double-blind placebo controlled crossover study of drug effects on movement disorder and dementia. Mov Disord. 1990;5(3):263–265. doi: 10.1002/mds.870050317. [DOI] [PubMed] [Google Scholar]
- Gonzalez V, Cif L, Biolsi B, Garcia-Ptacek S, Seychelles A, Sanrey E, Descours I, Coubes C, de Moura AM, Corlobe A, James S, Roujeau T, Coubes P. Deep brain stimulation for Huntington's disease: long-term results of a prospective open-label study. J Neurosurg. 2014;121(1):114–122. doi: 10.3171/2014.2.JNS131722. [DOI] [PubMed] [Google Scholar]
- Goodman AO, Murgatroyd PR, Medina-Gomez G, Wood NI, Finer N, Vidal-Puig AJ, Morton AJ, Barker RA. The metabolic profile of early Huntington's disease--a combined human and transgenic mouse study. Exp Neurol. 2008;210(2):691–698. doi: 10.1016/j.expneurol.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Graham J, Levick D, Schreiber R. AMDIS Case Conference: Intrusive Medication Safety Alerts. Applied clinical informatics. 2010;1(1):68–78. doi: 10.4338/ACI-2010-03-CR-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Grohmann R, Koch R, Schmidt LG. Extrapyramidal symptoms in neuroleptic recipients. Agents Actions Suppl. 1990;29:71–82. doi: 10.1007/978-3-0348-7292-8_7. [DOI] [PubMed] [Google Scholar]
- Grove VE, Jr, Quintanilla J, DeVaney GT. Improvement of Huntington's disease with olanzapine and valproate. N Engl J Med. 2000;343(13):973–974. doi: 10.1056/NEJM200009283431316. [DOI] [PubMed] [Google Scholar]
- Groves M, van Duijn E, Anderson K, Craufurd D, Edmondson MC, Goodman N, van Kammen DP, Goodman L. An International Survey-based Algorithm for the Pharmacologic Treatment of Obsessive-Compulsive Behaviors in Huntington's Disease. PLoS Curr. 2011;3 doi: 10.1371/currents.RRN1261. Rrn1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber D, Kuhn AA, Schoenecker T, Kopp UA, Kivi A, Huebl J, Lobsien E, Mueller B, Schneider GH, Kupsch A. Quadruple deep brain stimulation in Huntington's disease, targeting pallidum and subthalamic nucleus: case report and review of the literature. J Neural Transm (Vienna) 2014;121(10):1303–1312. doi: 10.1007/s00702-014-1201-7. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol Dis. 2004;17(3):455–461. doi: 10.1016/j.nbd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nature Reviews Neuroscience. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, Como P, Zimmerman C, Lin M, Zhang L, Ulug AM, Beal MF, Matson W, Bogdanov M, Ebbel E, Zaleta A, Kaneko Y, Jenkins B, Hevelone N, Zhang H, Yu H, Schoenfeld D, Ferrante R, Rosas HD. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2'dG. Neurology. 2006;66(2):250–252. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Schifitto G, Oakes D, Bredlau AL, Meyers CM, Nahin R, Rosas HD. The CREST-E study of creatine for Huntington disease: A randomized controlled trial. Neurology. 2017;89(6):594–601. doi: 10.1212/WNL.0000000000004209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama H, Billin AN, Okamoto Y, Kinoshita M, Asano S. Expression profiling of Peroxisome proliferator-activated receptor-delta (PPAR-delta) in mouse tissues using tissue microarray. Histochemistry and cell biology. 2007;127(5):485–494. doi: 10.1007/s00418-007-0279-5. [DOI] [PubMed] [Google Scholar]
- Ho AK, Gilbert AS, Mason SL, Goodman AO, Barker RA. Health-related quality of life in Huntington's disease: Which factors matter most? Mov Disord. 2009;24(4):574–578. doi: 10.1002/mds.22412. [DOI] [PubMed] [Google Scholar]
- Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2003;100(4):2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington's, Disease, Collaborative, Research, Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- HuntingtonStudyGroup. Safety and tolerability of the free-radical scavenger OPC-14117 in Huntington's disease. Neurology. 1998;50(5):1366–1373. doi: 10.1212/wnl.50.5.1366. [DOI] [PubMed] [Google Scholar]
- HuntingtonStudyGroup. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology. 2001;57(3):397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- HuntingtonStudyGroup. Dosage effects of riluzole in Huntington's disease: a multicenter placebo-controlled study. Neurology. 2003;61(11):1551–1556. doi: 10.1212/01.wnl.0000096019.71649.2b. [DOI] [PubMed] [Google Scholar]
- HuntingtonStudyGroup. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66(3):366–372. doi: 10.1212/01.wnl.0000198586.85250.13. [DOI] [PubMed] [Google Scholar]
- HuntingtonStudyGroup. Randomized controlled trial of ethyl-eicosapentaenoic acid in Huntington disease: the TREND-HD study. Arch Neurol. 2008;65(12):1582–1589. doi: 10.1001/archneur.65.12.1582. [DOI] [PubMed] [Google Scholar]
- HuntingtonStudyGroup. A randomized, double-blind, placebo-controlled trial of pridopidine in Huntington's disease. Mov Disord. 2013;28(10):1407–1415. doi: 10.1002/mds.25362. [DOI] [PubMed] [Google Scholar]
- Imarisio S, Carmichael J, Korolchuk V, Chen CW, Saiki S, Rose C, Krishna G, Davies JE, Ttofi E, Underwood BR, Rubinsztein DC. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412(2):191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- Inc. BP. RO289 | Biomedtracker 2017 [Google Scholar]
- Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509–1523. doi: 10.1586/ern.11.149. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Orman J. Tetrabenazine therapy of dystonia, chorea, tics, and other dyskinesias. Neurology. 1988;38(3):391–394. doi: 10.1212/wnl.38.3.391. [DOI] [PubMed] [Google Scholar]
- Jia H, Kast RJ, Steffan JS, Thomas EA. Selective histone deacetylase (HDAC) inhibition imparts beneficial effects in Huntington's disease mice: implications for the ubiquitin-proteasomal and autophagy systems. Hum Mol Genet. 2012;21(24):5280–5293. doi: 10.1093/hmg/dds379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Wang Y, Morris CD, Jacques V, Gottesfeld JM, Rusche JR, Thomas EA. The Effects of Pharmacological Inhibition of Histone Deacetylase 3 (HDAC3) in Huntington's Disease Mice. PLoS One. 2016;11(3):e0152498. doi: 10.1371/journal.pone.0152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Albertz J, Guo Z, Peng Q, Rudow G, Troncoso JC, Ross CA, Duan W. Neuroprotective effects of PPAR-gamma agonist rosiglitazone in N171-82Q mouse model of Huntington's disease. J Neurochem. 2013;125(3):410–419. doi: 10.1111/jnc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells AP, Henry RA, Connor B. AAV-BDNF mediated attenuation of quinolinic acid-induced neuropathology and motor function impairment. Gene Ther. 2008;15(13):966–977. doi: 10.1038/gt.2008.23. [DOI] [PubMed] [Google Scholar]
- Koller WC, Trimble J. The gait abnormality of Huntington's disease. Neurology. 1985;35(10):1450–1454. doi: 10.1212/wnl.35.10.1450. [DOI] [PubMed] [Google Scholar]
- Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, Artates JW, Weiss A, Cheng SH, Shihabuddin LS, Hung G, Bennett CF, Cleveland DW. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Spada AR, Taylor JP. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat Rev Genet. 2010;11(4):247–258. doi: 10.1038/nrg2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. Huntington's disease: underlying molecular mechanisms and emerging concepts. Trends Biochem Sci. 2013;38(8):378–385. doi: 10.1016/j.tibs.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landwehrmeyer GB, Dubois B, de Yebenes JG, Kremer B, Gaus W, Kraus PH, Przuntek H, Dib M, Doble A, Fischer W, Ludolph AC. Riluzole in Huntington's disease: a 3-year, randomized controlled study. Ann Neurol. 2007;62(3):262–272. doi: 10.1002/ana.21181. [DOI] [PubMed] [Google Scholar]
- Lazar AS, Panin F, Goodman AO, Lazic SE, Lazar ZI, Mason SL, Rogers L, Murgatroyd PR, Watson LP, Singh P, Borowsky B, Shneerson JM, Barker RA. Sleep deficits but no metabolic deficits in premanifest Huntington's disease. Ann Neurol. 2015;78(4):630–648. doi: 10.1002/ana.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard DP, Kidson MA, Brown JG, Shannon PJ, Taryan S. A double blind trial of lithium carbonate and haloperidol in Huntington's chorea. Aust N Z J Psychiatry. 1975;9(2):115–118. doi: 10.3109/00048677509159834. [DOI] [PubMed] [Google Scholar]
- Lima WF, Prakash TP, Murray HM, Kinberger GA, Li W, Chappell AE, Li CS, Murray SF, Gaus H, Seth PP, Swayze EE, Crooke ST. Single-stranded siRNAs activate RNAi in animals. Cell. 2012;150(5):883–894. doi: 10.1016/j.cell.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119(1):121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell autonomous neurodegenerative disease. Nat Neurosci. 2007;10(11):1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sendon Moreno JL, Garcia Caldentey J, Trigo Cubillo P, Ruiz Romero C, Garcia Ribas G, Alonso Arias MA, Garcia de Yebenes MJ, Tolon RM, Galve-Roperh I, Sagredo O, Valdeolivas S, Resel E, Ortega-Gutierrez S, Garcia-Bermejo ML, Fernandez Ruiz J, Guzman M, Garcia de Yebenes Prous J. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington's disease. J Neurol. 2016;263(7):1390–1400. doi: 10.1007/s00415-016-8145-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Sendon Moreno JL, Garcia-Caldentey J, Regidor I, del Alamo M, Garcia de Yebenes J. A 5-year follow-up of deep brain stimulation in Huntington's disease. Parkinsonism Relat Disord. 2014;20(2):260–261. doi: 10.1016/j.parkreldis.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Lu XH, Yang XW. “Huntingtin holiday”: progress toward an antisense therapy for Huntington's disease. Neuron. 2012;74(6):964–966. doi: 10.1016/j.neuron.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucetti C, Del Dotto P, Gambaccini G, Dell' Agnello G, Bernardini S, Rossi G, Murri L, Bonuccelli U. IV amantadine improves chorea in Huntington's disease: an acute randomized, controlled study. Neurology. 2003;60(12):1995–1997. doi: 10.1212/01.wnl.0000068165.07883.64. [DOI] [PubMed] [Google Scholar]
- Maeder ML, Gersbach CA. Genome-editing Technologies for Gene and Cell Therapy. Mol Ther. 2016;24(3):430–446. doi: 10.1038/mt.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen I, Hornstein E. Oligonucleotide-based therapy for neurodegenerative diseases. Brain Res. 2014;1584:116–128. doi: 10.1016/j.brainres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Martinez T, Wright N, Lopez-Fraga M, Jimenez AI, Paneda C. Silencing human genetic diseases with oligonucleotide-based therapies. Hum Genet. 2013;132(5):481–493. doi: 10.1007/s00439-013-1288-1. [DOI] [PubMed] [Google Scholar]
- Mastrokolias A, Ariyurek Y, Goeman JJ, Duijn Ev, Roos RA, Mast RCvd, Ommen GBv, Dunnen JTd, Hoen PAt, Roon-Mom WMv. Huntington's disease biomarker progression profile identified by transcriptome sequencing in peripheral blood. European Journal of Human Genetics. 2015;23(10):1349–1356. doi: 10.1038/ejhg.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A. 1998;95(15):8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland KN, Cha JH. Molecular biology of Huntington's disease. Handb Clin Neurol. 2011;100:25–81. doi: 10.1016/B978-0-444-52014-2.00003-3. [DOI] [PubMed] [Google Scholar]
- McGarry A, McDermott M, Kieburtz K, de Blieck EA, Beal F, Marder K, Ross C, Shoulson I, Gilbert P, Mallonee WM, Guttman M, Wojcieszek J, Kumar R, LeDoux MS, Jenkins M, Rosas HD, Nance M, Biglan K, Como P, Dubinsky RM, Shannon KM, O'Suilleabhain P, Chou K, Walker F, Martin W, Wheelock VL, McCusker E, Jankovic J, Singer C, Sanchez-Ramos J, Scott B, Suchowersky O, Factor SA, Higgins DS, Jr, Molho E, Revilla F, Caviness JN, Friedman JH, Perlmutter JS, Feigin A, Anderson K, Rodriguez R, McFarland NR, Margolis RL, Farbman ES, Raymond LA, Suski V, Kostyk S, Colcher A, Seeberger L, Epping E, Esmail S, Diaz N, Fung WL, Diamond A, Frank S, Hanna P, Hermanowicz N, Dure LS, Cudkowicz M. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology. 2017;88(2):152–159. doi: 10.1212/WNL.0000000000003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Patry M, Ragland N, Lowden PA, Goodman J, Minnich J, Zahasky B, Park L, Leeds J, Howland D, Signer E, Tobin AJ, Brunner D. Comprehensive behavioral testing in the R6/2 mouse model of Huntington's disease shows no benefit from CoQ10 or minocycline. PLoS One. 2010;5(3):e9793. doi: 10.1371/journal.pone.0009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M, Benn CL, Franklin SA, Smith DL, Woodman B, Marks PA, Bates GP. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington's disease. PLoS One. 2011;6(11):e27746. doi: 10.1371/journal.pone.0027746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A, Price BH, Menear M, Lepage M. Brain imaging and cognitive dysfunctions in Huntington's disease. J Psychiatry Neurosci. 2006:21–29. [PMC free article] [PubMed] [Google Scholar]
- Murlidharan G, Samulski RJ, Asokan A. Gene Therapy of CNS Disorders Using Recombinant AAV Vectors. 2016:9–32. [Google Scholar]
- Murphy SM, Puwanant A, Griggs RC. Unintended effects of orphan product designation for rare neurological diseases. Ann Neurol. 2012;72(4):481–490. doi: 10.1002/ana.23672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance MA. Genetic testing of children at risk for Huntington's disease. US Huntington Disease Genetic Testing Group. Neurology. 1997;49(4):1048–1053. doi: 10.1212/wnl.49.4.1048. [DOI] [PubMed] [Google Scholar]
- NINDS. NINDS Common Data Elements 2017 [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Follenzi A, Athanasiadou R, Miyawaki T, Greally JM, Bennett MVL, Zukin RS. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A. 2012:E962–971. doi: 10.1073/pnas.1121568109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos PC, Aylward EH, Ross CA, Johnson HJ, Magnotta VA, Juhl AR, Pierson RK, Mills J, Langbehn DR, Paulsen JS. Cerebral cortex structure in prodromal Huntington disease. Neurobiol Dis. 2010;40(3):544–554. doi: 10.1016/j.nbd.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novina CD, Sharp PA. The RNAi revolution. Nature. 2004:161–164. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- O'Suilleabhain P, Dewey RB., Jr A randomized trial of amantadine in Huntington disease. Arch Neurol. 2003;60(7):996–998. doi: 10.1001/archneur.60.7.996. [DOI] [PubMed] [Google Scholar]
- Oliveira JM. Mitochondrial bioenergetics and dynamics in Huntington's disease: tripartite synapses and selective striatal degeneration. J Bioenerg Biomembr. 2010;42(3):227–234. doi: 10.1007/s10863-010-9287-6. [DOI] [PubMed] [Google Scholar]
- Paleacu D, Anca M, Giladi N. Olanzapine in Huntington's disease. Acta Neurol Scand. 2002;105(6):441–444. doi: 10.1034/j.1600-0404.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- Patel SV, Tariot PN, Asnis J. L-Deprenyl augmentation of fluoxetine in a patient with Huntington's disease. Ann Clin Psychiatry. 1996;8(1):23–26. doi: 10.3109/10401239609149087. [DOI] [PubMed] [Google Scholar]
- Paulsen JS. Cognitive impairment in Huntington disease: diagnosis and treatment. Curr Neurol Neurosci Rep. 2011;11(5):474–483. doi: 10.1007/s11910-011-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Long JD, Ross CA, Harrington DL, Erwin CJ, Williams JK, Westervelt HJ, Johnson HJ, Aylward EH, Zhang Y, Bockholt HJ, Barker RA. Prediction of manifest Huntington's disease with clinical and imaging measures: a prospective observational study. Lancet Neurol. 2014;13(12):1193–1201. doi: 10.1016/S1474-4422(14)70238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson GW. Lioresal in Huntington's disease. Dis Nerv Syst. 1976;37(8):465–467. [PubMed] [Google Scholar]
- Pazos MR, Sagredo O, Fernandez-Ruiz J. The endocannabinoid system in Huntington's disease. Curr Pharm Des. 2008;14(23):2317–2325. doi: 10.2174/138161208785740108. [DOI] [PubMed] [Google Scholar]
- Peiris JB, Boralessa H, Lionel ND. Clonazepam in the treatment of choreiform activity. Med J Aust. 1976;1(8):225–227. [PubMed] [Google Scholar]
- Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, Kassis I, Vaknin-Dembinsky A, Ben-Hur T, Offen D, Abramsky O, Melamed E, Karussis D. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients With Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016;73(3):337–344. doi: 10.1001/jamaneurol.2015.4321. [DOI] [PubMed] [Google Scholar]
- Priller J, Ecker D, Landwehrmeyer B, Craufurd D. A Europe-wide assessment of current medication choices in Huntington's disease. Mov Disord. 2008;23(12):1788. doi: 10.1002/mds.22188. [DOI] [PubMed] [Google Scholar]
- Prundean A, Youssov K, Humbert S, Bonneau D, Verny C. A phase II, open-label evaluation of cysteamine tolerability in patients with Huntington's disease. Mov Disord. 2015;30(2):288–289. doi: 10.1002/mds.26101. [DOI] [PubMed] [Google Scholar]
- Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, Hersch S, Vaddadi KS, Sword A, Horrobin DF, Manku M, Murck H. Ethyl-EPA in Huntington disease: a double-blind, randomized, placebo-controlled trial. Neurology. 2005;65(2):286–292. doi: 10.1212/01.wnl.0000169025.09670.6d. [DOI] [PubMed] [Google Scholar]
- Quinn N, Marsden CD. A double blind trial of sulpiride in Huntington's disease and tardive dyskinesia. J Neurol Neurosurg Psychiatry. 1984;47(8):844–847. doi: 10.1136/jnnp.47.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready RE, Mathews M, Leserman A, Paulsen JS. Patient and caregiver quality of life in Huntington's disease. Mov Disord. 2008;23(5):721–726. doi: 10.1002/mds.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilmann R, Leavitt BR, Ross CA. Diagnostic criteria for Huntington's disease based on natural history. Mov Disord. 2014;29(11):1335–1341. doi: 10.1002/mds.26011. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(15):5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveley MA, Dursun SM, Andrews H. A comparative trial use of sulpiride and risperidone in Huntington's disease: a pilot study. J Psychopharmacol. 1996;10(2):162–165. doi: 10.1177/026988119601000213. [DOI] [PubMed] [Google Scholar]
- Riley BE, Orr HT. Polyglutamine neurodegenerative diseases and regulation of transcription: assembling the puzzle. Genes Dev. 2006;20(16):2183–2192. doi: 10.1101/gad.1436506. [DOI] [PubMed] [Google Scholar]
- Roos RA, Buruma OJ, Bruyn GW, Kemp B, van der Velde EA. Tiapride in the treatment of Huntington's chorea. Acta Neurol Scand. 1982;65(1):45–50. doi: 10.1111/j.1600-0404.1982.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Doros G, Gevorkian S, Malarick K, Reuter M, Coutu JP, Triggs TD, Wilkens PJ, Matson W, Salat DH, Hersch SM. PRECREST: a phase II prevention and biomarker trial of creatine in at-risk Huntington disease. Neurology. 2014;82(10):850–857. doi: 10.1212/WNL.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10(4):204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- Ross CA, Becher MW, Colomer V, Engelender S, Wood JD, Sharp AH. Huntington's disease and dentatorubral-pallidoluysian atrophy: proteins, pathogenesis and pathology. Brain pathology (Zurich, Switzerland) 1997;7(3):1003–1016. doi: 10.1111/j.1750-3639.1997.tb00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangamo. Sangamo tries its hand with zinc fingers for Huntington's: Spoonful of Medicine 2012 [Google Scholar]
- Saran BM, Klein MS, Benay EM. Clinical evaluation of amantadine and haloperidol in Huntington's chorea. J Clin Psychiatry. 1980;41(6):221. [PubMed] [Google Scholar]
- Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95(1):55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington's disease transgenic mouse model. Neurosci Lett. 2001;315(3):149–153. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- Schoenfeld M, Myers RH, Cupples LA, Berkman B, Sax DS, Clark E. Increased rate of suicide among patients with Huntington's disease. J Neurol Neurosurg Psychiatry. 1984;47(12):1283–1287. doi: 10.1136/jnnp.47.12.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab LC, Garas SN, Drouin-Ouellet J, Mason SL, Stott SR, Barker RA. Dopamine and Huntington's disease. Expert Rev Neurother. 2015;15(4):445–458. doi: 10.1586/14737175.2015.1025383. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Sesok S, Bolle N, Kobal J, Bucik V, Vodusek DB. Cognitive function in early clinical phase huntington disease after rivastigmine treatment. Psychiatr Danub. 2014;26(3):239–248. [PubMed] [Google Scholar]
- Shannon KM, Fraint A. Therapeutic advances in Huntington's Disease. Mov Disord. 2015;30(11):1539–1546. doi: 10.1002/mds.26331. [DOI] [PubMed] [Google Scholar]
- Shen YC. Lamotrigine in motor and mood symptoms of Huntington's disease. World J Biol Psychiatry. 2008;9(2):147–149. doi: 10.1080/15622970701332520. [DOI] [PubMed] [Google Scholar]
- Shoulson I, Odoroff C, Oakes D, Behr J, Goldblatt D, Caine E, Kennedy J, Miller C, Bamford K, Rubin A, et al. A controlled clinical trial of baclofen as protective therapy in early Huntington's disease. Ann Neurol. 1989;25(3):252–259. doi: 10.1002/ana.410250308. [DOI] [PubMed] [Google Scholar]
- Skotte NH, Southwell AL, Ostergaard ME, Carroll JB, Warby SC, Doty CN, Petoukhov E, Vaid K, Kordasiewicz H, Watt AT, Freier SM, Hung G, Seth PP, Bennett CF, Swayze EE, Hayden MR. Allele-specific suppression of mutant huntingtin using antisense oligonucleotides: providing a therapeutic option for all Huntington disease patients. PLoS One. 2014;9(9):e107434. doi: 10.1371/journal.pone.0107434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Matson S, Matson WR, Cormier K, Del Signore SJ, Hagerty SW, Stack EC, Ryu H, Ferrante RJ. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington's disease mice. Biochim Biophys Acta. 2006;1762(6):616–626. doi: 10.1016/j.bbadis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Song JH, Huang CS, Nagata K, Yeh JZ, Narahashi T. Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1997;282(2):707–714. [PubMed] [Google Scholar]
- Southwell AL, Franciosi S, Villanueva EB, Xie Y, Winter LA, Veeraraghavan J, Jonason A, Felczak B, Zhang W, Kovalik V, Waltl S, Hall G, Pouladi MA, Smith ES, Bowers WJ, Zauderer M, Hayden MR. Anti-semaphorin 4D immunotherapy ameliorates neuropathology and some cognitive impairment in the YAC128 mouse model of Huntington disease. Neurobiol Dis. 2015;76:46–56. doi: 10.1016/j.nbd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Squitieri F, Cannella M, Porcellini A, Brusa L, Simonelli M, Ruggieri S. Short-term effects of olanzapine in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(1):69–72. [PubMed] [Google Scholar]
- Squitieri F, Cannella M, Simonelli M, Sassone J, Martino T, Venditti E, Ciammola A, Colonnese C, Frati L, Ciarmiello A. Distinct brain volume changes correlating with clinical stage, disease progression rate, mutation size, and age at onset prediction as early biomarkers of brain atrophy in Huntington's disease. CNS Neurosci Ther. 2009;15(1):1–11. doi: 10.1111/j.1755-5949.2008.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek LM, Yang W, Angus S, Sardi PS, Hayden MR, Hung GH, Bennett CF, Cheng SH, Shihabuddin LS. Antisense oligonucleotide-mediated correction of transcriptional dysregulation is correlated with behavioral benefits in the YAC128 mouse model of Huntington's disease. J Huntingtons Dis. 2013;2(2):217–228. doi: 10.3233/JHD-130057. [DOI] [PubMed] [Google Scholar]
- Stewart JT. Adverse behavioral effects of amantadine therapy in Huntington's disease. South Med J. 1987;80(10):1324–1325. doi: 10.1097/00007611-198710000-00032. [DOI] [PubMed] [Google Scholar]
- Stewart JT. Treatment of Huntington's disease with clonazepam. South Med J. 1988;81(1):102. doi: 10.1097/00007611-198801000-00029. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Carta A, Berardelli A, Antonini A, Argenta M, Formica A, Agnoli A. Effects of terguride in patients with Huntington's disease. Clin Neuropharmacol. 1989;12(5):435–439. doi: 10.1097/00002826-198910000-00008. [DOI] [PubMed] [Google Scholar]
- Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Carlozzi N, Duff K, Beglinger LJ, Langbehn DR, Johnson SA, Biglan KM, Aylward EH. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25(1):1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugars KL, Brown R, Cook LJ, Swartz J, Rubinsztein DC. Decreased cAMP response element-mediated transcription: an early event in exon 1 and full-length cell models of Huntington's disease that contributes to polyglutamine pathogenesis. J Biol Chem. 2004;279(6):4988–4999. doi: 10.1074/jbc.M310226200. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, Borowsky B, Landwehrmeyer B, Frost C, Johnson H, Craufurd D, Reilmann R, Stout JC, Langbehn DR. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12(7):637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Sammut S, Menniti FS, Schmidt CJ, West AR. Inhibition of Phosphodiesterase 10A Increases the Responsiveness of Striatal Projection Neurons to Cortical Stimulation. J Pharmacol Exp Ther. 2009;328(3):785–795. doi: 10.1124/jpet.108.146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, West AR. Review: Modulation of striatal neuron activity by cyclic nucleotide signaling and phosphodiesterase inhibition. Basal Ganglia. 2013;3(3):137–146. doi: 10.1016/j.baga.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager U, Andre R, Lahiri N, Magnusson-Lind A, Weiss A, Grueninger S, McKinnon C, Sirinathsinghji E, Kahlon S, Pfister EL, Moser R, Hummerich H, Antoniou M, Bates GP, Luthi-Carter R, Lowdell MW, Bjorkqvist M, Ostroff GR, Aronin N, Tabrizi SJ. HTT-lowering reverses Huntington's disease immune dysfunction caused by NFkappaB pathway dysregulation. Brain. 2014;137(Pt 3):819–833. doi: 10.1093/brain/awt355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RA, Lazarowski ER, Damian VA, Masliah E, La Spada AR. PGC-1alpha Rescues Huntington's Disease Proteotoxicity by Preventing Oxidative Stress and Promoting TFEB Function. Sci Transl Med. 2012;4(142):142ra197. doi: 10.1126/scitranslmed.3003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddadi KS, Soosai E, Chiu E, Dingjan P. A randomised, placebo-controlled, double blind study of treatment of Huntington's disease with unsaturated fatty acids. Neuroreport. 2002;13(1):29–33. doi: 10.1097/00001756-200201210-00011. [DOI] [PubMed] [Google Scholar]
- van Duijn E, Kingma EM, van der Mast RC. Psychopathology in verified Huntington's disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441–448. doi: 10.1176/jnp.2007.19.4.441. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Pearson J, Bailey CD, Rogers DA, Johnson GV, Hayden MR, Leavitt BR. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J Neurochem. 2005;95(1):210–220. doi: 10.1111/j.1471-4159.2005.03357.x. [DOI] [PubMed] [Google Scholar]
- van Vugt JP, Siesling S, Vergeer M, van der Velde EA, Roos RA. Clozapine versus placebo in Huntington's disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35–39. doi: 10.1136/jnnp.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, Hespel P, Dom R. Creatine supplementation in Huntington's disease: a placebo-controlled pilot trial. Neurology. 2003;61(7):925–930. doi: 10.1212/01.wnl.0000090629.40891.4b. [DOI] [PubMed] [Google Scholar]
- Verhagen Metman L, Morris MJ, Farmer C, Gillespie M, Mosby K, Wuu J, Chase TN. Huntington's disease: a randomized, controlled trial using the NMDA-antagonist amantadine. Neurology. 2002;59(5):694–699. doi: 10.1212/wnl.59.5.694. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington's disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, Cui L, Beyer RP, Easley CN, Smith AC, Krainc D, Luquet S, Sweet IR, Schwartz MW, La Spada AR. Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell Metab. 2006;4(5):349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10(8):816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- Yu D, Pendergraff H, Liu J, Kordasiewicz HB, Cleveland DW, Swayze EE, Lima WF, Crooke ST, Prakash TP, Corey DR. Single-stranded RNAs use RNAi to potently and allele-selectively inhibit mutant huntingtin expression. Cell. 2012;150(5):895–908. doi: 10.1016/j.cell.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadori D, Klivenyi P, Vamos E, Fulop F, Toldi J, Vecsei L. Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies. J Neural Transm. 2009;116(11):1403–1409. doi: 10.1007/s00702-009-0263-4. [DOI] [PubMed] [Google Scholar]
- Zappacosta B, Monza D, Meoni C, Austoni L, Soliveri P, Gellera C, Alberti R, Mantero M, Penati G, Caraceni T, Girotti F. Psychiatric symptoms do not correlate with cognitive decline, motor symptoms, or CAG repeat length in Huntington's disease. Arch Neurol. 1996;53(6):493–497. doi: 10.1001/archneur.1996.00550060035012. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol. 2007;81(5-6):294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293(5529):493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90(3):905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]