Abstract
We utilized meta-analysis to compare tandem autologous (TA) hematopoietic SCT (auto-HSCT) or single auto-HSCT followed by reduced intensity conditioning (RIC), allogeneic (AR) hematopoietic SCT in the upfront management of patients with multiple myeloma (MM). A comprehensive search strategy of published and unpublished reports utilized the following entry criteria: newly diagnosed patients, first autologous transplantation in both arms, use of an RIC regimen and assignment to TA or AR based exclusively on the availability of an HLA matched donor. Six trials were identified yielding 1192 subjects in TA and 630 in AR. Patients in AR had higher likelihoods of TRM (relative risk (RR) = 3.3, 95% confidence interval (CI) = 2.2–4.8) and CR (RR = 1.4, 95% CI = 1.1–1.8). OS was not different in the first 36 months (hazard ratio (HR) = 1.15, 95% CI = 0.91–1.45) or after (HR = 0.74, 95% CI = 0.53–1.04) 36 months from assignment. Similar findings were seen for PFS. When compared with TA in the upfront management of MM, AR is associated with higher TRM and CR without improvement in PFS or OS.
Keywords: meta-analysis, multiple myeloma, allogeneic transplantation, autologous transplantation, HLA
INTRODUCTION
Autologous hematopoietic SCT (auto-HSCT) has a defined role in the upfront treatment of multiple myeloma (MM).1–5 Some trials, but not all, have shown an advantage for tandem autologous (TA) transplantation over a single procedure.6,7 The main challenge remains the durability of response, and nearly all patients eventually relapse.
Allogeneic transplantation carries the potential benefit of graft vs myeloma (GVM) effect. Such effect is confirmed by the lower risk of recurrence after allogeneic transplantation when compared with autologous transplantation and the occurrence of responses to donor lymphocyte infusion.8–10
The early experience with conventional (myeloablative) allogeneic transplantation was plagued by a very high risk of TRM,5,11,12 that in some series approached 50%. RIC allogeneic transplantation may prevent some of the short-term complications associated with transplantation, reduce the risk of TRM and yet provide GVM effect, but it has limited cytoreduction potential. Therefore, the combination of a cytoreductive autologous transplantation followed shortly after by an RIC allograft has been proposed as a strategy to achieve deep remissions and long-term disease control through the GVM effect.13,14
There have been multiple prospective trials that assigned younger, newly diagnosed MM patients to TA or the combination of autologous and RIC allogeneic transplantation (AR) based on the availability of an HLA-matched donor (biological assignment). These trials yielded conflicting results so it remains unclear which strategy is preferred in younger patients with suitable donors.
We performed a systematic review of the literature and meta-analysis of all known prospective trials comparing autologous with autologous plus RIC allogeneic transplantation in newly diagnosed MM to test if the approach including RIC allogeneic transplantation provides advantage in achievement of response, PFS or OS.
MATERIALS AND METHODS
Entry criteria
We included studies meeting all the following entry criteria: prospective trial, inclusion only of newly diagnosed patients (typically after conventional induction therapy), subjects undergoing first autologous transplantation in both arms, allocation to reduced intensity allogeneic transplantation or a second autologous transplantation based exclusively on the availability or not of an HLA matched donor (biological assignment), conditioning regimen in the allogeneic transplantation arm meeting the center for international blood and marrow transplant research criteria for reduced intensity,15 and report of OS and/or PFS for both arms. EFS end points were treated as equivalent to PFS end points whenever the reported EFS definition matched the PFS definition.
Identification of studies
We utilized a comprehensive search strategy to capture all available relevant data, published or unpublished. A search in MEDLINE (PubMed) was performed on 30 September 2011 utilizing the terms ‘allogeneic’ and ‘myeloma’ or ‘allograft’ and ‘myeloma’. There was no filtering for type of publication, language, country of publication or year of publication. The manuscripts resulting from this search were manually reviewed at the title and abstract level to capture studies possibly meeting the entry criteria. The manuscripts appearing to meet the entry criteria were retrieved and fully reviewed. The reference lists of included manuscripts were also screened for published and unpublished similar trials potentially meeting the entry criteria. In addition to published trials, we manually reviewed the abstracts presented since 2001 until end of 2011 at the American Society of Hematology, American Society of Clinical Oncology, American Society of Blood and Marrow Transplantation, European Group for Blood and Marrow Transplantation and European Hematology Association Annual Meetings. For trials reported in a meeting abstract not yet followed by a published manuscript, the authors were contacted to obtain additional data not present in the body of the abstract. For trials with more than one report, all the available reports were reviewed in detail to obtain the most accurate and updated data set.
Outcomes
The primary objective of this study was comparing OS as reported for the intention to treat (ITT) population. Secondary objectives were to compare: PFS for the ITT population, OS and PFS for the population that completed the assigned treatment (CT), rate of TRM and rate of complete response to therapy (CR).
Statistical methods
The recommended summary statistics for trials with time-to-event end points are the (log transformed) HR and corresponding 95% CIs.16 Accordingly, our primary analytical objective was to construct meta-analytical HRs comparing survival experiences between autologous plus reduced intensity allogeneic transplantation and TA transplantation. It has been suggested that the excessive upfront TRM associated with allogeneic transplantation would be overcome by the reduction in the risk of late relapse.17 This phenomenon presents itself in the data by way of intersecting survival curves (that is, a violation of proportional hazards), which we observed in a number of studies. To accommodate this issue, we constructed HRs and corresponding 95% CIs for time-to-event outcomes separately for the first 36 months of follow-up (early period), and for follow-up beyond 36 months (late period). We chose to divide the analysis at 36 months since in all instances where survival curves intersected, such intersection happened at or before 36 months. Furthermore, 36 months were deemed a sufficient amount of time to capture all TRM. Finally, before analysis and to achieve data uniformity across studies, survival curves were adjusted as necessary so that time 0 corresponds to trial enrollment (for ITT analyses) or time of second transplant (for CT analyses).
For each study, we used the reported Kaplan–Meier survival curves to construct estimates of log HRs and their respective variances for both early and late follow-up periods using the method put forth by Parmar et al.16 To briefly summarize their approach as applied to our analysis, for a given study we divided the total follow-up time into consecutive, non-overlapping time intervals and, within each sub-interval, estimated the probability of survival, the number at risk and the number of events. In all studies, we assumed uniform censoring. We used sub-intervals of 3 (or 6) months in width for follow-up before (or after) 48 months, due to the sparseness of observed events beyond 48 months. We then estimated the log HR and variance within each sub-interval and combined estimates across sub-intervals to construct a study-specific log HR for early and late follow-up periods. When necessary, we aggregated sub-intervals to yield a minimum of one event in each arm, an approach that we found resulted in improved numerical stability of the estimates. Estimates were then pooled across studies to provide meta-analytic early and late follow-up HR estimates and corresponding 95% CIs for OS and PFS end points for both the ITT and CT populations.
To evaluate our method’s performance, for each study reporting an HR, we used our approach to construct a study-specific HR and corresponding 95% CI for the entire follow-up period. We then informally compared the constructed estimates with those reported in the literature to assess accuracy. The constructed estimates closely resembled those in the published reports with only slight attenuation toward the null. Ninety-five percent CIs constructed using our approach were only slightly wider than those reported in individual studies, which is expected since there is a loss of information in estimating the HR based on the Kaplan–Meier curve rather than individual patient data (Supplementary Table 1).
For the binary end points TRM and CR, data were combined across studies using fixed and random-effects meta-analytical methods to construct overall estimates of RR and their 95% CIs. For studies reporting percentages without raw frequencies, the number of patients who experienced TRM and CR was estimated from available data (reported percent and total sample size). Study heterogeneity was assessed via Cochran’s Q statistic,18 with P<0.05, indicating a random-effects model should be used to construct pooled estimates in order to account for study heterogeneity. For each end point, we reported the value of Higgin’s I2 index,19 a quantitative measure of the percent of overall variation attributable to study-to-study heterogeneity. Fixed models used the Mantel-Haenszel method20 and random-effects models followed the DerSimonian-Laird method21 for calculating RR summary estimates and 95% CIs.
Potential publication biases for all meta-analyses were assessed via funnel plots, although formal hypothesis tests were not conducted as this is not a recommended practice for meta-analyses with fewer than 10 studies.22 For all forest plots, the size of the plotting symbol for a given study is proportional to the study’s weight in that particular analysis, with higher weights implying smaller variability. Study weights are time period (early or late) specific for meta-analyses of time-to-event end points. Specifically, the weighted contribution of the same study can vary for early vs late time periods due to differing numbers of events, numbers at risk and follow-up times. If a study had no events in either the AR or TA arms in the late period, then no study-specific HR estimate was available, and that study did not contribute to the pooled estimate for that period.
For the analysis of time-to-event end points (OS and PFS), we wrote our own analysis code using R v.2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).23 For the analysis of binary end points, we used the ‘meta’ package in R.24 For all analyses, TA was the reference treatment arm.
Methodology and findings are reported in conformity with the PRISMA statement.25
RESULTS
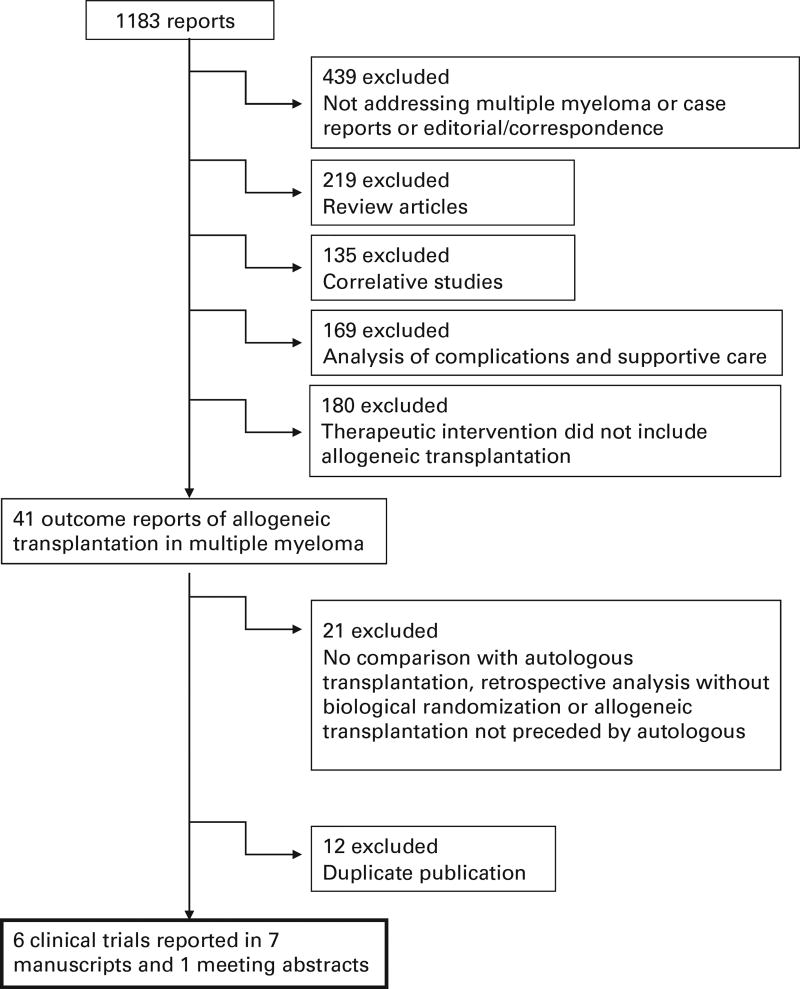
Utilizing the comprehensive search strategy as described above we identified 1183 reports in our initial screening. Figure 1 displays the reason for exclusion of the reports not meeting all the eligibility criteria. Overall, there were eight relevant reports that provided information about six clinical trials17,26–33 that met eligibility criteria for inclusion in the analysis. There were 1192 patients analyzed in the TA arm and 630 patients in the AR arm. Three trials included only patients considered as ‘high risk’.28,30,31,33 The trial reported by Krishnan et al.29 provided separate reports in the same paper for ‘high-risk’ and ‘standard-risk’ patients so it can be interpreted as two parallel trials with identical design, raising to 4 the number of trials reporting outcomes for high-risk patients, although the definition of high-risk diverged among trials (Table 1). In all but one trial, patients were assigned to allogeneic transplant only if they had an HLA identical sibling donor. The trial reported by Knop et al.28 also assigned patients with an unrelated HLA identical donor to the AR arm. In all the analyses described below, there were no clear indications of publication bias noted upon visual examination of funnel plots (Supplementary Figures 1–3).
Figure 1.
Flow chart showing the outcomes of the comprehensive search strategy to identify studies meeting entry criteria for the meta-analysis.
Table 1.
Characteristics of the studies included
| Authors | Cooperative group |
Population | Age criteria |
Follow-up (months) |
N | Median age | Conditioning | GVHD prophylaxis |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| TA | AR | TA | AR | Auto | RIC | ||||||
| Bjorkstrand | EBMT | Newly diagnosed MM patients | <70 | 61 | 249 | 108 | 57 | 54 | Mel200 | Flu + TBI 200 cGy | CSA + MMF |
| Garban, Moreau | IFM | Chr 13 del + β2 microglobulin >3 mg/L | <65 | 56 | 219 | 65 | 58 | 54 | Mel200, Mel220 | Bu + Flu + ATG | CSA + MTX |
| Giaccone, Bruno | NA | Newly diagnosed MM patients | ≤65 | 86 | 82 | 80 | 54 (mean) | 54 (mean) | Mel200, Mel100–200 | TBI 200 cGy | CSA + MMF |
| Knop | DSMM | Chr 13 del by FISH | ≤60 | 41 | 73 | 126 | 56 | 52 | Mel200 | Flu + Mel + /− ATG | NA |
| Krishnan (SR) | BMT-CTN | Not meeting criteria for high risk | ≤70 | 40 | 436 | 189 | 55 | 53 | Mel200 | TBI 200 cGy | CSA + MMF |
| Krishnan (HR) | BMT-CTN | Chr13 del by metaphase cytogenetics or β2 microglobulin >4 mg/L | ≤70 | 40 | 48 | 37 | 57 | 51 | Mel200 | TBI 200 cGy | CSA + MMF |
| Rosinol | PETHEMA | Not achieving CR/nCR after first autologous | <65 | 62 | 85 | 25 | 55 | 52 | Mel200 or CVB | Flu + Mel | CSA + MTX |
Abbreviations: AR = autologous + reduced intensity allogeneic transplantation; ATG = antithymocyte globulin; BMT-CTN = Blood and Marrow Transplant Clinical Trials Network; Bu = busulfan; CVB = cyclophosphamide, etoposide, BCNU; DSMM = Deutsche Studiengruppe Multiples Myelom; EBMT = European BMT; Flu = fludarabine; HR = high risk; IFM = Intergroupe Francophone du Myelome; Mel200 = Melphalan 200 mg/m2; MM = multiple myeloma; MMF = mycophenolate mofetil; NA, not applicable; PETHEMA = Programa para el Estudio de la Terapéutica en Hemopatía Maligna; SR = standard risk; TA = tandem autologous transplantation; TBI = total body irradiation.
Treatment-related mortality
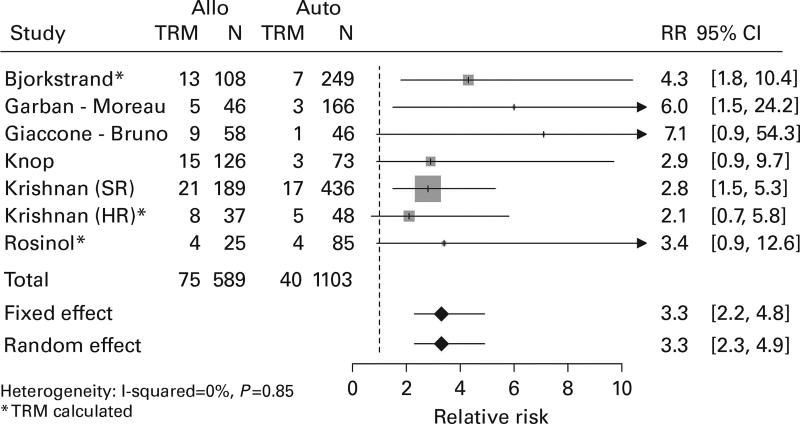
All studies provided sufficient information to contribute to the TRM analysis, and each indicated higher TRM with relative risks greater than one. Overall, patients assigned to AR had more than three times the probability of death from treatment complications than did patients assigned to TA (RR = 3.3, 95% CI = 2.2–4.8; Figure 2).
Figure 2.
Forest plot comparing TRM between the arms.
Complete response
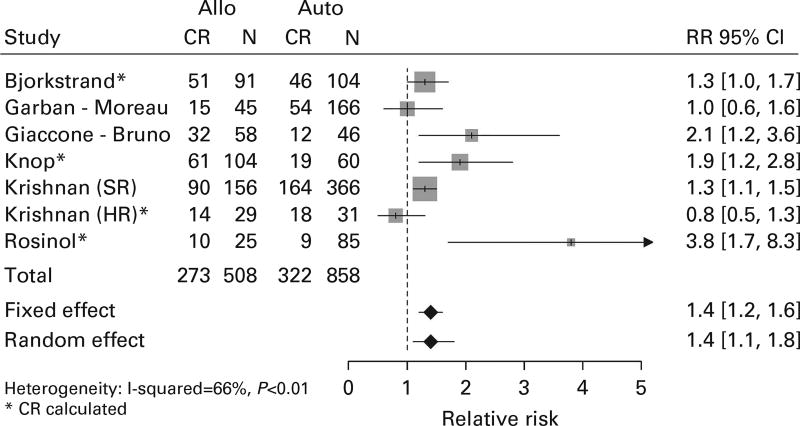
The achievement of complete response is a validated surrogate for PFS, OS and a necessary step to achieve cure in MM.34 Individual study results were mixed, with five studies indicating an improved CR rate. Overall, there was higher likelihood of obtaining CR in AR than in TA (RR = 1.4, 95% CI = 1.1–1.8; Figure 3).
Figure 3.
Forest plot comparing incidence of CR between the arms.
Progression-free survival
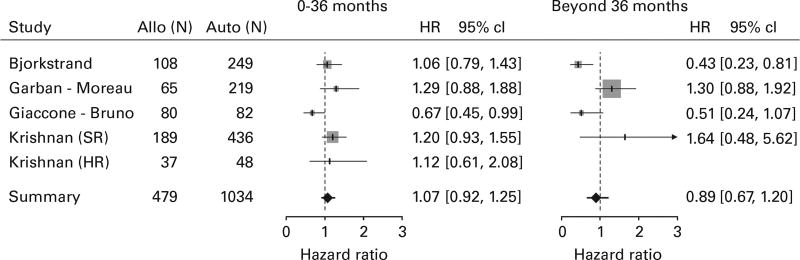
Five trials provided sufficient information for ITT PFS analysis with 1034 subjects in the TA arm and 479 subjects in the AR arm (Figure 4). Four of five studies had HR estimates greater than one in the early period, favoring the TA arm, and two of four in the late period. There was no significant difference between arms in the early period (HR = 1.07, 95% CI = 0.92–1.25) or in the late period (HR = 0.89, 95% CI = 0.67–1.20). When the same analysis was repeated for studies reporting PFS for CT population (Supplementary Figure 4), again no significant difference between arms was found in the early (HR = 0.89, 95% CI = 0.72–1.09) or late period (HR = 0.91, 95% CI = 0.71–1.16). Three of five studies had HR estimates less than one in the early period, favoring the AR arm, and three of four in the late period.
Figure 4.
Forest plot comparing PFS for the ITT population between the arms.
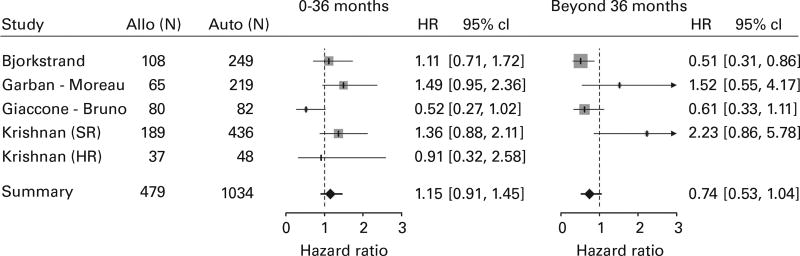
OS
Like PFS, four trials (including both standard-risk and high-risk comparisons in the study by Krishnan et al.) contributed 1034 subjects in the TA arm and 479 subjects in the AR arm for the ITT OS analysis (Figure 5). Three of five comparisons had HR estimates greater than one in the early period, favoring the TA arm, and two of four in the late period. No significant difference between the arms could be found in the risk of death of any cause in the early period (HR = 1.15, 95% CI = 0.91–1.45) or in the late period (HR = 0.74, 95% CI = 0.53–1.04). When the same comparison was made utilizing the CT population (Supplementary Figure 5), similar results were found both in the early (HR = 1.14, 95% CI = 0.79–1.64) and in the late period (HR = 0.59, 95% CI = 0.31–1.12).
Figure 5.
Forest plot comparing OS for the ITT population between the arms.
DISCUSSION
The possible superiority of autologous followed by RIC sibling allogeneic transplantation was suggested in a 2007 study by Bruno et al.26,27 Despite its relatively long follow-up, this study was met by skepticism, driven, among other factors, by the inconsistency in dose of melphalan in the conditioning regimen, the high drop-out rate between first and second transplant and the exquisitely poor performance of the control arm. A diverging result emerged from the larger comparison between the Intergroupe Francophone du Myelome (IFM) studies IFM99-03 and IFM99-0432,33 in a high-risk population, raising further questions about the appropriateness of upfront AR transplantation in MM. More recently, the results of the two largest trials addressing this question have been published. While the trial conducted by the European BMT (EBMT) centers17 found superior PFS for the autologous/RIC allogeneic arm at 60 months, the north-American Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) study29 did not find superiority for either approach in standard-risk or in high-risk patients. Of interest, the BMT-CTN trial has the highest number of patient but has been reported with relatively short follow-up. It is possible that differences may emerge in future updates. We understand that the plurality of clinical trials addressing this question, the divergence in results and conclusions, the recent report of results from large studies and the lack of ongoing trials make a meta-analysis timely and necessary.
One important trial excluded from the present analysis is the HOVON-50. In this trial, patients with newly diagnosed MM were randomized to induction with one of two different regimens (VAD or TAD) followed by auto-HSCT. After transplantation, patients were assigned to maintenance with IFN-α or thalidomide. Patients with an HLA identical sibling could be enrolled in the HOVON-54, a phase II trial of reduced intensity allogeneic transplantation without post-transplant maintenance. Even though a donor vs no donor analysis has been published,35 this trial did not meet entry criteria for the present analysis due to no intent to proceed with second auto-HSCT for the majority of patients without sibling donor, the lack of information on a systematic treatment assignment based on availability or not of a donor, and variability in time for enrollment in HOVON-54, including enrollment of patients who were already receiving maintenance therapy.35
The present study confirms the higher rate of TRM in patients assigned to AR. Nevertheless, the overall rate of TRM was only 12.7% in AR (vs 3.6% in TA), verifying that this approach is safer than fully myeloablative allogeneic transplantation in MM.5,11,12
Our study found higher likelihood of CR among subjects in AR. In fact, most trials,17,26–28 including some that did not find superiority of AR in PFS or OS,29,30 acknowledge the lower risk or disease recurrence in AR, confirming the existence of GVM effect. A formal meta-analysis of risk of relapse was not possible since this outcome was not reported for most of the trials included. The fact that this study did not find PFS superiority for AR despite higher rate of CR indicates that the GVM, although present, is insufficient to overcome the penalty of excessive TRM associated with AR, even if the first 36 months of follow-up are excluded from the analysis.
In the trials analyzed, AR is considered as experimental while TA is the standard conventional approach. The results of the present analysis do not support the adoption of the experimental approach. However, it also did not show clear superiority of TA. It is possible that a subgroup of patients with specific individual or disease-related characteristics may have a clear advantage with one of the approaches. It is well established that patients with high-risk myeloma, defined by either chromosome abnormalities, high levels of β2 microglobulin, or inadequate response to induction therapy perform poorly after TA transplantation.36,37 However, when the studies that included only patients with high-risk disease were meta-analyzed, there was still no advantage for AR in either PFS or OS (results not shown). Therefore, if there is a subgroup of patients for which the AR approach is beneficial, such subgroup remains unidentified.
Meta-analyses have limitations (for example, the risk of multiple types of bias) and are not perfect substitutes for properly designed and powered prospective trials, yet they are an invaluable source of evidence with their own set of strengths, particularly higher statistical power (than individual studies) and potentially better generalization of findings. Despite well-defined entry criteria, there are still notable dissimilarities among the included studies in regards to population included (unselected, standard risk or high risk), definition of high-risk MM, conditioning regimens utilized and strategies for GVHD prophylaxis. Two of the trials also included post TA transplantation maintenance therapy. IFM99-04 assessed maintenance with a murine anti-IL-6 MoAb, a strategy that proved itself ineffective. The BMT-CTN trial randomized patients undergoing TA transplantation to observation or thalidomide + dexamethasone maintenance, but unfortunately there was an excessively high rate of treatment discontinuation in the maintenance arm and the two arms (maintenance and observation) were merged in the analysis. Bias assessments in this meta-analysis were limited in scope and challenging to interpret due to the small number of studies and the division of time-to-event end points into two periods.
In summary, this meta-analysis of all available prospective trials with biological assignment indicates that single autologous followed by RIC allogeneic hematopoietic SCT is not superior to tandem auto-HSCT in patients with newly diagnosed MM. Substantial innovative measures are necessary to either reduce the TRM and/or enhance the GVM effect before allogeneic transplantation can be re-assessed in this setting.
Supplementary Material
Acknowledgments
The research presented in this article was supported in part by the Biostatistics Shared Resource as part of the Hollings Cancer Center at the Medical University of South Carolina which is funded by a Cancer Center Support Grant P30 CA138313.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
This study was presented at the 38th European Group or EBMT annual meeting in Geneva, Switzerland in 03 April 2012.
Supplementary Information accompanies the paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Blade J, Rosinol L, Sureda A, Ribera JM, Diaz-Mediavilla J, Garcia-Larana J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–3759. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 5.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 6.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 7.Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 8.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. [PubMed] [Google Scholar]
- 9.Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000;18:3031–3037. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 10.Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4364. doi: 10.1182/blood-2003-11-3862. [DOI] [PubMed] [Google Scholar]
- 11.Gahrton G, Tura S, Ljungman P, Belanger C, Brandt L, Cavo M, et al. Allogeneic bone marrow transplantation in multiple myeloma. European Group for Bone Marrow Transplantation. N Engl J Med. 1991;325:1267–1273. doi: 10.1056/NEJM199110313251802. [DOI] [PubMed] [Google Scholar]
- 12.Gahrton G, Svensson H, Cavo M, Apperly J, Bacigalupo A, Bjorkstrand B, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983–93 and 1994–8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–216. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 13.Kroger N, Schwerdtfeger R, Kiehl M, Sayer HG, Renges H, Zabelina T, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100:755–760. doi: 10.1182/blood-2002-01-0131. [DOI] [PubMed] [Google Scholar]
- 14.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 15.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011;29:3016–3022. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 18.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Chichester (UK): 2008. [Google Scholar]
- 23.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 24.Schwarzer G. Meta-Analysis with R. R package version 1.6-0 ed. 2010 [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 27.Giaccone L, Storer B, Patriarca F, Rotta M, Sorasio R, Allione B, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood. 2011;117:6721–6727. doi: 10.1182/blood-2011-03-339945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knop S, Liebisch P, Hebart H, Holler E, Engelhardt M, Bargou RC, et al. Allogeneic stem cell transplant versus tandem high-dose melphalan for front-line treatment of deletion 13q14 myeloma—an interim analysis of the German DSMM V Trial. ASH Annu Meet Abstr. 2009;114:51. [Google Scholar]
- 29.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, 3rd, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3593. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 31.Moreau P, Garban F, Attal M, Michallet M, Marit G, Hulin C, et al. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood. 2008;112:3914–3915. doi: 10.1182/blood-2008-07-168823. [DOI] [PubMed] [Google Scholar]
- 32.Moreau P, Hullin C, Garban F, Yakoub-Agha I, Benboubker L, Attal M, et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99-04 protocol. Blood. 2006;107:397–403. doi: 10.1182/blood-2005-06-2573. [DOI] [PubMed] [Google Scholar]
- 33.Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 34.Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–3146. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 35.Lokhorst HM, van der Holt B, Cornelissen JJ, Kersten MJ, van Oers M, Raymakers R, et al. Donor versus no donor comparison of newly diagnosed myeloma patients included in the HOVON 50 multiple myeloma study. Blood. 2012;119:6219–6225. doi: 10.1182/blood-2011-11-393801. [DOI] [PubMed] [Google Scholar]
- 36.Gertz MA, Lacy MQ, Dispenzieri A, Greipp PR, Litzow MR, Henderson KJ, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and-17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106:2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR, et al. Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood. 2010;115:2348–2353. doi: 10.1182/blood-2009-07-235531. quiz 2560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.