Abstract
Aims
To determine the frequency of increasing levels of stress hyperglycemia and its associated complications in surgery patients without a history of diabetes.
Methods
We reviewed hospital outcomes in 1971 general surgery patients with documented preoperative normoglycemia [blood glucose (BG) <140 mg/dL] who developed stress hyperglycemia (BG >140 mg/dL or >180 mg/dL) within 48 h after surgery between 1/1/2010 and 10/31/2015.
Results
A total of 415 patients (21%) had ≥1 episode of BG between 140 and 180 mg/dL and 206 patients (10.5%) had BG > 180 mg/dL. The median length of hospital stay (LOS) was 9 days [interquartile range (IQR) 5,15] for BG between 140 and 180 mg/dL and 12 days (IQR 6,18) for BG > 180 mg/dL compared to normoglycemia at 6 days (IQR 4,11), both p < 0.001. Patients with BG 140–180 mg/dL had higher rates of complications with an odds ratio (OR) of 1.68 [95% confidence interval (95% CI) 1.15–2.44], and those with BG > 180 mg/dL had more complications [OR 3.46 (95% CI 2.24–5.36)] and higher mortality [OR 6.56 (95% CI 2.12–20.27)] compared to normoglycemia.
Conclusion
Increasing levels of stress hyperglycemia are associated with higher rates of perioperative complications and hospital mortality in surgical patients without diabetes.
Keywords: Inpatient hyperglycemia, General surgery, Stress hyperglycemia, Hospital hyperglycemia, Hospital mortality, Hospital complications
1. Introduction
Hyperglycemia in hospitalized patients with and without a history of diabetes is reported in up to 40% of critically ill patients and 32% of general medicine and surgery patients.1,2 Hospital hyperglycemia is commonly found in the setting of known diabetes, previously undiagnosed diabetes, and due to acute medical illness or surgery. The term stress hyperglycemia describes transient elevations in blood glucose in patients without a history of diabetes that occur during acute illness or stress.3–5
Several observational studies in mixed medicine and surgery populations have reported higher morbidity and mortality in patients with newly-recognized hyperglycemia during hospitalization when compared to those with known diabetes.6–12 In general surgery, the development of perioperative hyperglycemia is associated with up to a 4-fold increase in complications and a 2-fold increase in death compared to patients maintaining normoglycemia.12–14 Different blood glucose limits have been utilized to define stress hyperglycemia in the literature, with some studies using a BG > 140 mg/dL and other a BG > 180 mg/dL.12–15 In addition, most studies have failed to clearly define glycemic control prior to surgery, potentially leading to the misclassification of patients by diabetes status.12–14 To our knowledge, no previous studies have focused on clinical outcomes associated with increasing degrees of stress hyperglycemia in general surgery patients without a history of diabetes and documented preoperative normoglycemia. Accordingly, we analyzed data on perioperative glycemic control, associated complications and mortality in normoglycemic patients undergoing non-cardiac general surgery over a 5-year period.
2. Materials and methods
2.1. Study design
We performed a retrospective observational study of 1971 patients without a history of diabetes undergoing non-cardiac surgery at four university-affiliated hospitals in Atlanta, GA: Emory University, Emory University Midtown, Emory University St. Joseph’s, and Emory University Johns Creek Hospitals. Patients without a history of diabetes and with a preoperative BG of < 140 mg/dL on no antihyperglycemic medications prior to undergoing non-cardiac surgery were included in the study. The study was approved by the Emory Institutional Review Board.
2.2. Data source
De-identified individual level patient data was collected from the Emory University Clinical Data Warehouse from 1/1/2010 to 10/31/2015. This system collects data from the hospital electronic medical records, billing systems and laboratory and pathology departments. Demographic and anthropometric measures were collected for each subject. Prior diabetes status was determined by ICD-9 coding, and by hemoglobin A1c (HbA1c) when available (HbA1c ≥ 6.5% was considered diagnostic of diabetes). Preoperative comorbidities were also identified by ICD-9 codes during admission dates, with comorbidity burden assessed using the Charlson comorbidity index.16 The time period for the data analysis preceded implementation of ICD-10 coding. Glycemic control was assessed, both preoperatively and postoperatively, by point of care glucose testing and serum glucose values. Glycemic control parameters were assessed for up to 10 days postoperatively, or until time of discharge if occurring prior to postoperative day 10.
2.3. Outcomes
Stress hyperglycemia was defined as BG > 140 mg/dL and further stratified into groups according to severity of postoperative hyperglycemia with BG values between 140 and 180 mg/dL, and BG > 180 mg/dL. ICD-9 codes generated during the hospital stay, but not present during admission, were used to calculate the frequency of complications including: acute myocardial infarction, stroke, wound infection, pneumonia, urinary tract infection (UTI), sepsis, acute respiratory failure and acute renal failure. Acute renal failure was defined as an increase in creatinine by ≥0.5 mg/dL between admission and any time during hospitalization. Hospital mortality was assessed using hospital records during the time of surgical admission. Incidence of both composite of complications and hospital mortality were adjusted for age, gender, BMI, race, and Charlson score in the multivariate analysis.
2.4. Statistical analysis
We used descriptive statistics to characterize the baseline characteristics of the study population. We summarized continuous variables by mean ± standard deviation (SD) or median with interquartile range (IQR), and discrete variables by count and percentage. Stress hyperglycemia was categorized as BG between 140 and 180 mg/dL or BG > 180 mg/dL during the first 48 h after surgery and was compared to postoperative normoglycemia (BG < 140 mg/dL) as the reference group. We compared continuous variables between or among groups by using the nonparametric Kruskal-Wallis tests and compared discrete variables by using Chi-square tests (or Fisher’s Exact tests if needed). We also employed univariate and multivariate logistic regression to determine the association of stress hyperglycemia with hospital complications and mortality. p values < 0.05 were considered as significant. We performed the statistical analysis using SAS (v9.4).
3. Results
Hospital glycemic control data was analyzed for 1971 patients undergoing non-cardiac general surgery who had no prior documented history of diabetes. During the study period, there were a total of 11,692 patients undergoing general non-cardiac surgery. Of them, 9690 patients had missing pre- or post-operative glucose values within 48 h after surgery. A total of 2002 patients had a pre-operative BG < 140 mg/dL; of them, 31 patients were excluded due to HbA1c values > 6.5% leaving a total 1971 patients included in the analysis. The clinical characteristics of the patients are shown in Table 1, grouped by post-operative glucose levels. There was no significant association between gender or BMI and the development of stress hyperglycemia; however, there was a significant positive association with increasing age (p = 0.003) and Caucasian race (p < 0.001) and the development of stress hyperglycemia. There was no significant difference observed in Charlson comorbidity indices between groups (p = 0.12). Among major surgical types included in this analysis, patients undergoing neurosurgery were more likely to develop stress hyperglycemia postoperatively.
Table 1.
Baseline patient characteristics stratified by post-operative glycemic control.
| Normoglycemia
|
Stress hyperglycemia
|
p-Value | ||
|---|---|---|---|---|
| <140 mg/dL | 140–180 mg/dL | >180 mg/dL | ||
| # patients | 1350 | 415 | 206 | |
| Age, years | 56.2 ± 18.5 | 59.3 ± 17.2 | 59.0 ± 17.6 | 0.003 |
| Male gender, n (%) | 640 (47) | 208 (50) | 101 (49) | 0.6 |
| BMI, kg/m2 | 26.3 ± 6.9 | 27.1 ± 7.0 | 26.2 ± 7.5 | 0.14 |
| Race, n (%) | <0.001 | |||
| Caucasian | 715 (53) | 279 (67) | 138 (67) | |
| African-American | 635 (47) | 136 (33) | 68 (33) | |
| Charlson score | 2.4 ± 2.0 | 2.4 ± 2.0 | 2.6 ± 1.9 | 0.12 |
| Type of surgery, n (%) | ||||
| General surgery | 207 (62) | 86 (26) | 42 (12) | |
| Orthopedics | 89 (66) | 30 (22) | 15 (11) | |
| Gastrointestinal | 523 (83) | 65 (10) | 39 (6) | |
| Neurosurgical | 92 (44) | 79 (38) | 39 (18) | |
| Thoracic | 201 (66) | 68 (22) | 35 (12) | |
| Other | 238 (66) | 87 (24) | 36 (10) | |
Legend: BMI (body mass index).
The incidence of stress hyperglycemia increased daily during the first 72 hour postoperatively (Fig. 1A). During the first 24 h after surgery, 25.2% of patients developed hyperglycemia to BG > 140 mg/dL, with 7.4% of these values exceeding a BG > 180 mg/dL. By 48 and 72 h, 31.5% and 34.6% of patients had a BG of >140 mg/dL, respectively. The percentage of BG values >180 mg/dL was observed in 10.5% and 12.5% of patients by 48 and 72 h, respectively. Most patients developed stress hyperglycemia during the first 24 h (25.2%), with fewer patients developing hyperglycemia during the second hospital day (6.3%), and only 3.1% of patients developing hyperglycemia during the third hospital day. By 48 hour postoperatively, a total of 415 patients (21%) had ≥1 episode of BG between 140 and 180 mg/dL and 206 patients (10.5%) had BG > 180 mg/dL.
Fig. 1.
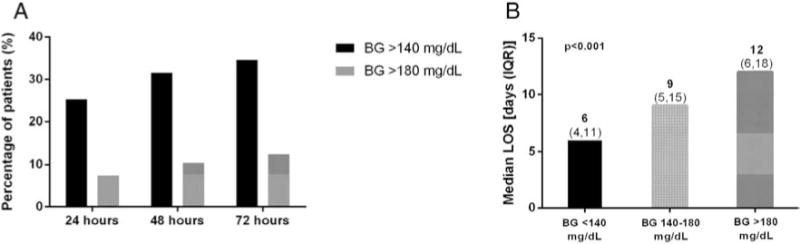
(A) Frequency of post-operative hyperglycemia. The frequency of post-operative hyperglycemia to BG values > 140 mg/dL and percentage of values > 180 mg/dL increased within the first 72 h of surgery. (B) Length of hospital stay by postoperative blood glucose levels. The development of stress hyperglycemia significantly increased the hospital length of stay (LOS), with average hospital days increasing with the degree of hyperglycemia.
An additional analysis of 175 (8.8%) patients who received corticosteroids both preoperatively and within 48 hour postoperatively was performed. Among them, 89 (51%) patients developed postoperative hyperglycemia; 56 (33%) patients with a BG between 140 and 180 mg/dL, and 31 (18%) patients with a BG > 180 mg/dL (p < 0.001).
The development of stress hyperglycemia was associated with an increase in length of hospital stay (LOS) (Fig. 1B). Patients maintaining normoglycemia in the perioperative period had a median LOS of 6 days (IQR 4,11). Those with hyperglycemia postoperatively had an increase in median LOS; 9 days (IQR 5,15) for BG values 140–180 mg/dL and 12 days (IQR 6,18) for BG > 180 mg/dL (p < 0.001).
Patients who developed stress hyperglycemia also experienced significantly more postoperative complications, including stroke, pneumonia, acute respiratory failure and acute renal failure, as shown in Fig. 2. After adjusting for age, gender, BMI, race, and Charlson score, patients with postoperative hyperglycemia had increased odds of developing complications. Odds ratios for composite of complications were 1.68 (95% CI 1.15–2.44) for patients with BG values between 140 and 180 mg/dL and 3.46 (95% CI 2.24–5.36) for those with BG > 180 mg/dL (Fig. 3A).
Fig. 2.
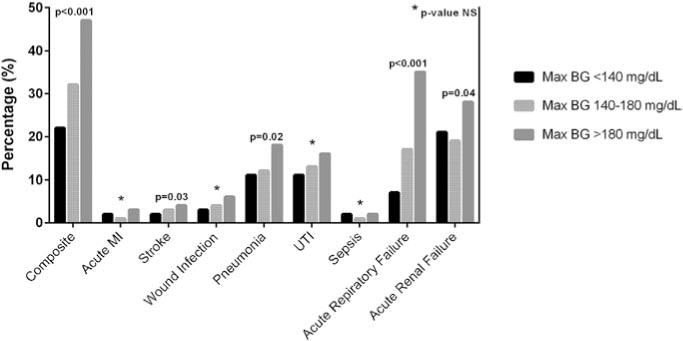
Hospital complications by postoperative glucose values. Patients developing stress hyperglycemia, both to BG values 140–180 mg/dL and >180 mg/dL, had significantly higher frequency of stroke, pneumonia, acute respiratory failure and acute renal failure, as well as an overall higher occurrence of a composite of hospital complications.
Fig. 3.
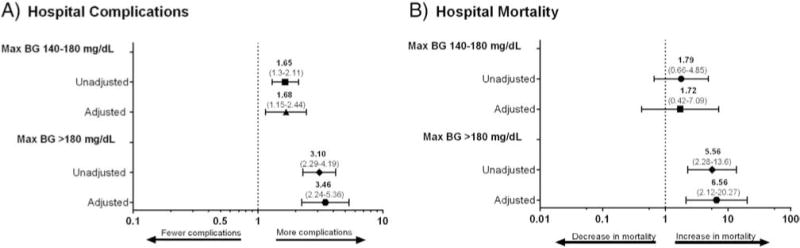
(A) Stress hyperglycemia and complications. Crude odds ratios (OR), and model adjusted for age, gender, BMI, race, and Charlson score for the association of stress hyperglycemia categories and composite of complications (acute MI, stroke, wound infection, pneumonia, UTI, sepsis, acute respiratory failure and acute renal failure). (B) Stress hyperglycemia and mortality. Crude odds ratios (OR), and model adjusted for age, gender, BMI, race, and Charlson score for the association of stress hyperglycemia categories with mortality.
The presence of stress hyperglycemia was associated with increased hospital mortality (Fig. 3B). Patients with postoperative BG 140–180 mg/dL had higher odds for mortality [OR 1.72 (95% CI 0.42–7.09)]; however, the data did not reach statistical significance. In contrast, patients developing postoperative BG levels > 180 mg/dL had a significantly higher mortality [OR 6.56 (95% CI 2.12–20.27)] when compared to those with normoglycemia.
4. Discussion
The present study examined the prevalence and clinical importance of varying degrees of stress hyperglycemia during the perioperative period in patients without diabetes and documented preoperative normoglycemia undergoing general surgery. We report that, among patients without diabetes who had normoglycemia prior to surgery, 32% of patients developed hyperglycemia to BG > 140 mg/dL and 10% of patients to BG > 180 mg/dL within the first 48 h of surgery. Stress hyperglycemia was associated with increased rates of complications at both BG levels; however, even after adjustment for multiple confounders, those with maximum BG > 180 mg/dL experienced over a 3-fold increase in complications and 6-fold increase in mortality in the fully adjusted model compared to those maintaining normoglycemia.
The present findings are consistent with prior studies in patients with hyperglycemia undergoing general non-cardiac surgery. Among them, stress hyperglycemia was reported in over 50% of patients both with and without diabetes, and was associated with increased rates of surgical complications and higher mortality in those without a prior history of diabetes.11–13 Frisch et al. reported that 30-day mortality increased for patients without a history of diabetes in proportion to severity of hyperglycemia after non-cardiac surgery compared to patients with normoglycemia and with a history of diabetes.11 The Surgical Care and Outcomes Assessment Program, which included patients with and without diabetes, found that hyperglycemia was seen in 29% of patients within 2 days following surgery and was associated with increased postoperative complications compared to non-hyperglycemic patients.14 Similarly, identification of patients with stress hyperglycemia is important in critically-ill and cardiac surgery patients because previous randomized controlled studies have shown that treatment of stress hyperglycemia in patients without diabetes reduces complications and improves overall outcomes.17,18 Van den Berghe et al.17 and Umpierrez et al.18 showed that, while intensive insulin treatment of critically-ill and coronary artery bypass surgical patients did not impact outcomes for patients with diabetes, those with stress hyperglycemia had significant improvement in complications targeting lower glucose values (80–110 mg/dL and 110–140 mg/dL, respectively) when compared to higher glucose targets.17,18
Several mechanisms of the impact of stress hyperglycemia on vascular function and wound healing have been proposed. Stress hyperglycemia results from metabolic and hormonal changes associated with the acute response to injury and stress.4,19 Acute illness, surgery, or trauma may raise levels of counter-regulatory hormones such as glucagon, cortisol, epinephrine, and growth hormone. This results in changes in carbohydrate metabolism leading to increased hepatic glucose production and insulin resistance.20,21 Stress hyperglycemia leads to increased oxidative stress and formation of pro-inflammatory cytokines including tumor necrosis factor-α, Interleukin-1 and Interleukin-6.22–25 Human and animal studies suggest that episodes of hyperglycemia may directly promote thrombosis and vascular injury through increased platelet activation and reduced fibrinolysis.26,27 Thus, it seems that stress hyperglycemia leads to both direct and indirect effects on inflammation and vascular injury, resulting in increased hospital complications seen in the current and prior studies.
We acknowledge several limitations to this study. This is a retrospective analysis that relies on electronic hospital data, primarily ICD9 coding, to report on comorbidities and complications. Only diagnoses present at the time of admission were reported for comorbidities. However, it is possible that some comorbidities were not identified on admission and may have erroneously been considered as complications. Diagnosis of diabetes was based on ICD-9 coding on admission, and because HbA1c testing is not done for all hospitalized patients, it is possible that some patients with both diagnosed and undiagnosed diabetes were included in the analyses. To help address this, patients who had HbA1c data available during admission were excluded if HbA1c was ≥6.5%, even if diabetes had not been diagnosed by ICD-9 codes. In addition, we do not know if patients with persistent hyperglycemia during multiple days developed higher rates of complications compared to subjects with a single episode of hyperglycemia. Finally, due to the observational design of the study, it is not possible to determine whether stress hyperglycemia is a direct contributor to complications or simply a marker of stress for higher risk individuals.
5. Conclusion
The present study demonstrated that among patients without a history of diabetes with normoglycemia prior to surgery, the development of stress hyperglycemia to BG values >140 mg/dL and >180 mg/dL is associated with increased hospital complications and mortality compared to patients maintaining normoglycemia (<140 mg/dL). Higher rates of perioperative complications and mortality were observed in patients with BG > 180 mg/dL compared to patients with BG between 140 and 180 mg/dL. These findings highlight the importance of obtaining perioperative information regarding glycemic control in general surgery patients. The association of perioperative glucocorticoid administration with the development of stress hyperglycemia also emphasizes the need for further assessment of patients receiving these medications. It remains uncertain whether stress hyperglycemia directly leads to complications, or if it is simply a marker of increased stress and inflammation. Future studies targeting treatment and prevention of stress hyperglycemia are needed to determine whether it plays a direct role in clinical outcomes.
Acknowledgments
Funding
This work was supported by the Jacobs Family Research Fund. Partial data from this trial were presented at the American Diabetes Association meeting in June, 2016 and at the Southern Society for Clinical Investigation Southern Regional Meeting in February, 2017.
Disclosure summary
The present study was supported by an unrestricted grant from the Jacobs Family Research Fund (to Emory University and GEU). GEU is partly supported by research grants from the Public Health Service (grants UL1 TR002378 from the Clinical and Translational Science Award program and 1P30DK111024-01 from the National Institutes of Health and National Center for Research Resources). GEU has received unrestricted research support for inpatient studies (to Emory University) from Merck, Novo Nordisk, AstraZeneca, Boehringer Ingelheim, and Sanofi. FJP and PV have received consulting fees from Boehringer Ingelheim and Merck.
Footnotes
GD, MF, DRU, SH, JSH, LP, and SJ declared no conflicts of interest.
Authors’ contributions
GEU is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. GD, MF and GEU wrote the initial research proposal. GD, MF, SH and GEU wrote the manuscript. DRU, FJP, JSH, PV, and SJ reviewed/edited the research proposal and manuscript and contributed to the discussion. LP conducted the statistical analysis.
References
- 1.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–8. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 2.Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853–61. doi: 10.4158/EP11042.OR. [DOI] [PubMed] [Google Scholar]
- 3.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17(1):107–24. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 5.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–97. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 6.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 7.Szekely A, Levin J, Miao Y, Tudor IC, Vuylsteke A, Ofner P, et al. Impact of hyperglycemia on perioperative mortality after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2011;142(2):430–437.e431. doi: 10.1016/j.jtcvs.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Ascione R, Rogers CA, Rajakaruna C, Angelini GD. Inadequate blood glucose control is associated with in-hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery. Circulation. 2008;118(2):113–23. doi: 10.1161/CIRCULATIONAHA.107.706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37(12):3001–9. doi: 10.1097/CCM.0b013e3181b083f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez CE, Mok KT, Ata A, Tanenberg RJ, Calles-Escandon J, Umpierrez GE. Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care. 2013;36(12):4091–7. doi: 10.2337/dc12-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33(8):1783–8. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97–103. doi: 10.1097/SLA.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buehler L, Fayfman M, Alexopoulos AS, Zhao L, Farrokhi F, Weaver J, et al. The impact of hyperglycemia and obesity on hospitalization costs and clinical outcome in general surgery patients. J Diabetes Complicat. 2015;29(8):1177–82. doi: 10.1016/j.jdiacomp.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8–14. doi: 10.1097/SLA.0b013e31827b6bbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Investigators N-SS. Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–9. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 18.Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, et al. Randomized Controlled Trial of Intensive Versus Conservative Glucose Control in Patients Undergoing Coronary Artery Bypass Graft Surgery: GLUCO-CABG Trial. Diabetes Care. 2015;38(9):1665–72. doi: 10.2337/dc15-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrokhi F, Smiley D, Umpierrez GE. Glycemic control in non-diabetic critically ill patients. Best Pract Res Clin Endocrinol Metab. 2011;25(5):813–24. doi: 10.1016/j.beem.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan TM. The permissive effects of glucocorticoid on hepatic gluconeogenesis. Glucagon stimulation of glucose-suppressed gluconeogenesis and inhibition of 6-phosphofructo-1-kinase in hepatocytes from fasted rats. J Biol Chem. 1984;259(12):7426–32. [PubMed] [Google Scholar]
- 21.McMahon M, Gerich J, Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988;4(1):17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- 22.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 23.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 24.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–86. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 25.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130(1):43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 26.Gresele P, Guglielmini G, De Angelis M, Ciferri S, Ciofetta M, Falcinelli E, et al. Acute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with type II diabetes mellitus. J Am Coll Cardiol. 2003;41(6):1013–20. doi: 10.1016/s0735-1097(02)02972-8. [DOI] [PubMed] [Google Scholar]
- 27.Pandolfi A, Giaccari A, Cilli C, Alberta MM, Morviducci L, De Filippis EA, et al. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol. 2001;38(2):71–6. doi: 10.1007/s005920170016. [DOI] [PubMed] [Google Scholar]


