Immunodeficiency can be associated with acute lymphoblastic leukemia (ALL) in various ways. ALL can be a feature of various primary immunodeficiencies including, but not limited, to X-linked agammaglobulinemia, chromosomal breakage disorders such as ataxia telangiectasia, and GATA2 haploinsufficiency.[1], [2], [3], [4] ALL also can be secondary to an infection such as human immunodeficiency virus. A congenital leukemia such as ALL can lead to abnormal newborn screening results for severe combined immunodeficiency.5 This could be due to dilution of naïve T cells compared with the proportion of leukemic cells. In addition, ALL can lead to immunodeficiency secondary to chemotherapy. Very few cases or case series of severe immunodeficiency with extended follow-up have been described after ALL.[6], [7]
We present a case of transient severe immunodeficiency secondary to therapy for infantile ALL with remission over time. We obtained written informed consent of the parents for this report. The patient was born by emergency cesarean section to nonconsanguineous parents at 39 weeks 4 days of gestation and noted to have leukemia cutis (blue macular rash) and a white blood cell count of 514,000/µL with 94% blasts. She had no family history of immunodeficiency or malignancies. At further evaluation she was diagnosed with congenital pre-B ALL (CD19+, CD10−/aberrant CD15+) on her first day of life. Immunophenotyping showed cells positive for CD34, CD38, CD19, CD22, HLA-DR, CD15, TdT, CD45, CD11b (8%), and CD7 (7%). Fluorescent in situ hybridization showed reciprocal translocation t(4;11) with KMT2A rearrangement, previously known as mixed lineage leukemia gene rearrangement. This rearrangement and her very young age suggested a high-risk ALL with poor prognosis.8 Cytogenetic testing was negative for trisomy 21, ETV6/RUNX1 and BCR/ABL1 gene fusion, and p16 gene deletion, and Poseidon chromosome 4 (D4Z1) and 10 (D10Z1) centromere probes showed a normal signal pattern. She was treated according to the Children's Oncology Group ALL clinical treatment trial protocol AALL0631, Arm C for high risk (age + mixed lineage leukemia rearrangement). This protocol included vincristine, daunorubicin, cyclophosphamide, cytarabine (Ara-C), asparaginase, methylprednisolone, triple intrathecal therapy (methotrexate, Ara-C, and hydrocortisone), granulocyte colony-stimulating factor, etoposide, and lestaurtinib. She was in complete remission at 1.5 months of age. She was started on continuation chemotherapy at 6 months of age with a plan to complete therapy at 2 years of age.
At 17 months of age, she developed a rash that occurred monthly and showed improvement with transient intravenous immunoglobulin therapy over the next 2 months. At 21 months, after a dose of intravenous methotrexate, her rash became significantly worse, with erythroderma of her extremities, face, and scalp with overlying thick yellow hyperkeratosis that mostly spared her trunk (Fig 1 ). Her chemotherapy was discontinued at 22 months. At 23 months, she was hospitalized for feeding intolerance, vomiting, diarrhea, and worsening skin rash and eventually transferred to a tertiary care center. During her hospital course, she had multiple infections including Klebsiella septic shock (5 months of age), methicillin-sensitive Staphylococcus aureus sepsis, Clostridium difficile colitis (owing to failure of her prolonged course of oral vancomycin, she received a fecal transplant from a parent for 2 episodes at 17 and 22 months of age), Staphylococcus epidermis conjunctivitis (21 months), CLABSI with Enterococcus fecalis, Staphylococcus epidermis, Klebsiella species, and Candida parapsilosis (23 months of age), otitis externa, and viral (coronavirus) bronchiolitis (24 months of age). Other complications included neutropenic fevers, persistent vomiting, diarrhea, hypertension, and pulmonary edema.
Figure 1.
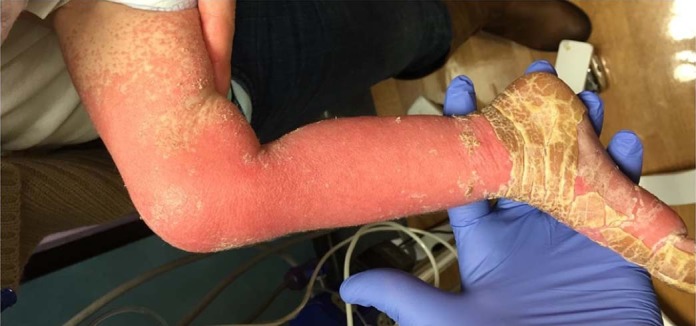
Lower extremity dermatologic findings in a patient with transient severe immunodeficiency secondary to acute lymphoblastic leukemia and its therapy.
She had an extensive workup during her hospital stay. At 22 months of age, a punch biopsy specimen of her rash showed changes compatible with subacute cytotoxic dermatitis with features of erythema multiforme and confluent upper dermal necrosis. At 23 months of age, she was noted to have hypogammaglobulinemia (immunoglobulin G 208 mg/dL) and lymphopenia (lowest absolute lymphocyte count 120/μL). She had an esophagogastroduodenoscopy and colonoscopy at 25 months of age that showed graft-vs-host disease-like findings. Her laboratory findings over the clinical course are presented in eTable 1. Telomere length studies were normal. Whole exome sequencing failed to show pathogenic variants in genes associated with severe combined immunodeficiency or other known primary immunodeficiencies or ALL. She had 3 heterozygous variants in genes that were not related to primary immunodeficiencies or ALL.
She was started on corticosteroids, intravenous immunoglobulin at 1 g/kg, and sirolimus. Clinical improvement was noticed 2 to 3 days after starting the therapy. Corticosteroids dose was tapered over few months. She was started on fungal and Pneumocystis jirovecii pneumonia prophylaxis. Her immunoglobulin therapy was switched from intravenous to subcutaneous infusions. She was monitored closely for infections. Her rash, vomiting, and diarrhea continued to show improvement. Her lymphopenia showed improvement over the next 10 months. At 32 months of age, her subcutaneous immunoglobulin was discontinued. Immune laboratory results returned to normal by 34 months of age. She is currently doing well at 4 years of age.
This case demonstrates that ALL and its therapy can be associated with complications of severe combined immune dysfunction. Prompt recognition, treatment, and supportive care can lead to recovery from transient severe immunodeficiency. Most patients with ALL have immune reconstitution after chemotherapy within 6 months.9 A study describing immune dysfunction at 6 months after therapy in 23 patients with ALL or acute myeloid leukemia noted lymphopenia in 5% and hypogammaglobulinemia in 25%.10 However, clinical immunologic features were not described in these patients.10 A case series by Geerlinks et al6 described severe immunodeficiency with leukemia, but the patients did not recover or needed hematopoietic stem cell transplantation for immune recovery. In addition, a case study of children with ALL and their immunologic status described the rate of immune recovery.7 However, none of these cases were as severe as the present case. We acknowledge the limitation of our case report. This child might have a genetic abnormality that had not yet completely manifested and could have been missed at exome sequencing. This case exemplifies that implementation of medical therapy including immunoglobulin infusions and prophylactic antimicrobial regimen with isolation of the patient can result in resolution of the severe immunodeficiency symptoms while awaiting spontaneous T-cell reconstitution without needing immune reconstitution through stem cell transplantation.
Acknowledgments
We thank the patient and her family for their cooperation.
Footnotes
Disclosures: Dr Sullivan is a consultant to the Immune Deficiency Foundation and Elsevier, has received grants (to institution) from the National Institutes of Health, and has received royalties from UpToDate.
Funding Sources:National Institutes of Health award number T32HD043021.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2017.12.023.
Supplementary Data
eTable 1.
Laboratory Findings and Timeline
| Birth | 23 months | 25 months | 27 months | 28 months | 30 months | 32 months | 34 months | 40 months | |
|---|---|---|---|---|---|---|---|---|---|
| WBCC (×103/µL) | 358.1 | 4.3 | 5.4 | 3.67 | 2.7 | 4 | 4.2 | 9.04 | 9.74 |
| ALC (×103/µL) | 3.58 | 0.12 | 0.5 | 0.6 | 0.4 | 1.2 | 1.6 | 2.32 | 3.25 |
| ANC (×103/µL) | 10.74 | 3.65 | 4.2 | 1.96 | 1.4 | 2.1 | 2.1 | 5.47 | 5.55 |
| Immunoglobulin G (mg/dL) | 612 | 208 | 1,290 | 1,560 | 1,050 | 963 | 553 | 774 | |
| CD3+ T cells (mm3) | 39 | 24 | 163 | 500 | 1,069 | 1,650 | 2,375 | ||
| CD4+ T cells (mm3) | 37 | 18 | 130 | 354 | 750 | 1,138 | 1,627 | ||
| CD8+ T cells (mm3) | 2 | 0 | 28 | 137 | 303 | 488 | 748 | ||
| CD19+ B cells (mm3) | 23 | 132 | 156 | 225 | 335 | 441 | 586 | ||
| CD16+CD56+ NK cells (mm3) | 519 | 485 | 235 | 144 | 192 | 186 | 260 | ||
| CD19+CD27+IgM− B cells (mm3) | 6 | ||||||||
| CD4+/45RA+ T cells (mm3) | 24 | 17 | 57 | 250 | 622 | 926 | 1,413 | ||
| Mitogen assay | <5% | decreased | normal | normal | normal | ||||
| Antigen assay | absent | decreased |
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; IgM, immunoglobulin M; NK, natural killer; WBCC, white blood cell count.
References
- 1.Bielorai B., Fisher T., Waldman D. Acute lymphoblastic leukemia in early childhood as the presenting sign of ataxia-telangiectasia variant. Pediatr Hematol Oncol. 2013;30:574–582. doi: 10.3109/08880018.2013.777949. [DOI] [PubMed] [Google Scholar]
- 2.Hoshino A., Okuno Y., Migita M. X-linked agammaglobulinemia associated with B-precursor acute lymphoblastic leukemia. J Clin Immunol. 2015;35:108–111. doi: 10.1007/s10875-015-0127-7. [DOI] [PubMed] [Google Scholar]
- 3.Koegel A.K., Hofmann I., Moffitt K. Acute lymphoblastic leukemia in a patient with MonoMAC syndrome/GATA2 haploinsufficiency. Pediatr Blood Cancer. 2016;63:1844–1847. doi: 10.1002/pbc.26084. [DOI] [PubMed] [Google Scholar]
- 4.van der Werff Ten Bosch J., van den Akker M. Genetic predisposition and hematopoietic malignancies in children: primary immunodeficiency. Eur J Med Genet. 2016;59:647–653. doi: 10.1016/j.ejmg.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Kwan A., Abraham R.S., Currier R. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312:729–738. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geerlinks A.V., Issekutz T., Wahlstrom J.T. Severe, persistent, and fatal T-cell immunodeficiency following therapy for infantile leukemia. Pediatr Blood Cancer. 2016;63:2046–2049. doi: 10.1002/pbc.26108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosmidis S., Baka M., Bouhoutsou D. Longitudinal assessment of immunological status and rate of immune recovery following treatment in children with ALL. Pediatr Blood Cancer. 2008;50:528–532. doi: 10.1002/pbc.21327. [DOI] [PubMed] [Google Scholar]
- 8.Cooper S.L., Brown P.A. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62:61–73. doi: 10.1016/j.pcl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Tilburg C.M., van Gent R., Bierings M.B. Immune reconstitution in children following chemotherapy for haematological malignancies: a long-term follow-up. Br J Haematol. 2011;152:201–210. doi: 10.1111/j.1365-2141.2010.08478.x. [DOI] [PubMed] [Google Scholar]
- 10.Perkins J.L., Harris A., Pozos T.C. Immune dysfunction after completion of childhood leukemia therapy. J Pediatr Hematol Oncol. 2017;39:1–5. doi: 10.1097/MPH.0000000000000697. [DOI] [PubMed] [Google Scholar]


