Abstract
Sulfide:quinone oxidoreductase (SQR) is the primary sulfide-oxidizing enzyme found in all three domains of life. Of the six phylogenetically distinct types of SQR, four have representatives that have been biochemically characterized. The genome of Chlorobaculum tepidum encodes three SQR homologs. One of these, encoded by CT1087, is a type VI SQR that has been previously shown to be required for growth at high sulfide concentrations and to be expressed in sulfide-dependent manner. Therefore, CT1087 was hypothesized to be a high sulfide adapted SQR. CT1087 was expressed in Escherichia coli with an N-terminal His-tag (CT1087NHis6) and purified by Ni-NTA chromatography. CT1087NHis6 was active and contained FAD as a strongly bound cofactor. The measured kinetic parameters for CT1087NHis6 indicate a low affinity for sulfide and a high enzymatic turnover rate consistent with the hypothesis for its function inferred from genetic and expression data. These are the first kinetic data for a type VI SQR and have implications for structure-function analyses of all SQR's.
Keywords: sulfide:quinone oxidoreductase, Chlorobaculum tepidum, sulfur metabolism, energy metabolism, Sulfur2016
Hydrogen sulfide, a toxic gas, is consumed by microbes; here, one type of the key sulfide oxidation enzyme is shown to function very rapidly at high substrate concentrations suggesting that this group may be key for sulfide tolerance.
INTRODUCTION
Sulfide is naturally produced from the biodegradation of organic matter and a byproduct or waste material of various industrial operations (National Research Council 1979). Sulfide-oxidizing microbes have the potential to remediate these waste streams (Basu, Clausen and Gaddy 1996; Chung, Huang and Li 1997; Nishimura and Yoda 1997; Henshaw and Zhu 2001) by oxidizing sulfide to elemental sulfur or sulfate. The first step of sulfide oxidation for energy conservation in many organisms is carried out by the enzyme sulfide:quinone oxidoreductase (SQR, E.C. 1.8.5.4). SQR is a member of the flavoprotein disulfide reductase protein family, also known as the glutathione reductase family that also includes flavocytochrome c sulfide dehydrogenase (FCSD), lipoamide dehydrogenase, thioredoxin reductase and mercuric ion reductase (Williams 1992). SQRs are found in all three domains of life and they have been classified into six types based on sequence and structural analyses (I–VI, Fig. 1, (Marcia et al. 2010a)) with proposed names SqrA-F along with SqrX to denote a sub-group of type IV SQR's (Gregersen, Bryant and Frigaard 2011). However, only members of type I, II, III and V SQRs have been biochemically characterized (Arieli, Padan and Shahak 1991; Arieli et al. 1994; Griesbeck et al. 2002; Brito et al. 2009; Marcia et al. 2009; Cherney et al.2010, 2012; Zhang and Weiner 2014).
Figure 1.
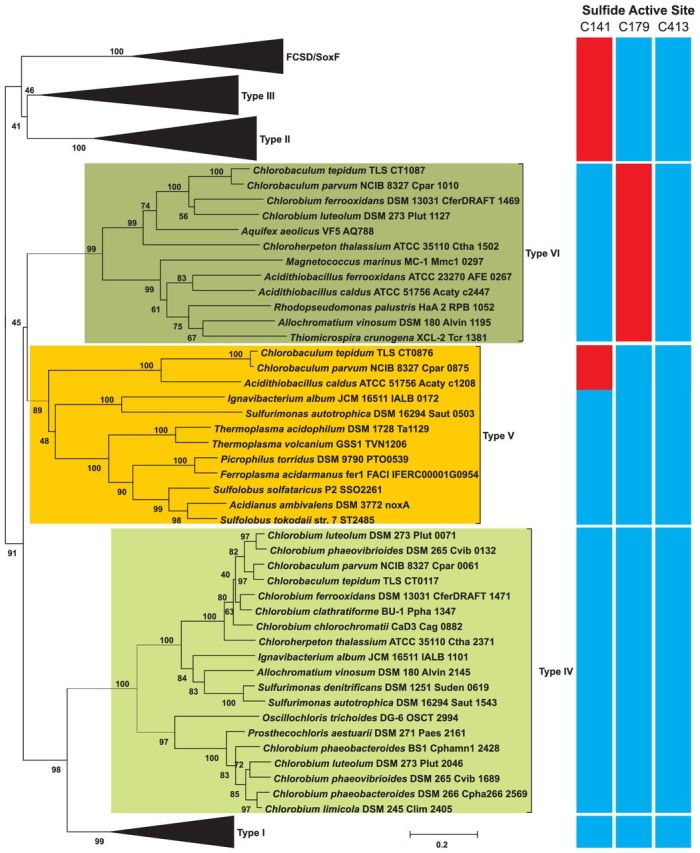
Phylogenetic relationships of SQR types I–VI, flavocytochrome c sulfide dehydrogenase and SoxF amino acid sequences with a heat map indicating the conservation of proposed active site cysteine residues numbered according to the CT0117 sequence: blue = conserved, red = not conserved. Values at nodes are the percentage of 1000 bootstrap runs where the node was present.
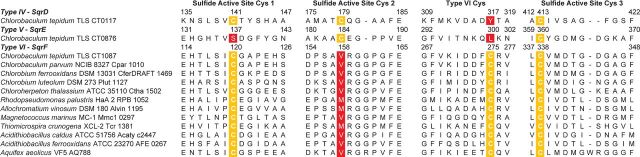
Green sulfur bacteria (family Chlorobiaceae) are anaerobic anoxygenic phototrophic bacteria, many of which utilize sulfide as an electron donor for photosynthesis. All genome-sequenced members of the Chlorobiaceae possess a gene encoding a type IV SQR, while many possess genes encoding additional SQR types. The genome of Chlorobaculum tepidum encodes three different SQR homologs: type IV-CT0117, type V-CT0876 and type VI-CT1087 (Eisen et al. 2002). Sequence comparisons have produced SQR fingerprint sequences (Griesbeck and Hauska 2000) including three cysteine residues that are highly conserved in the flavoprotein disulfide reductase family that structural and site-directed mutagenesis studies have indicated are important for SQR function (Fig. 1). The sulfide active site differs between the three SQR homologs. CT0117 conserves all three redox active cysteine residues, while CT0876 lacks the first and CT1087 lacks the second (Fig. 1). CT1087 was initially predicted to be non-functional since the second conserved cysteine was essential for SQR activity in Rhodobacter capsulatus (Griesbeck et al. 2002). However, a C. tepidum CT1087 mutant strain had a growth defect at high (>4 mM) sulfide concentrations and had lower in vitro SQR activity in crude membrane preparations compared to the wild-type strain (Chan, Morgan-Kiss and Hanson 2009). Furthermore, CT1087 mRNA abundance increased within 30 min of sulfide addition to C. tepidum growing on thiosulfate, making it the most highly expressed of the three SQR genes (Eddie and Hanson 2013) suggesting a role for the CT1087 gene product in sulfide metabolism.
To better understand the physiological role of CT1087, we produced and purified histidine-tagged CT1087 in Escherichia coli. The purified enzyme was active and displayed high velocity and turnover number coupled with a poor affinity for sulfide. Together, these observations indicate that the type VI SQR from C. tepidum has been selected to rapidly oxidize high sulfide concentrations, consistent with the available mutant phenotype and gene regulation data.
MATERIALS AND METHODS
Phylogenetic Analysis of SQR
A phylogenetic tree of SQR, FCSD and SoxF protein sequences was made using the Neighbor-Joining method using MEGA5 (Tamura et al. 2011). Evolutionary distances were computed using the Poisson correction method.
Construction of pET16b_CT1087NHis6
CT1087 was PCR amplified using DreamTaq DNA Polymerase (Thermo Fisher Scientific, Waltham, MA) with primers CT1087-NdeI-F (5′- CATATGAAAAAAGTACTGATTCTTGGTGGAGGTATTGCCGGTGTTGC-3′) and CT1087-BamHI-R (5′-AGCCGGATCCGATCACATCCCCGGAATCCTCGGAATG-3′) and cloned (TOPO-TA cloning kit, Invitrogen, Carlsbad, CA) after ExoSAP-IT® (Affymetrix, Santa Clara, CA) treatment. Clones were recovered in E. coli EC100D (Epicentre, Madison, WI) by plating on LB with 50 μg ml−1 kanamycin, 40 μg ml−1 X-Gal and 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 37°C. The CT1087-insert was confirmed in white colonies by colony PCR with M13F (5′- GTAAAACGACGGCCAGT-3′) and M13R (5′-AACAGCTATGACCATG-3′) and Sanger sequencing using the same primers. The CT1087 insert was gel purified (QIAprep Spin Mini, QIAGEN, Hilden, Germany) after NdeI and BamHI (FastDigest®, Thermo Scientific, Waltham, MA) digestion and mixed with NdeI and BamHI digested pET16b (Novagen, Madison, WI) for ligation (Rapid DNA Ligation Kit, Thermo Scientific, Waltham, MA). The pET16b_CT1087 expression construct was recovered in E. coli EC100D with selection on LB plates with 100 μg ml−1 ampicillin at 37°C. After the pET16b_CT1087 construct was confirmed by HindIII digestion it was transferred to E. coli BL21(DE3) pLysS with selection on LB plates containing 100 μg ml−1 ampicillin and 20 μg ml−1 chloramphenicol.
Expression and Purification of CT1087NHis6
Escherichia coli BL21(DE3) pLysS carrying pET16b_CT1087 was grown in LB supplemented with 0.4% (w/v) glucose, 20 mM 3-(4-Morpholino)propane sulfonic acid and 25 mM sodium nitrate at pH = 7.8. Starter cultures were grown aerobically in 10 mL of medium in 15 mL screw cap tubes (Fisher Scientific, Waltham, MA) with 100 μg ml−1 ampicillin and 20 μg ml−1 chloramphenicol overnight at 37°C shaking at 250 rpm. Expression was performed in 250 mL narrow mouth glass bottles sealed with butyl rubber septa caps (Fisher Scientific, Waltham, MA) with medium made anoxic by cooling with loosened caps in an anaerobic chamber (1-2% H2 + 98–99% N2 atmosphere, Coy Laboratory Products Inc., Grass Lake, MI) after autoclaving. The headspace was exchanged and pressurized to 10 psi with 5% CO2 + 95% N2 that had been passed through heated copper filings. Expression cultures (4 × 250 mL bottles) were inoculated to an OD600 of 0.01 and incubated shaking at 37°C until an OD600 of 0.3 when IPTG was added to 0.4 mM. One hour later, sulfide was added to a final concentration of 5 mM from a pH-neutralized stock solution (Siefert and Pfennig 1984) and cells harvested 18–20 h later.
All buffer solutions used were stirred under vacuum for >1 h in an anaerobic chamber at least 24 h prior to use. All transfers, cell disruption and purification steps were carried out in an anaerobic chamber. Cells were collected by centrifugation (30 000 × g, 10 min, 4°C) in 250 mL centrifuge bottles with o-ring sealing lids (Beckman-Coulter, Pasadena, CA). Cell pellets were resuspended in 100 mM Tris(hydroxymethyl)aminomethane-hydrochloric acid (Tris-HCl) pH 7.4 and recentrifuged. Cells were suspended in lysis buffer (100 mM Tris-HCl pH 7.4, 100 μg mL−1 Lysozyme, SigmaFAST protease inhibitor cocktail EDTA-free) and sonicated using a model 450 sonifier equipped with a microtip (Output control 4, 40% Duty cycle, 1 min, on ice, 5 cycles; Branson Ultrasonics, Danbury, CT). Lysate was clarified by centrifugation (10 000 × g, 10 min, 4°C) in centrifuge tubes with o-ring sealing lids (Beckman Coulter, Pasadena, CA). The supernatant was fractionated into membrane (pellet) and soluble (supernatant) fractions by ultracentrifugation (175 000 × g, 1 h, 4°C). The membrane fraction was suspended in 100 mM Tris-HCl pH 7.4 and collected by ultracentrifugation (175 000 × g, 30 min, 4°C). Proteins were solubilized from the membrane by suspending the membrane fraction in 100 mM Tris-HCl pH 7.4, 300 mM NaCl, 0.4 M sucrose, 10 mM MgCl2 6H2O and 25 mM sodium cholate for 30 min at room temperature followed by ultracentrifugation (175 000 × g, 30 min, 4°C). The supernatant containing solubilized membrane proteins was then loaded on a Ni-NTA agarose (QIAGEN, Hilden, Germany) column on a BioLogic Low Pressure (LP) Liquid Chromatography system connected to a laptop with LP Data View software (BIO-RAD, Hercules, CA). Elution profiles were monitored at 280 nm.
Bound CT1087NHis6 was eluted from the column with the solubilization buffer above containing 250 mM imidazole and either 1.0% (w/v) sodium dodecyl sulfate (SDS) or Triton X-100 (TX-100) as noted. Imidazole was removed from TX-100 purified CT1087NHis6 by dialysis (30 kDa MWCO) against solubilization buffer containing 1.0% (w/v) TX-100. Purified CT1087NHis6 was transferred to 2.0 mL cryo tubes with o-ring sealing lids (Fisher Scientific, Waltham, MA) that were then placed into a 250 mL centrifuge bottle with an o-ring sealing lid in the anaerobic chamber before storage at −20°C. Protein concentrations were determined by Bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA).
SQR Characterization and Assay
Size exclusion chromatography was performed on a Sephacryl S-200 HR Column (GE Life Sciences, Pittsburgh, PA) equilibrated in solubilization buffer containing 1.0% (w/v) TX-100. Cytochrome c (12.4 kDa), carbonic anhydrase (29 kDa), BSA (66 kDa), alcohol dehydrogenase (150 kDa), β-amylase (200 kDa) and blue dextran (2000 kDa) were used as calibration standards (Sigma-Aldrich, St. Louis, MO).
All assays and absorption spectra were collected on a DU7400 UV-Vis spectrophotometer equipped with a Peltier temperature controlled cell holder (Beckman Coulter, Pasadena, CA). Solutions for SQR activity were made anoxic as above. SQR activity assays were performed in 1 mL 10 mM Tris-HCl pH 7.4 containing 100 μM decylubiquinone (dUQ) in septum-screw cap quartz cuvettes (Starna Cells, Atascadero, CA) at 47°C, which is the optimum growth temperature for C. tepidum. dUQ reduction was monitored by the change in absorbance at 275 nm using the extinction coefficient (ɛ = 9.2 × 10−3 l μmol−1 dUQ) determined under SQR assay conditions. Assays were initiated by adding sulfide to a final assay concentration of 1 mM from a pH neutralized stock solution (Siefert and Pfennig 1984). Non-enzymatic reduction of dUQ by sulfide was measured by omitting protein from the assay mixture.
The kinetic parameters of CT1087 were determined for sulfide by varying the concentration of sulfide (0.25–6.00 mM) in the assay. SQR activity dependence on dUQ with 1 mM sulfide was examined from 75–200 μM, the solubility limit of dUQ in assay buffer. Kinetic parameters were determined using the Excel Solver Add-on in Microsoft Excel 2011 (Microsoft, Redmond, WA).
SDS-PAGE and Immunoblotting
Fractions were analyzed by SDS-PAGE (5% stacking/15% resolving). TX-100 was removed from protein samples using Bio-Beads® SM (BIO-RAD, Hercules, CA). Protein samples were boiled in loading dye containing 20 mM Tris-HCl pH 6.8, 1% (w/v) SDS, 8% (v/v) glycerol, 0.1 mg mL−1 bromophenol blue and 1% (v/v) β-mercaptoethanol and equal amounts loaded on duplicate gels. One gel was stained with Bio-Safe Coomassie (BIO-RAD, Hercules, CA) and the other was used for immunoblotting.
For immunoblotting, gels were equilibrated in 25 mM Tris-HCl, 19.2 mM glycine and 20% (v/v) methanol and transferred to Immobilon-FL membrane (Millipore, Billerica, MA) using a Mini Trans-Blot® Cell (BIO-RAD, Hercules, CA). The primary antibody was 1:2000 diluted Mouse Anti-His4 (α-His4, QIAGEN, Hilden, Germany) and the secondary antibody was 1:5000 diluted Goat Anti-Mouse alkaline phosphatase conjugate (BIO-RAD, Hercules, CA). The membrane was developed using ECF substrate (GE Healthcare Life Sciences, Pittsburgh, PA) and imaged on a MD Typhoon® 6500 Variable Mode Imager (GE Healthcare Life Sciences, Pittsburgh, PA) with 526 nm excitation and 532 nm emission.
Statistical Analyses
All P-values were calculated using two-way, unpaired Student's t-test by making comparisons of data sets consisting of three or more replicates in Microsoft Excel 2011.
RESULTS
Purification of CT1087NHis6
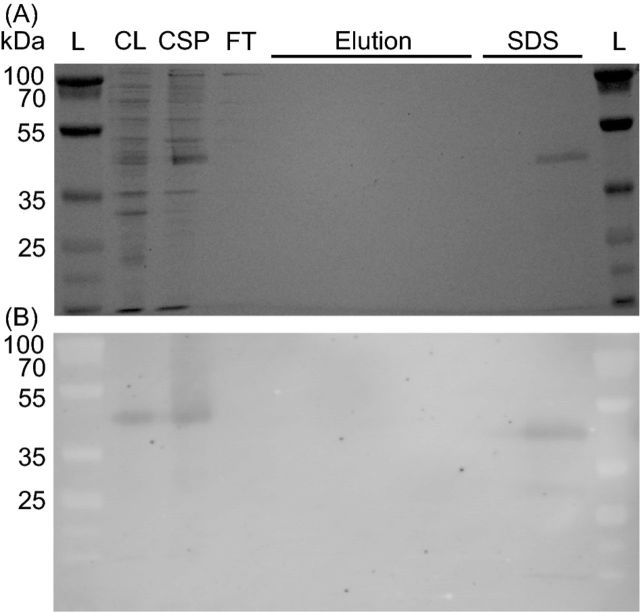
To test whether or not the CT1087 gene product possessed SQR activity, it was cloned and overexpressed in E. coli as an N-terminal His-tag fusion, CT1087NHis6. Expression, purification and assays were carried out under strictly anoxic conditions. SQR activity dramatically increased in crude lysates of IPTG-induced (1.0–2.0 μmol dUQ reduced (mg protein)−1 min−1) cultures relative to uninduced (<0.1 μmol dUQ reduced (mg protein)−1 min−1) cultures. This correlated with the appearance of a protein cross-reactive with α-His4 antibody that could be solubilized from the membrane fraction with sodium cholate (Fig. 2). Initial attempts to purify this protein resulted in a complete loss of activity and His-tagged protein at the affinity purification step. Washing the Ni-NTA column with SDS resulted in the elution of a single protein with a His-tag suggesting that CT1087NHis6 was tightly bound on the column. The addition of 1% (w/v) TX-100 in the Ni-NTA elution buffer allowed for the purification of CT1087NHis6 (Fig. 3). Typical purifications by this protocol yielded ∼0.7 mg of active enzyme per liter of expression culture (Table 1).
Figure 2.
CT1087NHis6 binds tightly to a Ni-NTA column. (A) Coomassie stained SDS-PAGE gel. (B) Immunoblot with α-His4 antibody. Lane labels: L = MW ladder, CL = crude lysate, CSP = cholate solubilized membrane protein, FT = Ni-NTA agarose column flow through during binding, Elution = fractions following imidazole elution, SDS = fractions following addition of 1% SDS to elution buffer.
Figure 3.
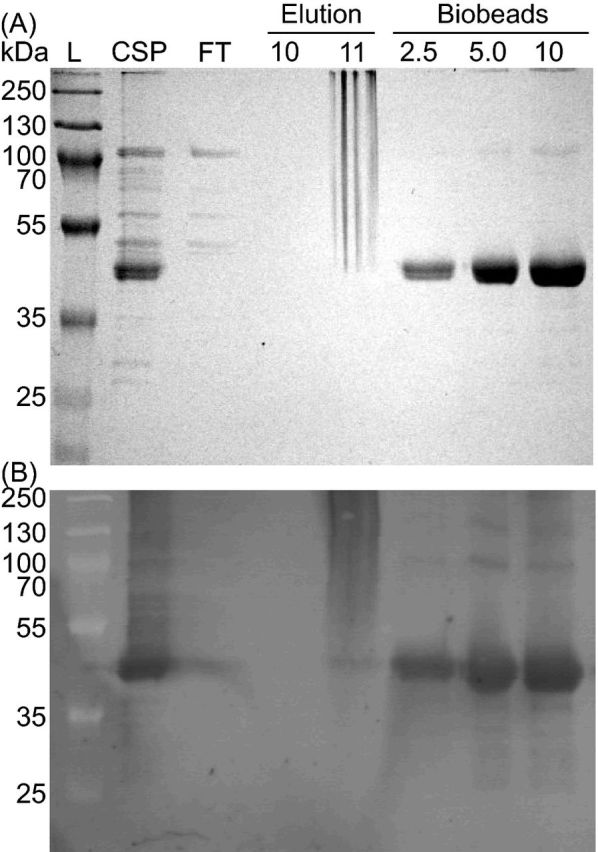
Purification of CT1087NHis6 is enabled by the addition of TX-100 to Ni-NTA elution buffer. (A) Coomassie stained SDS-PAGE gel. (B) Immunoblot with α-His4 antibody. Lane labels: L = MW ladder, CSP = cholate solubilized membrane protein, FT = Ni-NTA agarose column flow through during binding, Elution = fractions following elution with buffer containing TX-100, Biobeads = the indicated amounts of protein (μg) from elution fraction 11 after removal of TX-100.
Table 1.
Purification of CT1087NHis6 from E. coli.
| Specific activity | Total units | |||
|---|---|---|---|---|
| Fraction | Protein (mg) | (μmol dUQ mg protein−1 min−1) | (μmol dUQ min−1) | Yield (%) |
| Crude lysate | 45.9 ± 6.9 | 1.6 ± 0.6 | 76.4 ± 39.8 | 100 |
| Solubilized membrane | 1.9 ± 0.9 | 6.8 ± 3.0 | 13.6 ± 9.3 | 16.7 ± 6.7 |
| CT1087NHis6 | 0.7 ± 0.1 | 12.7 ± 1.5 | 7.1 ± 1.3 | 10.9 ± 4.9 |
Characterization of CT1087NHis6
SDS-PAGE and immunoblotting of CT1087NHis6 yielded faint bands at approximately 100 and 130 kDa that may indicate the formation of dimers and trimers (Fig. 3). However, size exclusion chromatography of purified CT1087NHis6 indicated a molecular mass of 43.5 kDa, similar to the predicted monomeric mass of 45.8 kDa. Purified CT1087NHis6 contained two absorption maxima at 366 nm and 455 nm similar to those observed for previously purified SQRs (Brito et al. 2009; Marcia et al. 2010b). Comparing the absorption spectrum of CT1087NHis6 to a solution of pure FAD at an equal concentration (∼1.6 μM) indicated the purified enzyme appeared was 75% occupied by FAD after dialysis. Purified CT1087NHis6 could be stored under anoxic conditions with no significant change in SQR specific activity for up to 110 h (data not shown).
CT1087NHis6 Kinetic Parameters
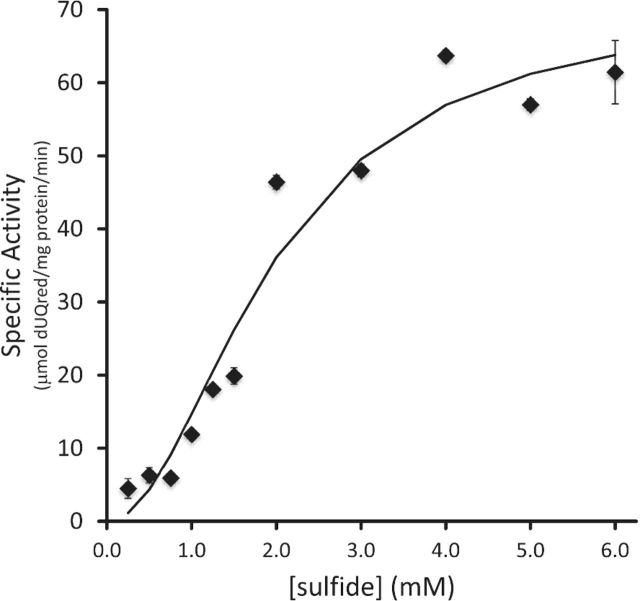
The enzymatic kinetics of purified CT1087NHis6 for sulfide as a substrate were determined for three independent preparations of enzyme (Fig. 4). While CT1087NHis6 as purified was apparently not fully occupied with FAD, the addition of FAD to reactions or pre-incubation of enzyme with FAD did not affect the enzymatic activity (data not shown). Therefore, the enzyme was used as purified. SQR specific activity did not increase until sulfide concentrations >0.75 mM were used, giving the saturation curve a distinct sigmoidal shape indicating non-Michaelis–Menten kinetics. The Hill equation (equation 1) (Bezeau and Endrenyi 1986; Sun et al. 2014) with a Hill coefficient (n) of 2 provided the best fit.
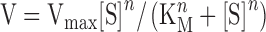 |
(1) |
With this coefficient, CT1087NHis6 displayed a KM of 1.95 mM for sulfide, Vmax of 71 μmol dUQred (mg protein)−1 min−1, and a Kcat of 54 sec−1. When compared to available kinetic parameters for other SQRs, CT1087NHis6 is the most similar to type II SQRs that have similarly high Vmax, poor affinity for sulfide, and high Kcat (Table 2).
Figure 4.
Analysis of CT1087NHis6 specific activity with respect to sulfide concentration. The line indicates the fit based on the Hill equation with parameters noted in the text. Error bars are equal to one standard deviation of three or more replicates.
Table 2.
Biochemically characterized SQRs and their kinetic properties.
| Organism | Type | Vmaxa | KMb | Kcatc | Reference |
|---|---|---|---|---|---|
| Acidithiobacillus ferrooxidans | I | 30–50 | 42 | 31.59 | Wakai et al. (2007) |
| Aquifex aeolicus | I | 58.8 | 5.94 | 46.5 | Marcia et al. (2010b) |
| Oscillatoria limnetica | I | 1.89 | 8 | 1.50 | Arieli et al. (1994) |
| Rhodobacter capsulatus | I | 50–55 | 5 | 41.13 | Griesbeck et al. (2002) |
| Geobacillus stearothermophilus | II | 1 | 3100 | 0.75 | Shibata, Suzuki and Kobayashi (2007) |
| Pseudomonas putida | II | 1.2 | 0.85 | Shibata and Kobayashi (2006) | |
| Arenicola marina | II | 1.5–5.6 | 23 | 3.02 | Theissen and Martin (2008) |
| Homo sapiens | II | 85.06 | 315 | 65 | Jackson, Melideo and Jorns (2012) |
| Schizosaccharomyces pombe | II | 81.6 | 2000 | 52 | Vande Weghe and Ow (1999) |
| Urechis unicintus | II | 1.53 | 40.3 | 1.29 | Ma et al. (2011) |
| Caldivirga maquilingensis | III | 0.8 | 77 | 0.6 | Lencina et al. (2013) |
| Acidianus ambivalens | V | 0.47 | 2 | 0.37 | Brito et al. (2009) |
| Chlorobaculum tepidum CT1087NHis6 | VI | 71 | 1950 | 54 | – |
μmol dUQred mg protein−1 min−1.
μM.
s−1.
A saturation curve for dUQ was attempted, but the low solubility of dUQ prevented an accurate estimation of the kinetic parameters. At the highest concentration of dUQ tested (200 μM), CT1087NHis6 exhibited a specific activity of 130 μmol dUQred mg protein−1 min−1, suggesting a higher Vmax than was estimated in the sulfide saturation curve.
DISCUSSION
The purification and characterization of CT1087NHis6 is the first in vitro analysis of a type VI SQR. Like previously characterized SQRs, purified CT1087NHis6 contained FAD as a cofactor, tightly bound to the enzyme, possibly by a covalent bond to the first conserved cysteine (C120) in the sulfide active site as seen in SQRs from Aquifex aeolicus (Marcia et al. 2009) and Acidianus ambivalens (Brito et al. 2009).
The sulfide saturation curve for CT1087NHis6 best fit the Hill equation with a Hill coefficient of n = 2. The Hill equation was initially developed to model the effect of cooperative binding on hemoglobin function (Hill 1910). When the Hill coefficient is equal to 1, typical Michaelis–Menten kinetics are observed. A Hill coefficient >1 indicates positive cooperativity (Bezeau and Endrenyi 1986; Sun et al. 2014) due to allosteric activation, e.g. Glucokinase (Kamata et al. 2004), or positive cooperativity in multimeric enzymes, e.g. aspartate transcarbamoylase and hemoglobin (Goodey and Benkovic 2008), where binding of substrate to one subunit increases the binding affinity of adjacent subunits. Since SQR has been reported as both homodimers (Brito et al. 2009; Cherney et al. 2010) and homotrimers (Marcia et al. 2009), binding of sulfide to one subunit of an SQR multimer may increase the affinity for sulfide in another subunit. CT1087NHis6 appears to be a monomer as isolated casting doubt on this interpretation. However, the SQR from A. ambivalens also behaved as a monomer in size exclusion chromatography, but the crystal structure indicated a homodimeric structural unit leaving positive cooperativity as possibility (Brito et al. 2009). Clearly, additional structural data will be required to address this question and the protocol for CT1087NHis6 purification outlined here should facilitate this aim.
CT1087NHis6 is only one of three SQRs biochemically characterized to date with a KM for sulfide in the millimolar range (Table 2). These are clearly preliminary parameter estimates given the FAD content of as purified CT1087NHis6. However, we would expect increased Vmax, decreased KM and increased Kcat in a fully occupied enzyme, which would make the values even more extreme than those reported here. Other low-affinity SQRs are from Geobacillus stearothermophilus (Shibata, Suzuki and Kobayashi 2007) and Schizosaccharomyces pombe (Vande Weghe and Ow 1999), which display a KM for sulfide of 3.1 mM and 2.0 mM, respectively. CT1087 contains a valine (V158) at the position of the second SQR active-site cysteine inferred from SQR structures and from site-directed mutagenesis studies with the R. capsulatus enzyme (Griesbeck et al. 2002), which might explain the poor sulfide affinity of CT1087NHis6. However, both the G. stearothermophilus and S. pombe SQRs have cysteine at the equivalent position suggesting that loss of the second conserved cysteine is not the sole controller of sulfide affinity in SQR. Overall, the kinetic parameters of CT1087NHis6 most closely resemble those of the S. pombe enzyme.
We inspected alignments of type VI SQRs to identify candidates that might replace the proposed second active site cysteine in this family (Fig. 5). There is one cysteine residue that is uniquely conserved among type VI SQRs at position 275 in CT1087 (Fig. 5). This residue is clearly a high priority candidate for site directed mutagenesis to understand if it is critical for the observed kinetics and biological role of CT1087.
Figure 5.
Condensed amino acid sequence alignment focusing on predicted active site cysteines for SQR sequences from C. tepidum (CT0117-type IV CT0876-type V, CT1087-type VI) and others that phylogenetically cluster as type VI. Sequences are identified by organism name and strain followed by the locus tag for the gene encoding the SQR. Cysteine residues are indicated by white text on a yellow background. Non-conservative substitutions at Cys residues are indicated by white text on a red background.
In conclusion, the enzymatic kinetics of CT1087NHis6 indicate that CT1087 is a high-sulfide adapted SQR that operates most efficiently in a range from 1 to 5 mM sulfide. The CT1087 gene is required for growth of C. tepidum above 4 mM sulfide and sulfide starts to become inhibitory to growth of wild-type C. tepidum around 6 mM (Chan, Morgan-Kiss and Hanson 2009), which is when CT1087NHis6 specific activity becomes saturated. This work shows that the loss of the second conserved cysteine in the sulfide active site does not affect the ability of CT1087 to oxidize sulfide and calls into question the suggested role of this residue as part of the SQR catalytic site, at least for type VI SQRs. Most organisms that have type VI SQRs also have either a type I (e.g A aeolicus, Magnetococcus marinus and Rhodopseudomonas palustris) or type IV SQR (e.g. Allochromatium vinosum and Chlorobium ferrooxidans). Therefore, we predict that type VI SQRs will likely contribute to high sulfide tolerance in these organisms.
FUNDING
This work was supported by the U.S. National Science Foundation [MCB 0919682 to T.E.H.] and utilized infrastructure resources provided by the National Institutes of Health National Center for Research Resources [2 P20 RR016472-09].
Conflict of interest. None declared.
REFERENCES
- Arieli B, Padan E, Shahak Y. Sulfide-induced sulfide-quinone reductase activity in thylakoids of Oscillatoria limnetica. J Biol Chem. 1991;266:104–11. [PubMed] [Google Scholar]
- Arieli B, Shahak Y, Taglicht D, et al. Purification and characterization of sulfide-quinone reductase, a novel enzyme driving anoxygenic photosynthesis in Oscillatoria limnetica. J Biol Chem. 1994;269:5705–11. [PubMed] [Google Scholar]
- Basu R, Clausen EC, Gaddy JL. Biological conversion of hydrogen sulfide into elemental sulfur. Environ Prog. 1996;15:234–8. [Google Scholar]
- Bezeau M, Endrenyi L. Design of experiments for the precise estimation of dose-response parameters: the Hill equation. J Theor Biol. 1986;123:415–30. doi: 10.1016/s0022-5193(86)80211-9. [DOI] [PubMed] [Google Scholar]
- Brito JA, Sousa FL, Stelter M, et al. Structural and functional insights into sulfide:quinone oxidoreductase. Biochemistry. 2009;48:5613–22. doi: 10.1021/bi9003827. [DOI] [PubMed] [Google Scholar]
- Chan L-K, Morgan-Kiss RM, Hanson TE. Functional analysis of three sulfide:quinone oxidoreductase homologs in Chlorobaculum tepidum. J Bacteriol. 2009;191:1026–34. doi: 10.1128/JB.01154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney MM, Zhang Y, James MNG, et al. Structure-activity characterization of sulfide:quinone oxidoreductase variants. J Struct Biol. 2012;178:319–28. doi: 10.1016/j.jsb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Cherney MM, Zhang Y, Solomonson M, et al. Crystal structure of sulfide:quinone oxidoreductase from Acidithiobacillus ferrooxidans: insights into sulfidotrophic respiration and detoxification. J Mol Biol. 2010;398:292–305. doi: 10.1016/j.jmb.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Chung Y, Huang C, Li C. Removal characteristics of H2S by Thiobacillus novellus ch 3 biofilter in autotrophic and mixotrophic environments. J Environ Sci Heal A. 1997;32:1435–50. [Google Scholar]
- Eddie BJ, Hanson TE. Chlorobaculum tepidum TLS displays a complex transcriptional response to sulfide addition. J Bacteriol. 2013;195:399–408. doi: 10.1128/JB.01342-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JA, Nelson KE, Paulsen IT, et al. The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium. P Natl Acad Sci USA. 2002;99:9509–14. doi: 10.1073/pnas.132181499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodey NM, Benkovic SJ. Allosteric regulation and catalysis emerge via a common route. Nat Chem Biol. 2008;4:474–82. doi: 10.1038/nchembio.98. [DOI] [PubMed] [Google Scholar]
- Gregersen LH, Bryant DA, Frigaard N-U. Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck C, Hauska G. Biological sulfide oxidation: sulfide-quinone reductase (SQR), the primary reaction. Recent Res Dev Microbiol. 2000;4:179–203. [Google Scholar]
- Griesbeck C, Schütz M, Schödl T, et al. Mechanism of sulfide-quinone reductase investigated using site-directed mutagenesis and sulfur analysis. Biochemistry. 2002;41:11552–65. doi: 10.1021/bi026032b. [DOI] [PubMed] [Google Scholar]
- Henshaw P, Zhu W. Biological conversion of hydrogen sulphide to elemental sulphur in a fixed-film continuous flow photo-reactor. Water Res. 2001;35:3605–10. doi: 10.1016/s0043-1354(01)00082-3. [DOI] [PubMed] [Google Scholar]
- Hill A V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol. 1910;40:4–7. [Google Scholar]
- Jackson MR, Melideo SL, Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51:6804–15. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- Kamata K, Mitsuya M, Nishimura T, et al. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. 2004;12:429–38. doi: 10.1016/j.str.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Lencina AM, Ding Z, Schurig-Briccio LA, et al. Characterization of the type III sulfide:quinone oxidoreductase from Caldivirga maquilingensis and its membrane binding. BBA-Bioenergetics. 2013;1827:266–75. doi: 10.1016/j.bbabio.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-B, Zhang Z-F, Shao M-Y, et al. Sulfide:quinone oxidoreductase from echiuran worm Urechis unicinctus. Mar Biotechnol (NY) 2011;13:93–107. doi: 10.1007/s10126-010-9273-3. [DOI] [PubMed] [Google Scholar]
- Marcia M, Ermler U, Peng G, et al. The structure of Aquifex aeolicus sulfide:quinone oxidoreductase, a basis to understand sulfide detoxification and respiration. P Natl Acad Sci. 2009;106:9625–30. doi: 10.1073/pnas.0904165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcia M, Ermler U, Peng G, et al. A new structure-based classification of sulfide:quinone oxidoreductases. Proteins. 2010a;78:1073–83. doi: 10.1002/prot.22665. [DOI] [PubMed] [Google Scholar]
- Marcia M, Langer JD, Parcej D, et al. Characterizing a monotopic membrane enzyme. Biochemical, enzymatic and crystallization studies on Aquifex aeolicus sulfide:quinone oxidoreductase. Biochim Biophys Acta. 2010b;1798:2114–23. doi: 10.1016/j.bbamem.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Yoda M. Removal of hydrogen sulfide from an anaerobic biogas using a bio-scrubber. Water Sci Technol. 1997;36:349–56. [Google Scholar]
- National Research Council Hydrogen Sulfide Baltimore, MD: University Park Press; 1979. 1–9 [Google Scholar]
- Shibata H, Kobayashi S. Characterization of a HMT2-like enzyme for sulfide oxidation from Pseudomonas putida. Can J Microbiol. 2006;52:724–30. doi: 10.1139/w06-022. [DOI] [PubMed] [Google Scholar]
- Shibata H, Suzuki K, Kobayashi S. Menaquinone reduction by an HMT2-like sulfide dehydrogenase from Bacillus stearothermophilus. Can J Microbiol. 2007;53:1091–100. doi: 10.1139/W07-077. [DOI] [PubMed] [Google Scholar]
- Siefert E, Pfennig N. Convenient method to prepare neutral sulfide solution for cultivation of phototrophic sulfur bacteria. Arch Microbiol. 1984;139:100–1. [Google Scholar]
- Sun W, Vallooran JJ, Zabara A, et al. Controlling enzymatic activity and kinetics in swollen mesophases by physical nano-confinement. Nanoscale. 2014;6:6853–9. doi: 10.1039/c4nr01394h. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen U, Martin W. Sulfide: quinone oxidoreductase (SQR) from the lugworm Arenicola marina shows cyanide- and thioredoxin-dependent activity. FEBS J. 2008;275:1131–9. doi: 10.1111/j.1742-4658.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- Vande Weghe JG, Ow DW. A fission yeast gene for mitochondrial sulfide oxidation. J Biol Chem. 1999;274:13250–7. doi: 10.1074/jbc.274.19.13250. [DOI] [PubMed] [Google Scholar]
- Wakai S, Tsujita M, Kikumoto M, et al. Purification and characterization of sulfide:quinone oxidoreductase from an acidophilic iron-oxidizing bacterium, Acidithiobacillus ferrooxidans. Biosci Biotech Bioch. 2007;71:2735–42. doi: 10.1271/bbb.70332. [DOI] [PubMed] [Google Scholar]
- Williams CH., Jr Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase-a family of flavoenzyme transhydrogenases. Chem Biochem Flavoenzymes. 1992;3:121–211. [Google Scholar]
- Zhang Y, Weiner JH. Characterization of the kinetics and electron paramagnetic resonance spectroscopic properties of Acidithiobacillus ferrooxidans sulfide:quinone oxidoreductase (SQR) Arch Biochem Biophys. 2014;564:110–9. doi: 10.1016/j.abb.2014.09.016. [DOI] [PubMed] [Google Scholar]