Abstract
A cDNA encoding a protein of 36 kDa, polymerase delta-interacting protein 1 (PDIP1), that interacts with the small subunit (p50) of DNA polymerase δ (pol δ) was identified in a two-hybrid screen of a HepG2 cDNA library by using p50 as bait. The interaction of PDIP1 with p50 was confirmed by pull-down assays, and a similar assay was used to demonstrate that PDIP1 interacts directly with the proliferating cell nuclear antigen (PCNA). PCNA and p50 bound to PDIP1 simultaneously, and PDIP1 stimulated pol δ activity in vitro in the presence, but not the absence, of PCNA, suggesting that PDIP1 also interacts functionally with both p50 and PCNA. Subcellular localization studies demonstrated that PDIP1 is a nuclear protein that colocalizes with PCNA at replication foci. A putative PCNA-binding motif was identified within the C terminus of PDIP1, and a synthetic peptide containing this PCNA-binding motif was shown to bind PCNA by far-Western analysis. Northern analysis demonstrated that PDIP1 mRNA is present in a wide variety of human tissues. PDIP1 was found to be highly homologous to a previously identified protein, B12 [Wolf, F. W., Marks, R. M., Sarma. V., Byers, M. G., Katz, R. W., Shows, T. B. & Dixit, V. M. (1992) J. Biol. Chem. 267, 1317–1326], one of the early response genes induced by tumor necrosis factor α. PDIP1 synthesis can also be induced by tumor necrosis factor α and by IL-6, cytokines essential for liver regeneration after loss of hepatic tissue. It is suggested that PDIP1 provides a link between cytokine activation and DNA replication in liver as well as in other tissues.
The proliferating cell nuclear antigen (PCNA) is a highly conserved protein that has been identified in all eukaryotes as well as in Archaeobacteria (1, 2). It is a multifunctional protein that participates in a variety of essential cellular processes, including DNA replication, DNA repair, and cell-cycle control, by interacting with proteins involved in these processes. Many of these proteins have been shown to contain a consensus PCNA-binding motif, initially identified in p21 (3), that specifically binds at the interdomain connecting loop of PCNA (4), suggesting that PCNA may coordinate DNA replication with DNA repair as well as with cell-cycle progression by functioning as a regulatory target (5). Although the best understood function of PCNA is its role as a processivity factor for DNA polymerase δ (pol δ) (6, 7), where the PCNA homotrimer encircles DNA and serves as a sliding clamp to tether the DNA polymerase to its template/primer for highly processive DNA synthesis (8), the mechanism by which the two proteins interact is not well understood. We have demonstrated that the processivity of the recombinant 125-kDa catalytic subunit of human pol δ (p125) is not responsive to PCNA (9), whereas coexpression of the catalytic and small (p50) subunits of pol δ results in the formation of a recombinant heterodimer whose processivity is markedly stimulated by PCNA, like the native pol δ heterodimer isolated from calf thymus (p125/p50). This observation suggests that the small subunit is required for functional interaction of pol δ with PCNA, although it is not clear whether PCNA directly interacts with p50 or whether the binding of p50 to p125 leads to interaction of the heterodimer with PCNA (10).
Recently, a third subunit of pol δ (Cdc27) was identified in Schizosaccharomyces pombe (11), by virtue of its interaction with the small subunit of pol δ, Cdc1 (homolog of the mammalian 50-kDa subunit). Cdc27 was subsequently found to bind PCNA by means of a consensus PCNA-binding motif (12), suggesting that it mediates the interaction of the p125/p50 heterodimer with PCNA through its interaction with the 50-kDa subunit. A functional homolog of Cdc27 was also identified in Saccharomyces cerevisiae (Pol32), primarily on the basis of the presence of a PCNA-binding motif highly homologous to that of S. pombe Cdc27 (13). It is believed that, in addition to their roles in mediating the interaction of PCNA and pol δ, both Cdc27 and Pol32 function as dimerization factors for pol δ, a requirement for concurrent replication of the leading and lagging strands at the replication fork (12, 13). A putative third subunit of mammalian pol δ, p66, has been isolated from mouse (14) and calf thymus tissues (15) by affinity chromatography, but it is not yet clear whether mammalian p66 is functionally analogous to Cdc27 and Pol32.
To understand the mechanisms by which DNA replication is regulated during cellular growth and differentiation, it is essential to understand how pol δ interacts with various replication, repair, or cell-cycle proteins at the molecular level. Because p50 has been implicated as a modulator of the interaction between pol δ and PCNA, either directly or indirectly (10), we undertook to identify other proteins that interact with p50 by the yeast two-hybrid system. Here, we report the identification and characterization of a protein that is inducible by the cytokines tumor necrosis factor α (TNF-α) and IL-6 and that specifically interacts with both the 50-kDa subunit of human pol δ and PCNA.
Materials and Methods
Materials.
Yeast two-hybrid vectors pGBT9, pGAD424, and yeast strain Y153 were kindly provided by Stanley Fields (University of Washington, Seattle). A HepG2 cDNA library constructed in pPC86 was from Earl W. Davie (University of Washington). Escherichia coli strain JMB9 was a gift of Erbei Bi (University of Pennsylvania, Philadelphia). MCF7 and HepG2 cells were from the American Type Culture Collection. Fetal bovine thymus pol δ was purified as described (6). Recombinant human p50 and PCNA were purified according to Zhou et al. (10). Restriction endonucleases were from New England Biolabs. T4 DNA ligase, bacterial alkaline phosphatase, the large fragment of DNA polymerase I, FBS, and human IL-6 were from GIBCO/BRL. Polyclonal antibodies to glutathione S-transferase-pol delta-interacting protein 1 (GST-PDIP1) and p50 were raised in rabbits. Human autoantisera to PCNA were a generous gift of Irving Kushner (Cleveland Metropolitan General Hospital). Monoclonal anti-PCNA antibody (PC10), monoclonal anti-BrdUrd antibody (BU33), rhodamine- and FITC-conjugated secondary antibodies, BrdUrd, and cycloheximide were from Sigma. Peroxidase-conjugated secondary antibodies were from Pierce. PDIP1 peptide (SIVYATEKKQTKVEFPEARIFE), mutated PDIP1 peptide (SIVYATEKKQTKAEAPEARIFE), and p21 peptide (GRKRRQTSMTDFYHSKRRLIFS) were synthesized by Research Genetics (Huntsville, AL). [α-32P]dCTP and [3H]dTTP were from ICN. Poly(dA)≈2500 and oligo(dT)12–18 were from Midland Certified Reagents (Midland, TX).
Two-Hybrid Screen and Cloning.
The TRP1 gene (PstI–PvuII fragment) in pGBT9 was replaced with the LEU2 gene (PstI–PvuII fragment) from pGAD424 to produce pGBT9(Leu). The p50 coding sequence, obtained by PCR using pGEX-4T-1-p50 (16) as template, was inserted into pGBT9(Leu) at the BamHI site. This plasmid, designated pGBT9(Leu)-p50, was used as bait to screen a HepG2 cDNA library that was constructed in pPC86 (17). Yeast reporter strain Y153 was transformed simultaneously with pGBT9(Leu)-p50, and the cDNA library and His+ colonies were screened for β-galactosidase activity by using filter lift and liquid culture assays. Plasmids were retrieved from His+, LacZ+ colonies according to Hoffman and Winston (18) and selected in E. coli JMB9 according to Dower et al. (19) and Sambrook et al. (20). To eliminate possible false positives, plasmids containing PDIP1 cDNA, rescued from yeast transformants, were retransformed into yeast reporter strain Y153 either alone or paired with pGBT9(Leu)-p50 or other pGBT9 constructs.
Sequence Analysis.
DNA was sequenced by using dideoxynucleoside triphosphates and Sequenase 2.0 according to the manufacturer's specifications (United States Biochemical). DNA and protein sequence analyses and homology searches were performed with the BLAST program.
Northern Blotting.
Total RNA was isolated from HepG2 cells by using an RNeasy Midi kit (Qiagen), and Northern blotting was performed (20) with randomly 32P-labeled PDIP1 cDNA as probe. A membrane containing RNA from several human tissues (CLONTECH) was also probed for PDIP1 mRNA. PDIP1 mRNA was normalized with β-actin mRNA as standard and quantitated by using National Institutes of Health IMAGE version 1.62 software.
Chromosomal Localization of the PDIP1 Gene.
Fluorescence in situ hybridization (FISH) mapping (21) of the PDIP1 gene was performed by SeeDNA Biotech (Windsor, ON, Canada) using PDIP1 cDNA as probe.
Plasmid Construction and Protein Purification.
The pPC86 plasmid carrying PDIP1 cDNA was partially digested with SalI and NotI, and the 1.6-kb insert was isolated and subcloned into the SalI–NotI site on the expression plasmid pGEX-4T-2 (Amersham Pharmacia). Bacterially expressed GST-PDIP1 fusion protein was purified as described in Smith and Johnson (22) with an additional Q-Sepharose step. GST protein was similarly prepared as a control.
GST Pull-Down Assay.
GST-PDIP1 fusion protein or GST was mixed with 1 μg of p50, 1 μg of PCNA, or both in binding buffer (50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/10% glycerol/1 mM EDTA/0.05% Nonidet P-40/5 mM DTT and protease inhibitors) at 4°C for 2 h. Glutathione-Sepharose 4B beads (20 μl; Amersham Pharmacia) were added, and mixing was continued for 1 h. The beads were washed three times with binding buffer; bound proteins were eluted with SDS gel loading buffer and resolved on SDS/PAGE. Proteins were detected by Western analysis using the appropriate antibodies and Supersignal enhanced chemiluminescent substrate (Pierce).
Far-Western Analysis.
Peptides or proteins were slot blotted onto a poly(vinylidene difluoride) membrane (Bio-Rad), blocked with 5% BSA in 100 mM potassium phosphate buffer, pH 7.5/20 mM Tris/60 mM NaCl and either 0.25% Tween-20 or 0.25% Nonidet P-40. The membrane was incubated overnight at 5°C in the above buffers containing PCNA (3 μg/ml) and washed with the appropriate buffers; PCNA binding was detected with PC10 antibody and Supersignal substrate.
Subcellular Localization and Colocalization.
MCF7 cells were grown on glass coverslips in DMEM/FBS. The coverslips were placed in six-well dishes and immediately processed for indirect immunofluorescence. For single antibody labeling, cells were fixed in 2% formaldehyde for 30 min at room temperature and permeabilized with 90% ethanol/PBS for 30 min at 4°C. If double antibody labeling was used, cells were extracted with 0.1% Triton X-100 before fixation (23). Cells were incubated with primary antibodies for 60 min; after washing, rhodamine- or FITC-conjugated secondary antibodies were added and incubated for 30 min at room temperature. Samples were viewed with a laser scanning confocal microscope (Multiprobe 2001, Molecular Dynamics). To identify replication foci, MCF7 cells were pulse labeled with BrdUrd as described in Bravo and Macdonald-Bravo (24) and processed for indirect immunofluorescence as described above.
DNA Polymerase Assay.
DNA synthesis using poly(dA)/oligo(dT) as template/primer was performed as described (3).
TNF-α and IL-6 Treatment of HepG2 Cells.
HepG2 cells were grown to ≈70% confluence in DMEM with 10% FBS. For TNF-α treatment, cells were first treated with 10 μg/ml cycloheximide for 30 min, then with 20 ng/ml recombinant human TNF-α (R & D Systems) for various times. Total RNA isolation and Northern blotting were as described above. For IL-6 treatment, cells were treated with 500 units/ml human IL-6 for various times and then extracted with SDS gel loading buffer without dye. The amount of total protein was determined by Dc protein assay (Bio-Rad), and equal amounts of protein for each time point were loaded onto SDS gels for Western analysis.
Results
Cloning of the p50-Binding Protein and Chromosomal Localization of the Gene.
To identify proteins that interact with the small subunit of pol δ, a yeast two-hybrid screen of a HepG2 cDNA library with p50 as bait was carried out. Screening of ≈1 × 106 independent transformants resulted in the identification of a 1.6-kb cDNA with an ORF of 1,098 bp followed by ≈500 nucleotides of 3′-untranslated sequence. This cDNA would potentially encode a polypeptide of 329 aa, starting from the first methionine codon, with a calculated molecular mass of 36,395 Da (Fig. 1). The protein was called PDIP1. Homology searches of GenBank databases with the deduced amino acid sequence revealed that PDIP1 shares a high degree of homology (62% identity) with a previously identified protein of 36 kDa, the B12 protein (25), whose function is not known (data not shown). B12 is one of the primary response genes induced by the cytokine TNF-α in vascular endothelial cells. Interestingly, there are putative message destabilization signals in the 3′-untranslated regions of both PDIP1 and B12 mRNAs, i.e., AUUUA surrounded by stretches of A and/or U (26), suggesting that the expression of both mRNAs may be regulated.
Figure 1.
Nucleotide and deduced amino acid sequences of PDIP1 cDNA. The nucleotide (left) and amino acid (right) numberings are indicated. The amino acid sequence begins with the first in-frame methionine. The stop codon is indicated with an asterisk. Two AUUUA mRNA destabilization signals, which begin at nucleotides 1380 and 1583 in the 3′-untranslated region, are underlined, and the polyadenylation signal is in bold. The PCNA-binding motif (amino acids 249–255) is also in bold.
The PDIP1 gene was assigned to human chromosome 16 at 16p11.2 by fluorescence in situ hybridization mapping (data not shown). This location is distinct from that of the B12 gene, which has been mapped to human chromosome 17 at 17q22–q23 (25). Thus, B12 and PDIP1 are products of separate genes. The location of the PDIP1 gene was further established by identification of a PCR-based sequence-tagged site from chromosome 16 that is identical to a cDNA fragment of the PDIP1 gene.
Expression of PDIP1 in Human Tissues.
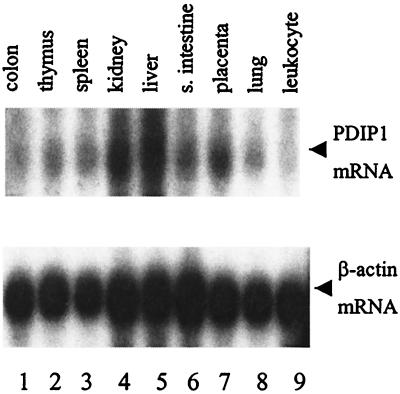
Northern blot analysis of total RNA from HepG2 cells detected a single band of ≈1.8 kb, which included 100–200 nucleotides of poly(A) tail (data not shown), consistent with the size of the isolated PDIP1 cDNA (1.6 kb). This finding suggests that the cloned cDNA is unlikely to be missing substantial 5′ sequence. To determine the tissue distribution of PDIP1, Northern analysis was carried out on blots containing mRNAs isolated from nine different normal human tissues. The results (Fig. 2) show that PDIP1 mRNA is expressed in all of the tissues examined, with the highest levels detected in liver and kidney, i.e., 2- to 3-fold higher than the other tissues examined.
Figure 2.
Analysis of PDIP1 mRNA expression by multiple-tissue Northern blotting. A preblotted membrane with mRNAs from nine different human tissues was probed with a 1.6-kb PDIP1 cDNA, randomly labeled with [α-32P]dCTP as described in Materials and Methods. After stripping, the membrane was reprobed with a 2.0-kb cDNA of a β-actin gene fragment. PDIP1 mRNA levels were normalized to β-actin mRNA and quantitated by using National Institutes of Health IMAGE version 1.62 software.
Interaction of PDIP1 with p50.
To confirm the interaction between PDIP1 and p50 observed in vivo with the yeast two-hybrid system, a recombinant GST-PDIP1 fusion protein was overexpressed in E. coli, purified to apparent homogeneity by a two-step procedure, and tested for its ability to bind recombinant human p50. As shown in Fig. 3, incubation of either GST or GST-PDIP1 with p50, followed by recovery on glutathione-agarose beads, indicated that the GST-PDIP1 fusion protein interacted with p50 in vitro. No interaction was observed between p50 and GST, and, as expected, doubling the amount of the GST-PDIP1 input led to an increased pull-down of recombinant p50. These results suggest that PDIP1 interacts with p50 both in vivo and in vitro.
Figure 3.
Interaction of PDIP1 with p50. The indicated amounts of purified GST or GST-PDIP1 were mixed with 1 μg of p50 and processed as described in Materials and Methods. p50 was detected by Western blotting with rabbit anti-p50 as primary antibody. Lanes 1–3, GST; lanes 4–6, GST-PDIP1.
PDIP1 Interacts with PCNA.
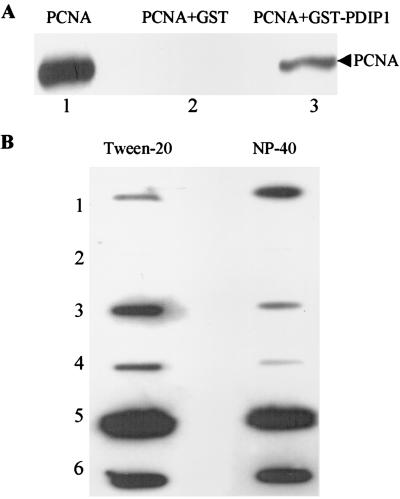
Because p50 is required for functional interaction of PCNA with pol δ, and PDIP1 directly interacts with p50, there is a possibility that PDIP1 might also interact with PCNA. To examine this possibility, we used GST-PDIP1 pull-down assays. As shown in Fig. 4A, when equal amounts of purified GST and GST-PDIP1 were incubated with PCNA, only GST-PDIP1 pulled down PCNA. This suggests that PDIP1 interacts not only with p50 but also with PCNA.
Figure 4.
Interaction of PDIP1 with PCNA. (A) Pull-down assay. GST or GST-PDIP1 was mixed with human PCNA and processed as described in Materials and Methods. PCNA was detected with an anti-PCNA monoclonal antibody (PC10). Lane 1, PCNA control; lane 2, GST + PCNA; lane 3, GST-PDIP1 + PCNA. (B) Far-Western analysis. Peptides or proteins bound to poly(vinylidene difluoride) membranes were overlayed with PCNA as described in Materials and Methods. The binding buffer used for the membrane on the left contained 0.25% Tween-20, and the binding buffer used for the membrane on the right contained 0.25% Nonidet P-40. Slot 1, 30 μg of wild-type PDIP1 peptide; slot 2, 30 μg of mutated PDIP1 peptide; slot 3, 30 μg of wild-type p21 peptide; slot 4, 6 μg of wild-type p21 peptide; slot 5, 500 ng of p21 protein; slot 6, 30 ng of PCNA. Bound PCNA was detected with PC10.
Inspection of the PDIP1 sequence for possible PCNA-binding domains identified a potential PCNA-binding motif (QTKV-EFP) located at amino acids 249–255 at the C terminus of the protein (Fig. 1). However, instead of the canonical 8-aa motif (Q1xxM4xxF7Y8) identified earlier in p21 (3), Met and Tyr are replaced by Val and Pro, respectively, in the 7-aa motif in PDIP1. To determine whether this sequence indeed represents a PCNA-binding motif, far-Western analysis was used. A 22-aa synthetic peptide corresponding to the putative PCNA-binding motif of PDIP1 was synthesized, as well as a double mutant form of the oligopeptide in which Val and Phe were replaced by Ala residues. A peptide representing the PCNA-binding domain of p21 was used as a positive control (27). These peptides, along with p21 and PCNA proteins, were slot blotted to a poly(vinylidene difluoride) membrane, incubated with PCNA, and probed with a monoclonal antibody against PCNA. The results (Fig. 4B) showed that the synthetic PDIP1 peptide did interact with PCNA, but not the double mutant peptide, suggesting that PDIP1 binds to PCNA through this motif and that V4 and/or F7 are critical residues. As expected, p21 protein and the peptide representing the PCNA-binding domain of p21 also interacted with PCNA. It is interesting to note that the relative affinities for PCNA of the p21 oligopeptide and the PDIP1 oligopeptide depend on the nonionic detergent used in the reaction buffer. The binding affinity of p21 peptide to PCNA is greater than that of PDIP1 peptide in the presence of 0.25% Tween-20, whereas the reverse is true in the presence of 0.25% Nonidet P-40, suggesting the possibility that PDIP1 might compete with p21 for binding to PCNA in vivo.
Simultaneous Binding of PDIP1 to p50 and PCNA.
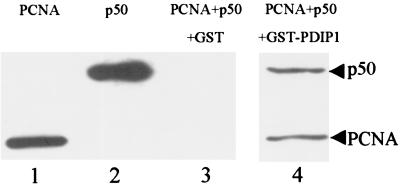
As shown in Fig. 5, when GST-PDIP1 was incubated with PCNA and p50, both proteins were pulled down by GST-PDIP1 fusion protein but not by GST, suggesting that PDIP1 can interact with both PCNA and p50 simultaneously. Furthermore, because p50 and PCNA do not compete for binding to PDIP1 (data not shown), it is likely that PCNA and p50 bind to separate sites on PDIP1. This would suggest that PDIP1 might mediate an interaction between PCNA and the small subunit of pol δ.
Figure 5.
PDIP1 simultaneously binds to PCNA and p50. PCNA and p50 were mixed with GST-PDIP1 or GST and processed as described in Materials and Methods. Lane 1, PCNA control; lane 2, p50 control; lane 3, GST, p50, and PCNA; lane 4, GST-PDIP1, p50, and PCNA. PCNA was detected with PC10 as primary antibody, and p50 was detected with rabbit anti-p50 as primary antibody.
Subcellular Localization of PDIP1 and Its Presence at Replication Foci.
The subcellular distribution of PDIP1 was examined by indirect immunofluorescence and confocal microscopy. When MCF7 cells were fixed and incubated with rabbit antisera to PDIP1, followed by rhodamine-conjugated goat anti-rabbit IgG, PDIP1 was found to be located within nuclei (Fig. 6A). Detergent (0.1% Triton X-100) treatment of cells before fixation and indirect immunofluorescence has been shown to extract nucleoplasmic PCNA and to leave behind only PCNA molecules bound at replication foci, defined by pulse-labeling cells with BrdUrd (23, 24). By the use of similar techniques, we found that PCNA is bound at replication foci in S-phase MCF-7 cells (data not shown) and that PDIP1 colocalizes with PCNA at replication foci (Fig. 6B). These results are consistent with a role for PDIP1 in the process of DNA replication.
Figure 6.
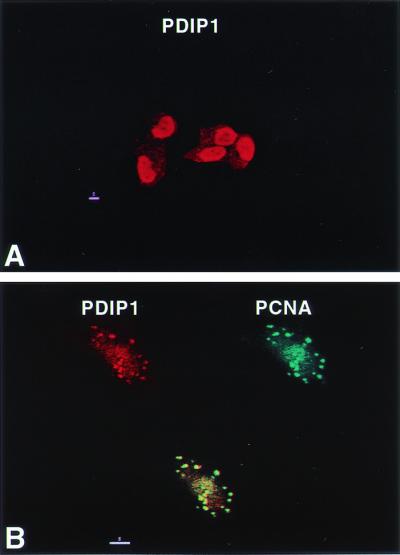
Nuclear localization of PDIP1 and colocalization with PCNA at replication foci. (A) MCF7 cells were grown on glass coverslips, fixed, and permeabilized as described in Materials and Methods. The cells were then incubated with anti-PDIP1 antibody for 1 h, washed, incubated with rhodamine-conjugated goat anti-rabbit IgG for 30 min, and visualized by confocal microscopy. (B) MCF7 cells grown on glass coverslips were extracted with 0.1% Triton X-100 before fixation. Primary antibodies were rabbit anti-PIDP1 and human anti-PCNA; secondary antibodies were rhodamine-conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-human IgG. Red, PDIP1; green, PCNA; yellow, merged image.
Recombinant PDIP1 Stimulates PCNA-Dependent pol δ Activity.
As an initial attempt to understand the role of PDIP1 in DNA synthesis, the effects of PDIP1 on the DNA polymerase activity of pol δ were examined both in the presence and in the absence of PCNA (Fig. 7). In the absence of PCNA, GST-PDIP1 had little or no effect on the activity of pol δ. However, in the presence of PCNA, GST-PDIP1 stimulated the activity 2- to 3-fold. GST had no effect either in the presence or in the absence of PCNA. These results, along with the results of the binding studies, suggest that PDIP1 might modulate the interaction of PCNA with pol δ.
Figure 7.
PDIP1 stimulates PCNA-dependent pol δ activity. BSA, GST, or GST-PDIP1 was preincubated with 0.3 unit of calf thymus pol δ at 4°C for 30 min, then assayed for DNA polymerase activity as described in Materials and Methods. Gray bars, activity in the absence of PCNA; black bars, activity in the presence of PCNA.
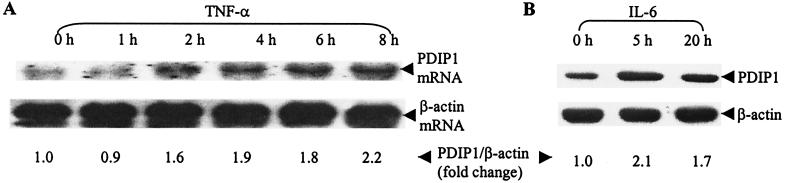
Induction of PDIP1 by TNF-α and IL-6.
PDIP1 shares a high degree of amino acid sequence homology with the B12 protein, a member of a family of TNF-α-induced early response genes (25). Moreover, PDIP1 was obtained by screening a cDNA library constructed from hepatocytes that had been primed to proliferate by IL-6, a mediator of TNF-α activation. To determine whether the synthesis of PDIP1 is induced by these cytokines, we measured PDIP1 mRNA and protein levels in HepG2 cells as a function of time after cytokine administration. As shown in Fig. 8A, PDIP1 mRNA levels, as determined by Northern blotting, were increased 1.6-fold at 2 h and 2.2-fold after 8 h of incubation with TNF-α. This is similar to the degree of induction of B12 mRNA by TNF-α (25). PDIP1 protein levels were found to be increased 2.1-fold 5 h after the addition of IL-6 to HepG2 cells (Fig. 8B), suggesting that the stimulation of PDIP1 synthesis by TNF-α is mediated via an IL-6 pathway.
Figure 8.
Induction of PDIP1 expression in HepG2 cells by TNF-α and IL-6. (A) HepG2 cells were treated with 20 ng/ml TNF-α (in the presence of 10 μg/ml cycloheximide) for the indicated times. Total RNA was isolated and subjected to Northern blotting as described in Materials and Methods and in the legend of Fig. 2. Thirty micrograms of total RNA was loaded for each lane. (B) HepG2 cells were treated with recombinant human IL-6 at a final concentration of 500 units/ml for the indicated times. Cells were lysed, and 30 μg of total protein from each sample was loaded for SDS/PAGE followed by Western blotting. PDIP1 and β-actin were detected with a rabbit anti-PDIP1 and a monoclonal antibody to β-actin, respectively. Protein and mRNA levels were normalized by using β-actin as standard and quantitated by using National Institutes of Health IMAGE version 1.62 software. The fold change in PDIP1 mRNA and protein levels relative to those of β-actin are given at each time point.
Discussion
In the present study, we have identified a human protein, PDIP1, that interacts with the small subunit of pol δ. This interaction was initially detected in a yeast two-hybrid screen and confirmed by GST-pull-down experiments. It was further demonstrated that PDIP1 interacts with PCNA and that PDIP1 can simultaneously bind both p50 and PCNA. In addition to physical interactions, PDIP1 also functionally interacts with these two proteins, as evidenced by the stimulation of pol δ activity by PDIP1 in the presence, but not in the absence, of PCNA. PDIP1 was shown to be a nuclear protein by indirect immunofluorescence and to colocalize at replication foci with PCNA, suggesting a role for the protein in DNA replication.
Similar to S. pombe Cdc27, the third subunit of pol δ (11, 12), PDIP1 binds simultaneously to p50 and PCNA and, like Pol32 of S. cerevisiae (13), interacts functionally with both p50 and PCNA. However, PDIP1 shares only 20% identity with the mouse homolog of the pol δ third subunit, p66 (14), and does not appear to be an integral component of pol δ because it does not copurify with the enzyme from calf thymus (unpublished observation). Thus, PDIP1 is unlikely to be a homolog of Cdc27.
The binding of Cdc27 to PCNA is mediated through a consensus PCNA-binding motif identified at the C terminus of Cdc27 (12). We have also identified a PCNA-binding motif (QTKV-EFP) at amino acids 249–255 in the C-terminal region of PDIP1. This motif is similar but not identical to the consensus PCNA-binding motif [Q1xx(M/I/L)4xx(F/H)7(Y/F)8] originally identified in p21 and subsequently in numerous PCNA-binding proteins (1–3). The presence of Val at the fourth position of the PDIP1 motif represents a conservative substitution that has recently been identified in a consensus PCNA-binding motif by screening of a random peptide library for PCNA-binding sequences (28). Pro at the eighth position has not been seen in other PCNA-binding motifs; however, the hydrophobicity of proline is very similar to that of Met (−0.99 vs. −0.96) (29), and Met has been shown to substitute for Phe in the seventh position in Xenopus DNA methyltransferase (30). It is interesting to note that the PDIP1 PCNA-binding motif is very similar to the sequence (QARL-PF) identified in the 31-aa PCNA-binding site of the large subunit of human CAF-1, which also contains a Pro but at the seventh position of the consensus PCNA-binding motif (31). The relevance of this similarity is not apparent at present.
The precise role of PDIP1 in DNA replication/repair is not known at present. Because it can interact with p50 and PCNA simultaneously and stimulates pol δ activity only in the presence of PCNA, PDIP1 could function to modulate the interaction of PCNA with pol δ, similar to the postulated role of the third subunit of pol δ, but in a different context. For example, whereas the binding of an oligopeptide containing the Cdc27 PCNA-binding motif to PCNA is readily inhibited by competition with an oligopeptide containing the p21 PCNA-binding motif (12), the PDIP1 oligopeptide can bind PCNA more tightly than the p21 oligopeptide under certain in vitro conditions. This finding suggests the possibility that p21 may not compete with PDIP1 for binding to PCNA in vivo, i.e., PDIP1 might be involved in DNA replication or repair when high levels of p21 are present in the cell (e.g., in the presence of DNA damage).
In addition to PDIP1, at least two other proteins have been shown to interact with the small subunit of pol δ. These include the third subunit of pol δ (S. pombe Cdc27, S. cerevisiae Pol32, mammalian p66) and the Werner syndrome protein (WRN) (32). Interestingly, all three proteins also interact with PCNA. These findings suggest that the small subunit may function as an adaptor through which various replication proteins interact with pol δ, as previously proposed (10). A similar function for the small subunit of pol δ has also recently been suggested (33).
TNF-α was originally identified as a mediator of necrosis of certain tumor cells and has now been shown to have a wide array of biological activities, such as apoptosis, proliferation, B cell activation, and some inflammatory responses (34). Probably the best-studied pathway for the induction of cell proliferation by TNF-α is liver regeneration after partial hepatectomy (35). It has been shown that the activation of hepatocyte proliferation by TNF-α is mediated through an IL-6-dependent pathway, leading to induction of the transcription factor Stat3 and ultimately to DNA synthesis (36). It was not entirely unanticipated that PDIP1 could be induced by TNF-α and IL-6, as it was identified in a screen of a cDNA library constructed from hepatocytes that had been primed to proliferate by IL-6. However, PDIP1 is not restricted to hepatic tissues, as we have detected its mRNA in all human tissues examined thus far.
Our findings that PDIP1 is inducible by TNF-α and IL-6, that it physically and functionally interacts with both the small subunit of pol δ and PCNA, and that it colocalizes with these proteins at replication foci, when taken together, suggest that PDIP1 may play an important role in the regulation of DNA replication by TNF-α and IL-6. They further suggest that PDIP1 is a distal target of TNF-α activation and a link between cytokine activation and DNA replication.
The B12 protein, which shares 62% sequence identity with PDIP1, is induced by TNF-α in endothelial cells (25). The presence of the PDIP1 PCNA-binding motif (QTKV-EFP) in the B12 protein (amino acids 268–274) implies that it also interacts with PCNA and suggests the intriguing possibility of a conserved TNF-α-induced regulatory mechanism mediated through a family of proteins interacting with PCNA.
Acknowledgments
We gratefully acknowledge the assistance of Dr. Lilly Bourguignon and Mr. Youwei Chen, Department of Cell Biology and Anatomy, University of Miami, in indirect immunofluorescence and confocal microscopy. This work was supported by National Institutes of Health Grant DK26206 and in part by funds from the Sylvester Comprehensive Cancer Center.
Abbreviations
- pol δ
polymerase δ
- PCNA
proliferating cell nuclear antigen
- TNF-α
tumor necrosis factor α
- GST
glutathione S-transferase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF401315).
References
- 1.Warbrick E. BioEssays. 2000;22:997–1006. doi: 10.1002/1521-1878(200011)22:11<997::AID-BIES6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Tsurimoto T. Front Biosci. 1999;4:D849–D858. doi: 10.2741/tsurimoto. [DOI] [PubMed] [Google Scholar]
- 3.Warbrick E. BioEssays. 1998;20:195–199. doi: 10.1002/(SICI)1521-1878(199803)20:3<195::AID-BIES2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Gulbis J M, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- 5.Waga S, Stillman B. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 6.Tan C-K, Castillo C, So A G, Downey K M. J Biol Chem. 1986;261:12310–12316. [PubMed] [Google Scholar]
- 7.Prelich G, Tan C-K, Kostura M, Mathews M B, So A G, Downey K M, Stillman B. Nature (London) 1987;326:517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- 8.Krishna T S, Kong X P, Gary S, Burgers P M J, Kuriyan J. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J-Q, Tan C-K, So A G, Downey K M. J Biol Chem. 1996;271:29740–29745. doi: 10.1074/jbc.271.47.29740. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J-Q, He H, Tan C-K, Downey K M, So A G. Nucleic Acids Res. 1997;25:1094–1099. doi: 10.1093/nar/25.6.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacNeill S A, Moreno S, Reynolds N, Nurse P, Fantes P A. EMBO J. 1996;15:4613–4628. [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds N, Warbrick E, Fantes A, MacNeill S. EMBO J. 2000;19:1108–1118. doi: 10.1093/emboj/19.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerik K J, Li X, Pautz A, Burgers P M. J Biol Chem. 1998;273:19747–19755. doi: 10.1074/jbc.273.31.19747. [DOI] [PubMed] [Google Scholar]
- 14.Hughes P, Tratner I, Ducoux M, Piard K, Baldacci G. Nucleic Acids Res. 1999;27:2108–2114. doi: 10.1093/nar/27.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo J, Liu L, Leon A, Mazloum N, Lee M Y. Biochemistry. 2000;39:7245–7254. doi: 10.1021/bi0000871. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Tan C-K, Mcmullen B, Downey K M, Davie E W, So A. Genomics. 1995;29:179–186. doi: 10.1006/geno.1995.1229. [DOI] [PubMed] [Google Scholar]
- 17.Chevray P M, Nathans D. Proc Natl Acad Sci USA. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman C S, Winston F A. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 19.Dower W J, Miller J F, Ragsdale C W. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 21.Heng H H, Tsui L C. Methods Mol Biol. 1994;33:35–49. doi: 10.1385/0-89603-280-9:35. [DOI] [PubMed] [Google Scholar]
- 22.Smith D B, Johnson K S. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Hannon G J, Beach D, Stillman B. Curr Biol. 1996;6:189–199. doi: 10.1016/s0960-9822(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 24.Bravo R, Macdonald-Bravo H. J Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf F W, Marks R M, Sarma V, Byers M G, Katz R W, Shows T B, Dixit V M. J Biol Chem. 1992;267:1317–1326. [PubMed] [Google Scholar]
- 26.Chen C-Y, Shyu A B. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 27.Warbrick E, Lane D P, Glover D M, Cox L S. Curr Biol. 1995;5:275–282. doi: 10.1016/s0960-9822(95)00058-3. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Zhang P, Liu L, Lee M Y W T. Biochemistry. 2001;40:4512–4520. doi: 10.1021/bi010103+. [DOI] [PubMed] [Google Scholar]
- 29.Creighton T E. Protein. 2nd Ed. San Francisco: Freeman; 1993. pp. 153–154. [Google Scholar]
- 30.Warbrick E, Heatherington W, Lane D P, Glover D M. Nucleic Acids Res. 1998;26:3925–3932. doi: 10.1093/nar/26.17.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moggs J, Grandi P, Quivy J-P, Jonsson Z O, Hubscher U, Becker P B, Almouzni G. Mol Cell Biol. 2000;20:1206–1218. doi: 10.1128/mcb.20.4.1206-1218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szekely A M, Chen Y-H, Zhang C, Oshima J, Weissman S M. Proc Natl Acad Sci USA. 2000;97:11365–11370. doi: 10.1073/pnas.97.21.11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Patel A H, Zhou S, Linn S. J Biol Chem. 1997;272:32337–32344. doi: 10.1074/jbc.272.51.32337. [DOI] [PubMed] [Google Scholar]
- 34.Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E, Denecker G, Depuydt B, De Valck D, De Wilde G, et al. J Inflamm. 1995;47:67–75. [PubMed] [Google Scholar]
- 35.Fausto N. J Hepatol. 2000;32, Suppl. 1:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 36.Cressman D E, Greenbaum L E, DeAngelis R A, Ciliberto G, Furtly E E, Poli B, Taub R. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]