Abstract
Today, we understand peptide transmitters to be signaling molecules that modulate neural activity. However, in 1982, little was known about neuropeptides and their role in neural communication. The influential 1982 paper by Jan and Jan reported definitive evidence that a presynaptically-released neuropeptide evokes postsynaptic responses in an identified cholinergic synapse, thereby fueling a new era in neuroscience.
Keywords: Neuropeptides, peptide transmitters, LHRH, neurotransmission, signaling molecules
Main text
Neuropeptides are short polypeptides synthesized and secreted by neurons, whose functional and molecular diversity contribute to a wide range of modulatory effects in vertebrate and invertebrate nervous systems1. They constitute an important form of neural communication, which complements conventional neurotransmission, mediated by small molecule amino acid-based neurotransmitters, such as glutamate, glycine, and γ-aminobutyric acid (GABA). Amino acid neurotransmitters, however, are generally much better understood2,3. This stems in part from the diversity and complexity of neuropeptide-mediate transmission, but also from basic differences in terms of spatial distribution – both intracellularly and extracellularly. First, unlike amino acid neurotransmitters, synthesis and release of neuropeptides is independent of specific synaptic specialization1. Second, while amino acid neurotransmitters diffuse only tens of nanometers from their release site before being rapidly degraded, neuropeptides can diffuse multiple microns, and by targeting G-protein coupled receptors with nanomolar affinities, they can maintain long extracellular half-life1 (Fig. 1). Progress in understanding the role of neuropeptides in modulating neural function began in the 1970s and reached a turning point with the publication of Jan and Jan (1982)4. This paper presented the first conclusive, direct evidence that presynaptic activity leads to release of the neuropeptide luteinizing hormone-releasing hormone (LHRH)-like peptide, and that postsynaptic action of this neuropeptide induces a delayed, slow postsynaptic potential.
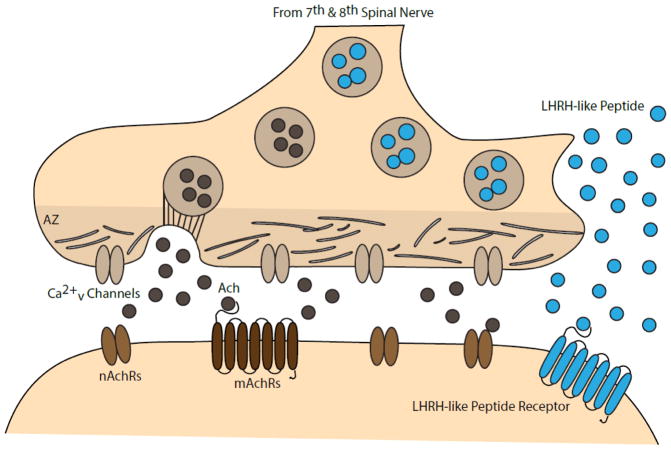
Fig. 1. Ganglionic Synapse.
Preganglionic bouton releases acetylcholine (Ach), activating nicotinic acetylcholine receptors (nAchRs) and muscarinic acetylcholine receptors (mAchRs). LHRH-like peptide is co-released, its function putatively mediated by the LHRH-like peptide receptor. According to Cao and Peng (1998), docking of acetylcholine-filled vesicles occurs at the presynaptic active zone (AZ), whereas release of LHRH-like peptide occurs away from the AZ15.
At the time of this publication, it was known that neural stimulation can evoke peptide release and that exogenous addition of neuropeptides can elicit a neural response. For example, substance P’s action as a neuromodulator was inferred from its expression pattern and from its release upon neural stimulation5. There was also evidence that the biophysical characteristics of fast, amino acid neurotransmission differed from neuropeptide-mediated modulation. For example in 1968, Nishi and Koketsu characterized a non-cholinergic, delayed, and slow postsynaptic potential that differed from the fast, excitatory postsynaptic potential (EPSP) or inhibitory postsynaptic potentials previously described6. Katayama and North (1978) showed that iontophoretic administration of substance P onto ganglion cells of guinea pig myenteric plexus induced postsynaptic depolarizing potentials with a delayed time course and lasting 10–100 seconds7. This delayed, slow postsynaptic potential became the hallmark for neuropeptide-mediated modulation and the subject of many studies. However, the precise physiological distinction between amino acid neurotransmission and neuropeptide-mediated modulation had yet to be established in specific neurons and synapses.
Jan and Jan used the bullfrog sympathetic ganglion to isolate peptidergic synapses and identify the role of LHRH-like peptide in mediating the delayed, slow postsynaptic potential4. Exploiting its electrophysiological and pharmacological accessibility, Jan and Jan used the bullfrog paravertebral sympathetic ganglion as a model system for understanding neuropeptide-mediated transmission. Preganglionic nerve fibers extend a dense network of presynaptic boutons onto ganglion cells. The high density of peptidergic synapses allowed the authors to perform reliable electrophysiological recordings of postsynaptic responses induced by preganglionic nerve stimulation. Three types of postsynaptic potentials were recorded; a fast EPSP, a slow EPSP, and a late, slow EPSP. The fast EPSP and slow EPSP were eliminated by perfusion of nicotinic and muscarinic inhibitors, respectively. In this way, the authors isolated the late, slow EPSP and demonstrated that it was elicited by a molecule other than acetylcholine.
The authors radio-labeled a high density of LHRH-like molecule found in each sympathetic ganglion and used gel filtration chromatography to demonstrate that the substance had a molecular weight of 1000 g/mol, suggesting that the molecule is a peptide whose structure closely matches mammalian LHRH protein. By quantifying radio-labeled LHRH-like peptide, the authors revealed that nerve stimulation of the preganglionic chains induced release of LHRH-like peptide in a calcium-dependent fashion. Iontophoretic injection of LHRH-like peptide onto the ganglion cell surface caused changes in membrane resistance and permeability, as well as eliciting a slow depolarization similar to the delayed, slow EPSP induced by preganglionic nerve stimulation. To determine if the electrically- and exogenous LHRH-like peptide-evoked EPSPs were the same, the authors altered the holding potential and showed that the amplitude and time constants for depolarization and decay of both EPSPs were indistinguishable. These experiments suggested, albeit indirectly, that the LHRH-like peptide secreted in response to preganglionic stimulation was the intercellular signal inducing the postsynaptic delayed, slow EPSP.
To definitively and directly test this hypothesis, the authors tested the effects of LHRH agonists and antagonists on postsynaptic potentials. Agonists of the mammalian LHRH receptor robustly increased the amplitude of the LHRH-like peptide evoked EPSP, suggesting that both mammalian LHRH and the LHRH-like peptide of the bullfrog sympathetic ganglion share a structurally-similar receptor. In the pivotal experiment of this paper, the authors tested the effect of multiple LHRH receptor antagonists on the delayed, slow EPSP. Bath application of LHRH antagonists eliminated both the electrically-stimulated EPSP and the LHRH-evoked EPSP. Not only do these results imply a shared receptor for mammalian LHRH and LHRH-like peptide, but they left no question that LHRH-like peptide does indeed mediate the late, slow EPSP at this synapse. In this elegant experiment, the authors finally closed the loop between exogenous peptide-mediated and electrically-mediated postsynaptic response in the sympathetic ganglion of the bullfrog. To explore whether other neuropeptides may be involved in triggering this late, slow EPSP, the authors applied substance P and LHRH-like peptide and showed that there was no cross-desensitization of the LHRH-like peptide and substance P induced depolarization. This indicated that substance P and LHRH-like peptide modulation function through orthogonal molecular pathways and that LHRH-like peptide is responsible for the delayed, slow EPSP induced by preganglionic electrical stimulation.
Jan and Jan proposed that in order for novel signaling molecules, such as neuropeptides, to qualify as neurotransmitters or modulators of neural communication, they must share similarities with the already-established amino acid neurotransmitters. Under this view, LHRH-like peptide could be classified as a transmitter if there was evidence that (1) it was expressed and synthesized by preganglionic boutons, (2) it was released in a calcium dependent fashion and degraded after synaptic release, (3) it evoked changes in the biophysical membrane properties of a postsynaptic cell, and (4) its actions could be regulated by agonists or antagonists. In this influential paper, Jan and Jan provided evidence that nearly all of these criteria were satisfied. Later studies definitively confirmed that LHRH-like peptide is indeed a co-transmitter with acetylcholine in the bullfrog sympathetic ganglion8,9.
Today, further work has provided insight into how the relationships between multiple neurotransmitters and neuromodulators contribute to responses of postsynaptic neurons. Examples range from the vertebrate central nervous system, including co-release of serotonin-GABA-substance P and co-release of glutamate-dopamine, to the co-transmission of pigment dispersing factor neuropeptide and acetylcholine in invertebrate circadian clock neurons. These neural circuits have benefitted from profound insight into the co-existence of neuropeptide-neurotransmitter release and the relevance of this relationship10–12. However, despite the wealth of information characterizing peptide function in various neural circuits, it lags far behind our understanding of amino acid neurotransmission in determining the functional properties of neural circuits, especially in regard to co-transmission13. For many neuropeptides, such as with peptide transmitters in the mammalian cortex, it remains unclear how their co-existence and co-release with fast-acting amino acids at a single synapse affects postsynaptic responses, and even less how this relationship contributes to the dynamics of a neural circuit13,14. With current advances in genetic targeting and manipulation, electrophysiological techniques, and optics, we are now primed to make substantial large-scale progress at addressing the functional significance of the phenomenon conclusively established by Jan and Jan over 35 years ago.
Acknowledgments
M.N.N. was supported in part by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (R01NS091070) and by the National Institute of General Medical Sciences, NIH (R01GM098931). A.D.G.-S. was supported by the Kavli Institute for Neuroscience and by NIGMS, NIH (5T32GM100884).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 3.Hughes J, et al. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- 4.Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol (Lond) 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iversen LL, Jessell T, Kanazawa I. Release and metabolism of substance P in rat hypothalamus. Nature. 1976;264:81–83. doi: 10.1038/264081a0. [DOI] [PubMed] [Google Scholar]
- 6.Nishi S, Koketsu K. Analysis of slow inhibitory postsynaptic potential of bullfrog sympathetic ganglion. J Neurophysiol. 1968;31:717–728. doi: 10.1152/jn.1968.31.5.717. [DOI] [PubMed] [Google Scholar]
- 7.Katayama Y, North RA. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978;274:387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- 8.Kuffler SW, Sejnowski TJ. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol (Lond) 1983;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones SW. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J Physiol (Lond) 1985;366:63–87. doi: 10.1113/jphysiol.1985.sp015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi C, et al. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuello AC. Co-Transmission: Proceedings of a Symposium held at Oxford during the 50th Anniversary Meeting of the British Pharmacological Society; Springer; 1982. [Google Scholar]
- 12.Broussard JI. Co-transmission of dopamine and glutamate. J Gen Physiol. 2012;139:93–96. doi: 10.1085/jgp.201110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger AJ, Mulder N, Saunders A, Sabatini BL. Cotransmission of acetylcholine and GABA. Neuropharmacology. 2016;100:40–46. doi: 10.1016/j.neuropharm.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao YJ, Peng YY. Activation of nicotinic receptor-induced postsynaptic responses to luteinizing hormone-releasing hormone in bullfrog sympathetic ganglia via a Na+-dependent mechanism. Proc Natl Acad Sci USA. 1998;95:12689–12694. doi: 10.1073/pnas.95.21.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]