Abstract
Despite the similarities observed between the fragile X syndrome (FXS) and autism spectrum disorder (ASD) phenotypes, few studies have compared their behavioral profiles outside of ASD symptomatology. In the present study, we sought to compare lexical and grammatical abilities in these two conditions. Comparisons of language abilities in both of these conditions are particularly interesting because both conditions are characterized by difficulties navigating social interactions. Results suggest that although both FXS and ASD are associated with language difficulties, there are important differences between the two conditions in terms of the language profiles observed and the factors influencing language when considering children of similar developmental levels. Theoretical implications are discussed.
Keywords: Fragile X syndrome, Autism spectrum disorder, Language
Introduction
Language facilitates an individual’s ability to effectively socialize with other people (Homer and Tamis-LeMonda 2005) and to organize information, sharpen categorical boundaries, and efficiently access and use information from others (e.g., Donald 1991; Vygotsky 1986; Waxman and Markow 1995). More generally, language skills support learning and psychosocial outcomes across a variety of domains (e.g., Venter et al. 1992). Language, however, is not a unitary ability, but rather a set of interrelated component abilities with the development of each influenced by different factors. This is particularly apparent when considering children with neurodevelopmental disorders, who can demonstrate different degrees of impairment across components. Thus, research on language development in children with neurodevelopmental disorders affords an opportunity to understand how language is modified by genetic and environmental factors. Furthermore, such research facilitates the development of language interventions for these children.
ASD is a multifactorial behaviorally defined disorder (Ronald and Hoekstra 2011) that is heterogeneous in etiology (American Psychiatric Association 2013). In contrast, FXS, the most common inherited form of intellectual disability, results from the silencing of the FMR1 gene, which controls production of fragile X mental retardation protein (FMRP; Bassell and Warren 2008). Despite these etiological differences, there are many similarities between these disorders.
Direct comparisons of individuals with FXS+ASD and individuals with ASD suggest comparable behavioral symptoms. For example, similar weaknesses in pragmatic language, including odd/limited gesture use, difficulties following the rules governing reciprocal communication, atypical rate/intonation, and atypical gaze and facial expressions are seen in boys with FXS+ASD and boys with ASD (e.g., Klusek et al. 2014; McDuffie et al. 2015). In terms of neurobiology, abnormalities in the GABAergic signaling system have been implicated in both FXS and ASD (Coghlan et al. 2012). Furthermore, the actions of many of the ASD susceptibility genes identified to date are controlled by FMRP, further reinforcing links between FXS and ASD (Iossifov et al. 2012). In fact, symptoms of ASD are frequently observed in individuals with FXS, with as many as 60% of males warranting a comorbid diagnosis of ASD, making FXS the most common genetic cause of ASD (e.g., Bailey et al. 1998; Clifford et al. 2007; Demark et al. 2003; Hall et al. 2010; Harris et al. 2008). In contrast, FXS accounts for only 2–6% of all cases of ASD (e.g., Cohen et al. 2005; Geschwind 2011; Betancur 2011).
Important differences, however, have been noted between the two disorders at both the symptom level and in the factors associated with similar symptomatology (Abbeduto et al. 2014). For example, on average, the severity of ASD symptomatology is milder in FXS than in ASD, even for children with FXS who meet diagnostic criteria for ASD (e.g., McDuffie et al. 2015; Wolff et al. 2012). Moreover, there is evidence to suggest that the developmental trajectory of ASD symptomatology is different in FXS and nonsyndromic ASD (Thurman et al. 2015a). These data suggest there may be differences in the paths by which similar symptoms develop across the two conditions.
It is important to note, however, that little is known about the similarities and differences in aspects of these phenotypes outside of the behavioral symptoms core to the ASD diagnosis. Empirical research focused on the similarities and differences across these two conditions in other domains of functioning, particularly those that are detrimental to adaptive functioning, is important to clarify the nature of the relationship between these two conditions so as to provide insights into the complex developmental mechanisms leading to their phenotypic weaknesses.
Structural language is frequently noted as problematic in FXS (Abbeduto and Hagerman 1997) and, although no longer part of the criteria for diagnosis in ASD, there is evidence of structural language difficulties in individuals with ASD (e.g., Kjelgaard and Tager-Flusberg 2001). In fact, delays in vocabulary acquisition are often the first recognized symptom for young children with ASD (e.g., Tager-Flusberg et al. 2009) and language impairment remains a specifier for the ASD diagnosis. A comparison of language abilities in ASD and FXS is important given that both conditions are characterized by difficulties navigating reciprocal social interactions, a developmental pattern that can be expected to negatively influence the ways in which language is learned and used. The information gained from this cross-condition comparison will clarify the nature of language difficulties observed in individuals with these disorders and provide insight into the vulnerabilities associated with language development more generally (Rice et al. 2005). Moreover, this line of research can elucidate the role of FMRP in language development and identify shared behavioral targets for potential shared psychopharmacological and behavioral interventions in FXS and ASD. In the present study, we focus on vocabulary and grammar when considering language in these two conditions.
Fragile X Syndrome
Many aspects of the FXS phenotype can negatively affect language, such as intellectual disability and behavioral challenges (e.g., Cornish et al. 2007; Cordeiro et al. 2011; Kau et al. 2000; Harris et al. 2008; Hessl et al. 2009; Scerif et al. 2012). Not surprisingly, language difficulties, relative to chronological age and/or nonverbal cognitive ability, are common in males with FXS (Abbeduto et al. 2007).
Receptive Language
Males with FXS demonstrate receptive language skills well below chronological age expectations (Madison et al. 1986; Paul et al. 1987; Sudhalter et al. 1992). It is not clear, however, whether receptive lexical knowledge is comparable to, exceeds, or lags behind nonverbal cognitive achievements. Indeed, findings have been inconsistent across studies likely due to differences in sample characteristics (e.g., age range) and the approach used to assess and quantify lexical knowledge, as well as the fact that some studies combined data from males and females (Abbeduto et al. 2003; Madison 1986; Price et al. 2007; Roberts et al. 2007; Sudhalter et al. 1992). In addition, results of some studies were based on the use of age-equivalent scores, which have poor psychometric properties (i.e., these scores do not actually reflect an equal-interval scale), and therefore must be interpreted cautiously (e.g., Abbeduto et al. 2003; Price et al. 2007).
In terms of receptive grammar, some studies of males with FXS have suggested that level of receptive grammar is consistent with nonverbal cognitive ability (Abbeduto et al. 2003; Paul et al. 1987), whereas other studies (e.g., Oakes et al. 2013; Price et al. 2007; Roberts et al. 2001) have found below-MA levels of receptive grammar. Again, these inconsistencies are likely the result of sample and methodological differences.
Expressive Language
The expressive language skills of males with FXS, as a group, are delayed relative to chronological age expectations (e.g., Madison et al. 1986; Sudhalter et al. 1992). Although expressive language has been found to be more impaired than receptive language (Roberts et al. 2001), it is unclear whether this asymmetry characterizes both vocabulary and grammar.
Findings are inconsistent for the lexical domain. Some early studies reported higher scores on expressive than on receptive measures of vocabulary for males with FXS (Madison et al. 1986; Sudhalter et al. 1991), although Paul et al. (1987) found no differences between these domains in a sample of adult males with FXS who were institutionalized. Differences across studies in the types of words included on the assessments, the ways in which lexical knowledge was measured, and participant characteristics likely contributed to these differing results. More recently, Roberts et al. (2007) found that boys with FXS produced fewer different words relative to a cognitively matched group of typically developing boys, indicating delays relative to nonverbal cognitive level.
Early studies of expressive grammar have been inconsistent with regard to whether achievements are delayed relative to nonverbal cognitive-level expectations in FXS (e.g., Paul et al. 1984; Ferrier et al. 1991). More recently, however, several studies have reported delays in expressive grammatical ability beyond that expected for nonverbal mental age in boys with FXS (e.g., Estigarribia et al. 2011; Price et al. 2008; Roberts et al. 2007).
Autism Spectrum Disorder
There is an abundance of research documenting qualitative difficulties in social-communication in ASD. In comparison, research on lexical and grammatical abilities, particularly that which considers the ASD population as a whole, is more limited. Evidence suggests that the majority of individuals with ASD display language difficulties relative to CA- and/or nonverbal cognitive level expectations (e.g., Boucher 2012; Klinger et al. 2002). In fact, delays in language are often the first recognized symptom of developmental difficulty for children with ASD (and an estimated 25% of individuals with ASD develop little to no functional language; e.g., Boucher 2012; Klinger et al. 2002; Tager-Flusberg et al. 2009). Because childhood structural language abilities are a strong predictor of long-term outcomes (e.g., Szatmari et al. 2009; Tager-Flusberg et al. 2005), elucidating the full range of language difficulties in individuals with ASD is important.
Receptive Language
Because of the large variation in language outcomes in individuals with ASD, a single specific ASD structural language profile has not been identified; however, many children with ASD demonstrate receptive language difficulties (Boucher 2012). For example, Kjelgaard and Tager-Flusberg (2001) reported that in their sample of children with ASD between 4 and 14 years (n = 89), 26.8% obtained receptive vocabulary scores within the normal range (standard scores ≥85), 12.2% obtained scores in the borderline range (standard scores 70–84), and 61% obtained scores in the language difficulty range (standard scores <70). Kjelgaard and Tager-Flusberg also found that receptive vocabulary was strongly correlated with IQ.
Kjelgaard and Tager-Flusberg (2001) also found that standard scores indexing receptive vocabulary abilities (PPVT M = 85.57) tended to be higher than standard scores indexing knowledge of syntax and semantics (CELF-Receptive M = 70.89). That being said, the authors pointed out that their inclusion criteria restricted their sample to participants who were able to complete their measures of syntax and semantics, thereby resulting in a substantially higher functioning group of children relative to the ASD population. Furthermore, these findings contrast with those of Jarrold et al. (1997), who reported comparable vocabulary and grammatical levels in children with ASD.
Expressive Language
Although expressive language skills have often been described as more advanced than comprehension skills in ASD (Ellis et al. 2010; Luyster et al. 2008; Mitchell et al. 2006), some studies have failed to confirm this difference (e.g., Jarrold et al. 1997). Importantly, most studies finding a profile of expressive exceeding receptive language have utilized age-equivalent scores, which, due to their poor psychometric properties (i.e., these scores do not actually reflect an equal-interval scale), complicate interpretation of results. In addition, delayed echolalia and stereotyped utterances may lead to an over estimation of expressive language ability. Finally, test scores indicating an expressive advantage may reflect a measurement artifact rather than a difference in the true levels of receptive and expressive ability (e.g., Jarrold et al. 1997).
In fact, Kjelgaard and Tager-Flusberg (2001), utilizing standard scores on co-normed measures, did not find a receptive-expressive difference in vocabulary. Except for a single-word naming subtest, these authors also found no expressive-receptive difference for CELF subtests tapping higher order knowledge of semantics and syntax that involve integration across language domains and require significant working memory capacity.
Kover et al. (2013) utilized a cross-sectional developmental trajectories approach (Thomas et al. 2009) to provide a more nuanced understanding of the association between receptive and expressive vocabulary in relation to nonverbal cognition for a group of 4- to 11-year-olds with ASD compared to a younger group of typically developing children (Kover et al. 2013). Kover et al. first found, at both the individual and group levels, no significant difference in receptive and expressive vocabulary scores when using co-normed measures. There was, however, a reduced likelihood for boys with ASD to demonstrated an advantage in receptive relative to expressive vocabulary in comparison to TD boys (Kover et al. 2013), even when controlling for nonverbal cognitive ability. Follow-up analyses indicated that, although receptive vocabulary abilities increased more slowly than did expressive vocabulary, this discrepancy was not observed when nonverbal cognitive levels were controlled. Taken together, this study failed to document a greater receptive than expressive language difficulty, but did find that development of receptive language in ASD differs in some ways from that of TD children.
Fragile X Syndrome Compared to Autism Spectrum Disorder
To date, little is known about how the profiles of lexical and grammatical abilities compare across FXS and ASD. Recently, McDuffie et al. (2013) reported that, after controlling for levels of nonverbal cognition, boys with FXS demonstrated significantly better receptive and expressive vocabularies than did boys with ASD between 4 and 10 years of age. It is important to note, however, that McDuffie et al. did not take into account between-group differences in ASD symptom severity; thus, the between-group differences in lexical ability could potentially be attributed to a less severe presentation of ASD symptomatology, on average, for males with FXS.
Present Study
In the present study, we sought to expand our understanding of the FXS and ASD phenotypes by exploring the similarities and differences in the lexical and grammatical skills of boys with FXS and boys with ASD, skills that have a significant impact on long-term outcomes. The aims of the study were:
To determine whether lexical and grammatical abilities are equally impaired in males with FXS and males with ASD after controlling for age, NVIQ, and overall ASD symptom severity. We hypothesized that there are potential between-group differences in the lexical and/or grammatical abilities of boys with FXS or ASD.
To determine whether the predictors of lexical and grammatical abilities are similar for males with FXS and males with nonsyndromic ASD. Numerous developmental factors have been shown to influence language development, including both social affective and restricted and repetitive behavior symptomatology of ASD (e.g., Thurman et al. 2015a, b). We hypothesized that there may be differences in the developmental factors predicting language abilities between these two conditions.
Method
Participants
Participants were males with FXS and males with nonsyndromic ASD who were drawn from a larger longitudinal study examining word learning. Several publications (e.g., Benjamin et al. 2015; McDuffie et al. 2013, 2015; Thurman et al. 2015a, b) have resulted from this study, but none have addressed the research questions addressed in this paper. Participants were recruited nationally and were assessed at one of two university sites (University of California, Davis and University of Wisconsin, Madison). The following inclusion criteria, based on parent report, were utilized in the larger project: (a) native English speaker; (b) able to comply with simple instructions (e.g., “Give me the ball”); (c) speech is the primary means of communication; (d) production of approximately 10 different words spontaneously within the prior month; (e) no sensory or physical impairments that would limit participation in project activities; and (f) lives at home with biological mother who is a fluent English speaker. In addition, participants were tested by project staff and found to have a pure tone, air conduction threshold of 30 dB HL or better in each ear (averaged across 500, 1000, and 2000 Hz). Approval from the Institutional Review Boards of the participating university sites, as well as parental informed consent, was obtained.
Documentation of a diagnosis of FMR1 full mutation (i.e., >200 CGG repeats, with our without mosaicism) was obtained for all participants with FXS. For participants with nonsyndromic ASD, the community diagnosis was confirmed through administration of both the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2007) and the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al. 2003) using the caseness criteria proposed by Risi and colleagues (Risi et al. 2006). Using these criteria, an ASD diagnosis was considered verified if the participant received an ADOS calibrated severity score of at least 4, and met one of the three following benchmarks on the ADIR: (1) met the autism cutoff for the ADI-R Social Reciprocity Domain and either the Communication or Repetitive Behavior domains; (2) came within one point of the cutoffs for both Social Reciprocity and Communication Domains; or (3) met the autism cutoff on either the Social Reciprocity or Communication domains and came within two points of the cut-off on the other domain. Project staff who administered both the ADOS and the ADI-R had completed research reliability training.
In addition, participants with nonsyndromic ASD: (1) entered the study with an existing community diagnosis of ASD; (2) had parent-provided documentation of prior genetic testing ruling out FXS; and (3) had other obvious syndromic causes of ASD (e.g., Rett syndrome, tuberous sclerosis) ruled out via dysmorphology and neurological examinations conducted by a project physician.
Participant Selection for the Current Study
The samples included in the present study overlap with those of other previously published studies (Benjamin et al. 2015; McDuffie et al. 2013, 2015; Thurman et al. 2015a, b). Consideration for inclusion in the present study required participants with FXS or nonsyndromic ASD to have a nonverbal IQ score less than or equal to 85, as this cut-off is inclusive of essentially all males with FXS (Hessl et al. 2009), resulting in the exclusion of 6 children with FXS (3 IQ data not available, 3 IQ scores > 85) and 20 children with nonsyndromic ASD (3 IQ data not available and 17 IQ scores > 85).
These inclusionary and exclusionary criteria resulted in the final sample of 51 males with FXS and 36 males with nonsyndromic ASD. Descriptive statistics for the samples are presented in Table 1. The groups were matched on chronological age (U = 895.00, z = −0.20, p = .84, r = .02, s2ratio = 1.16, range 4.02–10.99). Of the 51 males with FXS, 39 participants met study criteria (described previously) for a comorbid diagnosis of ASD. With regard to the 12 participants with FXS who did not meet research criteria for a diagnosis of ASD, two participants did not meet criteria on either diagnostic instrument (ADOS or ADI-R), six participants met criteria on only one diagnostic instrument (4 ADI-R only, 2 ADOS only), and four participant met criteria on the ADOS but were missing ADI-R data. The group with FXS and the group with nonsyndromic ASD differed significantly in terms of nonverbal IQ (U = 1193.00, z = 2.37, p = 0.01, r = 0.25) and overall ASD symptom severity score (U = 1429, z = 4.47, p < 0.001, r = 0.48). For participants with FXS, the racial/ethnic composition of the sample was 82% Caucasian, 8% Hispanic, 4% Asian, 2% African-American, and 4% Native American. For the participants with nonsyndromic ASD, the racial/ethnic composition of the sample was 67% Caucasian, 11% Hispanic, 11% African-American, 8% Asian, and 3% Native American.
Table 1.
Descriptive statistics (Mean, SD, and Range) for participant groups
| FXS all participants (n = 51) | FXS + ASD only (n = 39) | Nonsyndromic ASD (n = 36) | |
|---|---|---|---|
| Chronological age | 7.52 (2.02; 4.06–10.63) | 7.55 (2.08; 4.06–10.63) | 7.43 (1.87; 4.02–10.99) |
| Nonverbal IQ SS | 58.63 (13.51; 36–83) | 56.38 (13.20; 36–83) | 65.69 (12.32; 40–85) |
| Receptive vocabulary SS | 67.92 (15.92; 25–99) | 65.54 (15.92; 25–99) | 60.81 (21.22; 20–99) |
| Expressive vocabulary SS | 66.26 (16.25; 20–103) | 63.55 (16.25; 20–103) | 61.40 (23.56; 20–96) |
| Receptive grammar SS | 59.84 (7.69; 55–83) | 60.13 (8.32; 55–83) | 60.09 (10.44; 55–99) |
| Expressive grammar SS | 55.56 (11.07; 40–81) | 53.92 (10.49; 40–81) | 56.50 (14.19; 40–89) |
| Overall autism symptom severity | 6.18 (2.09; 2–10) | 6.56 (1.70; 4–10) | 8.17 (1.54; 4–10) |
| Social Affective Symptom Severity | 5.92 (2.30; 2–10) | 6.23 (2.10; 2–10) | 7.81 (1.69; 3–10) |
| Restricted and repetitive behavior symptom severity | 7.18 (1.72; 1–10) | 7.44 (1.48; 5–10) | 8.31 (1.39; 5–10) |
Assessment Measures
The Leiter International Performance Scale—Revised
The Leiter International Performance Scale – Revised (Leiter-R; Roid and Miller 1997) is a nonverbally administered standardized assessment of nonverbal intelligence. The subtests comprising the Brief IQ were administered; namely, Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. The Brief IQ standard score from the Leiter-R was utilized in the present study. The mean IQ for the Leiter-R standardization sample is 100, with a standard deviation of 15.
Autism Diagnostic Observation Schedule
The ADOS (Lord et al. 2007) is a semi-structured play-based interaction in which a trained examiner creates specific interactive contexts to observe reciprocal social interaction skills as well as the presence of repetitive behaviors. One of four ADOS modules is administered based upon the participant’s expressive language level. In the current project, participants received modules 1, 2, or 3. The Social Affect Calibrated Severity Scores (SA-CSS) and the Restricted and Repetitive Behavior Calibrated Severity Scores (RRB-CSS), which allow comparisons across different modules, provided by Hus et al. (2014) were utilized. Within the present study, 22 Module 1s (10 FXS, 12 ASD), 46 Module 2s (34 FXS, 12 ASD), and 19 Module 3s (7 FXS, 12 ASD) were administered.
Peabody Picture Vocabulary Test, Fourth Edition
The Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4; Dunn and Dunn 2007) is an individually administered, norm-referenced measure of receptive vocabulary. During this assessment, the participant is instructed to select the picture, from a four-picture array, that matches the vocabulary word spoken by the examiner. The PPVT-4 yields raw scores, standard scores relative age-normed performance, and growth scores. Depending on the research question, either standard scores or raw scores were used as the dependent measure for receptive vocabulary (see analysis plan). Approximately half of the participants in each group received Version A and half received Version B of this measure.
Expressive Vocabulary Test, Second Edition
The Expressive Vocabulary Test, Second Edition (EVT-2; Williams 2007) is an individually administered, norm referenced measure of expressive vocabulary. During this assessment, the participant provides a label or synonym for each color picture on the page of an easel book. The EVT-2 yields raw scores, standard scores relative to age-normed performance, and growth scores. Depending on the research question, either standard scores or raw scores were used as the dependent measure for expressive vocabulary (see analysis plan). Approximately half of the participants in each group received Version A and half received Version B of this measure.
Test for Reception of Grammar, Version 2
The Test for Reception of Grammar, Version 2 (TROG-2; Bishop 2003) is an individually administered, norm-referenced measure designed to assess comprehension of syntactic constructs that are marked by inflection, function words, and word order in English. The participant is instructed to select the picture, from a four picture array, that best represents the grammatical or lexical element contained in a target sentence produced by the examiner. The TROG-2 consists of items organized into blocks of four items; each block tests a single grammatical form. In the present study, depending on the research question, either standard scores or the number of individual items passed on the TROG-2 was used as the dependent measure for receptive grammar (see analysis plan).
Comprehensive Assessment of Spoken Language
The Syntax Construction subtest of the Comprehensive Assessment of Spoken Language (CASL; Carrow-Woolfolk 1995) assessed the production of words, phrases, and sentences that require the use of a variety of morphosyntactic rules (e.g., verb tense, plurals, interrogatives, pronouns). Within this task, the participant was instructed to respond to a picture by imitating the examiner, completing a sentence, answering a question designed to elicit a specific syntactic form, formulating a sentence to tell a story, and using a model sentence to generate a similar sentence. Depending on the research question, either standard scores or raw scores from this subtest were used as the dependent measure for expressive grammar (see analysis plan).
Analysis Plan
In order to provide some context regarding participant performance relative to CA expectations, preliminary analyses using standard scores were conducted to characterize within-group patterns of performance across language measures and to examine language performance relative to nonverbal cognition. Due to limitations from floor effects, nonparametric analyses were utilized.
Next, in order to determine if between-group differences in language ability existed between boys with FXS and boys with ASD, regression analyses for each language variable were conducted examining whether diagnostic group was a significant predictor of language ability after controlling for the influence of chronological age, nonverbal IQ, and overall severity of autism symptomatology. Finally, separate regression analyses for boys with FXS and boys with ASD were conducted and compared with the goal of identifying differential predictors of lexical and grammatical abilities in these neurodevelopmental disorders. All regression analyses used raw scores on the language measures as the dependent variables due to floor effects on the standard scores for receptive and expressive grammar. Models were inspected to ensure the appropriateness of regression analyses; a natural log transformation was utilized in the regression models for expressive grammar based on the suggestion of non-constant variance when examining the residuals, examination of the variance inflation factors (VIFs) and tolerance statistics for all regression models indicated that collinearity was not a concern. In the case of significant findings, follow-up analyses were conducted to determine if the same pattern of findings was obtained when the sample of participants with FXS included only those who also met research criteria for an ASD diagnosis (FXS+ASD).
Results
Preliminary Analyses: Within-Group Patterns of Standard Score Performance in FXS and ASD
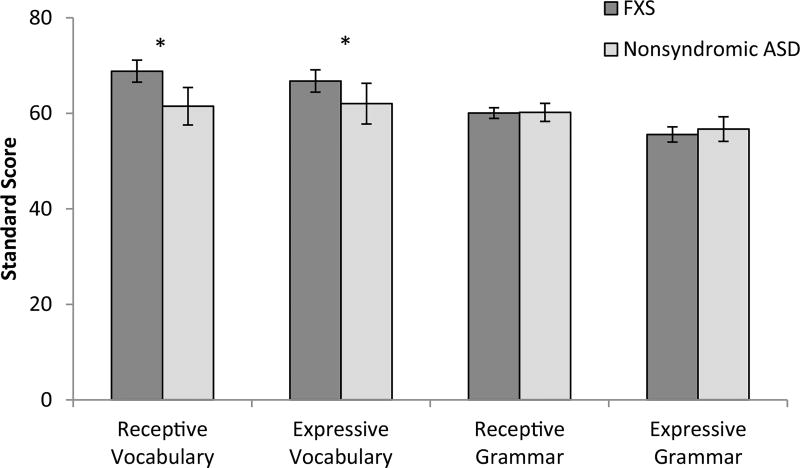
Preliminary analyses were first conducted to evaluate the pattern of standard score performance across language measures within each diagnostic group to provide context regarding the pattern of language performance relative to chronological age expectations. For boys with FXS, results of a nonparametric related-samples Friedman’s ANOVA indicated a significant difference in standard scores across language measures, χ2 = 48.83, p < 0.001. Pairwise comparisons were evaluated adjusting for multiple comparisons (αfw = 0.008). Comparisons of the PPVT vs. EVT (p = 0.30) and EVT versus TROG (p = 0.01) did not reach criterion for a significant difference. All other comparisons were significant, all ps < 0.007, with grammar scores lower than lexical scores and expressive grammar scores lower than receptive grammar scores; see Fig. 1. In addition, related-samples Wilcoxon signed rank tests (αfw = 0.0125) were conducted to evaluate language performance relative to nonverbal IQ. Results demonstrated that boys with FXS earned significantly higher receptive and expressive vocabulary standard scores (ps ≤ 0.001) and significantly lower expressive grammar standard scores (p = 0.005) than nonverbal IQ scores. Follow-up analyses including only boys with comorbid FXS+ASD yielded the same pattern of findings.
Fig. 1.
Mean standard scores as a function of language measures. Error bars represent standard errors. *p < 0.05
In contrast, for boys with ASD, the nonparametric related-samples Friedman’s ANOVA, did not indicate a significant difference in standard scores across language measures, χ2 = 7.64, p < 0.054; see Fig. 1. Comparisons of language relative to nonverbal cognitive performance (αfw = 0.0125) indicated that, despite the fact that comparisons across standard scores on the language measures indicated a relatively flat profile, expressive grammar standard scores were significantly lower than nonverbal IQ scores for boys with ASD (p ≤ 0.001). There was also a trend for receptive grammar scores to be lower than nonverbal IQ scores for boys with ASD (p = 0.013).
Between-Group Differences in Lexical and Grammatical Abilities
As mentioned previously, participants with FXS demonstrated significantly lower nonverbal IQ scores (more severely impaired) and overall lower ASD severity scores (less severely impaired) than did the participants with non-syndromic ASD. A series of multiple regression analyses were conducted to evaluate whether or not diagnostic group significantly contributed to the prediction of ability in each language domain after controlling for the effects of chronological age, nonverbal IQ, and overall ASD symptom severity.
With regard to vocabulary ability, we found that diagnostic group was a significant predictor even when chronological age, nonverbal IQ, and overall ASD symptom severity were included in the model. This combination of variables significantly predicted PPVT-4 raw scores, F(4,86) = 23.02, p < 0.001, with an R2adjusted value of 0.53. Chronological age, Leiter-R nonverbal IQ, and diagnostic group all significantly contributed to this prediction (see Table 2). Diagnostic group accounted for approximately 10% of the variance in PPVT-4 raw scores, with boys with FXS having PPVT-4 raw scores approximately 25 points higher than boys with nonsyndromic ASD when chronological age, nonverbal IQ, and ASD symptom severity were held constant. Similarly, the regression model for EVT-2 raw scores was also significant, F(4,84) = 26.05, p < 0.001, with an R2adjusted value of 0.54. Chronological age, Leiter-R nonverbal IQ, and diagnostic group significantly contributed to this prediction (see Table 2). Diagnostic group accounted for approximately 6% of the variance in EVT-2 raw scores, with boys with FXS demonstrating EVT-2 raw scores approximately 17 points higher than boys with nonsyndromic ASD when chronological age, nonverbal IQ, and ASD symptom severity were held constant. Finally, follow-up analyses, restricting the FXS sample to only boys with comorbid FXS+ASD yielded the same pattern of findings for both receptive and expressive vocabulary.
Table 2.
Linear regression analyses evaluating between-group differences in language ability
| B (unstandardized) | SEB | β | p value | |
|---|---|---|---|---|
| Receptive vocabulary—PPVT raw score | ||||
| Chronological age | 12.90 | 1.47 | 0.78 | <0.001* |
| Nonverbal IQ | 1.51 | 0.23 | 0.63 | <0.001* |
| ASD symptom severity | 1.07 | 1.40 | 0.70 | 0.77 |
| Diagnostic group | −24.76 | 6.09 | −0.38 | <0.001* |
| Expressive vocabulary—EVT-2 raw score | ||||
| Chronological age | 10.72 | 1.13 | 0.81 | <0.001* |
| Nonverbal IQ | 1.28 | 0.18 | 0.66 | <0.001* |
| ASD symptom severity | 1.97 | 1.07 | 0.16 | 0.07 |
| Diagnostic group | −16.86 | 4.69 | −0.32 | 0.001* |
| Receptive grammar—TROG-2 total number of items correct1 | ||||
| Chronological age | 4.12 | 0.60 | 0.68 | <0.001* |
| Nonverbal IQ | 0.58 | 0.09 | 0.66 | <0.001* |
| ASD symptom severity | 0.14 | 0.57 | 0.03 | 0.80 |
| Diagnostic group | −2.57 | 2.48 | −0.11 | 0.30 |
| Expressive grammar—CASL syntax construction raw score | ||||
| Chronological age | 2.10 | 0.33 | 0.66 | <0.001* |
| Nonverbal IQ | 0.26 | 0.05 | 0.54 | <0.001* |
| ASD symptom severity | 0.23 | 0.32 | 0.08 | 0.47 |
| Diagnostic group | −1.96 | 1.38 | −0.16 | 0.16 |
Because of suggestion of some non-constant variance when examining the residuals, the model was also run utilizing a natural log transformation; the same pattern of findings was observed
p < .05
With regard to grammatical ability, diagnostic group was not a significant predictor when chronological age, nonverbal IQ, and overall ASD symptom severity were included in the model. This combination of variables significantly predicted the number of individual items passed on the TROG-2, F(4, 86) = 14.87, p < 0.001, with an R2adjusted value of 0.39. Chronological age and Leiter-R nonverbal IQ were the only significant contributors to this prediction (Table 2); that is, there was no effect of diagnostic group after controlling for the effects of the other predictor variables. Similarly, the regression model for the raw score from the CASL Syntax Construction subtest was also significant, F(4,83) = 11.17, p < 0.001, with an R2adjusted value of 0.33. Once again, no between-group differences were observed; chronological age and Leiter-R nonverbal IQ were the only significant contributors to this prediction (Table 2). Finally, restricting the FXS sample to only boys with comorbid FXS+ASD yielded the same pattern of findings for both receptive and expressive grammar. In sum, these findings suggest better lexical performance in boys with FXS, relative to boys with ASD, even after controlling for the effects of chronological age, nonverbal cognitive ability, and autism symptom-severity on language performance.
Comparison of the Predictors of Within-Syndrome Variation in Lexical and Grammatical Abilities in FXS and ASD
Next, a series of regression analyses were conducted separately for boys with FXS and boys with nonsyndromic ASD to determine whether the predictors of lexical and grammatical competence were similar across these disorders. Predictors were chronological age, nonverbal IQ, severity of ASD symptomatology in the social affective domain, and severity of ASD symptomatology in the restricted and repetitive behavior domain. We also show the associations among the predictor variables for both participants with FXS (Table 3) and participants with ASD (Table 4).
Table 3.
Correlations across regression predictors for boys with FXS
p < 0.05
Table 4.
Correlations across regression predictors for boys with non-syndromic ASD
p < 0.05
When considering boys with FXS (Table 5), significant regression models were obtained for receptive vocabulary ability (F(4,50) = 25.37, p < 0.001, R2adjusted = 0.66), expressive vocabulary ability (F(4, 49) = 32.17, p < 0.001, R2adjusted = 0.72), receptive grammar (F(4, 50) = 19.93, p < 0.001, R2adjusted = 0.60), and expressive grammar (F(4, 48) = 13.98, p < 0.001, R2adjusted = 0.52). A similar pattern of findings was also observed when models were conducted limiting the FXS group to only those categorized as FXS+ASD (receptive vocabulary R2adjusted = 0.66; expressive vocabulary R2adjusted = 0.71; receptive grammar R2adjusted = 0.53; expressive grammar R2adjusted = 0.47). Across all models, chronological age contributed the most to language ability followed by nonverbal IQ. Although accounting for a relatively small percent of variance, severity of ASD symptomatology in the social affective domain was a significant positive contributor to models of receptive vocabulary, expressive vocabulary, and receptive grammar, and severity of ASD symptomatology in the restricted and repetitive behavior domain was a significant negative contributor to the models of receptive vocabulary and grammar. Although some minor fluctuations in p-values were observed in analyses including in the FXS group only participants with FXS+ASD, all significant findings from the full sample remained significant or marginally significant.
Table 5.
Linear regression analyses for boys with fragile X syndrome predicting language ability
| B (unstandardized) | SEB | β | p value | |
|---|---|---|---|---|
| Receptive vocabulary—PPVT raw score | ||||
| Chronological age | 13.38 | 1.56 | 0.88 | <0.001* |
| Nonverbal IQ | 1.29 | 0.25 | 0.57 | <0.001* |
| Social affective symptom severity | 2.97 | 1.26 | 0.22 | 0.02* |
| Restricted and repetitive behavior symptom severity | −3.71 | 1.80 | −0.19 | 0.04* |
| Expressive vocabulary—EVT-2 raw score | ||||
| Chronological age | 11.41 | 1.13 | 0.94 | <0.001* |
| Nonverbal IQ | 0.98 | 0.18 | 0.53 | <0.001* |
| Social affective symptom severity | 1.90 | 0.92 | 0.18 | 0.04* |
| Restricted and repetitive behavior symptom severity | −2.45 | 1.33 | −0.15 | 0.07 |
| Receptive grammar—TROG-2 total number of items correct | ||||
| Chronological age | 3.79 | 0.57 | 0.74 | <0.001* |
| Nonverbal IQ | 0.44 | 0.09 | 0.57 | <0.001* |
| Social affective symptom severity | 1.31 | 0.46 | 0.29 | 0.006* |
| Restricted and repetitive behavior symptom severity | −2.05 | 0.65 | −0.30 | 0.003* |
| Expressive grammar—CASL syntax construction raw score (transformed)1 | ||||
| Chronological age | 0.42 | 0.06 | 0.83 | <0.001* |
| Nonverbal IQ | 0.04 | 0.01 | 0.53 | <0.001* |
| Social affective symptom severity | 0.04 | 0.05 | 0.10 | 0.41 |
| Restricted and repetitive behavior symptom severity | −0.09 | 0.07 | −0.13 | 0.22 |
Because of the presence of non-constant variance in the residuals, this model data transformed using a natural log transformation
p < .05
For boys with nonsyndromic ASD (Table 6), significant regression models were also observed for receptive vocabulary ability (F(4, 35) = 6.20, p = 0.001, R2adjusted = 0.37), expressive vocabulary (F(4,34) = 8.93, p < 0.001, R2adjusted = 0.48), receptive grammar (F(4,35) = 6.22, p = 0.001, R2adjusted = 0.37), and expressive grammar (F(4,34) = 3.40, p = 0.02, R2adjusted = 0.22). Interestingly, the regression models generated for boys with nonsyndromic ASD explained approximately half the variance in language ability as did the regression models generated for boys with FXS. Furthermore, although chronological age was the largest contributor to language models for boys with FXS (followed by nonverbal IQ), the largest contributor to all models of language ability in boys with nonsyndromic ASD was nonverbal IQ followed by chronological age. Also in contrast to the boys with FXS, ASD symptom scores were not significant predictors for any language measure.
Table 6.
Linear regression analyses for boys with nonsyndromic ASD predicting language ability
| B (unstandardized) | SEB | β | p value | |
|---|---|---|---|---|
| Receptive vocabulary—PPVT raw score1 | ||||
| Chronological age | 10.17 | 2.77 | 0.57 | 0.001* |
| Nonverbal IQ | 1.85 | 0.42 | 0.68 | <0.001* |
| Social affective symptom severity | −0.26 | 2.73 | −0.01 | 0.92 |
| Restricted and repetitive behavior symptom severity | 1.93 | 3.58 | 0.08 | 0.59 |
| Expressive vocabulary—EVT-2 raw score1 | ||||
| Chronological age | 7.56 | 2.10 | 0.51 | 0.001* |
| Nonverbal IQ | 1.68 | 0.31 | 0.76 | <0.001* |
| Social affective symptom severity | 0.44 | 2.05 | 0.03 | 0.83 |
| Restricted and repetitive behavior symptom severity | 4.97 | 2.69 | 0.25 | 0.07 |
| Receptive grammar—TROG-2 total number of items correct1 | ||||
| Chronological age | 3.69 | 1.15 | 0.50 | 0.003* |
| Nonverbal IQ | 0.81 | 0.17 | 0.72 | <0.001* |
| Social affective symptom severity | −1.38 | 1.13 | −0.17 | 0.23 |
| Restricted and repetitive behavior symptom severity | 0.66 | 1.49 | 0.07 | 0.66 |
| Expressive grammar—CASL syntax construction raw score (transformed)2 | ||||
| Chronological age | 0.20 | 0.11 | 0.32 | 0.08 |
| Nonverbal IQ | 0.06 | 0.02 | 0.63 | 0.001* |
| Social affective symptom severity | −0.009 | 0.10 | −0.01 | 0.93 |
| Restricted and repetitive behavior symptom severity | 0.07 | 0.14 | 0.09 | 0.60 |
One potential outlier was observed in the model. Results obtained after removing this outlier were similar to those reported here
Because of the presence of non-constant variance in the residuals, this model data transformed using a natural log transformation
p < .05
Discussion
In the present study, we sought to expand our understanding of the similarities and differences between the FXS and ASD phenotypes by comparing lexical and syntactic abilities across these conditions, as well as the factors influencing these abilities in each of these neurodevelopmental disorders. This is a particularly important comparison given the frequent references to the neurobehavioral overlap between these two conditions. Despite their similarities, a number of important differences have been documented between the FXS and ASD phenotypes. Little is known, however, about the extent to which similarities and differences are observed across these two conditions in domains of functioning beyond the behavioral symptoms diagnostic for ASD.
Results from the present study indicate that although both FXS and ASD are associated with language difficulties, there are important differences between the two conditions in terms of the language profiles and the factors influencing language. Boys with ASD demonstrated receptive and expressive grammar skills that were significantly weaker than their level of nonverbal cognitive ability and a relatively flat profile across receptive and expressive vocabulary and grammar. In contrast, for the boys with FXS, lexical skills were stronger than grammar skills and receptive grammar was stronger than expressive grammar. Comparisons to nonverbal cognitive ability in FXS, demonstrated that vocabulary skills and receptive grammar were areas of relative strength, with performance at or above levels of nonverbal cognitive ability, whereas expressive grammar skills were weaker than nonverbal cognitive expectations. Importantly, these patterns remained even when the FXS group was restricted to only individuals who met criteria for FXS+ASD. These findings, therefore, extend to the full range of affectedness in FXS.
Previous studies have been inconsistent as to whether individuals with FXS demonstrate delays in the syntactic domain that are beyond those expected given nonverbal cognitive or lexical performance (e.g., Abbeduto et al. 2003; Ferrier et al. 1991; Oakes et al. 2013; Price et al. 2008). These inconsistencies may reflect differences across development and/or variability in the methods used to assess syntactic abilities across studies. Our study is the not the first to find receptive grammar skills to be impaired to a lesser degree than expressive grammar skills in FXS (Roberts et al. 2001). Although an area of strength relative to expressive grammar, it is important to remember that receptive grammar is nonetheless impaired relative to age expectations in FXS. In a recent study by Oakes et al. (2013), it was observed that adolescents with FXS continued to show a weakness in grammatical comprehension relative to TD children matched on nonverbal cognitive ability. Oakes et al. proposed that individuals with FXS might have particular difficulty in processing sentences that are highly taxing of auditory sequential memory as well as those that lack lexical supports for grammatical processing. In expression, a number of recent studies have documented impairments in individuals with FXS relative to cognitively matched TD peers (e.g., Finestack and Abbeduto 2010; Finestack et al. 2013; Kover et al. 2012). Moreover, some researchers have suggested that individuals with FXS are better able to manage syntactic demands of expressive tasks that are highly structured, draw heavily on vocabulary knowledge, and minimize social demands (e.g., Finestack et al. 2013). Thus, future research should make finer distinctions within language than made in the present study to identify an even more nuanced language profile.
Intervention activities that incorporate these characteristics may scaffold the spoken language performance of individuals with FXS and optimize opportunities for successful spoken communication. For example, McDuffie et al. (2016) recently reported data on a spoken language intervention for adolescents with FXS in which the authors embedded the intervention in the context of shared storytelling activities involving each boys and his mother. In the intervention, the participants were afforded frequent and repeated structured opportunities to practice using spoken language in a situation that provided extensive social scaffolding and visual supports for the production of spoken language.
Although common, structural language difficulties are not a core characteristic of the ASD phenotype (Kjelgaard and Tager-Flusberg 2001). Given the present study’s focus on a between-group comparison of FXS and ASD, the sample of children with ASD included in the present study is relatively low functioning (IQ < 85). Thus, it is not surprising to observe language difficulties in our ASD sample. Unlike the data reported by Kjelgaard and Tager-Flusberg, our data failed to demonstrate significant differences in achievement across language domains, revealing instead that delays in vocabulary and grammar were commensurate with one another as were delays in receptive and expressive language skills. Our findings, however, are consistent with those of Jarrold et al. (1997). Given the heterogeneity observed within the ASD phenotype and the fact that our sample was largely a lower functioning group, it is important to recognize the limits of the generalizability of our findings. Our findings also indicated that syntactic abilities, but not vocabulary abilities, were delayed relative to nonverbal cognitive performance in children with ASD.
The present findings, therefore, suggest the need for language intervention services for children with ASD, particularly those with cognitive impairments. More importantly, the findings suggest that interventions may need to provide different degrees and types of support for learning syntactic and lexical information because the level of impairment in these two domains appears to be somewhat different for children with ASD.
Between-Group Patterns of Language Performance
The present results also demonstrated that boys with ASD displayed a weakness in vocabulary skills relative to boys with FXS. More specifically, after controlling for the effects of chronological age, nonverbal IQ, and the severity of ASD symptomatology in both the social affective and restricted and repetitive behavior domains, the FXS group demonstrated receptive vocabulary raw scores that were 25 points higher, and expressive vocabulary raw scores that were 17 points higher, than those of the ASD group. In terms of syntactic ability, no between-group differences were observed after controlling for the effects of chronological age, nonverbal IQ, and ASD symptom severity. Once again, these findings also remained when the FXS group was restricted to only those who met criteria for FXS+ASD.
Efficient word learning requires the use of multiple strategies across a variety of contexts, with many of these strategies being social in nature. In addition to language difficulties, individuals with FXS or ASD often demonstrate some level of difficulty in the ability to notice, interpret, and respond to other people’s social signals and to use these signals in the service of word learning. Thus, there are multiple avenues by which word learning could be compromised in these populations. Our results indicate that during this developmental period, males with FXS demonstrate a “lexical advantage” over males with ASD at comparable developmental levels and that this between-group difference remains even after accounting for between-group differences in ASD symptomatology, suggesting that there are additional word learning differences between these two groups.
Interestingly, there is literature regarding between-group patterns of performance of boys with FXS and boys with ASD as they acquire new words under controlled experimental conditions. For example, when using a fast-mapping paradigm to examine the initial stages of learning a word, McDuffie et al. (2013) found that despite having lower levels of nonverbal cognitive ability, boys with FXS demonstrated better performance than did boys with ASD in terms of learning object labels. Importantly, the boys with FXS also had less severe social impairments than did the boys with ASD, raising the possibility of social-cognitive contributions to word learning deficits of children with ASD. In addition, between-group differences in the ability to use social cues, such as a speaker’s direction of gaze (Benjamin et al. 2015) and a speaker’s emotional reactions (Thurman et al. 2015a, b), to disambiguate the novel object that the speaker intended to label have also been considered. In these studies, although initial analyses demonstrated the presence of some between-group differences favoring the FXS group in terms of overall levels of performance, no group differences were observed after controlling for ASD symptom severity, consistent with the social origins of the word learning challenges associated with ASD, although participants with ASD were more apt to utilize the emotional reaction of disappointment to infer an intended referent (Thurman et al. 2015a, b). Such findings suggest that additional research on the use of social cues as a foundation for word learning may be useful in understanding the lexical disadvantage of children with ASD relative to children with FXS.
More generally, McDuffie et al. (2015) observed that, even when matching individuals with FXS or ASD on overall levels of ASD symptom severity, some symptom-level differences favoring the FXS group remained within the area of social reciprocity. It may be that these social-affective strengths allow those with FXS to accumulate more successful learning experiences over time, as compared to those with ASD, thereby facilitating vocabulary acquisition. If this interpretation is correct, interventions for ASD might do well to incorporate ways of highlighting or facilitating identification and use of social cues to word meaning.
Within-Group Predictors of Language Skills
Across all regression models and language domains for boys with FXS, the identified predictors explained more than half the total variance in language ability. Chronological age contributed the most to language ability, uniquely explaining approximately 50% of the variance accounted for in lexical ability and 30% of the variance accounted for in grammatical ability. The second largest contributor in all models was nonverbal IQ, uniquely explaining approximately 15% of the variance accounted for in all models of language ability. In contrast, the language models generated for boys with ASD were observed to explain less than half of the amount of variance in language ability as was explained in boys with FXS. Moreover, nonverbal IQ, rather than chronological age, was the most robust contributor for ASD, uniquely explaining approximately 20% of the variance accounted for in all models. In addition, ASD symptomatology was a significant, albeit small, contributor to language skills in individuals with FXS but not in individuals with ASD. More specifically, severity of social affective ASD symptomatology was a significant positive contributor to all language models in FXS except expressive grammar and severity of restricted and repetitive behavior ASD symptomatology was a significant negative predictor to models of receptive vocabulary and receptive grammar. All findings for boys with FXS remained largely the same when the FXS sample was restricted to only those who met criteria for FXS+ASD.
Taken together, these findings suggest that there are differences in the mechanisms underlying language acquisition between FXS and ASD. For those with FXS, we generally see, at least between 4 and 10 years of age, that the primary factor influencing language development is age; that is, as children with FXS grow older and have more exposure to language learning opportunities, they also acquire more language competence. This is not the case for individuals with ASD of the same age and general developmental level. For boys with ASD, at least for those with an IQ under 85, we see that extent of delay in nonverbal cognition plays a key role in language development. Nonverbal cognition also contributes in FXS, but in a less important way than chronological age. The predictive value of ASD symptomatology was seen only for FXS, further reinforcing the notion that the mechanisms of language development differ between the two conditions.
Note that the regression findings do not mean that social impairments are unimportant for language learning in ASD. Indeed, as we have already discussed, social impairments may contribute to the differences in vocabulary achievements across FXS and ASD. Instead, the findings suggest that cognitive rather than social impairments are at the heart of differences in language skill among individuals with nonsyndromic ASD. Identifying the specific cognitive skills contributing to successful language learning in ASD will thus be critical to the development of appropriate interventions, which may need to include cognitive targets. Furthermore, our models indicate that a substantial proportion of the variance in the language abilities of the boys with ASD remained unaccounted for; identification of other factors contributing to the variation in language performance in ASD will also be critical to the development of appropriate interventions.
Interestingly, although not true for boys with ASD, severity of social affective ASD symptomatology predicted most domains of language development in FXS, such that those who were more affected in terms of social affective symptomatology were observed to have higher language scores. Although this finding may seem counterintuitive, there are reasonable explanations. The ADOS social affective domain provides a general assessment of the persistent deficits in social communication and social interaction across multiple contexts associated with a diagnosis of ASD. Although these deficits can be clearly assessed in children with limited language abilities, it may be that, for individuals with FXS, increased language performance facilitates the identification of difficulties within this domain. That is, boys with FXS may have more difficulty with higher-level social expectations, such as situations that rely on verbal communicative competence than with lower level social expectations, such as use of gesture and affect in social interactions. Thus, for FXS, social affective deficits as assessed by the ADOS may simply be more detectable in those with more advanced language skills. It is important to note that the ADOS severity scores were designed to have little to no influence of language performance; our data indicate that this is true for ASD but does not extend to FXS+ASD.
In the present study, children with FXS who displayed fewer repetitive behaviors were observed to demonstrate better language skills, at least in terms of receptive vocabulary and grammar. Once again, this was not observed to be the case for participants with ASD. The ADOS repetitive behavior domain is considered to provide a general assessment of a broad heterogeneous category of behaviors characterized by repetitive motor movements, preoccupation with parts of objects, rigid adherence to non-functional routines, and preoccupation with restricted patterns of interest (American Psychiatric Association 2013). In typically developing children, these types of behaviors are commonly observed early in development and are believed to scaffold motor movements (Thelen 1981), facilitate the development of mastery motivation and self-regulation (Kopp 1982; Jennings 2002), and serve to alleviate anxiety (Evans et al. 1997). Furthermore, the frequency of these behaviors is theorized to decrease with age in typically developing children as goal-directed behaviors increase (Thelen 1981). In line with this theory, researchers have postulated that as children with developmental delays gain language, allowing them to better communicate with those around them, the need for these repetitive behaviors decreases (e.g., Bishop et al. 2006; Ray-Subramanian and Weismer 2012). Furthermore, there is evidence that, as expressive vocabulary standard score performance improves in boys with FXS, the severity of repetitive behaviors lessens (Thurman et. al. 2015a, b). Although our findings did not reach the criterion for statistical significance, we did observe a trend in raw score performance supporting this finding.
Limitations and Future Directions
We believe empirical research focused on disentangling the similarities and differences across the FXS and ASD phenotypes in domains frequently implicated in both conditions would help clarify the nature of the relationship between these disorders and provide valuable insights into the complex developmental mechanisms that lead to the impairments associated with each disorder. Importantly, this line of research is still in its early stages. Development reflects a complex interplay among genetic, developmental, and environmental factors that interact reciprocally over time. Thus, it is vital that we continue to explore the mechanisms underlying structural language development and gain insight into how these relations work across development. The present project only considered a small number of predictors likely to influence language development. Certainly, other important characteristics warrant consideration (e.g., maternal education, auditory memory), particularly in boys with ASD. An additional limitation of the present study is that only boys were included. It is important for the same relations to be considered in girls with FXS or ASD. These comparisons would add important information to our understanding of these phenotypes particularly in the higher IQ range where most of the girls with FXS are represented. In addition, due to the present study’s focus on comparisons between FXS and ASD, the boys with ASD included in the study were relatively low functioning (NVIQs < 85). Investigations focused on structural language abilities in individuals with ASD across the full IQ range are vital, especially given the present findings regarding the importance of nonverbal IQ in language learning. A number of additional areas warrant investigation. For example, the tools used to assess vocabulary in the present study consider primarily concrete vocabulary. Relational words (e.g., verbs and adjectives) and closed-class words (e.g., prepositions, pronouns, and conjunctions) require the same kind of reasoning that is central to grammatical learning (Dale et al. 2000). Given the potential differences observed between lexical and syntactic skills, it would be interesting to consider the profile of language performance as a function of word type. Finally, although the ADOS severity scores were designed to have little influence from developmental factors, such as language ability, this does not appear to have been as successful for FXS+ASD. Future studies should consider a more thorough investigation of the psychometric properties of the ADOS in FXS and potentially other genetic causes of ASD.
Conclusions
Findings from the present study demonstrate that although there are a number of similarities between the FXS and ASD phenotypes, including language difficulties, the profiles of language performance and the mechanisms underlying their development differ. Boys with FXS demonstrated a lexical advantage relative to boys with ASD, even after controlling for the effects of age, nonverbal IQ, and ASD symptom severity. Identifying the factors leading to this discrepancy may provide additional insights into intervention targets likely to improve lexical skills in boys with ASD. For example, some symptom-level differences within the social-affective domain remain between boys with FXS and boys with ASD even when considering groups matched on overall ASD severity level (McDuffie et al. 2015). These social-affective strengths may allow those with FXS to accumulate more successful learning experiences over time, as compared to those with ASD, thereby facilitating vocabulary acquisition.
In addition, not only were there differences in the factors predicting language performance in these groups, but also our models predicted about half the variance in language abilities in ASD than observed in FXS. Thus, to some extent, different treatment approaches are likely warranted to address the language difficulties with FXS and with ASD. Elucidating the routes by which lexical or syntactic development occurs in boys with FXS or ASD can facilitate the development of methods by which children with these neurodevelopmental disorders can access a more optimal range of learning opportunities and provide insight into the complex processes that underlie language development.
Acknowledgments
This research was supported by grants R01 HD054764 and U54 HD079125 from the National Institute of Child Health and Human Development. We wish to thank the children and their families for their participation in this study. We also thank David Benjamin, Susan Harris, Beth Goodlin-Jones, Claire Hauser, Sara Armson, Eileen Haebig, Ashley Oakes, and Cecilia Compton for assisting with data collection; Susen Schroeder for coordinating all study visits; and Danielle Harvey for assisting with data analyses. Leonard Abbeduto has received financial support to develop and implement outcome measures for clinical trials from F. Hoffman-LaRoche, Ltd., Roche TCRC, Inc. and Neuren Pharmaceuticals Limited. Randi J. Hagerman has received funding from Novartis, Roche Pharmaceuticals, Alcobra and Seaside Therapeutics to carry out treatment studies in fragile X syndrome and ASD. She has also consulted with Roche/Genetech, Zynerba, and Novartis regarding treatment studies in fragile X syndrome.
Author Leonard Abbeduto has received financial support to develop and implement outcome measures for clinical trials from F. Hoffman-LaRoche, Ltd., Roche TCRC, Inc. and Neuren Pharmaceuticals Limited.
Footnotes
No other authors have financial disclosures to make.
Author’s Contribution AJT conceived of the study, participated in its design and coordination, performed the statistical analysis, and drafted the manuscript; AM participated in the design, coordination, and interpretation of the data; RH conceived of the larger study from which data were drawn and participated in interpretation of the data; CJ participated in the design of the study and; LA conceived of the larger study from which data were drawn and participated in the design, coordination, and interpretation of the data. All authors helped to draft the manuscript, and read and approved the final manuscript.
Conflict of interest Authors Angela John Thurman, Andrea McDuffie, and Cynde K. Josol declare that they has no conflict of interest.
References
- Abbeduto L, Brady N, Kover ST. Language development and fragile X syndrome: Profiles, syndrome-specificity, and within-syndrome differences. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:36–46. doi: 10.1002/mrdd.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, Hagerman RJ. Language and communication in fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3:313–322. [Google Scholar]
- Abbeduto L, McDuffie A, Thurman AJ. The fragile X syndrome-autism comorbidity: What do we really know? Frontiers in Genetics. 2014;5:355. doi: 10.3389/fgene.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, Murphy MM, Cawthon SW, Richmond EK, Weissman MD, Karadottir S, O’Brien A. Receptive language skills of adolescents and young adults with Down syndrome or fragile X syndrome. American Journal on Mental Retardation. 2003;108:149–160. doi: 10.1352/0895-8017(2003)108<0149:RLSOAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Abbeduto L, Murphy MM, Cawthon SW, Richmond EK, Weissman MD, Karadottir S, O’Brien A. Receptive language skills of adolescents and young adults with Down or fragile X syndrome. American Journal on Mental Retardation. 2003;108:149–160. doi: 10.1352/0895-8017(2003)108<0149:RLSOAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Abbeduto L, Warren SF, Conners FA. Language development in Down syndrome: From the prelinguistic period to the acquisition of literacy. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:247–261. doi: 10.1002/mrdd.20158. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Bailey DB, Jr, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin DP, McDuffie A, Thurman AJ, Kover ST, Mastergeorge AM, Hagerman RJ, Abbeduto L. Effect of speaker gaze on word learning in fragile X syndrome and autism. Journal of Speech, Language, and Hearing Research. 2015;58:383–395. doi: 10.1044/2015_JSLHR-L-14-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Research. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Test for Reception of Grammar: TROG-2 Version 2. London: Psychological Corporation; 2003. [Google Scholar]
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychology. 2006;12:247–267. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Boucher J. Research review: Structural language in autistic spectrum disorder characteristics and causes. Journal of Child Psychology and Psychiatry. 2012;53:219–233. doi: 10.1111/j.1469-7610.2011.02508.x. [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Test for Auditory Comprehension of Language—Revised. Austin: Pro-Ed.; 1985. [Google Scholar]
- Carrow-Woolfolk E. Oral and Written Language Scales. Circle Pines: American Guidance Service; 1995. [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summary. 2014;63:1–21. [PubMed] [Google Scholar]
- Clifford S, Dissanyake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and permutation. Journal of Autism and Developmental Disorders. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neuroscience and Biobehavioral Reviews. 2012;36:2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, Héron D. Specific genetic disorders and autism: Clinical contribution toward their identifications. Journal of Autism and Developmental Disorders. 2005;35:103–116. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. Journal of Neurodevelopmental Disorders. 2011;3:57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Scerif G, Karmiloff-Smith A. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex. 2007;43:672–685. doi: 10.1016/s0010-9452(08)70497-0. [DOI] [PubMed] [Google Scholar]
- Dale PS, Dionne G, Eley TC, Plomin R. Lexical and grammatical development: A behavioural genetic perspective. Journal of Child Language. 2000;27:619–642. doi: 10.1017/s0305000900004281. [DOI] [PubMed] [Google Scholar]
- Demark JL, Feldman MA, Holden JJ. Behavioral relationship between autism and fragile X syndrome. American Journal on Mental Retardation. 2003;108:314–326. doi: 10.1352/0895-8017(2003)108<314:BRBAAF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Donald M. Origins of the Modern Mind: Three Stages in the Evolution of Culture and Cognition. Cambridge: Harvard University Press; 1991. [Google Scholar]
- Dunn DM, Dunn LM. Peabody Picture Vocabulary Test, Fourth Edition. Minneapolis. Minneapolis: Pearson; 2007. [Google Scholar]
- Ellis Weismer S, Lord C, Elser A. Early language patterns of toddlers on the autism spectrum compared to toddlers with developmental delay. Journal of Autism and Developmental Disorders. 2010;40:1259–1273. doi: 10.1007/s10803-010-0983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estigarribia B, Roberts JE, Sideris J, Price J. Expressive morphosyntax in boys with fragile X syndrome with and without autism spectrum disorder. International Journal of Language and Communication Disorders. 2011;46:216–230. doi: 10.3109/13682822.2010.487885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Leckman JF, Carter A, Reznick JS, Henshaw D, King RA, Pauls D. Ritual, habit, and perfectionism: The prevalence and development of compulsive-like behavior in normal young children. Child Development. 1997;68:58–68. [PubMed] [Google Scholar]
- Ferrier LJ, Bashir AS, Meryash DL, Johnston J, Wolff P. Conversational skills of individuals with fragile—X syndrome: A comparison with autism and Down syndrome. Developmental Medicine and Child Neurology. 1991;33:776–788. doi: 10.1111/j.1469-8749.1991.tb14961.x. [DOI] [PubMed] [Google Scholar]
- Finestack LH, Abbeduto L. Expressive language profiles of verbally expressive adolescents and young adults with Down syndrome or fragile X syndrome. Journal of Speech, Language, and Hearing Research. 2010;53:1334–1348. doi: 10.1044/1092-4388(2010/09-0125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finestack LH, Sterling AM, Abbeduto L. Discriminating Down syndrome and fragile X syndrome based on language ability. Journal of Child Language. 2013;40:244–265. doi: 10.1017/S0305000912000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends in Cognitive Sciences. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in fragile X syndrome: A category mistake? Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:921–933. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, et al. Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Nguyen D, Green C, Chavez A, Tassone F, Hagerman R, et al. A solution to limitations of cognitive testing in children with intellectual disabilities: The case of fragile X syndrome. Journal of Neurodevelopmental Disorders. 2009;1:33–45. doi: 10.1007/s11689-008-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer BD, Tamis-LeMonda CS. The Development of Social Cognition and Communication. Mahway: Lawrence Erlbaum Associates, Inc.; 2005. [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disabilities. 2014;44:2400–2412. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Wilson RK. De novo gene disruptions in children on the autism spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C, Boucher J, Russell J. Language profiles in children with autism: Theoretical and methodological implications. Autism. 1997;1:57–76. [Google Scholar]
- Jennings KD. Mastery motivation and the formation of self-concept from infancy through early. In: Finn JD, editor. Mastery Motivation: Children’s Investigation, Persistence and Development. London: Routledge; 2002. pp. 36–54. [Google Scholar]
- Kau ASM, Reider EE, Payne L, Meyer WA, Freund L. Early behavioral signs of psychiatric phenotypes in fragile X syndrome. American Journal on Mental Retardation. 2000;105:266–299. doi: 10.1352/0895-8017(2000)105<0286:EBSOPP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger GF, Dawson G, Renner P. Autistic Disorder. In: Mash EJ, Barkley RA, editors. Child Psychopathology. Second. New York: Guilford Press; 2002. pp. 409–454. [Google Scholar]
- Klusek J, Martin GE, Losh M. A comparison of pragmatic language in boys with autism and fragile X syndrome. Journal of Speech, Language, and Hearing Research. 2014;57:1692–1707. doi: 10.1044/2014_JSLHR-L-13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Kover ST, McDuffie A, Abbeduto L, Brown WT. Effects of sampling context on spontaneous expressive language in males with fragile X syndrome or Down syndrome. Journal of Speech, Language, and Hearing Research. 2012;55:1022–1038. doi: 10.1044/1092-4388(2011/11-0075). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, McDuffie AS, Hagerman RJ, Abbeduto L. Receptive vocabulary in boys with autism spectrum disorder: Cross-sectional developmental trajectories. Journal of Autism and Developmental Disorders. 2013;43:2696–2709. doi: 10.1007/s10803-013-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 2007. [Google Scholar]
- Luyster RJ, Kadlec MB, Carter A, Tager-Flusberg H. Language assessment and development in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1426–1438. doi: 10.1007/s10803-007-0510-1. [DOI] [PubMed] [Google Scholar]
- Madison LS, George C, Moeschler JB. Cognitive functioning in the fragile-X syndrome: A study of intellectual, memory and communication skills. Journal of Mental Deficiency Research. 1986;30:129–148. doi: 10.1111/j.1365-2788.1986.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM. Advances in research on the fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:96–106. doi: 10.1002/1098-2779(2000)6:2<96::AID-MRDD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Kover ST, Hagerman R, Abbeduto L. Investigating word learning in fragile X syndrome: A fast-mapping study. Journal of Autism and Developmental Disorders. 2013;43:1676–1691. doi: 10.1007/s10803-012-1717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Machalicek W, Bullard L, Nelson S, Mello M, Tempero-Feigles R, Abbeduto L. A spoken-language intervention for school-aged boys with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2016;121:236–265. doi: 10.1352/1944-7558-121.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, Abbeduto L. Symptoms of autism in males with fragile X syndrome: A comparison to nonsyndromic ASD using current ADI-R scores. Journal of Autism and Developmental Disorders. 2015;45:1925–1937. doi: 10.1007/s10803-013-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, John AE. Vocabulary abilities of children with Williams syndrome: Strengths, weaknesses, and relation to visuospatial construction ability. Journal of Speech, Language, and Hearing Research. 2008;51:967–982. doi: 10.1044/1092-4388(2008/071). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Methodological issues in group-matching design: α levels for control variable comparisons and measurement characteristics of control and target variables. Journal of Autism and Developmental Disorders. 2004;34:7–17. doi: 10.1023/b:jadd.0000018069.69562.b8. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, Bryson S. Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics. 2006;27:S69–S78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Oakes A, Kover ST, Abbeduto L. Language comprehension profiles of young adolescents with fragile X syndrome. American Journal of Speech-Language Pathology. 2013;22:615–626. doi: 10.1044/1058-0360(2013/12-0109). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra BA, Willemson R. A fragile X balance: FMR1 expression levels. Human Molecular Genetics. 2003;12:249–457. doi: 10.1093/hmg/ddg298. [DOI] [PubMed] [Google Scholar]
- Paul R, Cohen DJ, Breg R, Watson M, Herman S. Fragile X syndrome: Its relation to speech and language disorders. Journal of Speech and Hearing Disorders. 1984;49:326–336. doi: 10.1044/jshd.4903.328. [DOI] [PubMed] [Google Scholar]
- Paul R, Dykens E, Leckman F, Watson M, Breg WR, Cohen DJ. A comparison of language characteristics of mentally retarded adults with fragile X syndrome and those with nonspecific mental retardation and autism. Journal of Autism and Developmental Disorders. 1987;17:457–468. doi: 10.1007/BF01486963. [DOI] [PubMed] [Google Scholar]
- Price J, Roberts J, Vandergrift N, Martin G. Language comprehension in boys with fragile X syndrome and boys with Down syndrome. Journal of Intellectual Disability Research. 2007;51:318–326. doi: 10.1111/j.1365-2788.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- Price JR, Roberts JE, Hennon EA, Berni MC, Anderson KL, Sideris J. Syntactic complexity during conversation of boys with fragile X syndrome and Down syndrome. Journal of Speech, Language, and Hearing Research. 2008;51:3–15. doi: 10.1044/1092-4388(2008/001). [DOI] [PubMed] [Google Scholar]
- Ray-Subramanian CE, Weismer SE. Receptive and expressive language as predictors of restricted and repetitive behaviors in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42:2113–2120. doi: 10.1007/s10803-012-1463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Warren SF, Betz SK. Language symptoms of developmental language disorders: An overview of autism, Down syndrome, fragile X, specific language impairment, and Williams syndrome. Applied Psycholinguistics. 2005;26:7–27. [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mirrett P, Burchinal M. Receptive and expressive communication development of young males with fragile X syndrome. American Journal on Mental Retardation. 2001;106:216–230. doi: 10.1352/0895-8017(2001)106<0216:RAECDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Price J, Barnes E, Nelson L, Burchinal M, Hooper SR. Receptive vocabulary, expressive vocabulary, and speech production of boys with fragile X syndrome in comparison to boys with Down syndrome. American Journal on Mental Retardation. 2007;112:177–193. doi: 10.1352/0895-8017(2007)112[177:RVEVAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter International Performance Scale—Revised. Wood Dale: Stoelting; 1997. [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. American Journal of Medical Genetics Part B. 2011;156:255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism diagnostic interview—Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Scerif G, Longhi E, Cole V, Karmiloff-Smith A, Cornish K. Attention across modalities as a longitudinal predictor of early outcomes: The case of fragile X syndrome. Journal of Child Psychology and Psychiatry. 2012;53:614–650. doi: 10.1111/j.1469-7610.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- Sudhalter V, Maranion M, Brooks P. Expressive semantic deficit in the productive language of males with fragile X syndrome. American Journal of Medical Genetics. 1992;43:65–71. doi: 10.1002/ajmg.1320430110. [DOI] [PubMed] [Google Scholar]
- Sudhalter V, Scarborough HS, Cohen IL. Syntactic delay and pragmatic deviance in the language of fragile X males. American Journal of Medical Genetics. 1991;38:493–497. doi: 10.1002/ajmg.1320380270. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Bryson S, Duku E, Vaccarella L, Zwaigenbaum L, Bennett T, Boyle MH. Similar developmental trajectories in autism and Asperger syndrome: from early childhood to adolescence. Journal of Child Psychology and Psychiatry. 2009;50:1459–1467. doi: 10.1111/j.1469-7610.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Paul R, Lord C. Language and communication in autism. Handbook of Autism and Pervasive Developmental Disorders. 2005;1:335–364. [Google Scholar]
- Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, et al. Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2009;52:643–652. doi: 10.1044/1092-4388(2009/08-0136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Rhythmical behavior in infancy: An ethological perspective. Developmental Psychology. 1981;17:237–257. [Google Scholar]
- Thomas MS, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. Journal of speech, language, and hearing research. 2009;52:336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Kover ST, Hagerman RJ, Abbeduto L. Autism symptomatology in boys with fragile X syndrome: A cross-sectional developmental trajectories comparison with nonsyndromic autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015a;45:2816–2832. doi: 10.1007/s10803-015-2443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Kover ST, Hagerman RJ, Channell MM, Mastergeorge A, et al. Use of emotional cues for lexical learning: A comparison of autism spectrum disorder and fragile X syndrome. Journal of Autism and Developmental Disorders. 2015b;45:1042–1061. doi: 10.1007/s10803-014-2260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter A, Lord C, Schopler E. A follow—up study of high—functioning autistic children. Journal of Child Psychology and Psychiatry. 1992;33:489–597. doi: 10.1111/j.1469-7610.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Vygotsky LS. Thought and language. Cambridge: MIT Press; 1986. [Google Scholar]
- Waxman RS, Markow DB. Words as invitations to form categories: Evidence from 12-to 13-month-old infants. Cognitive Psychology. 1995;29:257–302. doi: 10.1006/cogp.1995.1016. [DOI] [PubMed] [Google Scholar]
- Williams KT. Expressive Vocabulary Test. 2. Minneapolis: Pearson; 2007. [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1324–1332. doi: 10.1016/j.jaac.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]